Abstract
The management of CLL has undergone unprecedented changes over the last decade. Modern targeted therapies are incorporated into clinical practice. Unfortunately, patients have begun to develop resistance or intolerance to multiple classes. Symptomatic patients previously treated with a BTK inhibitor and venetoclax represent a new and rapidly growing unmet need in CLL. Here we define unmet needs in a modern treatment context. We also critically review the literature for PI3K inhibitors and chemoimmunotherapy and lack of data to support their utility following BTK inhibitors and venetoclax. Finally, we suggest opportunities to ensure the continued innovation for patients with CLL.
Keywords: CLL/SLL, BTK, ibrutinib, venetoclax, PI3K
Introduction: A Transformation in the treatment of CLL/SLL
The management paradigm for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has undergone unprecedented changes over the last decade resulting in radically improved outcomes for patients (1,2). The previous cornerstone of treatment, cytotoxic chemotherapy, resulted in remission for many patients but also short and long-term treatment-related morbidities (3). For patients with poor risk disease biology these remissions were short lived (4,5). By comparison, patients can now expect to be treated sequentially with targeted therapies that are both better tolerated, orally administered, and markedly more efficacious (6–10). Despite the dramatically improved outcomes modern targeted therapies have yielded, these agents have now been incorporated into routine clinical practice for a long enough time that patients have begun to develop resistance or intolerance to multiple classes (11–17). These patients represent a new and rapidly growing frontier of unmet medical need in CLL/SLL. Ensuring continued progress for patients with CLL/SLL will require increased focus on this emerging group of patients treated with multiple classes of targeted therapy. Here, we focus on how best to define unmet needs in a modern treatment context and suggest opportunities for key stakeholders/caregivers to ensure the continued innovation our patients deserve.
An Unprecedented Decade of Drug Development in CLL/SLL
Three new classes of targeted agents have been approved for CLL/SLL globally in the last 10 years. These include the BTK inhibitors (ibrutinib, acalabrutinib, zanubrutinib), a BCL2 inhibitor (venetoclax), and the phosphoinositide-3-kinase (PI3Ki) inhibitors (idelalisib and duvelisib) (6–8,10). Of these three classes, BTK inhibitors and venetoclax-based therapy, sometimes combined with anti-CD20 antibodies, are supplanting chemoimmunotherapy (CIT), while PI3K inhibitors are typically reserved for later lines of treatment (1,18–20). While ibrutinib and venetoclax were initially approved in CLL/SLL based on the results from single arm studies in high-risk patients, use of these agents since then has been primarily guided by randomized Phase 3 clinical trials (7,10,11,21–24). Although these studies have provided insights into the efficacy and safety of these drugs, critical evaluation of these studies also reveals important areas of ongoing uncertainty.
Ibrutinib-based therapy has demonstrated superior outcomes (either PFS, OS or both) compared to those seen with ofatumumab, chlorambucil, fludarabine/cyclophosphamide/rituximab (FCR), bendamustine/rituximab (BR) and chlorambucil/obinutuzumab (CO) in five Phase 3 studies (22,25–28). The more selective BTK inhibitor acalabrutinib has demonstrated superior outcomes over those seen with CO and investigators choice of idelalisib/rituximab or BR in two randomized Phase 3 studies (23,29). Venetoclax-based therapy has demonstrated superior outcomes over those seen with CO and BR in 2 randomized studies (21,30). Finally, idelalisib/rituximab and duvelisib have demonstrated improvements over those seen with rituximab and ofatumumab, respectively (31,32). With the exception of the ASCEND study (acalabrutinib vs. idelalisib/rituximab) (29), common among these 11 randomized trials is a comparison of modern targeted therapy to chemotherapy, an anti-CD20 antibody, or both, as well as inclusion of patients naïve to targeted therapies.
While CIT (FCR, BR, or CO) are still acceptable options in certain patients, most patients in North America and Western Europe are initially managed with either a BTK inhibitor, utilizing a treat-to-progression strategy, or the combination of venetoclax-obinutuzumab for a fixed duration of 12 months (Figure 1, Table 1) (18,33). In the next line of therapy, patients typically are either treated with a BTK inhibitor or venetoclax (either as continuous monotherapy or as 24-month fixed duration with rituximab), typically switching to the class of agent not used in the front-line setting, depending on the efficacy and tolerability of that agent (18,33). In contemporary practice, BTK inhibitor and venetoclax-based therapy, administered in either sequence, collectively define standard first- and second-line treatment (34). Although the clinical activity of BTK inhibitors and venetoclax was established in pivotal studies that included patients naïve to the other drug class, smaller prospective Phase 2 studies and real-world data demonstrate previously unrecognized incomplete cross-resistance and intolerance between BTK inhibitors and venetoclax, allowing them to be administered in either order with sequential benefit (35–40). In addition, emerging data suggests that some patients may benefit from retreatment with a venetoclax-based regimen although large prospective cohort data supporting this practice are not yet available (41). Collectively, we estimate two classes of highly effective approved targeted therapy agents typically provide patients with 10 or more years of effective disease control. However, neither class of agent is given with curative intent and some patients will derive less profound benefit due to early progression or intolerance. Therefore, ultimately many patients treated with a BTK inhibitor and venetoclax will still require subsequent therapy. Efforts to extend clinical benefit and provide time off therapy have focused on combinations of BTK inhibitors and venetoclax, which are now currently being evaluated in multiple Phase 3 studies (NCT04608318, NCT03701282, NCT03836261, NCT03737981, NCT03462719). Although there is optimism that patients will enjoy long remissions after combined BTK inhibitor and venetoclax therapy administered on a fixed-duration schedule, limited information exists regarding the sequencing of novel agents following such combinations or the clinical efficacy of retreatment strategies.”
