Abstract
Mitochondrial dynamics, such as fusion and fission, play a critical role in maintaining cellular metabolic homeostasis. The molecular mechanisms underlying these processes include fusion proteins (Mitofusin 1 [MFN1], Mitofusin 2 [MFN2], and optic atrophy 1 [OPA1]) and fission mediators (mitochondrial fission 1 [FIS1] and dynamin-related protein 1 [DRP1]), which interact with each other to ensure mitochondrial quality control. Interestingly, defects in these proteins can lead to the loss of mitochondrial DNA (mtDNA) integrity, impairment of mitochondrial function, a severe alteration of mitochondrial morphology, and eventually cell death. Emerging evidence has revealed a causal relationship between dysregulation of mitochondria dynamics and age-associated type 2 diabetes, a metabolic disease whose rates have reached an alarming epidemic-like level with the majority of cases (59%) recorded in men aged 65 and over. In this sense, fragmentation of mitochondrial networks is often associated with defects in cellular energy production and increased apoptosis, leading, in turn, to excessive reactive oxygen species release, mitochondrial dysfunction, and metabolic alterations, which can ultimately contribute to β-cell dysfunction and insulin resistance. The present review discusses the processes of mitochondrial fusion and fission and their dysfunction in type 2 diabetes, with special attention given to the therapeutic potential of targeting mitochondrial dynamics in this complex metabolic disorder.
Keywords: Aging, Men, Mitochondria, Type 2 diabetes
INTRODUCTION
Aging is defined as a decrease in the regenerative and reparative potential of organs and tissues. This reduction leads to a time-dependent failure of several molecular mechanisms and a decreased physiological reserve in response to stress known as homeostenosis [1]. Currently, there are around 703 million persons aged at least 65 years worldwide, of which 125 million are 80 or over, and this number is forecast to double by 2050. This unprecedented aging of the world’s population is a major contributor to the age-associated diabetes epidemic. Interestingly, men show slightly higher rates of diabetes than women, representing 59% of cases in those aged 65 and over. Type 2 diabetes (T2D) is the most common type of diabetes reported in these subjects, accounting for more than 9 in 10 cases (90%) [2,3]. In this sense, emerging evidence reveals that aging processes are closely related to metabolic disorders such as T2D and cardiovascular diseases [4]. An increase in visceral adiposity and decline in lean body mass that often accompanies aging may contribute to these chronic conditions [5]. Aging can also lead to an inadequate β-cell functional mass compensation or impaired β-cell function, thus inducing a decrease in insulin sensitivity and an impairment of metabolic profile [6]. Consistent with the reported diabetes prevalence, it has been revealed that androgen deficiency during aging predisposes men to increased insulin resistance, and, if the deficiency is severe, additional β-cell dysfunction and diabetes development can occur [7]. Moreover, decreased high density lipoprotein cholesterol and high triglyceride levels have been associated with reduced testosterone levels in male subjects [8,9]. Similarly, a relationship between a decline in testosterone levels and an increase in insulin resistance is documented [10].
The aforementioned age-dependent features are closely related to oxidative stress. In this regard, changes in cellular homeostasis and redox state and overproduction of reactive oxygen species (ROS) have been described in the pathogenesis of T2D and associated cardiovascular issues. Particularly, hyperglycaemia-induced mitochondrial superoxide overproduction seems to promote various mechanisms, such as increased concentration of cytokines and prostanoids, accumulation of nitric oxide (NO), and advanced glycation-end products (AGE), increased protein kinase C (PKC), and activation of the polyol-sorbitol pathway, diacylglycerol (DAG) and xanthine oxidoreductase (XOR), which lead to endoplasmic reticulum (ER) stress, mitochondrial dysfunction and β-cell apoptosis, thus highlighting even further the pivotal role of ROS and oxidant-derived tissue injury in the pathogenesis and evolution of T2D [11].
Of note, increased plasma XOR activity is more common among men than among women in the general T2D population [12], and leads to a more pronounced ROS production and endothelial dysfunction. In line with these data, our group has shown that the altered mitochondrial function and oxidative stress observed in 280 T2D male patients was related to low testosterone levels, which induced a higher total and mitochondrial ROS production, undermined antioxidant defences, and altered subclinical atherosclerotic markers measured in leukocytes [13].
It is well known that the main source of ROS is mitochondria, double membrane organelles involved in such fundamental cellular functions as redox balance, calcium regulation and signalling, as well as the triggering/regulation of apoptosis. Not surprisingly, cells have developed multiple quality control mechanisms to guarantee that mitochondria function properly [14], and fusion and fission cycles are the most relevant. These complex dynamical processes include repairing transformations of the mitochondrial architecture, such as morphological changes, intracellular mitochondrial distribution and density, and their movement along the cytoskeleton in order to preserve cell integrity, adapt to metabolic changes, and protect against cell death [15]. While mitochondrial fusion physically merges the membranes and components of two originally distinct mitochondria (one damaged and the other non-damaged), fission involves separating the mitochondrial membranes to reorganize the damaged components and meet their requirements. Mitochondrial fusion and division dynamics continually counterbalance each other [16]. However, alteration of this equilibrium can induce mitochondrial morphology. Specifically, excess fusion can lead to elongated mitochondrial tubules, while increased fission can result in fragmented mitochondria. These events can induce oxidative stress and halt energy production, as well as triggering abnormal signalling pathways that contribute to numerous metabolic abnormalities, including enhanced hepatic gluconeogenesis, activation of inflammatory proteins and further exacerbation of mitochondrial deterioration. Prolonged oxidative status and chronic inflammation also cause β-cell dysfunction and apoptosis, thus reducing insulin sensitivity and glucose tolerance of target tissues, which are recognized risk factors for age-associated diabetes and its related complications [17,18].
In light of this evidence, the present review explores how mitochondrial dynamic dysfunction orchestrates metabolic alteration under diabetic conditions and places particular attention on the therapeutic potential of targeting these processes.
SURVEY METHODS
A literature search was conducted to collect the current knowledge about the pathophysiological implication of mitochondrial dynamics dysfunction in age-associated T2D. Electronic databases such as Google Scholar, PubMed, Scopus, and Web of Science were used to collect information regarding the topic. The keywords used were: mitochondrial dynamics, T2D, fusion, fission, and oxidative stress.
