Abstract
Purpose
During epididymal sperm maturation, spermatozoa acquire progressive motility through dynamic protein modifications. However, the relationship between sequential protein modifications during epididymal sperm maturation and sperm motility and fertility has not yet been investigated. This study investigated whether sequential changes in fertility-related protein expression including that of enolase 1 (ENO1), ubiquinol-cytochrome c reductase core protein 1 and 2 (UQCRC1 and UQCRC2), and voltage-dependent anion channel 2 (VDAC2) in spermatozoa during epididymal maturation are related to bovine sperm motility. Moreover, we found that mitochondrial metabolism is closely related to fertility-related proteins. Therefore, we investigated how the sequential modification of mitochondrial proteins during epididymal maturation regulates sperm motility.
Materials and Methods
To determine the differential protein expression in caput and cauda epididymal spermatozoa from low and high motility bulls, western blot analysis was performed. Moreover, signaling pathways were identified to understand the mechanisms of regulation of sperm motility through the differential protein expression associated with fertility-related proteins.
Results
We found that ENO1 was substantially higher in the caput spermatozoa from low motility bulls than the caput and cauda spermatozoa from high motility bulls. However, ENO1 expression in low motility bull spermatozoa was downregulated to a level comparable to that in the high motility bull spermatozoa during epididymal maturation. Moreover, there was a lack of modification of mitochondrial proteins, including glutathione peroxidase 4 and NADH:Ubiquinone Oxidoreductase Core Subunit S8, in low motility bull spermatozoa during epididymal maturation, whereas active changes were detected in high motility bull spermatozoa.
Conclusions
Irregular modifications of mitochondrial proteins during epididymal sperm maturation may increase excessive ROS production and premature activation of spermatozoa during epididymal maturation. Consequently, spermatozoa may lose their motility by the earlier consumption of their energy source and may be damaged by ROS during epididymal maturation, resulting in a decline in sperm motility and bull fertility.
Keywords: Epididymis, Fertility, Proteome, Sperm maturation, Sperm motility
INTRODUCTION
The epididymis is a highly segmented organ which is composed of four main distinct regions, the initial segment, caput, corpus, and cauda epididymides. After leaving the testis, spermatozoa undergo a series of morphological, biochemical, and physiological changes in the epididymis to acquire progressive motility and fertilizing ability, a process called sperm maturation [1]. In the epididymis, epithelial cells such as narrow cells, principal cells, basal cells, and clear cells, contribute to sperm maturation by secretion or reabsorption of proteins [2,3]. Because spermatozoa are transcriptionally and translationally inactive cells, proteome profiling in spermatozoa is modified through the uptake, remodelling, and post-translational modification [4]. Recently, proteomic tools have been developed and applied to identify the proteomes in mammalian spermatozoa, which has greatly increased our understanding of sperm fertility [4,5,6]. Furthermore, many comparative proteomic studies of spermatozoa in the caput, corpus, and cauda epididymides have been conducted to identify the maturation-related proteomes which elucidate the key roles of sperm maturation in sperm fertility [4,7,8]. In particular, lipid localization and glycosylation-related proteins are abundant in spermatozoa from the caput and corpus epididymis, whereas sperm-egg recognition and motility associated proteins are enriched in the cauda epididymis [4]. These reports indicate that the presence of specific proteins along the epididymis may provide unique and specialized conditions for sperm maturation (depending on the segment or region) and they play an important role in sperm fertility. In previous studies, comprehensive proteomic studies were conducted to identify fertility-related proteins in bovine [6] and porcine [5] spermatozoa. Especially, enolase 1 (ENO1), ubiquinol-cytochrome c reductase core protein 1 and 2 (UQCRC1 and UQCRC2), and voltage-dependent anion channel 2 (VDAC2) were closely related to male fertility in the bovine.
Although sperm motility and motion parameters have a lower relationship with male fertility than other male fertility indicators [9,10] such as spermoocyte penetration [11], capacitation status [10], cervical mucus [12], and proteome and transcriptome markers [6,13], approximately 60% of idiopathic male infertility is closely related to motility defects. Motility is therefore the most important factor in male infertility [14]. Sperm motility has traditionally and routinely been used as a preliminary indicator of potential fertility in humans and animals.