Figure 1:
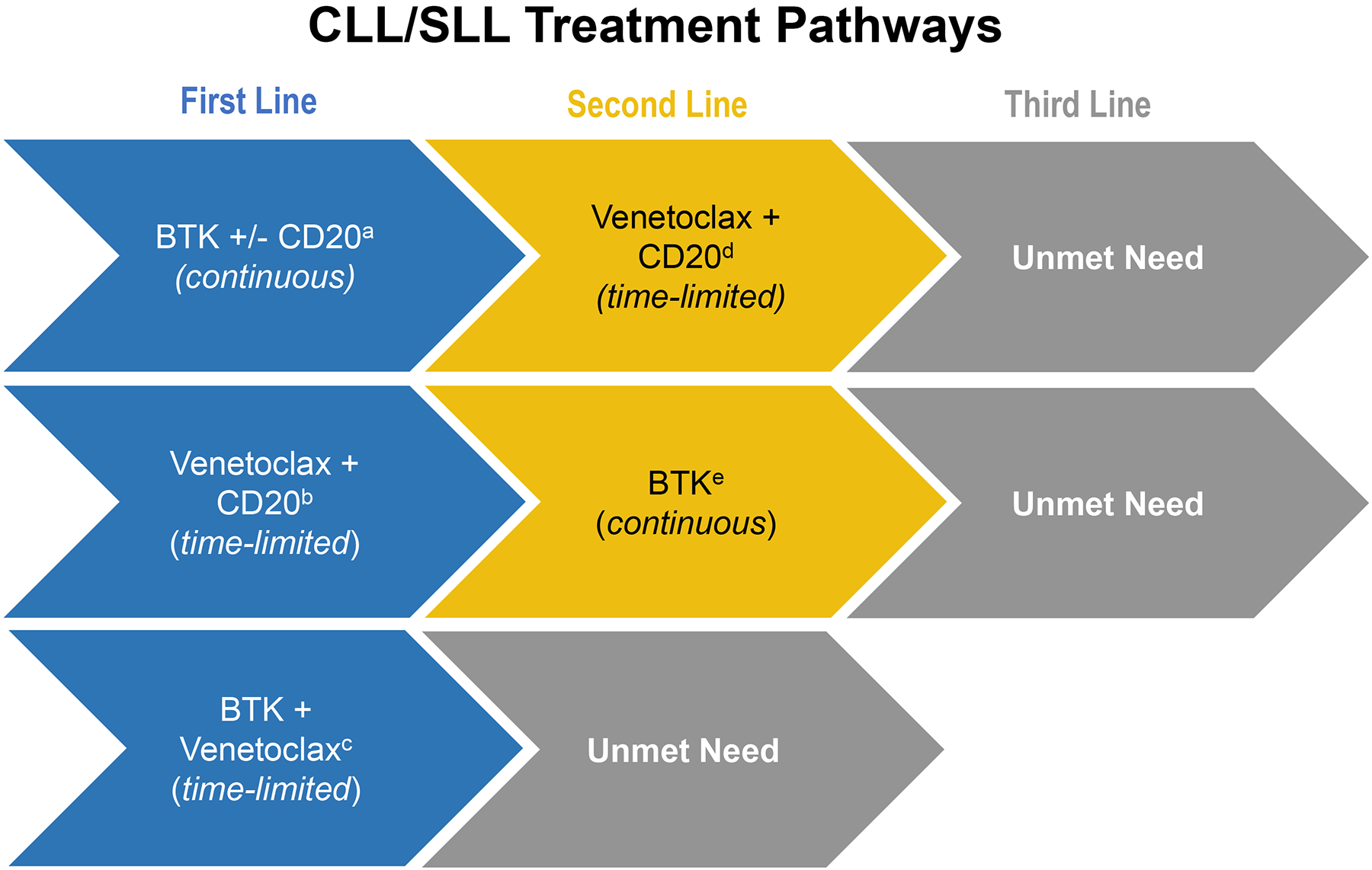
Chemotherapy free sequencing algorithms for patients with CLL/SLL in modern clinical practice with current unmet needs
aRESONATE-2 (2016); ELEVATE TN (2019); ILLUMINATE (2019); E1912 (2020)
bCLL-14 (2019)
cGLOW; CAPTIVATE (both ongoing)
dMURANO (2018)
eRESONATE (2014); ASCEND (2019)
Table 1:
Randomized Studies of Covalent BTK Inhibitors, Venetoclax, and PI3K Inhibitors
| Study Name | Disease Setting | Active arm | Comparator | Prior BTK % | Prior BCL2 % | Pts, N | Hazard Ratio |
|---|---|---|---|---|---|---|---|
| RESONATE26 | R / R | Ibrutinib | CD20 | 0 | 0 | 391 | 0.22 |
| RESONATE26 | R / R (17p) | Ibrutinib | CD20 | 0 | 0 | 127 | 0.25 |
| HELIOS11 | R / R | Ibrutinib + Chemo + CD20 | Chemo + CD20 | 0 | 0 | 578 | 0.20 |
| RESONATE-225 | 1L | Ibrutinib | Chemo | 0 | 0 | 269 | 0.16 |
| iLLUMINATE22 | 1L | Ibrutinib + CD20 | Chemo + CD20 | 0 | 0 | 229 | 0.23 |
| E191228 | 1L | Ibrutinib + CD20 | FCR | 0 | 0 | 529 | 0.34 |
| ASCEND29 | R / R | Acalabrutinib | Chemo + CD20 OR PI3K + CD20 | 0 | 0 | 310 | 0.31 |
| ELEVATE-TN23 | 1L | Acalabrutinib Acalabrutinib + CD20 |
Chemo + CD20 | 0 | 0 | 535 | 0.20 0.10 |
| MURANO30 | R / R | Venetoclax + CD20 | Chemo + CD20 | <2% | 0 | 389 | 0.17 |
| CLL-14 | 1L | Venetoclax + CD20 | Chemo + CD20 | 0 | 0 | 432 | 0.35 |
| NA | R/R | Idelalisib + CD20 | CD20 | 0 | 0 | 220 | 0.15 |
| DUO31 | R/R | Duvelisib | CD20 | 0 | 0 | 319 | 0.52 |
Marked Progress but Important Knowledge Gaps
Despite the many randomized Phase 3 trials that have led to these current treatment paradigms, significant unanswered questions remain. Head-to-head comparisons of BTK inhibitors to venetoclax-based approaches have been launched (NCT04608318, NCT05057494), although results are not available. Similarly, comparisons among BTK inhibitors have been undertaken and are recently reported as abstracts at the ASCO and EHA 2021 meetings. Importantly, the studies evaluating head-to-head comparisons of modern targeted therapies may not address key outstanding questions in terms of efficacy due to their study designs / primary endpoints which include non-inferiority