STUDY SELECTION CRITERIA
All articles fulfilled the following specific eligibility criteria: (1) reviews and original articles concerning the close relationship between mitochondrial dynamics dysfunction in the onset of diabetes and its associated complications; (2) reports of in vivo, in vitro, or human studies; (3) reviews, articles, and original works written in English; and (4) papers published between 2000 and 2021.
PHYSIOLOGICAL MITOCHONDRIAL DYNAMICS: FUSION AND FISSION
As previously mentioned, cellular energy homeostasis is controlled by key double-membrane–bound subcellular organelles known as mitochondria, which function within an interconnected reticulum that branches, fragments and fuses. The highly dynamic behaviour of mitochondria determines their morphology and intracellular distribution, and allows the cell to adapt to physiological conditions and the energy demands of a given moment.
Mitochondria regularly undergo essential events known as fusion and fission, which are critical in maintaining mitochondrial homeostasis in response to environmental or metabolic stresses, and are implicated in autophagy, apoptosis and cell division [19,20].
Fusion mitigates stress by mixing the contents of partially damaged mitochondria by way of complementation. For example, in cells with a highly active metabolism, like muscle cells, mitochondria fuse and form long networks that connect the peripheral areas rich in O2 with the interior of the muscle fibre, which is poor in O2, thereby transmitting the membrane potential along the mitochondrial filaments, contributing to the dissipation of energy and allowing the production of adenosine triphosphate (ATP) in different cell locations [21]. Concurrently, fission helps to create new mitochondria, facilitating cell death during high levels of oxidative stress and exerting quality control by enabling the removal of damaged mitochondria [20,22].
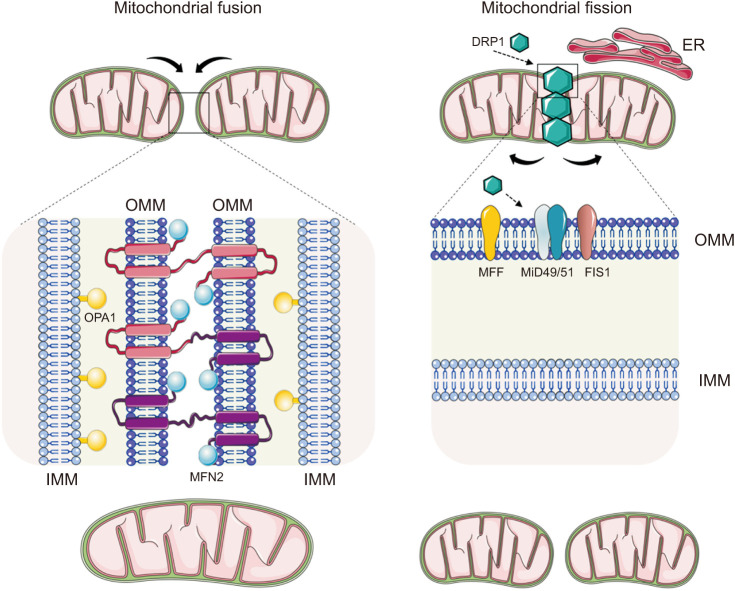
The proteins involved in fusion/fission processes are guanosine triphosphatases (GTPases), a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it into guanosine diphosphate (GDP). They also divide and fuse the two lipid bilayers that surround mitochondria (Fig. 1). Mitochondrial fusion is primarily controlled by three GTPases: Mitofusin (MFN1) 1 and 2 [23], and optic atrophy 1 (OPA1) [24]. While MNF1 and 2 orchestrates the fusion of the outer mitochondrial membrane (OMM), the fusion of the inner mitochondrial membrane (IMM) is mediated by OPA1 protein [24]. These molecular actors are capable of interacting intermitochondrially. Specifically, the C-terminal coiled-coil region of Mfn1 and Mfn2 mediates the mutual tethering of mitochondria through homo- or heterotypic complexes formed between adjacent mitochondria, while OPA1 helps to maintain the mitochondrial cristae morphology [24]. Of note, these events induce conformational changes that drive GTP hydrolysis by MFN1 molecules, leading to the fusion of the two OMMs. Similarly, IMM fusion is a result of right-turned helical assemblies of OPA1, which seems to be activated somehow by MFN1 [25].
Fig. 1. Physiological mitochondrial dynamics: fusion and fission. When mitochondria fuse, their matrix materials intermix, creating elongated organelles. Mitofusin (MFN) 1 and 2 orchestrate mitochondrial fusion of the outer mitochondrial membrane (OMM), while fusion of the inner mitochondrial membrane (IMM) is mediated by optic atrophy 1 (OPA1) protein. Specifically, the C-terminal coiled-coil region of Mfn1 and Mfn2 mediates tethering between mitochondria through homo- or heterotypic complexes formed between adjacent mitochondria and OPA1 helps to maintain mitochondrial cristae morphology. A cytosolic dynamin-related protein 1 (DRP1) and fission protein 1 (FIS1) mediate mitochondrial fission. The process begins when DRP1 molecules are activated and move from the cytosol to the OMM, where they assemble and form a ring-shaped structure that constricts the mitochondrial tubule in order to mediate fission. Integral mitochondrial dynamics protein 51 kD (MiD51) and mitochondrial dynamics protein 49 kD (MiD49), along with MFF and FIS1, act as receptors that recruit DRP1 to the mitochondrial surface. ER: endoplasmic reticulum.
On the other hand, a cytosolic GTPase dynamin-related protein 1 (DRP1) and fission protein 1 (FIS1) mediate fission process [26]. The process begins when a number units of the DRP1 (approximately 100) [27] are recruited to the sites of fractionation in the OMM, where they assemble and form a ring-shaped structure that constricts mitochondrial membranes in a GTP-dependent manner, thus leading to separation of the mitochondria. Four receptors have been identified as recruiters of DRP1 to the mitochondrial surface: mitochondrial dynamics proteins of 51 and 49 kDa (MiD51 and MiD49), mitochondrial fission factor (MFF), and FIS1 [28,29]. Among these, MFF seems to be the primary receptor, and its overexpression results in increased fission. In contrast, FIS1 plays a minor role in DRP1 recruitment, while MiD51 and MiD49 recruit inactive forms of DRP1 [30]. Of note, DRP1 activity at the OMM is also mediated by interactions with alternative mitochondrial effectors and accessory proteins such as GDAP1 (ganglioside induced differentiation-associated protein 1) and MTP18, a mitochondrial protein of 18 kDa, which induce fragmentation of the mitochondrial network if blocked or overexpressed [31,32]. In addition, mitochondrial fission may trigger the release of cytochrome C, which eventually induces apoptotic cell death [33].
Fusion and fission processes serve as quality control mechanisms in mitochondrial function and homeostasis. Of note, the fusion process, in addition to transmission of potential membrane from areas with high bioavailability of O2 to those poor in O2 with the aim of modulating the production of ATP, also favours the exchange of material (mitochondrial DNA [mtDNA], metabolites, substrates, lipids, etc.) between mitochondria, allowing intact mitochondria to complement damaged ones. Concurrently, the fission process facilitates the distribution and inheritance of mitochondria during cell division, favours the segregation and elimination of mitochondrial components damaged by mitophagy, and contributes to the apoptosis process by regulating the release into the cytosol of components of the intermembrane space. In this way, mitochondrial dynamics is a complex process that depends on the needs of each moment and requires a balance between fission and fusion. In fact, the fragmentation of mitochondrial networks, either due to lack of fusion or excess fission, is often associated with defects in cellular energy production or increased apoptosis, so any state that alters the fusion/fission equilibrium can culminate in excessive ROS release, mitochondrial dysfunction, and altered metabolism, which may ultimately contribute to the pathogenesis of T2D and obesity. In this sense, several studies have revealed that MFN1 or DRP1 deficiency in the liver protects mice from insulin resistance and high fat diet-induced obesity [34,35]. Moreover, Mfn1 genetic ablation leads to an enhanced mitochondrial respiration capacity and a highly fragmented mitochondrial network in the liver and myocardium [34,36] in diabetic humans and animals. Interestingly, the liver of Mfn1 knockout mice exhibits a more active complex I and a preference for using lipids as the main energy source in order to protect against insulin resistance [34]. In cultured cells, inhibition of mitochondrial fusion has been shown to reduce mtDNA, mtDNA-encoded proteins and membrane integrity, consequently altering oxidative phosphorylation, since fusion-deficient mitochondria cannot exchange their contents [37].
These results confirm that mitochondrial dynamics are key players in insulin signalling and glucose metabolism regulation, and that interruption of fusion/fission cycles is critical to the pathogenesis of obesity, T2D and metabolic abnormalities.
MITOCHONDRIAL DYNAMICS AND MITOPHAGY
The maintenance of a healthy mitochondrial population is critical for cell energy homeostasis, survival and proper functions. Damaged mitochondria are removed through a regulated process called mitophagy, a form of selective macroautophagy [38]. Macroautophagy is a genetically programmed mechanism of degradation where a double membrane vesicle called the autophagosome surrounds the damaged cellular components and fuses with a lysosome in order to facilitate content degradation. Autophagy of damaged mitochondria is known as mitophagy [39]. Depending on cell physiological conditions, mitophagy can be classified as basal mitophagy, programmed mitophagy, or stress-induced mitophagy. Normally, basal mitophagy is necessary for metabolic requirements, cellular homeostasis and mitochondrial turnover; programmed mitophagy is required for differentiation and development processes, such as allophagy and maturation of cardiomyocytes and erythrocytes; and stress-induced mitophagy is directly mediated by stimuli such as hypoxia, loss of mitochondrial membrane potential (MMP), starvation and oxidative stress, and in order to reduce high amounts of mitochondria and the O2 consumption and ROS generated by damaged mitochondria. One of the most studied mechanisms of mitophagy is that mediated by the ubiquitin ligase PARKIN and phosphatase and tensin homologue (PTEN)-induced putative kinase 1 (PINK1) [40,41].
In healthy mitochondrial conditions, PINK1 is constitutively transported by TIM/TOM complex to the inner membrane, where it is immediately clipped by matrix processing peptidases and a series of proteases, including the mitochondrial-processing protease (MPP) and the inner membrane presenilin-associated rhomboid-like protease, which rapidly degrades it within the cytosol. In aged or damaged mitochondria, loss of MMP impedes importation of PINK1 into the inner membrane, thereby promoting intact PINK1 to stabilize on the mitochondrial outer membrane where it interacts with the TOM complex [42]. Accumulating evidence suggests that PINK1 acts as a molecular signal of damaged mitochondria and that its accumulation leads to the translocation of PARKIN from the cytosol to the organelle [42,43]. As a result, activated PARKIN polyubiquitinates specific proteins on the OMM, thus forming ubiquitin chains that act as “eat me signals” and are further phosphorylated by PINK1. These events lead to the recruitment of five autophagosomal microtubule-associated protein 1A/1B-light chain 3 (LC3) interacting region (LIR)-containing autophagy adapters: TAX1 binding protein 1 (TAX1BP1), Optineurin (OPTN), sequestosome-1 (p62/SQSTM1), neighbour of BRCA1 gene 1 (NBR1), and nuclear domain 10 protein 52 (NDP52), which, in turn, deliver the damaged mitochondria to lysosomes for degradation [41].
Several studies have highlighted the fact that alteration of fission and fusion protein expression can modify mitophagy. Overexpression of a dominant negative isoform of DRP1 (DRP1K38A) or knockdown of FIS1 (by siRNA) in INS1 cells was shown to induce reduction of ER mass inside the autophagosome, undermining, in turn, mitochondrial autophagy [44]. Similarly, studies performed in the same cultured cells provided evidence that overexpression of DRP1 and the subsequent stimulation of mitochondrial fission facilitate mitochondrial fragmentation and elimination under various proapoptotic stimuli, including high fat and high glucose treatments [45]. On the other hand, knockdown of Drp1 prevented ROS formation under increased glucose levels and markedly decreased hyperglycaemia-induced apoptosis in HL-1 [46] and H2c9 [47] cardiac cells. Several interesting studies performed in INS1 and HeLa cells also indicated that elevated expression of Fis1 causes mitochondrial fragmentation by reducing the organelle’s mass [48,49]. As a result, impaired glucose-stimulated insulin secretion and cellular ATP levels were observed. Furthermore, inhibition of fission and overexpression of Opa1 have been related with a reduction of mitophagy rate by ~65% to 75% in INS1 cells [44]. In line with these findings, posttranslational modifications of fusion and fission proteins have been shown to directly modulate the activity, localization and stability of molecules. These posttranslational modifications include conjugation of small ubiquitin-like modifier proteins, proteolysis, acetylation, phosphorylation, O-linked-N-acetyl-glucosamine glycosylation and ubiquitination [26,50,51,52,53]. Interestingly, studies performed in transgenic mice revealed that ablation of MFN2 prevented depolarization-induced translocation of PARKIN to the mitochondria and impaired mitophagy [50], thus promoting the accumulation of damaged mitochondria and cardiomyopathy [54]. Future research needs to clarify the underlying mechanisms of mitochondrial dynamics and how their dysfunction can provoke alteration of cellular metabolic homeostasis.