During epididymal transit, the formation of disulphide bonds and thiol oxidization of cytoskeletal and structurally related proteins is increased in the sperm tail and may be related to the stability of the sperm flagellum for subsequent sperm motility [15]. How the sequential protein changes that occur in spermatozoa during transit in the epididymis regulate sperm motility has not yet been thoroughly investigated.
Sperm motility is preliminarily determined during epididymal maturation. We hypothesized that the changes in these fertility-related proteins in spermatozoa may be differentially expressed between high and low motile spermatozoa. Therefore, this study investigated how the sequential changes in fertility-related proteins (including ENO1, VDAC2, UQCRC1, and UQCRC2) in bovine spermatozoa during epididymal maturation are associated with sperm motility. Moreover, these fertility-related proteins are closely associated with mitochondrial metabolism pathways (Table 1). Therefore, the mechanisms of the regulation of sperm motility through differential protein expression associated with mitochondrial metabolism during epididymal maturation were identified.
Table 1. Summary of the GO biological processes based on fertility-related proteins in bovine spermatozoa.
| GO ID | Description | FDR | Gene |
|---|---|---|---|
| GO:0006122 | Mitochondrial electron transport, ubiquinol to cytochrome c | <0.01 | UQCRC1, UQCRC2 |
| GO:0046034 | ATP metabolic process | <0.05 | ENO1, UQCRC1, UQCRC2 |
GO: Gene Ontology, FDR: false discovery rate.
MATERIALS AND METHODS
1. Ethical statement
All procedures were performed according to the guidelines for the ethical treatment of animals approved by the Institutional Animal Care and Use Committee of Chung-Ang University, Seoul, Korea (Approval No. 2015-00056).
2. Sample preparation
Caput and cauda spermatozoa were cryopreserved in Tris-egg yolk buffer (containing 250 mM Tris, 88.5 mM citric acid, 68.8 mM glucose, and 20% egg yolk) as described by Yoon et al [16]. Briefly, the caput and cauda epididymis were dissected from testis. Spermatozoa from caput epididymis were collected through small incisions with a scalpel in a phosphate buffered saline (PBS), and the cauda epididymis was back-flushed with PBS from end of the vas deferens. To remove the dead sperm and extender debris, frozen caput and cauda spermatozoa were centrifuged at 400×g for 20 minutes with a discontinuous Percoll density gradient of 500 µL of 45% to 60% Percoll for caput spermatozoa and 45% to 90% Percoll for cauda spermatozoa.
3. Sperm motility and motion kinematics
Sperm motility (%) from 20 bulls was assessed by computer-assisted semen analysis (CASA, SAIS-PLUS 10.1; Medical Supply, Seoul, Korea). Briefly, Percoll-separated spermatozoa were washed and resuspended with Tyrode albumin lactated pyruvate medium containing 100 mM NaCl, 3.1 mM KCl, 2.0 mM CaCl·2H2O, 0.4 mM MgCl·6H2O, 0.3 mM Na2HPO4·12H2O, 21.6 mM sodium lactate, 25 mM NaHCO3, and 1.0 mM sodium pyruvate. Sperm cells were then incubated for 30 minutes at 37℃ in an atmosphere of 5% CO2. A 10 µL aliquot of the semen sample was placed in a pre-heated Makler chamber (Makler, Haifa, Israel), which was kept warm at 38.5℃ during the procedure. After 30 seconds, sperm motility and motion kinematics including hyperactivation (HYP), curvilinear velocity (VCL), straight line velocity (VSL), and average path velocity (VAP) were evaluated. Spermatozoa observed with VCL >80 µm/s, linearity (LIN) <65%, and an amplitude of lateral head displacement >6.5 µm were considered as possessing high HYP. According to their motility (%), semen samples were divided into two groups: a high motility group of three bulls with the highest motility (≥70% motility, n=3 bulls) and a low motility group of three bulls with lowest motility (<70% motility, n=3 bulls). The average motility for the high and low motility bulls was 75.99%±2.31% and 55.88%±3.51%, respectively (p<0.05, Table 2).
