Everyday we make decisions about what to seek out, whether it is a tasty sandwich, money, or someone to spend time with. Similarly, we also have to decide what or who to avoid. The ease with which we do this relies on a region of the brain sitting directly above the eyes known as the orbitofrontal cortex (OFC). Scientists and neurologists first determined that this part of the brain was important for these abilities by studying people who lacked a properly functioning OFC. People with OFC damage often make disastrous life choices, such as pouring all of their money into ill-advised schemes or breaking the law on a lark. In addition to making poor decisions, they are frequently inappropriate, impulsive, and unable to socially navigate the world. In some cases the impairment is so great that they have been described as having “acquired sociopathy”.
While these individuals have provided vital insights into what this part of the brain might be necessary for, patient observations alone cannot tell the full story of OFC function. Many open questions remain about how OFC shapes our behavior and the underlying neural mechanisms involved. In this primer, we provide an overview of our understanding of OFC function as it has evolved from basic and clinical research, as well as the theories that have guided it. To begin, we will review important features of anatomy and connectivity that define OFC.
What constitutes OFC?
The OFC consists of a large swath of cortex on the ventral side of the frontal lobes. Precisely where its boundaries lie is uncertain, in part because “OFC” is a general anatomical label, similar to geographical terms like Southeast Asia or Middle East: it references a region, but everyone’s idea of what constitutes it is slightly different. For instance, the cortex of the gyrus rectus is sometimes considered part of OFC proper, part of the ventromedial prefrontal cortex (vmPFC), or part of a distinct region labeled medial OFC. For reasons such as this, it is important to clarify what we mean when we refer to OFC. For the purposes of this review, we will use the term OFC to refer to cortical areas that lie exclusively on the ventral surface of the frontal cortex in primates (Figure 1). In rodents this roughly approximates medial, lateral, and ventral orbital subfields. Of note, the region just above the medial aspects of OFC, the ventromedial prefrontal cortex (vmPFC), is often involved in cases of incidental OFC damage in humans, leading to a tendency to lump these two areas together when discussing their function. However, they connect to largely different parts of the brain, so we will be clear when referring to OFC alone or with vmPFC.
Figure 1.
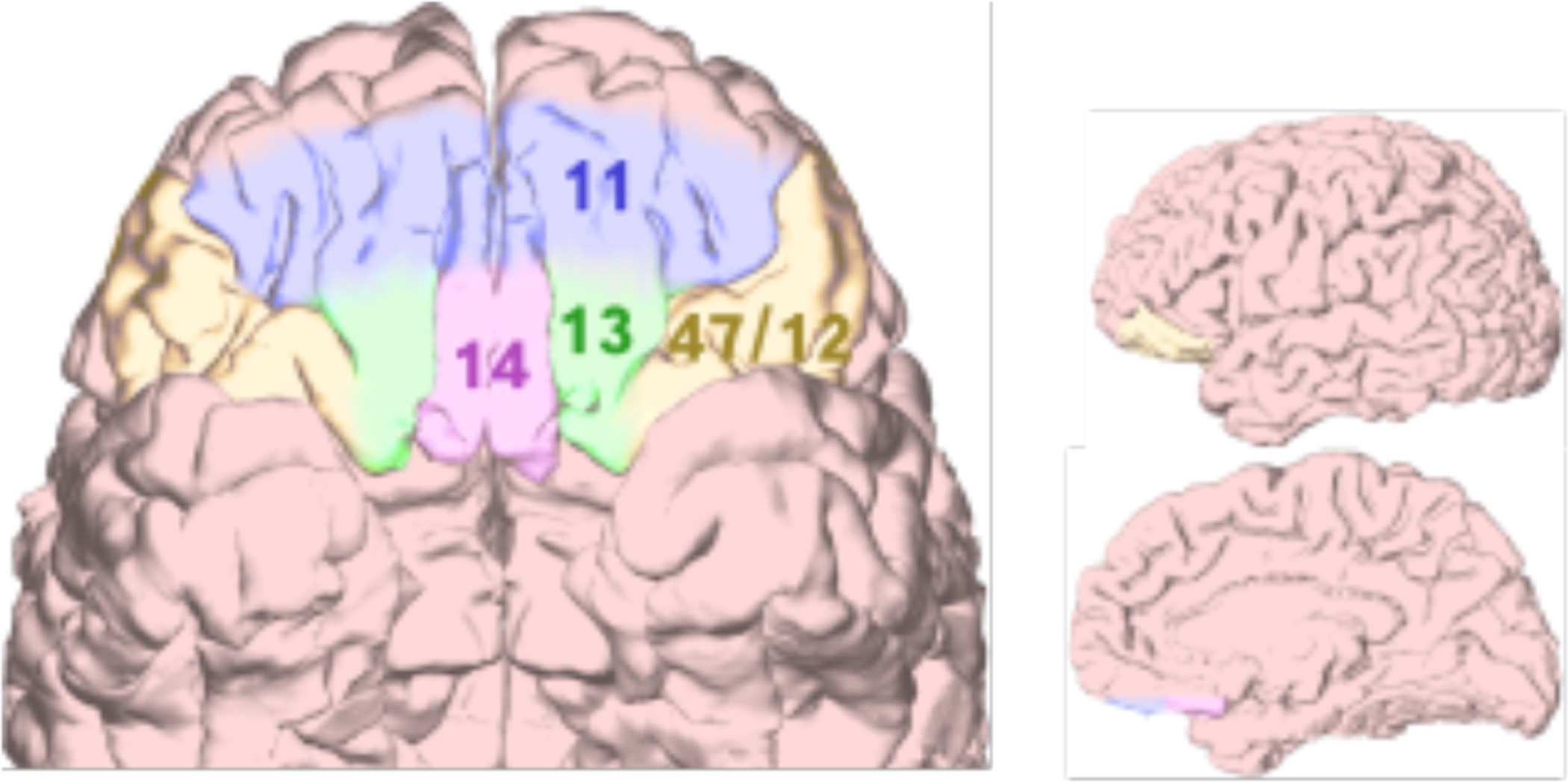
Subregions of the human OFC. Ventral, lateral, and medial brain views are shown with OFC regions highlighted. While various parcellations of OFC into subregions have been proposed, most include divisions into areas 11, 13, 14, and 47/12.
