Abstract
CD44 exerts anti-senescence effects in many disease models. We examined senescence in tendinopathy and the effect of CD44 on senescence-associated secretory phenotypes (SASPs). Senescent markers were determined in human tendinopathic long head of bicep (LHB) and normal hamstring tendons. CD44 gene transfer in rat tendinopathic tenocytes stimulated with interleukin (IL)-1β and a rat Achilles tendinopathy model were performed using lentiviral vectors. Expression levels of p53, p21, and p16 and senescence-associated β-galactosidase (SA-β-gal) activity were positively correlated with the severity of human tendinopathy and were higher in rat and human tendinopathic tenocytes than in normal controls. CD44 overexpressed tenocyte transfectants exhibited reduced levels of IL-6, matrix metalloproteinases (MMPs), cyclooxygenase (COX)-2, p53, p21, p16, SA-β-gal, and phospho-nuclear factor (NF)-κB, whereas their collagen type I alpha 1 (COL1A1) and tenomodulin (tnmd) levels were increased when compared with control transfectants under IL-1β-stimulated conditions. In the animal model, CD44 overexpression lowered the ultrasound and histology scores and expression levels of the senescent and SASP markers COX-2 and phospho-NF-κB. Bromodeoxyuridine (BrdU)- and tnmd-positive cell numbers were increased in the LVCD44-transduced tendinopathic tendons. Senescence is positively correlated with tendinopathic severity, and CD44 overexpression may protect the tendinopathic tendons from SASPs via anti-inflammation and maintenance of extracellular matrix homeostasis.
Keywords: tendinopathy, CD44, lentiviral vector, gene therapy, senescence-associated secretory phenotypes
Graphical abstract

Senescence is positively correlated with tendinopathic severity. Lentiviral-vector-mediated CD44 gene therapy protects tendinopathic tendons from senescence and senescence-associated secretory phenotypes in concert with anti-inflammation and maintenance of extracellular matrix homeostasis. This study provides new insights into the development of pharmaceutical therapeutics targeting CD44 to prevent cell senescence in tendinopathy.
Introduction
The exact pathophysiology of tendinopathy remains unclear and has been attributed to multiple factors, including mechanical overuse,1 chronic inflammation,2 and aging.3 Cell senescence is an irreversible process in which cell-cycle arrest is attributed to the activation of p16INK4a or p53/p21cip stress-responsive signaling pathways.4 Senescent cells have particular characteristics, such as excess secretion of matrix-degrading enzymes and pro-inflammatory cytokines,5,6 designated senescence-associated secretory phenotypes (SASPs)4 and expression of lysosomal senescence-associated β-galactosidase (SA-β-gal).7 Interestingly, many of these SASP cytokines and enzymes, including interleukin (IL)-6 and matrix metalloproteinase (MMP)-1 and -3, are implicated in the pathogenesis of tendinopathy.8,9 IL-1β, one of the main initiators of tendinopathy, induces IL-6, IL-1β, cyclooxygenase (COX)-2, and MMP-1, -3, and -13 gene expression in human tendon cells and reduces the expression levels of tenomodulin (tnmd) and type 1 and 3 collagen in injured tendon-derived cells.10,11 Furthermore, IL-1β and dexamethasone, two factors that contribute to tendinopathy, have been shown to increase human tenocyte senescence, either alone or in combination.12 Therefore, our first hypothesis was that senescence is positively correlated with the progression of tendinopathy.
CD44 is a principal cell-surface receptor for hyaluronan (HA), which is the major component of almost all types of mammalian extracellular matrix (ECM).13 At present, the cellular and molecular mechanisms underlying CD44-related cell senescence in tendinopathy remain elusive. HA-mediated CD44 signaling pathways have been shown to promote keratinocyte proliferation and migration, cell-cell adhesion, and differentiation in aged epidermis.14 A cancer-related study further pointed out that HA triggers anti-senescence signals through CD44 in human leukemic cell lines.15 Our previous studies showed that CD44 mediated HA-induced downregulation of MMP-1 and -3 expression in IL-1β-stimulated tenocytes and that inhibition of the CD44 pathway using an antagonizing antibody induced pro-inflammatory cytokine and MMP expression in rat tendinopathic tenocytes.16,17 Therefore, our second hypothesis was that CD44 protects tenocytes from cell senescence under tendinopathic conditions.
Two specific aims were set to prove the hypotheses: (1) to investigate senescence in tendinopathy and (2) to examine the role of CD44 in SASPs in tendinopathy models. A lentivirus-based gene regulatory system for CD44 overexpression was used. Our results showed that senescence was positively correlated with the severity of tendinopathy, and CD44 overexpression could protect tendinopathic tendons from SASPs in concert with anti-inflammation and maintenance of ECM homeostasis.
