Abstract
Purpose
There is no definite treatment method for chronic pelvic pain syndrome (CPPS). The purpose of this study was to compare and assess the effectiveness and safety of low-intensity extracorporeal shockwave therapy (Li-ESWT) versus placebo treatment in CPPS IIIb patients.
Materials and Methods
Thirty participants with CPPS IIIb were included and randomized in this prospective, double-blind, placebo-controlled study. Li-ESWT was performed at the perineum without anesthesia once per week for 8 weeks. CPPS-related symptoms were evaluated using the National Institutes of Health-chronic prostatitis symptom index (NIH-CPSI). Pain and erectile function were appraised using the Visual Analogue Scale (VAS) and International Index of Erectile Function-Erectile Function (IIEF-EF), respectively. The Global Efficacy Assessment Question (GEAQ) was also assessed. The parameters were evaluated immediately after the last Li-ESWT treatment and 4 weeks after Li-EWST treatment.
Results
Fifteen subjects each in the Li-ESWT and placebo groups completed this study. Amelioration of NIH-CPSI total, pain, and quality of life score in the Li-ESWT group was found compared to the placebo group (p=0.002, 0.02, 0.001, respectively). Improvement of the VAS score was observed in the Li-ESWT group (p=0.002). The differences in the GEAQ “Yes” responses were also significant in the Li-ESWT group. No patients experienced side effects related to ESWT during therapeutic period or follow-up duration.
Conclusions
Results indicated that Li-ESWT improved the NIH-CPSI score, pain, and the quality of life in CPPS IIIb patients. Li-ESWT could be an effective alternative treatment modality for CPPS IIIb.
Keywords: Chronic pelvic pain syndrome, Human, Low-intensity extracorporeal shock wave therapy
INTRODUCTION
Chronic pelvic pain syndrome (CPPS) is categorized as category III by the National Institutes of Health (NIH). It is defined as unspecific, poorly localized pelvic inconvenience or tenderness without certain infection or other definite pathology for at least 3 of the prior 6 months [1]. CPSS is categorized into either NIH IIIa or NIH IIIb prostatitis according to the presence or absence of white blood cells in the prostatic fluid or semen. CPPS is a common problem in male, with the mean incidence of roughly 10% for different ages [2]. Its prevalence ranged from approximately 2% to 10%, with a whole life prevalence of approximately 9% to 16% [3]. Symptoms include inflammation of the prostate, penile, pelvic, perineal pain, voiding dysfunction such as frequent voiding and/or sense of residual urine, and a variable degree of sex-like pain during or after ejaculation and erectile dysfunction (ED) [4,5].
Since CPPS represents various symptoms from likely multiple etiologies, various ranges of therapy have been studied. Furthermore, uncertain etiologies affect the availability of unanimous therapy at present. Antibiotics, anti-inflammatory agents, analgesics, α-blockers, 5 α-reductase inhibitors as monotherapy or combined treatment have been suggested and assessed for CPPS treatment with variable treatment success rates [6,7]. Non-medical treatments such as electromagnetic therapy, acupuncture, massage in prostate, physiotherapy, thermal therapy, neuromodulatory therapy, or life-style modifications could be used as second-line therapy for CPPS patients. Other invasive procedures such as intraprostatic injection, transcutaneous electrical nerve stimulation, and radical prostatectomy have been reported [8,9]. However, none of these treatment modalities showed significant successful effects. Therefore, new therapeutic methods are mandatory.
Recently, several clinical studies reported that low-intensity extracorporeal shockwave therapy (Li-ESWT) can significantly relieve pelvic pain, voiding symptoms for CPPS patients [10,11,12]. Although several published data confirmed that Li-ESWT might be an effective treatment for CPPS patients, few studies have investigated its role in Asians, particularly Korean CPPS patients. Therefore, we conducted a prospective, randomized, double-blind clinical trial to investigate and assess the efficacy and safety of ESWT for the treatment of CPPS IIIb in Korea.
MATERIALS AND METHODS
1. Participants
This clinical study was designed as a prospective-randomized, double-blind, placebo-controlled study. All CPPS-confirmed participants were allocated randomly to treat Li-ESWT or placebo group: two groups in a 1:1 ratio.
The patients were included if Category III b chronic prostatitis patients had symptoms for at least 3 months and no proof of infection in urinary and seminal culture studies. The exclusion criteria of this study were the following: 1) use of another treatment modality at the beginning of the study; 2) Category IIIa CPPS after lower urinary tract localization studies; 3) prior prostate surgery; 4) prostate specific antigen (PSA) >4 ng/mL; 5) history of pelvic surgery; 6) pelvic radiation therapy; 7) any other urological condition related to lower urinary tract symptoms such as urethral stricture, or bladder stones, any neurological disease; and 8) non-indication to maintain this study. Participants were also excluded if PSA >4 ng/mL was reported during primary screening, and if prostate biopsy was performed preferentially to eliminate potential prostate cancer risk. At screening, the diagnosis of CPPS IIIb contained a full medical history, physical examination, NIH-chronic prostatitis symptom index (NIH-CPSI) questionnaire, PSA measurement, microscopic analysis and microbiological culture before and after prostate massage, and prostate secretions. Eligible participants were randomly seperated into two groups. The first group contained 15 patients who received Li-ESWT therapy. The second group contained 15 patients who were treated with placebo therapy.
2. Interventions
A study assistant randomly allocated patients via block randomization using a table of random numbers. All participants were requested to stop using pain reducing medications, such as nonsteroidal anti-inflammatory drugs and a-blockers, for two weeks before the first treatment with Li-ESWT. All patients were treated with electromagnetic Li-ESWT (MT 2000H; Urontech Korea, Hwaseong, Korea) once per week for 8 weeks in an outpatient hospital without general or local anesthesia. The shockwave applicator was smoothly located directly on the ultrasound transmission gel over the skin of the perineum at six different regions (500 shocks per site with a total of 3,000 shocks) at each treatment session. We virtually divided six regions between the anus and scrotum for including the entire prostatic and pelvic floor region. The areas divided virtually are decided according to the focus geometry of the probe head. The energy setting was a frequency of 3 Hz and a maximum total energy flow density of 0.25 mJ/mm2. For the placebo treatment, the identical probe as that in Li-ESWT was utilized, except the energy was set to 0 during each procedure, and a similar noise was transmitted to the patients during the procedure. The existing shock wave number and intensity used were amended in a previous study [10]. The machine applied for the present study was an electromagnetic shock wave device with a wide focused shockwave source. Since the focal zone of this device was deeper and wider, this wide range of focused shock waves could be covered in the prostate from the perineal area without difficulty.
3. Outcome measurement
Follow-ups were executed by an independent observer, who had no data about the clinical study protocol, and performed during outpatient clinic visits at baseline, immediately finishing the last ESWT treatment, and 4 weeks after completing the ESWT course. Clinical symptoms of the patients were evaluated using the NIH-CPSI score, International Index of Erectile Function-Erectile Function (IIEF-EF) score, Visual Analogue Scale (VAS) score, and the Global Efficacy Assessment Question (GEAQ). CPPS-related complaints were investigated using the NIH-CPSI score. The IIEF-EF score was used as a potency function. The intensity of pain was assessed using the VAS score. The primary endpoint was the average changes in NIH-CPSI total score between baseline and 4 weeks after treatment compared with both groups. The change in NIH-CPSI pain score, NIH-CPSI urinary score, NIH-CPSI quality of life (QoL) score, IIEF-EF, VAS, and GEAQ were assessed as secondary endpoints.
4. Statistical analysis
Continuous variables were represented as mean±standard deviation. Independent samples t-test and Mann-Whitney test were used to compare the continuous variables between both groups. Paired t-test or Wilcoxon signed rank test were used to compare the variables within each group. To evaluate group mean differences in the changes from baseline in all continuous variables, analyses of covariance were used with change from baseline at follow-up as the dependent variable and baseline value of the dependent variable and treatment group as covariates. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). p<0.05 was considered a statistical significance level.
The number of patients evaluated in this study was based on the results of a previous study [11]. Given that information from Li-ESWT could be efficient for CPPS patients, we measured the sample number (n=30) using G*power software (ver. 3.1) with one tailed test, 1:1 allocation, α error=0.05, and β=0.8.
5. Ethics statement
Ethical approval for the fulfillment of the study was granted by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital and Korea University Guro Hospital (approval number: XC19DEDI0048) after permission by the Korean Ministry of Food and Drug Safety. Informed consent was confirmed by the IRB, and before any procedures were executed, informed consent was obtained from each subject. This study was performed according to the principles of the Declaration of Helsinki and the ethical principles of Good Clinical Practice guidelines. The protocol was registered in Clinical Research Information Service (https://cris.nih.go.kr/cris, KCT identifier: KCT0004441).
RESULTS
A total of 34 patients participated in this study. Fifteen and nineteen patients were randomly allocated into the Li-ESWT and placebo groups, respectively. Four participants in the placebo group were not included due to withdrawal of consent during the therapy period (Fig. 1). The clinical and demographic data of the patients in both groups are described in Table 1. No significant differences in age, body weight, height, body mass index, NIH-CPSI score, VAS, and serum PSA level were observed between both groups at baseline. IIEF-EF score showed a statistical difference between groups (p=0.023).
Fig. 1. Patient disposition. Li-ESWT: low-intensity extracorporeal shockwave therapy.
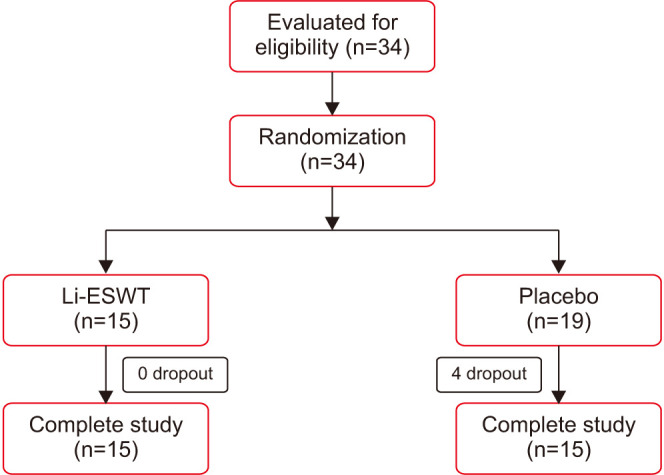
Table 1. Dermographic characteristics of the patients.
| Variable | Li-ESWT group (n=15) | Placebo group (n=19) | p-value |
|---|---|---|---|
| Age (y) | 58.4±8.4 | 56.5±5.1 | 0.636 |
| Height (cm) | 172.0±4.5 | 172.5±6.7 | 0.783 |
| Body weight (kg) | 72.0±8.2 | 70.2±9.3 | 0.564 |
| BMI (kg/m2) | 24.3±4.7 | 23.6±3.1 | 0.357 |
| PSA (ng/mL) | 1.03±0.83 | 0.81±0.59 | 0.507 |
| Symptoms duration (mo) | 33.0±38.9 | 49.3±48.7 | 0.515 |
| NIH-CPSI total score | 27.1±4.8 | 24.5±5.9 | 0.478 |
| NIH-CPSI pain score | 13.0±3.6 | 11.9±3.3 | 0.375 |
| NIH-CPSI urinary score | 5.0±3.2 | 3.9±2.7 | 0.434 |
| NIH-CPSI QoL score | 9.1±2.1 | 8.3±2.3 | 0.618 |
| IIEF-EF | 11.3±10.7 | 20.3±9.6 | 0.023 |
| VAS | 6.5±2.5 | 5.8±1.9 | 0.556 |
Li-ESWT: low-intensity extracorporeal shockwave therapy, BMI: body mass index, PSA: prostate specific antigen, NIH-CPSI: National Institutes of Health-chronic prostatitis symptom index, QoL: quality of life, IIEF-EF: International Index of Erectile Function-Erectile Function, VAS: Visual Analogue Scale.
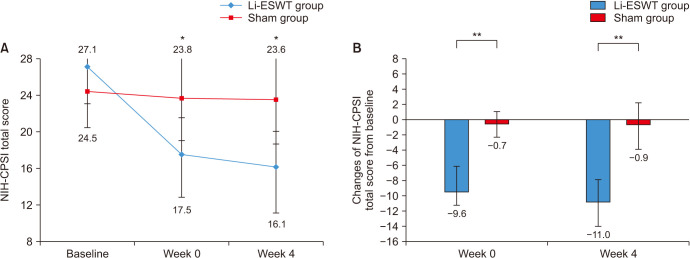
The baseline NIH-CPSI total score in the Li-ESWT and placebo groups did not show a statistically significant difference (27.1±4.8 vs. 24.5±5.9, p=0.478). No significant differences in baseline NIH-CPSI pain, urinary, and QoL subdomain scores were also observed between the groups (Table 2). Compared to the initial scores, significant improvements (decrease) in the NIH-CPSI total score, NIH-CPSI pain, NIH-CPSI urinary, and NIH-CPSI QoL subdomain scores in the Li-ESWT group were detected at the end of the last ESWT and 4 weeks after ESWT (Table 2, Fig. 2). However, there was no significant improvement in the NIH-CPSI total score, pain, urinary, and QoL subdomain scores in the placebo group. IIEF-EF score was improved for Li-ESWT group in the finishing treatment immediately and 4 weeks after treatment compared to baseline (p<0.05) (Table 2). Nevertheless, although the IIEF-EF score at baseline was higher in the placebo treatment, there was statistical difference between the groups. The baseline mean scores of VAS were 6.5±2.5 and 5.8±1.9, respectively. For the Li-ESWT group, there was a significant improvement in the VAS score after treatment compared to baseline, with a significant difference in VAS score between both groups (Table 2). The rates of the GEAQ “Yes” response in the Li-ESWT and placebo groups were 100% and 15.4%, respectively. No significant difference from baseline values and between both groups was observed also for the 4-week follow-up visit PSA (p=0.589) (Table 2).
Table 2. Variables at baseline, immediate, 4 weeks after treatment in patient in Li-ESWT and placebo.
| Variable | Baseline | F/U (immediately) | F/U (Week 4) | |
|---|---|---|---|---|
| NIH-CPSI total | ||||
| Li-ESWT | 27.1±4.8 | 17.5±4.1* | 16.1±4.2* | |
| Placebo | 24.5±5.9 | 23.8±3.9 | 23.6±3.1 | |
| p-value for between group | 0.478 | 0.003 | 0.002 | |
| NIH-CPSI pain | ||||
| Li-ESWT | 13.0±3.6 | 7.5±3.7* | 7.1±5.0* | |
| Placebo | 11.9±3.3 | 11.8±4.1 | 11.4±4.5 | |
| p-value for between group | 0.375 | 0.006 | 0.02 | |
| NIH-CPSI urinary | ||||
| Li-ESWT | 5.0±3.2 | 3.6±2.9* | 3.3±3.1* | |
| Placebo | 3.9±2.7 | 3.7±3.2 | 4.1±3.2 | |
| p-value for between group | 0.434 | 0.849 | 0.081 | |
| NIH-CPSI QoL | ||||
| Li-ESWT | 9.1±2.2 | 6.4±2.5* | 5.7±2.3* | |
| Placebo | 8.7±2.2 | 8.3±2.3 | 8.1±2.1 | |
| p-value for between group | 0.618 | 0.042 | 0.001 | |
| IIEF-EF | ||||
| Li-ESWT Changes of NIH-CPSI | 11.3±10.7 | 15.1±10.1* | 14.0±11.4* | |
| Placebo total score from baseline | 20.3±9.6 | 20.3±10.0 | 17.2±11.3 | |
| p-value for between group | 0.023 | 0.043 | 0.019 | |
| VAS | ||||
| Li-ESWT | 6.5±2.5 | 2.9±1.6* | 2.7±1.9* | |
| Placebo | 5.8±1.9 | 5.1±2.2 | 5.3±2.3 | |
| p-value for between group | 0.556 | 0.005 | 0.002 | |
| PSA | ||||
| Li-ESWT | 1.03±0.83 | - | 0.99±0.75 | |
| Placebo | 0.81±0.59 | - | 0.81±0.62 | |
| p-value for between group | 0.507 | - | 0.589 | |
Li-ESWT: low-intensity extracorporeal shockwave therapy, F/U: follow-up, NIH-CPSI: National Institutes of Health-chronic prostatitis symptom index, QoL: quality of life, IPSS: International Prostate Symptom Score, IIEF-EF: International Index of Erectile Function-Erectile Function, VAS: Visual Analogue Scale, PSA: prostate specific antigen, −: not available.
*p<0.05 compared to baseline.
Fig. 2. (A) Mean and change off NIH-CPSI total score for patients treated with Li-ESWT or placebo treatment at baseline, week 0, and week 4. Asterisk means p<0.001 compared with baseline. (B) Comparison of the mean NIH-CPSI total score changes from baseline. Double asterisk mean p<0.001. NIH-CPSI: National Institute Health-chronic prostatitis symptom index.
No adverse effects related to Li-ESWT like gross hematuria, ecchymosis, and hematospermia were detected in any of the patients during the study period.
DISCUSSION
Li-ESWT, which is a minimally invasive treatment, has been recently adapted in the treatment of urologic diseases such as ED [13], Peyronie's disease (PD), and CPPS. For ED, the Asia-Pacific Society for Sexual Medicine commented that Li-ESWT ameliorated erectile function score and penile hemodynamic parameters in patients with vasogenic ED. However, the long-term effect of Li-ESWT was controversial. They recommended that Li-ESWT should be used in patients with mild-moderated vasogenic ED [14]. For PD, although Li-ESWT in PD seems to be effective in terms of penile pain and erectile function, the effect on penile curvature and plaque size is questionable. However, a recently reported study on the effect of Li-ESWT in PD, Li-ESWT improved significantly erectile function and penile pain every week for six weeks. Moreover, penile curvature and plaque size were statistical significantly reduced [15].
Treatment of patients with difficulty in CPPS is one of the most troublesome and challenging matters in urology. Most of the available treatment options are limited to symptomatic treatments and do not treat the underlying reason. Therefore, it is necessary to find an easily applied treatment modality with proven effectiveness and safety. Although the precise mechanisms of shock wave therapy are currently under investigation, shock wave therapy may ameliorate CPPS symptoms through several mechanisms, such as nociceptor hyperstimulation, nitric oxide synthesis induction, passive muscle tone decrease, interruption of nerve impulses, and rising of local microvascularization [16,17,18].
Zimmerman et al [19] initially represented the efficacy of Li-ESWT in CPPS patients in 2008. The authors represented significant ameliorations in pain and QoL after Li-ESWT. Although urinary condition improved no statistically significant difference was observed. According to positive preliminary results, a prospective-randomized, double-blind study was performed by the same study group. The authors observed that a total of 30 patients in the Li-ESWT group showed significant amelioration of pain, QoL, and urinary symptoms after Li-ESWT in comparison with the placebo group. The therapeutic effect of Li-ESWT persisted until 12 weeks. The author concluded that the strengths of Li-ESWT are the following: its inexpensive and easy application, repetition of treatment as often as required, and low probability of any side effects.
Regarding the long-term effect of Li-ESWT, the results of several studies were controversial. The data from Moayednia et al [20] showed no statistically significant difference between the ESWT and placebo groups in NIH-CPSI pain, urinary, and QoL scores after a 24-week follow-up duration. We think that applying ESWT for only 4 weeks may negatively affect its long-term effects. However, another study comparing the efficiency of combined Li-ESWT+triple therapy (anti-inflammatory, α-blocker, and muscle relaxant) with triple therapy only showed different results [11]. The triple therapy+Li-ESWT group showed the most significant improvement in total and prostatic pain NIH-CPSI scores. Furthermore, the triple therapy+Li-ESWT group of subjects represented significantly better outcomes than the triple therapy group in all subdomains of NIH-CPSI scores at 36 weeks after the beginning of treatment. Moreover, significant improvement in NIH-CPSI scores after 36 weeks of treatment is identifiable in mostly every score excluding QoL and urinary compared to the initial NIH-CPSI score. This result means that triple therapy combined with Li-ESWT showed better long-term effects than triple therapy alone. The authors commented that Li-ESWT could be a significantly important treatment option for CPPS patients. However, longer treatment duration and proper treatment machine application are mandatory.
Our study group performed an in vivo study using a prostatitis rat model to confirm the mechanism of Li-ESWT for chronic prostatitis before starting a clinical study [21]. We found that Li-ESWT reduced COX-2 by inhibiting the TLR3-NFκB pathway in an experimental model. Additionally, the TRAF2 regulator in ERK1/2 inhibition significantly decreased inflammation. This signaling event co-operatively facilitated inflammation with different levels of the expression of interleukin-1β, interleukin-6, and other inflammatory molecular markers via different stimulation models.
After confirming the mechanism of Li-ESWT for treating CPPS, we performed a clinical study in Korea for the first time. Furthermore, we performed the first study of Li-ESWT for the treatment of ED in Korea [22]. We confirmed the effectiveness of electromagnetic Li-ESWT for ED therapy. The present study using the same electromagnetic Li-ESWT machine showed that Li-ESWT relieved pain and urinary symptoms and ameliorated the quality of life in CPPS patients. Li-ESWT significantly decreased the NIH-CPSI total score and the effect persisted for 4 weeks after Li-ESWT. These results are comparable with those of the aforementioned published studies. The significant amelioration in NIH-CPSI QoL subdomain score was most likely a consequence of the substantial improvement in pain. Additionally, pain intensity was the strongest independent predictor of QoL in CPPS patients [23].