for PFS in the ELEVTAE R/R and overall response rate in the ALPINE study. Additionally, in terms of safety these studies demonstrate differences in safety profiles which do not definitely favor any agent over another (acalabrutinib vs. ibrutinib, zanubrutinib vs. ibrutinib) with the notable exception of cardiovascular events. (42,43) Collectively, these data gaps mean that the optimal sequencing and selection among available BTK inhibitors and venetoclax remain undefined (44). Perhaps most importantly, we still do not understand the true efficacy of available therapies in the patient population increasingly seen in our clinics today – namely those who have been previously treated with BTK inhibitors, venetoclax, or both. Indeed, it is remarkable to note that only 9 of 921 (~1%) patients treated on 6 recent randomized studies in relapsed/refractory CLL/SLL were previously treated with at least one targeted therapy and likely none on a truly contemporary chemotherapy-free treatment (41). In short, while the randomized datasets upon which we base our current practice have yielded an impressive armamentarium of agents among which to select for our patients, these same studies have not taught us the true efficacy of these agents in the relapsed/refractory patient population that constitute the majority of patients currently seen in everyday practice.
Available therapy for patients previously exposed to both BTK inhibitors and venetoclax generally include the PI3K inhibitors, cytotoxic chemotherapy, alemtuzumab, single agent anti-CD20 antibodies and allogeneic stem cell transplantation (in highly selected patients) (45). Unfortunately, studies evaluating these agents or their combinations have been exclusively conducted in patients naïve to BTK inhibitors and venetoclax and as a result their safety and especially their efficacy in patients treated on a pathway that includes a BTK inhibitor and venetoclax remains unknown. Data from limited published anecdotal and retrospective case series suggest these available therapies have limited efficacy following treatment with BTK inhibitors, venetoclax, or both (20,38,46).