Anatomy and Organization of OFC
All mammals have an area that resembles the human OFC in terms of the anatomical connections sent and received from other brain areas. OFC receives highly processed sensory information, information about current bodily states such as hunger and thirst, as well as inputs from areas that process high-level emotional and social information (Figure 2A). The main outputs connect to the medial striatum, mediodorsal thalamus, and other parts of the prefrontal cortex. Together, this suite of connections is believed to enable neurons in OFC to encode associations between sensory stimuli in the external world and internal states related to emotionally relevant events. These signals can then be integrated into ongoing cognitive operations in other parts of PFC and beyond.
Figure 2.
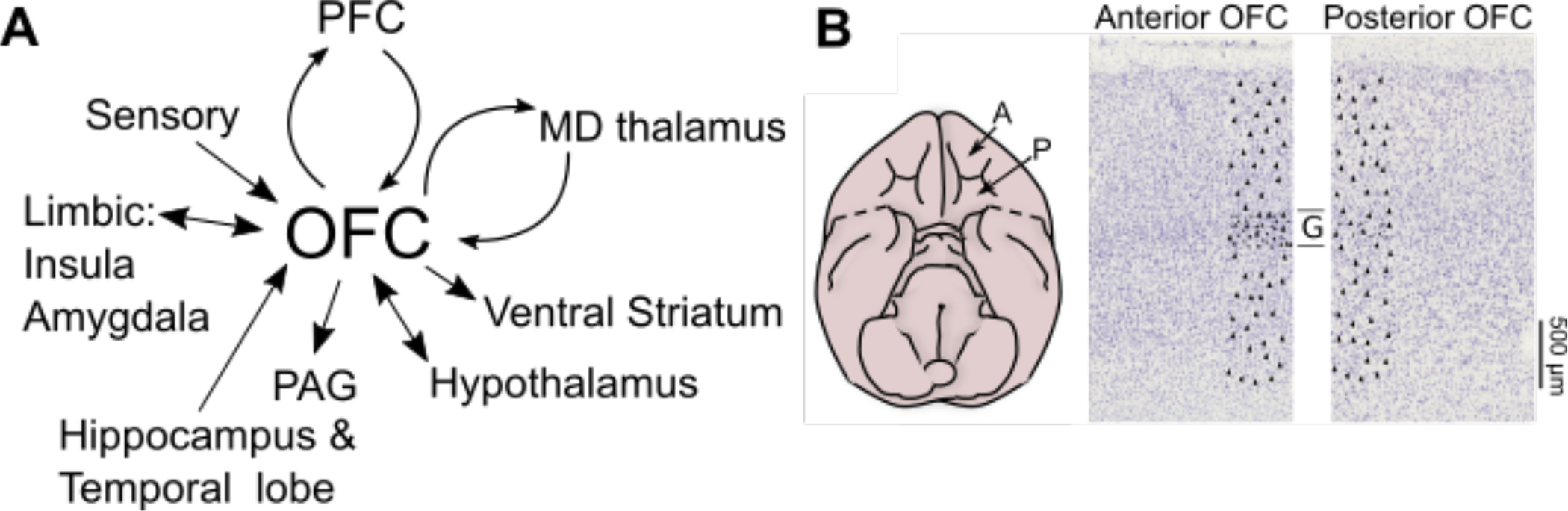
OFC anatomy and connectivity. (A) The major cortical and subcortical connections with OFC. PFC = prefrontal cortex, MD = mediodorsal, PAG = periaqueductal gray. (B) The first panel is a ventral view of the monkey brain with anterior temporal lobes removed to show OFC. A = Anterior, P = Posterior. The second panel is two thionin-stained sections of monkey OFC, oriented with the cortical surface at the top. There are higher cell densities in the middle layer (G) of the anterior section.
Despite similar connectivity, there are marked differences in OFC structure and complexity across species. Starting in the early twentieth century, anatomists painstakingly mapped and quantified how neurons are distributed in the human cortex. They showed that OFC includes a host of distinct subregions (Figure 1) that are characterized by differences in the cellular anatomy of cortical layers, (Figure 2B). Analysis of monkey OFC revealed that it possesses similar subregions as in humans, but the rodent OFC doesn’t have the same diversity. In particular, there are no parts of rodent OFC that anatomists describe as granular, referring to cortex that contains small pyramidal shaped ‘granule cells’ in the middle cortical layers. In primate OFC there is a gradient from posterior regions that are agranular like the rodent, to middle, so-called dysgranular regions that have sparse granule cells, to fully granular cortex anteriorly (Figure 2B). This progression is similar to what is seen in humans and likely reflects the fact that humans and monkeys have a more recent common ancestor than rodents. The pattern of cellular organization across species has led some to suggest that the rodent OFC is most similar to posterior agranular areas in primates, and that the more anterior regions appeared later in evolution. It is important to note, however, that the OFC of monkeys is not simply a smaller version of the human’s. Recent analyses suggest that there are parts of anterior OFC in human that do not have clear homologues in monkeys.