Results
Cellular senescence in human tendons
Our previous study revealed that CD44 expression was positively correlated with the histological grade of tendinopathy.16 To examine the role of cellular senescence and create a correlation with CD44 during disease progression, we detected SA-β-gal activity and expression, as well as several senescent markers in normal hamstrings (5 men and 5 women; median age: 28 years; mean ± standard error of the mean [SEM]: 30.1 ± 3.2) and tendinopathic long head of bicep (LHB) tendons (3 men and 9 women; median age: 70 years; mean ± SEM: 67.8 ± 2.1). All histological sections in the hamstring had normal grades, and the sections in LHB had moderate to severe tendinopathy grades. Senescent phenotypes were observed in the tendinopathic tendon compared with the normal grade areas (Figure 1A). The expression of senescent markers, including p53, p21, p16, and SA-β-gal, was strongly correlated with tendinopathy severity (r > 0.8, p < 0.0001; Figure 1B). Furthermore, higher SA-β-gal activity was observed in tendinopathic LHB tendons than in normal hamstring tendons (Figure 1C).
Figure 1.
Expression levels of p21, p53, and p16 in tendon tissues of normal hamstring (Ham) tendon during anterior cruciate ligament reconstruction and long head of bicep (LHB) with tendinopathic changes during surgical intervention
(A and B) Representative figures of hematoxylin and eosin (H&E) and immunohistochemistry (IHC) staining for senescence-associated β-galactosidase (SA-β-gal), p53, p21, and p16 in tendon tissues of normal Ham (n = 10) and LHB (n = 12) tendons with tendinopathic changes (moderate to severe) during surgical intervention. Scale bars represent 20 μm in ×400 magnifications. Arrow heads indicate positive cells. (B) Harvested tissues from Ham, moderate, and severe LHB were subjected to IHC staining, and the p53-, p21-, p16-, and SA-β-gal-positive cells were counted and normalized with hematoxylin-counterstained total cells. Values are represented as the mean ± standard error of the mean (SEM). Spearman correlation rank test was used. (C) Representative figures of SA-β-gal activity in human tendon tissues from the normal Ham and LHB tendons. 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was used for nuclear staining. Arrow heads indicate SA-β-gal and DAPI double-positive cells. Scale bars represent 20 μm in ×400 magnifications. The blue-boxed areas are higher-magnification views of the red-boxed areas. (D) Representative figures of immunoblotting for p53, p21, and p16 expression levels (n = 2) and SA-β-gal activity (n = 1) in tenocytes from Ham and LHB tendons. Results are representative of at least two independent experiments. Scale bars represent 200 μm in ×40 magnifications.
Cellular senescence in human and rat tendinopathic tenocytes
Rat primary tendinopathic tenocytes were collected from Achilles tendons treated with collagenase for 1 week, as described previously.16 Human primary tenocytes were isolated from normal hamstring and tendinopathic LHB tendons. In human LHB tenocytes, SA-gal-positive cells and the expression of p53, p16, and p21 were significantly higher than those in normal hamstring tendons (Figure 1D). As published previously,16 we established a cell model in which tenocytes were isolated from Achilles tendons of Sprague-Dawley rats and treated (T) or untreated (Un) with an intratendinous injection of collagenase I for 1 week. In treated tenocytes, SA-β-gal-positive cells (Figure 2A) and the expression of p53, p16, and p21 were significantly higher than in untreated control tenocytes, as determined by immunoblotting and quantitative reverse transcription polymerase chain reaction (qRT-PCR) (Figures 2B and 2C).
Figure 2.
Expression levels of CD44, P21, p53, p16, and SA-β-gal activity in rat primary tenocytes
Tenocytes were isolated from the Achilles tendons of Sprague-Dawley rats treated (T) or untreated (Un) with intratendinous injection of collagenase I (10 lambda/rat, 1.5 mg/lambda) for 1 week. (A) Primary tenocytes were subjected to SA-β-gal activity assay. The black arrows indicate SA-β-gal-positive cells. Scale bars represent 50 μm in ×100 magnifications. SA-β-gal-positive cells were counted and quantitated (n = 3). (B) Expression levels of p53, p21, and p16 were determined via immunoblotting. (C) Expression levels of p53 (n = 5), p21 (n = 6), and p16 (n = 6) were determined via quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Values are represented as the mean ± SEM. The between-the-group differences were assessed using the Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Results are representative of at least two independent experiments.
