Abstract
Purpose
Some evidence suggests that male infertility increases the risk of cardiovascular diseases (CVDs). However, the evidence in Asian populations is relatively scarce. The aim of this study is to determine whether male infertility increases the risk of CVDs.
Materials and Methods
We used inpatient and outpatient data for the years 2000 to 2015 from the Taiwanese Longitudinal Health Insurance Database. We enrolled 7,016 males over 18 years old and diagnosed with male infertility. Of these, 2,326 matched our inclusion criteria and were assigned to the study group. For each infertility patient, four comparison patients were frequency-matched by age and index date to form a control cohort comprising 9,304 patients. Cox proportional hazards analysis was used to estimate the association between male infertility and CVDs.
Results
After a 15-year follow-up, the incidence rate of CVDs was higher in the infertility group than the control group (1,460.23 and 1,073.70 per 100,000 person-years, respectively). The Cox proportional hazards regression analysis revealed that the adjusted HR for CVDs was 1.472 for the infertility group (95% CI, 1.288–1.683; p<0.001) relative to the control group. The Kaplan–Meier analysis of the cumulative incidence of CVDs in the two groups showed that the cumulative risk curve for CVDs was significantly higher for the infertility group than the control group.
Conclusions
This study shows that men with infertility have a higher risk of developing incident CVDs. In the future, healthcare providers should pay attention to these patients because of their higher health risks.
Keywords: Cardiovascular diseases, Fertility, Male infertility
INTRODUCTION
The World Health Organization (WHO) defines infertility as a failure to clinically conceive after at least 12 months of regular and unprotected sexual intercourse [1], and it can be associated with either sex. According to the 2020 annual report on Taiwan's National Health Insurance (NHI) claim system, over the past decade the rate of medical visits for male infertility has increased 1.6-fold [2]. Increasing evidence has shown that the Charlson comorbidity index is significantly higher for infertile males than for fertile males [3], indicating that men with infertility have a higher risk of chronic diseases in later life. Other studies have found that infertile males are at a higher risk of cardiovascular diseases (CVDs) in particular [4,5], regardless of education level, income and race/ethnicity. In addition, male infertility has been identified as a risk factor for obesity, hypogonadism and mental illness, all of which are also associated with CVDs. Therefore, it is possible that male infertility is an antecedent of CVDs. CVDs are a group of disorders of the heart and blood vessels. Since CVDs are typically severe and cause the highest mortality rate among chronic diseases [6], it is important to examine the contribution of male infertility to the risk of CVDs.
Although previous researchers have investigated the association between male infertility and CVDs, there remain two gaps in the literature. First, the majority of these studies have focused on Caucasian populations, and the evidence for Asian populations is relatively limited [7]. Second, the follow-up period is generally relatively short, with an average of 3 to 5 years. There is still limited evidence concerning the risk of development of CVDs in infertile males in the long term. Therefore, the purpose of this study is to investigate whether men with infertility in an Asian population have a higher incidence of CVDs in the long term using a population-based cohort study.
MATERIALS AND METHODS
1. Data sources
We used data from Taiwan's National Health Insurance Research Database (NHIRD). The NHI program was launched in 1995 and includes almost 99% of the 23 million residents of Taiwan and most of the clinics in the country [8]. Diagnoses in the NHIRD were coded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). To protect the privacy of the participants, the identities of individuals were anonymized. We used inpatient and outpatient data for the years of 2000 to 2015 from the Longitudinal Health Insurance Database (LHID) in Taiwan. The LHID contains all longitudinal claims data for approximately two million individuals who were randomly selected from the NHI program.
2. Study design and participantse
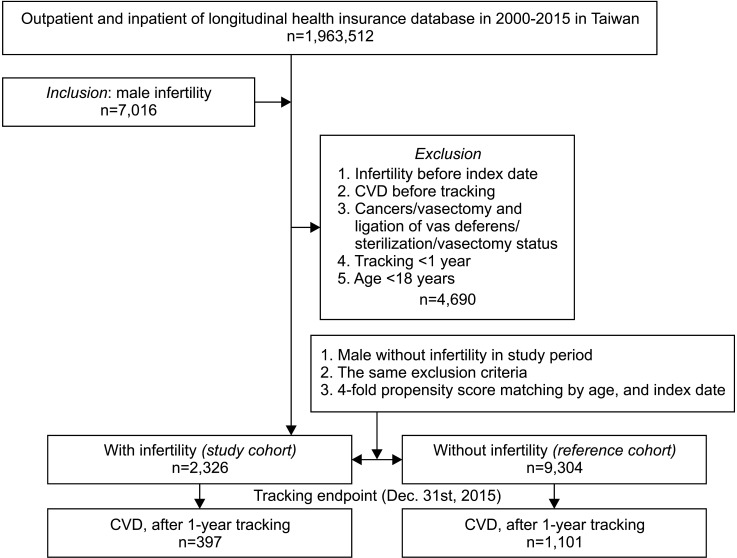
This study is a retrospective matched cohort study. We recruited males aged over the age of 18 years who had been diagnosed with male factor infertility (ICD-9-CM code: 606), including azoospermia (ICD 9-CM-606.0), oligospermia (ICD-9-CM: 606.1), infertility due to extra testicular causes (ICD-9-CM: 606.8), and male infertility unspecified (ICD-9-CM: 606.9) and used data from the LHID from 1 January 2000 to 31 December 2015. We excluded patients diagnosed with infertility before the year 2000; patients who were diagnosed with cancer (ICD-9-CM: 140–209); patients who received vasectomy or ligation of the vas deferens (ICD-9-OP: 63.70–63.73); and patients who had a status of Sterilization (ICD-9-CM: V25.2) or vasectomized (ICD-9-CM: V26.52). In addition, patients who had been diagnosed with hypertension (ICD-9-CM: 401–405), ischemic heart disease (ICD-9-CM: 410–414), stroke (ICD-9-CM: 430–438), or other forms of heart disease including heart failure, arrhythmia, heart valve problems (ICD-9-CM: 420–429) before the year 2000 or their first visit for infertility were also excluded. We enrolled a control cohort comprising four participants for every infertility patient, and these control cohort participants were selected by 1:4 matched ages and index dates with infertility patients.