Interestingly, improvement of IIEF-EF in the Li-ESWT group was observed in this study. Zimmerman et al [10] explained that improvement of QoL attained by Li-ESWT in patients had a positive effect on their sexual function. Furthermore, local application of Li-ESWT in the perineal area could positively affect erectile function [24]. However, no statistical difference in IIEF-EF between both groups was observed, except at baseline. We believe that more high-quality studies are mandatory to elucidate the effect of Li-ESWT on erectile function in CPPS patients.
The main strong point of this study was the design of the study as a prospective, randomized, double-blind, placebo-controlled study. Another strong point lies in the fact that ESWT for CPPS was performed first in Asia, specifically in Korea. However, the present study had some limitations. First, the sample size was small. Therefore, it is difficult to compare the effectiveness of Li-ESWT for different causes of CPPS. Second, the follow-up duration was only 1 month. Hence, it is mandatory to perform further large-scale and long-term studies. Furthermore, comparing the effectiveness of other types of Li-ESWT and confirming a proper therapeutic protocol are also essential for further studies.
CONCLUSIONS
Li-ESWT decreased the NIH-CPSI total score, showing clinical and therapeutic effects. This could be an efficient alternative therapeutic modality for CPPS IIIb. Further studies with long-term follow-up duration, large sample sizes, and comprehensive evaluation designs are warranted to appraise the effects of Li-ESWT on CPPS IIIb.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant HI19C0310).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: KSK, DGM, SWK.
- Data curation: KSK, WJB, STA, DGM, SWK.
- Formal analysis: KSK, DGM, SWK.
- Funding acquisition: KSK, DGM, SWK.
- Investigation: KSK, DGM, SWK.
- Methodology: KSK, DGM, SWK.
- Project administration: KSK, DGM, SWK.
- Resources: KSK, DGM, SWK.
- Supervision: YSC, WJB, HJC, USH, SHH, JYL.
- Validation: KSK, DGM, SWK.
- Visualization: KSK, DGM, SWK.
- Writing — original draft: KSK, DGM, SWK.
- Writing — review & editing: KSK, DGM, SWK.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/A0GVDE.
References
- 1.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 2.Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001;165:842–845. [PubMed] [Google Scholar]
- 3.Krieger JN, Lee SW, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents. 2008;31 Suppl 1:S85–S90. doi: 10.1016/j.ijantimicag.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magistro G, Wagenlehner FM, Grabe M, Weidner W, Stief CG, Nickel JC. Contemporary management of chronic prostatitis/chronic pelvic pain syndrome. Eur Urol. 2016;69:286–297. doi: 10.1016/j.eururo.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 5.Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis. 2016;19:132–138. doi: 10.1038/pcan.2016.8. [DOI] [PubMed] [Google Scholar]
- 6.Engeler DS, Baranowski AP, Dinis-Oliveira P, Elneil S, Hughes J, Messelink EJ, et al. European Association of Urology. The 2013 EAU guidelines on chronic pelvic pain: is management of chronic pelvic pain a habit, a philosophy, or a science? 10 Years of development. Eur Urol. 2013;64:431–439. doi: 10.1016/j.eururo.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Tuğcu V, Taşçi AI, Fazlioğlu A, Gürbüz G, Ozbek E, Sahin S, et al. A placebo-controlled comparison of the efficiency of triple- and monotherapy in category III B chronic pelvic pain syndrome (CPPS) Eur Urol. 2007;51:1113–1117. doi: 10.1016/j.eururo.2006.09.036. discussion 1118. [DOI] [PubMed] [Google Scholar]
- 8.Schneider MP, Tellenbach M, Mordasini L, Thalmann GN, Kessler TM. Refractory chronic pelvic pain syndrome in men: can transcutaneous electrical nerve stimulation help? BJU Int. 2013;112:E159–E163. doi: 10.1111/bju.12005. [DOI] [PubMed] [Google Scholar]
- 9.Chopra S, Satkunasivam R, Aron M. Feasibility of robotic radical prostatectomy for medication refractory chronic prostatitis/chronic pelvic pain syndrome: initial results. Indian J Urol. 2016;32:238–241. doi: 10.4103/0970-1591.185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann R, Cumpanas A, Miclea F, Janetschek G. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome in males: a randomised, double-blind, placebo-controlled study. Eur Urol. 2009;56:418–424. doi: 10.1016/j.eururo.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Pajovic B, Radojevic N, Dimitrovski A, Vukovic M. Comparison of the efficiency of combined extracorporeal shock-wave therapy and triple therapy versus triple therapy itself in Category III B chronic pelvic pain syndrome (CPPS) Aging Male. 2016;19:202–207. doi: 10.1080/13685538.2016.1197899. [DOI] [PubMed] [Google Scholar]
- 12.Mykoniatis I, Kalyvianakis D, Zilotis F, Kapoteli P, Fournaraki A, Poulios E, et al. Evaluation of a low-intensity shockwave therapy for chronic prostatitis type IIIb/chronic pelvic pain syndrome: a double-blind randomized sham-controlled clinical trial. Prostate Cancer Prostatic Dis. 2021;24:370–379. doi: 10.1038/s41391-020-00284-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Cho MC, Cho SY, Chung H, Rajasekaran MR. Novel emerging therapies for erectile dysfunction. World J Mens Health. 2021;39:48–64. doi: 10.5534/wjmh.200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung E, Lee J, Liu CC, Taniguchi H, Zhou HL, Park HJ. Clinical practice guideline recommendation on the use of low intensity extracorporeal shock wave therapy and low intensity pulsed ultrasound shock wave therapy to treat erectile dysfunction: the Asia-Pacific Society for Sexual Medicine position statement. World J Mens Health. 2021;39:1–8. doi: 10.5534/wjmh.200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Mauro M, Russo GI, Della Camera PA, Di Maida F, Cito G, Mondaini N, et al. Extracorporeal shock wave therapy in Peyronie's disease: clinical efficacy and safety from a single-arm observational study. World J Mens Health. 2019;37:339–346. doi: 10.5534/wjmh.180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, et al. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide. 2005;12:89–96. doi: 10.1016/j.niox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Wess OJ. A neural model for chronic pain and pain relief by extracorporeal shock wave treatment. Urol Res. 2008;36:327–334. doi: 10.1007/s00240-008-0156-2. [DOI] [PubMed] [Google Scholar]
- 18.Marszalek M, Berger I, Madersbacher S. Low-energy extracorporeal shock wave therapy for chronic pelvic pain syndrome: finally, the magic bullet? Eur Urol. 2009;56:425–426. doi: 10.1016/j.eururo.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann R, Cumpanas A, Hoeltl L, Janetschek G, Stenzl A, Miclea F. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: a feasibility study and the first clinical results. BJU Int. 2008;102:976–980. doi: 10.1111/j.1464-410X.2008.07742.x. [DOI] [PubMed] [Google Scholar]
- 20.Moayednia A, Haghdani S, Khosrawi S, Yousefi E, Vahdatpour B. Long-term effect of extracorporeal shock wave therapy on the treatment of chronic pelvic pain syndrome due to non bacterial prostatitis. J Res Med Sci. 2014;19:293–296. [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon SH, Zhu GQ, Kwon EB, Lee KW, Cho HJ, Ha US, et al. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFκB pathway in a prostatitis rat model. Prostate. 2019;79:1498–1504. doi: 10.1002/pros.23880. [DOI] [PubMed] [Google Scholar]
- 22.Kim KS, Jeong HC, Choi SW, Choi YS, Cho HJ, Ha US, et al. Electromagnetic low-intensity extracorporeal shock wave therapy in patients with erectile dysfunction: a sham-controlled, double-blind, randomized prospective study. World J Mens Health. 2020;38:236–242. doi: 10.5534/wjmh.190130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripp DA, Curtis Nickel J, Landis JR, Wang YL, Knauss JS CPCRN Study Group. Predictors of quality of life and pain in chronic prostatitis/chronic pelvic pain syndrome: findings from the National Institutes of Health chronic prostatitis cohort study. BJU Int. 2004;94:1279–1282. doi: 10.1111/j.1464-410X.2004.05157.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalyvianakis D, Memmos E, Mykoniatis I, Kapoteli P, Memmos D, Hatzichristou D. Low-intensity shockwave therapy for erectile dysfunction: a randomized clinical trial comparing 2 treatment protocols and the impact of repeating treatment. J Sex Med. 2018;15:334–345. doi: 10.1016/j.jsxm.2018.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/A0GVDE.