Abstract
Shift work is associated with increased risk for vascular disease, including stroke- and cardiovascular-related mortality. However, evidence from these studies is inadequate to distinguish the effect of altered circadian rhythms in isolation from other risk factors for stroke associated with shift work (e.g., smoking, poor diet, lower socioeconomic status). Thus, the present study examined the diathetic effects of exposure to shifted LD cycles during early adulthood on circadian rhythmicity, inflammatory signaling and ischemic stroke pathology during middle age, when stroke risk is high and outcomes are more severe. Entrainment of circadian activity was stable in all animals maintained on a fixed light:dark 12:12 cycle but was severely disrupted during exposure to shifted LD cycles (12hr advance/5d). Following treatment, circadian entrainment in the shifted LD group was distinguished by increased daytime activity and decreased rhythm amplitude that persisted into middle-age. Circadian rhythm desynchronization in shifted LD males and females was accompanied by significant elevations in circulating levels of the inflammatory cytokine IL-17A and gut-derived inflammatory mediator lipopolysaccharide (LPS) during the post-treatment period. Middle-cerebral artery occlusion, 3 months after exposure to shifted LD cycles, resulted in greater post-stroke mortality in shifted LD females. In surviving subjects, sensorimotor performance, assessed 2- and 5-days post-stroke, was impaired in males of both treatment groups, whereas in females, recovery of function was observed in fixed but not shifted LD rats. Overall, these results indicate that early exposure to shifted LD cycles promotes an inflammatory phenotype that amplifies stroke impairments, specifically in females, later in life.
Keywords: Circadian rhythm dysregulation, Stroke pathology, Middle cerebral artery, Inflammation, IL-17A, Lipopolysaccharide
Highlights
-
•
Early exposure to shifted LD cycles alters circadian entrainment of the activity rhythm that persists into middle age.
-
•
In conjunction with circadian dysregulation, shift work-like schedules promote the induction of key inflammatory mediators.
-
•
In females, exposure to shift work-like schedules amplifies functional impairments caused by strokes arising later in life.
-
•
Circadian dysregulation during shift work is a hysteretic risk factor in the overall severity of ischemic strokes.
-
•
Shift work-related circadian dysregulation affects stroke outcomes independent of lifestyle vascular disease risk factors.
1. Introduction
The regulation and light-dark entrainment of circadian rhythms is mediated by the master pacemaker in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus and by peripheral clocks throughout the body. This hierarchical network of cell-autonomous clocks plays an important role in human health by coordinating local tissue- and cell-specific processes so as to occur at the “right time” relative to each other and to the external environment. Desynchronization of these circadian clocks and dysregulation of their output rhythms are known to occur in response to shift work, jet lag and workplace or social influences that commonly impose highly irregular schedules on our sleep-wake patterns, mealtimes and other body processes.
Using genetic mutations in core clock genes or shift work-like paradigms, basic research studies have implicated circadian rhythm dysregulation in various human health disorders, including vascular disease and related risk factors such as metabolic syndrome, obesity, diabetes and inflammation (Westgate et al., 2008; Scheer et al., 2009; Huang et al., 2011a, Huang et al., 2011b; Xu et al., 2014; Griffin et al., 2019). In Clock mutant mice, genetic dysregulation of the core clock mechanism produces alterations in circadian rhythmicity that are accompanied by notable risk factors for cardiovascular disease including obesity and metabolic syndrome (Marcheva et al., 2010; Huang et al., 2011a, Huang et al., 2011b). In a similar manner, chronic modulation of circadian rhythms through periodic reversal of the light-dark cycle to simulate shift work schedules expedites the development of coronary pathology and congestive heart failure in cardiomyopathic hamsters (Penev et al., 1998). Importantly, epidemiological studies have provided supportive evidence implicating circadian rhythm dysregulation, due to shift work, as a risk factor for both cardio-and cerebrovascular disease (Rosa et al., 2019). Meta-analysis of cardiovascular disease in shift workers, including subjects in the Nurses Health Study and several Danish cohort studies, indicates that cardiovascular-related mortality and morbidity risk were at least 20% higher in shift workers than those with no shift work experience, with a further increase in risk of 7.1% for every additional five years of exposure (Torquati et al., 2018). The susceptibility of the vascular system to circadian rhythm disruption was corroborated by a Swedish study showing that stroke- and cardiovascular-related mortality, but not general mortality due to all causes, was significantly increased among male shift workers as compared to day workers (Karlsson et al., 2005). Furthermore, nurses performing shift work for at least 5 years were distinguished by significant increases in risk of ischemic stroke relative to non-shift workers (Brown et al., 2009).
Because shift work is associated with more prevalent risk factors for vascular disease, such as smoking, poor diet and lower socioeconomic status, these epidemiological studies provide limited opportunity to resolve the extent to which circadian rhythm dysregulation by shift work schedules differentially contributes to cardiovascular and stroke pathology. Recent studies with animal models have provided an important complement to epidemiological observations by using chronic shifts of the light-dark (LD) cycle to determine whether shift work-like modulation of circadian rhythms alone amplifies the extent of stroke-induced brain injury and functional impairment. In this regard, our recent study indicates that circadian rhythm dysregulation in response to chronic shifts (12hr advance/5d) of the LD cycle increases the severity of stroke outcomes in 5–7 month old rats (Earnest et al., 2016). Similar to the key findings of the Swedish epidemiological study, exposure to these shifted LD cycles amplified sex differences in stroke impairments such that circadian rhythm dysregulation was accompanied by high rates of post-stroke mortality in males, but not females. Relative to age demographics for shift work and stroke risk, our study was congruent with the chronology of the exposure to the shifted LD paradigm (i.e., young adult) but not the age for increased stroke susceptibility. According to the 2004 Work Schedules and Work at Home survey, the proportion of shift workers in full- and part-time jobs was the highest in individuals between 16 and 24 years of age and the lowest in people 55 and older. In comparison, stroke risk has the opposite relation with age; ischemic strokes are most likely to occur in middle age whereas stroke risk is low in young individuals (Kelly-Hayes, 2010). Therefore, the present experiments coupled the shifted LD paradigm with subsequent exposure to the standard fixed LD cycle to: 1) first examine the effects shifted LD cycles on circulating levels of the gut-derived inflammatory mediator lipopolysaccharide (LPS) and the inflammatory cytokine IL-17A; and 2) then determine the “after” or diathetic effects of these shift work-like schedules during early adulthood on the pathophysiology of ischemic strokes occurring later at middle age, when stroke risk is high and outcomes are more severe.
2. Material and methods
2.1. Animals
Adult (5-month old) male (n = 28) and female (n = 36) Sprague Dawley rats were purchased from Harlan Laboratories. All animals were housed individually in cages equipped with running wheels to provide for continuous analysis of wheel-running activity.
Experiments used a chronic light-dark (LD) cycle shift paradigm that has been shown to be effective in desynchronizing circadian rhythms and in inducing pathological changes in cardiovascular physiology (Penev et al., 1998). After baseline acclimation under standard LD 12:12 conditions (lights-on at 0600hr) for about 2 weeks, animals (5-months old) were randomly divided into 2 groups (n = 13–18/treatment group) and exposed for 80 days to either this “fixed” LD 12:12 cycle or to a “shifted” LD 12:12 cycle (see Fig. 1). During exposure to the shifted LD paradigm, lights-on was advanced by 12hr every 5 days and these shifts in the LD cycle were repeated for 8 full cycles. At the conclusion of experimental LD cycle manipulations (“treatment period”), animals (8-months old) in both groups were exposed to the same standard LD 12:12 schedule (lights-on at 0600hr) for 3 additional months (“post-treatment period”) and then subjected to experimental ischemic stroke surgery (11-months old) at the same relative time during the circadian cycle (i.e., inactive phase; ≈ ZT 2–8). Saphenous blood was collected from all animals: 1) immediately before experimental LD cycle manipulations, and 2) following the conclusion of the experimental LD treatment interval (i.e., when both the fixed and shifted LD groups are on the same LD 12:12 schedule). Sensorimotor testing was performed 1d before as well as 2d and 5d after MCAo surgery at Zeitgeber Time (ZT) 5 (i.e., 5hr after lights-on) to assess functional deficits. At 5d post-MCAo, brains were processed for histological analysis of infarct volume. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal procedures used in this study were conducted in compliance with Animal Use Protocol # 2020–0044 as reviewed and approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Fig. 1.
Experimental Design. At 5-months of age, male and female rats were exposed for ≈3 months to fixed or shifted (12hr advance/5d) LD 12:12 cycles. Following this treatment period, animals (8-months old) in both groups were exposed to the same standard LD 12:12 cycle for 3 additional months and then subjected to experimental ischemic stroke surgery (at ≈11 months of age). Blood was collected from all animals: immediately before and after experimental LD cycle manipulations as indicated by the red arrows. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Analysis of wheel-running activity
Wheel-running activity was continuously recorded, stored in 10-min bins, graphically depicted in actograms, and analyzed using ClockLab data collection and analysis software (ActiMetrics). Entrainment and qualitative parameters of the activity rhythm were measured over the same interval for all animals. The onset of activity for a given cycle was identified as the first bin during which an animal attained 10% of peak running-wheel revolutions (i.e., intensity). In addition, Chi-square periodogram analysis was used to determine the amplitude of the rhythm in wheel-running activity.
2.3. Middle cerebral artery occlusion
Animals were subjected to stereotaxic surgery to occlude the left middle cerebral artery using endothelin-1 (ET-1) as described previously (Selvamani and Sohrabji, 2010; 2010b). Briefly, animals were anesthetized (ketamine/xylazine) and placed in a stereotaxic apparatus (Kopf). A midline incision was made on the scalp and a craniotomy was performed on the left side with a small drill using the following coordinates (relative to Bregma): +0.9 mm anteroposterior, +3.4 mm mediolateral, −8.5 mm dorsoventral. Endothelin (ET)-1 (American Peptide Company Inc, CA; 2 μl of 0.5 μg/μl) was injected at a rate of 0.5 μl per 30 s onto the middle cerebral artery. To minimize backflow, the syringe was maintained in place for 4 min following ET-1 administration. Rats were maintained on heating pads during the procedure and then placed under heating lamps during recovery. Post-stroke survival was carefully recorded at 24h intervals. All surviving animals were sacrificed at day 5 post-MCAo. At termination, the brain was rapidly removed and processed for TTC (Triphenyl Tetrazolium Chloride) staining to assess infarct volume.
2.4. Infarct volume
Infarct volume measurements were performed on animals terminated on day 5 post-stroke using previously described procedures (Selvamani and Sohrabji, 2010). Briefly, brain sections (2 mm) between −2.00 mm and +4.00 mm from Bregma were incubated in a 2% TTC solution at 37 °C for 20 min and then photographed using a Nikon E950 digital camera attached to a dissecting microscope. Digitized images were coded and analyzed by an investigator blind to the code. Infarct volume was determined using the Quantity One software package (Bio-Rad CA) and expressed as a percentage of the volume of the contralateral (non-occluded) hemisphere.
2.5. Behavioral assays
Motor impairment following MCAo was assessed with the vibrissae-evoked forelimb placement task as well as the adhesive tape test, using procedures reported by Selvamani and Sohrabji (2010a) and Balden et al. (2012). The vibrissae-elicited forelimb placement test was used both before and after MCAo surgery. Animals were subjected to same-side and cross-midline placing trials elicited by stimulating the ipsi- and contra-lesional vibrissae. During the same-side forelimb placing trials, the animal was gently held such that all four limbs were free to move. The animal's ipsi-lesional vibrissae were brushed against the edge of a table to elicit a forelimb placing response, which typically involved the forelimb ipsi-lateral to the stimulated vibrissae. Ten trials were performed before the same task was repeated for the contra-lesional vibrissae. In the cross-midline placing trials, the animal was held gently by the upper body such that the ipsi-lesional vibrissae lie perpendicular to the tabletop and the forelimb on that side is gently restrained as the vibrissae was brushed on the top of the table to evoke a response from the contralateral limb and vice versa. Between each trial, the animal was allowed to rest all four limbs briefly on the tabletop to help relax its muscles. Trials in which the animal seemed to struggle or make premature forelimb movements were not counted. Scoring during the trials was done by an experimenter blind to the animal's treatment group and was based on a 4-point scale (0–3), with a score of 3 representing brisk forward and upward movement that ended in the paw pads making a flat, full contact with the tabletop.
The adhesive tape test, which is commonly used in MCAo stroke models as a more sensitive indicator of sensorimotor deficits (Zhang et al., 2000), was also performed both before and after surgery. Two pieces of adhesive-backed foam tape (1 x ½”) were used as a tactile stimulus attached to the palmar surface of the paw of each forelimb. For each forelimb, the latency time to remove each stimulus (tape) from the forelimbs was recorded during three trials per day for each forepaw. Animals were allowed to rest for 1 min between sessions, and each test session had a maximum time limit of 120 s.
2.6. ELISA assays
Circulating levels of the gut-derived inflammatory mediator LPS and the proinflammatory cytokine IL-17A were determined using ELISA assays. Serum LPS levels were quantitatively measured using a double-sandwich ELISA method (MyBiosource, USA) and colorimetric detection system. Serum and standard samples (100μl/well) were assayed in duplicate. Assay sensitivity ranged from 15.6 to 1000 ng/ml. Plates were read at 450 nm in a plate reader (BioTek), and sample measurements were interpolated from the standard curve.
As described previously (Bake et al., 2014), serum levels of the cytokine IL-17A were measured in blood samples from fixed and shifted LD animals using a multiplexed magnetic bead immunoassay (Milipore Corp. MA) and a Bio-Plex suspension array system (Bio-Rad Laboratories,CA) following manufacturer's protocols. Cytokine levels were normalized to total protein content.
2.7. Statistical analysis
Quantitative measurements of daily wheel-running activity were analyzed by two-way ANOVA separately for each sex to evaluate LD treatment (fixed, shifted) and phase (treatment, post-treatment period) differences. A simple linear regression model was used to analyze in body weight gain during the LD treatment phase and group differences were assessed by comparing the slope. Kaplan-Meier survival plots were used to assess post-stroke mortality, and group differences were assessed by Log Rank Chi square test. Infarct volume and sensorimotor behavior were assessed further in all surviving subjects. For behavioral tests, a two-way ANOVA coded for repeated measure was used for each group, comparing values obtained pre- and post-stroke. For infarct volume analysis, an unpaired t-test was performed. In each case, group differences were considered significant at p < 0.05.
3. Results
3.1. Effect of shifted LD cycles on circadian rhythm of wheel-running activity
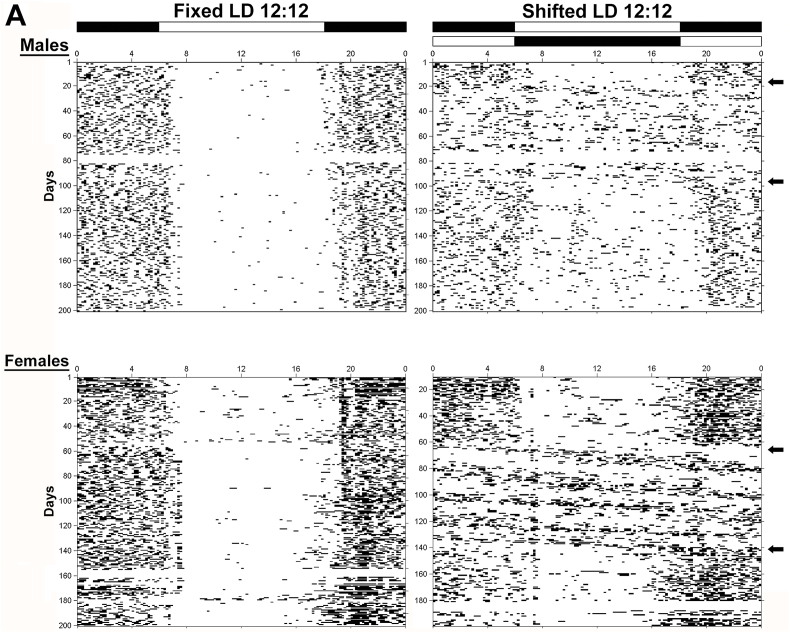
Throughout baseline exposure to the standard LD 12:12 cycle, all animals exhibited stable entrainment of the circadian rhythm of wheel-running activity. During exposure to experimental lighting conditions, the activity rhythms of all male and female rats in the fixed LD group were stably entrained to the LD 12:12 cycle (Fig. 2, left panel) such that their daily onsets of activity consistently occurred around 5–20 min after lights-off (1800hr). In comparison, male and female rats exposed to shifted LD paradigm (Fig. 2, right panel) were marked by desynchronized rhythms of wheel-running behavior in which the phase relationship between the onset of activity and lights-off varied greatly following each shift of the LD cycle. When exposed to the same LD 12:12 schedule (lights-on at 0600hr) during the post-treatment phase of the experiment, photoentrainment of the activity rhythm was maintained in fixed LD animals and was restored in the shifted LD group. The post-treatment pattern of entrainment remained virtually the same in fixed LD rats with most wheel-running activity occurring during the night but was altered in the shifted LD group such that activity was diffusely distributed throughout the day as well (Fig. 2).
Fig. 2.
Circadian patterns of wheel-running behavior in adult female and male rats exposed to fixed or shifted LD cycles. Representative records of wheel-running activity in adult male (top panel) and female (bottom panel) rats that were maintained in a fixed LD 12:12 cycle (left) or exposed to a shifted (12hr/5d) LD 12:12 cycle (right). Actograms are plotted over a 24-h period. The open and closed bars at the top respectively signify the timing of the light and dark phase in the fixed and shifted LD 12:12 cycles. Arrows on the right denote the interval when exposure to the shifted LD cycles was initiated (“treatment” phase) and when shifted LD animals were returned to the same regular LD 12:12 schedule as the fixed LD group (post-treatment phase).
Further differences in circadian behavior between LD treatment groups were established by separately analyzing the amplitude of the activity rhythm and total daily wheel-running activity during the LD “treatment” (fixed, shifted) phase (30-102d), and the subsequent “post-treatment” phase (120-192d) of the study when both groups were exposed to the same fixed LD cycle. In both shifted LD males and females, the amplitude of the activity rhythm during the treatment phase was significantly (p < 0.05) decreased in comparison with fixed LD animals of the same sex (Fig. 3A). During the post-treatment phase, the amplitude of the activity rhythm increased in both the fixed and shifted LD groups relative to treatment phase values. Despite these post-treatment increases in the amplitude of the activity rhythm, the effect of shifted LD cycles persisted into middle age such that rhythm amplitude in shifted LD males and females was significantly (p < 0.05) decreased by 22–35% in comparison with fixed LD animals of the same sex. In contrast to its effects on the entrainment and amplitude of the activity rhythm, exposure to shifted LD cycles had no significant effect on the total amount of daily wheel-running behavior during either the treatment or post-treatment phases relative to that observed in fixed LD controls over the same intervals (Fig. 3B). Similar to the sex differences reported previously in our and other published studies (Schull et al., 1989; Hagenauer et al., 2011; Earnest et al., 2016), daily activity levels (wheel revolutions/24hr) during and after LD cycle manipulations were 10-fold greater in female subjects than in their male counterparts within each treatment group. Consistent with the general patterns of circadian entrainment observed when both groups were maintained on same fixed LD cycle, further analysis during the post-treatment phase revealed that daytime activity in shifted LD males and females was significantly (p < 0.05) increased relative to fixed LD animals of the same sex (Fig. 3C).
Fig. 3.
Effects of experimental LD cycles on circadian entrainment and other properties of the rhythm in wheel-running activity. (A) Rhythm amplitude and (B) total daily wheel-running activity (wheel revolutions/24hr) of adult male (top) and female (bottom) rats: during LD “treatment” (fixed, shifted) phase (TX; day 30–102), and the subsequent post-treatment phase (Post-TX; day 120–192) when both groups were exposed to the same fixed LD cycle. (C) Daytime activity (light counts/day) in male (top) and female (bottom) rats following exposure to fixed or shifted LD cycles (Post-TX; day 120–192). Bars depict mean values (+SEM). Asterisks indicate significant differences (p < 0.05) between the fixed and shifted LD groups in the amplitude of the rhythm in wheel-running activity.
3.2. Effect of shifted LD cycles on body weight and inflammatory mediators
In both females and males, no significant differences were evident in the body weights of fixed and shifted LD rats at the onset of experimental lighting conditions (Fig. 4). During LD cycle manipulations, rats in both groups exhibited progressive increases in body weight. In males, there were no significant differences in body weight gain between the fixed and shifted LD groups over time, as determined by the slope in a simple regression model (fixed males: 2.37; shifted males: 3.58, p = 0.6). However, weight gain in females was significantly greater in fixed LD rats than in shifted LD controls (slope: fixed females: 4.09; shifted females: 1.98; p = 0.03).
Fig. 4.
Effects of experimental LD cycles on body weight. Graphs depict weekly determinations (mean ± SEM) of body weight in adult male (top) and female (bottom) rats during exposure to fixed ( ) or shifted (
) or shifted ( ) LD 12:12 cycles and immediately prior to ET-1-induced MCAo surgery. Simple linear regression analysis of LD treatment group differences indicates weight gain was significantly greater in fixed, than in shifted, LD females (*p = 0.03).
) LD 12:12 cycles and immediately prior to ET-1-induced MCAo surgery. Simple linear regression analysis of LD treatment group differences indicates weight gain was significantly greater in fixed, than in shifted, LD females (*p = 0.03).
