Abstract
Background
Invasive mold diseases (IMDs) cause severe illness, but public health surveillance data are lacking. We describe data collected from a laboratory-based, pilot IMD surveillance system.
Methods
During 2017–2019, the Emerging Infections Program conducted active IMD surveillance at 3 Atlanta-area hospitals. We ascertained potential cases by reviewing histopathology, culture, and Aspergillus galactomannan results and classified patients as having an IMD case (based on European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group [MSG] criteria) or a non-MSG IMD case (based on the treating clinician’s diagnosis and use of mold-active antifungal therapy). We described patient features and compared patients with MSG vs non-MSG IMD cases.
Results
Among 304 patients with potential IMD, 104 (34.2%) met an IMD case definition (41 MSG, 63 non-MSG). The most common IMD types were invasive aspergillosis (n = 66 [63.5%]), mucormycosis (n = 8 [7.7%]), and fusariosis (n = 4 [3.8%]); the most frequently affected body sites were pulmonary (n = 66 [63.5%]), otorhinolaryngologic (n = 17 [16.3%]), and cutaneous/deep tissue (n = 9 [8.7%]). Forty-five (43.3%) IMD patients received intensive care unit–level care, and 90-day all-cause mortality was 32.7%; these outcomes did not differ significantly between MSG and non-MSG IMD patients.
Conclusions
IMD patients had high mortality rates and a variety of clinical presentations. Comprehensive IMD surveillance is needed to assess emerging trends, and strict application of MSG criteria for surveillance might exclude over one-half of clinically significant IMD cases.
Keywords: invasive mold disease, surveillance, antifungal drugs, invasive aspergillosis, mucormycosis
Invasive mold diseases (IMDs) impose a substantial burden on the US health care system and cause severe patient morbidity and mortality [1–4]. Mortality rates associated with IMD can exceed 50%, depending on the pathogen involved and host characteristics [5]. Invasive aspergillosis is the most common form of IMD, followed by mucormycosis, fusariosis, and scedosporiosis [6, 7]. IMD can involve a variety of body sites, and classic IMD risk factors include hematologic malignancy (HM), hematopoietic stem cell transplant (HSCT), solid organ transplant (SOT), and other forms of severe immunosuppression [8–11].
Recent reports have documented several noteworthy trends in IMD epidemiology, including greater recognition of IMD in patients who lack classic risk factors. IMD is increasingly reported in postsurgical patients and in influenza or COVID-19 patients receiving intensive care unit (ICU)–level care [12–16]. The overall incidence of IMD in the United States appears to be rising, with invasive aspergillosis hospitalization rates increasing annually by 3% and mucormycosis-related hospitalization rates doubling during 2000–2013 [17]. Increasing IMD incidence might be because of the growing number of persons living with immunosuppression [18] or because of enhanced detection through non-culture-based diagnostic methods, such as Aspergillus galactomannan antigen (GM) testing [17] and sequencing of cell-free plasma to detect fungal DNA [19].
Despite the public health significance of IMDs, IMD surveillance data are lacking for several reasons. First, distinguishing colonization from invasive disease is clinically challenging, and definitive IMD diagnosis requires histopathologic samples or cultures from sterile body sites; however, these tests lack sensitivity and often require invasive procedures that might be contraindicated in certain patient populations [20]. Also, comprehensive IMD public health surveillance might require ascertainment of cases from a variety of data sources, a process that is burdensome because of the need for manual review of free text from histopathology or radiology reports [21, 22]. Finally, public health surveillance relies on clear case classification criteria, which is challenging given the diagnostic complexity and heterogenous spectrum of IMD.
To facilitate studies of invasive fungal diseases in at-risk patients, the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (MSG) developed and validated a case definition for use in clinical trials involving transplant and immunocompromised patients [8]. MSG case definitions are useful for categorizing and describing IMD in major studies of patients with cancer and transplantation [3, 7]; however, these definitions might lack the sensitivity desired for public health IMD surveillance because they are less suitable for ICU patients and involve highly specific MSG host and clinical factors that are frequently absent in patients without cancer or transplantation [8, 23].
To address the need for comprehensive IMD public health surveillance, we created a pilot surveillance program involving both the MSG case definition and a surveillance case definition (non-MSG case) intended to capture the broad spectrum of patients who might experience IMD. We conducted active, laboratory-based surveillance in Atlanta, Georgia, through the Georgia Emerging Infections Program (EIP), which is part of a network of US state public health departments, academic institutions, clinical laboratories, and health care providers that collaborate with Centers for Disease Control and Prevention (CDC) to monitor emerging infectious diseases [24]. We describe the pilot IMD surveillance system and summarize the demographics, clinical data, health care utilization, and outcomes of patients identified during the system’s 2017–2019 pilot phase.
METHODS
During February 1, 2017, to December 31, 2019, the EIP conducted IMD surveillance at 3 Atlanta metropolitan area hospital systems (1 academic, 1 federal, 1 county). Surveillance personnel used a standardized case report form to collect demographic and clinical data from medical and laboratory records.
Ascertainment of Potential IMD Cases
During the pilot phase, we restricted surveillance to persons residing within the Atlanta metropolitan statistical area. We defined potential IMD cases as the presence of at least 1 of the following positive mold tests: a positive GM (defined as an index value ≥0.5 on a serum or bronchoalveolar lavage sample), a histopathology specimen with mold elements or evidence of mold angioinvasion, or a positive mold culture. We chose the GM index value cutoff of 0.5 (rather than the EORTC/MSG 2020–proposed cutoff of 1.0) to increase the surveillance system’s sensitivity for ascertaining potential cases. We limited histopathology and culture specimens to those meeting certain body site– and species-specific criteria intended to exclude specimens likely representing colonization or environmental contamination (eg, Penicillium spp. in a sputum sample) or noninvasive mold disease (eg, mold isolated from hair or nails). A full list of inclusion criteria for mold culture and histopathology specimens is available in the Supplementary Data. We considered subsequent positive mold tests obtained from the same patient within 60 days of incident specimen collection (earliest positive mold test meeting inclusion criteria) as part of the same potential case.