The two pivotal studies with the PI3K inhibitors, idelalisib and duvelisib, illustrate the challenge of applying data generated from these studies to a treatment context now dominated by use of BTK inhibitors and venetoclax (6,31). As mentioned, no patients in these randomized studies were previously treated with a BTK inhibitor or venetoclax. Moreover, both studies utilized single anti-CD20 antibody monotherapies as the comparator arm, a treatment approach known to have limited efficacy in CLL/SLL. Consequently, the results of these studies cannot be utilized to guide treatment decisions in patients previously treated on a chemotherapy free paradigm (41). Additionally, several real-world data sets suggest that the PI3K inhibitor class has limited effectiveness in patients who are venetoclax naïve and previously treated with BTK inhibitors (possibly due to cross resistance) or have been treated with both a BTK inhibitor and venetoclax in earlier lines of therapy. Specifically, in 17 patients who had been exposed to a prior BTK inhibitor and venetoclax, the ORR was 47% with median PFS of only 5 months (38).
The adoption of currently approved PI3K inhibitors into contemporary treatment has also been severely limited by significant immune-mediated toxicities and infectious complications resulting in high discontinuation rates. In the Phase 3 study which compared acalabrutinib to the PI3K inhibitor idelalisib with rituximab, 47% of the 119 patients who received idelalisib plus rituximab discontinued treatment prior to disease progression due to adverse events (median time on therapy 11.5 months) (29). Consistent with these clinical trial data, real-world data similarly confirm discontinuation rates with PI3K inhibitors ranging from 40–95%, again predominantly due to AEs. While real-world data are not yet available for duvelisib, the discontinuation rate from the Phase 3 DUO trial was 77%, with 27% of patients discontinuing due to adverse events (31). Given these collective data, we do not consider PI3K inhibitors a sufficiently effective standard option for patients with disease previously treated with BTK and venetoclax. As the number of CLL patients treated with venetoclax and BTK inhibitors will continue to increase with time, novel effective therapies for this patient population are urgently needed.
Another key data gap is the lack of any studies that have evaluated CIT following progression on either BTK inhibitors or BTK inhibitors / venetoclax; neither clinical trial data nor real-world data exist to answer this question. Again, anecdotal experience and small real-world series suggest that CIT would have an extremely limited role in non-transformed patients previously treated with multiple targeted therapies in an earlier line of therapy; CIT is used with transformed disease (Richter syndrome) (47). As with patients previously treated with CIT, these patients tend to have more aggressive disease characterized by resistance mutations or TP53 aberrancy, the latter predicts for reduced efficacy of CIT (48). Furthermore, older prospective Phase 2 trials and randomized trials of CIT in the second or later treatment line setting after prior CIT have typically shown relatively modest PFS (i.e., on the order of two years or less) (49,50). In the absence of convincing data at this time, we cannot counsel or encourage the use of chemotherapy or CIT as an established standard of care (particularly if the CLL clone has acquired poor risk molecular/genetic features) for patients treated with one or more targeted therapies as earlier lines of therapy. As efficacy of CIT following BTK inhibitors or BTK inhibitors / venetoclax is not available, prospective studies will need to be conducted to assess if CIT is a valid standard of care or as a control arm in future randomized studies in this setting.
Conclusions: Addressing the Unmet Need
After a decade of unprecedented innovation driven largely by the adoption of BTK inhibitors and venetoclax that have collectively transformed the treatment landscape and outcomes for patients with CLL/SLL, we are now seeing a growing population of patients who are in need of new therapeutic options following treatment with both of these novel targeted agents. These patients lack therapeutic options with proven efficacy and safety following treatment with a BTK inhibitor and venetoclax and, as such, constitute the vanguard of contemporary unmet need for this disease. Achieving the next round of breakthroughs for these patients will once again require close collaboration between all key stakeholders, including the patient community, clinical investigators, the pharmaceutical industry and global regulatory bodies. This progress begins by simply acknowledging that after a decade of enormous progress, we once again have CLL/SLL patients with unmet medical need. These patients can be readily identified and should be preferentially enrolled into clinical trials. Progress will certainly require biologic insight into resistance mechanisms to BTK inhibitors and venetoclax. Equally importantly, however, progress will also require consensus on key aspects of new drug development for this patient population. Importantly, as new agents are developed in this population, we must determine what absolute effect size is meaningful, as measured using objective endpoints. The urgency is real, but so is the promise. We believe that further dramatic progress in CLL therapy is not only possible but imminent – the time for collective action is now.
Translational relevance statement.