Because circadian rhythm dysregulation promotes a persistent proinflammatory condition (Xu et al., 2014; Kim et al., 2018) and inflammation contributes to the extent of brain injury and functional deficits in ischemic stroke, we next examined the effects of the shifted LD paradigm on the cytokine IL-17A and endotoxin LPS, which act synergistically to amplify the proinflammatory signaling cascade and activate immune cells (Waisman et al., 2015; Kawanokuchi et al., 2008) and contribute to the severity of ischemic stroke outcomes (Park et al., 2020; El-Hakim et al., 2021). Serum levels of IL-17A (Fig. 5A) and LPS (Fig. 5B) were analyzed at baseline (pre-treatment) and immediately after exposure to experimental lighting conditions (post-treatment). In males, circulating levels of IL-17A were elevated in both fixed and shifted LD rats over time compared to their respective baseline values (F(1,16):8.919; p = 0.0087) (Fig. 5A). However, planned post-hoc comparisons revealed that post-treatment IL-17A levels were significantly elevated by 2.3-fold in the shifted group (p = 0.0447) relative to baseline values but were not significantly different from basal IL-17A levels in fixed LD rats (p = 0.8345). In females, a similar effect was observed in which IL-17A levels were elevated in both groups after exposure to experimental LD cycles (fixed or shifted) (F(1,16):29.81; p = 0.0001), and post-treatment levels of this proinflammatory cytokine were significantly increased by 2.8-fold over baseline in shifted LD group (p = 0.0007), but not in fixed LD females.
Fig. 5.
Shifted LD cycles alter circulating levels of the inflammatory cytokine IL-17A and lipopolysaccharide (LPS). Serum levels (pg/ml) of IL-17A (A) and LPS (B) in male (left) and female (right) rats exposed to fixed or shifted LD cycles. Histograms depict mean IL-17A and LPS levels (+SEM) in saphenous blood collected immediately before (PRE) and after (POST) experimental LD cycle manipulations. *, p < 0.05; **, p < 0.01.
With regard to circulating levels of endotoxin LPS, the patterns observed in response to experimental lighting conditions were similar to that of IL-17A (Fig. 5B). Consistent with previous evidence for sex differences in serum levels of these inflammatory mediators (El-Hakim et al., 2021), both IL-17A and LPS were considerably higher in males, irrespective of treatment (fixed, shifted) and timing (pre-vs post-treatment). In males, post-treatment LPS levels were elevated over time in both treatment groups (F(1,36): 11.08; p = 0.0020). Furthermore, planned post-hoc comparisons indicated that after experimental LD treatments LPS was significantly elevated in the shifted LD group relative to its baseline (p = 0.0044) but showed no significant differences in the fixed group when compared to basal levels (p = 0.6601). In females, a significant interaction effect was observed (F(1,22):17.55; p = 0.0004) between the pre- and post-treatment values, and planned comparisons indicate that LPS was significantly elevated in the shifted LD group (p = 0.0044), but not in the fixed group (p = 0.2595). Collectively, these data suggest that shifted LD cycles may promote a basal inflammatory state, which is a notable risk factor for cardiovascular disease.
3.3. Effect of shifted LD cycles on ischemic stroke outcomes
The effects of circadian rhythm dysregulation were manifested in stroke-induced pathological outcomes that, in turn, were marked by sex differences. Kaplan-Meier survival plots indicate that while post-stroke mortality and survival time were similar in fixed LD males and females, the rate of mortality was significantly higher in females as compared to males in the shifted LD group (Fig. 6A). Among male subjects, the incidence of mortality in response to MCAo-induced stroke was lower, ranging from 12.5% in the fixed LD rats to 8.3% in the shifted LD group with all mortalities collectively occurring on day 1 after surgery in both treatment groups. In comparison with males, post-stroke mortality was much higher among females, with the greatest incidence (46.7%) in the shifted LD group.
Fig. 6.
Effects of experimental LD cycles on MCAo-induced mortality and infarct size. (A) Kaplan-Meier survival plots following MCAo-induced stroke in adult (female left panel) and male (right panel) rats exposed to fixed or shifted LD 12:12 cycles. The individual plots depict the conditional probability of survival over post-surgery days 0–5. Asterisk indicates the rate of mortality was significantly (p < 0.05) increased in females relative to that found in males in the shifted LD group. (B) Representative TTC-stained sections (top) illustrating MCAo-induced cortical and striatal infarcts (pale, unstained) in the left hemisphere of adult male (left) and female (right) rats exposed to fixed (n = 18) or shifted (n = 18) LD 12:12 cycles. The approximate borders of infarcted tissue are indicated by the dotted black outline on each section. Bar graphs (top) depict infarct volumes (cortex and striatum) normalized to the contralateral hemisphere.
Subsequent analysis of the extent of infarct volume and functional impairment in surviving subjects revealed sex differences in the impact of circadian rhythm dysregulation on ischemic stroke outcomes. Based on quantitative analysis of TTC staining in brain sections, cortical and striatal infarction was observed in all surviving animals in both LD treatment groups, ranging in volume from 25 to 30% when normalized to the contralateral, non-occluded hemisphere. At 5 days after ET-1-induced MCAo, exposure to shifted LD cycles had no significant effect on stroke volume in females or males relative to that found in fixed LD controls (Fig. 6B).No significant differences in infarct volume were observed between male and female rats in either the fixed or shifted LD groups.
Functional deficits associated with MCAo-induced infarction were first assessed using the vibrissae-evoked paw placement task. Forelimb placement in response to vibrissae stimulation was completely accurate in all animals prior to stroke (Fig. 7A). Following MCAo, performance on this sensorimotor task was unchanged with the ipsi-lesional paw as expected (data not shown) in both males and females. On the contra-lesional paw, post-stroke functional deficits were evident such that forelimb placement at 2d after stroke was significantly impaired (p < 0.05) in all animals relative to pre-stroke performance in males (main effect of time (F(2,31):9.901; p = 0.0005) and females (F(2,32): 6.103 p = 0.0057), and the extent of the contra-lesional impairment on this task was comparable in both fixed LD and shifted LD groups. However, this impairment of sensorimotor function was temporary as males and females of both treatment groups showed no significant differences in contra-lesional forelimb placement at 5d after stroke relative to pre-stroke (-1d) values.