Initial insights into OFC function from neuropsychology
Early investigations into OFC function focused mainly on observations from patients with brain damage and similar lesions made experimentally in animals. For instance, in the era when frontal lobotomies were performed to treat psychiatric patients, a small number of “orbital leucotomies” were also carried out. Frontal lobotomies or leucotomies were operations that severed the brain’s connections to and from the frontal lobes. The orbital version was more selective, targeting only the connections in the vicinity of OFC in an attempt to reduce psychosis with fewer side effects than a full frontal lobotomy. Among the small number of patients that received them, doctors reported increased extroversion, restlessness, and euphoria, while aggressive or impulsive tendencies worsened. Even though these procedures were done in patients with psychiatric problems, the effects were consistent with those of incidental OFC damage in healthy individuals, in that there was an overall reduction of emotional and behavioral inhibition. Those who were pathologically inhibited improved, but those with tendencies to be cruel or impulsive became more so.
Based on these observations one prominent theory suggested that OFC was critical for exerting “inhibitory control” over behavior. In other words, using knowledge of a situation and likely consequences of an action to control impulsive responses. This hypothesis was appealing because it was consistent with the phenotype of social and behavioral dis-inhibition observed in humans, and could be operationalized to study animal behavior. As in humans, lesions of OFC in animals produced behavior that could be described as dis-inhibited. For example, studies in monkeys where large lesions of the orbital area were made found inappropriate emotional behavior, such as disregarding threatening stimuli, as well as learning impairments that were largely due to an inability to inhibit responses. This was especially true in a paradigm called reversal learning. In this paradigm, subjects’ become highly practiced at performing one response, for instance selecting one of a pair of visual images, to gain a food reward. Then the contingencies are unexpectedly reversed so the practiced response becomes unrewarded and the new response leads to food instead (Figure 3A). Studies in species ranging from rodents to carnivores to monkeys to humans all reported deficits in reversal learning tasks following OFC damage, with subjects perseverating on previously learned responses. Thus, studies of reversal learning in subjects with large lesions contributed to the idea that OFC was important for exerting inhibitory control.
Figure 3.
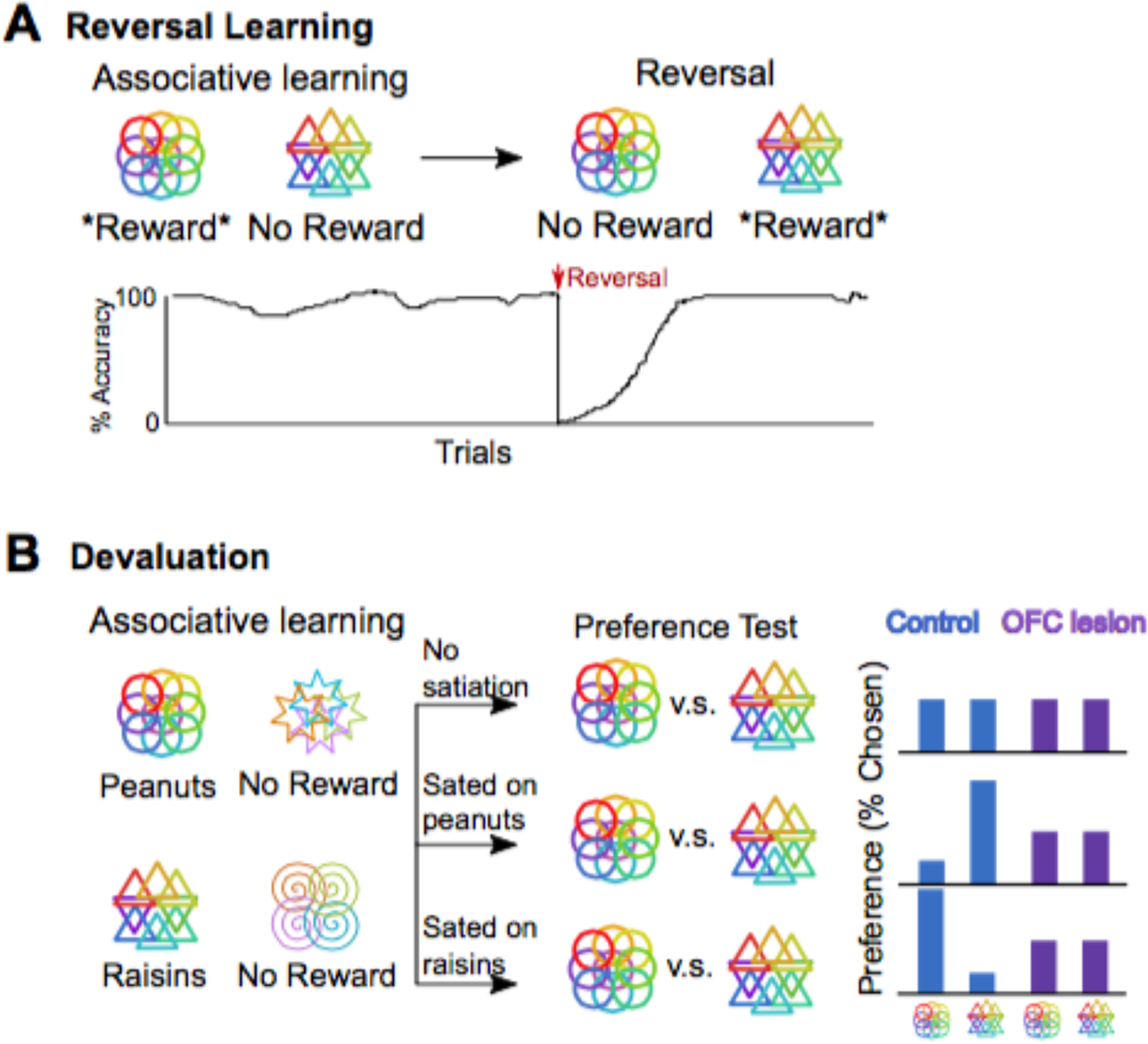
Two classic tests of OFC function. (A) In reversal learning tasks, subjects first learn stimulus-reward associations. In this case, selecting circles will lead to reward and selecting triangles will lead to no reward. Next, these associations are reversed, so that the triangles predict reward and the circles predict no reward. The bottom panel shows typical performance of a normal subject. Once the first association is learned, they have a high accuracy for selecting the rewarding stimulus. At the onset of the reversal, performance drops below chance because the subject is not warned that contingencies have changed and they continue to select the circles, expecting this stimulus to be rewarded. With trial and error, they learn to stop selecting circles and instead select triangles to receive reward. (B) In devaluation tasks, subjects first learn a series of stimulus-reward associations. In this case, selecting circles will lead to peanuts, triangles will lead to raisins, stars and spirals will lead to no reward. After learning, the subject is given a preference test in which their options are stimuli that predict different types of rewards that are equally preferred. If they are not sated, they will choose circles or triangles with equal probability. If they are sated on peanuts, they will prefer to have raisins, and should select triangles. If they are sated on raisins, they will prefer peanuts and should select circles. OFC damage impairs devaluation, and abolishes satiety-specific preferences.
