Abstract
The interest in nutritional strategies that may counteract the deleterious oxidative effects induced by strenuous exercises is remarkable. Herein, the impact of white tea (Camellia sinensis) (WT), a polyphenol-rich beverage, on antioxidant status in endurance-trained rats after one session of exhaustive exercise were evaluated. Male Wistar rats were divided into groups, which received: control groups - water, and testing groups - WT1 (0.25%; w/v) or WT2 (0.5%; w/v). Drinks were consumed, ad libitum, for 5 or 10 weeks, concomitantly with the running training. Exhaustive running tests were applied before and after the experimental periods. WT intake increased the serum antioxidant capacity of rats in a dose-dependent manner (P < 0.001), which was unaccompanied by the activity of endogenous antioxidant enzymes SOD, GPx, and GR, and GSH content. Inflammatory markers in serum [IL-1β (P = 0.004) and IL-6 (P = 0.001)] could be downregulated by tea intake. In liver tissue, lower levels of lipid oxidation (P < 0.05) and improved antioxidant defenses (SOD, GPx, GR, and GSH, P < 0.05) were related to the consumption of WT in both doses, supporting protective effects in this responsible metabolic organ. In conclusion, long-term consumption of WT could be a promising adjuvant to exercise-stress management, emphasizing its ability to regulate antioxidant responses and prevent oxidative tissue damage.
Keywords: Camellia sinensis, Antioxidants, Oxidative stress, Phenolic compounds, Performance, Exercise
Graphical abstract
Highlights
-
•
White tea intake improved antioxidant status of blood and liver of runner rats.
-
•
White tea intake promoted protective effect against liver lipid peroxidation after an exhaustive exercise.
-
•
Long term white tea intake did not enhance physical performance.
1. Introduction
Reactive oxygen and nitrogen species (RONS) are unstable atoms and molecules which can easily react with biocompounds leading to oxidative damage and total loss of cellular function. Exhaustive sessions of physical exercise or even intense training load allied to periods of insufficient recovery for athletes result in a non-negligible RONS generation ratio (Peake et al., 2007; Vezzoli et al., 2016). Excessive exercise-induced oxidative stress may increase the athlete's vulnerability to delayed fatigue, inflammation, infections, and overtraining syndrome, which cause the attention to sports medicine (Fatouros et al., 2010; Tanskanen et al., 2010; Radak et al., 2017). In endurance exercises, RONS are produced through the aerobic cellular metabolism in significant amounts due to the increased oxygen consumption. Additionally, the exercise-induced muscle damage caused by the mechanical disruption of the myofibrils structure triggers immune cells that release pro-inflammatory cytokines and RONS (Haramizu et al., 2013; Mason et al., 2016). In its turn, RONS exacerbates muscle damage and attacks other tissues by oxidative reactions, and triggers more immune cells. RONS may also influence pro-inflammatory cytokine production because activated redox-sensitive signal transduction pathways show a close relationship between inflammation and oxidative stress (Peake et al., 2007).
It is hypothesized that antioxidant and anti-inflammatory nutritional strategies may help accelerate or support the recovery after exercise sessions and contribute to improving performance (Haramizu et al., 2013; Buchwald-Werner et al., 2018; Bowtell and Kelly, 2019). Despite the popular use of antioxidant supplements, the position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports for physically active individuals is the intake of a diet based on natural antioxidant-rich foods due to an extensive body of pieces of evidence with no reported adverse effects on performance parameters (Pingitore et al., 2015; Close et al., 2016; Thomas et al., 2016). Furthermore, previous studies have reported that polyphenols-rich foods may work in favor of keeping body redox homeostasis by suppressing RONS directly, preventing oxidation of biomolecules, or even by up-regulating the endogenous antioxidant defenses (Liu et al., 2017; Bowtell and Kelly, 2019). These defenses include enzymes like superoxide dismutase [SOD], catalase [CAT], glutathione peroxidase [GPx], glutathione reductase [GR], and other antioxidant compounds like reduced glutathione [GSH]. Often, modulating cellular anti-oxidative systems also reduces the oxidative stress-induced inflammatory response (Cazarin et al., 2015; Peña-Oyarzun et al., 2018).
In this way and thinking about the polyphenols-rich foods, the most ingested drink in the world (excluding water) is tea (Camellia sinensis (L.) Kuntze), represented by black tea in the first place. White tea (WT) differs from other tea types by being produced from young shoots and leaves of the plant, which undergoes minimal processing (Alcazar et al., 2007). Several biologic properties of WT, such as antioxidant, anti-inflammatory, anti-viral, anti-carcinogenic, and DNA protection, are attributed to its high content of polyphenols, especially catechins (Hajiaghaalipour et al., 2015; Pastoriza et al., 2017; Sanlier et al., 2018), besides gallic acid is also present in large amounts (Damiani et al., 2014; Lin et al., 2017).
Although WT is one of the less-studied varieties of tea, some studies retracted higher antioxidant effects to WT than green tea, leading to an increased search for this tea type (Koutelidakis et al., 2009; Pastoriza et al., 2017). However, there are no reports in the literature about WT as an antioxidant dietary strategy in the sports context to the best of our knowledge. Therefore, the present study aimed to evaluate the influence of long-term WT intake on oxidative and inflammatory parameters associated with strenuous exercise in trained rats.
2. Materials and methods
2.1. Camellia sinensis and aqueous extract preparation
The plant (C. sinensis) product was purchased from a local market in São Paulo-SP, Brazil. The WT was prepared using 1g of the dried plant subjected to infusion in 200 mL of previously boiled water (or 0.5%; w/v) and homogenized over 15 min using a magnetic stirrer and vacuum-filtered through qualitative filter paper. For each analysis, extractions were carried out in triplicate. In addition, teas were prepared every two days to offer to the animals based on previous WT antioxidant stability tests performed in our laboratory.
2.1.1. Spectrophotometric analysis of white tea bioactive compounds and antioxidant capacity
All the analyses were performed in the Synergy HT microplate reader (BioTek, Winooski, USA), which was coupled to the Gen5™ data software program (version 2.0). With adaptations, the total reducing capacity was determined using the Folin–Ciocalteu reagent described by Swain and Hillis (1959). Briefly, WT or standard solutions were mixed with distilled water and Folin–Ciocalteau reagent and allowed to rest for 3 min, followed by the addition of a 20% (w/v) sodium carbonate solution. The mixture was kept in the dark for 120 min. The absorbance was measured at 725 nm, and the results were expressed in gallic acid equivalents (mg GAE/L).
The oxygen radical absorbance capacity (ORAC) assay was carried out by adding WT or standard solutions, fluorescein, and AAPH to black microplates. Phosphate buffer (pH 7.4) was used as a diluent, and Trolox was used as standard. The readings were done in the following fluorescent filters: excitation wavelength at 485 nm and emission wavelength at 520 nm; for 80 min every 1 min, at 37 °C (Ou et al., 2013). Results were expressed as Trolox equivalent (μmol TE)/L.
The ferric reducing antioxidant power (FRAP) assay was performed according to Benzie and Strain (1996). In brief, WT or standard solutions was mixed with working FRAP reagent [acetate buffer, TPTZ in an HCl solution, and FeCl3] in a microplate, incubated in the dark for 30 min at 37 °C, cooled to room temperature (24 °C) and then absorbance was read at 595 nm. Results were expressed in Trolox equivalent (μmol TE)/L.
2.1.2. Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analyses of white tea
A stock solution at 1 g/L of each standard was prepared and diluted in dimethyl sulfoxide. For the calibration curve, an intermediate solution (1 μg/mL) was obtained by diluting the stock solution of each standard in ascorbic acid (2.5 mg/mL) + EDTA (0.5 mg/mL)/MeOH (1:1). This diluent was chosen as previously described in the literature due to its antioxidant and metal scavenger properties, retarding standard degradation (Zhou et al., 2003). Then, six calibration points were diluted with the same diluent, containing 1.0, 10.0, 25.0, 50.0, 75.0, and 100.0 ng/mL of each standard. The samples were diluted appropriately with the concentration in ascorbic acid (1.25 mg/mL) + EDTA (0.25 mg/mL), mixed for 1 min, and centrifuged for 5 min at 10000 rpm before being injected into the UPLC-MS/MS system.
The LC-MS/MS analysis was carried out using an ultra-performance liquid chromatography system (Waters Xevo I-Class, Milford, USA) coupled to a tandem mass spectrometry detector with an electrospray source ionization (ESI) in positive mode. The compounds separation was carried out on a BEH C18 column (50 mm × 2.1 mm x 1.7 μm, Waters, Milford, USA) at a 0.8 mL/min flow rate. The mobile phase consisted of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in methanol). The linear gradient was from 2 to 100% of solvent B over 3.5 min, and the column oven was kept at 40 °C. The instrument control and data processing were performed by MassLynx software (Waters Co.) version 4.1. Samples were analyzed in MRM mode, according to parameters described in Table S1 (Supplementary material). The following parameters were set for the MS operating conditions: capillary voltage 2.0 kV, source temperature 150 °C, desolvation temperature 550 °C, cone gas flow 20 L/h and desolvation gas flow 900 L/h. All sample solutions were prepared and analyzed in triplicate.
2.2. In vivo experiment
2.2.1. Animals and diet
The experiment was previously approved by the Ethics Committee on Animal Experiments (CEUA/UNICAMP #3 595-1/14, Campinas-SP, Brazil). All procedures followed the Guide for the Care and Use of Laboratory Animals and the institutional ethical guidelines. In addition, all sections of this report adhere to the ARRIVE Guidelines for reporting animal research.
Sixty-three male Wistar rats (Rattus norvegicus, 3-weeks old) obtained from the Multidisciplinary Center for Biological Research at UNICAMP (CEMIB) were maintained in individual cages under controlled conditions of temperature (22 °C ± 2), humidity (60–70%), and inverted light-dark cycle (12/12 h). Food (commercial diet for rodents - Nuvilab®) and drink were supplied under free access throughout the experimental period, and the intake was monitored three times a week and the weight gain once a week. The tea offered to the animals was prepared every two days based on previous WT antioxidant stability tests in our laboratory.
2.2.2. Treadmill training and exhaustive test protocols
The exercise protocol used was adapted from Hohl et al. (2009), which developed an exercise's impact rat model considering it would not be appropriate to use human subjects for these studies. A motorized treadmill without inclination containing 12 individual lanes was used in a dark room with minimum light. A shock grid at the back of the treadmill provided a mild shock (1.5 mA) if the rat's pace went below the treadmill rate. Animals were subjected to 10 experimental weeks of running training, divided into two phases of 5 weeks each, as shown in Table 1. Phase 1 consisted of an acquisition period to improve fitness, characterized by a progressive increase in speed and running time, while in phase 2, the pacing parameters remain constant. Thus, we distinguish three groups of experiments based on training time: time 0 (T0), time 5 (T5), and time 10 (T10). T0 was not subjected to the training protocol, and it was used as the control group, while T5 and T10 experiments represent phase 1 concluded and phase 1 + phase 2 concluded, respectively. The animals that refused to run during the experiment were excluded from the study (n = 5).
Table 1.
Exercise training protocol.
| Experimental weeks | Exercise training phases | Running speed (m/min) | Running time (min) | Number of daily sessions | Number of weekly sessions |
|---|---|---|---|---|---|
| 0 | Acclimation | 12.0 | 10 | 1 | 2 |
| 1 | 1 | 14.0 | 20 | 1 | 3 |
| 2 | 1 | 15.5 | 30 | 1 | 3 |
| 3 | 1 | 17.0 | 40 | 1 | 3 |
| 4 | 1 | 18.5 | 50 | 1 | 3 |
| 5 | 1 | 20.0 | 60 | 1 | 3 |
| 6–10 | 2 | 20.0 | 60 | 1 | 3 |
An exhaustive running test was applied at the end of each training period (T0, T5, and T10), always 72 h after the last training session, with animals euthanized immediately afterward. The test started at an initial speed of 12 m/min, and it was increased by 1 m/min every 2 min until it reached 20 m/min. After that, the speed was increased by 2 m/min every 3 min until the animals reached exhaustion, which was defined as when animals were unable to sustain the exercise, and then it was rescued. Time to reach exhaustion was recorded and expressed in min. For the tests, the animals were randomly selected and received a different identification to ensure the blinding evaluation by the researchers.
2.2.3. Experimental groups
The tea was prepared in two concentrations to be tested in the animal experiment: 0.25% (w/v) of WT and 0.5% (w/v) of WT, which were named White tea 1 (WT1) and White tea 2 (WT2), respectively.
After acclimatization on the treadmill, the rats were randomly distributed into four groups accordingly to Fig. 1 and appropriately ordered in close cages. The WT1 and WT2 groups received tea instead of water throughout the experimental period. Sedentary control (SC) groups were not subjected to the training protocol, just to the acclimatization on the treadmill (Fig. 1). This group was considered to ensure that the growth and maturation stage of the rats did not influence the results. All groups performed the exhaustive running test immediately before euthanasia.
Fig. 1.
Overview of experimental design. T0 experiment consists in one subgroup (n = 7): SC; T5 experiment consists in four subgroups (n = 7 each): SC, EC, WT1 and WT2; and T10 experiment consist in four subgroups (n = 7 each): SC, EC, WT1 and WT2. SC: Sedentary control group; EC: Exercise control group; WT1: Exercise training + WT1 group; WT2: Exercise training + WT2 group.