Table 2. Comparison of sperm motility and motion kinematics between high and low fertility bulls.
| Parameter | High motility (%) | Low motility (%) |
|---|---|---|
| Motility (%) | 79.55±2.23 | 55.88±3.51* |
| HYP (%) | 1.97±1.07 | 0.67±0.34 |
| VCL (μm/s) | 115.00±4.47 | 98.17±1.88* |
| VSL (μm/s) | 70.72±0.37 | 52.62±1.49* |
| VAP | 70.12±1.16 | 54.43±1.85* |
| LIN | 60.12±1.91 | 53.60±0.99* |
| BCF (Hz) | 15.50±0.47 | 17.98±0.11* |
| Wobble | 61.15±2.16 | 55.41±1.41* |
| Dance | 613.46±38.00 | 438.82±19.48* |
| ALH (μm) | 5.32±0.13 | 4.44±0.13* |
Values are presented as mean±standard error of the mean.
HYP: hyperactivation, VCL: curvilinear velocity, VSL: straight line velocity, VAP: average path velocity, LIN: linearity, BCF: beat-cross frequency, ALH: mean amplitude of head lateral displacement.
*Superscripts indicate significant differences between the high and low motility spermatozoa, as determined using Student’s t-test at a significance level of p<0.05.
4. Western blot
To determine the differential protein expression in caput and cauda epididymal spermatozoa from low and high motility bulls, western blot analysis was performed as previously described [10]. Briefly, sperm pellets were homogenized in 2×Laemmli sodium dodecyl sulphate (SDS) sample buffer containing 5% mercaptoethanol. Approximately 1×106 cells were loaded into each lane and SDS-polyacrylamide gel electrophoresis was conducted. The proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Amersham, Piscataway, NJ, USA). Antibodies against ENO1, UQCRC1, UQCRC2, NADH:Ubiquinone Oxidoreductase Core Subunit S8 (NDUFS8), VDAC2, cytochrome c (CYC), NDUFS2, Parkinsonism-associated deglycase (PARK7), glutathione peroxidase 4 (GPX4), and glucose transporter 3 (GLUT3) were used. Alpha-tubulin was used as the loading control. All antibodies were purchased from Abcam (Abcam, Cambridge, MA, USA). Protein expression was detected by chemiluminescence and the expression level was quantified by Image J software (Version 1.52a; National Institutes of Health, Bethesda, MD, USA).
5. Signaling pathways
Based on the fertility-related proteins, ENO1, VDAC2, UQCRC1, and UQCRC2, signaling pathways were identified by g:Profiler, Cytoscape (Version 3.8.2), and EnrichmentMap according to the protocols of Reimand et al [17] to explore the molecular functions associated with sperm maturation. Also, Pathway Studio program (Elsevier, Amsterdam, The Netherlands) was used to identify the protein-protein interaction and cellular regulation.
6. Statistical analysis
Statistical analyses were performed using GraphPad Prism (Version, 9.0.0; GraphPad, San Diego, California, USA). Sperm motility and motion kinematics between the high and low motility groups was analysed using a Student’s t-test for normally distributed data. Two-way analysis of variance with a Šidák post hoc test was used to analyse the differences in protein expression of spermatozoa from the caput and cauda epididymides between the high and low motility groups (p<0.05). Data were presented as mean±standard error of the mean.
RESULTS
1. Differences in sperm motility and motion kinematics between high and low motility bull spermatozoa
After Percoll separation, sperm motility (%) and motion kinematics of cauda spermatozoa were investigated by CASA to divide the sperm into two groups on the basis of their motility (Table 2). Sperm motility (%) was higher in the high motility group (79.55%±2.23%) than the low motility group (55.88%±3.51%) (p<0.05). Similarly, motion kinematics including VCL, VSL, VAP, LIN, wobble, dance, and mean amplitude of head lateral displacement in high motility spermatozoa were higher than in low motility spermatozoa (p<0.05, Table 2).
2. Differential expression of fertility-related proteins between high and low motility bull spermatozoa during epididymal maturation
To explore whether the differentially expressed proteins between the caput and cauda epididymides are related to sperm motility, fertility-related protein expression in the caput and cauda spermatozoa was compared in high and low motility spermatozoa. There were no significant differences in protein expression of UQCRC1, UQCRC2, and VDAC2 between caput and cauda spermatozoa, regardless of sperm motility (Fig. 1). ENO1 was higher in the caput spermatozoa from low motility bulls than the caput and cauda spermatozoa from high motility bulls (Fig. 1A, p<0.05). However, the expression of ENO1 in low motility bull spermatozoa was downregulated to a level comparable to that in the high motility bull spermatozoa during epididymal maturation (Fig. 1A). To understand the comprehensive roles of fertility-related proteins in sperm motility regulation, we identified the signaling pathways associated with the fertility-related proteins, ENO1, UQCRC1, UQCRC2, and VDAC2. Gene Ontology analysis indicated that the biological processes of mitochondrial electron transport, ubiquinol to CYC, and the ATP metabolic pathway were most highly enriched in fertility-related proteins (Fig. 1F, Table 1).
Fig. 1. Changes in fertility-related protein expression between high and low motility bull spermatozoa during epididymal maturation. Density of (A) ENO1, (B) UQCRC1, (C) UQCRC2, and (D) VDAC2 proteins in the high and low motility bull spermatozoa following epididymal maturation. Statistics show the ratio of the normalized protein expression to caput spermatozoa in the high motility group. Data are presented as mean±standard error of the mean. *Superscripts indicate significant differences in the protein expression of spermatozoa from the caput and cauda epididymides between the high and low motility groups as determined by two-way ANOVA with a Šidák post hoc test (p<0.05). (E) Western blot image of ENO1, UQCRC1, UQCRC2, and VDAC2 proteins. (F) Summarized signaling pathways based on fertility-related proteins in bovine spermatozoa. Cellular processes were illustrated using the Pathway Studio program. Mot: motility, CP: spermatozoa from the caput epididymis, CD: spermatozoa from the cauda epididymis.
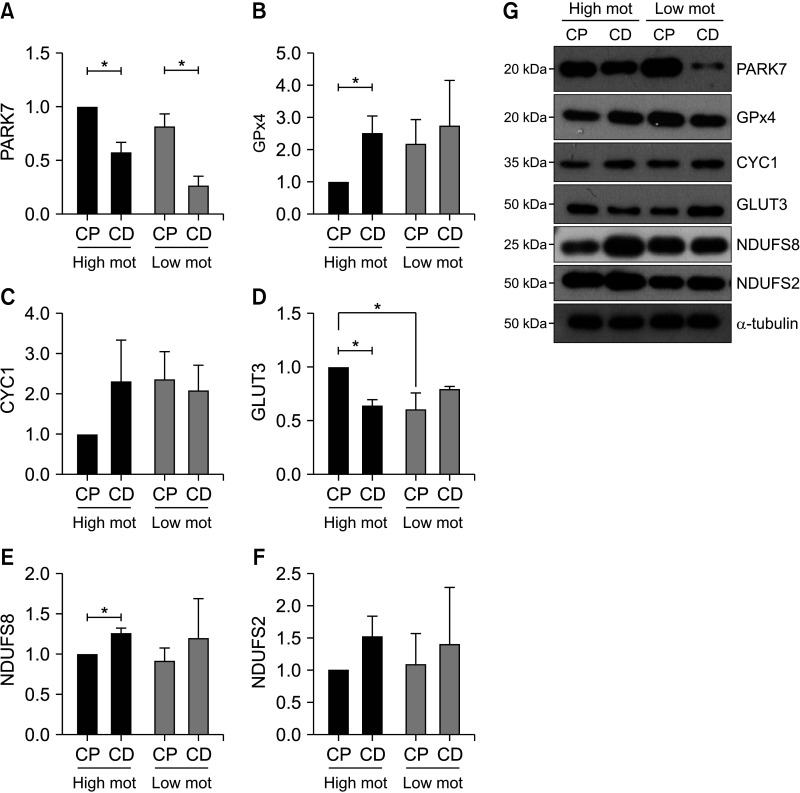
3. Different expression of mitochondrial ATP metabolism-related proteins in spermatozoa during epididymal maturation according to motility
Based on the fertility-related signaling pathways, we explored the sequential changes in proteins PARK7, CYC1, GLUT3, NDUFS8, NUDFS2, and GPX4, which are closely related to mitochondrial oxidative phosphorylation (OXPHOS) and ATP production in spermatozoa [14,18,19]. PARK7 protein expression was downregulated in spermatozoa during epididymal maturation, regardless of sperm motility (Fig. 2A). There was no significant difference in CYC1 and NDUFS2 protein expression in spermatozoa during epididymal maturation between high and low motility bulls (Fig. 2C, 2F). It is noteworthy that GPX4, GLUT3, and NDUFS8 protein expression was significantly changed only in high motility bull spermatozoa, whereas there were no differences in low motility bull spermatozoa during epididymal maturation. GPX4 and NDUFS8 protein expression in high motility bull spermatozoa was significantly increased following epididymal maturation (Fig. 2B, 2E). GLUT3 protein was more abundant in the caput spermatozoa from the high motility group; however, the expression in high motility bull spermatozoa was downregulated to a level comparable to that in the low motility bull spermatozoa during epididymal maturation (Fig. 2D).