Related to these ideas, a second hypothesis suggested that the OFC was important for interpreting changes in bodily state during emotional experiences, such as rapid heart beat or “butterflies” in the stomach. From this view, lack of inhibitory control and emotional disruption could be explained by an inability to use changes in bodily state as warning cues to inhibit risky or unwanted actions. This notion came from the finding that patients with OFC/vmPFC damage do not show characteristic changes in heart rate or skin conductance that usually accompany viewing emotionally charged pictures or encountering a risky situation. Researchers suggested that a key deficit in these patients was an inability to generate an appropriate “feeling”, in this case heightened arousal, which the healthy subjects might use to guide their behavior away from bad options and toward good. The idea that OFC creates these anticipatory responses was called the somatic marker hypothesis, a reference to the notion that what we call feelings have an origin in somatic (bodily) changes.
While there may, indeed, be strong links between OFC and emotion, follow-up studies suggested that the deficits indicative of somatic markers were not exclusively related to bodily responses. For instance, decision-making deficits in a gambling task that elicited somatic responses were more likely caused by the need for subjects to reverse their choices when one gamble appeared better at first but turned out to be a losing option. Indeed, when patients with OFC damage were tested on this task, they failed to switch responses appropriately, but could perform well when there was no reversal built in. While the somatic marker hypothesis appears less likely, the view that the OFC is important for inhibitory control persists in the literature. This idea has been an important waypoint in our understanding of OFC function, but ultimately deficits in inhibitory control are better understood as a description of the effects of OFC damage on behavior, and not a good characterization of the key functions that this part of the brain subserves.
Specific predictions for goal-directed behavior, not inhibitory control
Strong indications that OFC function was poorly defined by the theory of inhibitory control came from a number of sources. These supported a new view that OFC is involved in encoding or storing specific stimulus-reward associations that promote adaptive behavior, rather than inhibiting inappropriate responses per se. The first evidence was from studies that measured the activity of neurons in OFC when animals performed reversal learning tasks. If the key role of OFC were inhibiting incorrect responses as predicted by the inhibitory control theory, one would expect neurons to be most active at times when inhibition was required. However, this was not the case. OFC tends to be activated by affective outcomes – rewards or punishments – and the stimuli that predict them. When reversals occur, the activity of neurons in OFC tracks the new associations. Neuroimaging studies of humans performing similar tasks also found responses in OFC mainly to rewards and reward-predicting stimuli, and interestingly, more lateral parts of the inferior frontal convexity outside of the OFC do show activations related to inhibitory control.
These results dovetailed with other sources suggesting that OFC is not required for inhibiting responses, but is critical for goal directed behavior. Learning theory refers to behaviors that are based on the current motivational state of the animal and knowledge of a particular outcome (or goal) that is expected as “goal directed”. In this case, knowledge of the outcome means its sensory features, motivational value, quantities, and so forth. Having this knowledge means that responses are flexible, and can change when circumstances change. This is in contrast to habitual behaviors that are reflexive and not guided by knowledge of the outcome. For example, imagine that you hear in the news that there is a health scare associated with your favorite food. If your behavior is goal directed, the next time you are at the supermarket you won’t buy that food, as you know it has the potential to make you sick. By contrast, if your behavior is habitual, you will buy food as you always do, without considering that you’ll likely decide not to eat it later.