Case Classification and Laboratory Testing
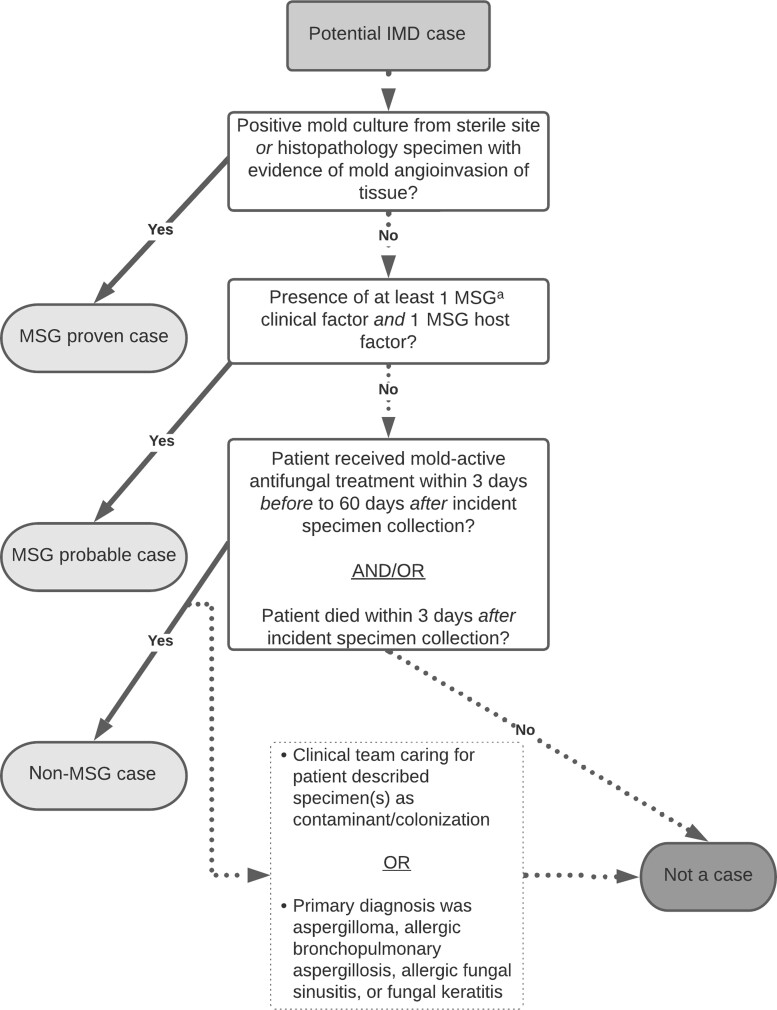
Using 2020 revised MSG case criteria, we classified potential cases as proven (based on the presence of a positive culture from a normally sterile body site or histopathologic IMD evidence) or probable (based on the presence of ≥1 MSG host factor, ≥1 MSG clinical factor, and mycologic evidence in an immunocompromised patient) [8]. We also applied a non-MSG case category to capture clinically significant IMDs that did not meet the MSG case definition (probable or proven) (Figure 1). We defined a non-MSG case based on the diagnosis of the treating clinicians and patient receipt of mold-active antifungal therapy. We defined mold-active antifungal therapy as receipt of treatment (ie, not prophylaxis) with amphotericin B, anidulafungin, caspofungin, flucytosine, isavuconazole, itraconazole, micafungin, posaconazole, or voriconazole within 3 days before to 60 days after incident specimen collection. Patients who died within 3 days after receipt of their first positive mold test were considered to have a non-MSG case, regardless of antimold antifungal therapy receipt, because those patients might have died before the clinical team had the opportunity to start antifungal treatment. If the clinical team caring for a patient with a non-MSG case indicated that the patient’s positive mold tests likely represented laboratory contamination or colonization, then the case was recategorized as a noncase; this exclusion step accounted for instances when a patient initially received empiric IMD treatment that was later discontinued because the clinical team ultimately identified an alternative diagnosis. We also classified potential cases as noncases if the patient’s primary diagnosis was allergic bronchopulmonary aspergillosis, aspergilloma, allergic fungal sinusitis, or fungal keratitis. We did not perform full chart abstractions and case classifications for patients with cystic fibrosis, as mold colonization is particularly common among patients with this disease [25], but we archived these patients’ records for potential future chart review.
Figure 1.
Flow diagram depicting classification scheme for potential cases of invasive mold disease (IMD) in an active surveillance system. aPotential cases of IMD were classified using the 2020 European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (MSG) consensus definitions of invasive fungal diseases and based on clinician diagnosis and patient receipt of mold-active antifungal therapy (non-MSG IMD cases; https://academic.oup.com/cid/article/71/6/1367/5645434).
Isolates from mold specimens were speciated by inpatient and outpatient clinical, reference, or commercial laboratories serving the surveillance population according to each facility’s current practices, and identifications for available isolates were confirmed by the CDC’s Mycotic Diseases Branch reference laboratory using DNA sequencing [26, 27]. For available A. fumigatus isolates, the CDC evaluated azole resistance using previously described methods [28, 29]. For IMD patients whose only positive fungal diagnostic test was Aspergillus GM, we presumed that the patient had aspergillosis.
Data Analysis
We restricted analyses to index IMD cases, defined as the first case per patient within the analytic period, and index potential IMD cases for patients who did not have an IMD. We combined IMD patients with MSG-proven and MSG-probable cases for analyses because of small sample size. We described demographic features, clinical characteristics, health care utilization, outcomes, diagnostic tests, and mold types identified for IMD patients with MSG cases, IMD patients with non-MSG cases, and non-IMD patients. We calculated 90-day all-cause mortality by matching surveillance data with data from the Georgia Department of Public Health Vital Statistics registry. We used Fisher exact tests for categorical variables and the Kruskal-Wallis H test for continuous variables (α = 0.05) to make comparisons between IMD patients vs non-IMD patients and between IMD patients with MSG vs non-MSG cases.
Patient Consent
Collection of human subject data was determined by the CDC to be routine public health surveillance and was not subject to CDC institutional review board approval.