2.2.4. Biologic sampling
The animals were anesthetized intraperitoneally with ketamine chloride and xylazine chloride (300 and 30 mg/kg, respectively), and euthanized by exsanguination through cardiac puncture. Blood samples were collected in tubes lacking EDTA and centrifuged at 3,000 g for 15 min to obtain serum. The whole liver, heart, gastrocnemius, and soleus muscles were immediately removed after euthanasia, cleaned with saline solution (0.9%), and weighed. Samples were stored at −80 °C until analyses. The liver homogenate was prepared in a 50 mmol/L phosphate buffer (pH 7.4) solution using a manual homogenizer (MA102/Mini, Marconi, Piracicaba, SP, Brazil). The protein concentrations were determined using the Bradford method (Bradford, 1976).
2.3. Antioxidant analyses in serum and liver
All the analyses were performed using a spectrophotometer (Synergy HT, Biotek, Winooski, VT, USA) and Gen5™ 2.0 data analysis software.
2.3.1. Total antioxidant potential in serum
Serum proteins were precipitated using ethanol: deionized water (2:1, v/v) and 0.75 mol/L metaphosphoric acid, previously the antioxidant analysis (Leite et al., 2011). The hydrophilic ORAC assay was performed as previously described from WT (see section 1.1.1.).
2.3.2. Endogenous antioxidant systems in serum and homogenate liver
SOD activity was quantified using the method described by Winterbourn et al. (1975). Samples and working solution (0.1 mmol hypoxanthine, 0.07 U xanthine oxidase, and 0.6 mmol NTB in PB at 1:1:1 proportions) were added to a microplate, and the kinetic reaction was monitored at 560 nm for 10 min.
GR activity was measured by monitoring the decrease in absorbance at 340 nm for 10 min, according to Carlberg and Mannervik (1985). GPx activity was determined as described by Flohé and Günzler (1984). The sample was mixed with reduced glutathione (10 mmol/L), NADPH (4 mmol/L), and GR (1 U), and the decrease in absorbance was monitored after the addition of H2O2 (0.25 mmol/L) at 365 nm for 10 min.
Reduced thiol (GSH) content was determined in serum and liver homogenate protein-free using DTNB (5,5′- dithiobis-(2-nitrobenzoic acid)), the Ellman's reagent (Ellman, 1959). GSH was used as the external standard, and colors' samples were read at 412 nm.
2.3.3. Lipid peroxidation
The thiobarbituric acid reactive substances (TBARS) level in the liver was measured according to the method described by Ohkawa et al. (1979), with some modifications. First, the liver homogenate was mixed with 8.1% sodium dodecyl sulfate (SDS) and a working reagent (TBA, 20% acetic acid, and 5% sodium hydroxide) using a vortex. After 60 min of heating (95 °C), samples were chilled in ice and then centrifuged at 10,000 g for 10 min (4 °C). The absorbance of the supernatant was read at 532 nm in a microplate using 1.1.3.3-tetraethoxypropane as standard.
2.4. Inflammatory biomarkers
The interleukin (IL) 6 and IL-1β levels in serum were measured using commercial Elisa kits (Peprotech, Ribeirão Preto-SP, Brazil) following the manufacturer's instructions.
2.5. Statistic treatment
Student's t-test was performed to compare averages between two groups in the biological assays, and One-way analysis of variance (ANOVA) was followed by the Tukey test to compare more than two groups. The difference was considered to be statistically significant when P ≤ 0.05. For outlier identification the method presented by Anbarasi et al. (2011) was used. Statistical analyses were carried out using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) software.
3. Results
3.1. Chemical characterization of white tea
The reducing capacity determined by the Folin assay in WT was 92.78 ± 1.34 mg GAE/L, and the antioxidant capacity according to ORAC and FRAP assays were 1,758.04 ± 94.41 μmol TE/L and 2,024.21 ± 15.95 μmol TE/L, respectively. The phenolic compounds identified and quantified in WT through the use of the UPLC-MS/MS method were gallic acid (3.94 mg/L), quercetin (0.06 mg/L), and catechin (0.02 mg/L).
3.2. Food and drink intake, body and tissue weight
The WT offered to the animals, as well as the exercise training did not influence food and drink intake (data not shown). Considering a median daily intake of 39.92 ± 3.24 mL of tea by WT1 animals and 42.56 ± 3.89 mL of tea by WT2 animals, it was estimated the ingestion of 3.70 mg and 1.97 mg of phenolic compounds per day, respectively, based on Folin assay of WT.
There was no difference in body weight in T5 or T10 groups throughout the experimental weeks (Fig. 2). Gastrocnemius muscle, soleus muscle, and liver relative weights (g/100g total body weight) were also statistically similar in T5 or T10 groups (Fig. 3A, B, and C). However, a significant increase (P = 0.0035) in the relative heart weight (g/100g total body weight) was observed in all trained groups compared to the sedentary control group in T10 (Fig. 3D).
Fig. 2.
The curve of body weight throughout the experimental weeks. Values expressed as mean ± SEM. (A) T5 experiment; (B) T10 experiment. SC: Sedentary control group; EC: Exercise control group; WT1: Exercise + WT1 group; WT2: Exercise + WT2 group. No statistic differences were observed between groups (n = 5–7) at the same experimental
week by one-way ANOVA followed by a Tukey test, P > 0.05.
Fig. 3.
Tissues weight. Values expressed as mean ± SEM. SC: Sedentary control group; EC: Exercise control group; WT1: Exercise training + WT1 group; WT2: Exercise training + WT2 group; bw: Body weight. The subgroups (n = 5–7) were compared at the same experimental group (T5 or T10). # indicates significant differences compared to the SC group according to Student's t-test (1 sign = P < 0.05, and 2 sign = P < 0.01).
3.3. Performance on treadmill running test
As shown in Fig. 4, the time to exhaustion of the sedentary groups was lower than the trained groups at both periods (T5 and T10). It means that the groups were submitted to different workloads (volume and intensity) and exposed to different levels of RONS in the exhaustion test (Gargallo et al., 2018). As expected, the exercise control group took longer to reach exhaustion compared to the sedentary group, showing that the training protocol was efficient in improving the endurance ability of the rats. The WT intake did not promote an additional gain in endurance capacity. Although the WT1 group showed a lower exhaustion time compared with the EC group in the T5 experiment, after 5 weeks of maintenance of the exercise workload, the WT1 group exhibited similar performance to the EC group, as observed in T10.
Fig. 4.
Results from the exhaustion test. Values expressed as mean ± SEM. SC: Sedentary control group; EC: Exercise control group; WT1: Exercise training + WT1 group; WT2: Exercise training + WT2 group. The subgroups (n = 5–7) were compared at the same experimental group (T5 or T10). # indicates significant differences from the SC group and * indicates significant differences from the EC group according to Student's t-test (2 sign = P < 0.01; and 3 sign = P < 0.001).
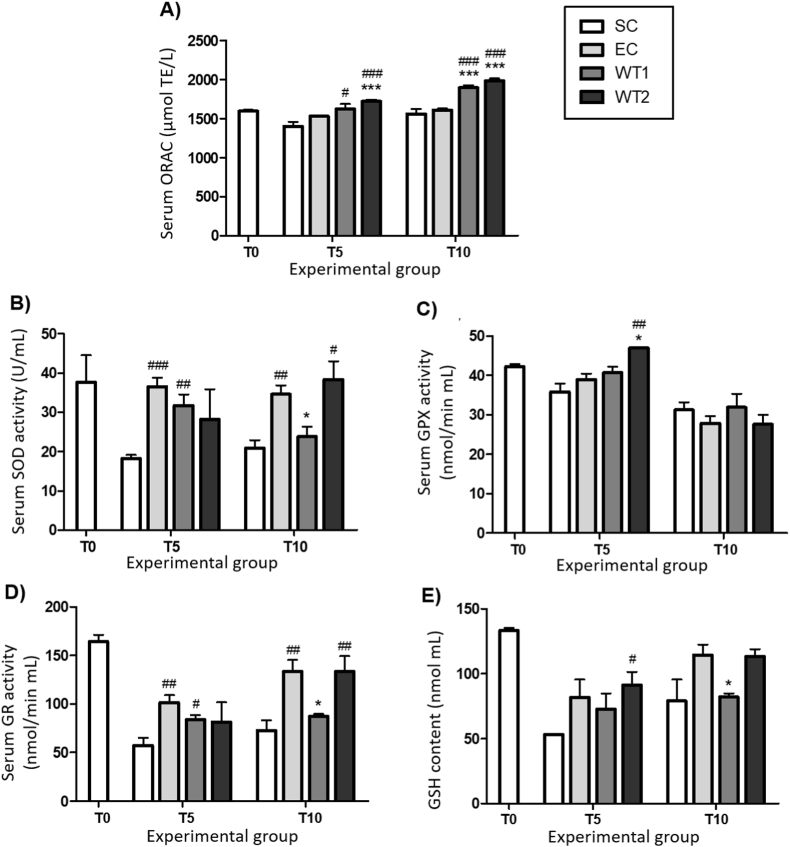
3.4. Serum antioxidant parameters
According to the ORAC assay, the WT1 group presented higher serum antioxidant capacity compared to the control group after 10 weeks of WT intake (Fig. 5A). WT2 showed a similar effect independent of the duration of the intake (5 or 10 weeks). Also, a positive correlation (R2 = 0.79) should be established between the concentration of tea and the serum antioxidant capacity of the T10 groups.
Fig. 5.
Serum antioxidant status and antioxidant defense system. Values expressed as mean ± SEM. (A) ORAC; (B) SOD activity; (C) GPx activity; (D) GR activity; and (E) GSH content. SC: Sedentary control group; EC: Exercise control group; WT1: Exercise training + WT1 group; WT2: Exercise training + WT2 group. The subgroups (n = 4–7) were compared at the same experimental group (T5 or T10). # indicates significant differences from the SC group and * indicates significant differences from the EC group according to Student's t-test (1 sign = P < 0.05; 2 sign = P < 0.01; and 3 sign = P < 0.001).
In T5, the serum's antioxidant enzyme activities (SOD, GPx, and GR) were statistically similar among WT and EC groups (Fig. 5B, C, and D). However, in T10, the WT1 group presented the lower activity of these three enzymes compared to the EC group. Regarding the thiol content after five weeks, the WT2 group showed higher GSH levels compared to the trained and sedentary control groups, at 17.0% and 23.7% range, respectively (Fig. 5E), but in T10 was no difference among the groups.
3.5. Liver antioxidant parameters and lipid peroxidation
A long-term WT intake reduced lipid peroxidation in the liver (39.0% for WT1 and 22.1% for WT2) compared to the EC group (Fig. 6A). Interestingly, liver MDA values of both WT groups did not differ from the sedentary group even though it was submitted at a higher workload in the exhaustion test (Fig. 4).
Fig. 6.
Liver lipid peroxidation and antioxidant defense system. Values expressed as mean ± SEM. (A) TBARS; (B) SOD activity; (C) GSH content; (D) GPx activity; and (E) GR activity. SC: Sedentary control group; EC: Exercise control group; WT1: Exercise training + WT1 group; WT2: Exercise training + WT2 group. The subgroups (n = 3–7) were compared at the same experimental group (T5 or T10). # indicates significant differences from the SC group and * indicates significant differences from the EC group according to Student's t-test (1 sign = P < 0.05; 2 sign = P < 0.01; and 3 sign = P < 0.001).
In the T5 experiment, WT intake did not cause a significant impact on SOD activity and GSH content in the liver (Fig. 6B and C). However, GPx activity was increased in WT groups (in 29.6% for WT1 and 44.5% for WT2) and also GR activity (WT2) in comparison to the water trained group (Fig. 6D and E).
In the T10 experiment, despite the GPx activity exhibiting a dependent dose-response pattern to the WT concentrations, WT1 seemed to be the most effective dose to improve liver antioxidant defenses. The WT1, but not WT2, had improved liver SOD, GPx, and GR activities and liver GSH content compared to the control trained group.
3.6. Serum inflammatory biomarkers
The results of inflammatory cytokines in serum are presented in Fig. 7. Trained control groups exhibited higher IL-1β levels than sedentary control groups (16.6% in T5 and 38.4% in T10). The highest workload sustained in the exhaustion test was possibly related to more significant muscle damage and subsequent higher inflammatory response in the EC group compared to the SC group (Koch et al., 2014). WT intake attenuated this increase when ingested for 5 weeks (20.1% to WT1 and 17.3% to WT2) and 10 weeks (7.6% to WT1). Regards the serum IL-6 levels it was statistically similar between the trained and sedentary control groups. Between the treated groups, the ingestion of WT2 for 10 weeks promoted the lowest IL-6 level compared to the trained group with water ingestion.
Fig. 7.
Inflammatory markers in serum. Values expressed as mean ± SEM. (A) IL-1β level. (B) IL-6 level. SC: Sedentary control group; EC: Exercise control group; WT1: Exercise training + WT1 group; WT2: Exercise training + WT2 group. The subgroups (n = 3–7) were compared at the same experimental group (T5 or T10). # indicates significant differences from the SC group and * indicates significant differences from the EC group according to Student's t-test (2 sign = P < 0.01; and 3 sign = P < 0.001).
4. Discussion
Natural extracts rich in bioactive compounds, such as phenolic compounds, have been extensively explored as food or dietary supplements for sports nutrition (Shanely et al., 2014; Fuster-Muñoz et al., 2016; Buchwald-Werner et al., 2018; Bowtell and Kelly, 2019). Black and green tea from C. sinensis are included in the top richest dietary sources for total polyphenols in the Phenol-Explorer database, based on the comparison among 452 foods (Perez-Jimenez et al., 2010). Concerning C. sinensis teas, previous studies presented that WT has higher contents of polyphenols, for example, gallic acid (Zhao et al., 2011), some catechins (Sun et al., 2019), some flavonol glycosides, and flavone glycosides (Dai et al., 2017) compared with green and black teas. However, there are few studies in the literature about the functional application of the WT compared to the last ones. Thus, the present study investigated the effect of a long-term WT intake on antioxidant and inflammatory responses after an exhaustive exercise in healthy trained animals.
The most abundant phenolic compounds present in WT were gallic acid, quercetin, and catechin, according to UPLC-MS/MS analyses. Catechins have been identified as the major class of polyphenols in WT in many studies, whose antioxidant effects are attributed (Damiani et al., 2014; Tan et al., 2017; Sanlier et al., 2018). The (−)-epigallocatechin and (−)-epigallocatechin gallate were the catechins found in higher concentrations in WT by Tan et al. (2017) and Damiani et al. (2014). While in the present study, the monomer catechin was found in higher amounts.
Different results about the chemical constituents profile of teas were observed in previous studies and required attention to the variety, region, manufacturing procedure of the tea plant, and also the extraction methods and solvents applied (Zhang et al., 2018; Perez-Burillo et al., 2018). During the extraction process, for example, the structural transformation from epi-catechins [(−)-epicatechin, (−)-epicatechin gallate, (−)-epigallocatechin, and (−)-epigallocatechin gallate] to their corresponding trans-catechins [(+)-catechin, (−)-catechin gallate, (+)-gallocatechin, and (−)-gallocatechin gallate] can occur, as well as the hydrolysis of gallic acid (degallation) from catechins. In this way, time-temperature specific conditions can influence these molecular changes, which significantly change the bioactivity of these compounds (Lin et al., 2017). Despite that, the present study's focus was to mimic the domestic way of preparing WT to drink as a sustainable and practical dietary polyphenol source and evaluate its effect on the experimental exercise model, especially in recovering the oxidative damages generated after the exhaustion.
In the biologic assay, the first interesting finding was related to the heart: treadmill exercise training for 10 weeks increased myocardial mass. Typically, cardiac hypertrophy may reflect a physiological or pathological condition. Endurance exercises promote morphological alterations in the heart, such as enlargement and proliferation of cardiomyocytes and angiogenesis in response to the redistribution of blood and energy requirements (Ma and Liao, 2019). This cardiac remodeling is directly related to enhanced cardiac function and cardioprotective effects promoted by exercise (Wang et al., 2017). Similar results were observed in rodents submitted to a swimming protocol for 8 weeks (Ramasamy et al., 2015) or a voluntary wheel running for 13 weeks (Copes et al., 2015).
It is clear that regular exercise training also results in total antioxidant capacity improvement (Radak et al., 2017; Wang et al., 2017). Nevertheless, intense training can increase ROS production, favoring fatigue and decreasing performance during training and competitions (He et al., 2016). In the current study, trained animals that received WT exhibited higher serum antioxidant potential, in a dose-dependent manner, than the trained control. It might be attributable to the association between the great antioxidant status and controlled oxidative stress levels, pre- and during the exhaustive exercise, respectively (Jowko et al., 2015). A previous study reported that flavanol and its metabolites can be found in the human plasma until 8 h following the ingestion of 500 mL of green tea (containing 648 μmol of flavanols) (Stalmach et al., 2009). Hence, circulating WT polyphenols and their derivates may have acted directly as a RONS scavenger or as an electron donator contributing, at least partially, to the great blood antioxidant status immediately after the running session. Importantly, this result can positively influence helping muscle recovery after pieces of training and competitions.
Regarding the antioxidant enzymes, we found no correlations among SOD, GR, and GPx activities and the total antioxidant capacity in serum. These results indicate that the improvement in the serum antioxidant status is related to the exogenous antioxidants ingested by the WT and not by the endogenous antioxidant system.
According to the hormeses theory, disruption of body redox homeostasis by RONS generation is essential to regulate and improve defense adaptations when repeated activation, as occurs under moderate-intensity exercise training (Pingitore et al., 2015; Radak et al., 2017). In contrast, exhaustive exercises are not generally associated with a regular stimulus and generate various deleterious physiological events, impairing health parameters and physical performance (Pingitore et al., 2015; Oláh et al., 2015).
Exhaustive exercise induces muscle damage and RONS production, which triggers a series of inflammatory responses. Importantly, dietary bioactive compounds have been shown to modulate cytokine production and downregulate inflammatory pathways activated by them (Shanely et al., 2014; Mohamed et al., 2016). Here, after an acute exhaustive running, a long-term WT intake was able to counteract the augment in some systemic pro-inflammatory cytokines, like IL-1β and IL-6. In another recent study, aqueous tea polyphenols (300 mg/kg/day) supplementation for 4 weeks effectively reduced inflammation and tissue damage among rats submitted to an exhaustive swimming exercise (Liu et al., 2017). Haramizu et al. (2013) had pointed as the mechanism of polyphenols to decrease the inflammation observed in exercise could be related to blocking the release of inflammatory cytokines by inactivating the nuclear factor-κB (NF-κB) pathway and neutrophil and macrophage decrease infiltration in the muscle. So, linking the inflammatory and antioxidant responses in serum, we suggest a favorable antioxidant status and lower cell damage levels promoted by WT. However, further experimental studies are required to clarify the role of WT polyphenols in exercise-induced muscle damage and inflammation.
Accumulated urea, inflammation, and hepatic damage markers were previously reported following exhaustive exercises (Huang et al., 2010). Commonly, little attention is directed to liver health in exercised individuals because of the lack of clinical symptoms when in low grade (Tang et al., 2016). In the present study, MDA, one of the many aldehydes formed as a secondary product during the lipid peroxidation process, was used as a complementary biomarker to evaluate exercise-induced oxidative stress in the liver. Animals that received WT for 10 weeks presented lower MDA levels than the water control, indicating potential protection against the oxidation of the cellular unsaturated lipid constituents. In agreement, other studies have reported the benefic effects of polyphenols-rich foods to counteract the oxidative damage after high-intensity exercise sessions (Allgrove et al., 2011; Jowko et al., 2015; Fuster-Muñoz et al., 2016). Moreover, as a target organ of polyphenols metabolism, hepatic damage markers could evidence no toxicological risk of WT in rats in both doses administered (Hu et al., 2018).
In this study, WT efficiently increases the endogenous antioxidant defense system activity in hepatic tissue after 5 weeks (GPx and GR) and 10 weeks (SOD, GPx, GR, and GSH) of WT intake. These finds can be associated with the effect of the natural antioxidants, e.g., catechins, in the activation of the transcription factor Nrf2 [nuclear factor (erythroid-derived 2) related factor), the master regulator of the expression of the antioxidant enzymes (Upadhyay and Dixit, 2015; Wang et al., 2015).
Liver GPx, which activity was highest in all WT groups than in its respective trained controls, is particularly engaged in reducing H2O2 to water and lipid hydroperoxides (LOOH) to the corresponding alcohols (Lushchak, 2012). LOOH are the primary products of lipid peroxidation, and its decomposition results in interrupting the propagation of the lipid peroxidation process and, consequently, MDA formation, as observed here (Ayala et al., 2014). Thus, the peroxidative damage inhibition in the liver after 10 weeks of WT intake may be attributed partially to the rise of the GPx enzyme and, therefore, the rise of the antioxidant glutathione system.
GSH, together with other non-enzymatic antioxidant substances, is responsible for reducing RONS and other antioxidants in their oxidized form, for complexing metal ions, as well as being adjuvant in repairing processes of damaged cells (Lushchak, 2012). When it is oxidized, glutathione is regenerated to GSH in the presence of NADPH, and the oxidative pentose phosphate cycle (OPPC) delivers the major source of cellular NADPH. Under exhaustive endurance exercise and muscle glycogen depletion conditions, the ability of OPPC to generate NADPH is likely to be attenuated because is relies on glucose-6-phosphate, an important OPPC substrate (Makarov et al., 2006; Lushchak, 2012). This compromises the regeneration of glutathione and the maintenance the redox homeostasis during the exercise, favoring the delayed fatigue (Li et al., 2013; Vezzoli et al., 2016; Radak et al., 2017). OPPC is active in liver rather than in the muscle, which means greater GSH levels in this organ. Considering GSH can cross the plasma membrane cells, the preservation or improvement in liver GSH content, as observed after chronic WT intake, should be especially important due to liver GSH can supply skeletal muscle and other organs that are exposed to higher RONS levels during exhaustive exercise (Lushchak, 2012; Vezzoli et al., 2016).
Based on the information mentioned above, it seems that exercise training is responsible for many physiological adaptive responses by the organism. However, in exhaustive exercise sessions, improving the antioxidant defenses with additional dietary antioxidant sources may be a suitable noninvasive tool for reducing oxidative damage and critical inflammation without compromising performance. Nevertheless, additional analyses are required to confirm some hypotheses raised in this study, especially cellular damage/integrity and muscle recovery markers after exercise. Moreover, comparisons with different studies are challenging since the experimental models differ regarding animal species, biomarkers, type and magnitude of exercises, training level of individuals and supplementation regime, factors that directly influence the results, and may to be considered.
In conclusion, the present study reveals that long-term WT intake may play a role in supporting a favorable antioxidant status of endurance-trained rats after an acute exhaustive exercise. Although the circulating inflammatory markers have been reduced after WT intake, the mechanisms involved in the effect of the polyphenols-rich foods in counteracting the inflammation in this situation need to be better explored in future research. WT intake effectively counteracts lipid peroxidation in hepatic tissue and enhances endogenous antioxidant defenses, suggesting a protective effect on oxidative damage.
Conflict of interest statement
The authors declare there are no competing interests.
CRediT authorship contribution statement
Patrícia Berilli: Conceptualization, Formal analysis, Investigation, Writing – original draft, Visualization. Gustavo Bernardes Fanaro: Term, Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Project administration. Jéssica Piva Santos: Investigation. Felix Guillermo Reyes Reyes: Investigation. Amadeu Hoshi Iglesias: Investigation. Marcella Reis: Investigation. Cínthia Baú Betim Cazarin: Writing – review & editing, Supervision. Mário Roberto Maróstica Junior: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) -Finance Code 001; National Council for Scientific and Technological Development (403328/2016-0; 301496/2019-6) and FAPESP (2015/50333-1; 2018/11069-5; 2015_13320-9)”. MRMJ acknowledges Red Iberomericana de Alimentos Autoctonos Subutilizados (ALSUB-CYTED, 118RT0543).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.crphys.2022.06.002.
Contributor Information
Patrícia Berilli, Email: pbberilli@gmail.com.
Gustavo Bernardes Fanaro, Email: gbfanaro@gmail.com.
Jéssica Piva Santos, Email: jeh.piva@gmail.com.
Felix Guillermo Reyes Reyes, Email: reyesfgr@gmail.com.
Amadeu Hoshi Iglesias, Email: amadeu_iglesias@apexsci.com.br.
Marcella Reis, Email: marcella_reis@apexsci.com.br.
Cínthia Baú Betim Cazarin, Email: cbetim@unicamp.br.
Mário Roberto Maróstica Junior, Email: mmarostica@unicamp.br.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alcazar A., Ballesteros O., Jurado J.M., Pablos F., Martin M.J., Vilches J.L., Navalon A. Differentiation of green, white, black, oolong, and pu-erh teas according to their free amino acids content. J. Agric. Food Chem. 2007;55:5960–5965. doi: 10.1021/jf070601a. [DOI] [PubMed] [Google Scholar]
- Allgrove J., Farrell E., Gleeson M., Williamson G., Cooper K. Regular dark chocolate consumption's reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metabol. 2011;21:113–123. doi: 10.1123/ijsnem.21.2.113. [DOI] [PubMed] [Google Scholar]
- Anbarasi M.S., Ghaayathri S., Kamaleswari R., Abirami I. Outlier detection for multidimensional medical data. Int. J. Comput. Sci. Inf. Technol. 2011;2:512–516. [Google Scholar]
- Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014:360438–360469. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (Frap) as a measure of "antioxidant power": the Frap assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bowtell J., Kelly V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med. 2019;49:3–23. doi: 10.1007/s40279-018-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchwald-Werner S., Naka I., Wilhelm M., Schütz E., Schoen C., Reule C. Effects of lemon verbena extract (Recoverben®) supplementation on muscle strength and recovery after exhaustive exercise: a randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018;15:1–10. doi: 10.1186/s12970-018-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Academic Press; 1985. Glutathione Reductase. Methods Enzymol. [DOI] [PubMed] [Google Scholar]
- Cazarin C.B.B., Da Silva J.K., Colomeu T.C., Batista Â.G., Meletti L.M.M., Paschoal J.A.R., Bogusz Junior S., Braga P.A.D.C., Reyes F.G.R., Augusto F., De Meirelles L.R., Zollner R.D.L., Maróstica Júnior M.R. Intake of Passiflora edulis leaf extract improves antioxidant and anti-inflammatory status in rats with 2,4,6-trinitrobenzenesulphonic acid induced colitis. J. Funct.Foods. 2015;17:575–586. doi: 10.1016/j.jff.2015.05.034. [DOI] [Google Scholar]
- Close G.L., Hamilton D.L., Philp A., Burke L.M., Morton J.P. New strategies in sport nutrition to increase exercise performance. Free Radic. Biol. Med. 2016;98:144–158. doi: 10.1016/j.freeradbiomed.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Copes L.E., Schutz H., Dlugosz E.M., Acosta W., Chappell M.A., Garland T. Effects of voluntary exercise on spontaneous physical activity and food consumption in mice: results from an artificial selection experiment. Physiol. Behav. 2015;149:86–94. doi: 10.1016/j.physbeh.2015.05.025. [DOI] [PubMed] [Google Scholar]
- Dai W., Xie D., Lu M., Li P., Lv H., Yang C., Peng Q., Zhu Y., Guo L., Zhang Y., Tan J., Lin Z. Characterization of white tea metabolome: comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017;96:40–45. doi: 10.1016/j.foodres.2017.03.028. [DOI] [PubMed] [Google Scholar]
- Damiani E., Bacchetti T., Padella L., Tiano L., Carloni P. Antioxidant activity of different white teas: comparison of hot and cold tea infusions. J. Food Compos. Anal. 2014;33:59–66. doi: 10.1016/j.jfca.2013.09.010. [DOI] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fatouros I.G., Chatzinikolaou A., Douroudos Ii, Nikolaidis M.G., Kyparos A., Margonis K., Michailidis Y., Vantarakis A., Taxildaris K., Katrabasas I., Mandalidis D., Kouretas D., Jamurtas A.Z. Time-course of changes in oxidative stress and antioxidant status responses following a soccer game. J. Strength Condit Res. 2010;24:3278–3286. doi: 10.1519/Jsc.0b013e3181b60444. [DOI] [PubMed] [Google Scholar]
- Flohé L., Günzler W.A. Academic Press; 1984. Assays of Glutathione Peroxidase. Methods Enzymol. [DOI] [PubMed] [Google Scholar]
- Fuster-Muñoz E., Roche E., Funes L., Martínez-Peinado P., Sempere J.M., Vicente-Salar N. Effects of pomegranate juice in circulating parameters, cytokines, and oxidative stress markers in endurance-based athletes: a randomized controlled trial. Nutrition. 2016;32:539–545. doi: 10.1016/j.nut.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Gargallo P., Colado J.C., Juesas A., Hernando-Espinilla A., Estan-Capell N., Monzo-Beltran L., Garcia-Perez P., Cauli O. The effect of moderate- versus high-intensity resistance training on systemic redox state and Dna damage in healthy older women. Biol. Res. Nurs. 2018;20:205–217. doi: 10.1177/1099800417753877. [DOI] [PubMed] [Google Scholar]
- Hajiaghaalipour F., Kanthimathi M.S., Sanusi J., Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, Ht-29, activates caspases and protects Dna of normal cells against oxidative damage. Food Chem. 2015;169:401–410. doi: 10.1016/j.foodchem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Haramizu S., Ota N., Hase T., Murase T. Catechins suppress muscle inflammation and hasten performance recovery after exercise. Med. Sci. Sports Exerc. 2013;45:1694–1702. doi: 10.1249/Mss.0b013e31828de99f. [DOI] [PubMed] [Google Scholar]
- He F., Li J., Liu Z., Chuang C.-C., Yang W., Zuo L. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016;7:1–10. doi: 10.3389/fphys.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl R., Ferraresso R.L., De Oliveira R.B., Lucco R., Brenzikofer R., De Macedo D.V. Development and characterization of an overtraining animal model. Med. Sci. Sports Exerc. 2009;41:1155–1163. doi: 10.1249/Mss.0b013e318191259c. [DOI] [PubMed] [Google Scholar]
- Hu J., Webster D., Cao J., Shao A. The safety of green tea and green tea extract consumption in adults – Results of a systematic review. Regul. Toxicol. Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Huang C.C., Lin W.T., Hsu F.L., Tsai P.W., Hou C.C. Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur. J. Appl. Physiol. 2010;108:557–566. doi: 10.1007/s00421-009-1247-7. [DOI] [PubMed] [Google Scholar]
- Jowko E., Dlugolecka B., Makaruk B., Cieslinski I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur. J. Nutr. 2015;54:783–791. doi: 10.1007/s00394-014-0757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A.J., Pereira R., Machado M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Interact. 2014;14:68–77. [PubMed] [Google Scholar]
- Koutelidakis A.E., Argyri K., Serafini M., Proestos C., Komaitis M., Pecorari M., Kapsokefalou M. Green tea, white tea, and Pelargonium purpureum increase the antioxidant capacity of plasma and some organs in mice. Nutrition. 2009;25:453–458. doi: 10.1016/j.nut.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Leite A.V., Malta L.G., Riccio M.F., Eberlin M.N., Pastore G.M., Marostica Junior M.R. Antioxidant potential of rat plasma by administration of freeze-dried jaboticaba peel (Myrciaria jaboticaba Vell Berg) J. Agric. Food Chem. 2011;59:2277–2283. doi: 10.1021/jf103181x. [DOI] [PubMed] [Google Scholar]
- Li J., Ward K.M., Zhang D., Dayanandam E., Denittis A.S., Prendergast G.C., Ayene I.S. A bioactive probe of the oxidative pentose phosphate cycle: novel strategy to reverse radioresistance in glucose deprived human colon cancer cells. Toxicol. Vitro. 2013;27:367–377. doi: 10.1016/j.tiv.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Xia G., Liu S. Modeling and comparison of extraction kinetics of 8 catechins, gallic acid and caffeine from representative white teas. LWT--Food Sci. Technol. 2017;83:1–9. doi: 10.1016/j.lwt.2017.04.028. [DOI] [Google Scholar]