Fig. 7.
MCAo-induced impairment of sensorimotor performance in adult female and male rats exposed to fixed or shifted LD cycles. Sensorimotor performance was examined 1 day before (PRE) and at 2 days (2d) and 5 days (5d) after MCAo surgery using the (A) vibrissae evoked forelimb placement task (VIB) and (B) adhesive tape test (ART) in male (left) and female (right) rats exposed to fixed or shifted LD cycles. Histograms depict average values (+SEM) for percent correct responses for the ipsi-lesional (IPSI) and contra-lesional (CONTRA) paw before and after stroke (A) and for latency in seconds to remove tape from the forepaw (B). *, p < 0.05 (PRE vs 2d; PRE vs 5d).
Analysis of sensorimotor deficits using the adhesive tape test revealed treatment group differences in females but not males (Fig. 7B). In males, MCAo-induced functional impairment was clearly apparent in fixed and shifted LD animals, such that latency to tape removal was significantly longer at both 2d and 5d after stroke (main effect of stroke: F(2,31): 50.81; p = 0.0001). However, there were no significant differences in the extent of impairment between the fixed and shifted LD groups. Similarly in females, stroke significantly affected latency of tape removal (main effect of stroke: F(2,34): 55.55; p = 0.0001), but the extent of impairment after stroke differed between the fixed and shifted LD groups (interaction effect F(2,34): 4.181; p = 0.0238). In the fixed LD group, the latency for tape removal was elevated at 2d post-stroke but was not different from pre-stroke (-1d) values at 5d whereas in shifted LD rats, there was a significant increase in latency at both 2d and 5d after stroke.
4. Discussion
Epidemiological studies have implicated circadian rhythm dysregulation, due to shift work, as a risk factor for cardiovascular disease and stroke (Karlsson et al., 2005; Torquati et al., 2018; Rosa et al., 2019). However, evidence from these studies provides inadequate opportunity to uncouple the impact of altered circadian rhythms, per se, from other life-style variables associated with shift work that also pose significant risk for these vascular diseases. The advantage of this preclinical study is that the impact of circadian rhythm dysregulation by shift work-like paradigms alone can be precisely isolated. Here we report that chronic shifts of the LD cycle induce persistent alterations in circadian rhythmicity that are accompanied by elevated levels of the inflammatory mediators, Il-17A and LPS. Even after intervening entrainment to a stable LD cycle for 3 months, MCAo-induced functional impairments were exacerbated in animals in the shifted LD group.
Age demographics on shift workers and stroke risk are important considerations in further development of animal models to study the link between shift work-induced circadian rhythm dysregulation and ischemic stroke outcomes. In our previous study (Earnest et al., 2016), the pathological effects of MCAo-induced stroke immediately after exposure to shifted LD cycles were exacerbated in young adult rats (i.e., 5–7mo) at a human age equivalent of when the highest proportion of shift workers in full- and part-time jobs (i.e., 22.3–34.6% at 16–24 years of age) in the 2004 Work Schedules and Work at Home survey but when stroke risk is low. Importantly, the present findings indicate that the effects of early exposure to shifted LD cycles persist during aging and exacerbate functional impairments in response to ischemic strokes that occur later in life (i.e., middle age). Even after intervening exposure to a stable LD cycle, circadian desynchronization during early adulthood both increased the rate of post-stroke mortality and decreased recovery from stroke-induced sensorimotor deficits in shifted LD females. Thus, current evidence for the diathetic effects of exposure to shifted LD cycles during early adulthood on the pathological outcomes of ischemic strokes during middle age is compatible with epidemiological observations on the implications of shift work-related circadian dysregulation in cerebrovascular disease (Karlsson et al., 2005; Brown et al., 2009).
This study also offers insight into how the persistent or diathetic effects of circadian dysregulation following exposure to shifted LD cycles differ from its acute impact. Our previous work (Earnest et al., 2016), where stroke was induced in young adults immediately after experimental LD cycle treatments, resulted in extremely high rates of mortality (70%) in shifted LD males but not females. However, the present study, where stroke occurred in middle-aged animals after intervening exposure to a stable LD cycle for 3 months, yielded contrasting results in which male mortality was low in both treatment groups and not significantly different. Instead, there was a remarkable reversal of outcomes such that among shifted LD animals, mortality in females was significantly increased in comparison to males, suggesting that circadian dysregulation during early adulthood may affect aging-associated resiliency in females. Moreover, the collective results of these studies demonstrate how circadian dysregulation interacts with other nonmodifiable risk factors to differentially exacerbate stroke outcomes in males during early adulthood and in females at middle age.
Despite the lack of treatment effects on stroke volume among both male and female stroke survivors, it is noteworthy that exposure to shifted LD cycles during early adulthood still had an impact on MCAo-induced impairment of sensorimotor function, especially in females. This uncoupling between infarct volume and the extent of sensory motor impairment has been similarly noted in other studies, indicating that correlations between sensory motor performance and the extent of infarction are high in the early acute phase (i.e., 24h post MCAo) (Rogers et al., 1997; Wen et al., 2017) but are lost in the late acute phase (7 days post MCAo) (Hunter et al., 2000). Age, which significantly impacts brain infarction and behavioral deficits, is another variable where studies using aged rats indicate that these two outcomes may be poorly correlated (Turner et al., 2016). The observed increase in sensorimotor impairment in shifted LD females is consistent with our previous work indicating that the resilience of young females in response to MCAo-induced stroke is lost at middle-age (Selvamani and Sohrabji, 2010). Intriguingly, fixed LD females show recovery on the adhesive removal test at 5d post stroke whereas the shifted LD group does not, suggesting that even among survivors, there is stroke-associated impairment. A critical future direction for this research is to follow the long-term recovery in response to the effects of shift work schedules on stroke, especially in view of the evidence that stroke is a leading risk factor for depression and dementia (Liebetrau et al., 2008; Pinkston et al., 2009; Makin et al., 2013).