Studies of animals with OFC lesions showed that, in addition to having problems inhibiting responses, they were also unable to remember the motivational value and sensory features of the rewards that they might receive for making a choice. That is, they were not goal-directed. This inability to remember the identity of unique goals has been classically shown using reinforcement devaluation paradigms (Figure 3B). Here a subject learns that different stimuli predict two unique outcomes, for instance two different foods such as peanuts and raisins. When the subject is sated on one food (e.g. peanuts) but not the other, and offered a choice between the two, healthy subjects will adaptively choose the option that leads to the food they were not sated on. Therefore, they use knowledge of their internal state to update the food’s value so they now prefer raisins, when previously they may have liked peanuts and raisins equally. By contrast, neither humans nor animals with damage to OFC show this preference, suggesting OFC plays an important role in updating the value of potential outcomes following satiation. At the circuit level, interactions between OFC and amygdala are critical for normal devaluation, and disruption of these connections can cause similar behavioral deficits. Functional neuroimaging studies have similarly found that when a specific food is devalued through satiety, the OFC responses to that food are altered, likely reflecting the change in motivational value, and this is associated with concomitant devaluation related changes in amygdala activity. In contrast, food responses in gustatory cortex are unchanged, since this area represents sensory features of the food, rather than its motivational value.
How, then, can we reconcile the goal-directed and inhibitory control hypotheses of OFC function? Interestingly, recent work has suggested that we may not have to. The large experimental lesions that produced impairments in reversal learning and reinforcer devaluation were ablations that removed the OFC. This approach not only damages neurons in the target area but also white matter nearby that contains axons traveling to other brain areas. The collateral white matter damage can be avoided with excitotoxic lesions that only damage cells in the target area, but leave fibers passing through intact. When monkeys were given large excitotoxic OFC lesions instead of ablations, they performed normally on reversal learning tasks, but were still impaired in reinforcer devaluation. This indicates that, at least in primates, OFC is required for updating and storing information about outcomes that will follow choices, not for inhibitory control associated with reversal learning. It is important to point out that these results differ from classic observations in human patients, where damage is physical, similar to the experimental ablations, and therefore involved neurons as well as adjacent white matter. Indeed the orbital leucotomies described earlier specifically targeted these white matter connections near OFC to alter people’s behavior. Thus, inhibitory control deficits seen in humans after lesions or leucotomies involving OFC are likely the consequence of damage to the nearby white matter not the OFC itself. Instead, a primary function of neurons in OFC is computing specific sensory and motivational information about the result of potential choice.
Decision-making – computation and comparison of options
If OFC is necessary to predict the likely outcomes of a particular choice, it stands to reason that individuals with damage to OFC would have difficulties making decisions. This is because, incorrect or absent predictions about the consequences of possible actions would lead to erratic choices. There have been many studies of humans and laboratory animals making simple choices that reveal aspects of OFC activity that might play an important role in decision-making. First, the activity of neurons in monkey OFC is related to the subjective evaluation of choice options. For example, given a set of 3 images where image 3 predicts the receipt of a large reward, 2 a small reward and 1 an aversive stimulus, the activity of a sizable proportion of OFC neurons will have graded responses to images 1, 2, and 3 (Figure 4A). This pattern of activity is described as encoding value because it defines a scale from aversive to rewarding. These neural responses also integrate value across qualitatively different rewards or punishments, and can incorporate the costs, such as time or effort required to obtain a reward or avoid a punishment. Value signals are also influenced by factors such as risk or uncertainty. This integrated and subjective nature of these value signals has led many to view these OFC responses from the perspective of economic choice. This concept of value is distinct from processes such as motivation or attention, that are related to the intensity of a stimulus, but aversive and rewarding stimuli may be similarly salient. Salience encoding has been reported in widespread areas outside of the OFC, including sensory areas and premotor cortex.
Figure 4.
