Summary
The most frequent genetic aberration leading to infant ALL (iALL) is the chromosomal translocation t(4;11), generating the fusion oncogenes KMT2A:AFF1 and AFF1:KMT2A, respectively. KMT2A-r iALL displays a dismal prognosis through high relapse rates and relapse-associated mortality. Relapse occurs frequently despite ongoing chemotherapy and without the accumulation of secondary mutations. A rational explanation for the observed chemo-resistance and satisfactory treatment options remain to be elucidated. We found that elevated ICOSLG expression level at diagnosis was associated with inferior event free survival (EFS) in a cohort of 43 patients with t(4;-11) iALL and that a cohort of 18 patients with iALL at relapse displayed strongly increased ICOSLG expression. Furthermore, co-culturing t(4;11) ALL cells (ICOSLGhi) with primary T-cells resulted in the development of Tregs. This was impaired through treatment with a neutralizing ICOSLG antibody. These findings imply ICOSLG (1) as a relapse-predicting biomarker, and (2) as a therapeutic target involved in a potential immune evasion relapse-mechanism of infant t(4;11) ALL.
Subject areas: Health sciences, Immunology, Cancer
Graphical abstract
Highlights
-
•
Early growth response 3 (EGR3) is a direct transactivator of the immune checkpoint gene ICOSLG
-
•
high ICOSLG expression at diagnosis is predictive for ALL relapse
-
•
EGR3 and ICOSLG expressions are relapse-associated
-
•
expression of ICOSLG on t(4;11) ALL cells leads to the rapid expansion of Tregs
Health sciences; Immunology; Cancer
Introduction
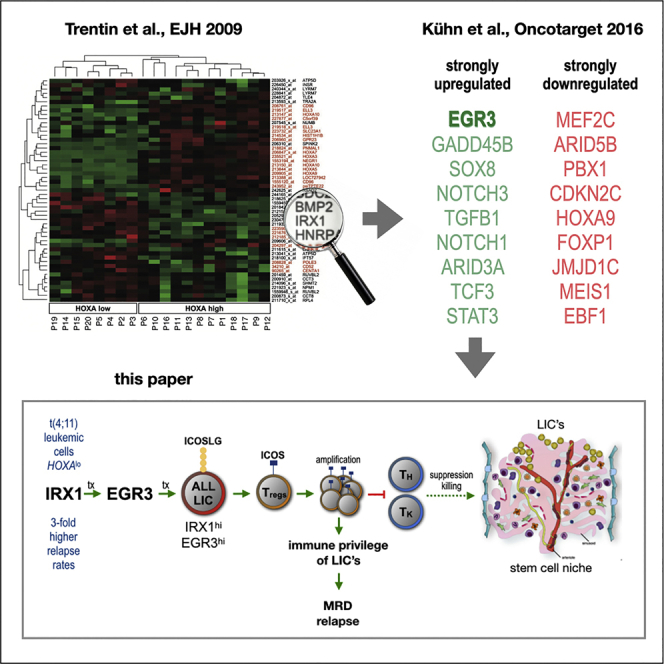
KMT2A-rearrangements (KMT2A-r) occur in 70-80% of all infant acute lymphoblastic leukemias (iALL) (Pieters et al., 2007, 2019). Among these, the translocation t(4;11) generating the fusion oncogenes KMT2A::AFF1 and AFF1::KMT2A is the most frequent, accounting for about 49% of all KMT2A-r iALL cases (Meyer et al., 2018). KMT2A-r is a negative risk factor in iALL with a 4-year event-free survival (4-year-EFS) of only 37% among KMT2A-r cases and 36% among t(4;11) cases, respectively. By comparison, patients with KMT2A-germline achieve a 4-year-EFS of 74% (Pieters et al., 2007). The typical clinical picture of KMT2A-r iALL is that patients initially reach complete remission (CR) during induction therapy but relapse months later despite continuous chemotherapy (Pieters et al., 2007). This results in a 3-year post-relapse overall survival (3-year-OS) of only 17% for patients with t(4;11), with OS defined as the time from relapse to death (Driessen et al., 2016). Designated studies indicate the absence of significant accumulation or selection of secondary mutations in the dominant leukemic clone between the timepoints of diagnosis and relapse (Andersson et al., 2015; Agraz-Doblas et al., 2019). Thus, an explanation for how therapy resistance is achieved remains an open question.
Our group previously identified the C2H2 zinc finger transcription factors Early growth response 1, 2, and 3 (EGR1, EGR2, EGR3) as downstream mediators of the transcription factor Iroquois homeobox 1 (IRX1) (Kühn et al., 2016). Relatively higher transcription of IRX1 was observed in patients with iALL presenting a low HOXA gene expression (Trentin et al., 2009). This IRX1hi/HOXAlo patient group displayed an increased relapse rate with dismal outcome (Stam et al., 2010; Kang et al., 2012). EGR1, EGR2, EGR3, and their murine homologs have been described to be involved in cell cycle inhibition and stemness of hematopoietic stem cells (HSC) (Min et al., 2008; Cheng et al., 2015; Li et al., 2017). Additionally, EGR3 expression in murine T- and B-cells is known to restrict immune activity and prevent autoimmunity (Safford et al., 2005; Anderson et al., 2006; Collins et al., 2008; Li et al., 2012; Parkinson et al., 2014).
We were able to demonstrate that the zinc finger transcription factor Early growth response 3 (EGR3) binds directly to the promoter of the ICOSLG gene and upregulates ICOSLG protein. ICOSLG is an immune checkpoint expressed by antigen-presenting cells (APC) as well as non-lymphoid cells including mesenchymal stem cells, endothelial cells, fibroblasts, and tumor cells (Swallow et al., 1999; Khayyamian et al., 2002; Martin-Orozco et al., 2010; Zhang et al., 2016). ICOSLG belongs to the B7 family of costimulatory immune receptors with partial sequence identity of CD80/CD86 and binds ICOS (Coyle and Gutierrez-Ramos, 2001). ICOS belongs to the CD28 superfamily of immune receptors, is expressed at low levels in resting memory T-cell subsets, but becomes upregulated through ICOSLG binding (Hutloff et al., 1999; Khayyamian et al., 2002). The interaction between ICOSLG and ICOS has been shown to lead to the development of Tregs in healthy bone marrow as well as in several tumor microenvironments with clinical implications in melanoma, glioblastoma, breast cancer and acute myeloid leukemia (AML) (Martin-Orozco et al., 2010; Faget et al., 2012; Lee et al., 2017; Han et al., 2018; Iwata et al., 2019).
In summary, we report here that high gene expression of ICOSLG at initial diagnosis was associated with inferior EFS in a cohort of 43 infant patients with t(4;11) ALL and that a cohort of 18 patients with infant ALL (iALL) at relapse displayed strongly increased EGR3 and ICOSLG transcription levels. Upregulation of ICOSLG expression in t(4;11) ALL cells caused increased development of regulatory T-cells (Tregs) when co-cultured with primary T-cells. The development of Tregs was impaired through the treatment with a neutralizing α-ICOSLG antibody.
Thereby, we provide a potential relapse mechanism that could explain how KMT2A-r leukemia cells reach therapy resistance. Consequently, our study not only suggests ICOSLG as a relapse-predicting prognostic biomarker, but identifies the ICOSLG/ICOS interaction as a putative therapeutic target in infant t(4;11) ALL.
Results
Early growth response 3 upregulates ICOSLG through direct promoter binding in an early growth response 3-overexpressing t(4;11) SEM cell model
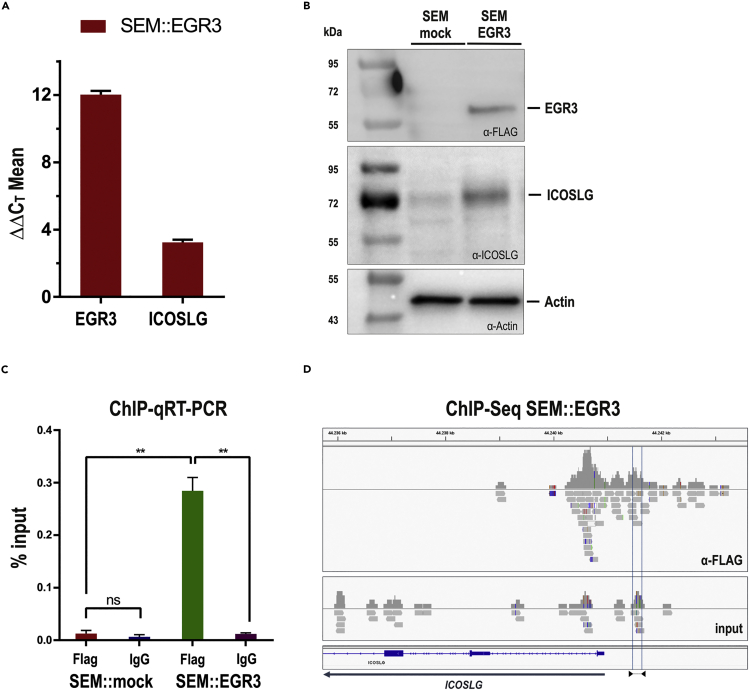
The initial observation that IRX1 is upregulated in patients with t(4;11) iALL that display a HOXAlo signature prompted us to investigate the role of IRX1 in further detail. Overexpression of IRX1 resulted in the transcriptional upregulation of HOXB4 and the Early growth response genes EGR1-3 (Kühn et al., 2016). We cloned the EGR3 open reading frame (ORF) fused to a C-terminal FLAG tag into our sleeping beauty vector system (Kowarz et al., 2015). This vector construct was stably integrated into the genome of the t(4;11) cell line SEM (SEM::EGR3), and transgene expression was induced by the addition of Doxycycline. An empty vector was used for the generation of a stable control cell line (SEM::mock). This cell culture model system enabled us to investigate the effects of EGR3 overexpression in a t(4;11) pro-B cellular context. Quantitative real-time PCR (qPCR) confirmed transgene expression and revealed strong upregulation of ICOSLG by EGR3 (ΔΔCT = 3.24 ± 0.16) at the transcription level (Figure 1A). We used Western blot experiments to confirm the EGR3 protein expression and the ICOSLG upregulation on the protein level (Figure 1B).
Figure 1.
EGR3 upregulates ICOSLG through direct promoter binding in an EGR3-overexpressing t(4;11) SEM cell model
(A) qRT-PCR proved the transcriptional upregulation of EGR3 (ΔΔCT = 12.03 ± 0.22) and ICOSLG (ΔΔCT = 3.24 ± 0.16) 48h after the transgene induction of the SEM::EGR3 cell model.
(B) Western blotting of 20 μg protein lysate confirmed the upregulation of EGR3 (FLAG-tagged) and ICOSLG on the protein level. SEM::mock showed a slight ICOSLG band owing to the basal expression of ICOSLG on SEM cells.
(C and D) Chromatin immunoprecipitation followed by qRT-PCR (C) and sequencing (D) displayed the direct binding of EGR3 towards the ICOSLG promoter area. ChIP-qRT-PCR was analyzed through percent input calculation followed by two-tailed unpaired t tests with Welch’s correction to compare the percent input values of SEM:EGR3 α-FLAG with SEM::EGR3 IgG (p = 0.0082), SEM::mock α-FLAG with SEM::mock IgG (p = 0.4452), and SEM::mock α-FLAG with SEM::EGR3 α-FLAG (p = 0.0063). The location of the primer pair used for ChIP-qRT-PCR is displayed as blue lines in the ICOSLG promoter area and indicated below with triangles accordingly.
Considering that EGR3 is a zinc finger transcription factor we aimed to investigate whether EGR3 binds directly to the ICOSLG promoter or acts as an indirect ICOSLG activator. For that purpose, we performed chromatin immunoprecipitation (ChIP) followed by qRT-PCR and ChIP-Seq. ChIP-qRT-PCR and percent input calculation of the SEM::EGR3 cell model demonstrated that EGR3 binds directly to the promoter of the ICOSLG gene (Figure 1C). This initial result was later corroborated by ChIP-Seq (Figure 1D).