- Liu L., Wu X., Zhang B., Yang W., Li D., Dong Y., Yin Y., Chen Q. Protective effects of tea polyphenols on exhaustive exercise-induced fatigue, inflammation and tissue damage. Food Nutr. Res. 2017;61:1–7. doi: 10.1080/16546628.2017.1333390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak V.I. Glutathione homeostasis and functions: potential targets for medical interventions. J. Amino Acids. 2012:1–26. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Liao Y. Noncoding Rnas in exercise-induced cardio-protection for chronic heart failure. EBioMedicine. 2019;46:532–540. doi: 10.1016/j.ebiom.2019.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov P., Kropf S., Wiswedel I., Augustin W., Schild L. Consumption of redox energy by glutathione metabolism contributes to hypoxia/reoxygenation-induced injury in astrocytes. Mol. Cell. Biochem. 2006;286:95–101. doi: 10.1007/s11010-005-9098-y. [DOI] [PubMed] [Google Scholar]
- Mason S.A., Morrison D., Mcconell G.K., Wadley G.D. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic. Biol. Med. 2016;98:29–45. doi: 10.1016/j.freeradbiomed.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Mohamed S., Lamya N., Hamda M. Effect of maximal versus supra-maximal exhausting race on lipid peroxidation, antioxidant activity and muscle-damage Biomarkers in long-distance and middle-distance runners. Asian J. Sports Med. 2016;7:1–8. doi: 10.5812/asjsm.27902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Oláh A., Németh B.T., Mátyás C., Horváth E.M., Hidi L., Birtalan E., Kellermayer D., Ruppert M., Merkely G., Szabó G., Merkely B., Radovits T. Cardiac effects of acute exhaustive exercise in a rat model. Int. J. Cardiol. 2015;182:258–266. doi: 10.1016/j.ijcard.2014.12.045. [DOI] [PubMed] [Google Scholar]
- Ou B., Chang T., Huang D., Prior R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (Orac) using fluorescein as the fluorescence probe: first Action 2012.23. J. AOAC Int. 2013;96:1372–1376. doi: 10.5740/jaoacint.13-175. [DOI] [PubMed] [Google Scholar]
- Pastoriza S., Mesias M., Cabrera C., Rufian-Henares J.A. Healthy properties of green and white teas: an update. Food Funct. 2017;8:2650–2662. doi: 10.1039/c7fo00611j. [DOI] [PubMed] [Google Scholar]
- Peake J.M., Suzuki K., Coombes J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007;18:357–371. doi: 10.1016/j.jnutbio.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Peña-Oyarzun D., Bravo-Sagua R., Diaz-Vega A., Aleman L., Chiong M., Garcia L., Bambs C., Troncoso R., Cifuentes M., Morselli E., Ferreccio C., Quest A.F.G., Criollo A., Lavandero S. Autophagy and oxidative stress in non-communicable diseases: a matter of the inflammatory state? Free Radic. Biol. Med. 2018;124:61–78. doi: 10.1016/j.freeradbiomed.2018.05.084. [DOI] [PubMed] [Google Scholar]
- Perez-Burillo S., Gimenez R., Rufian-Henares J.A., Pastoriza S. Effect of brewing time and temperature on antioxidant capacity and phenols of white tea: relationship with sensory properties. Food Chem. 2018;248:111–118. doi: 10.1016/j.foodchem.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Perez-Jimenez J., Neveu V., Vos F., Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010;64(Suppl. 3):S112–S120. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- Pingitore A., Lima G.P., Mastorci F., Quinones A., Iervasi G., Vassalle C. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31:916–922. doi: 10.1016/j.nut.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Radak Z., Ishihara K., Tekus E., Varga C., Posa A., Balogh L., Boldogh I., Koltai E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017;12:285–290. doi: 10.1016/j.redox.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S., Velmurugan G., Shanmugha Rajan K., Ramprasath T., Kalpana K. Mirnas with apoptosis regulating potential are differentially expressed in chronic exercise-induced physiologically hypertrophied hearts. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlier N., Atik I., Atik A. A minireview of effects of white tea consumption on diseases. Trends Food Sci. Technol. 2018;82:82–88. doi: 10.1016/j.tifs.2018.10.004. [DOI] [Google Scholar]
- Shanely R.A., Nieman D.C., Zwetsloot K.A., Knab A.M., Imagita H., Luo B., Davis B., Zubeldia J.M. Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav. Immun. 2014;39:204–210. doi: 10.1016/j.bbi.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Stalmach A., Troufflard S., Serafini M., Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009;53:S44–S53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- Sun L., Xu H., Ye J., Gaikwad N.W. Comparative effect of black, green, oolong, and white tea intake on weight gain and bile acid metabolism. Nutrition. 2019;65:208–215. doi: 10.1016/j.nut.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Swain T., Hillis W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tan J., Engelhardt U.H., Lin Z., Kaiser N., Maiwald B. Flavonoids, phenolic acids, alkaloids and theanine in different types of authentic Chinese white tea samples. J. Food Compos. Anal. 2017;57:8–15. doi: 10.1016/j.jfca.2016.12.011. [DOI] [Google Scholar]
- Tang Y., Li J., Gao C., Xu Y., Li Y., Yu X., Wang J., Liu L., Yao P. Hepatoprotective effect of quercetin on endoplasmic reticulum stress and inflammation after intense exercise in mice through phosphoinositide 3-kinase and nuclear factor-kappa B. Oxid. Med. Cell. Longev. 2016:1–12. doi: 10.1155/2016/8696587. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen M., Atalay M., Uusitalo A. Altered oxidative stress in overtrained athletes. J. Sports Sci. 2010;28:309–317. doi: 10.1080/02640410903473844. [DOI] [PubMed] [Google Scholar]
- Thomas D.T., Erdman K.A., Burke L.M. Position of the Academy of nutrition and Dietetics, Dietitians of Canada, and the American College of sports medicine: nutrition and athletic performance. J. Acad. Nutr. Diet. 2016;116:501–528. doi: 10.1016/j.jand.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Upadhyay S., Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015:1–15. doi: 10.1155/2015/504253. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzoli A., Dellanoce C., Mrakic-Sposta S., Montorsi M., Moretti S., Tonini A., Pratali L., Accinni R. Oxidative stress assessment in response to ultraendurance exercise: thiols redox status and Ros production according to duration of a competitive race. Oxid. Med. Cell. Longev. 2016:1–13. doi: 10.1155/2016/6439037. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Xu M., Li W., Li X., Zheng Q., Niu X. Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-Ar-nnos-No activation. PLoS One. 2017;12:1–23. doi: 10.1371/journal.pone.0179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Wang Y., Wan X., Yang C.S., Zhang J. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015;283:65–74. doi: 10.1016/j.taap.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Winterbourn C.C., Hawkins R.E., Brian M., Carrell R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975;85:337–341. [PubMed] [Google Scholar]
- Zhang C., Suen C.L.-C., Yang C., Quek S.Y. Antioxidant capacity and major polyphenol composition of teas as affected by geographical location, plantation elevation and leaf grade. Food Chem. 2018;244:109–119. doi: 10.1016/j.foodchem.2017.09.126. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen P., Lin L., Harnly J.M., Yu L.L., Li Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using Uplc/Dad/Ms. Food Chem. 2011;126:1269–1277. doi: 10.1016/j.foodchem.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Chiang H., Portocarrero C., Zhu Y., Hill S., Heppert K., Jayaratna H., Davies M., Janle E., Kissinger P. Investigating the stability of Egcg in aqueous media. Curr. Sep. 2003;20:83–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.