Overexpression of CD44 by lentiviral vectors in vitro reduces the SASPs in rat tendinopathic tenocytes
Rat tendinopathic tenocytes were transduced with lentiviral vectors expressing CD44 (LVCD44) to detect alterations in SASPs. Tenocyte transfectants in which CD44 was overexpressed displayed decreased protein levels of p53, p21, p16, MMP-1 and -3, COX-2, and phospho-NF-κB/NF-κB, whereas their collagen type I alpha 1 (COL1A1) and tnmd levels were increased when compared with control transfectants (LVSin) under IL-1β-stimulated conditions, as determined by immunoblotting (Figure 3A). Furthermore, mRNA levels of IL-6, MMP-1, and COX-2 were lower, whereas tnmd and COL1A1 levels were higher, in LVCD44-transduced tenocyte transfectants than in their LVSin-transduced counterparts in response to IL-1β stimulation (Figure 3D). The levels of IL-6 in the supernatant of LVCD44-transduced tenocyte transfectants were lower than those in LVSin-transduced control transfectants (Figure 3B). Furthermore, these transfectants exhibited reduced SA-β-gal activity in response to IL-1β stimulation (Figure 3C).
Figure 3.
Expression levels of CD44, matrix degrading enzymes, inflammatory mediators, extracellular matrix (ECM)-related molecules, and senescent markers in LVCD44-transduced rat primary tenocytes
(A) Immunoblotting for CD44, matrix metalloproteinase-1 (MMP)-1 and -3, cyclooxygenase-2 (COX-2), phospho-nuclear factor-κB (p-NF-κB), NF-κB, collagen type I alpha 1 (COL1A1), tenomodulin (tnmd), p53, p21, and p16 expression levels in LVSin- (the control vector), and LVCD44-transduced tenocytes. LVCD44- and LVSin-transduced tenocytes were stimulated with IL-1β (10 ng/mL) or left unstimulated for 5 days (except for phospho-NF-κB, which was stimulated for 30 min). (B) Supernatant was isolated and subjected to enzyme-linked immunosorbent assay (ELISA) for detecting IL-6 levels (n = 3). (C) The underlying tenocytes were subjected to SA-β-gal activity assay. SA-β-gal was identified and counted in five high-power fields (200×) to determine the average percentages of SA-β-gal-positive cells corresponding to total cells (n = 6). (D) qRT-PCR to determine CD44, IL-6, MMP-1, COX-2, COL1A1, and tnmd expression levels. LVCD44- and LVSin-transduced tenocytes were stimulated with IL-1β (10 ng/mL) or left unstimulated for 24 h (n = 3). Results are representative of at least three independent experiments. Scale bars represent 50 μm in ×100 magnifications. Values are represented as the mean ± SEM. The difference within groups were assessed using one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison tests. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See Figure 2 for other definitions.
Overexpression of CD44 by lentiviral vectors in vivo reduces the SASPs and clinical signs in a rat tendinopathy model
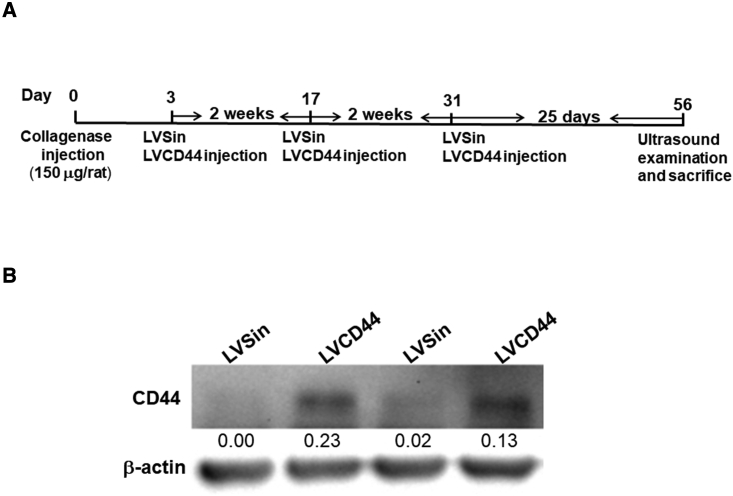
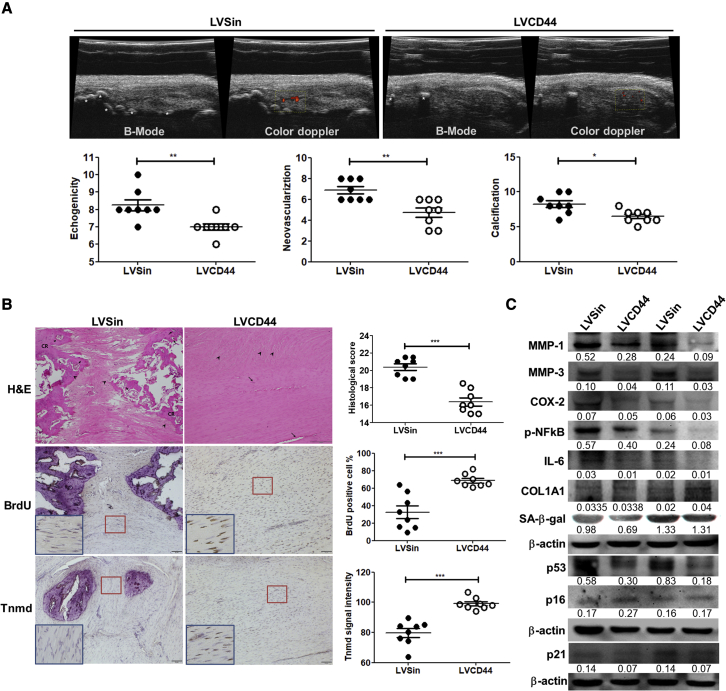
Figure 4A shows the experimental procedures used in the animal study. We showed that CD44 levels were overexpressed in the rat Achilles tendons receiving LVCD44 injection compared with LVSin 7 days later (Figure 4B). The expression of virus-borne CD44 could hardly be detected in the LVCD44-treated tendons compared with their LVSin-injected counterparts 14 days after injection (data not shown). Therefore, three consecutive injections at 2-week intervals were administered in our animal experiments (Figure 4A). Eight weeks after collagenase injection, hallmarks of tendinopathy, including neovascularization and calcification, were observed in both the LVCD44 and LVSin groups (Figure 5A). However, the LVCD44-injected tendinopathic tendons had significantly lower scores for ultrasound features, including echogenicity, neovascularization, and calcification, than