Comorbidities were listed to compare the baseline characteristics of the two groups. These included diabetes mellitus (DM; ICD-9-CM: 250), hyperlipidemia (ICD-9-CM: 272), chronic kidney disease (CKD; ICD-9-CM: 585), chronic obstructive pulmonary disease (COPD; ICD-9-CM: 491, 492, 496), Scrotal varices (ICD-9-CM: 456.4), obesity (ICD-9-CM: 278, V77.8, 783.1), depression (ICD-9-CM: 296.2, 296.3, 296.82, 300.4, 311), anxiety (ICD-9-CM: 293.84, 300.0, 313.0) and manic disorder (ICD-9-CM: 296.0,296.1, 296.4–296.8). We also included risk factors for CVDs, such as alcoholic psychoses (ICD-9-CM: 291.0–291.3, 291.5, 291.8, 291.81, 291.89, 291.9, 303.00–303.93, 305.00–305.03, V11.3), tobacco use disorder (ICD-9-CM: 305.1) and personal history of tobacco use (ICD-9-CM: V15.82). The study cohort included a total of 2,326 males ≥18 years old who had been diagnosed with infertility and 9,304 control participants without infertility who were matched by age and index date (Fig. 1). We used the first date of infertility diagnosis as the index date.
Fig. 1. Flowchart regarding the participant selection and study design. CVD: cardiovascular disease.
3. Covariates
The covariates included were age, marital status, level of education (<12 years or ≥12 years), monthly insurance premium income (Taiwan New Dollars [TWD]<18,000; TWD 18,000–34,999; or TWD ≥35,000), geographical area of residence (northern, central, southern, or eastern Taiwan, or the outlying islands), season in which diagnosis was made, level of care (medical center, regional hospital, or local hospital) and level of urbanization at place of residence. In Taiwan NHRI publications, the final covariate is divided into four categories according to population density and role in the politics, economy and culture of Taiwan. Level 1 is the most urbanised and is defined as a population of >1,250,000. Level 4 is the least urbanised and is defined as a population of <149,999 [9].
4. Outcome measures
All study participants were followed from index date until the development of CVDs including hypertension (ICD-9-CM: 401–405), ischemic heart disease (ICD-9-CM: 410–414), cerebrovascular disease (ICD-9-CM: 430–438), or any other form of heart disease (ICD-9-CM: 420–429) or until the end of the study period. According to ICD9, male infertility can be divided into four types: azoospermia (ICD 9-CM-606.0), oligospermia (ICD-9-CM: 606.1), infertility due to extra testicular causes (ICD-9-CM: 606.8), and male infertility unspecified (ICD-9-CM: 606.9). In the subgroup analysis, we analyzed the relationship between different types of male infertility and CVD according to the ICD 9.
5. Statistical analysis
All statistical analyses were performed using SPSS for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). Chi-squared tests and t-tests were used to examine the distributions of the categorical and continuous variables, respectively. The Fisher's exact test for categorical variables was used to test for differences between the two cohorts. Multivariate Cox proportional hazards regression analysis was used to compare the risk of developing CVDs between the two groups. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox models. We evaluated the cumulative incidence of CVDs in males with infertility using Kaplan–Meier analysis and the log-rank test. The level of statistical significance was set at a two-tailed p-value of 0.05.
6. Ethics statement
The present study protocol was reviewed and approved by the institutional review board of Tri-Service General Hospital (TSGH IRB No. B-109–35). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
1. Baseline characteristics of the study population
There were 2,326 men with infertility in the infertility cohort and 9,304 male patients in the control cohort (Fig. 1), with a similar age distribution. The infertility group had somewhat lower rates of DM and COPD. A larger proportion of the infertility patients lived in northern Taiwan, resided in areas with level 1 urbanization and sought help in medical centers than in the control group (Table 1).
Table 1. Characteristics of participants in the baseline.