In CLL, while outcomes for patients have been dramatically improved, patients treated with prior BTK and BCL2 inhibitor-based therapy represent a new population with significant unmet need. Despite improved outcomes, the two most common reasons for discontinuation of these agents include drug resistance or intolerance. To date, no agent has clearly demonstrated efficacy in patients with double refractory CLL (i.e. resistant to both BTKi and venetoclax). Nearly all of the CLL patients enrolled on prior randomized studies of BTK inhibitors, PI3K inhibitors and venetoclax were conducted in patients who were BTK inhibitor and BCL2 inhibitor naïve and therefore do not represent patients in clinical practice - defining the true unmet need in CLL. While randomized studies have an important role in generating evidence for CLL therapies, we also recognize that to our knowledge nearly all randomized Phase 3 studies conducted in CLL in the last 10 years have utilized chemotherapy, an anti-CD20 antibody, or the combination of these agents as the comparator arm. Here we offer our perspective on how best to define unmet needs in a modern treatment context. We review the literature with a focus on agents such as PI3K inhibitors and chemoimmunotherapy and lack of data to support their utility following BTK inhibitors and venetoclax. Finally, we suggest opportunities for key stakeholders to ensure the continued innovation our patients deserve. We believe this perspective will help to initiate a conversation among our community and key stakeholders as to how to best and most rapidly advance innovation for our patients in need.
Acknowledgement:
The authors would like to thank Dr. David Hyman for his comments and insight and LOXO Oncology for editorial support in submitting this manuscript, which did not include writing. We also thank Ms. Camilla Pena and Dr. Julia Aronson for academic and editorial support.
Disclosure of Conflict of Interest:
A.R. Mato reports grants and personal fees from, and data safety monitoring board member for TG Therapeutics; grants and personal fees from Loxo Oncology (a wholly owned subsidiary of Eli Lilly), Genentech, AbbVie, AstraZeneca, Adaptive, Pharmacyclics, Nurix, Genmab, and Curio Sciences; grants from Sunesis, Regeneron, Pfizer, Aprea, Aptose, and DTRM; non-financial support from NCCN, CLL society, and Lymphoma Research Foundation; and grants from and steering committee for Verastem, during the conduct of the study. M.S. Davids reports grants and personal fees from AbbVie, Ascentage Pharma, Genentech, MEI Pharma, Novartis, Pharmacyclics, TG Therapeutics, Verastem, and AstraZeneca; personal fees from Adaptive Biotechnologies, BeiGene, Celgene, Eli Lilly, Gilead Sciences, Janssen, Merck, Research to Practice, Syros Pharmaceuticals; personal fees from Zentalis; and grants from Surface Oncology, outside the submitted work. J. Sharman reports personal fees from AbbVie, Acerta Pharma, AstraZeneca, Genentech, Pharmacyclics, Sunesis, and TG Therapeutics. L. Roeker reports minority ownership interest in AbbVie and Abbott Laboratories; personal fees from Vaniam Group and Janssen Biotech; and grant funding from the American Society of Hematology, outside of the submitted work. N. Kay serves in the advisory boards of Abbvie, Astra Zeneca, Cytomx Therapy, Dava oncology, Juno Theraputics, Oncotracker, Pharmacyclics and Targeted Oncology, in the Data Safety Monitoring Committee of Agios Pharm, AstraZeneca, BMS –Celgene, Cytomx Therapeutics, Janssen, Morpho-sys, Rigel and Jassen, research funding to the institution from Abbvie, Acerta Pharma, Bristol Meyer Squib, Celgene, Genentech, MEI Pharma, Pharmacyclics, Sunesis, TG Therapeutics, and Tolero Pharmaceuticals. A. Kater reports research funding from a Dutch Research Council (NWO) VIDI grant and an European Research Council (ERC) Consolidator grant, Janssen, Roche/Genentech, AstraZeneca, Abbvie, and BMS, Advisory board activity with BMS, AstraZeneca, Janssen, Abbvie, and LAVA therapeutics, and speakers fee from Abbvie. K. Rogers reports research funding from AbbVie, Genentech, Novartis, and Janssen, advisory board fee for Acerta Pharma, Pharmacyclics, AbbVie, Genentech, Innate Pharma, and AstraZeneca, and travel funds from AstraZeneca. M. Thompson has no conflicts to report. J. Rhodes reports honorarium from AstraZeneca and advisory board fee from Abbie, Genentech, Pharmacyclics, TG Therapeutics, and Verastem. A. Goy reports other from Acerta, Celgene, Constellation, Infinity, Infinity Verastem, Janssen, Karyopharm, Pharmacyclics, Elsevier’s PracticeUpdate Oncology, Gilead, Michael J Hennessey Associates, INC., OncLive Peer Exchange, Physcians Education Resource, LLC., Xcenda, Genentech, Hoffman La Roche, Genentech-Hoffman La Roche, AstraZeneca, MorphoSys and Incyte, COTA, Genomic Testing Cooperative, Kite Pharma, Novartis, Rosewell Park, and Vincera and personal fees from Celgene, Janssen, Pharmacyclics, Elsevier’s PracticeUpdate Oncology, Gilead, Michael J Hennessey Associates, INC, OncLive Peer Exchange, Physcians Education Resource, LLC., Xcenda, AstraZeneca, MorphoSys and Incyte, COTA, Genomic Testing Cooperative, Kite Pharma, Novartis, and Rosewell Park, and non-financial support Vincera outside the submitted work. A. Skarbnik reports research support from Acerta Pharma; research support and personal fees from AbbVie, AstraZeneca, Celegene, Janssen, Pharmacyclics, and Verastem; personal fees from Genentech, Gilead, Kite Pharma, Jazz Pharmaceuticals, Novartis, Beigene, and Seattle Genetics; received a drug for an investigator-initiated trial from Bristol-Myers Squibb, outside of the submitted work; and owns stock in COTA Healthcare. S.J. Shuster reports personal fees from Loxo Oncology during the conduct of the study; personal fees from AlloGene, AstraZeneca, BeiGene, Genentech, F Hoffmann-LaRoche, Juno/Celgene, Loxo Oncology, Nordic Nanovector, Novartis, Tessa Therapeutics; and grants from Novartis, Genentech, F Hoffman-La Roche, outside the submitted work. C.S. Tam reports grants and personal fees from Janssen, BeiGene, and AbbVie; and personal fees from Pharmacyclics, outside the submitted work. T.A. Eyre reports personal fees from Roche, Loxo Oncology, AbbVie, Janssen, Beigene, AstraZeneca, and Kite; and personal fees from and consultancy, board of directors, or advisory for Gilead, outside the submitted work. S. O’Brien reports consulting with Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc., Vaniam Group LLC., Abbvie, Alexion, Verastem, Juno Therapeutics, Vida Ventures, Autolus, Johnson and Johnson, Merck, BMS, NOVA Research Company, Gilead, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis, and research funding from Kite, Regeneron, Acerta, Caribou, Gilead, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis. C. Nabhan reports employment and stocks with Caris. N. Lamanna reports grants from Loxo Oncology during the conduct of the study; and grants and personal fees from, and consultancy, board of directors, or advisory for AbbVie, AstraZeneca, BeiGene, and Genentech; personal fees from and consultancy, board of directors, or advisory committees for Celgene, Gilead, Janssen, and Pharmacyclics; and grants from Juno, Octernal, Verastem, TG Therapeutics, MingSight, and Octapharma, outside the submitted work. C. Sun reports research grants from Genmab. M. Shadman reports consulting, advisory Boards, steering committees or data safety monitoring committees with Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, and Atara Biotherapeutics, research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, and Atara Biotherapeutics. J.M. Pagel reports consultancy with Loxo Oncology, AstraZeneca, Gilead, and BeiGene, during the conduct of the study. C. Ujjaini reports research funding from abbvie, Pharmacyclics, AZ, LOXO, Adaptive biotech, and Kite/Gilead, consulting fee from Atara, Epizyme, pharmacylics, TG therapeutics, abbvie, and Genentech. D. Brander reports consulting/advisory fee from AbbVie, Genentech, Pharmacyclics, Pfizer, TG Therapeutics, and Verastem, grants paid to institution/site PI clinical trials from AbbVie, ArQule, Ascentage, AstraZeneca, BeiGene, DTRM, Genentech, Juno/Celgene/BMS, Loxo Oncology, MEI Pharma, Novaris, Pharmacyclics, and TG Therapeutics. C.C. Coombs reports personal fees and honoraria and payment to institution for clinical trial from Loxo Oncology, during the conduct of the study; personal fees from Novartis, AbbVie, Genentech, MEI Pharma, and Octapharma; and payment to institution for clinical trial from Gilead, H3 Biomedicine, and Incyte, outside the submitted work. N. Jain reports research funding from Pharmacyclics, AbbVie, Genentech, AstraZeneca, BMS, Pfizer, ADC Therapeutics, Incyte, Servier, Cellectis, Adaptive Biotechnologies, Precision Biosciences, Aprea Therapeutics, Fate Therapeutics, and Kite, advisory board / honoraria from Pharmacyclics, Janssen, AbbVie, Genentech, AstraZeneca, Adaptive Biotechnologies, Servier, Precision Biosciences, Beigene, TG Therapeutics, ADC Therapeutics, Cellectis, and BMS. C.Y. Cheah reports personal fees from Loxo Oncology during the conduct of the study; grants and personal fees from Roche and Bristol Myers Squibb; personal fees from Takeda, AstraZeneca, Ascentage Pharma, and TG Therapeutics; grants from AbbVie; and personal fees from Janssen and Gilead, outside the submitted work. J.R. Brown reports personal fees from AbbVie, Acerta/AstraZeneca, Astellas, BeiGene, Catapult Therapeutics, Dynamo Therapeutics, Genentech/Roche, Gilead, Janssen, Juno/Celgene, Novartis, Octapharma, Pfizer, Pharmacyclics, Redx, Sunesis, Teva, TG Therapeutics, MEI Pharma, Nextcea, Rigel, and Kite; consultancy from Morphosys and Invectys; grants from Sun; and grants and personal fees from Verastem and Loxo Oncology, outside the submitted work. J. Seymour reports advisory board activity with AbbVie, Acerta, AstraZeneca, BMS/Celgene, Genetich, Gilead, Janssen, MeiPharma, Morphosys, Roche, Sunesis and Takeda, research funding from Abbvie, BMS/Celgene, Janssen and Roche, and speaker’s bureau activity with Abbvie, BMS/Celgene, and Roche. J.A. Woyach reports personal fees from Loxo Oncology during the conduct of the study; and personal fees from Janssen, Pharmacyclics, AstraZeneca, AbbVie, and ArQule; and grants from Pharmacyclics, Janssen, Morphosys, Karyopharm, Verastem, and AbbVie, outside the submitted work.