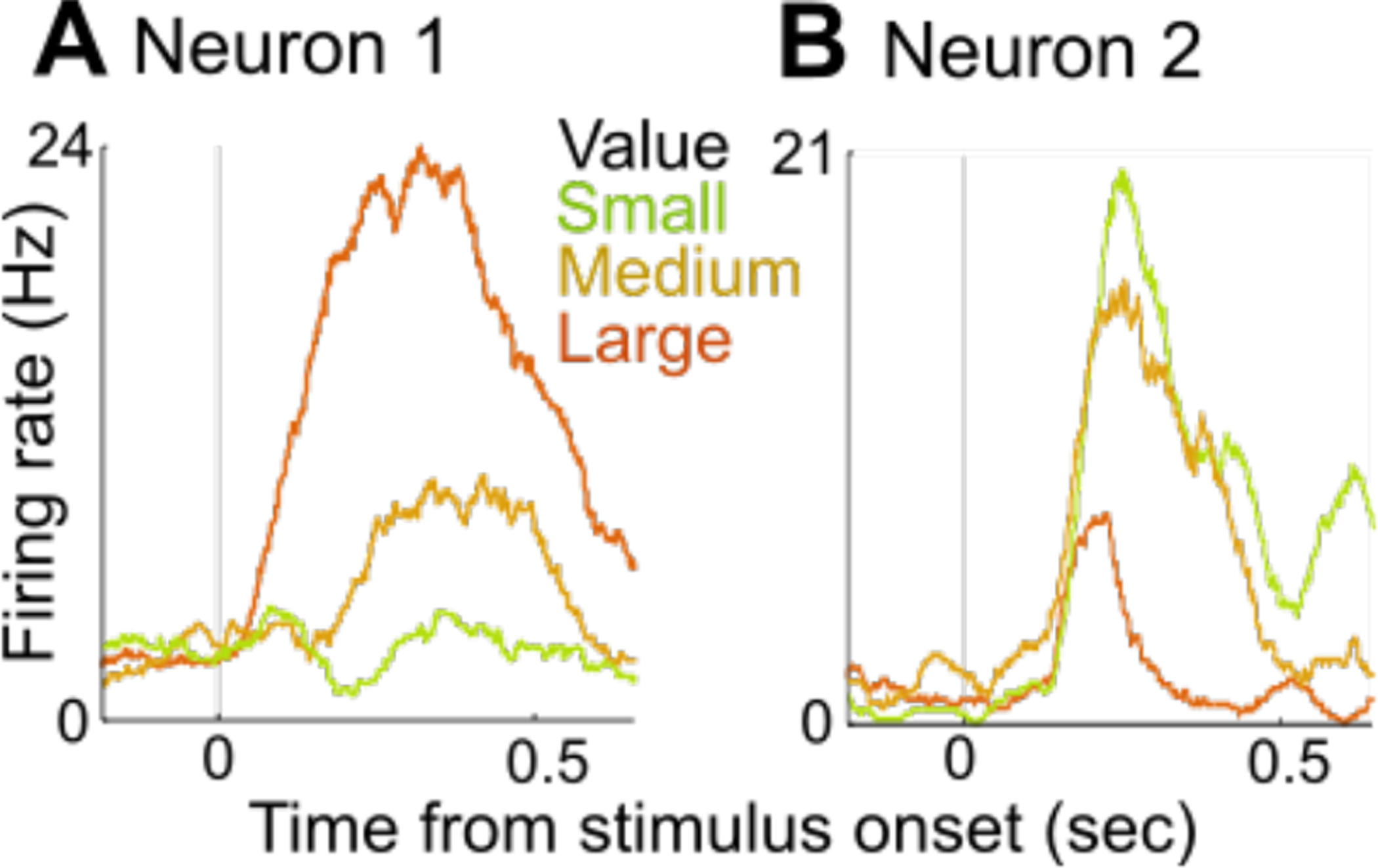
Value-coding neurons in monkey OFC. On each trial, the subject saw a stimulus at time 0 that predicted a small, medium, or large reward. The lines show all the average neuronal activity on all trials of each type, aligned to the time the stimulus appeared. (A) Neuron 1 responded more to stimuli predicting large rewards (B) Neuron 2 responded more to stimuli predicting small rewards. Neurons with both types of responses are present in approximately equal proportions in monkey OFC.
What are these value signals in OFC important for? One prominent theory suggests that computing the value of different options makes it possible to directly compare dissimilar goals by representing them on a common scale. For instance, say you have to choose between three lotteries that offer different probabilities of winning $100, at 80%, 50% and 20%. This choice is easy because the options are discernable on the common scale. However, the problem gets harder when the options are dissimilar; say comparing between an apple, two oranges, and bunch of raisins. OFC is important for making these types of value-based decisions, which depend on preference (e.g. apples, oranges, or raisins better?), but not perceptual decisions, which depend on interpreting sensory information (e.g. is this a picture of an apple, orange, or raisin?). The idea of a common scale for comparison is also appealing because it explains situations where we make suboptimal decisions. For example, in the lottery above, humans and animals are prone to suboptimal choices when one option is much worse than the other two. If the probabilities are 80%, 70% and 5%, then the difference between 70% and 80% seems small and 70% is chosen more often than if the options are 80%, 70% and 60%. This is because the value scale is spread out by the presence of the 5% option, making it more difficult to tell the difference between the higher options. In humans and monkeys, the ability to choose optimally in this situation depends on the most medial portion of OFC.
What are the mechanisms by which OFC makes these comparisons? In the case of perceptual decisions elsewhere in the brain, a process called mutual inhibition is believed to be important. Here, separate populations of neurons respond to different categories of stimuli, and the activity of one population directly inhibits the activity of the other, such that this simple network computation could determine which type of stimulus is being perceived. There is some evidence that mutual inhibition also occurs in the most medial areas of OFC, but is not the case in other OFC areas, which are nonetheless thought to be important for choice behavior. Instead, the majority of OFC neurons encode the subjective value of choice options on a single value scale, meaning there are not separate populations of neurons that respond to each option, making it impossible for these neurons to implement mutual inhibition. Of note, when the choice involves qualitatively different outcomes, a smaller population of neurons does encode the value of each option, or ‘offer’, nonetheless mutual inhibition does not appear to be a key computation in most of OFC. More recent ideas suggest that the choice computation may involve the dynamic representation of different options by ensembles of neurons in OFC, because each option can be transiently represented in OFC as the subject makes a choice. This process may be closely related to attention, and emerging research has shown that value signals in OFC are influenced by which stimuli are currently being attended. From this view, one possibility is that the mechanisms of comparison in OFC involve the rapid fluctuations of our internal focus between different possibilities being considered.
OFC, learning, memory, and other higher cognitive functions
Similar to decision-making, learning and memory would be significantly affected if one could not accurately predict outcomes. Indeed, there appears to be a central role for OFC in learning, particularly when subjects must continually update which stimuli will lead to desired outcomes and which will not. Neurons in the OFC track available rewards and punishments, signaling their quality, quantity and other associated features. Lesions of the OFC, particularly in rats, render them unable to learn and quickly update stimulus reward associations. In humans and monkeys, learning impairments following OFC damage are most notable when the relationship between stimuli and rewards is probabilistic, i.e. there is not a one-to-one mapping between choosing a stimulus and getting a reward. Neural activity in OFC also tracks probabilities of getting a reward when they change over time. Interestingly, learning in probabilistic settings and storing specific information about an outcome appear to depend on different parts of OFC. Areas 11 and 13 in the central OFC are necessary for updating and storing outcome specific information, but not learning probabilistic associations. In contrast, area 12 on the inferior-lateral convexity is important for learning from probabilistic feedback but not storing sensory specific information. This dichotomy largely agrees with surveys of the neural activity across these two areas.
Neural recordings in animals, human imaging, and lesion studies have also highlighted a role for OFC in signaling higher order representations related to reward and other abstract information. Specifically, neurons in OFC have been found to signal rules, strategies, conflict between courses of action, information about future rewards, secondary reinforcers, and even hypothetical rewards, i.e. rewards that would have been obtained if another choice had been made. Similarly activations in human OFC/vmPFC have been reported during the experience of regret and other higher order constructs. One recent idea that attempted to synthesize all of these data suggests that the core function of OFC is to represent a “cognitive map” of the current environment in relation to current goals. Note that the cognitive map in OFC is different from what has been described in hippocampus, where neurons strongly represent physical space. The cognitive map in OFC appears organized around goals and desired outcomes rather than space as in hippocampus, and would tell you when it’s appropriate to make a given choice. This OFC map would be learned and accessed by other brain areas as the animal interacts with the environment. For example, prediction errors signaled by the activity of dopamine neurons in the midbrain depend on expectations that originate from these putative maps in OFC. Currently, this view accommodates the available data, but further efforts are needed to flesh out specifics of how such maps might be constructed and used.
A role for OFC in emotion regulation, social behavior, and psychiatric disorders
Navigating the social world relies on many of the same prediction and decision-making functions ascribed to OFC. Not surprisingly, damage to the OFC in humans is associated with a host of changes in social behavior and at the extreme these have presentations similar to sociopathy. In animals, lesions of the OFC are associated with increased aggression and reduced fear-related behavior. The hypotheses about the role of OFC in emotion have recently been revised in monkeys based on comparisons between ablation and excitotoxic lesions. Monkeys with OFC ablations that damage both gray and white matter exhibit blunted emotional responses to anxiety provoking stimuli, such as snakes. However, when excitotoxic lesions are made, monkeys show heightened emotional responses, indicating an inability to inhibit fearful emotions consistent with anxiety. Human neuroimaging studies also support a role for OFC in emotion regulation. Activity in area 12 on the inferior-lateral convexity correlates with the ability to regulate emotion by reappraising emotionally charged images, for instance by imagining a less emotional narrative for a violent scene, when instructed.
This relationship of OFC to emotional regulation can be extrapolated to social contexts. For instance the aggression and emotional dis-inhibition following lesion may come about because of a reduced ability to predict the consequences of interacting with others. Indeed, fMRI studies in humans find that OFC is differentially activated by faces when social information is or is not important. In macaques, neurons in OFC signal the presence of faces and socially relevant features of conspecifics, such as identities, expressions, and social rank of the other individual. These factors are important for determining how to interact socially with others, and loss of this information could lead to dysfunctional social interactions.
Social context, such as the presence of another individual or who that individual is can also influence the meaning of rewards and how they are processed in OFC. For instance, when macaques play interactive games with each other, OFC neurons are modulated by reward depending on whether another animal is present and will also receive a reward. Similarly, OFC activity is modulated by situations where people have to decide between different courses of action based on social context.
Ultimately these effects on emotional and social behavior are intimately tied to the cognitive functions supported by OFC. Together, the data indicate that OFC is critical for interfacing emotion and cognition, and as such, OFC dysfunction is linked to cognitive-emotional disorders. Abnormal structure or function in OFC has been noted in a wide range of psychiatric patient populations, including individuals with depression, anxiety, obsessive-compulsive disorder, psychopathic and sociopathic disorders, and substance use disorders. In obsessive-compulsive disorder (OCD) there are unregulated negative emotions that drive the need to perform certain rituals to excess. Both structural and functional changes in OFC, as well as its interactions with the striatum, correlate with these symptoms. OFC dysfunction is implicated in drug addiction, where a series of maladaptive stimulus-reward associations are learned and drive compulsive drug seeking. Recent research even suggests that substances such as cocaine can directly cause changes in local circuit activity through epigenetic modifications and this degrades OFC function, perhaps diminishing goal-directed behavior and increasing the tendency toward habitual drug seeking. Psychopathic traits, including violence and lack of emotional reactivity and empathy, have been tied to impaired OFC/vmPFC – amygdala interactions, which may disrupt the ability to learn about social rewards. These and many other examples illustrate how disruptions in the core functions of OFC and associated circuits can result in myriad psychiatric syndromes.