Fig. 2. Changes in mitochondrial protein expression between high and low motility bull spermatozoa during epididymal maturation. Density of (A) PARK7, (B) GPX4, (C) CYC1, (D) GLUT3, (E) NUDFS8, and (F) NDUFS2 proteins in high and low motility bull spermatozoa following epididymal maturation. Statistics show the ratio of the normalized protein expression to caput spermatozoa in the high motility group. Data are presented as mean±standard error of the mean. *Superscripts indicate significant differences in the protein expression of spermatozoa from caput and cauda epididymides between the high and low motility groups, as determined by two-way ANOVA with a Šidák post hoc test (p<0.05). (E) Western blot image of PARK7, GPX4, CYC1, GLUT3, NDUFS8, and NDUFS2 proteins. Mot: motility, CP: spermatozoa from the caput epididymis, CD: spermatozoa from the cauda epididymis.
DISCUSSION
Following advances in the field of proteomics, comprehensive and comparative studies between immature and mature spermatozoa have been conducted to understand the complex mechanisms of sperm maturation in the epididymis [4,7,8]. These studies reported that the composition of the plasma membrane in spermatozoa is remodelled, depending on extracellular factors that are synthesized and secreted by epithelial cells of the epididymis in a region-dependent manner [20]. Following the addition of proteins to the spermatozoa during epididymal transit, spermatozoa acquire functional abilities, including capacitation, sperm-egg fusion, and motility [4]. Golgi structures, membrane organization, peroxisomes, and cytoskeletal related proteins are removed from spermatozoa during transit out of the caput [4].
Many studies have reported that protein modifications in spermatozoa during epididymal transit fundamentally confer both motility and fertilizing-ability to sperm. Especially, it is well documented that sperm-egg associated proteins including acrosin, fertilin, izumo sperm-egg fusion, and sperm adhesion molecule 1 undergo the proteolytic cleavage processes during the epididymal maturation [21]. Moreover, protein phosphorylation of spermatozoa during epididymal transit is one of the major protein modification which plays an important role in sperm motility acquisition through the regulation of cAMP-PKA and calcium signaling pathways in spermatozoa during the epididymal maturation [22]. Although these studies provide important information regarding the role of protein modification in sperm motility and fertility during epididymal maturation, the molecular and biochemical events which regulate sperm motility during maturation remain poorly understood. Previous study reported that ENO1, VDAC2, UQCRC1, and UQCRC2 proteins were differentially expressed between high and low fertility bull spermatozoa [6]. Because approximately 60% of idiopathic male infertility is closely associated with defects in sperm motility [15], we investigated how the sequential changes in fertility-related protein expression in spermatozoa during epididymal maturation might regulate sperm motility. We found that only one fertility-related protein, ENO1, was significantly different between high and low motility bull spermatozoa. Although ENO1 was more abundant in the caput spermatozoa from low motility bulls than in high motility bulls, it was downregulated in the low motility bull spermatozoa during epididymal maturation, resulting in a comparable expression level of ENO1 between high and low motility bull spermatozoa.
ENO1 is a glycolytic enzyme which catalyses the conversion of 2-phosphoglycerate to phosphoenolpyruvate during glycolysis [23]. Glycolysis begins with a glucose molecule in the inner compartment of the sperm tail which is transferred from seminal plasma via glucose transporters [23]. We found that the glucose transporter, GLUT3, is more highly expressed in the caput spermatozoa from high motility bulls than in low motility bull spermatozoa, whereas in the cauda spermatozoa, the protein expression was comparable in both high and low motility bull spermatozoa. Previous study reported that the metabolic transition from glycolysis to OXPHOS in male germ cells occurs during spermatogenesis to support the higher rates of ATP production for male germ cell development [14].
Because the spermatozoa require enormous energy to undergo capacitation and fertilization, earlier activation of spermatozoa in the epididymis, called premature capacitation, is prevented by specific luminal environmental conditions [2,3]. Therefore, we hypothesize that decreased glucose transporter in the high motility bull spermatozoa may induce a smaller number of glucose transporters, maintaining static glycolysis, and consequently, preventing premature activation during epididymal maturation. Alternatively, higher expression of ENO1 in the caput spermatozoa may activate glycolysis, induce early activation of immature spermatozoa, and increase energy consumption for fertilization during maturation, resulting in a decline in sperm motility. ENO1 also plays an important role in defence against oxidative stress in spermatozoa [23].
Interestingly, we found that the mitochondrial electron transport and ATP metabolism-related signaling pathways are closely related to the fertility-related proteins. The major role of mitochondria in spermatozoa is ATP production through OXPHOS to maintain their motility. Moreover, mitochondria are closely associated with the regulation of cell death to maintain male fertility through reactive oxygen species (ROS) generation [14]. Following metabolic processes (including glycolysis and fatty acid oxidation), the tricarboxylic acid cycle is activated and acetyl coenzyme A (acetyl-CoA) is generated. Carbon dioxide (CO2) and reducing agents such as NADH and flavin adenine dinucleotide (FADH2) are produced by the acetyl-CoA oxidation, which work as fuel in the respiratory chain for the induction of sequential electron transfer in the inner mitochondrial membrane [24]. The ROS, such as superoxide (O2−) and hydrogen peroxide (H2O2), are produced by the incomplete reduction of oxygen by an electron [25]. A moderate ROS level in spermatozoa is essential for post-translational modification during capacitation and fertilization, while excessive ROS increase lipid peroxidation and DNA damage, with a consequent decline in sperm motility and fertility [26]. It is noteworthy that there was a lack of modification of the mitochondrial proteins (including GPX4 and NDUFS8) in the low motility bull spermatozoa during epididymal maturation, whereas active changes were detected in high motility bull spermatozoa. GPX4 is an antioxidant enzyme that regulates non-apoptotic cell death through lipid peroxidation. Especially in spermatozoa, soluble GPX4 is switched to an enzymatically inactive, oxidatively cross-linked, and insoluble structural protein during sperm maturation and is closely related to the structural stability of sperm chromatin [19]. GPX4 is also a major structural constituent of mitochondria which functions in spermatozoa to maintain male fertility through the antioxidant activity against mitochondrial ROS [18,19]. NDUF subunits (NDUFS) (i.e., respiratory complex I) are closely related to OXPHOS through the transport of respiratory electrons [14,27]. Moreover, downregulation of NDUFS (including NDUFS8) is associated with excessive ROS production, potentially contributing to mitochondria-dependent apoptosis in spermatozoa [28]. Similar to our previous review paper which summarized and established a new fertility-related signaling pathways based on the proteomic data from fertile and infertile patients in varied studies, suggested that male infertility may be traceable to defects in energy metabolism-related and mitochondrial-related proteins which is closely related to sperm motility and fertility in spermatozoa [14]. Moreover, some comparative omic studies between spermatogonial stem cells and spermatocytes/spermatids suggested that there were metabolic transition from glycolysis to OXPHOS during the spermatogenesis and sperm maturation to accelerate sperm cell differentiation and maturation following the transition in the testis [29,30]. Taken together, our data suggest that the increased expression of these mitochondrial proteins in spermatozoa during the epididymal maturation confers that motility and fertilizing ability through the reduction of ROS damage and the stabilization of the mitochondrial sheath during maturation (Fig. 3).