References:
- 1.Schiattone L, Ghia P, Scarfò L. The evolving treatment landscape of chronic lymphocytic leukemia. Curr Opin Oncol 2019;31(6):568–73 doi 10.1097/cco.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 2.Sengar M, Jain H, Rajendra A, Rengaraj K, Thorat J. Frontline Therapy of Chronic Lymphocytic Leukemia: Changing Treatment Paradigm. Curr Hematol Malig Rep 2020;15(3):168–76 doi 10.1007/s11899-020-00580-7. [DOI] [PubMed] [Google Scholar]
- 3.Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 2016;127(2):208–15 doi 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 4.Stilgenbauer S, Schnaiter A, Paschka P, Zenz T, Rossi M, Döhner K, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood 2014;123(21):3247–54 doi 10.1182/blood-2014-01-546150. [DOI] [PubMed] [Google Scholar]
- 5.Zenz T, Eichhorst B, Busch R, Denzel T, Häbe S, Winkler D, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol 2010;28(29):4473–9 doi 10.1200/jco.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 6.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014;123(22):3390–7 doi 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369(1):32–42 doi 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374(4):323–32 doi 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frustaci AM, Tedeschi A, Deodato M, Zamprogna G, Cairoli R, Montillo M. Duvelisib for the treatment of chronic lymphocytic leukemia. Expert Opin Pharmacother 2020;21(11):1299–309 doi 10.1080/14656566.2020.1751123. [DOI] [PubMed] [Google Scholar]
- 10.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374(4):311–22 doi 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser GAM, Chanan-Khan A, Demirkan F, Santucci Silva R, Grosicki S, Janssens A, et al. Final 5-year findings from the phase 3 HELIOS study of ibrutinib plus bendamustine and rituximab in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma 2020;61(13):3188–97 doi 10.1080/10428194.2020.1795159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D’Rozario J, et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the MURANO Phase III Study. J Clin Oncol 2020;38(34):4042–54 doi 10.1200/jco.20.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lama TG, Kyung D, O’Brien S. Mechanisms of ibrutinib resistance in chronic lymphocytic leukemia and alternative treatment strategies. Expert Rev Hematol 2020;13(8):871–83 doi 10.1080/17474086.2020.1797482. [DOI] [PubMed] [Google Scholar]
- 14.Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol 2019;94(12):1353–63 doi 10.1002/ajh.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ondrisova L, Mraz M. Genetic and Non-Genetic Mechanisms of Resistance to BCR Signaling Inhibitors in B Cell Malignancies. Front Oncol 2020;10:591577 doi 10.3389/fonc.2020.591577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J Clin Oncol 2019;37(16):1391–402 doi 10.1200/jco.18.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutre SE, Byrd JC, Hillmen P, Barrientos JC, Barr PM, Devereux S, et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv 2019;3(12):1799–807 doi 10.1182/bloodadvances.2018028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2021;32(1):23–33 doi 10.1016/j.annonc.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Eyre TA, Lamanna N, Roeker LE, Ujjani CS, Hill BT, Barr PM, et al. Comparative analysis of targeted novel therapies in relapsed, refractory chronic lymphocytic leukaemia. Haematologica 2021;106(1):284–7 doi 10.3324/haematol.2019.241539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol 2017;28(5):1050–6 doi 10.1093/annonc/mdx031. [DOI] [PubMed] [Google Scholar]
- 21.Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2020;21(9):1188–200 doi 10.1016/s1470-2045(20)30443-5. [DOI] [PubMed] [Google Scholar]
- 22.Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20(1):43–56 doi 10.1016/s1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 23.Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020;395(10232):1278–91 doi 10.1016/s0140-6736(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 2016;17(6):768–78 doi 10.1016/s1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 25.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 2015;373(25):2425–37 doi 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N Engl J Med 2014;371(3):213–23 doi 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol 2016;17(2):200–11 doi 10.1016/s1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 28.Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med 2019;381(5):432–43 doi 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol 2020;38(25):2849–61 doi 10.1200/jco.19.03355. [DOI] [PubMed] [Google Scholar]