RESULTS
Case Ascertainment
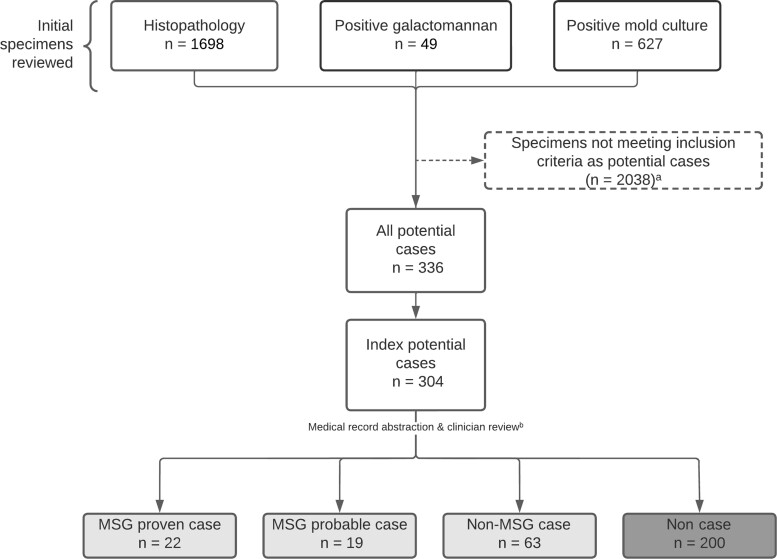
To ascertain cases, we reviewed 627 positive mold cultures, 1698 histopathology reports, and 49 positive GM test results collected from participating facilities during the analytic period (Figure 2). After applying exclusion criteria, we identified 304 patients with potential IMD cases; 104 (34.2%) patients had an IMD case, including 41 MSG cases (22 proven, 19 probable) and 63 non-MSG cases. The remaining 200 (65.8%) patients did not have an IMD case.
Figure 2.
Flow diagram depicting case ascertainment and classification in an active surveillance system for invasive mold disease (IMD)—Georgia, 2017–2019. aA full list of specimen inclusion and exclusion criteria is available in the Supplementary Data. bPotential cases of IMD were classified using the 2020 European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (MSG) consensus definitions of invasive fungal diseases and based on clinician diagnosis and patient receipt of mold-active antifungal therapy (non-MSG IMD cases; https://academic.oup.com/cid/article/71/6/1367/5645434).
Demographic Features
Demographic features did not differ significantly between IMD patients and non-IMD patients or by IMD case type. Among the 304 patients with potential cases, the median age (interquartile range) was 61 (50–70) years, 182 (59.9%) were male, 145 (47.7%) were Black, and 275 (90.5%) were non-Hispanic (Table 1).
Table 1.
Patient Demographic Characteristics, Underlying Conditions, Health Care Utilization, Treatment, and Outcomes in an Active Surveillance System for Invasive Mold Disease—Georgia, 2017–2019a
| Characteristic | Total (N = 304) | All IMD Cases vs Noncases | IMD Cases (MSG vs Non-MSG) | ||||
|---|---|---|---|---|---|---|---|
| All Cases (n = 104) | Noncases (n = 200) | P Value | MSG Case (n = 41) | Non-MSG Case (n = 63) | P Value | ||
| Demographic characteristics | |||||||
| Age, median (IQR), y | 61 (50–70) | 60 (46–66) | 62 (51–71) | .056 | 54 (44–67) | 62 (52–66) | .261 |
| Male sex | 182 (59.9) | 69 (66.3) | 113 (56.5) | .109 | 25 (61.0) | 44 (69.8) | .399 |
| Race | .301 | .162 | |||||
| Black | 145 (47.7) | 53 (51.0) | 92 (46.0) | .468 | 23 (56.1) | 30 (47.6) | .428 |
| White | 126 (41.4) | 40 (38.5) | 86 (43.0) | .464 | 12 (29.3) | 28 (44.4) | .150 |
| Otherb | 14 (4.6) | 7 (6.7) | 7 (3.5) | .250 | 5 (12.2) | 2 (3.2) | .109 |
| Unknown | 19 (6.3) | 4 (3.8) | 15 (7.5) | .318 | 1 (2.4) | 3 (4.8) | >.999 |
| Ethnicity | .950 | .657 | |||||
| Hispanic or Latino | 12 (3.9) | 4 (3.8) | 8 (4.0) | >.999 | 1 (2.4) | 3 (4.8) | >.999 |
| Not Hispanic or Latino | 275 (90.5) | 95 (91.3) | 180 (90.0) | .838 | 39 (95.1) | 56 (88.9) | .477 |
| Unknown | 17 (5.6) | 5 (4.8) | 12 (6.0) | .796 | 1 (2.4) | 4 (6.3) | .646 |
| Site of infection or colonization | .364 | .045 | |||||
| Pulmonary | 183 (60.2) | 66 (63.5) | 117 (58.5) | .459 | 23 (56.1) | 43 (68.3) | .220 |
| ENT | 46 (15.1) | 17 (16.3) | 29 (14.5) | .736 | 10 (24.4) | 7 (11.1) | .103 |
| Cutaneous or deep tissue | 41 (13.5) | 9 (8.7) | 32 (16.0) | .080 | 1 (2.4) | 8 (12.7) | .085 |
| Other body sitec | 34 (11.2) | 12 (11.5) | 22 (11.0) | >.999 | 7 (17.1) | 5 (7.9) | .211 |
| Clinical characteristics and risk factors for invasive mold diseased | |||||||
| MSG clinical and host factors | |||||||
| ≥1 clinical and host factor | 28 (9.2) | 28 (26.9) | 0 (0.0) | <.001 | 28 (68.3) | 0 (0.0) | <.001 |
| ≥1 host factor | 92 (30.3) | 61 (58.7) | 31 (15.5) | <.001 | 31 (75.6) | 30 (47.6) | .008 |
| ≥1 clinical factor | 53 (17.4) | 39 (37.5) | 14 (7.0) | <.001 | 31 (75.6) | 8 (12.7) | <.001 |
| Hematologic malignancy | 33 (10.9) | 25 (24.0) | 8 (4.0) | <.001 | 16 (39.0) | 9 (14.3) | .005 |
| Leukemia | 14 (4.6) | 11 (10.6) | 3 (1.5) | <.001 | 7 (17.1) | 4 (6.3) | .107 |
| Lymphoma | 15 (4.9) | 10 (9.6) | 5 (2.5) | .010 | 5 (12.2) | 5 (7.9) | .510 |
| Multiple myeloma | 2 (0.7) | 2 (1.9) | 0 (0.0) | .116 | 1 (2.4) | 1 (1.6) | >.999 |
| Other hematological malignancies | 3 (1.0) | 3 (2.9) | 0 (0.0) | .039 | 3 (7.3) | 0 (0.0) | .059 |
| Solid organ malignancy | 29 (9.5) | 8 (7.7) | 21 (10.5) | .539 | 2 (4.9) | 6 (9.5) | .475 |
| Hematopoietic stem cell transplant | 9 (3.0) | 8 (7.7) | 1 (0.5) | .001 | 5 (12.2) | 3 (4.8) | .259 |
| Solid organ transplant | 28 (9.2) | 21 (20.2) | 7 (3.5) | <.001 | 8 (19.5) | 13 (20.6) | >.999 |