Fig. 3. Scheme of the regulation of sperm motility via protein modifications during epididymal maturation. (A) Summarized cellular processes associated with both fertility- and mitochondrial-related proteins were established using the Pathway Studio program. Green arrows indicated activation of cellular processes while red arrows indicated inhibition of cellular processes. (B) Increased ENO1 protein in the caput spermatozoa may increase the consumption of ATP through activated glycolysis in the spermatozoa. Upregulation of GPX4 and NDUFS8 proteins in the spermatozoa may prevent reactive oxygen species (ROS) damage during epididymal maturation, whereas lack of GPX4 and NDUFS8 protein modifications in spermatozoa may increase ROS damage during epididymal maturation. Altogether, low motility may be determined by irregular protein modifications in spermatozoa during epididymal maturation, resulting in male infertility.
In this study, cryopreserved bovine spermatozoa were used due to the difficulty to obtain the non-pathological specimens from men with fertilizing ability. Therefore, we cannot exclude the possibility that low sperm motility may be derived from spermatozoa have more vulnerable to cryostress than those of spermatozoa with higher motility. Although our results indicated that low sperm motility may come from the lack of fertility- and mitochondrial-related protein modifications during the epididymal maturation, we suggested that further study with fresh semen samples is required for exactly and comprehensively understanding the mechanisms of male fertility.
CONCLUSIONS
Overall, low sperm motility may be determined by the aberrant modification of mitochondrial proteins during epididymal maturation. The irregular mitochondrial protein expression in spermatozoa during epididymal maturation may induce ROS damage and earlier activation and the consequent increase in energy consumption through glycolysis in spermatozoa, leading to a loss of sperm motility and consequently, a decrease in male fertility. Although this study may serve the preliminary mechanisms of bull fertility associated with sperm maturation, we suggest that the function tests of these proteins using agonist or antagonist chemicals, knock-out or knock-in models, will have to be required to fully understand the complexities of male fertility.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (NRF-2020R1C1C1003380). This research was supported by the Chung-Ang University Graduate Research Scholarship in 2021 (to BML).
- Conceptualization: YJP, MGP.
- Data curation: BML, YJP.
- Formal analysis: YJP.
- Funding acquisition: YJP.
- Investigation: YJP, WKP, BML, DYR.
- Methodology: YJP, MGP.
- Project administration: MGP.
- Resources: MGP, YJP.
- Software: YJP, MGP.
- Supervision: MGP.
- Validation: MGP, MSR.
- Visualization: YJP, BML.
- Writing – original draft: YJP, BML.
- Writing – review & editing: MSR, MGP.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/PABN5H.
References
- 1.Gervasi MG, Visconti PE. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology. 2017;5:204–218. doi: 10.1111/andr.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breton S, Ruan YC, Park YJ, Kim B. Regulation of epithelial function, differentiation, and remodeling in the epididymis. Asian J Androl. 2016;18:3–9. doi: 10.4103/1008-682X.165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park YJ, Battistone MA, Kim B, Breton S. Relative contribution of clear cells and principal cells to luminal pH in the mouse epididymis. Biol Reprod. 2017;96:366–375. doi: 10.1095/biolreprod.116.144857. Erratum in: Biol Reprod 2017;96:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skerget S, Rosenow MA, Petritis K, Karr TL. Sperm proteome maturation in the mouse epididymis. PLoS One. 2015;10:e0140650. doi: 10.1371/journal.pone.0140650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon WS, Oh SA, Kim YJ, Rahman MS, Park YJ, Pang MG. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci Rep. 2015;5:13821. doi: 10.1038/srep13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YJ, Kwon WS, Oh SA, Pang MG. Fertility-related proteomic profiling bull spermatozoa separated by percoll. J Proteome Res. 2012;11:4162–4168. doi: 10.1021/pr300248s. [DOI] [PubMed] [Google Scholar]