- 30.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med 2018;378(12):1107–20 doi 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 31.Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 2018;132(23):2446–55 doi 10.1182/blood-2018-05-850461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014;370(11):997–1007 doi 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wierda WG, Byrd JC, Abramson JS, Bilgrami SF, Bociek G, Brander D, et al. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 4.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18(2):185–217 doi 10.6004/jnccn.2020.0006. [DOI] [PubMed] [Google Scholar]
- 34.Mato AR, Barrientos JC, Ghosh N, Pagel JM, Brander DM, Gutierrez M, et al. Prognostic Testing and Treatment Patterns in Chronic Lymphocytic Leukemia in the Era of Novel Targeted Therapies: Results From the informCLL Registry. Clin Lymphoma Myeloma Leuk 2020;20(3):174–83.e3 doi 10.1016/j.clml.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampel PJ, Call TG, Ding W, Muchtar E, Kenderian SS, Wang Y, et al. Addition of venetoclax at time of progression in ibrutinib-treated patients with chronic lymphocytic leukemia: Combination therapy to prevent ibrutinib flare. Am J Hematol 2020;95(3):E57–e60 doi 10.1002/ajh.25690. [DOI] [PubMed] [Google Scholar]
- 36.Hillmen P, Rawstron AC, Brock K, Muñoz-Vicente S, Yates FJ, Bishop R, et al. Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study. J Clin Oncol 2019;37(30):2722–9 doi 10.1200/jco.19.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones JA, Mato AR, Wierda WG, Davids MS, Choi M, Cheson BD, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol 2018;19(1):65–75 doi 10.1016/s1470-2045(17)30909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mato AR, Roeker LE, Jacobs R, Hill BT, Lamanna N, Brander D, et al. Assessment of the Efficacy of Therapies Following Venetoclax Discontinuation in CLL Reveals BTK Inhibition as an Effective Strategy. Clin Cancer Res 2020;26(14):3589–96 doi 10.1158/1078-0432.Ccr-19-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molica S, Giannarelli D, Montserrat E. Comparison Between Venetoclax-based and Bruton Tyrosine Kinase Inhibitor-based Therapy as Upfront Treatment of Chronic Lymphocytic Leukemia (CLL): A Systematic Review and Network Meta-analysis. Clin Lymphoma Myeloma Leuk 2020. doi 10.1016/j.clml.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Rogers KA, Huang Y, Ruppert AS, Abruzzo LV, Andersen BL, Awan FT, et al. Phase II Study of Combination Obinutuzumab, Ibrutinib, and Venetoclax in Treatment-Naïve and Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol 2020;38(31):3626–37 doi 10.1200/jco.20.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roeker LE, Mato AR. Approaches for relapsed CLL after chemotherapy-free frontline regimens. Hematology Am Soc Hematol Educ Program 2020;2020(1):10–7 doi 10.1182/hematology.2020000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hillmen P, Brown JR, Eichhorst BF, Lamanna N, O’Brien SM, Qiu L, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol 2020;16(10):517–23 doi 10.2217/fon-2019-0844. [DOI] [PubMed] [Google Scholar]
- 43.Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan AAA, Furman RR, et al. First Results of a Head-to-Head Trial of Acalabrutinib versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia. J Clin Oncol 2021. 39:15_suppl, 7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin VS, Lew TE, Handunnetti SM, Blombery P, Nguyen T, Westerman DA, et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood 2020;135(25):2266–70 doi 10.1182/blood.2020004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rai KR, Freter CE, Mercier RJ, Cooper MR, Mitchell BS, Stadtmauer EA, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol 2002;20(18):3891–7 doi 10.1200/jco.2002.06.119. [DOI] [PubMed] [Google Scholar]
- 46.Mato AR, Nabhan C, Barr PM, Ujjani CS, Hill BT, Lamanna N, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood 2016;128(18):2199–205 doi 10.1182/blood-2016-05-716977. [DOI] [PubMed] [Google Scholar]
- 47.Khan M, Siddiqi R, Thompson PA. Approach to Richter transformation of chronic lymphocytic leukemia in the era of novel therapies. Ann Hematol 2018;97(1):1–15 doi 10.1007/s00277-017-3149-9. [DOI] [PubMed] [Google Scholar]
- 48.Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood 2011;117(12):3391–401 doi 10.1182/blood-2010-09-302174. [DOI] [PubMed] [Google Scholar]
- 49.Badoux XC, Keating MJ, Wang X, O’Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 2011;117(11):3016–24 doi 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2011;29(26):3559–66 doi 10.1200/jco.2010.33.8061. [DOI] [PubMed] [Google Scholar]