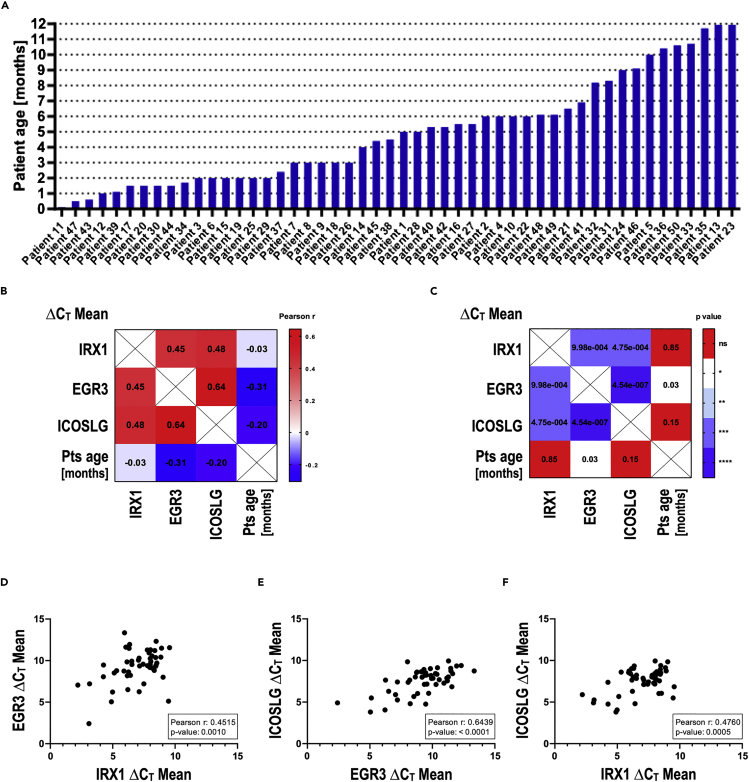
Iroquois homeobox 1, early growth response 3, and ICOSLG expressions correlate strongly in a cohort of 50 infant patients with t(4;11) pro-B ALL
In order to validate the findings of our experimental model system, we examined the gene expression of 50 infant patients with t(4;11) pro-B ALL using qRT-PCR. Patient characteristics are summarized in Table 1. The age distribution of the cohort showed that no distinct age group was overrepresented (Figure 2A). Plotting of the ΔCT values and subsequent Pearson correlation testing for the IRX1, EGR3, and ICOSLG expressions revealed that the ICOSLG expression strongly correlated with the expression of EGR3 and IRX1 (Figures 2B–2F). The highest Pearson R value for the EGR3-ICOSLG relation (Figure 2E) was in line with the observation that EGR3 binds directly to the ICOSLG promoter in the SEM cell model system (Figures 1C and 1D). According to our studies, IRX1 does not directly bind the EGR3 or ICOSLG promoter, in agreement with the relatively lower Pearson r values for these correlations (Figures 2D and 2F). In summary, gene expression analysis of patient samples verified that IRX1 upregulates EGR3, which subsequently transactivates ICOSLG in infant t(4;11) ALL, as suggested by our SEM::EGR3 cell culture model system.
Table 1.
Patient characteristics of the dx cohort (n = 50)
| Patient no. | Patient age [months] | Treatment protocol | sex | Time to event (last follow-up) [months] | HOXA9 ΔCT Mean |
IRX1 ΔCT Mean |
EGR3 ΔCT Mean |
ICOSLG ΔCT Mean |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.0 | Interfant-06 | F | (40.1) | 13.3592 | 6.1705 | 8.1850 | 7.6499 |
| 2 | 6.0 | / | F | / | 3.9204 | 7.7667 | 11.2758 | 8.0223 |
| 3 | 2.0 | Interfant-06 | F | (23.6) | 12.9546 | 7.1581 | 9.3618 | 7.1255 |
| 4 | 6.0 | Interfant-06 | M | (17.1) | 11.5783 | 7.9774 | 11.1637 | 7.8868 |
| 5 | 10.0 | / | M | / | 3.5974 | 8.3317 | 8.8214 | 6.0364 |
| 6 | 2.0 | Interfant-06 | M | (83.6) | 4.9698 | 9.5555 | 11.5618 | 6.8416 |
| 7 | 3.0 | Interfant-06 | F | (5.6) | 11.0093 | 5.1167 | 8.5196 | 5.6658 |
| 8 | 3.0 | Interfant-06 | F | (1.2) | 4.9163 | 6.4184 | 8.2271 | 4.8055 |
| 9 | 3.0 | Interfant-06 | M | (2.5) | 4.5976 | 9.0338 | 8.0114 | 9.8380 |
| 10 | 6.0 | Interfant-06 | F | 9.5 (10.7) | 5.0818 | 8.4812 | 12.3136 | 9.3984 |
| 11 | 0.1 | / | F | / | 8.6964 | 8.0250 | 11.2989 | 9.9469 |
| 12 | 1.0 | / | M | / | 12.3443 | 6.0576 | 11.6236 | 8.4245 |
| 13 | 12.0 | / | F | / | 2.2824 | 7.9734 | 9.1826 | 7.9894 |
| 14 | 4.0 | / | F | / | 11.4825 | 6.3166 | 11.4695 | 9.0590 |
| 15 | 2.0 | Interfant-06 | M | 6.0 (6.0) | 4.6973 | 5.9447 | 13.3440 | 8.7451 |
| 16 | 5.5 | Interfant-06 | M | 9.5 (15.0) | 6.2129 | 6.5399 | 9.5330 | 8.2498 |
| 17 | 1.5 | Interfant-06 | F | 10.0 (13.0) | 6.0703 | 4.9900 | 6.2132 | 4.0517 |
| 18 | 3.0 | Interfant-06 | F | 6.0 (11.5) | 2.0906 | 8.4791 | 9.4723 | 8.5023 |
| 19 | 2.0 | Interfant-06 | F | 10.5 (19) | 7.8537 | 3.1429 | 7.2056 | 5.2518 |
| 20 | 1.5 | Interfant-06 | M | 26.5 (41.0) | 4.4627 | 8.3685 | 11.2026 | 7.5564 |
| 21 | 6.5 | Interfant-06 | M | 24.0 (32.8) | 11.2673 | 5.3156 | 8.7817 | 8.6026 |
| 22 | 6.0 | Interfant-06 | F | 19.5 (23.5) | 11.5891 | 7.0636 | 10.0553 | 7.8697 |
| 23 | 12.0 | Interfant-06 | F | 32.0 (60.6) | 11.1226 | 4.2582 | 8.0600 | 7.3822 |
| 24 | 9.0 | Interfant-06 | M | (84.9) | 8.7665 | 2.2034 | 7.0569 | 5.8906 |
| 25 | 2.0 | Interfant-06 | F | (118.8) | 4.6017 | 7.1138 | 10.2925 | 7.1171 |
| 26 | 3.0 | Interfant-06 | M | 8.0 (108.5) | 13.0872 | 5.9083 | 8.7375 | 8.5604 |
| 27 | 5.5 | Interfant-06 | M | (9.0) | 5.9626 | 8.1536 | 9.1105 | 8.6464 |
| 28 | 5.0 | Interfant-06 | M | 17.0 (33.0) | 5.9922 | 4.8934 | 5.0456 | 3.8081 |
| 29 | 2.0 | Interfant-06 | F | 56.0 (58.6) | 5.3297 | 8.4461 | 9.3271 | 8.9127 |
| 30 | 1.5 | Interfant-06 | F | (146.1) | 5.8358 | 8.0518 | 9.8910 | 8.1978 |
| 31 | 8.3 | Interfant-06 | F | (109.3) | 2.2195 | 6.2132 | 6.5075 | 6.2880 |
| 32 | 8.2 | Interfant-06 | M | (95.9) | 1.7726 | 7.4956 | 7.2074 | 7.4143 |
| 33 | 10.7 | Interfant-06 | M | 8.8 (8.8) | 2.6953 | 3.0935 | 2.4138 | 4.9064 |
| 34 | 1.7 | Interfant-06 | F | (91.5) | 3.8579 | 7.1200 | 6.2540 | 7.6448 |
| 35 | 11.7 | Interfant-06 | F | (73.2) | 2.9311 | 7.9152 | 10.9273 | 7.2855 |
| 36 | 10.4 | Interfant-06 | F | (75.9) | 5.7541 | 8.8581 | 10.4144 | 9.1282 |
| 37 | 2.4 | Interfant-06 | F | (73.8) | 13.3331 | 6.1426 | 9.8438 | 9.0200 |
| 38 | 4.5 | Interfant-06 | M | 16.1 (63.5) | 4.6562 | 8.9152 | 11.4891 | 8.7484 |
| 39 | 1.1 | Interfant-06 | F | 3.3 (15.7) | 12.9071 | 6.3552 | 11.9464 | 9.3496 |
| 40 | 5.3 | Interfant-06 | M | (58.7) | 5.4284 | 8.4380 | 10.3705 | 8.3493 |
| 41 | 6.9 | Interfant-06 | F | (63.2) | 9.2472 | 8.5374 | 9.7053 | 6.0532 |
| 42 | 5.3 | Interfant-06 | M | (60.7) | 3.4737 | 7.4371 | 9.5640 | 7.1599 |
| 43 | 0.6 | Interfant-06 | F | 5.0 (11.9) | 7.9122 | 6.2711 | 8.6364 | 8.0174 |
| 44 | 1.5 | Interfant-06 | M | 17.3 (18.3) | 8.1333 | 8.0008 | 9.5745 | 7.1672 |
| 45 | 4.4 | Interfant-06 | M | 6.6 (10.8) | 4.2993 | 8.0016 | 10.2528 | 8.4346 |
| 46 | 9.1 | Interfant-06 | F | 39.7 (45.2) | 3.1663 | 7.7915 | 9.3985 | 8.2159 |
| 47 | 0.5 | Interfant-06 | F | 8.7 (13.3) | 6.2701 | 6.5276 | 9.9358 | 7.8396 |
| 48 | 6.1 | AIEOP-BFM ALL 2017 | F | 6.6 | 12.6734 | 4.2609 | 9.4741 | 4.7534 |
| 49 | 6.1 | AIEOP-BFM ALL 2017 | F | 8.3 | 4.5847 | 8.1496 | 10.5529 | 7.6922 |
| 50 | 10.6 | / | M | / | 5.0774 | 9.4705 | 5.1199 | 5.4875 |
All samples were taken from PB at the day of diagnosis. Gene expression values are indicated as the ΔCT mean of three technical replicates. F = female, M = male.
Figure 2.
IRX1, EGR3, and ICOSLG expressions correlate strongly in a cohort of 50 infant patients with t(4;11) pro-B ALL
(A) Patient age distribution. Patients of all infant age groups are represented, and no age group is overrepresented.
(B–F) Pearson correlation matrix visualizes the Pearson r values (B) and corresponding p values (C) for all correlations tested. The strong positive correlations between the expressions of EGR3-> IRX1 (D), ICOSLG-> EGR3 (E) and ICOSLG-> IRX1 (F) are obvious mapping each patient sample in the correlation plots.
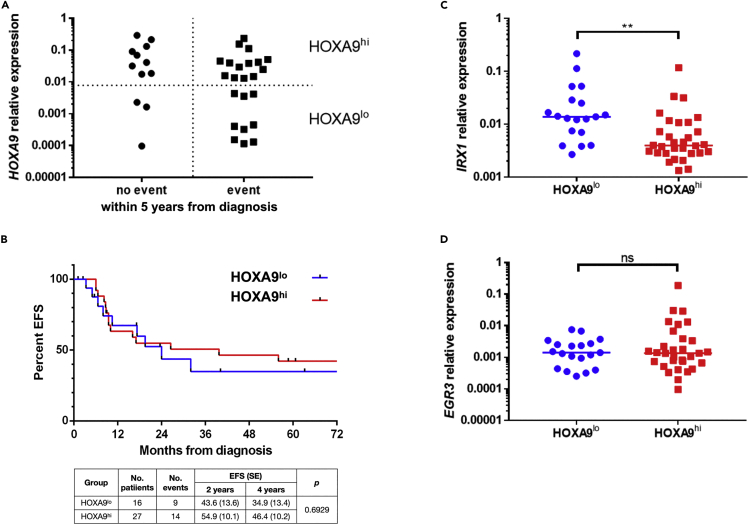
Patients with HOXA9lo show slightly inferior EFS than patients with HOXA9hi in a cohort of 43 infant patients with t(4;11) pro-B ALL
Patients with t(4;11) iALL were reported to cluster into a HOXAhi and a HOXAlo group (Trentin et al., 2009), with higher relapse rates in the HOXAlo group (Stam et al., 2010; Kang et al., 2012; Symeonidou and Ottersbach, 2021). We aimed to verify this finding and could demonstrate the clustering of patients with t(4;11) iALL into these two subgroups when analyzing the HOXA9 transcriptional profiles (19 pts with HOXA9lo, 31 pts with HOXA9hi). Within the cohort of 50 patients, outcome data were available for 43 pts. We divided the patients into a group that achieved no event within 5 years from diagnosis (n = 12), and a group that achieved an event within the same time frame (n = 23). The assignment of these patients to the respective HOXA9 transcription level revealed that patients with HOXA9lo were found in both groups (Figure 3A). Subsequent Kaplan-Meier analysis of all patients with the availabe outcome data (n = 43) demonstrated that the EFS disadvantage of patients with HOXA9lo was quite modest and did not reach statistical significance in a log rank test (Figure 3B). Consistent with the above-mentioned studies, patients with HOXA9lo displayed a higher median IRX1 expression level than patients with HOXA9hi (p = 0.0012) (Figure 3C). However, this was not the case for the EGR3 expression level (p = 0.5397) (Figure 3D) although we showed that the IRX1 and EGR3 expressions correlate strongly among the 50 patients (Figure 2D).
Figure 3.
Patients with HOXA9lo show slightly inferior EFS than patients with HOXA9hi in a cohort of 43 infant patients with t(4;11) pro-B ALL
(A) Patients were divided into a group that displayed no event within 5 years from diagnosis (n = 12), and a group with an event within the same time frame (n = 23). Subsequently, patients were assigned to the respective HOXA9 transcription level and divided into a HOXA9lo and a HOXA9hi subgroup.
(B) Kaplan-Meier curves of the patients with HOXA9lo vs HOXA9hi visualize the slightly inferior EFS of patients with HOXA9lo which did not reach statistical significance in a log rank test (p = 0.6929).
(C and D) Patients with HOXA9lo have a higher median IRX1 (p = 0.012) (C) but not EGR3 gene expression (p = 0.5397) (D) than patients with HOXA9hi. Significance was tested with two-tailed unpaired t tests.
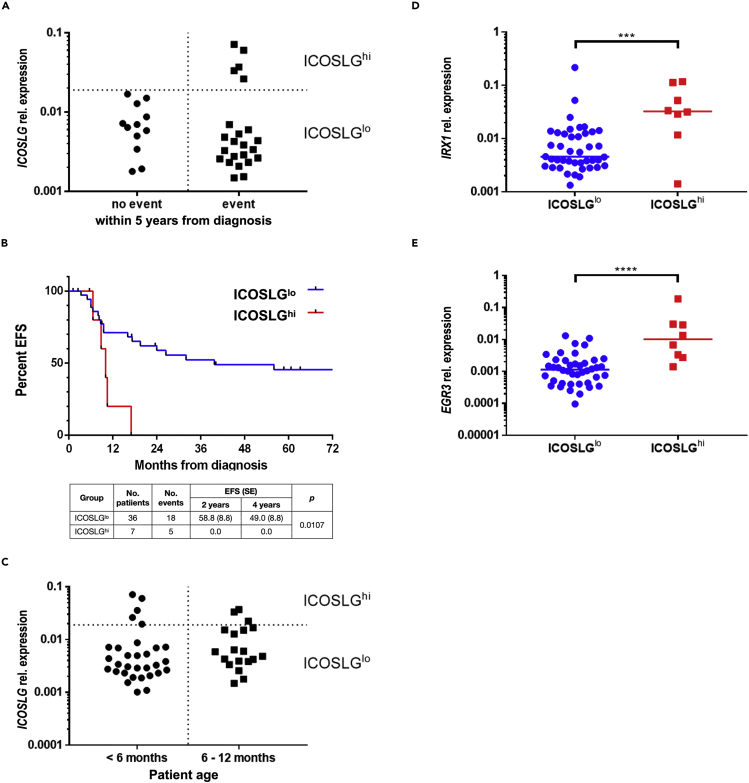
High ICOSLG expression is associated with inferior EFS in a cohort of 43 infant patients with t(4;11) pro-B ALL
We then focused on ICOSLG expression, hypothesizing it could contribute to therapy resistance and relapse rates. For that purpose, we assigned the patients of the “no event group” (n = 12) and the “event group” (n = 23) to their respective ICOSLG transcription levels and found the clustering of patients into an ICOSLGlo and an ICOSLGhi group (Figure 4A). Interestingly, the “no event group” consisted only of patients with ICOSLGlo, whereas the “event group” included an ICOSLGlo and an ICOSLGhi cluster. The Kaplan-Meier analysis of all patients with available outcome data (n = 43) clearly demonstrated inferior EFS of the ICOSLGhi group (n = 7; 2-year-EFS = 0.0%) compared to the ICOSLGlo group (n = 36; 2-year-EFS = 58.8 ± 8.5%) (Figure 4B). A log rank test confirmed that both curves were significantly different (p value = 0.0107). However, this analysis could be biased owing to the difference in group sizes, but was not biased owing to patient age as the ICOSLGhi group is composed of patients younger and older than 6 months (Figure 4C). In agreement with our previous data, we showed that the ICOSLGhi group (n = 8) of all patients (n = 50) possessed significantly elevated IRX1 and EGR3 transcription levels. (Figures 4D and 4E).
Figure 4.
High ICOSLG expression is associated with inferior EFS in a cohort of 43 infant patients with t(4;11) pro-B ALL
(A) Patients of the “no event group” (n = 12) and the “event group” (n = 23) were assigned to their respective ICOSLG transcription levels and divided into an ICOSLGlo and an ICOSLGhi subgroup.
(B) Kaplan-Meier curves and log rank test of the patients with ICOSLGlo vs ICOSLGhi visualize the clearly inferior EFS of patients with ICOSLGhi (p = 0.0107).
(C) Patients separated by age were assigned to the respective ICOSLG transcription level illustrating that the ICOSLGhi group was composed of patients younger and older than 6 months.
(D and E) The ICOSLGhi group (n = 8) of all patients (n = 50) displayed significantly elevated IRX1 (p = 0.0006) (D) and EGR3 (p < 0.0001) (E) transcription levels in two-tailed unpaired t tests.
High EGR3 and ICOSLG expressions are relapse associated
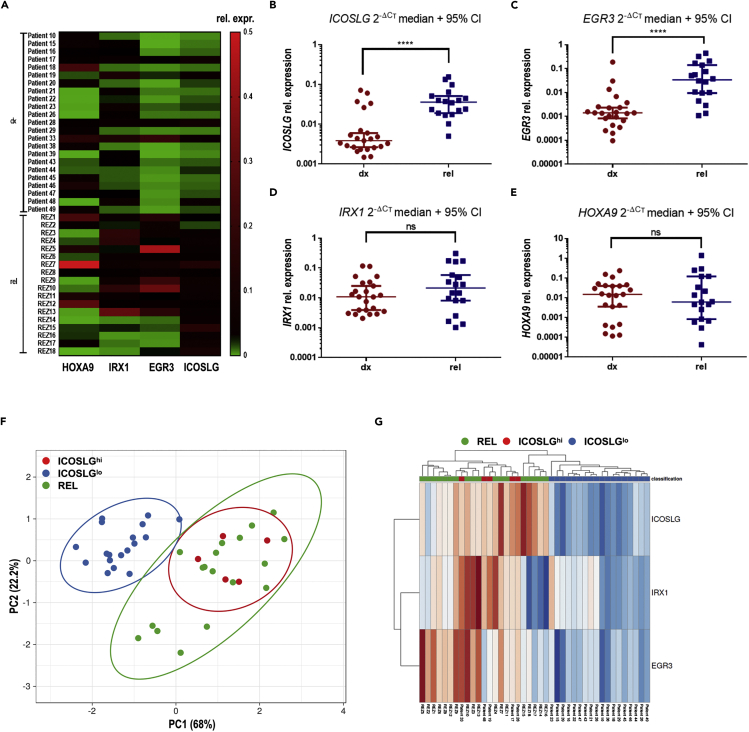
To examine whether the high expression of ICOSLG at diagnosis could be associated with relapse formation, we assessed the HOXA9, IRX1, EGR3, and ICOSLG gene expressions in a cohort of 18 relapsed pro-B iALL patient samples with different KMT2A-r partner genes taken at the time of relapse. These patients were an independent cohort, not related to the samples taken at diagnosis and patient characteristics are summarized in Table 2. We compared the gene expression data of the cohort at initial diagnosis (“dx”) with that one at relapse diagnosis (“rel”). To enable an appropriate comparison of both groups, we included only those patients of the dx cohort that achieved an event (n = 23). Thereby, we found that the rel cohort possessed a generally higher expression level of EGR3 and ICOSLG when compared to the dx cohort (Figure 5A). Notably, the ICOSLG expression of the rel cohort was mainly at the same level as the ICOSLGhi group of the dx cohort (Figure 5B). The higher EGR3 and ICOSLG expressions of the rel cohort compared to the dx cohort were statistically significant in unpaired t tests (p < 0.0001) (Figures 5B and 5C), whereas no clear difference became obvious regarding the IRX1 and HOXA9 expression comparison of both cohorts (Figures 5D and 5E). Subsequently, we performed a principal component analysis (PCA) of the IRX1, EGR3, and ICOSLG expression data of the dx and rel cohorts using ClustVis (Metsalu and Vilo, 2015). Patients were assigned to the dx ICOSLGhi group, dx ICOSLGlo group, or the rel cohort to display the clustering of the patients representing the grade of similarity of their gene expression profiles (Figure 5F). The dx ICOSLGhi group (red) clustered together and overlapped completely with the rel cohort (green), whereas the dx ICOSLGlo group (blue) clustered besides with only a marginal overlap. The coherent clustering of the dx ICOSLGhi group with the rel cohort against dx ICOSLGlo became also obvious regarding the PCA heatmap (Figure 5G). Thus, PCA revealed a high grade of similarity regarding IRX1, EGR3, and ICOSLG expressions between the group of patients with high ICOSLG expression at diagnosis and the relapsed cohort. Taken together, these results strongly suggest that the IRX1-EGR3-ICOSLG axis is involved in relapse formation, and that high ICOSLG expression level at diagnosis can be described as a predictor for subsequent relapse.
Table 2.
Patient characteristics of the rel cohort (n = 18)
| Patient no. | Age at primary diagnosis [months] | KMT2A-r partner | sex | HOXA9 ΔCT Mean |
IRX1 ΔCT Mean |
EGR3 ΔCT Mean |
ICOSLG ΔCT Mean |
|---|---|---|---|---|---|---|---|
| REZ1 | 1.3 | AFF1 | F | 2.016418 | 5.156435 | 2.832994 | 6.612465 |
| REZ2 | 2.9 | MLLT1 | M | 5.517492 | 6.952302 | 4.17735 | 7.647544 |
| REZ3 | 3.8 | AFF1 | F | 10.23312 | 2.528003 | 4.301431 | 5.388622 |
| REZ4 | 2.5 | AFF1 | F | 7.767597 | 2.621157 | 6.657438 | 4.662416 |
| REZ5 | 1.2 | AFF1 | F | 3.034274 | 6.716536 | 1.18892 | 5.421249 |
| REZ6 | 10.7 | MLLT1 | M | 7.759405 | 4.871906 | 5.178097 | 5.714579 |
| REZ7 | 4.3 | MLLT1 | M | −0.47106 | 4.344864 | 3.729839 | 2.899311 |
| REZ8 | 3.6 | MLLT3 | F | 6.206228 | 6.106342 | 4.742364 | 5.892561 |
| REZ9 | 9.1 | AFF1 | F | 10.72401 | 4.103871 | 2.276168 | 4.287584 |
| REZ10 | 0.7 | AFF1 | F | 8.609946 | 2.540371 | 1.63076 | 4.492456 |
| REZ11 | 4.4 | AFF1 | M | 3.047612 | 5.159082 | 6.565317 | 4.748623 |
| REZ12 | 4.2 | AFF1 | M | 1.789008 | 6.10047 | 5.030182 | 5.842515 |
| REZ13 | 2.0 | AFF1 | F | 10.53802 | 1.708041 | 2.584866 | 5.500404 |
| REZ14 | 3.1 | AFF1 | F | 11.73027 | 9.246894 | 8.439681 | 4.853207 |
| REZ15 | 7.6 | AFF1 | F | 7.390529 | 6.94658 | 7.833634 | 2.701138 |
| REZ16 | 6.7 | AFF1 | M | 7.300869 | 9.940318 | 9.541564 | 4.663118 |
| REZ17 | 5.3 | AFF1 | M | 4.871099 | 8.645488 | 9.86666 | 3.962195 |
| REZ18 | 7.2 | AFF1 | M | 14.54123 | 9.570634 | 6.724747 | 3.354452 |
All samples were taken from PB on the day of relapse diagnosis. Gene expression values are indicated as the ΔCT mean of three technical replicates. F = female, M = male
Figure 5.
High EGR3 and ICOSLG expressions are relapse associated
(A) HOXA9, IRX1, EGR3, and ICOSLG expression levels are displayed in a heatmap for the dx cohort (patients with event; n = 23) and the rel cohort (n = 18). High 2−ΔCT values indicating high expression are displayed in red.
(B–D) Unpaired t tests indicated significantly higher ICOSLG (p < 0.0001) (B) and EGR3 (p < 0.0001) (C) expression levels of the rel cohort compared to the dx cohort, whereas the HOXA9 and IRX1 expression levels did not significantly differ between both cohorts (pHOXA9 = 0.8374; pIRX1 = 0.2737) (E and D).
(F) Principal component analysis among IRX1, EGR3, and ICOSLG expression data with patients assigned to the rel cohort (green), or to the ICOSLGhi (red) or ICOSLGlo (blue) group of the dx cohort. PC1 represents 68%, PC2 22.2% of total variance, respectively.
(G) Heatmap of the PCA confirms the clustering of the ICOSLGhi group at diagnosis with the relapse cohort. High 2−ΔCT values indicating high transcription levels are displayed in red.
ICOSLG upregulation in SEM::EGR3 cells leads to the rapid development of Tregs upon co-culture with primary T-cells
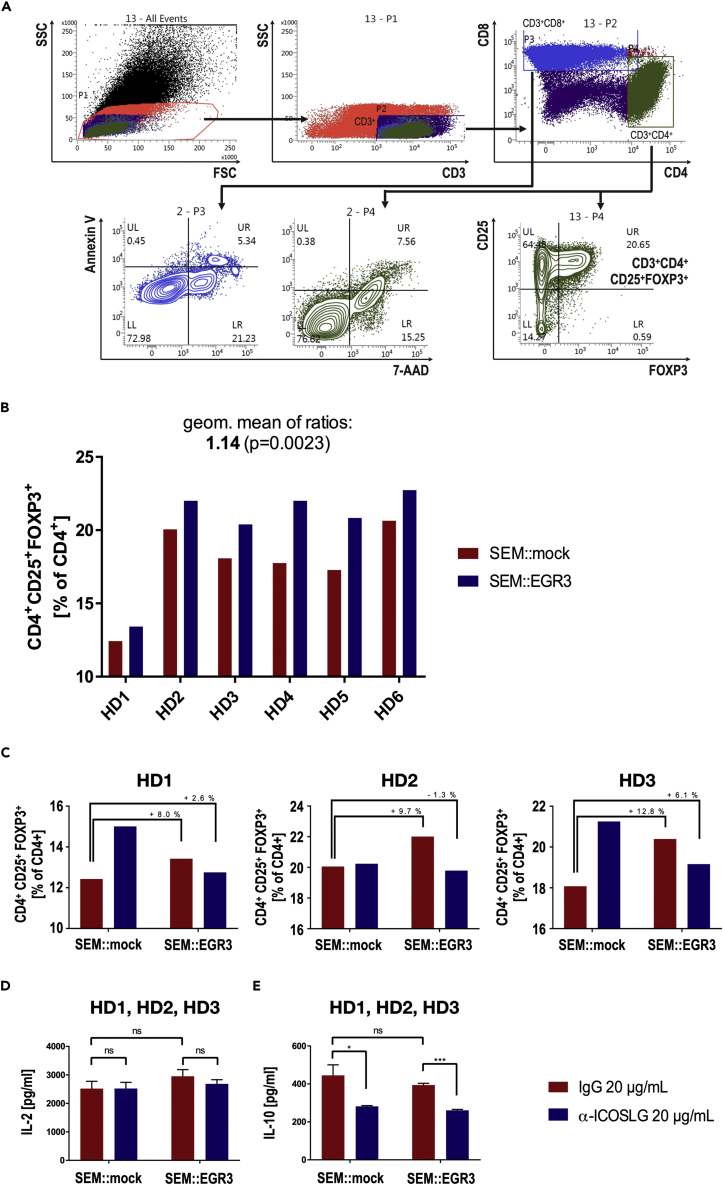
We then speculated that the causal relationship between ICOSLG expression and relapse in t(4;11) iALL could be the ICOSLG-mediated induction of Treg development which was previously described in different physiological and pathological contexts (Martin-Orozco et al., 2010; Lee et al., 2017; Han et al., 2018; Iwata et al., 2019). Accordingly, we examined this question in the t(4;11) pro-B cellular context using the established SEM::EGR3 cell line. For this purpose, primary human T-cells were co-cultured with either SEM::EGR3 or SEM::mock cells for 48h (50% SEM cells with 25% CD4+ and 25% CD8+ T-cells). The T-lymphocytes were isolated from peripheral blood mononuclear cells (PBMC) of healthy MHC-unmatched donors and were stimulated with coated α-CD2/-CD3/-CD28 beads for 16h before co-culture. This stimulation mimicked T-cell receptor (TCR) activation, and thus, enabled a TCR-independent immune activation. After 48h of co-culture, the percentage of CD4+CD25+FOXP3+ cells (Tregs) among CD4+ cells was quantified using flow cytometry. With six independent healthy donors (HD1-6), we observed an increase in the percentage of CD4+CD25+FOXP3+ Tregs in the SEM::EGR3 co-culture between 7.96 and 23.94% (geometric mean: 1.14 ± 0.009991, p value = 0.0023) compared to the SEM::mock co-culture (Figure 6B). Noteworthy, SEM::mock cells expressed low amounts of ICOSLG but were not ICOSLG-negative (see Figure 1B).
Figure 6.
ICOSLG upregulation in SEM::EGR3 cells leads to the rapid development of Tregs upon co-culture with primary T-cells
(A) Flow cytometry gating strategy of the co-cultured cells. Cells with low granularity (low SSC) were gated out of all cells. CD3+ cells were selected to subsequently separate CD4+ and CD8+ cells. Viability after co-culture of these populations was assed using Annexin V and 7-AAD staining to ensure that the majority of T-cells were alive throughout co-culture. CD25+FOXP3+ cells (Tregs) were quantified out of the CD4+ population.
(B) Percentages of CD4+CD25+FOXP3+ Tregs among CD4+ T-cells after co-culturing T-cells from six healthy donors (HD1-HD6) with SEM::mock or SEM::EGR3. SEM::EGR3 co-culture led to 7.96%-23.94% more Tregs than SEM::mock co-culture (geometric mean: 1.14 ± 0.009991, p = 0.0023 in a two-tailed ratio paired t test).
(C) Replication of the co-culture experiment of HD1-HD3 under the addition of 20 μg/mL mouse IgG1 isotype control or neutralizing monoclonal mouse α-human ICOSLG antibody demonstrated that EGR3-mediated Tregs induction could be impaired through ICOSLG antibody blockade.
(D and E) ELISAs of the co-culture supernatants depicted that ICOSLG antibody blockade has no effect on the IL-2 level (D) but strongly decreased the IL-10 level (SEM::mock: p = 0.0424; SEM::EGR3: p = 0.0002) (E) of the media. Significance was tested with multiple t tests.
To prove whether the ICOSLG/ICOS interaction was responsible for Treg development, we performed the experiment under the addition of 20 μg/mL mouse IgG1 isotype control or neutralizing monoclonal mouse α-human ICOSLG antibody to the media (HD1-3). α-ICOSLG but not IgG control antibody impaired the EGR3-mediated Treg development during co-culture. (Figure 6C). These experimental observations suggest that EGR3-expressing t(4;11) ALL cells rapidly induced the development of Tregs. This induction was most likely caused by the EGR3-mediated transactivation of ICOSLG, as the effect was not observed when the immune checkpoint was selectively blocked by a specific checkpoint inhibitor antibody. We also assessed the cytokine levels in the supernatant of the co-culture using appropriate ELISA tests. These experiments revealed that ICOSLG blockade strongly decreased the IL-10 level of both the SEM::mock and SEM::EGR3 co-culture, whereas the IL-2 levels were not affected (Figures 6D and 6E). All this indicated that ICOSLG blockade impaired the development of Tregs. However, for HD1 and HD3 we observed also a strong increase of Tregs in the SEM::mock co-culture through ICOSLG antibody blockade which anyway did not affect the decreased IL-10 level through ICOSLG blockade.
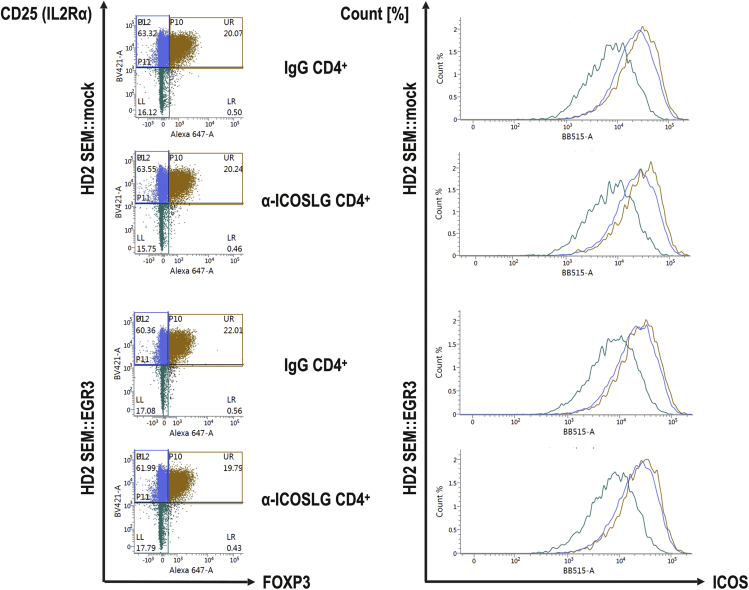
Tregs exhibit a higher ICOS surface expression than other CD4+ cells after co-culture
We hypothesized that a higher ICOS surface expression on Tregs compared to other T-cell subsets could be the reason for the preferential induction of Tregs through ICOSLG expression. This has already been reported for Tregs in the PB of healthy individuals (Duhen et al., 2012). Flow cytometry analyses revealed that CD25+FOXP3+ cells displayed a higher ICOS surface expression than CD25+FOXP3- and CD25−FOXP3- cells within the CD4+ subset (Figure 7). This was neither influenced by the co-cultured cell model (SEM::mock vs SEM::EGR3) nor by different antibody treatments (IgG vs α-ICOSLG). As a result, the higher ICOS surface expression of Tregs in comparison to other T-cell subsets was likely the explanation for the induction of Tregs through ICOSLG expression.
Figure 7.
Tregs exhibit a higher ICOS surface expression than other CD4+ cells after co-culture
Shown are the representative CD25->FOXP3 dot plots of the CD3+CD4+ subset of HD2. CD25+FOXP3+, CD25+FOXP3- and CD25−FOXP3- cells were gated and their respective ICOS surface expression were displayed in a comparative plot. CD25+FOXP3+ Tregs showed the highest ICOS expression, followed by CD25+FOXP3- cells. CD25−FOXP3- cells clearly display the lowest ICOS surface expression.
Discussion
Infant t(4;11) ALL is one of the cancers with the lowest mutational burden with approximately 1.3 non-silent mutations in the dominant leukemic clone besides the chromosomal translocation (Andersson et al., 2015; Agraz-Doblas et al., 2019; Brown et al., 2019). Secondary mutations are present in some patients at diagnosis but are the result of the dramatic cycling activity of the dominant clone and disappear once leukemia re-emerges as a relapse, indicating that the translocation is the only necessary leukemic driver (Agraz-Doblas et al., 2019). Based on our results, we propose that high ICOSLG expression at diagnosis and inferior EFS are causally linked, in that the ICOSLG-mediated induction of Treg development counteracts the immune recognition of leukemia cells. Through this mechanism, ALL cells could acquire a protective immune privilege in their bone marrow niche, enabling them to re-emerge as relapse despite ongoing chemotherapy. This mechanism is a possible explanation for relapse occurrence without the accumulation of additional mutations and clonal evolution.
This hypothesis implies that “fusion derived neoantigens” (FDNA) lead to T-cell priming and specific immune responses against the leukemic cells. Although cancers with a low mutational burden are generally regarded as barely immunogenic, ETV6::RUNX1 fusion-specific CD8+ T-cell responses have been reported in pediatric ALL (Zamora et al., 2019). Moreover, gene fusions are known to render T-cell responses in head and neck tumors with low mutational burden and minimal immune infiltration resulting in clinical response to immune checkpoint blockade (Yang et al., 2019). Therefore, it could be possible that also t(4;11)-derived neoantigens are immunogenic although this has not yet been demonstrated experimentally.
In pediatric ALL, the bone marrow (BM) is the predominant compartment where relapse originates (Tallen et al., 2010; Driessen et al., 2016). The BM provides the specialized niche in which HSC reside and give rise to all subsets of blood cells throughout life (Morrison and Scadden, 2014; Chen et al., 2016). In AML, leukemia stem cells (LSC) occupy the HSC niche and outcompete their healthy counterparts (Bonnet and Dick, 1997; Boyd et al., 2014; Medyouf, 2017; Kumar et al., 2018). In ALL, chemotherapy induces the formation of a protective niche by leukemia-initiating cells (LIC) that perturb hematopoiesis and lead to apoptosis of healthy HSC (Duan et al., 2014; Tang et al., 2018; Burt et al., 2019). In case of t(4;11) ALL, fetal liver-derived CD34+CD19− primitive B lineage-negative but lymphoid-restricted progenitor/stem cells have been identified as the most likely cellular origin of transformation (Hotfilder et al., 2005; Rice et al., 2021). However, it has been shown that not only CD34+ but also CD34− ALL cells including blasts at different stages of differentiation possess stem cell capabilities and may act as LIC when serially transplanted in immunocompromised mice (le Viseur et al., 2008; Aoki et al., 2015). Accordingly, in t(4;11) ALL stemness seems not to be restricted to a certain cell type, suggesting that leukemia cells at different stages of differentiation can home to and reside in the BM niche and subsequently contribute to relapse. Therefore, therapeutic approaches that interfere with leukemic niche formation hold promise to prevent relapse development, particularly in infant t(4;11) ALL.
Importantly, the BM is not only a primary but also a secondary immune organ as it is part of the lymphocyte recirculation network (Osmond, 1994). CD4+ and CD8+ T-cells enter the BM from the vasculature and become primed through BM-residing dendritic cells (DC) resulting in primary immune responses (Feuerer et al., 2001, 2003). Besides CD4+ and CD8+ T-cells, Tregs migrate to and accumulate in the BM through CXCL12/CXCR4 interactions (Zou et al., 2004). These Tregs have been shown to colocalize with HSC and provide an immune privilege to the niche (Fujisaki et al., 2011). Additionally, niche-associated Tregs are known to maintain HSC quiescence (Hirata et al., 2018, 2019). As healthy HSC depend on the colocalization with Tregs, it is highly likely that LSC/LIC do as well, considering especially that the recruitment of immune suppressive cells to avoid immune destruction is a common measure of several solid tumors and a hallmark of cancer (Hanahan and Weinberg, 2011).
Therefore, we speculate that ALL cell expression of ICOSLG facilitates the recruitment of Tregs to the BM niche. The ICOSLG/ICOS interaction is known to affect CD4+ T-cells to produce IL-10 resulting in immunosuppression (Figure 8) (Hutloff et al., 1999; Akbari et al., 2002; Löhning et al., 2003). Furthermore, the development of Tregs in the healthy bone marrow as well as in melanoma, glioblastoma, breast cancer and AML has been shown to strongly depend on the ICOSLG/ICOS interaction (Martin-Orozco et al., 2010; Faget et al., 2012; Lee et al., 2017; Han et al., 2018; Iwata et al., 2019). Accordingly, the here described identification of EGR3-mediated ICOSLG-expressing ALL cells, enabling increased Treg development is in line with previous observations.
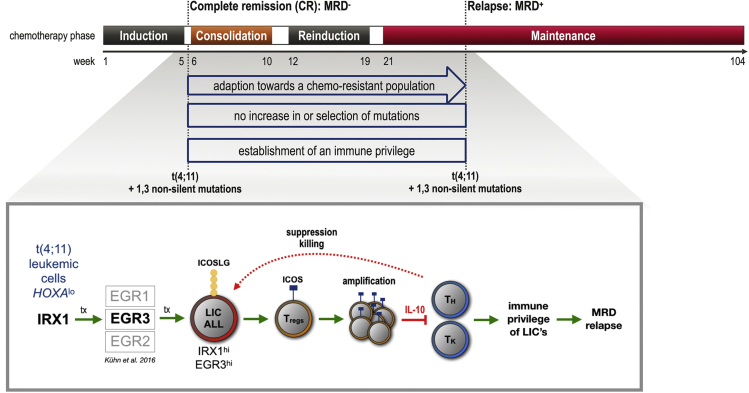
Figure 8.
Proposed immune-evasion relapse mechanism in t(4;11) ALL
Upper panel: Scheme of ALL chemotherapy. Although complete remission (CR) is frequently achieved after induction therapy, relapse occurs prevalently during consolidation, reinduction, or maintenance phase. Bottom panel: Infant t(4;11) ALL cells characterized by low HOXA transcription overexpress IRX1, which in turn causes the upregulation of several EGR transcription factors. EGR3 was shown to cause the transcriptional upregulation of ICOSLG. The interaction of ICOSLG on ALL cells with ICOS on T-cells initiates the development of regulatory T-cells (Tregs). The accumulation of Tregs in the bone marrow niche could provide ALL cells with an immune privilege allowing them to acquire therapy resistance and to develop minimal residual disease (relapse).
In our study, co-culture of SEM::EGR3 with adult T-cells leads to an enrichment of ICOS+FOXP3+ Tregs. In mice, the high surface expression of ICOS among Tregs determines a higher proliferative capability, longer survival, and stronger suppression compared to ICOS− Tregs (Chen et al., 2012). The higher suppressive activity of ICOS+ Tregs has also been observed in human studies (Vocanson et al., 2010). ICOS is expressed at low levels in all T-cell subsets but becomes upregulated upon ICOSLG binding (Hutloff et al., 1999; Khayyamian et al., 2002). The Tregs which developed in our co-culture setting showed the highest ICOS surface expression among the CD4+ T-cells mirroring the results of previous studies (Duhen et al., 2012). Therefore, we assume that the higher and constitutional ICOS surface expression of Tregs compared to non-Tregs could be a reason for their preferential proliferation during their co-culture with ICOSLG-expressing SEM cells. It is known that ICOS+ Tregs suppress other T-cells mainly through IL-10 secretion, whereas ICOS− Tregs secrete transforming growth factor-β (TGF-β) leading to weaker immunosuppression (Ito et al., 2008). In concordance, we show that ICOSLG antibody blockade leads to a strong decrease in the IL-10 level of the co-culture media supernatant. Intracellularly, ICOS activation promotes FOXP3 transcription, and in turn, FOXP3- and NFAT-dependent transactivation of IL10 and TGFB1 (Li and Xiong, 2020). Additionally, ICOS+FOXP3+ Tregs play an important role during fetal development and pregnancy as both cord blood and the newborn’s thymus already contain ICOS+FOXP3+ Tregs (Takahata et al., 2004; Ito et al., 2008). Furthermore, the newborn’s thymus contains more ICOS+FOXP3+ Tregs than adult peripheral blood and these Tregs express the thymus emigration marker CD31 which implies that the ICOS+ Treg subset is derived directly from the thymus and does not develop out of ICOS−FOXP3+ Tregs (Takahata et al., 2004; Ito et al., 2008). The presence of ICOS+ Tregs during fetal development is of special interest because it has been demonstrated by twin studies that the translocation t(4;11) arises in utero. (Gale et al., 1997; Wiemels et al., 1999; Greaves and Wiemels, 2003; Greaves et al., 2003). During pregnancy, the fetus is protected from the mother’s semiforeign immune system through placental trophoblast cells which surround fetal tissues. ICOSLG is highly expressed throughout pregnancy by the extravillous trophoblast cells (EVT) (Petroff et al., 2005) and has been validated as a mediator of tolerance at the fetomaternal interface during pregnancy (Riella et al., 2013). Furthermore, EVTs mediate the invasion and differentiation of decidual Tregs which suppress T-cell responses during pregnancy through the secretion of IL-10 (Aluvihare et al., 2004; Tilburgs et al., 2008; Du et al., 2014; Svensson-Arvelund et al., 2015; Salvany-Celades et al., 2019). Taken together, it is highly likely that the ICOSLG/ICOS interaction also provides an immune privilege to the fetomaternal interface. Recombining this knowledge with the results of our study leads to the suggestion that t(4;11) iALL depends on an immune privilege firstly to occur in utero and secondly to relapse in the BM. Accordingly, the ICOSLG-mediated Treg development in the leukemic BM niche could reflect the immune privilege during pregnancy, and thus could be a prerequisite for relapse development. Correspondingly, switching the immune-privileged leukemic BM niche toward immune-susceptibility holds promise to prevent relapse or to treat relapse-associated therapy resistance.
Here, we have shown that elevated ICOSLG expression at diagnosis is predictive for relapse development, and that targeting ICOSLG with a neutralizing mAb prevents the ICOSLG-mediated Treg induction in vitro. Based on these findings, we propose the clinical investigation of ICOSLG targeting therapies such as the α-ICOSLG fully human mAb Prezalumab. Prezalumab (AMG 557/MEDI 5872) has been tested clinically for the treatment of autoimmune diseases such as systemic lupus erythematosus (SLE) and active lupus arthritis in three phase I trials: NCT02391259, NCT00774943, and NCT01683695 (Sullivan et al., 2016; Cheng et al., 2018). In the first and second trial, 112 adult patients with mild SLE were enrolled and the safety and tolerability of single (1.8-210 mg s.c. or 18 mg i.v.) vs. multiple (6-210 mg s.c. every other week) dose administration of Prezalumab have been shown. Importantly, only non-neutralizing α-Prezalumab antibodies were observed. The third trial demonstrated the long-term (23 weeks) safety and tolerability of Prezalumab among 10 patients. However, Prezalumab failed to demonstrate clinical improvement in a phase IIa study (NCT02334306) for the treatment of primary Sjögren’s syndrome halting all further clinical investigation (Mariette et al., 2019; Pontarini et al., 2020).
Unfortunately, up to now, no studies were conducted to evaluate the safety of Prezalumab in infants or children with ALL. Regarding checkpoint inhibition in general, an interim analysis is available of the KEYNOTE-051 trial (NCT02332668), a phaseI/II study evaluating the safety and efficacy of Pembrolizumab (α-PD-L1) in children >6 months with advanced melanoma or PD-L1 positive solid tumor or lymphoma (Geoerger et al., 2020). Three patients aged 6 months-2 years, 22 aged 2-5 years, 25 aged 6-9 years, and 104 aged 10-17 years were enrolled. The study proved Pembrolizumab checkpoint inhibition safe in children as serious adverse events occurred only in 9% of patients (pyrexia, hypertension, and pleural effusion). Besides, another phase I/II trial proved the safety and tolerability of the PD-1 checkpoint inhibitor Nivolumab in children >1 year with lymphoma (Davis et al., 2020). These studies demonstrate that PD-1/PD-L1 immune checkpoint inhibition is safe in young children; however, the safety of ICOSLG-directed mAbs remains to be demonstrated in infants and children.
In summary, we provide a molecular rationale for the clinical evaluation of immune-based therapies targeting the ICOSLG/ICOS interaction for the treatment and prevention of relapse in infants with t(4;11) ALL. Further studies are needed to clarify whether t(4;11)-derived neoantigens lead to immune responses in infants and if that explains the potential demand of the disease for an immune-privileged site firstly to originate in utero and secondly to relapse in the BM. Even though these questions remain unanswered, we communicate our findings to enable a rapid translation into the clinical investigation.
Limitations of the study
The conclusion of this study has been obtained by in vitro studies, and thus, an appropriate mouse model is still missing. Also, the co-culture experiments with genetically modified t(4;11) cells, overexpressing the ICOSLG, were performed in vitro with a limited time window (48 h) and without MHC matching. In addition, we used a neutralizing commercial antibody against ICOSLG and not the clinically tested Prezalumab (AMGEN) because this antibody was not available to us.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat Anti-Rabbit IgG, polyclonal antibody, Horseradish Peroxidase conjugated (1:10000; WB) | abcam | Abcam Cat# ab6721, RRID: AB_955447 |
| DYKDDDDK Tag (D6W5B) Rabbit, monoclonal antibody, unconjugated (1/1,000 WB and ChIP) | Cell Signaling Technology | Cell Signaling Technology Cat# 14793, RRID: AB_2572291 |
| Rabbit Anti-Actin, polyclonal antibody, unconjugated (1/1,000 WB) | Sigma-Aldrich | Sigma-Aldrich Cat# A2066, RRID: AB_476693 |
| Rabbit Recombinant Anti-ICOS Ligand/ICOSL, monoclonal antibody, unconjugated (1/1,000 WB) | abcam | ab209262 RRID not found |
| Mouse Anti-Human CD3, monoclonal antibody, APC-H7 conjugated (flow cytometry) | BD | BD Biosciences Cat# 560176, RRID: AB_1645475 |
| Mouse Anti-Human CD4, monoclonal antibody, PE-Cy7 conjugated (flow cytometry) | BD | BD Biosciences Cat# 557852, RRID: AB_396897 |
| Mouse Anti-Human CD8, monoclonal antibody, BV510 conjugated (flow cytometry) | BD | BD Biosciences Cat# 563919, RRID: AB_2722546 |
| Mouse Anti-Human CD25 (IL-2 Receptor α), monoclonal antibody, BV421 conjugated (flow cytometry) | BD | BD Biosciences Cat# 564033, RRID: AB_2738555 |
| Mouse Anti-Human FoxP3, monoclonal antibody, AF647 conjugated (flow cytometry) | BD | BD Biosciences Cat# 561184, RRID: AB_10584328 |
| Mouse Anti-Human CD278 (ICOS), monoclonal antibody, BB515 conjugated (flow cytometry) | BD | BD Biosciences Cat# 564549, RRID: AB_2738840 |
| Mouse Anti-Human B7-h2, monoclonal antibody, neutralizing, unconjugated (neutralization assays) | RnD Systems | R and D Systems Cat# MAB165, RRID: AB_2122734 |
| Mouse IgG1 kappa isotype control, functional grade (neutralization assays) | Thermo Fisher | Thermo Fisher Scientific Cat# 16-4714-85, RRID: AB_470162 |
| Bacterial and virus strains | ||
| NEB® 5-alpha competent E. coli; DH5α™ derivative | NEB | NEB Cat# C2987 |
| Biological samples | ||
| see Tables | ||
| Chemicals, peptides, and recombinant proteins | ||
| Q5® High-Fidelity DNA Polymerase | NEB | NEB Cat# M0491L |
| SuperScript™ II Reverse Transcriptase | Invitrogen | Invitrogen 18064071 |
| Annexin V Pacific Blue | Invitrogen | Invitrogen A35122 |
| SfiI restriction enzyme | NEB | NEB Cat# R0123L |
| T4 DNA ligase | NEB | NEB Cat# M0202S |
| Clarity Max Western ECL Substrate | Bio-Rad | Bio-Rad Cat# 1705062 |
| Protease inhibitory cocktail tablets | Roche | Roche 4693116001 |
| Critical commercial assays | ||
| AmaxaTM Cell Line NucleofectorTM Kit R | Lonza | VCA-1001 |
| PierceTM BCA Protein Assay Kit (23225, thermo scientific) | Thermo Scientific | Thermo Scientific 23225 |
| IL-10 (human) ELISA Kit | Enzo | Enzo ADI-900-036 |
| IL-2 ELISA Kit (human) | Aviva | Aviva OKBB00179 |
| Accel-NGS® 2S plus DNA library kit | Swift Biosciences | Swift Biosciences 21024 |
| Swift Unique Dual Indexing Primer Kit | Swift Biosciences | Swift Biosciences X9096 |
| Accel-NGS 2S Truncated Adapters | Swift Biosciences | Swift Biosciences 28196 |
| Deposited data | ||
| All Chip-Seq data have been deposited in the NCBI Geobus database | GSE205652 | |
| Experimental models: Cell lines | ||
| SEM | DSMZ | ACC 546 |
| Oligonucleotides | ||
| GAPDH_fwd_short 5′-GGTCACCAGGGCTGC-3′ | this paper | N/A |
| GAPDH_rev_short 5′-CGTTCTCAGCCTTGA-3′ | this paper | N/A |
| ICOSLG_fwd 5′-TGACATCGGAGAGAGAGACAAG-3′ | this paper | N/A |
| ICOSLG_rev 5′-ACGCTTTTGAAAGGGCCTCA-3′ | this paper | N/A |
| EGR3.SfiI_fwd 5′-GGCCTCTGAGGCCACCATGAC CGGCAAACTCGCC-3′ |
this paper | N/A |
| EGR3cFLAG.SfiI_rev 5′-GGCCTGACAGGCCTTACTTATCG TCATCGTCCTTGTAGTC-3′ |
this paper | N/A |
| EGR3.SF1_fwd 5′-GGCCTAGCAAGACACCGC-3′ | this paper | N/A |
| HOXA9_fwd_short 5′-CCACGCTTGACACTCACACT-3′ | this paper | N/A |
| HOXA9_rev_short 5′-AGTTGGCTGCTGGGTTATTG-3′ | this paper | N/A |
| IRX1_fwd_short 5′-GGATCTCAGCCTCTTCTCG-3′ | this paper | N/A |
| IRX1_rev_short 5′-GTGGAGACCTGCGTGAGG-3′ | this paper | N/A |
| EGR3_fwd_short 5′-CAGCGACTCGGTAGTCCATT-3′ | this paper | N/A |
| EGR3_rev_short 5′-TAGGTCACGGTCTTGTTGCC-3′ | this paper | N/A |
| ICOSLG_fwd_short 5′-AGTCCGGAGACAGAGCTCAC-3′ | this paper | N/A |
| ICOSLG_rev_short 5′-CAGTCTGGGAGTCCATGCTC-3′ | this paper | N/A |
| ICOSLG_fwd_ChIP1 5′-ACCGGGACCCATGGCA-3′ | this paper | N/A |
| ICOSLG_rev_ChIP1 5′-CTCCCTCCTTCCAGCGTTC-3′ | this paper | N/A |
| ICOSLG_fwd_ChIP2 5′-GTGAGCCGGGGAAGGA-3′ | this paper | N/A |
| ICOSLG_rev_ChIP2 5′-ATGGGTCCCGGTGCCA-3′ | this paper | N/A |
| ICOSLG_fwd_ChIP3 5′-GCAGAGCCGAACTTTCCG-3′ | this paper | N/A |
| ICOSLG_rev_ChIP3 5′-TGGAGGCAGCCGTGTC-3′ | this paper | N/A |
| ICOSLG_fwd_ChIP4 5′-CCTCAGAGCCAGTGTTAAGC-3′ | this paper | N/A |
| ICOSLG_rev_ChIP4 5′-CGCATGCATGAATGGAAGGA-3′ | this paper | N/A |
| ICOSLG_fwd_ChIP5 5′-GCCCTGCCCAGCTCTT-3′ | this paper | N/A |
| ICOSLG_rev_ChIP5 5′-CAGGCCACGCTGAGAGAC-3′ | this paper | N/A |
| ICOSLG_fwd_ChIP6 5′-GCTCTCCAAGGCCAGCTG-3′ | this paper | N/A |
| ICOSLG_rev_ChIP6 5′-AAGCTGTCACTCCTGGTTCA-3′ | this paper | N/A |
| Recombinant DNA | ||
| pSBtet_Puro sleeping beauty vector backbone | Kowarz et al. (2015) | AddGene #60507 |
| EGR3 open reading frame | this paper | HEK293T cDNA |
| Sleeping beauty transposase plasmid pcGLobin-SB100XCO | Moudgil et al. (2020) | AddGene #154887 |
| Software and algorithms | ||
| GraphPad Prism 7.01 | GraphPad Software Inc. | GraphPad Prism 7.01 |
| ClustVis (Metsalu and Vilo, 2015) | open source | N/A |
| TrimGalore | open source | N/A |
| Bowtie2 | open source | N/A |
| SAMtools | open source | N/A |
| MACS3 | open source | N/A |
| R package “ChIPseeker” | open source | N/A |
| R package “encodeChIPqc” | open source | N/A |
| python tool “deepTools” | open source | N/A |
| Integrative Genomics Viewer (IGV) | open source | N/A |
| Other | ||
| Low Fluorescence PVDF Western Membrane | Abcam | Abcam Cat# ab133411 |
| Precision Plus Protein™ All Blue Standards; marker used for Western Blots with fluorescence detection | Bio-Rad | Bio-Rad Cat# 1610373EDU |
| Color Protein Standard Broad Range | NEB | NEB P7719S |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Rolf Marschalek (Rolf.marschalek@em.uni-frankfurt.de).
Materials availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Experimental model and subject details
Animals
Not applicable.
Cell culture
SEM cells (ACC 546) and isolated primary T-cells were cultivated under sterile conditions. SEM cells were maintained in RPMI 1640 (RPMI-HA, Capricorn Scientific) supplemented with 10% FBS (FBS-11A, Capricorn Scientific), 2 mM L-glutamine (STA-B, Capricorn Scientific), 100 U/ml penicillin and 100 μg/mL streptomycin (PS-B, Capricorn Scientific), preheated to 37°C prior to use. Primary T-cells were maintained in DMEM high glucose (DMEM-HPA, Capricorn Scientific) supplemented with 10% human plasma (derived from each donor), 2 mM L-glutamine (STA-B, Capricorn Scientific), 100 U/ml penicillin and 100 μg/ml streptomycin (PS-B, Capricorn Scientific), preheated to 37°C prior to use. Cells were kept at 37°C in 5% CO2 and a relative humidity of 95%. SEM cells were passaged twice a week with complete media exchange maintaining a density of ∼1 x 106 cells/mL. For this, cell suspension was centrifuged at 200 x g for 5 min and subsequently cell pellet was resuspended in new prewarmed media. T-cells were immediately activated following isolation as described below and underwent co-culture experiments, thus no passaging was necessary.
Co-culture study
1x106 SEM::EGR3 or SEM::mock were co-cultured for 48h with 5x105 CD4+ T-cells and 5x105 CD8+ T-cells in 1 mL of DMEM-HPA (Capricorn Scientific) supplemented with 10% human plasma (derived from each donor), 2 mM L-glutamine (STA-B, Capricorn Scientific), 100 U/ml penicillin and 100 μg/mL streptomycin (PS-B, Capricorn Scientific), preheated to 37°C prior to use. T-lymphocytes were isolated from the peripheral blood mononuclear cells (PBMC) of healthy donors using magnetic bead separation as described below. Prior to co-culture, isolated T-cells were stimulated with the T-cell Acitvation/Expansion Kit human (130-091-441, Mitlenyi Biotec) for 24h according to the manufacturer’s recommendations. SEM::EGR3 and SEM::mock were induced with 1 μg/mL Doxycycline 24h prior to co-culture and 20 μg/mL neutralizing monoclonal mouse α-human ICOSLG antibody (RnD Systems®) or mouse IgG1 isotype control (Invitrogen™) were added 2h prior to co-culture. After 48h of co-culture cells were collected and stained with FACS antibodies and supernatant immediately frozen in liquid nitrogen and stored for ELISA experiments.
Method details
Plasmid cloning
EGR3 open reading frame (ORF) was amplified from HEK293T cDNA with the primers EGR3.SfiI_fwd and EGR3cFLAG.SfiI_rev using Q5® High-Fidelity DNA Polymerase (NEB, M0491L). EGR3.SfiI_fwd adds the 5’-SfiI restriction site GGCCTCTGAGGCC upstream of ATG and EGR3cFLAG.SfiI_rev adds a FLAG (DYKDDDDK) tag to the ORF and the 3’-SfiI restriction site GGCCTGTCAGGCC downstream of TAA. Equipped with the SfiI restriction sites, the ORF was ligated into SfiI-digested pSBtet_Puro backbone (R0123L, NEB) using T4 DNA ligase (M0202S, NEB). The yielded plasmid pSBtet_EGR3cFLAG_Puro was sequence validated.
Establishment of the stable cell lines SEM::mock and SEM::EGR3
Electroporation of SEM cells was performed using the NucleofectorTM 2b device (AAB-1001, Lonza) together with the AmaxaTM Cell Line NucleofectorTM Kit R (VCA-1001) and the nucleofection program T-016 according to the manufacturer’s recommendations. 0.1 μg sleeping beauty transposase plasmid pcGLobin-SB100XCO together with 1.9 μg sleeping beauty plasmid (pSBtet_ohneLuc_Puro = SEM::mock; pSBtet_EGR3cFLAG_Puro = SEM::EGR3; pSBtet_Luc_GP = green fluorescence postive control) were electroporated. pSBtet_ohneLuc_Puro and pSBtet_EGR3cFLAG_Puro do not have a fluorescence marker in their backbone to avoid interference in multicolor flow cytometry. To monitor transfection and selection efficiency with a fluorescence microscope, pSBtet_Luc_GP containing a GFP marker in the backbone was treated equally and served as a reporter. Cells were selected six times (once to twice per week) with Puromycin 2 μg/mL for 24-48h until all reporter cells displayed green fluorescence. Proof of transgene expression was performed using PCR and subsequent gel electroporation, qRT-PCR, and western blot 48h after induction of transgene expression with Doxycycline 1 μg/mL.
Human T-cell isolation
PBMC were isolated from the blood of healthy adult donors using Ficoll-Paque density gradient centrifugation. Therefore, 15 mL blood were diluted with 20 mL PBS (PBS-1A, Capricorn Scientific) supplemented with 0.5% BSA and overlayed with Lymphocyte Separation Media (MS00ZZ1002, Biowest) followed by 30 min centrifugation at 400 g without break leading to separation of plasma and PBMC from remaining blood cells. Plasma was stored for T-cell culture media preparation. PBMC layer was moved to another tube and resuspended in 50 mL PBS supplemented with 0.5% BSA followed by centrifugation at 400 g for 5 min with break. Pellet was resuspended in 10 mL RBC lysis buffer for human liquid (J62990, Alfa Aesar) and 10 min incubated at room temperature to lyse remaining erythrocytes. Afterwards, cell suspension was washed with 30 mL PBS supplemented with 0.5% BSA and isolated PBMC were counted. CD4+ and CD8+ cells were magnetically separated using CD4 Micro-Beads, human (130-045-101, Miltenyi Biotec) or CD8 MicroBeads, human (130-045-201, Miltenyi Biotec), MS columns (130-042-201, Miltenyi Biotec) and OctoMACSTM separator (130-042-109, Miltenyi Biotec) according to the manufacturer’s protocols. Success of T-cell isolation was checked with flow cytometry prior to activation.
Gene expression analysis using qRT-PCR
RNA was extracted from ∼5x107 SEM::mock and SEM::EGR3 48h after transgene induction with 1 μg/mL Doxycycline. cDNA was synthesized out of 2 μg RNA using random hexamer N6 primers and SuperScript™ II Reverse Transcriptase (18064071, Invitrogen). PCR primers used in the study are summarized in STAR Methods. qRT-PCR was performed with the StepOnePlus system. ΔCT mean values were calculated out of triplicates using GAPDH expression as a reference, ΔΔCT values are related to the respective expression of SEM::mock.
Protein expression analysis using Western blot experiments
Protein was extracted from ∼5x107 SEM::mock and SEM::EGR3 48h after transgene induction with 1 μg/mL Doxycycline. Cells were lysed in 1 mL protein extraction buffer (150 mM NaCl, 10 mM Tris-HCl, 1mM EDTA, 1mM EGTA, 0.2 mM sodium ortho-vanadate, 0.2 mM PMSF, 1% Triton-X-100, 0.5% NP-40, freshly added protease inhibitory cocktail (4693116001, Roche) under constant agitation for 30 min at 4°C. Centrifugation at 400 g, 4°C enabled separation of the supernatant (= protein lysate) from cell debris. Protein lysate was quantified using PierceTM BCA Protein Assay Kit (23225, Thermo Scientific) according to the manufacturer’s recommendations. 20 μg protein lysate were electrophoretically separated using Mini-PROTEAN TGX Stain-Free Gels 4-15% (4568085, Bio-Rad) and Color Protein Standard Broad Range (P7719S, NEB). Proteins were blotted onto Low Fluorescence Western Memranes PVDF (ab133411, Abcam) in Towbin buffer (25 mM Tris, 192 mM Glycin, 15% methanol, 0.01% SDS, pH = 8.3) at 110 V for 70 min. Membranes were blocked using 5% BSA in TBS-T for 1h at room temperature and primary antibody incubation (1/1,000) performed o/n at 4°C. Membranes were washed with TBS-T and incubated with HRP-conjugated secondary antibody (1/10,000) for 1h at room temperature. Chemiluminescence detection was performed using Clarity™ Western ECL Substrate (170-5061, Bio-Rad) and ChemiDoc™ XRS+ system (1708265, Bio-Rad). Antibodies used for western blot are listed in STAR Methods.
Flow cytometry experiments
Cells were blocked and stained with FACS antibodies (BD Biosciences) according to the manufacturer’s recommendations. Half of the cell suspension was stained with α-CD3, -CD4, -CD8, Annexin V and 7-AAD to assess the viability of the T-cells after co-culture. The remaining cells were stained with α-CD3, -CD4, -CD8, -CD25, -ICOS, fixed, permeabilized and stained with α-FOXP3. Cells were analysed with a BD FACSVerseTM. The gating strategy is explained in Figure 6A. All antibodies used for this strategy are described in STAR Methods.
ELISA experiments
The IL-2 and IL-10 concentrations of the co-culture supernatants were assessed using IL-10 (human) ELISA Kit (ADI-900-036, Enzo) and IL-2 ELISA Kit (human) (OKBB00179, Aviva) according to the manufacturer’s recommendations.
Chromatin immunoprecipitation qRT-PCR and sequencing
2x107 SEM::EGR3 and SEM::mock were induced with 1 μg/mL Doxycycline for 48h followed by ChIP procedure as described by Kühn et al., (2016). The transcription factor binding site prediction tool JASPAR (Sandelin et al., 2004) was used to detect potential EGR3 binding sites 1 kb up- and downstream of the transcription start site of ICOSLG. Six primer pairs were used to cover the nine highest scoring potential binding sites in qRT-PCR. Primers used for ChIP-qRT-PCR are summarized in STAR Methods. Antibodies used for ChIP are described in STAR Methods. The ChIP-Seq library was prepared using the Accel-NGS® 2S plus DNA library kit (21024, Swift Biosciences) together with Swift Unique Dual Indexing Primer Kit (X9096, Swift Biosciences) and Accel-NGS 2S Truncated Adapters (28196, Swift Biosciences) according to the manufacturer’s recommendations with indexing by PCR (10 cycles). NGS was performed using the MiSeq system (SY-410-1003, Illumina) and the MiSeq Reagent Kit v2 (MS-102-2003, Illumina). The ChIP-Seq analysis was performed in several steps. As a first step, in order to remove adapters contamination, raw Illumina paired-end reads were processed with TrimGalore. In a second step, we mapped paired-end sequence reads against the human reference genome GRCh38 with Bowtie2. SAMtools was then used to convert the resulting sam files to bam files and to generate the index files. Once the chromatin immunoprecipitate (IP) and control (input) libraries have been aligned, we proceeded with the peak calling in order to determine the regions in which the IP signal is significantly higher than the input signal (=background). We performed the peak calling with the tool MACS3 with the option BAMPE active because of paired end reads. The result of the peak calling (narrowPeak file) was then used with the R package “ChIPseeker” to annotate the peaks to neighboring genes and genomics regions. We then performed a number of routine quality controls such as PCR bottleneck coefficient (PBC), fraction of reads in peaks (FriP) using the R package “encodeChIPqc” and fingerprint plot using “plotFingerprint” from the python tool “deepTools”. In the final step we used the functions “bamCoverage” and “bamCompare” from “deepTools” to generate signal files in the bigwig format. We generated either separate bigwig files for IP and control with “bamCoverage” or a single bigwig file in which we subtracted the background noise from the IP with “bamCompare”. Finally, the signal files, along with narrowPeak files and bam files, can be visualized using the Integrative Genomics Viewer (IGV). Data have been deposited in the NCBI Geobus database with the accession number GSE205652.
Patient gene expression study
Informed consent was obtained for all patients by the respective study centers that provided patient RNA. All patients displayed a pro-B phenotype and were diagnosed between 0 and 12 months of age (infants). RNA was extracted from blood at day of initial diagnosis (dx cohort, n = 50) or relapse diagnosis (rel cohort, n = 18) by the respective study centers. The dx cohort is solely composed of t(4;11) iALL cases, the rel cohort is composed of different translocations as indicated in Table 2 . cDNA was synthesized out of 1 μg RNA as described above. HOXA9, IRX1, EGR3 and ICOSLG gene expression was measured as triplicates using qRT-PCR (StepOnePlus system) and ΔCT mean values were calculated using GAPDH expression as a reference. Relative expressions were calculated as ratio (reference/target) = 2CT(GAPDH) - CT(target) = 2−ΔCT. Pearson correlation was calculated for the gene expression datasets IRX1- > EGR3, IRX1- > ICOSLG and EGR3- > ICOSLG using GraphPad Prism software. Clinical outcome of the patients was provided by the study centers.
Quantification and statistical analysis
Statistical tests were performed using GraphPad Prism 7.01 and settings are indicated in figure legends. Principal component analysis (PCA) was performed using ClustVis (Metsalu and Vilo, 2015) with patient sample ΔCT mean value assigned to the dx ICOSLGhi, dx ICOSLGlo or rel cohort, respectively. PCA was performed as singular value decomposition (SVD) and unit variance scaling for rows was applied. The PCA heatmap was generated with correlation as clustering distance for rows, average as clustering method for rows and columns, and Manhattan as clustering distance for columns.
Kaplan-Meier plotting and log rank test were performed using GraphPad Prism 7.01. EFS was defined as the time from diagnosis to first failure (induction failure, relapse, death, or second malignant neoplasm). Time was censored at last follow-up if no events had been observed. Curves were estimated with Kaplan-Meier, with SEs calculated according to Greenwood, and compared using the log rank test.
Acknowledgments
We thank all our collaborators for fruitful discussions and Birthe Fedders for sample composition and sending. This work has been funded in part by DFG grants MA 1876/12-1 and MA 1876/13-1.
Author contributions
MK and RM wrote the article; MK, HB, and RM planned and interpreted experiments; MK generated cell lines, performed qRT-PCR, Western blot, co-culture experiments, flow cytometry and ELISA; ALS performed plasmid cloning; CM, PL, and MK performed ChIP-Seq; CM and PL helped to write the article; AD and MK performed ChIP-qRT-PCR; JA, GC, CE, ACE, HC, MB, GiC, PDL, and MGV provided patient samples and outcome data, and helped to analyze clinical data; LD and HB provided blood samples and contributed to T-cell isolation; RM supervised the study and provided funding; all authors reviewed and approved the article.
Declaration of interests
The authors declare that they have no competing interests.
Published: July 15, 2022
Data and code availability
All experimental data or any additional information required to reanalyze the data reported in this paper are available from the Lead contact. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the Lead contact upon request.
References
- Agraz-Doblas A., Bueno C., Bashford-Rogers R., Roy A., Schneider P., Bardini M., Ballerini P., Cazzaniga G., Moreno T., Revilla C., et al. Unraveling the cellular origin and clinical prognostic markers of infant B-cell acute lymphoblastic leukemia using genome-wide analysis. Haematologica. 2019;104:1176–1188. doi: 10.3324/haematol.2018.206375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O., Freeman G.J., Meyer E.H., Greenfield E.A., Chang T.T., Sharpe A.H., Berry G., DeKruyff R.H., Umetsu D.T. Antigen-specific regulatory T cells develop via the ICOS–ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- Aluvihare V.R., Kallikourdis M., Betz A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Anderson P.O., Manzo B., Sundstedt A., Minaee S., Symonds A., Khalid S., Rodriguez-Cabezas M., Nicolson K., Li S., Wraith D., Wang P. Persistent antigenic stimulation alters the transcription program in T cells, resulting in antigen-specific tolerance. Eur. J. Immunol. 2006;36:1374–1385. doi: 10.1002/eji.200635883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A.K., Ma J., Wang J., Chen X., Gedman A.L., Dang J., Nakitandwe J., Holmfeldt L., Parker M., Easton J., et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat. Genet. 2015;47:330–337. doi: 10.1038/ng.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Watanabe T., Saito Y., Kuroki Y., Hijikata A., Takagi M., Tomizawa D., Eguchi M., Eguchi-Ishimae M., Kaneko A., et al. Identification of CD34+ and CD34− leukemia-initiating cells in MLL-rearranged human acute lymphoblastic leukemia. Blood. 2015;125:967–980. doi: 10.1182/blood-2014-03-563304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Boyd A.L., Campbell C.J., Hopkins C.I., Fiebig-Comyn A., Russell J., Ulemek J., Foley R., Leber B., Xenocostas A., Collins T.J., Bhatia M. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. J. Exp. Med. 2014;211:1925–1935. doi: 10.1084/jem.20140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Pieters R., Biondi A. How I treat infant leukemia. Blood. 2019;133:205–214. doi: 10.1182/blood-2018-04-785980. [DOI] [PubMed] [Google Scholar]
- Burt R., Dey A., Aref S., Aguiar M., Akarca A., Bailey K., Day W., Hooper S., Kirkwood A., Kirschner K., et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134:1415–1429. doi: 10.1182/blood.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Shen S., Gorentla B.K., Gao J., Zhong X.P. Murine regulatory T cells contain hyperproliferative and death-prone subsets with differential ICOS expression. J. Immunol. 2012;188:1698–1707. doi: 10.4049/jimmunol.1102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Y., Miyanishi M., Wang S.K., Yamazaki S., Sinha R., Kao K.S., Seita J., Sahoo D., Nakauchi H., Weissman I.L. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–227. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Hao S., Liu Y., Pang Y., Ma S., Dong F., Xu J., Zheng G., Li S., Yuan W., Cheng T. Leukemic marrow infiltration reveals a novel role for Egr3 as a potent inhibitor of normal hematopoietic stem cell proliferation. Blood. 2015;126:1302–1313. doi: 10.1182/blood-2015-01-623645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L.E., Amoura Z., Cheah B., Hiepe F., Sullivan B.A., Zhou L., Arnold G.E., Tsuji W.H., Merrill J.T., Chung J.B. Brief report: a randomized, double-blind, parallel-group, placebo-controlled, multiple-dose study to evaluate AMG 557 in patients with systemic lupus erythematosus and active lupus arthritis. Arthritis Rheumatol. 2018;70:1071–1076. doi: 10.1002/art.40479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Lutz M., Zarek P., Anders R., Kersh G., Powell J. Opposing regulation of T cell function by Egr-1/NAB2 and Egr-2/Egr-3. Eur. J. Immunol. 2008;38:528–536. doi: 10.1002/eji.200737157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle A.J., Gutierrez-Ramos J.C. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat. Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- Davis K.L., Fox E., Merchant M.S., Reid J.M., Kudgus R.A., Liu X., Minard C.G., Voss S., Berg S.L., Weigel B.J., Mackall C.L. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020;21:541–550. doi: 10.1016/S1470-2045(20)30023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen E.M.C., de Lorenzo P., CaMpbell M., Felice M., Ferster A., Hann I., VorA A., Hovi L., Escherich G., Li C.K., et al. Outcome of relapsed infant acute lymphoblastic leukemia treated on the interfant-99 protocol. Leukemia. 2016;30:1184–1187. doi: 10.1038/leu.2015.246. [DOI] [PubMed] [Google Scholar]
- Du M.-R., Guo P.F., Piao H.L., Wang S.C., Sun C., Jin L.P., Tao Y., Li Y.H., Zhang D., Zhu R., et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal–fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J. Immunol. 2014;192:1502–1511. doi: 10.4049/jimmunol.1203425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C.-W., Shi J., Chen J., Wang B., Yu Y.H., Qin X., Zhou X.C., Cai Y.J., Li Z.Q., Zhang F., et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell. 2014;25:778–793. doi: 10.1016/j.ccr.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Duhen T., Duhen R., Lanzavecchia A., Sallusto F., Campbell D.J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget J., Bendriss-Vermare N., Gobert M., Durand I., Olive D., Biota C., Bachelot T., Treilleux I., Goddard-Leon S., Lavergne E., et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 2012;72:6130–6141. doi: 10.1158/0008-5472.CAN-12-2409. [DOI] [PubMed] [Google Scholar]
- Feuerer M., Rocha M., Bai L., Umansky V., Solomayer E.F., Bastert G., Diel I.J., Schirrmacher V. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer. 2001;92:96–105. [PubMed] [Google Scholar]
- Feuerer M., Beckhove P., Garbi N., Mahnke Y., Limmer A., Hommel M., Hämmerling G.J., Kyewski B., Hamann A., Umansky V., Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat. Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- Fujisaki J., Wu J., Carlson A.L., Silberstein L., Putheti P., Larocca R., Gao W., Saito T.I., Celso C.L., Tsuyuzaki H., et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale K.B., Ford A.M., Repp R., Borkhardt A., Keller C., Eden O.B., Greaves M.F. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc. Natl. Acad. Sci. U S A. 1997;94:13950–13954. doi: 10.1073/pnas.94.25.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoerger B., Kang H.J., Yalon-Oren M., Marshall L.V., Vezina C., Pappo A., Laetsch T.W., Petrilli A.S., Ebinger M., Toporski J., et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020;21:121–133. doi: 10.1016/S1470-2045(19)30671-0. [DOI] [PubMed] [Google Scholar]
- Greaves M.F., Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat. Rev. Cancer. 2003;3:639–649. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- Greaves M.F., Maia A.T., Wiemels J.L., Ford A.M. Leukemia in twins: lessons in natural history. Blood. 2003;102:2321–2333. doi: 10.1182/blood-2002-12-3817. [DOI] [PubMed] [Google Scholar]