MITOCHONDRIAL DYNAMICS DYSFUNCTION IN TYPE 2 DIABETES
As mentioned previously, fusion and fission processes are essential in ensuring the functional efficiency of mitochondria and maintaining quality control. Disruption of mitochondrial dynamics can undermine function and leads to aging and several human diseases, including T2D and associated cardiovascular complications.
Growing evidence suggests that hyperglycaemia induces mitochondrial fragmentation in T2D, along with increased mitochondrial fission and reduced fusion [55]. A recent study by our group, performed in leukocytes from T2D patients [56], confirmed these findings. Indeed, we demonstrated that leukocyte-endothelial interaction in diabetic patients was associated with elevated fission-protein levels and lower fusion-protein levels, thus suggesting that mitochondrial dynamics are influenced by glycaemic control in T2D subjects [56].
In vivo studies performed in the skeletal muscle of leptin-deficient (ob/ob) mice, diet-induced obese C57BL/6 mice, and obese Zucker rats [57,58] have revealed an imbalance in mitochondrial fusion and fission events. Specifically, mitochondrial fission inhibition was found to regulate the insulin pathway in obese mice, while Drp1-dependent mitochondrial fission in both obese C57BL/6 and ob/ob mice was related to IR [57,58].
The reduction in mitochondria size often seen in skeletal muscle cells of T2D humans and animals has been linked to a lower activity of mitochondrial complex I and, in turn, an alteration in bioenergetic capacity [59]. Interestingly, two studies related changes in the mitochondrial morphology of diabetic muscle cells to a decrease in the expression of MFN2 [60,61]. In addition to helping to maintain the mitochondrial network, this fusion protein is involved in the regulation of metabolism, since it stimulates substrate oxidation and mitochondrial respiration [62].
On the other hand, MFN2 overexpression in Wistar diabetic rats has been shown to reduce lipid intermediates and neutralize the inhibition of the insulin pathway in the liver and muscle [63,64]. In this sense, expression levels of glucose transporter type 2 (GLUT2), phosphoinositide-3-kinase (PI3K), insulin receptor substrate 2 (IRS2) and insulin receptor (INSR) were found to increase after the recovery of MFN2 expression [64]. Similarly, MFN2-liver expression was related to increased expression of the INSR and activation of the PI3K/AKT2 pathway [64].
In light of the above-mentioned evidence, MFN2 has been proposed as a main player in diabetic mitochondrial dysfunction and in the progression of insulin resistance [58,65].
Moreover, mitochondrial dynamics play a central role in the regulation of β-cell insulin secretion function. In this regard, Schultz et al [66] confirmed that the fission protein FIS1 acts as a regulator in these cells and showed that FIS1 expression levels are related to insulin secretion stimulated by glucose. In addition, a possible role for mitochondrial fission in β-cell apoptosis has been pointed to. Molina et al [67] demonstrated that exposure to high levels of glucose and fatty acids promoted the fragmentation of mitochondria in β-pancreatic cells. When mitochondrial fission was inhibited, morphology and mitochondrial dynamics were conserved, and apoptosis of β-cells prevented.
Notably, a causal relationship has been highlighted between alteration of mitochondrial dynamics and ROS production under hyperglycaemic conditions [47]. Yu et al [47] observed that exposure to high glucose levels promoted rapid fragmentation of mitochondria, a critical step in the increase in ROS production induced by hyperglycaemia. However, imbalances in glucose homeostasis are not always a cause of the alteration of mitochondrial dynamics, but instead can be a consequence. In line with these findings, Sebastián et al [68] demonstrated impaired insulin signalling, glucose intolerance and an increase in hepatic gluconeogenesis in liver-specific Mfn2 KO mice. These results were corroborated when Mfn2 gene silencing resulted in increased expression of cAMP-response element-binding (CREB), a ubiquitous transcription factor that facilitates gluconeogenic molecule expression through the peroxisome proliferator-activated receptor gamma coactivator (PGC)-1 [69].
Accumulating evidence also indicates the involvement of OPA1 in diabetes [70]. Studies performed in mouse pancreatic β-cells revealed that Opa1 ablation, obtained through the Cre-loxP system, develops into hyperglycaemia by impairing insulin secretion and glucose-stimulated ATP production [71], which was associated with defects in the amount and activity of electron transport chain complex IV. The close relationship between Opa1 and mitochondrial insulin-stimulated energy metabolism was also highlighted by Parra et al [72], who demonstrated that Opa1 silencing blocks insulin-stimulated ATP synthesis, while insulin enhances OXPHOS and promotes mitochondrial fusion by increasing Opa1 and modulating the mTOR-NFκB signaling pathway in L6 skeletal muscle cells and cardiomyocyte cultures.
Although further investigation is necessary, the substantial existing evidence confirms mitochondrial dynamics dysfunction as the main triggering factor for T2D. In this context, knockout/knockdown of fusion and fission mediators as a therapeutic approach may have a strong impact on metabolic profiling.
TARGETING MITOCHONDRIAL DYNAMICS DYSFUNCTION IN TYPE 2 DIABETES
The direct targeting of mitochondrial fusion, fission and mitophagy has gained increasing attention among researchers interested in diabetes healthcare management. For example, growing evidence suggests that dynasore, a non-competitive inhibitor of dynamin GTPase activity, can prevent mitochondrial fission and oxidative stress and decrease myocardial infarct size, since DRP1, which is responsible for mitochondrial fission, is a dynamin-like protein. In this sense, Gao et al [73] showed that it limits cell damage and protects cardiac lusitropy in Langendorff-perfused mouse heart, a widely used model of myocardial function and responses to injury. Specifically, dynasore positively influences the energetics of diastolic dysfunction by maintaining intracellular ATP and mitochondrial morphology in stressed cells [73].
GTPase activity of DRP1 can also be selectively blocked by the mitochondrial division inhibitor-1 (mdivi-1), a cell-permeable quinazolinone compound known to attenuate mitochondrial division in mammalian cells and yeast [74]. In this regard, HeLa cells and cell-free murine liver mitochondrial preparations treated with mdivi-1 exhibit a decreased rate of phosphatidylserine exposure on their surface and decreased cytochrome C release following apoptosis induction [74], which is consistent with previous research employing other strategies to compromise DRP1 activity.
Interestingly, Jheng et al [57] demonstrated that mdivi-1 also attenuates palmitic acid-induced mitochondrial depolarization and fragmentation, reduction of insulin-stimulated glucose uptake, and ROS generation in mouse C2C12 myoblasts, in which positive effects were obtained in a time- and dose-dependent manner. In line with these results, other authors have confirmed that mdivi-1 rescues adult rat hippocampal neural stem cells from palmitate-induced lipotoxicity by stabilizing mitochondrial transmembrane potential and inhibiting mitochondrial intracellular ROS production [75]. Mdivi-1-treated cells also exhibited an inhibition of caspase-3 activation, absence of cytochrome c release, a reduction in pro-apoptotic protein Bax expression, and an increase in anti-apoptotic Bcl-2 expression.
Meanwhile, studies performed in streptozotocin (STZ)-induced diabetic ApoE-/- mice have shown that mdivi-1 (10 mg/kg, intraperitoneal, twice per week, 8 weeks) can improve endothelial function, an effect that was associated with decreased ROS production and mitochondrial fragmentation in aortic endothelial cells [57,76]. Results of the same experiment also demonstrated that mdivi-1 treatment attenuates diabetes-enhanced expression of vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1, two key players in all stages of atherosclerosis [57,76].
Although numerous studies have reported the short-term beneficial effects of mdivi-1 and, thus, its therapeutic potential to combat mitochondrial dynamics dysfunction in diabetes, there is other evidence that long-term treatment with mdivi-1 can inhibit mitochondrial function. Specifically, mdivi-1-treated myotubes display decreased mitochondrial mass, mtDNA content and membrane potential in a dose-dependent manner, showing altered mitochondrial biogenesis during myogenic differentiation [77].
Likewise, studies in vascular smooth muscle cells (VSMCs) exposed to platelet-derived growth factor-BB (PDGF)-induced mitochondrial fragmentation were performed to further investigate the beneficial effects of mdivi-1 [78]. In this case, the compound was able to prevent PDGF-induced cell proliferation through the downregulation of cyclin D1 and proliferating cell nuclear antigen (PCNA), two proteins that are crucial in cell cycle processes [79]. Cells treated for 24 hours with mdivi-1 revealed a largely preserved filamentous mitochondrial morphology when compared with control cells. In this way, the abilities of this compound has made it the focus of attention as a potential therapy for different pathologies [80,81].
Over the last few years, another novel compound has been shown to inhibit DRP1 GTPase activity. P110, a 7-amino acid peptide presenting a homology sequence between Fis1 and Drp1 [82], is capable of preserving mitochondrial integrity, improve cell viability and reduce programmed cell death. Particularly, studies performed in three different rat models of ischaemia-reperfusion injury (in vivo myocardial infarction model, ex vivo heart model and primary cardiomyocytes) have demonstrated that P110 treatment selectively inhibits FIS1/DRP1 interaction, improves bioenergetics and reduces mitochondrial fission [83,84].
It is widely accepted that neurodegenerative diseases, such as Huntington’s and Parkinson’s disease, are closely associated with excessive mitochondrial fission. Therefore, selective inhibitors of aberrant mitochondrial fission could constitute an interesting therapeutic approach to the management of these pathologies. In this regard, data reported by Qi et al [82] showed that P110 has a neuroprotective effect in a model of Parkinson’s disease in culture. It was able to reduce neurite loss of primary dopaminergic neurons, apoptosis and autophagic cell death, which led to an increase in neuronal cell viability. Similarly, P110 appears to inhibit mitochondrial fragmentation, improve MMP, and, subsequently, reduce ROS production [82]. Recent research in transgenic mice has also revealed a potential use of P110 in the prevention and treatment of Huntington’s disease, since it has been found to reduce neuropathology, motor deficits and mitochondrial dysfunction [85]. Immunochemistry and Western blot data analysis of the spinal cords of these animals harvested at 16 and 29 days demonstrated that inhibition of DRP1 reduces mitochondrial fission and raises the ratio of healthy oligodendrocytes [86]. Similarly, Joshi et al [87] observed altered mitochondrial dynamics in mice with amyotrophic lateral sclerosis (ALS) expressing the G93A SOD1 mutation, resulting in an accumulation of fragmented mitochondria and a marked decrease in mitochondrial length. Administration of P110 to these mice inhibited DRP1 translocation to mitochondria and prevented the interaction between DRP1 and FIS1, thus reducing mitochondrial fragmentation and slowing the progression of ALS pathology [87].
Another compound with positive effects on mitochondrial dynamics is 15-Oxospiramilactone (S3), a small natural molecule derived from spiramine A of Spiraea japonica [88]. As reported in an intriguing paper by Yue et al [88], S3 can induce oxidative respiration and mitochondrial re-networking in cells deficient in either Mfn1 or Mfn2 by promoting mitochondrial fusion [88]. Specifically, studies performed in HeLa cells overexpressing c-Myc-tagged Ubiquitination Proteasome System (USP) demonstrated that S3 targets a mitochondria-localized deubiquitinase USP30, which mediates deubiquitination of MFN1 and MFN2 and regulates mitochondrial morphology. Consequently, this effect can induce mitochondrial fusion by enhancing the non-degradative deubiquitination of MFN1/2 and increasing MFN1/2 activity [88].
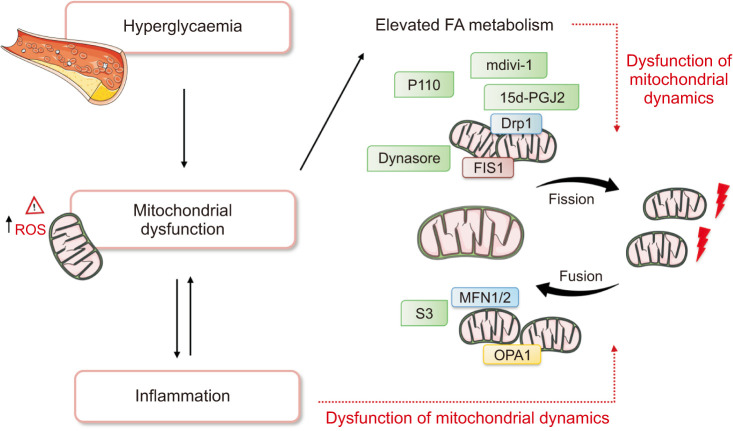
Last but not least, 15 deoxy prostaglandin J2 (15d-PGJ2) has attracted considerable attention in the field of mitochondrial plasticity modulators. A cyclopentenone prostaglandin produced in vivo during the resolution phase of inflammation, it has been shown to convert normal mitochondria into large interconnected and elongated mitochondria through covalent modification of Drp1 [89]. Treatment of HeLa cells and rat kidney proximal tubule cells (RPTC) with 15d-PGJ2 has been shown to induce mitochondrial elongation. Indeed, the number of fused and interconnected or moderately long mitochondria rose from <20% in untreated HeLa cells to ~60% and ~90% in cells treated with 1 µM and 2 µM of 15d-PGJ2, respectively [89]. These changes to mitochondrial morphology were specific to 15d-PGJ2, as they were not observed with the other prostaglandins tested. Of note, prolonged incubation of RPTC with 15d-PGJ2 resulted in increased degradation of the fusion protein OPA1, as well as ubiquitination and aggregation of newly synthesized OPA1. Concurrently, a reduction in Mfn1 and Mfn2 expression occurred, thus contributing to the formation of large swollen mitochondria with irregular cristae structure and reduced tubular rigidity [90]. The above mentioned compounds and their underlying mechanisms are shown in Fig. 2.
Fig. 2. Mitochondrial dynamics, fission and fusion is critical for maintaining several cellular mechanisms such as cell apoptosis, reactive oxygen species (ROS) generation and energy production. Hyperglycaemia has been shown to induce mitochondrial fragmentation in type 2 diabetes (T2D), along with increased mitochondrial fission and reduced fusion. This figure shows some potential compounds that target mitochondrial dynamics, and the underlying mechanisms by which they may be an effective strategy to prevent the development and progression of T2D. FA: fatty acids, mdivi-1: mitochondrial division inhibitor-1, DRP1: dynamin-related protein 1, FIS1: fission protein 1, MFN: Mitofusin, OPA1: optic atrophy 1.
Despite novel and exciting advances made in identifying and designing potent compounds that target mitochondrial dynamics, future investigation is vital to better understand the basis of such alterations and to target them more efficiently.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The pace of population ageing is increasing dramatically worldwide, as is the incidence of age-associated diseases. Aging is a complex and multifactorial process that reduces the regenerative and reparative potential of organs and tissues. This reduction has been related to insulin resistance and alteration or insufficient compensation of β-cell mass and function. Consequently, T2D has emerged as one of the leading global health problems related to aging, mainly in the male population, among which lipid abnormalities, oxidative stress and endothelial dysfunction are more pronounced. Accumulating evidence connects mitochondrial fission and fusion dynamics with diabetes and its related complications. In this sense, fragmentation of mitochondrial networks is often related to defects in cellular energy production and increased apoptosis, leading, in turn, to excessive ROS release, mitochondrial dysfunction, and metabolism alterations, which ultimately contribute to β-cell dysfunction and insulin resistance. This review has provided an overview of the functional and mechanistic aspects of mitochondrial dynamic dysfunction in T2D. Moreover, we have discussed how these processes have been identified as promising targets for therapy. Undoubtedly, other proteins implicated in mitochondrial dynamics need to be identified, and further in-depth experiments are crucial to clarify certain unresolved questions: “What are the cytotoxicity and pharmacokinetic profiles of DRP1 inhibitors?”; “How can we improve the poor solubility of mdivi-1 in water?”; “Can prolonged in vivo inhibition of mitochondrial fission critically affect other cellular processes?”
Acknowledgements
Servier Medical Art (https://smart.servier.com) was used to create the high-quality images.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This research was supported by an unrestricted grant from Menarini S.A and the European Regional Development Fund (ERDF “A way to build Europe”); UGP15-220 by FISABIO; PROMETEO/2019/027 by the Ministry of Education of the Valencian Regional Government; grants CIBERehd CB06/04/0071, PI19/0437 and PI19/00838 from the Carlos III Health Institute. PDP and FC are recipients of contracts from the Ministry of Education of the Valencian Regional Government (ACIF/2020/370 and GRISOLIAP/2019/091, respectively). AMM, ZAJ, CB and TV are recipients of PFIS, Sara Borrell and Miguel Servet contracts from the Carlos III Health Institute (FI17/00126, FI17/00144, CP19/00077 and CD19/00180, respectively). SLD is the recipient of an APOSTD/2020/145 fellowship from the Ministry of Innovation, University, Science and Digital Society of the Valencian Regional Government. MR is recipient of contract CPII16/00037 from the Carlos III Health Institute and the Ministry of Health of the Valencian Regional Government.
- Conceptualization: TV, PDP, FC, AMM, ZAJ, CGG, IR, ES, CB, SLD, VMV.
- Data curation: TV, PDP, FC, AMM, ZAJ, CGG, IR, ES, CB, SLD.
- Funding acquisition: MR, VMV.
- Investigation: TV, PDP, FC, AMM, ZAJ.
- Methodology: TV, PDP, FC, AMM.
- Visualization: CB, MR, VMV.
- Supervision: CB, MR, VMV.
- Project administration: MR, VMV.
- Writing—original draft preparation: TV, PDP, FC.
- Writing—review and editing: TV, CB, MR, VMV.
- All authors have read and agreed to the published version of the manuscript.