| Graft-vs-host disease within previous 90 d | 3 (1.0) | 2 (1.9) | 1 (0.5) | .270 | 1 (2.4) | 1 (1.6) | >.999 |
| Neutropenia within previous 30 d | 24 (7.9) | 20 (19.2) | 4 (2.0) | <.001 | 11 (26.8) | 9 (14.3) | .132 |
| Lymphopenia documented within previous 30 d | 79 (26.0) | 47 (45.2) | 32 (16.0) | <.001 | 23 (56.1) | 24 (38.1) | .106 |
| HIV | 20 (6.6) | 6 (5.8) | 14 (7.0) | .810 | 2 (4.9) | 4 (6.3) | >.999 |
| Advanced HIV | 13 (4.3) | 4 (3.8) | 9 (4.5) | >.999 | 2 (4.9) | 2 (3.2) | .646 |
| Chronic pulmonary disease | 101 (33.2) | 30 (28.8) | 71 (35.5) | .252 | 8 (19.5) | 22 (34.9) | .121 |
| COPD or asthma | 55 (18.1) | 14 (13.5) | 41 (20.5) | 5 (12.2) | 9 (14.3) | ||
| Chronic pulmonary disease besides COPD/asthmae | 48 (15.8) | 16 (15.4) | 32 (16.0) | 2 (4.9) | 14 (22.2) | ||
| Chronic pulmonary infectionsf | 14 (4.6) | 3 (2.9) | 11 (5.5) | 2 (4.9) | 1 (1.6) | ||
| Influenza | 3 (1.0) | 2 (1.9) | 1 (0.5) | .270 | 0 (0.0) | 2 (3.2) | .518 |
| Diabetes | 80 (26.3) | 36 (34.6) | 44 (22.0) | .020 | 13 (31.7) | 23 (36.5) | .677 |
| Cirrhosis | 5 (1.6) | 2 (1.9) | 3 (1.5) | >.999 | 0 (0.0) | 2 (3.2) | .518 |
| End-stage renal disease | 23 (7.6) | 12 (11.5) | 11 (5.5) | .069 | 6 (14.6) | 6 (9.5) | .533 |
| Autoimmune disease or inherited immunodeficiencyg | 22 (7.2) | 8 (7.7) | 14 (7.0) | .819 | 2 (4.9) | 6 (9.5) | .475 |
| Severe burn within previous 90 d | 7 (2.3) | 5 (4.8) | 2 (1.0) | .049 | 2 (4.9) | 3 (4.8) | >.999 |
| Medications within previous 90 d | |||||||
| Corticosteroids | 66 (21.7) | 41 (39.4) | 25 (12.5) | <.001 | 21 (51.2) | 20 (31.7) | .065 |
| Prolonged, high-dose corticosteroids (20 mg prednisone daily or bioequivalent, ≥1 wk) |
33 (10.9) | 21 (20.2) | 12 (6.0) | <.001 | 11 (26.8) | 10 (15.9) | .214 |
| Cytotoxic chemotherapy | 19 (6.2) | 14 (13.5) | 5 (2.5) | <.001 | 11 (26.8) | 3 (4.8) | .002 |
| Transplant immunosuppressive drugs | 44 (14.5) | 33 (31.7) | 11 (5.5) | <.001 | 14 (34.1) | 19 (30.2) | .673 |
| Other immunosuppressive drugs, including biologics and targeted/designer drugs | 17 (5.6) | 9 (8.7) | 8 (4.0) | .115 | 6 (14.6) | 3 (4.8) | .150 |
| Treatments and clinical outcomes | |||||||
| Mold-active antifungal drug therapyh | 113 (37.2) | 99 (95.2) | 14 (7.0) | <.001 | 36 (87.8) | 63 (100.0) | .008 |
| Amphotericin B | 21 (6.9) | 20 (19.2) | 1 (0.5) | <.001 | 12 (29.3) | 8 (12.7) | .044 |
| Echinocandin | 22 (7.2) | 18 (17.3) | 4 (2.0) | <.001 | 8 (19.5) | 10 (15.9) | .791 |
| Isavuconazole | 44 (14.5) | 40 (38.5) | 4 (2.0) | <.001 | 16 (39.0) | 24 (38.1) | >.999 |
| Itraconazole | 3 (1.0) | 2 (1.9) | 1 (0.5) | .270 | 1 (2.4) | 1 (1.6) | >.999 |
| Posaconazole | 20 (6.6) | 19 (18.3) | 1 (0.5) | <.001 | 9 (22.0) | 10 (15.9) | .448 |
| Voriconazole | 48 (15.8) | 42 (40.4) | 6 (3.0) | <.001 | 16 (39.0) | 26 (41.3) | .841 |
| Inpatient hospitalizationi | 187 (61.5) | 91 (87.5) | 96 (48.0) | <.001 | 37 (90.2) | 54 (85.7) | .559 |
| Admission to ICUi | 84 (27.6) | 45 (43.3) | 39 (19.5) | <.001 | 18 (43.9) | 27 (42.9) | >.999 |
| Died in hospital (among hospitalized patients) | 34 (18.2) | 26 (28.6) | 8 (8.3) | <.001 | 10 (27.0) | 16 (29.6) | .818 |
| 90-d mortality | 50 (16.4) | 34 (32.7) | 16 (8.0) | <.001 | 12 (29.3) | 22 (34.9) | .670 |
Abbreviations: ENT, ear, nose, and throat; ICU, intensive care unit; IMD, invasive mold disease; IQR, interquartile range; MSG, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group; NTM, nontuberculous mycobacterial infection.
Data are presented as No. (%) or median (interquartile range). P values were calculated using the Fisher exact test for categorical variables and the Kruskal-Wallis H test for continuous variables.
Among the 14 patients whose race was categorized as other, 9 were Asian, 3 were Native Hawaiian or other Pacific Islander, and 2 were American Indian/Alaska Native.
Among IMD patients, other sites of infection included central nervous system (n = 3), eye (n = 3), disseminated (n = 2), aortic valve (n = 1), and other/unspecified (n = 3).
Underlying medical conditions and treatments were present within 2 years before the date of incident mold specimen collection, unless otherwise specified. Patients could have multiple underlying conditions. Because diagnosis of an MSG-proven case is based solely on histopathology or culture data, patients with MSG-proven cases could lack MSG host or clinical risk factors.
Other chronic pulmonary diseases in IMD patients included bronchiectasis (n = 5), interstitial lung disease (n = 5), sarcoidosis (n = 2), pulmonary fibrosis (n = 1), bronchiolitis obliterans (n = 1), chronic respiratory failure from an unspecified cause (n = 1), and other (n = 1).
Three IMD patients had a diagnosis of NTM infection; 1 patient was diagnosed with both tuberculosis and NTM infection.
Among IMD patients, autoimmune conditions or inherited immunodeficiencies included rheumatoid arthritis (n = 3), antisynthetase syndrome (n = 1), primary biliary cholangitis (n = 1), primary immunodeficiency with T-cell defect (n = 1), mucous membrane pemphigoid (n = 1), unspecified connective tissue disease (n = 1), and scleroderma (n = 1).
Mold-active antifungal therapy was defined as receipt of treatment (ie, not prophylaxis) with amphotericin, anidulafungin, caspofungin, flucytosine, isavuconazole, itraconazole, micafungin, posaconazole, or voriconazole within 3 days before to 90 days after incident specimen collection. Among the 14 patients without an IMD case who received mold-active antifungal treatment, 4 had aspergilloma and 1 had allergic bronchopulmonary aspergillosis. For 8 patients, the mold-active antifungal treatment was stopped because the clinical team caring for the patient determined that the patient’s positive mold test(s) likely represented laboratory contamination or colonization; 1 patient received an echinocandin for invasive candidiasis but was not believed by the clinical team to have an IMD.
Inpatient hospitalization or ICU stay within 60 days after the date of incident specimen collection.
Infection Site
Site of infection or specimen collection did not differ significantly between IMD patients and non-IMD patients (P = .364). Among all patients with potential cases, the most frequently involved body sites were pulmonary (n = 183 [60.2%]), otorhinolaryngologic (n = 46 [15.1%]), and cutaneous or deep tissue (n = 41 [13.5%]). Among IMD patients, site of infection differed by MSG case status (P = .045): Compared with non-MSG IMD patients, MSG IMD patients less frequently had a pulmonary infection (n = 23 [56.1%] vs n = 43 [68.3%]) or cutaneous or deep tissue infection (n = 1 [2.4%] vs n = 8 [12.7%]) and more frequently had an otorhinolaryngologic infection (n = 10 [24.4%] vs n = 7 [11.1%]). Less frequent infection sites among all IMD patients included central nervous system (n = 3), ocular (n = 3), disseminated (n = 2), and endocarditis (n = 1).
Underlying Conditions, Previous Medications, Antifungal Treatment, and Outcomes
IMD patients were more likely than non-IMD patients to have ≥1 MSG host factor (n = 61 [58.7%] vs n = 31 [15.5%]; P < .001) or ≥1 MSG clinical factor (n = 39 [37.5%] vs n = 14 [7.0%]; P < .001), and MSG IMD patients were more likely than non-MSG IMD patients to have ≥1 MSG host factor (n = 31 [75.6%] vs n = 30 [47.6%]; P = .008) or ≥1 MSG clinical factor (n = 31 [75.6%] vs n = 8 [12.7%]; P < .001). Compared with non-MSG IMD patients, MSG IMD patients more frequently had HM (n = 16 [39.0%] vs n = 9 [14.3%]; P = .005) and had received cytotoxic chemotherapy (n = 11 [26.8%] vs n = 3 [4.8%]; P = .002) within the 60 days before IMD diagnosis.
Non-IMD patients were less likely than IMD patients to receive mold-active antifungal drugs (n = 14 [7.0%] vs n = 99 [95.2%]; P < .001); a higher percentage of non-MSG IMD patients were treated with mold-active antifungal therapy than MSG IMD patients (n = 63 [100.0%] vs n = 36 [87.8%]; P = .008). Compared with non-IMD patients, IMD patients had a higher rate of hospitalization (n = 91 [87.5%] vs n = 96 [48.0%]; P < .001), ICU admission (n = 45 [43.3%] vs n = 39 [19.5%]; P < .001), and 90-day all-cause mortality (n = 34 [32.7%] vs n = 16 [8.0%]; P < .001), but these outcomes did not differ significantly among IMD patients when compared by MSG case status.
Diagnostic Tests and International Classification of Diseases, 10th Revision, Codes
Among all IMD patients, 72 (69.2%) had ≥1 culture positive for mold growth, 39 (37.5%) had ≥1 positive serum or BAL GM test, and 29 (27.9%) had ≥1 histopathology specimen with fungal elements present (Table 2). For 7 (6.7%) IMD patients, histopathology was the only positive mold test among those used for case ascertainment.
Table 2.
Diagnostic Tests and ICD-10 Discharge Codes for Patients in an Active Surveillance System for Invasive Mold Disease—Georgia, 2017–2019a
| Diagnostic Test | Total (N = 304) | All IMD Cases vs Noncases | IMD Cases (MSG vs Non-MSG) | ||||
|---|---|---|---|---|---|---|---|
| Cases (n = 104) | Noncases (n = 200) | P Value | MSG Cases (n = 41) | Non-MSG Cases (n = 63) | P Value | ||
| Mold diagnostic tests used for case ascertainment | |||||||
| ≥1 culture positive for mold growth | 234 (77.0) | 72 (69.2) | 162 (81.0) | .031 | 23 (56.1) | 49 (77.8) | .029 |
| ≥1 positive serum or BAL GM test | 64 (21.1) | 39 (37.5) | 25 (12.5) | <.001 | 14 (34.1) | 25 (39.7) | .679 |
| ≥1 histopathology specimen with presence of fungal elements | 49 (16.1) | 29 (27.9) | 20 (10.0) | <.001 | 20 (48.8) | 9 (14.3) | <.001 |
| Histopathology was the only type of specimen used for case ascertainment that was positive | 22 (7.2) | 7 (6.7) | 15 (7.5) | >.999 | 6 (14.6) | 1 (1.6) | .014 |
| Culture was the only type of specimen used for case ascertainment that was positive | 197 (64.8) | 41 (39.4) | 156 (78.0) | <.001 | 9 (22.0) | 32 (50.8) | .004 |
| Galactomannan was the only type of specimen used for case ascertainment that was positive | 46 (15.1) | 23 (22.1) | 23 (11.5) | .018 | 10 (24.4) | 13 (20.6) | .809 |
| ICD-10 codes | |||||||
| ≥1 fungal ICD-10 discharge diagnosis code | 78 (25.7) | 63 (60.6) | 15 (7.5) | <.001 | 31 (75.6) | 32 (50.8) | .014 |
| B44.1 Other pulmonary aspergillosis | 21 (6.9) | 19 (18.3) | 2 (1.0) | 6 (14.6) | 13 (20.6) | ||
| B44.9 Aspergillosis, unspecified | 15 (4.9) | 10 (9.6) | 5 (2.5) | 1 (2.4) | 9 (14.3) | ||
| B48.8 Other specified mycoses | 14 (4.6) | 13 (12.5) | 1 (0.5) | 7 (17.1) | 6 (9.5) | ||
| B44.81 Allergic bronchopulmonary aspergillosis | 5 (1.6) | 0 (0.0) | 5 (2.5) | 0 (0.0) | 0 (0.0) | ||
| B44.89 Other forms of aspergillosis | 5 (1.6) | 4 (3.8) | 1 (0.5) | 4 (9.8) | 0 (0.0) | ||
| B44.0 Invasive pulmonary aspergillosis | 2 (0.7) | 2 (1.9) | 0 (0.0) | 2 (4.9) | 0 (0.0) | ||
| B43.0 Cutaneous chromomycosis | 1 (0.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | ||
| B44.7 Disseminated aspergillosis | 1 (0.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | ||
| B44.8 Other forms of aspergillosis | 1 (0.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | ||
| B46.0 Pulmonary mucormycosis | 1 (0.3) | 1 (1.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | ||
| B46.3 Cutaneous mucormycosis | 1 (0.3) | 1 (1.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | ||
| B49 Unspecified mycosis | 6 (2.0) | 5 (4.8) | 1 (0.5) | 4 (9.8) | 1 (1.6) | ||
| ≥2 ICD-10 codes | 5 (1.6) | 5 (4.8) | 0 (0.0) | 5 (12.2) | 0 (0.0) | ||
| No ICD-10 codes | 226 (74.3) | 41 (39.4) | 185 (92.5) | 10 (24.4) | 31 (49.2) | ||
Abbreviations: BAL, bronchoalveolar lavage; GM, Aspergillus galactomannan antigen; ICD-10, International Classification of Diseases, 10th Revision; IMD, invasive mold disease.
Data are presented as No. (%).
International Classification of Diseases, 10th Revision (ICD-10), discharge codes suggesting mold infection were more frequently documented for IMD patients than non-IMD patients (n = 63 [60.6%] vs n = 15 [7.5%]; P < .001). MSG IMD patients more frequently had fungal ICD-10 discharge codes than did non-MSG IMD patients (n = 31 [75.6%] vs n = 32 [50.8%]; P = .014). No patients in the surveillance system had received polymerase chain reaction (PCR) testing for mold; 3 IMD patients who died had an autopsy, 2 of whom demonstrated histopathologic evidence of IMD.
Molds Identified
Among IMD patients with a single mold genus identified, Aspergillus spp. (n = 66 [63.5%]) were most common; 33 patients were infected with Aspergillus fumigatus, 6 with non-fumigatus Aspergillus spp. and 27 with an unspecified Aspergillus species (Table 3). The Aspergillus species were unspecified because Aspergillus GM was the only positive test for mold (n = 23) or because the isolate was not identified beyond the genus level (n = 4). Molds identified less frequently among IMD patients included Mucorales (eg, Rhizopus, Mucor; n = 8 [7.7%]), Fusarium spp. (n = 4 [3.8%]), Paecilomyces spp. (n = 3 [2.9%]), and Scedosporium spp. (n = 2 [1.9%]); 3 (2.9%) IMD patients had >1 mold genus. Most pulmonary (n = 51 [77.3%]) and otorhinolaryngologic IMDs (n = 9 [52.9%]) involved Aspergillus spp., and other affected body sites involved a variety of mold genera (Supplementary Table 1). During the analytic period, the CDC confirmed the species identification for 120 isolates. All A. fumigatus isolates tested by the CDC (n = 43) were susceptible to itraconazole and voriconazole.
Table 3.
Mold Species or Genera Identified Among Patients in an Active Surveillance System for Invasive Mold Disease—Georgia, 2017–2019 a
| Mold Identified | Total (N = 304) | Case (n = 104) | Not a Case (n = 200) |
|---|---|---|---|
| Aspergillus spp. | 180 (59.2) | 66 (63.5) | 114 (57.0) |
| Aspergillus fumigatus | 82 (27.0) | 33 (31.7) | 49 (24.5) |
| Aspergillus spp. (non-fumigatus)b | 34 (11.2) | 6 (5.8) | 28 (14.0) |
| Aspergillus (species not specified)c | 64 (21.1) | 27 (26.0) | 37 (18.5) |
| Mucorales (eg, Rhizopus, Mucor) | 12 (3.9) | 8 (7.7) | 4 (2.0) |
| Fusarium spp. | 12 (3.9) | 4 (3.8) | 8 (4.0) |
| Paecilomyces spp. | 10 (3.3) | 3 (2.9) | 7 (3.5) |
| Penicillium spp. | 10 (3.3) | 0 (0.0) | 10 (5.0) |
| Scedosporium spp. | 9 (3.0) | 2 (1.9) | 7 (3.5) |
| Exophiala spp. | 8 (2.6) | 1 (1.0) | 7 (3.5) |
| Curvularia spp. | 7 (2.3) | 2 (1.9) | 5 (2.5) |
| Cladosporium spp. | 6 (2.0) | 0 (0.0) | 6 (0.0) |
| Exserohilum spp. | 4 (1.3) | 0 (0.0) | 4 (2.0) |
| Alternaria spp. | 3 (1.0) | 1 (1.0) | 2 (1.0) |
| Verruconis spp. | 3 (1.0) | 2 (1.9) | 1 (0.5) |
| Acremonium spp. | 2 (0.7) | 1 (1.0) | 1 (0.5) |
| >1 genusd | 9 (3.0) | 3 (2.9) | 6 (3.0) |
| Other mold spp.e | 8 (2.6) | 3 (2.9) | 5 (3.0) |
| Not available | 21 (6.9) | 8 (7.7) | 13 (6.5) |
Abbreviation: IMD, invasive mold disease.
Data are presented as No. (%).
Among IMD patients, non-fumigatus Aspergillus spp. included A. niger (n = 4), A. terreus (n = 1), and A. sydowii (n = 1).
Among these IMD patients, the species of Aspergillus was unavailable because the only positive mold specimen was an Aspergillus galactomannan antigen test (n = 23) or because the species result from the specimen was not available (n = 4).
Among IMD patients involving >1 genus of mold, combinations included Fusarium spp. and Penicillium spp.; Aspergillus fumigatus and Exserohilum rostratum; and Aspergillus fumigatus, Fusarium spp., and Scedosporium prolificans.
Among IMD patients, other molds identified included 1 each of Achrophialophora levis, Cladophialophora spp., and Scopulariopsis spp.
DISCUSSION
We report findings from a pilot IMD surveillance program designed to detect clinically significant IMD cases in 3 Atlanta-area hospitals during 2017–2019. Among patients with IMD, most (60.6%) did not meet the MSG case definition. Although MSG case classifications are an important tool for identifying IMD patients for clinical research, strict application of MSG criteria for surveillance purposes might exclude over one-half of clinically significant IMD cases. Only 58.7% of IMD patients (meeting either the MSG or surveillance case definition) had an MSG host factor, suggesting that classic risk factors might frequently be absent in this patient population. Rates of hospitalization (87.5%), ICU admission (43.3%), and all-cause 90-day mortality (32.7%) were similar among IMD patients, regardless of whether MSG case criteria were met. This finding suggests that our non-MSG case definition captured clinically significant IMD cases and that ongoing public health surveillance, employing a sensitive case definition, can further our understanding of IMD epidemiology.
Similar to data collected from SOT and HSCT centers during 2001–2006, approximately two-thirds of IMD cases in our investigation involved Aspergillus molds, of which the most commonly identified species was Aspergillus fumigatus [3, 7]. Analysis of administrative data sets from 2000–2013 found that the proportion of IMD caused by non-Aspergillus species has been increasing among highly immunosuppressed patients [17], and Aspergillus infections involving non-fumigatus species are increasingly reported [30, 31]. Monitoring these trends is important because non-fumigatus Aspergillus and non-Aspergillus molds have variable intrinsic antifungal drug resistance, and affected patients might therefore require different treatments [32–34]. The distribution of mold species causing IMD might vary based on patient characteristics, selective pressure exerted by antifungal prophylaxis practices, and geoclimatic factors [34, 35]. Comprehensive IMD surveillance is needed to identify emerging trends and guide clinical decision-making.
Influenza was uncommon (<2%) among IMD patients in our study, potentially reflecting a low prevalence of influenza-associated IMD or low rates of testing for IMD among influenza patients, a possibility we could not assess in our surveillance system. Previous studies of influenza-associated pulmonary aspergillosis have found widely varying disease incidence among different countries (7%–28%) [36]. Data for this report were collected before the COVID-19 pandemic began, but COVID-19-associated IMDs are an increasing domestic and global concern because of their association with poor outcomes [13, 37, 38]. IMDs associated with respiratory viral infections will require ongoing systematic surveillance.
Our findings and experience from developing this pilot program might provide useful insights to guide continued IMD surveillance efforts. We reviewed 1698 histopathology reports, which was the most labor-intensive aspect of the surveillance system; however, over a 3-year period, this process identified only 7 clinically significant cases that would not otherwise have been captured by GM or mold culture results. Ascertaining cases through histopathology free-text screening, as we did for this surveillance system, might be prudent in certain situations, such as during a health care–associated IMD outbreak investigation where the goal of case review might involve capturing all IMD cases that occurred in a well-circumscribed time period and setting [21]. However, this approach might not be necessary for public health surveillance, where the goal is to efficiently detect and monitor population-level IMD trends. Although ICD-10 codes might provide a simple mechanism to identify patients with IMD and have been useful when analyzing trends in large, administrative data sets [17], our data suggest that the sole use of ICD-10 codes for case ascertainment would have missed ∼25% of patients with MSG IMD cases and ∼50% of patients with non-MSG IMD cases. Review of mold-active antifungal drug treatment might also provide a useful tool for case ascertainment, but this approach may miss cases in patients who died before IMD was suspected and treated. No patients in the surveillance system had received PCR testing for mold, but this technology is increasingly being used for diagnosis [39], and future surveillance and epidemiology studies should consider newer diagnostic methods for case ascertainment.
Our findings have several notable limitations. This pilot surveillance program included patients residing in select Georgia counties and receiving care at 3 hospitals; therefore, we could not accurately calculate population- or facility-level IMD incidence. Further, our findings might not be generalizable to other parts of the country because of potential differences in the prevalence of underlying population risk factors and environmental differences that might affect local patient exposures to mold [35]. Another limitation is that we might have misclassified the involved mold type for a small proportion of IMD patients by presuming that patients whose only positive test was Aspergillus GM had aspergillosis, as invasive infections with other fungi (eg, Fusarium, Penicillium, Cryptococcus) may cause a positive GM test [40]. The final limitation to our findings relates to the sensitivity of case ascertainment and the specificity of case classifications. Our surveillance system likely misses clinically significant IMDs because of the low sensitivity of the tests used to diagnose these infections [20], potential gaps in clinician testing practices, and low frequency of autopsies [41]. Also, because our non-MSG case definition was intended to be sensitive in capturing clinically significant IMDs, some instances of colonization or contamination could have been misclassified as cases; however, we suspect that most non-MSG cases likely represented clinically significant IMD episodes, given that clinical suspicion from treating clinicians was strong enough to initiate IMD treatment and that these patients had comparable mortality to patients with MSG cases.
Despite its limitations, our pilot surveillance system demonstrated a feasible approach to public health IMD surveillance that will be further improved through future expansion and lessons learned. Our findings underscore the importance of considering IMD in patients without classic risk factors and highlight the possibility of robust IMD surveillance in the United States. To improve the generalizability of our surveillance system and to produce meaningful IMD incidence calculations, we eliminated county residency requirements beginning in 2020 and plan to expand the surveillance system to incorporate additional sites within Atlanta and other US states. This expanded system will be poised to assess regional differences in IMD incidence, identify emerging at-risk populations, evaluate treatment practices, and monitor trends over time.
Supplementary Material
Acknowledgments
The authors acknowledge Leota Amsterdam, Joy Gary, Jeannette Guarner, Ngoc Le, Zeyu Li, Meghan Light, Mitsuru Toda, Wun-Ju Shieh, and Samantha Williams.
Disclaimer. The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the US Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC-RFA-CK17-1701).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Ethical approval. This activity was reviewed by the CDC and was conducted in accordance with applicable federal law and CDC policy (see, eg, 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
Contributor Information
Jeremy A W Gold, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Andrew Revis, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA.
Stepy Thomas, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA; Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Lewis Perry, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA.
Rebekah A Blakney, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA.
Taylor Chambers, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA.
Meghan L Bentz, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Elizabeth L Berkow, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Shawn R Lockhart, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Colleen Lysen, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Natalie S Nunnally, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Alexander Jordan, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Hilary C Kelly, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Alejandro J Montero, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Monica M Farley, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA; Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Nora T Oliver, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA; Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Stephanie M Pouch, Georgia Emerging Infections, Atlanta, Georgia, USA; Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Andrew S Webster, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA; Georgia Emerging Infections, Atlanta, Georgia, USA; Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Brendan R Jackson, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Karlyn D Beer, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41:634–53. [DOI] [PubMed] [Google Scholar]
- 2. Webb BJ, Ferraro JP, Rea S, Kaufusi S, Goodman BE, Spalding J. Epidemiology and clinical features of invasive fungal infection in a US health care network. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 2010; 50:1091–100. [DOI] [PubMed] [Google Scholar]
- 4. Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 2019; 68:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lass-Flörl C, Cuenca-Estrella M. Changes in the epidemiological landscape of invasive mould infections and disease. J Antimicrob Chemother 2017; 72(Suppl 1):i5–11. [DOI] [PubMed] [Google Scholar]
- 6. Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis 2011; 17:1855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 8. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol 2011; 49:S7–12. [DOI] [PubMed] [Google Scholar]
- 10. Mantadakis E, Samonis G. Clinical presentation of zygomycosis. Clin Microbiol Infect 2009; 15:15–20. [DOI] [PubMed] [Google Scholar]
- 11. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis 2012; 54:S16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, Garcia P. COVID-19-associated mold infection in critically ill patients, Chile. Emerg Infect Dis 2021; 27:1454–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel A, Agarwal R, Rudramurthy SM, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis 2021; 27:2349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 2020; 46:1524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toda M, Benedict K, Jackson BR. Invasive aspergillosis after influenza and other viral respiratory infections among intensive care unit patients in a commercially insured population in the United States, 2013–2018. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dulski TM, DeLong M, Garner K, et al. Notes from the field: COVID-19-associated mucormycosis - Arkansas, July-September 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vallabhaneni S, Benedict K, Derado G, Mody RK. Trends in hospitalizations related to invasive aspergillosis and mucormycosis in the United States, 2000–2013. Open Forum Infect Dis 2017; 4:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA 2016; 316:2547–8. [DOI] [PubMed] [Google Scholar]
- 19. Hong DK, Blauwkamp TA, Kertesz M, Bercovici S, Truong C, Banaei N. Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn Microbiol Infect Dis 2018; 92:210–3. [DOI] [PubMed] [Google Scholar]
- 20. Lamoth F, Calandra T. Early diagnosis of invasive mould infections and disease. J Antimicrob Chemother 2017; 72:i19–28. [DOI] [PubMed] [Google Scholar]
- 21. Hartnett KP, Jackson BR, Perkins KM, et al. A guide to investigating suspected outbreaks of mucormycosis in healthcare. J Fungi (Basel) 2019; 5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park BJ, Chiller TM, Brandt ME, Warnock DW. Epidemiology of systemic fungal diseases: an overview. In: Kauffman CA, ed. Essentials of Clinical Mycology. Vol. 13. Springer; 2011:553. [Google Scholar]
- 23. Bassetti M, Azoulay E, Kullberg BJ, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin Infect Dis 2021; 72:S121– 7. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention . About EIP. Available at: https://www.cdc.gov/ncezid/dpei/eip/eip-about.html. Accessed 16 June 2021.
- 25. Pihet M, Carrere J, Cimon B, et al. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med Mycol 2009; 47:387–97. [DOI] [PubMed] [Google Scholar]
- 26. Lockhart SR, Pham CD, Gade L, et al. Preliminary laboratory report of fungal infections associated with contaminated methylprednisolone injections. J Clin Microbiol 2013; 51:2654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lysen C, Silva-Flannery L, Zaki SR, Gary JM, Lockhart SR. Performance evaluation of fungal DNA PCR amplification from formalin-fixed paraffin-embedded tissue for diagnosis: experience of a tertiary reference laboratory. Mycoses 2021; 64:603–11. [DOI] [PubMed] [Google Scholar]
- 28. Berkow EL, Nunnally NS, Bandea A, Kuykendall R, Beer K, Lockhart SR. Detection of TR(34)/L98H CYP51A mutation through passive surveillance for azole-resistant Aspergillus fumigatus in the United States from 2015 to 2017. Antimicrob Agents chemother 2018; 62:e02240-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beer KD, Farnon EC, Jain S, et al. Multidrug-resistant Aspergillus fumigatus carrying mutations linked to environmental fungicide exposure - three states, 2010–2017. MMWR Morb Mortal Wkly Rep 2018; 67:1064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steinbach WJ, Benjamin DKJR, Kontoyiannis DP, et al. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis 2004; 39:192–8. [DOI] [PubMed] [Google Scholar]
- 31. Krishnan S, Manavathu EK, Chandrasekar PH. Aspergillus flavus: an emerging non-fumigatus Aspergillus species of significance. Mycoses 2009; 52:206–22. [DOI] [PubMed] [Google Scholar]
- 32. Baddley JW, Marr KA, Andes DR, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J Clin Microbiol 2009; 47:3271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamoth F, Kontoyiannis DP. Therapeutic challenges of non-Aspergillus invasive mold infections in immunosuppressed patients. Antimicrob Agents Chemother 2019; 63:e01244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoenigl M, Salmanton-García J, Walsh TJ, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 2021; 21:e246– 57. [DOI] [PubMed] [Google Scholar]
- 35. Panackal AA, Li H, Kontoyiannis DP, et al. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin Infect Dis 2010; 50:1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz IS, Friedman DZP, Zapernick L, et al. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin Infect Dis 2020; 71:1760–3. [DOI] [PubMed] [Google Scholar]
- 37. Janssen NAF, Nyga R, Vanderbeke L, et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis. Emerg Infect Dis 2021; 27:2892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gangneux JP, Dannaoui E, Fekkar A, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med 2022; 10:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barnes RA, White PL, Morton CO, et al. Diagnosis of aspergillosis by PCR: clinical considerations and technical tips. Med Mycol 2018; 56:S60–72. [DOI] [PubMed] [Google Scholar]
- 40. Verweij PE, Mennink-Kersten MASH. Issues with galactomannan testing. Med Mycol 2006; 44:179–S83. [DOI] [PubMed] [Google Scholar]
- 41. Tejerina EE, Abril E, Padilla R, et al. Invasive aspergillosis in critically ill patients: an autopsy study. Mycoses 2019; 62:673–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.