- 7.Suryawanshi AR, Khan SA, Gajbhiye RK, Gurav MY, Khole VV. Differential proteomics leads to identification of domain-specific epididymal sperm proteins. J Androl. 2011;32:240–259. doi: 10.2164/jandrol.110.010967. [DOI] [PubMed] [Google Scholar]
- 8.Ijiri TW, Merdiushev T, Cao W, Gerton GL. Identification and validation of mouse sperm proteins correlated with epididymal maturation. Proteomics. 2011;11:4047–4062. doi: 10.1002/pmic.201100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon WS, Rahman MS, Lee JS, You YA, Pang MG. Improving litter size by boar spermatozoa: application of combined H33258/CTC staining in field trial with artificial insemination. Andrology. 2015;3:552–557. doi: 10.1111/andr.12020. [DOI] [PubMed] [Google Scholar]
- 11.Park YJ, Mohamed el-SA, Oh SA, Yoon SJ, Kwon WS, Kim HR, et al. Sperm penetration assay as an indicator of bull fertility. J Reprod Dev. 2012;58:461–466. doi: 10.1262/jrd.11-067h. [DOI] [PubMed] [Google Scholar]
- 12.O' Meara CM, Hanrahan JP, Prathalingam NS, Owen JS, Donovan A, Fair S, et al. Relationship between in vitro sperm functional tests and in vivo fertility of rams following cervical artificial insemination of ewes with frozen-thawed semen. Theriogenology. 2008;69:513–522. doi: 10.1016/j.theriogenology.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Pang WK, Ryu DY, Song WH, Rahman MS, Park YJ, et al. Porcine seminal protein-I and II mRNA expression in boar spermatozoa is significantly correlated with fertility. Theriogenology. 2019;138:31–38. doi: 10.1016/j.theriogenology.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Park YJ, Pang MG. Mitochondrial functionality in male fertility: from spermatogenesis to fertilization. Antioxidants (Basel) 2021;10:98. doi: 10.3390/antiox10010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ijiri TW, Vadnais ML, Huang AP, Lin AM, Levin LR, Buck J, et al. Thiol changes during epididymal maturation: a link to flagellar angulation in mouse spermatozoa? Andrology. 2014;2:65–75. doi: 10.1111/j.2047-2927.2013.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SJ, Kwon WS, Rahman MS, Lee JS, Pang MG. A novel approach to identifying physical markers of cryo-damage in bull spermatozoa. PLoS One. 2015;10:e0126232. doi: 10.1371/journal.pone.0126232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc. 2019;14:482–517. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad M, Moreno SG, Sinowatz F, Ursini F, Kölle S, Roveri A, et al. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol Cell Biol. 2005;25:7637–7644. doi: 10.1128/MCB.25.17.7637-7644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foresta C, Flohé L, Garolla A, Roveri A, Ursini F, Maiorino M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol Reprod. 2002;67:967–971. doi: 10.1095/biolreprod.102.003822. [DOI] [PubMed] [Google Scholar]
- 20.Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatti JL, Druart X, Guérin Y, Dacheux F, Dacheux JL. A 105- to 94-kilodalton protein in the epididymal fluids of domestic mammals is angiotensin I-converting enzyme (ACE); evidence that sperm are the source of this ACE. Biol Reprod. 1999;60:937–945. doi: 10.1095/biolreprod60.4.937. [DOI] [PubMed] [Google Scholar]
- 22.Dacheux JL, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2013;147:R27–R42. doi: 10.1530/REP-13-0420. [DOI] [PubMed] [Google Scholar]
- 23.Gitlits VM, Toh BH, Loveland KL, Sentry JW. The glycolytic enzyme enolase is present in sperm tail and displays nucleotide-dependent association with microtubules. Eur J Cell Biol. 2000;79:104–111. doi: 10.1078/S0171-9335(04)70012-6. [DOI] [PubMed] [Google Scholar]
- 24.Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10:387–399. doi: 10.1093/humupd/dmh034. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013;18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Han Y, Zhou T, Zhang R, Chen H, Chen S, et al. Mechanisms of ROS-induced mitochondria-dependent apoptosis underlying liquid storage of goat spermatozoa. Aging (Albany NY) 2019;11:7880–7898. doi: 10.18632/aging.102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lord T, Nixon B. Metabolic changes accompanying spermatogonial stem cell differentiation. Dev Cell. 2020;52:399–411. doi: 10.1016/j.devcel.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, et al. The neonatal and adult human testis defined at the single-cell level. Cell Rep. 2019;26:1501–1517.e4. doi: 10.1016/j.celrep.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/PABN5H.