References
- 1.Khan SS, Singer BD, Vaughan DE. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16:624–633. doi: 10.1111/acel.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care. 2017;40:440–443. doi: 10.2337/dci17-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr Physiol. 2018;9:1–58. doi: 10.1002/cphy.c170040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguayo-Mazzucato C. Functional changes in beta cells during ageing and senescence. Diabetologia. 2020;63:2022–2029. doi: 10.1007/s00125-020-05185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W, Morford J, Mauvais-Jarvis F. Emerging role of testosterone in pancreatic β-cell function and insulin secretion. J Endocrinol. 2019 doi: 10.1530/JOE-18-0573. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 9.Van Pottelbergh I, Braeckman L, De Bacquer D, De Backer G, Kaufman JM. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166:95–102. doi: 10.1016/s0021-9150(02)00308-8. [DOI] [PubMed] [Google Scholar]
- 10.Yeap BB, Chubb SA, Hyde Z, Jamrozik K, Hankey GJ, Flicker L, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the health in men study. Eur J Endocrinol. 2009;161:591–598. doi: 10.1530/EJE-09-0348. [DOI] [PubMed] [Google Scholar]
- 11.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017;13:3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- 12.Okuyama T, Shirakawa J, Nakamura T, Murase T, Miyashita D, Inoue R, et al. Association of the plasma xanthine oxidoreductase activity with the metabolic parameters and vascular complications in patients with type 2 diabetes. Sci Rep. 2021;11:3768. doi: 10.1038/s41598-021-83234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovira-Llopis S, Bañuls C, de Marañon AM, Diaz-Morales N, Jover A, Garzon S, et al. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic Biol Med. 2017;108:155–162. doi: 10.1016/j.freeradbiomed.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Jadiya P, Tomar D. Mitochondrial protein quality control mechanisms. Genes (Basel) 2020;11:563. doi: 10.3390/genes11050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott I, Youle RJ. Mitochondrial fission and fusion. Essays Biochem. 2010;47:85–98. doi: 10.1042/bse0470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer JN, Leuthner TC, Luz AL. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology. 2017;391:42–53. doi: 10.1016/j.tox.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai W, Jiang L. Dysregulated mitochondrial dynamics and metabolism in obesity, diabetes, and cancer. Front Endocrinol (Lausanne) 2019;10:570. doi: 10.3389/fendo.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman SB, Pineda FJ, Hardwick JM. Mitochondrial fission and fusion dynamics: the long and short of it. Cell Death Differ. 2008;15:1147–1152. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu SB, Pekkurnaz G. Mechanisms orchestrating mitochondrial dynamics for energy homeostasis. J Mol Biol. 2018;430:3922–3941. doi: 10.1016/j.jmb.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisner V, Lenaers G, Hajnóczky G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J Cell Biol. 2014;205:179–195. doi: 10.1083/jcb.201312066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandt T, Cavellini L, Kühlbrandt W, Cohen MM. A mitofusin-dependent docking ring complex triggers mitochondrial fusion in vitro. Elife. 2016;5:e14618. doi: 10.7554/eLife.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elgass K, Pakay J, Ryan MT, Palmer CS. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta. 2013;1833:150–161. doi: 10.1016/j.bbamcr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Michalska BM, Kwapiszewska K, Szczepanowska J, Kalwarczyk T, Patalas-Krawczyk P, Szczepański K, et al. Insight into the fission mechanism by quantitative characterization of Drp1 protein distribution in the living cell. Sci Rep. 2018;8:8122. doi: 10.1038/s41598-018-26578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12:565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tondera D, Czauderna F, Paulick K, Schwarzer R, Kaufmann J, Santel A. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J Cell Sci. 2005;118(Pt 14):3049–3059. doi: 10.1242/jcs.02415. [DOI] [PubMed] [Google Scholar]
- 32.Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clerc P, Ge SX, Hwang H, Waddell J, Roelofs BA, Karbowski M, et al. Drp1 is dispensable for apoptotic cytochrome c release in primed MCF10A and fibroblast cells but affects Bcl-2 antagonist-induced respiratory changes. Br J Pharmacol. 2014;171:1988–1999. doi: 10.1111/bph.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni SS, Joffraud M, Boutant M, Ratajczak J, Gao AW, Maclachlan C, et al. Mfn1 deficiency in the liver protects against diet-induced insulin resistance and enhances the hypoglycemic effect of metformin. Diabetes. 2016;65:3552–3560. doi: 10.2337/db15-1725. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Ishihara T, Ibayashi Y, Tatsushima K, Setoyama D, Hanada Y, et al. Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia. 2015;58:2371–2380. doi: 10.1007/s00125-015-3704-7. [DOI] [PubMed] [Google Scholar]
- 36.Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554–564. doi: 10.1161/CIRCULATIONAHA.113.008476. [DOI] [PubMed] [Google Scholar]
- 37.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 38.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bingol B, Sheng M. Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic Biol Med. 2016;100:210–222. doi: 10.1016/j.freeradbiomed.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Bader V, Winklhofer KF. PINK1 and Parkin: team players in stress-induced mitophagy. Biol Chem. 2020;401:891–899. doi: 10.1515/hsz-2020-0135. [DOI] [PubMed] [Google Scholar]
- 42.Sekine S, Youle RJ. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol. 2018;16:2. doi: 10.1186/s12915-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedorowicz MA, de Vries-Schneider RL, Rüb C, Becker D, Huang Y, Zhou C, et al. Cytosolic cleaved PINK1 represses Parkin translocation to mitochondria and mitophagy. EMBO Rep. 2014;15:86–93. doi: 10.1002/embr.201337294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ihenacho UK, Meacham KA, Harwig MC, Widlansky ME, Hill RB. Mitochondrial fission protein 1: emerging roles in organellar form and function in health and disease. Front Endocrinol (Lausanne) 2021;12:660095. doi: 10.3389/fendo.2021.660095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 49.Park KS, Wiederkehr A, Kirkpatrick C, Mattenberger Y, Martinou JC, Marchetti P, et al. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2008;283:33347–33356. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adaniya SM, O-Uchi J, Cypress MW, Kusakari Y, Jhun BS. Posttranslational modifications of mitochondrial fission and fusion proteins in cardiac physiology and pathophysiology. Am J Physiol Cell Physiol. 2019;316:C583–C604. doi: 10.1152/ajpcell.00523.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nan J, Zhu W, Rahman MS, Liu M, Li D, Su S, et al. Molecular regulation of mitochondrial dynamics in cardiac disease. Biochim Biophys Acta Mol Cell Res. 2017;1864:1260–1273. doi: 10.1016/j.bbamcr.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833:1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz-Morales N, Rovira-Llopis S, Bañuls C, Escribano-Lopez I, de Marañon AM, Lopez-Domenech S, et al. Are mitochondrial fusion and fission impaired in leukocytes of type 2 diabetic patients? Antioxid Redox Signal. 2016;25:108–115. doi: 10.1089/ars.2016.6707. [DOI] [PubMed] [Google Scholar]