Summary
While the fundamental functions of OFC are still a subject of intense research, our understanding has evolved from early patient observations to testable theories involving specific neural mechanisms. Overall, OFC lies at the interface of emotion and cognition. It plays a central role in our ability to make predictions about the likely consequences of potential actions so that we can make optimal decisions. When OFC processes go awry, the results manifest in a spectrum of cognitive-emotional disorders, with the particular syndrome depending on variables such as the subregion of OFC involved or associated circuit pathologies. Further research is still needed to address the many outstanding questions. Of particular impact will be those investigating the neural mechanisms that allow OFC to carry out its high-level functions, and how these processes go awry in psychological disorders. (2009)
Acknowledgements:
We would like to thank Denise Croote, Megan Fredericks, Alicia Izquierdo, Thorsten Kahnt and Johanna Crimins for invaluable feedback on earlier versions of this manuscript.
Footnotes
Competing interests: The authors declare no competing financial interests.
Reading list
- (1).Agustin-Pavon C, Braesicke K, Shiba Y, Santangelo AM, Mikheenko Y, Cockroft G, Asma F, Clarke H, Man MS, and Roberts AC (2012). Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol Psychiatry 72, 266–272. [DOI] [PubMed] [Google Scholar]
- (2).Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, and Hen R (2013). Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 340, 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Baxter MG, Parker A, Lindner CC, Izquierdo AD, and Murray EA (2000). Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience 20, 4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bechara A, Damasio H, and Damasio AR (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex 10, 295–307. [DOI] [PubMed] [Google Scholar]
- (5).Blair RJ (2010). Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. British Journal of Psychology 101, 383–399. [DOI] [PubMed] [Google Scholar]
- (6).Camille N, Tsuchida A, and Fellows LK (2011). Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. J Neurosci 31, 15048–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Eslinger PJ, and Damasio AR (1985). Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology 35, 1731–1741. [DOI] [PubMed] [Google Scholar]
- (8).Fellows LK, and Farah MJ (2005). Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex 15, 58–63. [DOI] [PubMed] [Google Scholar]
- (9).Glimcher PW, Camerer CF, Fehr E, Poldrack RA (2009) Neuroeconomics: Decision Making and the Brain. London, UK: Elsevier Academic Press. [Google Scholar]
- (10).Howard JD, and Kahnt T (2017). Identity-specific reward representations in orbitofrontal cortex are modulated by selective devaluation. J Neurosci. 37(10):2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Izquierod A (2017) Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making. J Neurosci 37(44):10529–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Morrison SE, and Salzman CD (2009). The convergence of information about rewarding and aversive stimuli in single neurons. Journal of Neuroscience 29, 11471–11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Neubert FX, Mars RB, Sallet J, and Rushworth MF (2015). Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc Natl Acad Sci U S A 112, E2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ongur D, Price JL. (2000) The organization of networks within orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10,206–219. [DOI] [PubMed] [Google Scholar]
- (15).Padoa-Schioppa C, and Assad JA (2006). Neurons in the orbitofrontal cortex encode economic value. Nature 441, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Passingham RE, and Wise SP (2012). The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight, 1 edn (Oxford University Press, USA: ). [Google Scholar]
- (17).Reitman F (1946). Orbital cortex syndrome following leucotomy. Am J Psychiatry 103, 238–241. [DOI] [PubMed] [Google Scholar]
- (18).Rich EL, and Wallis JD (2016). Decoding subjective decisions from orbitofrontal cortex. Nat Neurosci 19, 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rudebeck PH, Saunders RC, Lundgren DA, and Murray EA (2017). Specialized Representations of Value in the Orbital and Ventrolateral Prefrontal Cortex: Desirability versus Availability of Outcomes. Neuron 95, 1208–1220 e1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rudebeck PH, Saunders RC, Prescott AT, Chau LS, and Murray EA (2013). Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci 16, 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Schoenbaum G, Chiba AA, and Gallagher M (1998). Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci 1, 155–159. [DOI] [PubMed] [Google Scholar]
- (22).Stalnaker TA, Cooch NK, and Schoenbaum G (2015). What the orbitofrontal cortex does not do. Nat Neurosci 18, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Steiner AP, and Redish AD (2014). Behavioral and neurophysiological correlates of regret in rat decision-making on a neuroeconomic task. Nat Neurosci 17, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Strait CE, Blanchard TC, and Hayden BY (2014). Reward value comparison via mutual inhibition in ventromedial prefrontal cortex. Neuron In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Takahashi YK, Roesch MR, Wilson RC, Toreson K, O’Donnell P, Niv Y, and Schoenbaum G (2011). Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nat Neurosci 14, 1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Thorpe SJ, Rolls ET, and Maddison S (1983). The orbitofrontal cortex: neuronal activity in the behaving monkey. Experimental Brain Research 49, 93–115. [DOI] [PubMed] [Google Scholar]
- (27).Tsujimoto S, Genovesio A, and Wise SP (2012). Neuronal activity during a cued strategy task: comparison of dorsolateral, orbital, and polar prefrontal cortex. J Neurosci 32, 11017–11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wallis JD, Anderson KC, and Miller EK (2001). Single neurons in prefrontal cortex encode abstract rules. Nature 411, 953–956. [DOI] [PubMed] [Google Scholar]
- (29).Wilson RC, Takahashi YK, Schoenbaum G, and Niv Y (2014). Orbitofrontal cortex as a cognitive map of task space. Neuron 81, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]