In summary, the present study underscores the idea that shift work, independent of other lifestyle conditions such as diet, should be considered a risk factor that contributes to the overall severity of ischemic strokes occurring later in life. The two measures selected for analysis, circulating levels of IL-17A and endotoxin LPS, are both associated with chronic disease including obesity, hypertension and CNS inflammation (Harley et al., 2014; Waisman et al., 2015). While not an exhaustive list, it is worth noting that these inflammatory mediators are significantly elevated in the immediate aftermath of exposure to shifted LD cycles, in both males and females. LPS can be derived orally through certain foods or through microbial sources, namely gram-negative bacteria, that colonize the respiratory, urinary, and GI tracts as well as the mouth Colpin et al. (1981)]. The gut also houses a number of T cells, many subtypes of which produce IL-17A (Dubin and Kolls, 2008; Ogura et al., 2008). Therefore, we propose that circadian dysregulation associated with the shifted LD cycles itself may alter gut function, either through the expansion of IL-17A-producing T cells, or increased permeability of the gut epithelium and in turn, elevated LPS secretion, cooperatively resulting in a basal proinflammatory state. In future studies, it will thus be necessary to explore the potential role of gut pathophysiology in the mechanisms by which shift work-induced circadian dysregulation affects stroke severity and interacts with other nonmodifiable risk factors to modulate ischemic stroke outcomes.
Funding
This study was supported by NIH R21-NS106210 (DE) and NIH R01-NS074895 (FS).
CRediT authorship contribution statement
David J. Earnest: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Shaina Burns: Investigation, Resources, Data curation. Sivani Pandey: Investigation, Resources, Data curation. Kathiresh Kumar Mani: Methodology, Investigation, Resources, Data curation, Writing – original draft. Farida Sohrabji: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of interest
There are no potential competing interests (financial/personal) in which the authors’ professional judgment or objectivity was influenced with regard to the validity of research.
References
- Bake S., Selvamani A., Cherry J., Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balden R., Selvamani A., Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153(5):2420–2435. doi: 10.1210/en.2011-1783. Epub 2012 Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.L., Feskanich D., Sánchez B.N., Rexrode K.M., Schernhammer E.S., Lisabeth L.D. Rotating night shift work and the risk of ischemic stroke. Am. J. Epidemiol. 2009;169(11):1370–1377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpin G.G., Guiot H.F., Simonis R.F., Zwaan F.E. Bacillus cereus meningitis in a patient under gnotobiotic care. Lancet. 1981;2(8248):694–695. doi: 10.1016/s0140-6736(81)91025-4. [DOI] [PubMed] [Google Scholar]
- Dubin P.J., Kolls J.K. Th17 cytokines and mucosal immunity. Immunol. Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- Earnest D.J., Neuendorff N., Coffman J., Selvamani A., Sohrabji F. Sex differences in the impact of shift work schedules on pathological outcomes in an animal model of ischemic stroke. Endocrinology. 2016;157(7):2836–2843. doi: 10.1210/en.2016-1130. Epub 2016 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hakim Y., Mani K.K., Eldouh A., Pandey S., Grimaldo M.T., Dabney A., Pilla R., Sohrabji F. Sex differences in stroke outcome correspond to rapid and severe changes in gut permeability in adult Sprague-Dawley rats. Biol. Sex Differ. 2021;12(1):14. doi: 10.1186/s13293-020-00352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P., Dimitry J.M., Sheehan P.W., Lananna B.V., Guo C., Robinette M.L., Hayes M.E., Cedeño M.R., Nadarajah C.J., Ezerskiy L.A., Colonna M., Zhang J., Bauer A.Q., Burris T.P., Musiek E.S. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc. Natl. Acad. Sci. U.S.A. 2019;116(11):5102–5107. doi: 10.1073/pnas.1812405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer M.H., King A.F., Possidente B., McGinnis M.Y., Lumia A.R., Peckham E.M., Lee T.M. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm. Behav. 2011;60(1):46–57. doi: 10.1016/j.yhbeh.2011.03.001. Epub 2011 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley I.T., Stankiewicz T.E., Giles D.A., Softic S., Flick L.M., Cappelletti M., Sheridan R., Xanthakos S.A., Steinbrecher K.A., Sartor R.B., Kohli R., Karp C.L., Divanovic S. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59(5):1830–1839. doi: 10.1002/hep.26746. Epub 2014 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ramsey K.M., Marcheva B., Bass J. Circadian rhythms, sleep, and metabolism. J. Clin. Invest. 2011;121(6):2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ramsey K.M., Marcheva B., Bass J. Circadian rhythms, sleep, and metabolism. J. Clin. Invest. 2011;121(6):2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A.J., Hatcher J., Virley D., Nelson P., Irving E., Hadingham S.J., Parsons A.A. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39(5):806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Karlsson B., Alfredsson L., Knutsson A., Andersson E., Torén K. Total mortality and cause-specific mortality of Swedish shift- and dayworkers in the pulp and paper industry in 1952-2001. Scand. J. Work. Environ. Health. 2005;31(1):30–35. doi: 10.5271/sjweh.845. [DOI] [PubMed] [Google Scholar]
- Kawanokuchi J., Shimizu K., Nitta A., Yamada K., Mizuno T., Takeuchia H., Suzumura A. Production and functions of IL-17 in microglia. J. Neuroimmunol. 2008;194(1–2):54–61. doi: 10.1016/j.jneuroim.2007.11.006. Epub 2007 Dec 31. [DOI] [PubMed] [Google Scholar]
- Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J. Am. Geriatr. Soc. 2010;58(Suppl. 2):S325–S328. doi: 10.1111/j.1532-5415.2010.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-M., Neuendorff N., Alaniz R.C., Sun Y., Chapkin R.S., Earnest D.J. Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high fat diet on inflammation and metabolism. Faseb. J. 2018;32(6):3085–3095. doi: 10.1096/fj.201700784R. Epub 2018 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetrau M., Steen B., Skoog I. Depression as a risk factor for the incidence of first-ever stroke in 85-year-olds. Stroke. 2008;39(7):1960–1965. doi: 10.1161/STROKEAHA.107.490797. Epub 2008 May 1. [DOI] [PubMed] [Google Scholar]
- Makin S.D.J., Turpin S., Dennis M.S., Wardlaw J.M. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J. Neurol. Neurosurg. Psychiatry. 2013;84(8):893–900. doi: 10.1136/jnnp-2012-303645. Epub 2013 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B., Ramsey K.M., Buhr E.D., Kobayashi Y., Su H., Ko C.H., Ivanova G., Omura C., Mo S., Vitaterna M.H., Lopez J.P., Philipson L.H., Bradfield C.A., Crosby S.D., JeBailey L., Wang X., Takahashi J.S., Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H., Murakami M., Okuyama Y., Tsuruoka M., Kitabayashi C., Kanamoto M., Nishihara M., Iwakura Y., Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–636. doi: 10.1016/j.immuni.2008.07.018. Epub 2008 Oct 9. [DOI] [PubMed] [Google Scholar]
- Park M.J., Pilla R., Panta A., Pandey S., Sarawichitr B., Suchodolski J., Sohrabji F. Reproductive senescence and ischemic stroke remodel the gut microbiome and modulate the effects of estrogen treatment in female rats. Transl. Stroke Res. 2020;11:812–830. doi: 10.1007/s12975-019-00760-5. [DOI] [PubMed] [Google Scholar]
- Penev P.D., Kolker D.E., Zee P.C., Turek F.W. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am. J. Physiol. 1998;275(6):H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- Pinkston J.B., Alekseeva N., González Toledo E. Stroke and dementia. Neurol. Res. 2009;31(8):824–831. doi: 10.1179/016164109X12445505689643. [DOI] [PubMed] [Google Scholar]
- Rogers D.C., Campbell C.A., Stretton J.L., Mackay K.B. Correlation between motor impairment and infarct volume After permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28(10):2060–2066. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Rosa D., Terzoni S., Dellafiore F., Destrebecq A. Systematic review of shift work and nurses' health. Occup. Med. (Lond.) 2019;69(4):237–243. doi: 10.1093/occmed/kqz063. [DOI] [PubMed] [Google Scholar]
- Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. Epub 2009 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schull J., Walker J., Fitzgerald K., Hiilivirta L., Ruckdeschel J., Schumacher D., Stanger D., McEachron D.L. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol. Behav. 1989;46(3):341–346. doi: 10.1016/0031-9384(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Selvamani A., Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol. Aging. 2010;31(9):1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014. Epub 2008 Sep. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A., Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J. Neurosci. 2010;30(20):6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torquati L., Mielke G.I., Brown W.J., Kolbe-Alexander T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand. J. Work. Environ. Health. 2018;44(3):229–238. doi: 10.5271/sjweh.3700. [DOI] [PubMed] [Google Scholar]
- Turner R.C., DiPasquale K., Logsdon A.F., Tan Z., Naser Z.J., Huber J.D., Rosen C.L., Lucke-Wold B.P. The role for infarct volume as a surrogate measure of functional outcome following ischemic stroke. J. Syst. Integr. Neurosci. 2016;2(4) doi: 10.15761/JSIN.1000136. 10.15761/JSIN.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman A., Hauptmann J., Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129:625–637. doi: 10.1007/s00401-015-1402-7. Epub 2015 Feb 26. [DOI] [PubMed] [Google Scholar]
- Wen Z., Xu X., Xu L., Yang L., Xu X., Zhu J., Wu L., Jiang Y., Liu X. Optimization of behavioural tests for the prediction of outcomes in mouse models of focal middle cerebral artery occlusion. Brain Res. 2017;1665:88–94. doi: 10.1016/j.brainres.2017.04.001. Epub 2017 Apr 20. [DOI] [PubMed] [Google Scholar]
- Westgate E.J., Cheng Y., Reilly D.F., Price T.S., Walisser J.A., Bradfield C.A., FitzGerald G.A. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. Epub 2008 Apr 14. [DOI] [PubMed] [Google Scholar]
- Xu H., Li H., Woo S.-L., Kim S.-M., Neuendorff N., Guo X., Guo T., Qi T., Ji J.-Y., Alaniz R.C., Earnest D.J., Wu C. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J. Biol. Chem. 2014;289:16374–16388. doi: 10.1074/jbc.M113.539601. Epub 2014 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Chen J., Li Y., Zhang Z.G., Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J. Neurol. Sci. 2000;174:141–146. doi: 10.1016/s0022-510x(00)00268-9. [DOI] [PubMed] [Google Scholar]