| Variable | Infertility | p-value | ||
|---|---|---|---|---|
| With | Without | |||
| Overall | 2,326 (20.00) | 9,304 (80.00) | ||
| Age (y) | 30.60±6.45 | 30.60±6.67 | 0.893 | |
| Married | <0.001 | |||
| Without | 743 (31.94) | 4,665 (50.14) | ||
| With | 1,583 (68.06) | 4,639 (49.86) | ||
| Education (y) | <0.001 | |||
| <12 | 735 (31.60) | 4,664 (50.13) | ||
| ≥12 | 1,591 (68.40) | 4,640 (49.87) | ||
| Insured premium (TWD) | <0.001 | |||
| <18,000 | 1,614 (69.39) | 9,145 (98.29) | ||
| 18,000–34,999 | 369 (15.86) | 102 (1.10) | ||
| ≥35,000 | 343 (14.75) | 57 (0.61) | ||
| DM | <0.001 | |||
| Without | 2,296 (98.71) | 9,105 (97.86) | ||
| With | 30 (1.29) | 199 (2.14) | ||
| Hyperlipidemia | 0.243 | |||
| Without | 2,298 (98.80) | 9,129 (98.12) | ||
| With | 28 (1.20) | 175 (1.88) | ||
| CKD | 0.976 | |||
| Without | 2,308 (99.23) | 9,233 (99.24) | ||
| With | 18 (0.77) | 71 (0.76) | ||
| COPD | 0.001 | |||
| Without | 2,306 (99.14) | 9,169 (98.55) | ||
| With | 20 (0.86) | 135 (1.45) | ||
| Scrotal varices | <0.001 | |||
| Without | 1,906 (81.94) | 9,261 (99.54) | ||
| With | 420 (18.06) | 43 (0.46) | ||
| Obesity | 0.482 | |||
| Without | 2,326 (100.00) | 9,299 (99.95) | ||
| With | 0 (0.00) | 5 (0.05) | ||
| Depression | 0.186 | |||
| Without | 2,314 (99.48) | 9,271 (99.65) | ||
| With | 12 (0.52) | 33 (0.35) | ||
| Anxiety | 0.193 | |||
| Without | 2,326 (100.00) | 9,296 (99.91) | ||
| With | 0 (0.00) | 8 (0.09) | ||
| Manic disorder | 0.482 | |||
| Without | 2,320 (99.74) | 9,264 (99.62) | ||
| With | 6 (0.26) | 35 (0.38) | ||
| Alcoholic psychoses | 0.197 | |||
| Without | 2,315 (99.53) | 9,186 (98.73) | ||
| With | 11 (0.47) | 118 (1.27) | ||
| Tobacco use disorder | - | |||
| Without | 2,326 (100.00) | 9,304 (100.00) | ||
| With | 0 (0.00) | 0 (0.00) | ||
| Season | 0.999 | |||
| Spring (Mar–May) | 704 (30.27) | 2,816 (30.27) | ||
| Summer (Jun–Aug) | 519 (22.31) | 2,076 (22.31) | ||
| Autumn (Sep–Nov) | 583 (25.06) | 2,332 (25.06) | ||
| Winter (Dec–Feb) | 520 (22.36) | 2,080 (22.36) | ||
| Location | <0.001 | |||
| Northern Taiwan | 1,201 (51.63) | 3,875 (41.65) | ||
| Middle Taiwan | 508 (21.84) | 2,611 (28.06) | ||
| Southern Taiwan | 521 (22.40) | 2,389 (25.68) | ||
| Eastern Taiwan | 96 (4.13) | 428 (4.60) | ||
| Outlets islands | 0 (0.00) | 1 (0.01) | ||
| Urbanization level | <0.001 | |||
| 1 (the highest) | 1,206 (51.85) | 3,268 (35.12) | ||
| 2 | 851 (36.59) | 3,833 (41.20) | ||
| 3 | 105 (4.51) | 1,003 (10.78) | ||
| 4 (the lowest) | 164 (7.05) | 1,200 (12.90) | ||
| Level of care | <0.001 | |||
| Medical center | 1,065 (45.79) | 2,498 (26.85) | ||
| Regional hospital | 679 (29.19) | 2,843 (30.56) | ||
| Local hospital | 582 (25.02) | 3,963 (42.59) | ||
Values are presented as number (%) or mean±standard deviation.
TWD: Taiwan New Dollar, DM: diabetes mellitus, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease.
p-values are chi-square or Fisher exact test on category variables and t-test on continue variables.
2. Male infertility is associated with CVDs
During the follow-up period, the infertility group had a higher risk of CVDs and a higher prevalence of COPD, depression and anxiety than the control group (Supplement Table 1). At the end of the study, 397 men (17.07%) with infertility had developed CVDs, compared to 1,101 (11.83%) in the control group. The mean follow-up period was 11.72±11.64 years in the infertility cohort and 11.68±11.63 years in the control cohort (Supplement Table 2). The average time interval to CVDs was 8.13±4.58 years and 8.41±4.09 years for the infertility and control cohorts, respectively (Supplement Table 3).
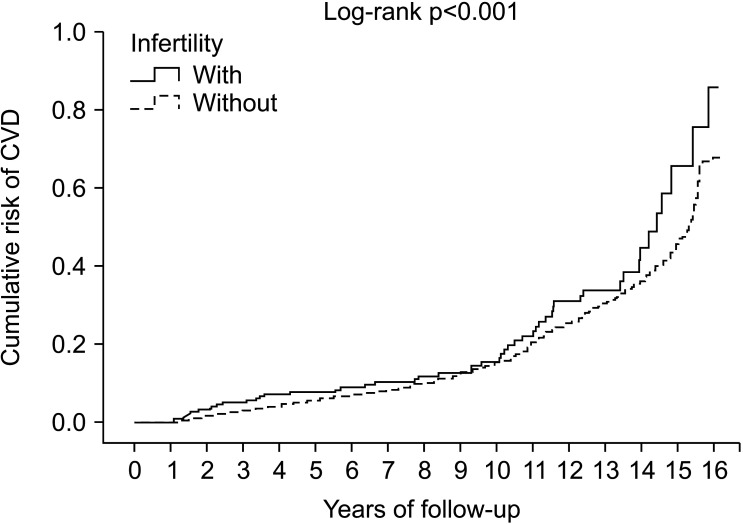
Compared with the control group, the infertile men had a higher incidence of CVDs (HR, 1.386; 95% CI, 1.227–1.565; p<0.001). After adjusting for age, marital status, level of education, and other covariates (list in Table 2), the Cox proportional hazards regression analysis revealed that the adjusted HR for CVDs was 1.472 for the infertility group (95% CI,1.288–1.683; p<0.001) relative to the control group (Table 2). The multivariable Cox regression model also showed that comorbidities such as DM, hyperlipidemia, CKD, Scrotal varices, and alcoholic psychoses were associated with an increased risk of CVDs (all p<0.001; Table 2). The Kaplan–Meier analysis of the cumulative incidence of CVDs in the two groups showed that the cumulative risk curve for CVDs was significantly higher for the infertility group than the control group (log-rank test, p<0.001; Fig. 2).