- Han Y., Dong Y., Yang Q., Xu W., Jiang S., Yu Z., Yu K., Zhang S. Acute myeloid leukemia cells express ICOS ligand to promote the expansion of regulatory T cells. Front. Immunol. 2018;9:2227. doi: 10.3389/fimmu.2018.02227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Furuhashi K., Ishii H., Li H.W., Pinho S., Ding L., Robson S.C., Frenette P.S., Fujisaki J. CD150high bone marrow Tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22:445–453.e5. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y., Kakiuchi M., Robson S.C., Fujisaki J. CD150high CD4 T cells and CD150high regulatory T cells regulate hematopoietic stem cell quiescence via CD73. Haematologica. 2019;104:1136–1142. doi: 10.3324/haematol.2018.198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotfilder M., Röttgers S., Rosemann A., Schrauder A., Schrappe M., Pieters R., Jürgens H., Harbott J., Vormoor J. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19− cells) Are Present in Primitive Lymphoid-Restricted CD34+CD19− Cells. Cancer Res. 2005;65:1442–1449. doi: 10.1158/0008-5472.CAN-04-1356. [DOI] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Ito T., Hanabuchi S., Wang Y.H., Park W.R., Arima K., Bover L., Qin F.X.F., Gilliet M., Liu Y.J. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata R., Hyoung Lee J., Hayashi M., Dianzani U., Ofune K., Maruyama M., Oe S., Ito T., Hashiba T., Yoshimura K., et al. ICOSLG-mediated regulatory T cell expansion and IL-10 production promote progression of glioblastoma. Neuro Oncol. 2019;22:333–344. doi: 10.1093/neuonc/noz204. noz204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Wilson C.S., Harvey R.C., Chen I.M., Murphy M.H., Atlas S.R., Bedrick E.J., Devidas M., Carroll A.J., Robinson B.W., et al. Gene expression profiles predictive of outcome and age in infant acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119:1872–1881. doi: 10.1182/blood-2011-10-382861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayyamian S., Hutloff A., Büchner K., Gräfe M., Henn V., Kroczek R.A., Mages H.W. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc. Natl. Acad. Sci. U S A. 2002;99:6198–6203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarz E., Löscher D., Marschalek R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 2015;10:647–653. doi: 10.1002/biot.201400821. [DOI] [PubMed] [Google Scholar]
- Kühn A., Löscher D., Marschalek R. The IRX1/HOXA connection: insights into a novel t(4;11)- specific cancer mechanism. Oncotarget. 2016;7:35341–35352. doi: 10.18632/oncotarget.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Garcia M., Weng L., Jung X., Murakami J.L., Hu X., McDonald T., Lin A., Kumar A.R., DiGiusto D.L., et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2018;32:575–587. doi: 10.1038/leu.2017.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-J., Kim S.N., Jeon M.S., Yi T., Song S.U. ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci. Rep. 2017;7:44486. doi: 10.1038/srep44486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Viseur C., Hotfilder M., Bomken S., Wilson K., Röttgers S., Schrauder A., Rosemann A., Irving J., Stam R.W., Shultz L.D., et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14:47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.-Y., Xiong X.-Z. ICOS+ Tregs: a functional subset of Tregs in immune diseases. Front. Immunol. 2020;11:2104. doi: 10.3389/fimmu.2020.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Miao T., Sebastian M., Bhullar P., Ghaffari E., Liu M., Symonds A., Wang P. The transcription factors Egr2 and Egr3 are essential for the control of inflammation and antigen-induced proliferation of B and T cells. Immunity. 2012;37:685–696. doi: 10.1016/j.immuni.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Wang Y., Cheng H., Liu G., Cheng T., Liu Y., Liu L. System modeling reveals the molecular mechanisms of HSC cell cycle alteration mediated by Maff and Egr3 under leukemia. BMC Syst. Biol. 2017;11:91. doi: 10.1186/s12918-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhning M., Hutloff A., Kallinich T., Mages H.W., Bonhagen K., Radbruch A., Hamelmann E., Kroczek R.A. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J. Exp. Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette X., Bombardieri M., Alevizos I., Moate R., Sullivan B., Noaiseh G., Kvarnström M., Rees W., Wang L., Illei G., et al. ACR Meeting Abstracts. 2019. A Phase 2a Study of MEDI5872 (AMG557), a Fully Human Anti-ICOS Ligand Monoclonal Antibody in Patients with Primary Sjögren’s Syndrome.https://acrabstracts.org/abstract/a-phase-2a-study-of-medi5872-amg557-a-fully-human-anti-icos-ligand-monoclonal-antibody-in-patients-with-primary-sjogrens-syndrome/ [Google Scholar]
- Martin-Orozco N., Li Y., Wang Y., Liu S., Hwu P., Liu Y.J., Dong C., Radvanyi L. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Cancer Res. 2010;70:9581–9590. doi: 10.1158/0008-5472.CAN-10-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H. The microenvironment in human myeloid malignancies: emerging concepts and therapeutic implications. Blood. 2017;129:1617–1626. doi: 10.1182/blood-2016-11-696070. [DOI] [PubMed] [Google Scholar]
- Metsalu T., Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C., BurmeisTer T., Gröger D., Tsaur G., Fechina L., Renneville A., Sutton R., Venn N.C., EMerenciano M., Pombo-de-Oliveira M.S., et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32:273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min I.M., Pietramaggiori G., Kim F.S., Passegué E., Stevenson K.E., Wagers A.J. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell. 2008;2:380–391. doi: 10.1016/j.stem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil A., Wilkinson M.N., Chen X., He J., Cammack A.J., Vasek M.J., Lagunas T., Jr., Qi Z., Lalli M.A., Guo C., et al. Self-reporting transposons enable simultaneous readout of gene expression and transcription factor binding in single cells. Cell. 2020;182:992–1008.e21. doi: 10.1016/j.cell.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D.G. Production and selection of B lymphocytes in bone marrow: lymphostromal interactions and apoptosis in normal, mutant and transgenic mice. Adv. Exp. Med. Biol. 1994;355:15–20. doi: 10.1007/978-1-4615-2492-2_3. [DOI] [PubMed] [Google Scholar]
- Parkinson R.M., Collins S.L., Horton M.R., Powell J.D. Egr3 induces a Th17 response by promoting the development of γδ T cells. PLoS One. 2014;9:e87265. doi: 10.1371/journal.pone.0087265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff M.G., Kharatyan E., Torry D.S., Holets L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am. J. Pathol. 2005;167:465–473. doi: 10.1016/S0002-9440(10)62990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R., Schrappe M., De Lorenzo P., Hann I., De Rossi G., Felice M., Hovi L., LeBlanc T., Szczepanski T., Ferster A., et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet (Lond, Engl) 2007;370:240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- Pieters R., De Lorenzo P., Ancliffe P., Aversa L.A., Brethon B., Biondi A., Campbell M., Escherich G., Ferster A., Gardner R.A., et al. Outcome of infants younger than 1 Year with acute lymphoblastic leukemia treated with the interfant-06 protocol: results from an international phase III randomized study. J. Clin. Oncol. 2019;37:2246–2256. doi: 10.1200/JCO.19.00261. [DOI] [PubMed] [Google Scholar]
- Pontarini E., Verstappen G.M., Grigoriadou S., Kroese F.G.M., Bootsma H., Bombardieri M. Blocking T cell co-stimulation in primary Sjögren's syndrome: rationale, clinical efficacy and modulation of peripheral and salivary gland biomarkers. Clin. Exp. Rheumatol. 2020;38:222–227. [PubMed] [Google Scholar]
- Rice S., Jackson T., Crump N.T., Fordham N., Elliott N., O’Byrne S., Fanego M.M.L., Addy D., Crabb T., Dryden C., et al. A human fetal liver-derived infant MLL-AF4 acute lymphoblastic leukemia model reveals a distinct fetal gene expression program. Nat. Commun. 2021;12:6905. doi: 10.1038/s41467-021-27270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riella L.V., Dada S., Chabtini L., Smith B., Huang L., Dakle P., Mfarrej B., D'Addio F., Adams L.T., Kochupurakkal N., et al. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am. J. Pathol. 2013;182:2204–2213. doi: 10.1016/j.ajpath.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford M., Collins S., Lutz M.A., Allen A., Huang C.T., Kowalski J., Blackford A., Horton M.R., Drake C., Schwartz R.H., Powell J.D. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat. Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- Salvany-Celades M., van der Zwan A., Benner M., Setrajcic-Dragos V., Bougleux Gomes H.A., Iyer V., Norwitz E.R., Strominger J.L., Tilburgs T. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019;27:2537–2547.e5. doi: 10.1016/j.celrep.2019.04.109. [DOI] [PubMed] [Google Scholar]
- Sandelin A., Alkema W., Engström P., Wasserman W.W., Lenhard B. JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004;32:D91–D94. doi: 10.1093/nar/gkh012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam R.W., Schneider P., Hagelstein J.A.P., van der Linden M.H., Stumpel D.J.P.M., de Menezes R.X., de Lorenzo P., Valsecchi M.G., Pieters R. Gene expression profiling–based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood. 2010;115:2835–2844. doi: 10.1182/blood-2009-07-233049. [DOI] [PubMed] [Google Scholar]
- Sullivan B.A., Tsuji W., Kivitz A., Peng J., Arnold G.E., Boedigheimer M.J., Chiu K., Green C.L., KAliyAperumAl A., Wang C., et al. Inducible T-cell co-stimulator ligand (ICOSL) blockade leads to selective inhibition of anti-KLH IgG responses in subjects with systemic lupus erythematosus. Lupus Sci. Med. 2016;3:e000146. doi: 10.1136/lupus-2016-000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson-Arvelund J., Mehta R.B., Lindau R., Mirrasekhian E., Rodriguez-Martinez H., Berg G., Lash G.E., Jenmalm M.C., Ernerudh J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 2015;194:1534–1544. doi: 10.4049/jimmunol.1401536. [DOI] [PubMed] [Google Scholar]
- Swallow M.M., Wallin J.J., Sha W.C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFα. Immunity. 1999;11:423–432. doi: 10.1016/S1074-7613(00)80117-X. [DOI] [PubMed] [Google Scholar]
- Symeonidou V., Ottersbach K. HOXA9/IRX1 expression pattern defines two subgroups of infant MLL-AF4-driven acute lymphoblastic leukemia. Exp. Hematol. 2021;93:38–43.e5. doi: 10.1016/j.exphem.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata Y., Nomura A., Takada H., Ohga S., Furuno K., Hikino S., Nakayama H., Sakaguchi S., Hara T. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp. Hematol. 2004;32:622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Tallen G., Ratei R., Mann G., Kaspers G., Niggli F., Karachunsky A., Ebell W., Escherich G., Schrappe M., Klingebiel T., et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J. Clin. Oncol. 2010:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- Tang C., Li M.H., Chen Y.L., Sun H.Y., Liu S.L., Zheng W.W., Zhang M.Y., Li H., Fu W., Zhang W.J., et al. Chemotherapy-induced niche perturbs hematopoietic reconstitution in B-cell acute lymphoblastic leukemia. J. Exp. Clin. Cancer Res. 2018;37:204. doi: 10.1186/s13046-018-0859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburgs T., Roelen D.L., van der Mast B.J., de Groot-Swings G.M., Kleijburg C., Scherjon S.A., Claas F.H. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- Trentin L., Giordan M., Dingermann T., Basso G., te Kronnie G., Marschalek R. Two independent gene signatures in pediatric t(4;11) acute lymphoblastic leukemia patients. Eur. J. Haematol. 2009;83:406–419. doi: 10.1111/j.1600-0609.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Vocanson M., Rozieres A., Hennino A., Poyet G., Gaillard V., Renaudineau S., Achachi A., Benetiere J., Kaiserlian D., Dubois B., Nicolas J.F. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J. Allergy Clin. Immunol. 2010;126:280–289.e7. doi: 10.1016/j.jaci.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Wiemels J.L., CazzaniGa G., Daniotti M., Eden O., Addison G., Masera G., Saha V., Biondi A., Greaves M. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354:1499–1503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- Yang W., Lee K.W., Srivastava R.M., Kuo F., Krishna C., Chowell D., Makarov V., Hoen D., Dalin M.G., Wexler L., et al. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat. Med. 2019;25:767–775. doi: 10.1038/s41591-019-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora A.E., Crawford J.C., Allen E.K., Guo X.J., Bakke J., Carter R.A., Abdelsamed H.A., Moustaki A., Li Y., Chang T.C., et al. Pediatric patients with acute lymphoblastic leukemia generate abundant and functional neoantigen-specific CD8 + T cell responses. Sci. Transl. Med. 2019;11:eaat8549. doi: 10.1126/scitranslmed.aat8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Luo Y., Qin S.L., Mu Y.F., Qi Y., Yu M.H., Zhong M. The clinical impact of ICOS signal in colorectal cancer patients. Oncoimmunology. 2016;5:e1141857. doi: 10.1080/2162402X.2016.1141857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Barnett B., Safah H., LaRussa V.F., Evdemon-Hogan M., Mottram P., Wei S., David O., Curiel T.J., Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that Traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All experimental data or any additional information required to reanalyze the data reported in this paper are available from the Lead contact. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the Lead contact upon request.