- 57.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 59.Genders AJ, Holloway GP, Bishop DJ. Are alterations in skeletal muscle mitochondria a cause or consequence of insulin resistance? Int J Mol Sci. 2020;21:6948. doi: 10.3390/ijms21186948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu L, Ding M, Tang D, Gao E, Li C, Wang K, et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics. 2019;9:3687–3706. doi: 10.7150/thno.33684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zorzano A, Liesa M, Palacín M. Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch Physiol Biochem. 2009;115:1–12. doi: 10.1080/13813450802676335. [DOI] [PubMed] [Google Scholar]
- 62.Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Wang C, Song G, Gan K, Kong D, Nie Q, et al. Mitofusion-2-mediated alleviation of insulin resistance in rats through reduction in lipid intermediate accumulation in skeletal muscle. J Biomed Sci. 2013;20:45. doi: 10.1186/1423-0127-20-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gan KX, Wang C, Chen JH, Zhu CJ, Song GY. Mitofusin-2 ameliorates high-fat diet-induced insulin resistance in liver of rats. World J Gastroenterol. 2013;19:1572–1581. doi: 10.3748/wjg.v19.i10.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filadi R, Pendin D, Pizzo P. Mitofusin 2: from functions to disease. Cell Death Dis. 2018;9:330. doi: 10.1038/s41419-017-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultz J, Waterstradt R, Kantowski T, Rickmann A, Reinhardt F, Sharoyko V, et al. Precise expression of Fis1 is important for glucose responsiveness of beta cells. J Endocrinol. 2016;230:81–91. doi: 10.1530/JOE-16-0111. [DOI] [PubMed] [Google Scholar]
- 67.Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sebastián D, Hernández-Alvarez MI, Segalés J, Sorianello E, Muñoz JP, Sala D, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 70.Quirós PM, Ramsay AJ, Sala D, Fernández-Vizarra E, Rodríguez F, Peinado JR, et al. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31:2117–2133. doi: 10.1038/emboj.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song WJ, Sereda S, et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol Biol Cell. 2011;22:2235–2245. doi: 10.1091/mbc.E10-12-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, et al. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFκB-Opa-1 signaling pathway. Diabetes. 2014;63:75–88. doi: 10.2337/db13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao D, Zhang L, Dhillon R, Hong TT, Shaw RM, Zhu J. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS One. 2013;8:e60967. doi: 10.1371/journal.pone.0060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim S, Kim C, Park S. Mdivi-1 protects adult rat hippocampal neural stem cells against palmitate-induced oxidative stress and apoptosis. Int J Mol Sci. 2017;18:1947. doi: 10.3390/ijms18091947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, et al. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes. 2017;66:193–205. doi: 10.2337/db16-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim B, Kim JS, Yoon Y, Santiago MC, Brown MD, Park JY. Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am J Physiol Regul Integr Comp Physiol. 2013;305:R927–R938. doi: 10.1152/ajpregu.00502.2012. [DOI] [PubMed] [Google Scholar]
- 78.Salabei JK, Hill BG. Mitochondrial fission induced by platelet-derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox Biol. 2013;1:542–551. doi: 10.1016/j.redox.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zerjatke T, Gak IA, Kirova D, Fuhrmann M, Daniel K, Gonciarz M, et al. Quantitative cell cycle analysis based on an endogenous all-in-one reporter for cell tracking and classification. Cell Rep. 2017;19:1953–1966. doi: 10.1016/j.celrep.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J, Hansen K, Edwards R, Van Houten B, Qian W. Mitochondrial division inhibitor 1 (mdivi-1) enhances death receptor-mediated apoptosis in human ovarian cancer cells. Biochem Biophys Res Commun. 2015;456:7–12. doi: 10.1016/j.bbrc.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai W, Wang G, Chwa J, Oh ME, Abeywardana T, Yang Y, et al. Mitochondrial division inhibitor (mdivi-1) decreases oxidative metabolism in cancer. Br J Cancer. 2020;122:1288–1297. doi: 10.1038/s41416-020-0778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126(Pt 3):789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian L, Neuber-Hess M, Mewburn J, Dasgupta A, Dunham-Snary K, Wu D, et al. Ischemia-induced Drp1 and Fis1-mediated mitochondrial fission and right ventricular dysfunction in pulmonary hypertension. J Mol Med (Berl) 2017;95:381–393. doi: 10.1007/s00109-017-1522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oliver D, Reddy PH. Dynamics of dynamin-related protein 1 in Alzheimer's disease and other neurodegenerative diseases. Cells. 2019;8:961. doi: 10.3390/cells8090961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo F, Herrup K, Qi X, Yang Y. Inhibition of Drp1 hyperactivation is protective in animal models of experimental multiple sclerosis. Exp Neurol. 2017;292:21–34. doi: 10.1016/j.expneurol.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joshi AU, Saw NL, Vogel H, Cunnigham AD, Shamloo M, Mochly-Rosen D. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol Med. 2018;10:e8166. doi: 10.15252/emmm.201708166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yue W, Chen Z, Liu H, Yan C, Chen M, Feng D, et al. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014;24:482–496. doi: 10.1038/cr.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mishra N, Kar R, Singha PK, Venkatachalam MA, McEwen DG, Saikumar P. Inhibition of mitochondrial division through covalent modification of Drp1 protein by 15 deoxy-Delta(12,14)-prostaglandin J2. Biochem Biophys Res Commun. 2010;395:17–24. doi: 10.1016/j.bbrc.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kar R, Mishra N, Singha PK, Venkatachalam MA, Saikumar P. Mitochondrial remodeling following fission inhibition by 15d-PGJ2 involves molecular changes in mitochondrial fusion protein OPA1. Biochem Biophys Res Commun. 2010;399:548–554. doi: 10.1016/j.bbrc.2010.07.108. [DOI] [PMC free article] [PubMed] [Google Scholar]