the LVSin-injected control tendons (p < 0.05; Figure 5A). LVCD44-treated tendons had significantly lower histological scores than their LVSin-treated counterparts (p < 0.05; Figure 5B). To assess whether the amelioration of tendinopathy was associated with senescence, inflammation, and SASPs, cell proliferation and the various markers depicted in Figure 3 were analyzed by immunohistochemistry and immunoblotting. Increased tnmd expression and BrdU-positive cells were observed in LVCD44-injected tendinopathic tendons (Figure 5B). The in vivo protein levels of MMP-1 and -3, COX-2, p-NF-κB, IL-6, SA-β-gal, p53, and p21 were significantly lower in the LVCD44 group than in the LVSin group (Figure 5C). Notably, the molecular weight of SA-β-gal is approximately 64 kDa, as proposed by Lee et al.7 COL1A1 expression was significantly higher in the LVCD44 group than in the control group. p16 expression remained unchanged between the two groups (Figure 5C). Taken together, these results indicated that CD44 plays a protective role in tendinopathy by modulating senescence, inflammation, and ECM homeostasis.
Figure 4.
Determination of infectivity of LVCD44 in rat tendons via immunoblotting
(A) Schematic representation of the procedures used for animal experiments. Three days after collagenase injection into rat Achilles tendons, three consecutive intratendinous injections of LVSin or LVCD44 (4×107 lentiviral particles) into their Achilles tendons in a 2-week interval were given according to the group names. Eight weeks after collagenase injection, the rats received ultrasound examination and were sacrificed for further histologic examination and immunoblotting analysis. (B) CD44 expression levels in the rat Achilles tendon receiving intratendinous injection of LVSin and LVCD44 for 7 days, as determined via immunobloting (n = 2). Results are representative of at least two independent experiments.
Figure 5.
Effects of CD44 overexpression in the collagenase-induced rat Achilles tendinopathy model
(A) Three days after the intratendinous collagenase injection, the rats randomly received three consecutive intratendinous injections of LVCD44 or LVSin within a 2-week interval. The ultrasound examination was done 2 months after collagenase injection. The semiquantitative scores of echogenicity, neovascularization, and calcification in B-mode and color doppler were measured and recorded. Yellow dashed square: region of interest for neovascularization. White asterisk: calcification. Values are represented as the mean ± SEM (n = 8). (B) H&E, histological score, tnmd, and BrdU immunohistochemical stainings and the quantitative analyses of LVSin- and LVCD44-treated tendinopathic tendon. Scale bars shown at ×100 magnification correspond to 50 and 200 μm. The blue-boxed areas are higher-magnification views of the red-boxed areas. CR, calcified region; arrowhead, chondrocyte-like cell; arrow, neovascularization. Values are represented as the mean ± SEM (n = 8). (C) Immunoblotting for MMP-1 and -3, COX-2, p-NF-kB, IL-6, COL1A1, SA-β-gal (64 kDa), p53, p21, and p16 expression levels in LVSin- and LVCD44-injected tendinopathic tendons (n = 2). Results are representative of at least three independent experiments. The between-the-group differences were assessed using the Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
In this study, we demonstrated that cellular senescence was positively correlated with the severity of tendinopathy in human tendons and was more evident in primary rat and human tendinopathic tenocytes than in controls. CD44 overexpression by a lentivirus-based gene transfer strategy showed a senolytic effect on rat tendinopathic tenocytes. Overexpression of CD44 in vivo ameliorates experimental tendinopathy. