Abstract
Omeprazole, a proton pump inhibitor (PPI), has widely been used to treat various gastrointestinal (GI) disorders. Notably, many clinical symptoms of GI disorders have been known to be associated with anxiety. In recent years, an exponentially increased number of subjects with abnormal ageing, neurological deficits, and psychiatric problems simultaneously exhibit GI dysfunctions as well as anxiety. Considering the fact, drugs that are used to treat GI disorders can be speculated to mitigate anxiety-related symptoms, and vice versa. Although, omeprazole treatment has been reported to result in development of anxiety and neurocognitive decline, ample reports suggest that omeprazole treatment is beneficial for the positive regulation of neuroplasticity. While underlying mechanisms of omeprazole-mediated neurological alterations remain obscure, the available scientific data on the omeprazole induced adverse effects in the brain appear to be inadequate, uncertain, and controversial. Hence, this study revisited the effect of omeprazole treatment on the degree of anxiety-like behaviours in a cysteamine hydrochloride (HCl) induced mouse model of GI disorder using open field test (OFT), light-dark box (LDB) test and elevated plus maze (EPM). Results revealed that omeprazole treatment mitigates anxiety-related behaviours in the cysteamine HCl induced animal model of GI disorder. Thus, this study assuredly supports and validates the anxiolytic properties of omeprazole. However, the adverse effects associated with inappropriate intake of omeprazole may not completely be excluded. Therefore, this study advocates the future direction in determining the long-term effects of omeprazole on the brain functions.
Keywords: Omeprazole, Anxiety, Open field test, Elevated plus maze, Proton pump inhibitor, Cysteamine hydrochloride
Omeprazole; Anxiety; Open field test; Elevated plus maze; Proton pump inhibitor; Cysteamine hydrochloride.
1. Introduction
Omeprazole is a potent proton pump inhibitor (PPI) that acts by specifically impeding the biochemical activity of the H+/K+-ATPase in the parietal cells and thereby, reducing the secretion of hydrochloric acid in the stomach [1, 2]. Considering its ulcer healing capacity in the stomach and duodenum, omeprazole has widely been implemented as an effective medication in the management of various gastrointestinal diseases including erosive esophagitis, peptic ulcers and gastrinomas [3, 4]. Besides, omeprazole has also been generally prescribed along with many antibiotics to treat infectious diseases that are known to have the risk of gastric acidity [4]. Notably, the combination of metronidazole, clarithromycin with omeprazole has been established as a highly beneficial therapeutic regime to heal ulcerative gastritis caused by Helicobacter pylori [5]. Moreover, omeprazole treatment also provides therapeutic aids against several clinical complications and surgical procedures that exhibit hemorrhagic episodes in the gastrointestinal tract [6]. Omeprazole is also used to treat clinical conditions like non-cardiac chest pain and dyspepsia regardless of esophageal erosion [7, 8]. Like most pharmacological agents, the possible side effects associated with the consumption of omeprazole include headache, nausea, vomiting, flatulence, and diarrhea. However, the clinical episodes of the side effects resulting from the omeprazole treatment appear to be very trivial and even most of the common side effects may recuperate with time. Nevertheless, some of the unstipulated adverse effects, which are caused by prolonged and improper intake of omeprazole cannot be completely ignored.
Recent reports indicated that long term and inappropriate courses of omeprazole treatment lead to neurocognitive impairments, depression and anxiety related problems [9, 10, 11, 12]. However, there exists some experimental proof that the omeprazole treatment can be beneficial in improving neurological functions [13, 14, 15, 16, 17]. Moreover, the recent outlooks strongly advocate that the available data claiming the omeprazole induced neurological defects appear to be highly subtle and inconclusive at this moment [18, 19, 20, 21]. Therefore, additional preclinical studies, epidemiological and clinical trials are required in order to determine and validate the potential relationship between omeprazole consumption and neurophysiological alterations. Among the different pharmacological aspects, understanding the effect of omeprazole on the regulation of anxiety in GI disorders has currently been an evolving scientific focus. Therefore, the present study revisited the effect of omeprazole treatment on the modulation of anxiety-related behaviours in a cysteamine HCl induced mouse model of GI disorder using animal behavioural paradigms such as open field test (OFT), light-dark box (LDB) test and elevated plus maze (EPM).
2. Materials and methods
2.1. Experimental animals and treatment
Four to five months old experimental adult BALB/c mice (N = 24) were randomly divided into four groups namely, (1) control (C) group (N = 6), (2) cysteamine HCl (CystM) group (N = 6), (3) omeprazole (OMP) group (N = 6) and (4) cysteamine HCl with omeprazole (CystM + OMP) group (N = 6). The animals were maintained in the standard condition at a temperature of about 22–24 °C in 12 hours of light and dark alternative cycles in the animal house, Bharathidasan University. Mice were provided with standard rodent feed and water of free access. The experimental animals in group 2 and 4 received intraperitoneal injection of 60 mg of cysteamine HCl per Kg body weight for 3 alternative days. Five days later animals in groups 3 and 4 were given 20 mg of omeprazole per Kg body weight through drinking water. The animals in the control group were given normal drinking water. After two weeks of omeprazole treatment, experimental mice in all groups were subjected to behavioural tests such as OFT, LDB and EPM for assessing the level of anxiety as described earlier [22]. All the animal experiments were conducted upon the approval of the Institutional Animal Ethics Committee (IAEC) (Ref NO. BDU/IAEC/P11/2019), Bharathidasan University under the regulation of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
2.2. Open field test (OFT)
OFT was executed to assess the effect of omeprazole on the general activity, exploration cum preference-based anxiety-like behaviours in the experimental animals. The OFT arena (120 cm × 120 cm) consists of 16 quadrants 30 cm each was digitally divided into the outer zone (red color) and inner zone (dark green) using the SMART 3.0 module, a video tracking software assisted with the computer (Pan lab, Harvard apparatus, Spain) (Figure 1). After habituation in the animal behavioral room, each mouse was taken from the home cage and carefully released into the center of the arena and allowed to explore freely for 5 minutes. The overall uninterrupted activities of animal were monitored and recorded using the SMART 3.0 video tracking software. Three trials of each 5 minutes were carried out for 3 consecutive days. The number of grids crossed, the time spent in the outer zone and inner zone by the mouse were assessed using SMART 3.0 tracking software. At the end of the test period, the animal was gently removed from the open arena and returned to its home cage. The open field apparatus was wiped with 70% ethanol and allowed to evaporate completely prior to every trial [22].
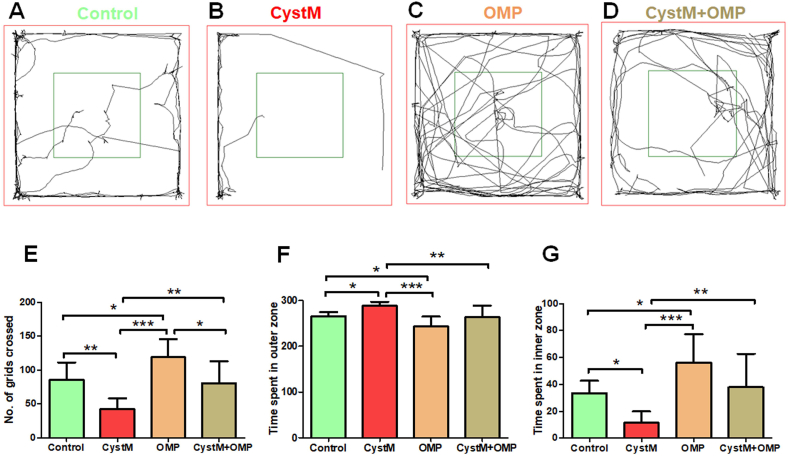
Figure 1.
Omeprazole improves exploration and reduced anxiety behaviours in cysteamine HCl treated animals in the open field arena. Representative video tracking image of an animal from the (A) control (C), (B) cysteamine HCl (CystM). (C) omeprazole (OMP) and (D) cysteamine HCl with omeprazole (CystM+OMP) treated groups.. The red square indicates the outer zone and the dark green square indicates the inner zone of the test arena. The bar graph data indicates the number of grids crossed (E) and, time spent in the outer zone (F) and inner zone (G) by the experimental mice of the control (C), cysteamine HCl (CystM), omeprazole (OMP) and cysteamine HCl with omeprazole (CystM + OMP) treated groups.
2.3. Light-dark box (LDB) test
The LDB test was applied to measure the unconditioned anxiety-responsive behaviour in experimental animals. The LDB apparatus consists of two compartments, one is the dark compartment and the other is the light compartment wherein digitally two rectangular zones were created using SMART 3.0 video tracking software. As the light compartment was marked with a red rectangle, the blue rectangle was designated to the dark compartment (Figure 2). Each animal was placed in the light compartment and allowed to move freely between the two compartments for 5 minutes. Three trials of each 5 minutes were carried out for 3 consecutive days. The time spent by each animal in the light compartment and the dark compartment was recorded and estimated by SMART 3.0 video tracking software. After each trial, the mouse was gently removed from the apparatus and replaced into their home cage and at the end, both the compartments were cleaned with 70% ethanol [22].
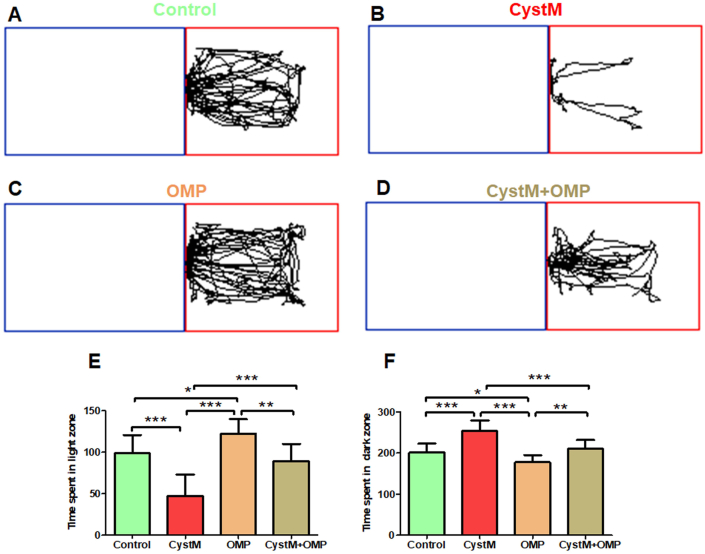
Figure 2.
Omeprazole reduces preference-based anxiety behaviours in cysteamine HCl treated animals in the light-dark box test. Representative video tracking image of an animal from the (A) control (C), (B) cysteamine HCl (CystM), (C) omeprazole (OMP) and (D) cysteamine HCl with omeprazole (CystM+OMP) treated groups in light-dark box test. The red rectangle indicates the light compartment and the blue rectangle represents the dark compartment. The bar graph data represents time spent in the light compartment (E) and the dark compartment (F) by the experimental mice in the control (C), cysteamine HCl (CystM), omeprazole (OMP) and cysteamine HCl with omeprazole (CystM + OMP) treated groups.
2.4. Elevated plus maze (EPM)
The elevated plus maze (EPM) test was used to evaluate the acrophobia-related anxiety-like behaviours in experimental animals. The apparatus consists of 4 arms connected in the middle in which 2 arms were closed by 30 cm high sidewalls and 2 open arms were left open without sidewalls. The whole four arms setup was 90 cm elevated from the ground level. Using the SMART 3.0 video tracking software, all four arms were digitally marked with different colors. Apparently, two closed arms were marked with blue and brown, and two open arms were marked in red and violet (Figure 3). Each animal was placed in the center of the apparatus and was allowed to explore the arms for a period of 5 minutes. Once the trial was completed, the animal was returned to the home cage. The arms and central area of EPM were cleaned with alcohol. Three trials of each 5 minutes were carried out for 3 consecutive days. Thus, movements and the time spent by the animal in each of the arms were recorded and calculated using the SMART 3.0 video tracking software [22].
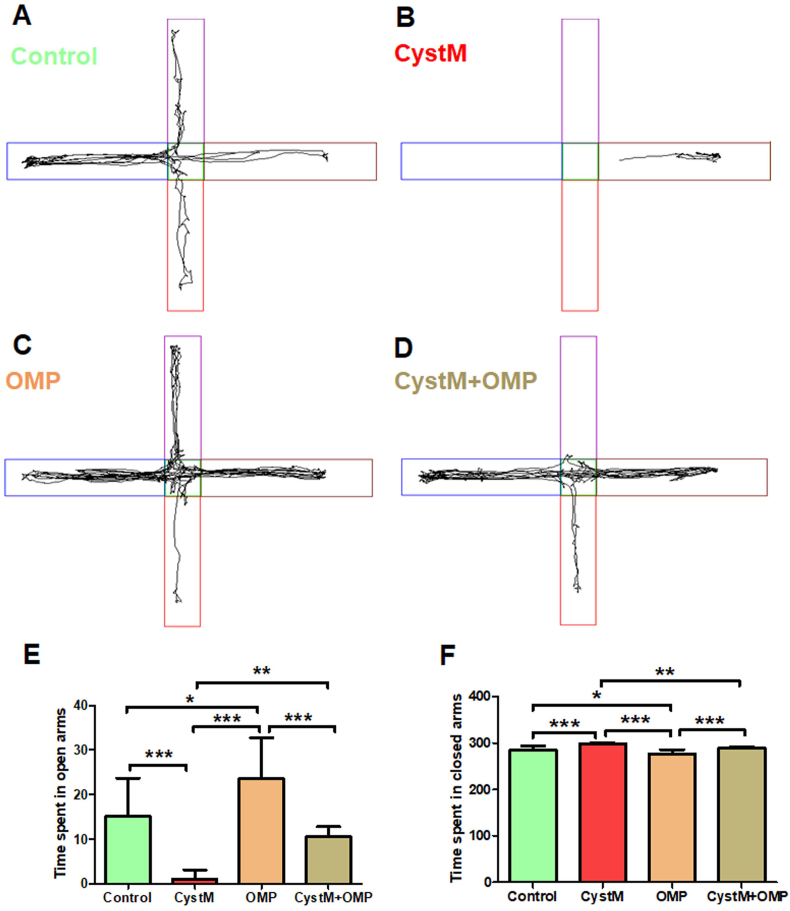
Figure 3.
Omeprazole treatment exhibit anxiolytic behaviors in cysteamine HCl treated mice in the elevated plus maze. Representative video tracking image of an animal from the (A) control (C), (B) cysteamine HCl (CystM), (C) omeprazole (OMP) and (D) cysteamine HCl with omeprazole (CystM+ OMP) treated groups during the elevated plus maze test. The open arms have been indicated by vertical red and violet rectangles and the closed arms have been indicated by horizontal blue and brown rectangles. The bar graph data represents time spent in open arms (E) and closed arms (F) by the animals in the control (C), cysteamine HCl (CystM), omeprazole (OMP) and cysteamine HCl with omeprazole (CystM + OMP) treated groups.
2.5. Statistical analysis
Results have been represented as Mean ± SD and the number of grids crossed in OFT and time spent in different zones by animals during OFT, LDB and EPM tests were assessed by one-way ANOVA followed by Tukey post-hoc comparison test. All the statistical analyses were carried out by Graph Pad Prism. The significant level was assumed at P < 0.05 unless otherwise indicated.
3. Results
3.1. Omeprazole treatment improved locomotion and reduced anxiety in cysteamine HCl treated animals in the open field test (OFT)
Results obtained from OFT revealed that the number of grids crossed by cysteamine HCl treated mice was drastically reduced when compared to mice in the control, omeprazole, cysteamine HCl with omeprazole groups. The number of grids crossed by omeprazole treated group was significantly increased when compared to that of mice in the control, cysteamine HCl and cysteamine HCl with omeprazole treated groups. Notably, the number of grids crossed by mice in the cysteamine HCl with omeprazole group was also notably increased than that of mice treated only with cysteamine HCl (C = 86 ± 26; CystM = 43 ± 16; OMP = 119 ± 27; CystM + OMP = 81 ± 33) (Figure 1). This result suggests that omeprazole treatment abolishes the cysteamine HCl induced locomotive and exploratory behavioural deficits in the experimental animals.
Considering the time spent in the outer zone of the open field arena by the experimental animals as a key measure of induced anxiety, the animals with reduced anxiety tend to explore more often the inner zone. During the open field experiment, mice in the cysteamine HCl treated group showed a tendency to stay mainly in the outer zone when compared to mice in the control, omeprazole, cysteamine HCl with omeprazole treated groups (C = 266 ± 9; CystM = 289 ± 8; OMP = 244 ± 21; CystM + OMP = 264 ± 25). As a result, the time spent in the inner zone by cysteamine HCl treated animals was considerably reduced when compared to mice in the control, omeprazole, cysteamine HCl with omeprazole treated groups. Notably, the time taken to explore the inner zone by mice in omeprazole treated group was significantly increased when compared to mice in control, cysteamine HCl and cysteamine HCl with omeprazole treated groups. Moreover, the time spent in the inner zone by cysteamine HCl with omeprazole treated mice was significantly increased than mice treated only with cysteamine HCl (C = 34 ± 9; CystM = 11 ± 9; OMP = 56 ± 21; CystM + OMP = 36 ± 25) (Figure 1). Hence, the results revealed from OFT indicate that the omeprazole treatment diminishes the cysteamine HCl induced anxiety-like behaviors in experimental animals.
3.2. Omeprazole treatment reduced unconditioned anxiety-like behavior in cysteamine HCl treated animals in the light-dark box (LDB) test
The results obtained from LDB test indicated that the time spent in the light compartment by the mice treated only with omeprazole was significantly increased than that of mice in the control, cysteamine HCl and cysteamine HCl with omeprazole treated groups (C = 99 ± 22; CystM = 47 ± 26; OMP = 123 ± 17; CystM + OMP = 89 ± 20). Notably, the time spent in the light compartment by the cysteamine HCl treated animals was significantly reduced as they preferred to stay in the dark compartment when compared to mice in control, omeprazole and cysteamine HCl with omeprazole treated groups. The time spent in the dark compartment by the mice treated only with omeprazole was considerably reduced in comparison with the cysteamine HCl and cysteamine HCl with omeprazole treated groups. Notably, mice in the cysteamine HCl with omeprazole treated group also spent more time in the light compartment than the dark compartment when compare to that of cysteamine HCl treated group (C = 201 ± 22; CystM = 253 ± 26; OMP = 177 ± 17; CystM + OMP = 211 ± 20) (Figure 2). Considering the results observed from the LDB test, it has become apparent that omeprazole treatment decreases cysteamine HCl induced anxiety-like symptoms in experimental animals.
3.3. Omeprazole treatment reduces acrophobia-related anxiety behaviors in cysteamine HCl treated animals upon the elevated plus maze (EPM) test
In EPM test, mice with reduced anxiety have been known to spend more time in exploring the open arms, whereas the animals with more anxiety will tend to stay in the closed arms. During the test, the mice in the cysteamine HCl treated group preferred to stay more time in the closed arms of EPM compared to that of mice in control, omeprazole and cysteamine HCl with omeprazole treated groups (C = 285 ± 8; CystM = 298 ± 2; OMP = 277 ± 9; CystM + OMP = 289 ± 2). Hence, the time spent by cysteamine HCl treated mice in the open arms was significantly reduced. Notably, the time spent in the open arms by mice treated only with omeprazole was significantly increased when compared to cysteamine HCl and cysteamine HCl with omeprazole treated groups. Also, the time spent in the open arms by cysteamine HCl with omeprazole treated mice was significantly increased than the mice treated only with cysteamine HCl (C = 15 ± 8; CystM = 2 ± 2; OMP = 23 ± 9; CystM + OMP = 11 ± 2) (Figure 3). Hence, the results obtained from EPM validates that the omeprazole treatment exhibits anxiolytic properties against the cysteamine HCl mediated anxiety in the experimental animals.
4. Discussion
The present study demonstrates that omeprazole treatment reduces anxiety-related behaviours in an experimental mouse model of GI disorder. Anxiety disorders are the foremost prevalent psychiatric disorders among the general population [23]. Ample scientific reports indicated that the clinical characteristics of anxiety and GI dysfunctions coexist to a more significant extent [24, 25]. To note, the pestilent symptoms of both anxiety and GI disorders have been mutually known to predispose and exacerbate the pathogenic signatures between them but the definitely combined etiology of these diseases remains obscure due to their co-morbid nature [24, 26, 27]. However, the experimental data on the shared biology and underlying synergistic pathomechanisms responsible for anxiety and GI disorders are highly limited. Indeed, it can be speculated that treatment options available for any one of the aforesaid disease conditions can be reciprocally beneficial to other. Nevertheless, in recent years, there has been a rising concern about the adverse effects of PPIs as some reports indicated that PPIs treatment has been associated with the alteration of the neuroplasticity and development of anxiety [9, 10, 11, 12].
Earlier, a case study indicated that a young girl who received omeprazole treatment along with some anticholinergic and serotonergic medications displayed alteration of the mental state [28]. Recently, Ali SB and his co-workers reported that the intraperitoneal administration of omeprazole in experimental rats induces anxiety, sedentary behavior, and neurocognitive impairments due to lowered levels of serotonin and decreased expression of the serotonin 1A receptor in the brain [9]. Interference of omeprazole with the biochemical pathways of serotonin and acetylcholine has been linked to the development of neurological defects and behavioral abnormalities [9, 29]. However, recent perspectives and meta-analyses advocate that the establishment of the association between the omeprazole and anxiety from the limited sources of data is inadequate and is of mere speculation [18, 19, 20, 21]. The available preclinical studies claim that the effect of omeprazole on the alteration of anxiety level needs to be reiterated and reproduced with appropriate experimental models. Furthermore, the detrimental effects of PPIs associated with anxiety in human situations need to be revisited in large scale assessment. Meanwhile, ample scientific records are also found for omeprazole mediating positive effects against neurological illness and neuroinflammation [13, 14, 15, 16, 17]. Chanchal SK et al. reported that two-weeks oral administration of omeprazole significantly reduced the expression of neuroinflammatory cytokines and oxidative stress in a chronic constriction injury induced rat model of neuropathic pain [13]. Omeprazole has also been reported to have antioxidant properties in different organs [30]. Özay R et al. demonstrated that omeprazole treatment has induced the antioxidant activities and reduced the apoptotic signals in the brains of the experimental rat models of traumatic brain injury [15].
Cysteamine HCl being a potent duodenal ulcerogenic agent, drastically increases the gastric acid and reduces alkaline phosphatase activity in mucosal cells that leads to induction of oxidative stress and inflammations mediating to the production of reactive oxygen species (ROS), which could be correlated to the development of anxiety in the experimental model of GI disorder [31, 32, 33]. Therefore, pharmacological neutralization of the oxidative stress and inflammations could be a formidable treatment strategy against anxiety. To note, the oral administration of omeprazole in healthy volunteers showed a reduction in the production of ROS from the activated circulating neutrophils [34, 35]. A colorimetric based study to determine the free radical scavenging activities of different PPIs indicated that omeprazole possess potent antioxidant activities [36]. Therefore, it can be speculated that omeprazole treatment might be involved in the reduction of cysteamine HCl mediated production of ROS. Recently, Yesudhas et al reported that the pharmacologically increased levels of antioxidant activities provides neuroprotection in the hippocampus and mitigates the innate anxiety-related behaviours in ageing experimental animals [22]. While omeprazole has been identified to cross the blood brain barrier (BBB), its anxiolytic effects observed in the present study could be due to induced antioxidant and neuroprotective properties in the brain [14, 16].
Serotonin plays an important role in controlling a variety of physiological functions like gut motility, mood, sleep and the brain development [37, 38]. Primarily, many anxiolytic drugs have been known to reduce the clinical severity of anxiety as they act as serotonin reuptake inhibitors [39]. Betari N and colleagues demonstrated that administration of omeprazole to experimental mice for 4 days significantly increased serotonin concentrations, both in the brain and serum, and reduced anxiety [40]. Besides, the administration of fluoxetine, a selective serotonin reuptake inhibitor type antidepressant, has been reported to reduce the central and peripheral levels of pro-inflammatory cytokines including IL-1β in a rat model of chronic mild stress (CMS) [41]. Interestingly, other antidepressants such as agomelatine and vortioxetine have been reported to promote the expression of the brain derived neurotropic factors (BDNF) in the hippocampus of experimental rats under chronic unpredictable mild stress (CUMS) condition. Considering the facts, it can be delineated that the anxiolytic effect of omeprazole may be due to its inhibitory actions on the levels of pro-inflammatory cytokines levels and supportive role in increased levels of neurotrophic factors including BDNF [42, 43]. Moreover, this study indicates that the omeprazole treatment reduces the innate level of anxiety in experimental mice during the exposure to the behavioural tasks regardless of cysteamine HCl treatment. Taken together, on one hand the anxiolytic effect of omeprazole might be related to the inhibition of neuroinflammatory molecules in the circulation and in the brain and on the other hand, it may play a supporting role in boosting the expression of neurotrophic factors and serotonin level in the brain. Therefore, future studies directed towards the underlying anxiolytic mechanisms of omeprazole at psycho-neuro-immunological aspects have become important. Further, establishment of the potential scientific link between PPIs and anxiety with large scale measurement in human situations and the interaction of omeprazole with other drugs during the combinational therapies appear to be an unmet need. The adverse effects of inappropriate intake of omeprazole may not be excluded and hence this study provokes the mandate importance of the future studies in determining the long-term effects of omeprazole at varying dosages along with other drugs.
5. Conclusion
Omeprazole has been a widely used important drug for the treatment of GI disorders. In contrast to few reports that highlighted anxiogenic events of omeprazole, the present study bestows the anxiolytic properties of omeprazole in control and a cysteamine HCl induced mouse model of GI disorder. Though the adverse effects of omeprazole might be due to the prolonged period, overdosage and the interaction with other medical parameters, a conclusion to its anxiogenic or anxiolytic properties require careful scientific attention. The underlying neurobiological alterations responsible for the behavioral outcome resulting from the omeprazole treatment needs to be reproduced and verified in different animal models of anxiety and GI disease. Therefore, the present study necessitates the future direction to reveal the effects of omeprazole on the abnormal cellular, biochemical and molecular biological aspect of anxiety.
Declarations
Author contribution statement
Harini Sri Rethinavel, Divya Bharathi Selvaraj and Jemi Feiona Vergil Andrews: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sathya Jeevitha Balakrishnan and Jerly Helan Mary Joseph: Analyzed and interpreted the data; Wrote the paper.
Mahesh Kandasamy: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Rashtriya Uchchatar Shiksha Abhiyan (RUSA) 2.0, Biological Sciences, Bharathidasan University (TN RUSA: 311/ RUSA (2.0) / 2018 dt. 02/12/2020). Mahesh Kandasamy has been supported by University Grants Commission-Faculty Recharge Programme (UGC-FRP), New Delhi, India. Divya Bharathi Selvaraj is the recipient of RUSA 2.0 project fellowship (Ref.No.BDU/RUSA 2.0/TRP/BS/Date 22/04/2021).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors acknowledge UGC-SAP and DST-FIST for the infrastructure of the Department of Animal Science, Bharathidasan University.
References
- 1.Shin J.M., Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J. Neurogastroenterol. Motil. 2013;19:25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin J.M., Sachs G. Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008;10:528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarpignato C., Gatta L., Zullo A., Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases – a position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah N., Gossman W. StatPearls. StatPearls Publishing; Treasure Island (FL): 2022. Omeprazole. (accessed March 12, 2022) [Google Scholar]
- 5.Yousfi M.M., el-Zimaity H.M., al-Assi M.T., Cole R.A., Genta R.M., Graham D.Y. Metronidazole, omeprazole and clarithromycin: an effective combination therapy for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1995;9:209–212. doi: 10.1111/j.1365-2036.1995.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 6.Lau J.Y., Sung J.J., Lee K.K., Yung M.Y., Wong S.K., Wu J.C., Chan F.K., Ng E.K., You J.H., Lee C.W., Chan A.C., Chung S.C. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N. Engl. J. Med. 2000;343:310–316. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 7.Chambers J., Cooke R., Anggiansah A., Owen W. Effect of omeprazole in patients with chest pain and normal coronary anatomy: initial experience. Int. J. Cardiol. 1998;65:51–55. doi: 10.1016/s0167-5273(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 8.Talley N.J., Meineche-Schmidt V., Paré P., Duckworth M., Räisänen P., Pap A., Kordecki H., Schmid V. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies) Aliment. Pharmacol. Ther. 1998;12:1055–1065. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 9.Ali S.B., Mahmood K., Saeed R., Salman T., Choudhary M.I., Haleem D.J. Elevated anxiety, hypoactivity, memory deficits, decreases of brain serotonin and 5-HT-1A receptors expression in rats treated with omeprazole. Toxicol. Res. 2021;37:237–248. doi: 10.1007/s43188-020-00060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laudisio A., Antonelli Incalzi R., Gemma A., Giovannini S., Lo Monaco M.R., Vetrano D.L., Padua L., Bernabei R., Zuccalà G. Use of proton-pump inhibitors is associated with depression: a population-based study. Int. Psychogeriatr. 2018;30:153–159. doi: 10.1017/S1041610217001715. [DOI] [PubMed] [Google Scholar]
- 11.Novotny M., Klimova B., Valis M. PPI long term use: risk of neurological adverse events? Front. Neurol. 2019;9:1142. doi: 10.3389/fneur.2018.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y.-H., Wintzell V., Ludvigsson J.F., Svanström H., Pasternak B. Proton pump inhibitor use and risk of depression and anxiety in children: nationwide cohort study. Clin. Transl. Sci. 2022 doi: 10.1111/cts.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanchal S.K., Mahajan U.B., Siddharth S., Reddy N., Goyal S.N., Patil P.H., Bommanahalli B.P., Kundu C.N., Patil C.R., Ojha S. In vivo and in vitro protective effects of omeprazole against neuropathic pain. Sci. Rep. 2016;6 doi: 10.1038/srep30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danzer M., Jocic M., Samberger C., Painsipp E., Bock E., Pabst M.-A., Crailsheim K., Schicho R., Lippe I.T., Holzer P. Stomach-brain communication by vagal afferents in response to luminal acid backdiffusion, gastrin, and gastric acid secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G403–411. doi: 10.1152/ajpgi.00308.2003. [DOI] [PubMed] [Google Scholar]
- 15.Özay R., Türkoğlu M.E., Gürer B., Dolgun H., Evirgen O., Ergüder B.İ., Hayırlı N., Gürses L., Şekerci Z. The protective effect of omeprazole against traumatic brain injury: an experimental study. World Neurosurg. 2017;104:634–643. doi: 10.1016/j.wneu.2017.04.136. [DOI] [PubMed] [Google Scholar]
- 16.Shin S.-H., Park Y., Park M.-H., Byeon J.-J., ill Lee B., Choi J., Shin Y.G. Profiling and identification of omeprazole metabolites in mouse brain and plasma by isotope ratio-monitoring liquid chromatography-mass spectrometric method. Life. 2020;10:115. doi: 10.3390/life10070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashioka S., Klegeris A., McGeer P.L. Proton pump inhibitors reduce interferon-γ-induced neurotoxicity and STAT3 phosphorylation of human astrocytes. Glia. 2011;59:833–840. doi: 10.1002/glia.21157. [DOI] [PubMed] [Google Scholar]
- 18.Azhar M., Fiedler L., Espinosa P.S., Hennekens C.H. Proton pump inhibitors and risk of dementia: a hypothesis generated but not adequately tested. Am. J. Alzheimers Dis. Other Demen. 2021;36 doi: 10.1177/15333175211062413. 15333175211062412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lochhead P., Hagan K., Joshi A.D., Khalili H., Nguyen L.H., Grodstein F., Chan A.T. Association between proton pump inhibitor use and cognitive function in women. Gastroenterology. 2017;153:971–979.e4. doi: 10.1053/j.gastro.2017.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen L.H., Lochhead P., Joshi A.D., Cao Y., Ma W., Khalili H., Rimm E.B., Rexrode K.M., Chan A.T. No significant association between proton pump inhibitor use and risk of stroke after adjustment for lifestyle factors and indication. Gastroenterology. 2018;154:1290–1297.e1. doi: 10.1053/j.gastro.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novotny M., Klimova B., Valis M. PPI long term use: risk of neurological adverse events? Front. Neurol. 2018;9:1142. doi: 10.3389/fneur.2018.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yesudhas A., Radhakrishnan R.K., Sukesh A., Ravichandran S., Manickam N., Kandasamy M. BOTOX® counteracts the innate anxiety-related behaviours in correlation with increased activities of key antioxidant enzymes in the hippocampus of ageing experimental mice. Biochem. Biophys. Res. Commun. 2021;569:54–60. doi: 10.1016/j.bbrc.2021.06.071. [DOI] [PubMed] [Google Scholar]
- 23.Bandelow B., Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015;17:327–335. doi: 10.31887/DCNS.2015.17.3/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roohafza H., Bidaki E.Z., Hasanzadeh-Keshteli A., Daghaghzade H., Afshar H., Adibi P. Anxiety, depression and distress among irritable bowel syndrome and their subtypes: an epidemiological population based study. Adv. Biomed. Res. 2016;5:183. doi: 10.4103/2277-9175.190938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones J.D., Dominguez B., Bunch J., Uribe C., Valenzuela Y., Jacobs J.P. A bidirectional relationship between anxiety, depression and gastrointestinal symptoms in Parkinson’s disease. Clin. Park. Relat. Disord. 2021;5 doi: 10.1016/j.prdoa.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah E., Rezaie A., Riddle M., Pimentel M. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2014;27:224–230. [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammad S., Chandio B., Soomro A.A., Lakho S., Ali Z., Ali Soomro Z., Shaukat F. Depression and anxiety in patients with gastroesophageal reflux disorder with and without chest pain. Cureus. 2019;11 doi: 10.7759/cureus.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerardi D.M., Murphy T.K., Toufexis M., Hanks C. Serotonergic or anticholinergic toxidrome: case report of a 9-year-old girl. Pediatr. Emerg. Care. 2015;31:846–850. doi: 10.1097/PEC.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R., Kumar A., Nordberg A., Långström B., Darreh-Shori T. Proton pump inhibitors act with unprecedented potencies as inhibitors of the acetylcholine biosynthesizing enzyme-A plausible missing link for their association with incidence of dementia. Alzheimers Dement. J. Alzheimers Assoc. 2020;16:1031–1042. doi: 10.1002/alz.12113. [DOI] [PubMed] [Google Scholar]
- 30.Lapenna D., de Gioia S., Ciofani G., Festi D., Cuccurullo F. Antioxidant properties of omeprazole. FEBS Lett. 1996;382:189–192. doi: 10.1016/0014-5793(96)00155-x. [DOI] [PubMed] [Google Scholar]
- 31.Puscas I., Coltau M., Maghiar A., Domuta G. Cysteamine, the most potent ulcerogenic drug known so far, powerfully activates carbonic anhydrase I, II and IV. In vitro and in vivo studies. Exp. Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 2000;52:431–435. [PubMed] [Google Scholar]
- 32.Japundzić I., Levi E. Mechanism of action of cysteamine on duodenal alkaline phosphatase. Biochem. Pharmacol. 1987;36:2489–2495. doi: 10.1016/0006-2952(87)90521-1. [DOI] [PubMed] [Google Scholar]
- 33.Szabo S. Duodenal ulcer disease. Animal model: cysteamine-induced acute and chronic duodenal ulcer in the rat. Am. J. Pathol. 1978;93:273–276. [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki M., Mori M., Miura S., Suematsu M., Fukumura D., Kimura H., Ishii H. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radic. Biol. Med. 1996;21:727–731. doi: 10.1016/0891-5849(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 35.Zedtwitz-Liebenstein K., Wenisch C., Patruta S., Parschalk B., Daxböck F., Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit. Care Med. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Abed M.N., Alassaf F.A., Jasim M.H.M., Alfahad M., Qazzaz M.E. Comparison of antioxidant effects of the proton pump-inhibiting drugs omeprazole. Esomeprazole, Lansoprazole, Pantoprazole, and Rabeprazole, Pharmacology. 2020;105:645–651. doi: 10.1159/000506232. [DOI] [PubMed] [Google Scholar]
- 37.Berger M., Gray J.A., Roth B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banskota S., Ghia J.-E., Khan W.I. Serotonin in the gut: blessing or a curse. Biochimie. 2019;161:56–64. doi: 10.1016/j.biochi.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Chu A., Wadhwa R. StatPearls. StatPearls Publishing; Treasure Island (FL): 2022. Selective serotonin reuptake inhibitors. (accessed March 12, 2022) [PubMed] [Google Scholar]
- 40.Betari N., Sahlholm K., Morató X., Godoy-Marín H., Jáuregui O., Teigen K., Ciruela F., Haavik J. Inhibition of tryptophan hydroxylases and monoamine oxidase-A by the proton pump inhibitor, omeprazole-in vitro and in vivo investigations. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.593416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y., Ho C.S., Liu X., Chua A.N., Wang W., McIntyre R.S., Ho R.C. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Y., Ho C.S., McIntyre R.S., Wang W., Ho R.C. Agomelatine-induced modulation of brain-derived neurotrophic factor (BDNF) in the rat hippocampus. Life Sci. 2018;210:177–184. doi: 10.1016/j.lfs.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y., Ho C.S., McIntyre R.S., Wang W., Ho R.C. Effects of vortioxetine and fluoxetine on the level of Brain Derived Neurotrophic Factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain Res. Bull. 2018;142:1–7. doi: 10.1016/j.brainresbull.2018.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.