Table 2. Cox regression analysis for the risk of CVDs with interaction of comorbidity.
| Variable | Crude HR | 95% CI | p-value | Adjusted HRa | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Infertility | |||||||
| Without | Reference | Reference | |||||
| With | 1.386 | 1.227–1.565 | <0.001 | 1.472 | 1.288–1.683 | <0.001 | |
| Age (y) | 1.153 | 1.146–1.161 | <0.001 | 1.110 | 1.102–1.117 | <0.001 | |
| Married | |||||||
| Without | 0.948 | 0.527–2.082 | 0.931 | 0.923 | 0.748–2.140 | 0.832 | |
| With | Reference | Reference | |||||
| Education (y) | |||||||
| <12 | Reference | Reference | |||||
| ≥12 | 1.332 | 0.651–2.067 | 0.815 | 1.250 | 0.570–1.968 | 0.755 | |
| Insured premium (TWD) | |||||||
| <18,000 | Reference | Reference | |||||
| 18,000–34,999 | 1.262 | 0.992–1.605 | 0.324 | 0.986 | 0.913–1.520 | 0.510 | |
| ≥35,000 | 0.942 | 0.685–1.296 | 0.352 | 0.828 | 0.585–1.171 | 0.512 | |
| Season | |||||||
| Spring | Reference | Reference | |||||
| Summer | 1.026 | 0.877–1.200 | 0.366 | 1.119 | 0.864–1.185 | -0.495 | |
| Autumn | 1.139 | 0.982–1.321 | 0.798 | 1.179 | 0.923–1.243 | 0.854 | |
| Winter | 1.336 | 1.148–1.556 | 0.018 | 1.228 | 0.954–1.432 | 0.094 | |
| Location | |||||||
| Northern Taiwan | Reference | Multicollinearity with urbanization level | |||||
| Middle Taiwan | 1.096 | 0.961–1.250 | 0.956 | Multicollinearity with urbanization level | |||
| Southern Taiwan | 1.076 | 0.941–1.230 | 0.732 | Multicollinearity with urbanization level | |||
| Eastern Taiwan | 1.703 | 1.406–2.063 | <0.001 | Multicollinearity with urbanization level | |||
| Outlets islands | 0.388 | 0.125–1.210 | 0.078 | Multicollinearity with urbanization level | |||
| Urbanization level | |||||||
| 1 (the highest) | 1.162 | 1.015–1.333 | 0.038 | 1.506 | 1.351–1.690 | 0.007 | |
| 2 | 1.048 | 0.920–1.196 | 0.249 | 1.429 | 1.299–1.581 | 0.009 | |
| 3 | 0.960 | 0.802–1.157 | 0.344 | 1.322 | 1.165–1.518 | 0.014 | |
| 4 (the lowest) | Reference | Reference | |||||
| Level of care | |||||||
| Medical center | 1.245 | 1.073–1.444 | 0.024 | 1.160 | 0.977–1.377 | 0.403 | |
| Regional hospital | 1.166 | 1.010–1.345 | 0.035 | 1.078 | 0.930–1.249 | 0.729 | |
| Local hospital | Reference | Reference | |||||
| Comorbidities (reference: without) | |||||||
| DM | 2.626 | 2.315–2.980 | <0.001 | 2.072 | 1.814–2.367 | <0.001 | |
| Hyperlipidemia | 2.513 | 2.053–3.077 | <0.001 | 1.954 | 1.588–2.403 | <0.001 | |
| CKD | 3.267 | 2.668–4.001 | <0.001 | 2.621 | 2.125–3.234 | <0.001 | |
| COPD | 1.318 | 0.912–1.905 | 0.395 | 1.278 | 0.881–1.853 | 0.386 | |
| Scrotal varices | 5.978 | 2.465–13.784 | <0.001 | 3.563 | 1.898–5.703 | <0.001 | |
| Obesity | 2.260 | 1.173–4.354 | 0.017 | 1.611 | 0.802–3.237 | 0.273 | |
| Depression | 1.150 | 0.472–1.230 | 0.139 | 1.079 | 0.465–1.232 | 0.171 | |
| Anxiety | 1.225 | 0.509–2.949 | 0.913 | 1.379 | 0.570–3.335 | 0.621 | |
| Manic disorder | 1.214 | 0.562–1.753 | 0.779 | 1.075 | 0.605–1.910 | 0.674 | |
| Alcoholic psychoses | 1.754 | 1.260–2.442 | 0.001 | 1.931 | 1.377–2.707 | <0.001 | |
| Tobacco use disorder | 0.000 | - | 0.570 | 0.000 | - | 0.858 | |
HR: hazard ratio, CI: confidence interval, TWD: Taiwan New Dollar, CVDs: cardiovascular diseases, DM: diabetes mellitus, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, -: not available.
aAdjusted variables listed in the table.
Fig. 2. Kaplan–Meier analysis for the cumulative risk of cardiovascular diseases (CVDs) among male aged 18 years old and over stratified by infertility with log-rank test.
The overall incidence rate of CVDs in the infertility group was 1,460.23 per 105 person-years (Table 3). Infertile males were associated with a higher risk of CVDs than fertile males (adjusted HR, 1.422–2.720; p<0.001; Table 3) regardless of whether comorbidities were present (DM, hyperlipidemia, CKD, COPD, obesity, depression, anxiety, and manic disorder) combined with marital status, level of education, geographical area of residence, the season in which the diagnosis was made, level of care, and level of urbanization at the place of residence.
Table 3. Incidence and HRs of CVDs for male infertility cohort compared with men without infertility cohort by demographic characteristics and comorbidity.
| Variable | Infertility | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| With | Without | With vs. Without (reference) | ||||||||
| Events | PYs | Ratea | Events | PYs | Ratea | Adjusted HRb | 95% CI | p-value | ||
| Overall | 397 | 27,187.45 | 1,460.23 | 1,101 | 102,542.67 | 1,073.70 | 1.472 | 1.288–1.683 | <0.001 | |
| Married | ||||||||||
| No | 172 | 11,424.82 | 1,505.49 | 599 | 54,201.09 | 1,105.14 | 1.490 | 1.303–1.703 | <0.001 | |
| Yes | 225 | 15,762.63 | 1,427.43 | 502 | 48,341.58 | 1,038.44 | 1.700 | 1.488–1.944 | <0.001 | |
| Education (y) | ||||||||||
| <12 | 157 | 11,526.29 | 1,362.10 | 598 | 56,753.84 | 1,053.67 | 1.430 | 1.251–1.635 | <0.001 | |
| ≥12 | 240 | 15,661.16 | 1,532.45 | 503 | 45,788.83 | 1,098.52 | 1.708 | 1.494–1.952 | <0.001 | |
| Insurance premium (TWD) | ||||||||||
| <18,000 | 306 | 19,229.26 | 1,591.33 | 1,028 | 100,213.52 | 1,025.81 | 1.921 | 1.680–2.196 | <0.001 | |
| 18,000–34,999 | 51 | 4,541.66 | 1,122.94 | 35 | 1,493.43 | 2,343.60 | 1.005 | 0.879–1.149 | 0.201 | |
| ≥35,000 | 40 | 3,416.53 | 1,170.78 | 38 | 835.72 | 4,546.98 | 0.400 | 0.350–0.458 | <0.001 | |
| DM | ||||||||||
| Without | 316 | 25,029.38 | 1,262.52 | 865 | 91,990.03 | 940.32 | 1.577 | 1.380–1.803 | <0.001 | |
| With | 81 | 2,158.07 | 3,753.36 | 236 | 10,552.64 | 2,236.41 | 2.044 | 1.788–2.336 | <0.001 | |
| Hyperlipidemia | ||||||||||
| Without | 363 | 26,455.89 | 1,372.09 | 1,024 | 99,119.39 | 1,033.10 | 1.584 | 1.386–1.811 | <0.001 | |
| With | 34 | 731.55 | 4,647.65 | 77 | 3,423.29 | 2,249.30 | 2.236 | 1.956–2.556 | <0.001 | |
| CKD | ||||||||||
| Without | 372 | 26,697.67 | 1,393.38 | 1,012 | 99,645.86 | 1,015.60 | 1.624 | 1.421–1.857 | <0.001 | |
| With | 25 | 489.77 | 5,104.41 | 89 | 2,896.81 | 3,072.34 | 1.913 | 1.673–2.186 | <0.001 | |
| COPD | ||||||||||
| Without | 321 | 23,260.49 | 1,380.02 | 1,071 | 100,513.26 | 1,065.53 | 1.494 | 1.307–1.708 | <0.001 | |
| With | 76 | 3,926.96 | 1,935.34 | 30 | 2,029.42 | 1,478.26 | 1.704 | 1.490–1.948 | <0.001 | |
| Scrotal varices | ||||||||||
| Without | 316 | 21,894.32 | 1,443.30 | 1,097 | 102,178.37 | 1,073.61 | 1.184 | 0.703–1.501 | 0.305 | |
| With | 81 | 5,293.12 | 1,530.29 | 4 | 364.30 | 1,097.99 | 2.495 | 1.722–3.187 | <0.001 | |
| Obesity | ||||||||||
| Without | 393 | 26,990.71 | 1,456.06 | 1,099 | 102,383.31 | 1,073.42 | 1.589 | 1.390–1.816 | <0.001 | |
| With | 4 | 196.74 | 2,033.15 | 2 | 159.36 | 1,254.99 | 3.713 | 3.248–4.244 | <0.001 | |
| Depression | ||||||||||
| Without | 387 | 26,970.78 | 1,434.89 | 1,080 | 100,866.54 | 1,070.72 | 1.598 | 1.398–1.827 | <0.001 | |
| With | 10 | 216.67 | 4,615.29 | 21 | 1,676.13 | 1,252.89 | 2.980 | 2.607–3.406 | <0.001 | |
| Anxiety | ||||||||||
| Without | 392 | 26,991.35 | 1,452.32 | 1,094 | 102,113.28 | 1,071.36 | 1.608 | 1.406–1.838 | <0.001 | |
| With | 5 | 196.10 | 2,549.78 | 7 | 429.40 | 1,630.19 | 1.721 | 1.505–1.967 | <0.001 | |
| Manic disorder | ||||||||||
| Without | 394 | 26,995.15 | 1,459.52 | 1,091 | 101,667.71 | 1,073.10 | 1.606 | 1.405–1.836 | <0.001 | |
| With | 3 | 192.30 | 1,560.06 | 10 | 874.97 | 1,142.90 | 1.986 | 1.738–2.271 | <0.001 | |
| Alcoholic psychoses | ||||||||||
| Without | 397 | 27,187.45 | 1,460.23 | 1,064 | 100,401.52 | 1,059.74 | 1.472 | 1.288–1.683 | <0.001 | |
| With | 0 | 0.00 | - | 37 | 2,141.15 | 1,728.04 | 0.000 | - | - | |
| Tobacco use disorder | ||||||||||
| Without | 397 | 27,187.45 | 1,460.23 | 1,101 | 102,482.17 | 1,074.33 | 1.472 | 1.288–1.683 | <0.001 | |
| With | 0 | 0.00 | - | 0 | 60.50 | 0.00 | - | - | - | |
| Stratified | Infertility | |||||||||
| With | Without | With vs. Without (reference) | ||||||||
| Events | PYs | Ratea | Events | PYs | Ratea | Adjusted HRb | 95% CI | p-value | ||
| Season | ||||||||||
| Spring | 81 | 9,364.27 | 864.99 | 249 | 31,429.24 | 792.26 | 1.428 | 1.249–1.632 | <0.001 | |
| Summer | 98 | 12,926.03 | 758.16 | 280 | 41,695.50 | 671.54 | 1.635 | 1.430–1.869 | <0.001 | |
| Autumn | 103 | 2,176.86 | 4,731.58 | 319 | 10,219.20 | 3,121.58 | 1.889 | 1.652–2.159 | <0.001 | |
| Winter | 115 | 2,720.29 | 4,227.50 | 253 | 19,198.74 | 1,317.79 | 2.687 | 2.350–3.071 | <0.001 | |
| Urbanization level | ||||||||||
| 1 | 172 | 9,364.27 | 1,836.77 | 359 | 31,429.24 | 1,142.25 | 1.784 | 1.561–2.040 | <0.001 | |
| 2 | 164 | 12,926.03 | 1,268.76 | 387 | 41,695.50 | 928.16 | 1.628 | 1.424–1.861 | <0.001 | |
| 3 | 24 | 2,176.86 | 1,102.50 | 98 | 10,219.20 | 958.98 | 1.571 | 1.374–1.796 | <0.001 | |
| 4 | 37 | 2,720.29 | 1,360.15 | 257 | 19,198.74 | 1,338.63 | 1.405 | 1.229–1.606 | <0.001 | |
| Level of care | ||||||||||
| Medical center | 186 | 12,222.48 | 1,521.79 | 352 | 31,905.87 | 1,103.25 | 1.689 | 1.477–1.931 | <0.001 | |
| Regional hospital | 152 | 11,033.59 | 1,377.61 | 486 | 47,624.24 | 1,020.49 | 1.552 | 1.358–1.774 | <0.001 | |
| Local hospital | 59 | 3,931.38 | 1,500.75 | 263 | 23,012.57 | 1,142.85 | 1.437 | 1.257–1.643 | <0.001 | |
Values are presented as number only or mean only.
HR: hazard ratio, CVD: cardiovascular diseases, PYs: person-years, Rate: incidence (per 105 PYs), CI: confidence interval, TWD: Taiwan New Dollars, DM: diabetes mellitus, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, -: not available.
aRate: incidence (per 105 PYs). bAdjusted variables listed in Table 2.
3. Association between specific male factor infertility and risk of CVDs
In the subgroup analysis, we analyzed the relationship between different types of male infertility and CVD according to the ICD 9. Compared with fertile men, men diagnosed with sperm defects (e.g., azoospermia and oligospermia) or other unexplained male infertility showed a higher risk of developing CVDs (adjusted HR, 1.365–1.556; all p<0.001; Supplement Table 4).
4. Association between male infertility and risk of specific CVDs
In the subgroup analysis, we found associations between male infertility and specific CVDs. After adjusting for the variables listed in Table 2, men diagnosed with infertility had a higher risk of developing hypertension (adjusted HR, 2.055; 95% CI, 1.688–2.532; p<0.001), ischemic heart disease (adjusted HR, 2.426; 95% CI, 1.773–3.320; p<0.001), stroke (adjusted HR, 2.261; 95% CI, 1.003–4.551; p=0.045) and other forms of heart disease (adjusted HR, 1.372; 95% CI, 1.091–1.668; p=0.002) than men in the control group (Supplement Table 5).
DISCUSSION
1. Association between male infertility and the risk of CVDs
To our knowledge, this is the first cohort study and the largest study of any type to investigate the association between male infertility and CVDs in the Taiwanese population. We found that males with infertility had an adjusted risk of CVDs—1.472 times that in the control group. After adjusting for confounding factors, the infertile males had a significantly higher risk for hypertension, coronary heart disease, stroke, and other types of heart diseases than the fertile males. This emphasis the importance of this topic.
We used data from Taiwan's NHIRD. Previous studies have found that the ICD-9 coding system has 92.4% specificity for predicting sperm abnormality parameters, with a positive predictive value of 85% [10]. Thus, these diagnosis codes are reliable for identifying infertile males.
2. Possible mechanisms for the increased risk of CVDs in males with infertility
At present, the pathophysiological mechanism for the association between male infertility and the increased risk of CVDs remains unclear. However, there are several clues that hint at the mechanism behind the association between the two. First, males with reproductive problems usually also suffer from problems such as testosterone deficiency [11] and erectile dysfunction (ED), and decreased testosterone levels can increase the risk of cardiovascular mortality [12]. Testosterone deficiency syndrome can also cause ED, which is also closely related to CVDs [13]. ED and coronary artery disease share pathophysiological mechanisms. They are both caused by endothelial dysfunction, which leads to atherosclerosis, peripheral vascular lesions and obstruction of blood flow [14]. A large systematic review and meta-analysis involving 154,794 individuals found that ED predicts cardiovascular events as an independent risk factor. Compared with those of men without ED, the CVD risk of ED patients was 43% higher and their risk of stroke was 34% higher [13]. This sheds some light on the relationship between male infertility and CVDs.
The association between male infertility and CVD may also be related to the quality of semen [15,16]. A long-term follow-up cohort study of 4,712 Danish men with infertility showed that the quality of semen is related to the incidence of CVD. The lower the total sperm count and the lower the sperm motility, the higher was the risk of CVD hospitalization [15]. Another study found that men with a low sperm count (<39 million/ejaculate) have higher body mass index, waist circumference, systolic blood pressure, low-density lipoprotein cholesterol, and triglycerides; lower high-density lipoprotein cholesterol and a higher prevalence of metabolic syndrome [16]. Our findings support this evidence. We have confirmed that semen quality plays an important role in the relationship between male infertility and CVD. However, previous studies have focused on Caucasian populations. This is the first cohort study and the largest study of any type to investigate the association between male infertility and CVD in the Taiwanese population. More studies are needed to confirm these important findings in the future.
Body mass index, waist circumference, blood pressure, and blood lipids are all also important indicators of CVDs, especially hypertension, ischemic heart disease, and stroke [17]. Our subgroup analysis also showed that infertile males were more likely to suffer from hypertension, ischemic heart disease, and stroke later in life than fertile males. This strengthens the support for the relationship between infertility and CVD.
Conversely, infertility often causes severe shock and mental health problems in the patients. The diagnosis process can be overwhelming, awkward, and stressful. Even as the patient undertakes treatment, the stress continues because he experiences anticipation of positive results and disappointment following negative pregnancy tests. This causes substantial emotional turmoil that tends to recur in cycles [18]. Stress as a result of traditional Chinese sociocultural ideas about having babies may result in a severe psychological burden [19]. Depression and anxiety are common problems in infertile Chinese men, and long-term mental stress is also a risk factor for CVDs [20]. When a person suffers from long-term stress or regularly experiences negative emotions, their amygdala activates the bone marrow to produce leukocytes, causing their body to secrete C-reactive proteins and aggravating artery inflammation [21]. This expedites the development of atherosclerosis and causes CVDs, and thus could be another reason why patients with infertility have a high risk for CVDs. However, more studies are required to further explore the mechanisms behind the relationship between male infertility and CVDs.
3. Comparison of our results with previous literature
Our finding that infertile males had a significantly higher risk for CVDs is similar to the findings of previous studies. One study using United States insurance claims data also explored male infertility and the risk of suffering from chronic diseases in later life [4]. After adjusting for confounding factors such as age, smoking, and obesity, and relative to a control group of male patients who had undergone vasectomy (in other words, there were few or no infertile males in this group), male patients with infertility had a 48% higher risk of future ischemic heart disease and a 16% higher risk of other heart diseases [4]. However, the risk of future cerebrovascular disease did not differ between the infertile and vasectomized males. The average follow-up period for that study was 3 to 3.28 years. The incidence increased significantly in infertile men with longer follow-up times (<3 years vs. >3 years). Another retrospective US study using Optum's deidentified Clinformatics Data Mart Database explored male infertility and the risk of the development of cardiovascular metabolic diseases in later life. The average follow-up period was 4.5 to 4.8 years, and this study also found that in comparison with vasectomized males, infertile males had a higher risk for future hypertension and heart disease [5].
The results of these two studies are similar to those of this study. However, these studies were limited to the use of insurance databases and could not follow-up patients if they were removed from that database. Therefore, the follow-up periods were shorter and the age at final observation tended to be lower. However, CVDs are chronic diseases, and a long follow-up period is necessary to verify whether they are going to occur. In our study, there was an average of 8.13±4.58 years between the diagnosis of infertility and the identification of CVDs, indicating that a long follow-up period is necessary to investigate this relationship thoroughly. Our study overcame this difficulty by sampling the entire Taiwanese population and using a long-term dataset with a mean follow-up period of 11.72 years. The results confirmed that for this Asian population, there is a real threat of CVDs occurring in males who are diagnosed with infertility. Thus, male infertility is a possible risk factor or biological marker for future health problems.
There has been a pattern of increasing CVD burden in recent years. In light of this and our results, we suggest that all countries should, while developing their CVD prevention and treatment programs and other health promotion policies, specifically target males diagnosed with infertility for CVD prevention measures. These patients should be informed of the importance of adjusting their lifestyles with the aim of actively preventing CVDs, and they should be provided with more effective consultation and subsequent treatment. In addition, further studies should be performed using subjects from Asian populations to investigate the long-term associations between male infertility and CVDs and other chronic diseases to explore the cause-and-effect relationships responsible for these associations.
Our study has several limitations. First, we know that prior studies used the presence or absence of children, the number of children, or semen analysis results to identify male infertility [16,22]. However, our study relies on the ICD codes to identify infertile men. we could not obtain detailed patient information (e.g., number of children or detailed semen analysis data results) and important data on cardiovascular risk factors such as physical activity or family history from the NHIRD. We attempted to use ‘tobacco use disorder’ (ICD-9-CM: 305.1) as a surrogate for smoker status and to control for smoking. Nonetheless, the study clearly shows that in the years after male patients were diagnosed with infertility, their risk of suffering from CVDs was higher than for healthy males, and this should not be ignored.
CONCLUSIONS
This study, based on a large cohort of the Taiwanese population, suggests that men with infertility have a higher risk of CVDs than fertile men. Stratified analysis shows that after adjusting for confounding factors, infertile males have a significantly higher risk for hypertension, coronary heart disease, stroke and other types of heart disease. The double threat of infertility and CVDs represents a substantial challenge to the health of such men. In the future, clinicians and healthcare providers should pay special attention to these patients because of their higher health risks.
ACKNOWLEDGEMENTS
This research was supported by the Tri-Service General Hospital Research Foundation (grant numbers TSGH-B-110-012).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: PCC, YJC, CCY, TTL.
- Data curation: CHC, CAS, WCC.
- Formal analysis: PCC, CHC, CAS, WCC.
- Funding acquisition: WCC.
- Methodology: PCC, CCH.
- Project administration: PCC, WCC.
- Resources: PCC, CCH, CCY, TTL.
- Software: CHC, CAS, WCC.
- Supervision: YJC, WCC.
- Validation: PCC, CCY, TTL, WCC.
- Visualization: CHC, CAS.
- Writing — original draft: PCC, CCH.
- Writing — review & editing: YJC, CCY, TTL, WCC.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.210098.
Characteristics of participants at the endpoint
Years of follow-up
Years to cardiovascular disease diagnosis
Subgroup analysis association between different type of infertility and risk of CVDs by using Cox regression
Cox regression in cardiovascular disease subgroup analysis
References
- 1.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Gender Equality Committee of the Executive Yuan. National health insurance infertility visit rate [Internet] Taipei City: Gender Equality Committee of the Executive Yuan; c2020. [cited 2020 Jun 29]. Available from: https://www.gender.ey.gov.tw/gecdb/Stat_Statistics_DetailData.aspx?sn=w9X33thXPLBiBr%2fwSMFPjQ%3d%3d&d=194q2o4%2botzoYO%2b8OAMYew%3d%3d . [Google Scholar]
- 3.Glazer CH, Bonde JP, Eisenberg ML, Giwercman A, Hærvig KK, Rimborg S, et al. Male infertility and risk of nonmalignant chronic diseases: a systematic review of the epidemiological evidence. Semin Reprod Med. 2017;35:282–290. doi: 10.1055/s-0037-1603568. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg ML, Li S, Cullen MR, Baker LC. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril. 2016;105:629–636. doi: 10.1016/j.fertnstert.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Kasman AM, Li S, Luke B, Sutcliffe AG, Pacey AA, Eisenberg ML. Male infertility and future cardiometabolic health: does the association vary by sociodemographic factors? Urology. 2019;133:121–128. doi: 10.1016/j.urology.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Global health estimates 2020: deaths by cause, age, sex, by country and by region, 2000-2019. [Internet] Geneva: World Health Organization; c2020. [cited 2021 Mar 11]. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death . [Google Scholar]
- 7.Del Giudice F, Kasman AM, Ferro M, Sciarra A, De Berardinis E, Belladelli F, et al. Clinical correlation among male infertility and overall male health: a systematic review of the literature. Investig Clin Urol. 2020;61:355–371. doi: 10.4111/icu.2020.61.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng TM. In: Six countries, six reform models: the healthcare reform experience of Israel, The Netherlands, New Zealand, Singapore, Switzerland and Taiwan: healthcare reforms "under the radar screen". Okma KGH, Crivelli L, editors. Singapore: World Scientific; 2009. Taiwan's national health insurance system: high value for the dollar; pp. 171–204. [Google Scholar]
- 9.Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4:1–22. [Google Scholar]
- 10.Khandwala YS, Zhang CA, Li S, Cullen MR, Eisenberg ML. Validity of claims data for the identification of male infertility. Curr Urol Rep. 2017;18:68. doi: 10.1007/s11934-017-0714-7. [DOI] [PubMed] [Google Scholar]
- 11.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 12.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15:1260–1271. doi: 10.1016/j.jsxm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Zhao B, Hong Z, Wei Y, Yu D, Xu J, Zhang W. Erectile dysfunction predicts cardiovascular events as an independent risk factor: a systematic review and meta-analysis. J Sex Med. 2019;16:1005–1017. doi: 10.1016/j.jsxm.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Jackson G, Boon N, Eardley I, Kirby M, Dean J, Hackett G, et al. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus. Int J Clin Pract. 2010;64:848–857. doi: 10.1111/j.1742-1241.2010.02410.x. [DOI] [PubMed] [Google Scholar]
- 15.Latif T, Kold Jensen T, Mehlsen J, Holmboe SA, Brinth L, Pors K, et al. Semen quality as a predictor of subsequent morbidity: a Danish cohort study of 4,712 men with long-term follow-up. Am J Epidemiol. 2017;186:910–917. doi: 10.1093/aje/kwx067. [DOI] [PubMed] [Google Scholar]
- 16.Ferlin A, Garolla A, Ghezzi M, Selice R, Palego P, Caretta N, et al. Sperm count and hypogonadism as markers of general male health. Eur Urol Focus. 2021;7:205–213. doi: 10.1016/j.euf.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Guadamuz JS, Durazo-Arvizu RA, Daviglus ML, Calip GS, Nutescu EA, Qato DM. Citizenship status and the prevalence, treatment, and control of cardiovascular disease risk factors among adults in the United States, 2011-2016. Circ Cardiovasc Qual Outcomes. 2020;13:e006215. doi: 10.1161/CIRCOUTCOMES.119.006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enache RG, Matei RS. Psychological and social consequences of infertility. Agora Psycho Pragmatica. 2016;10:62–72. [Google Scholar]
- 19.Logan S, Gu R, Li W, Xiao S, Anazodo A. Infertility in China: culture, society and a need for fertility counselling. Asian Pac J Reprod. 2019;8:1–6. [Google Scholar]
- 20.Yang B, Zhang J, Qi Y, Wang P, Jiang R, Li H. Assessment on occurrences of depression and anxiety and associated risk factors in the infertile Chinese men. Am J Mens Health. 2017;11:767–774. doi: 10.1177/1557988317695901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberg ML, Park Y, Hollenbeck AR, Lipshultz LI, Schatzkin A, Pletcher MJ. Fatherhood and the risk of cardiovascular mortality in the NIH-AARP Diet and Health Study. Hum Reprod. 2011;26:3479–3485. doi: 10.1093/humrep/der305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of participants at the endpoint
Years of follow-up
Years to cardiovascular disease diagnosis
Subgroup analysis association between different type of infertility and risk of CVDs by using Cox regression
Cox regression in cardiovascular disease subgroup analysis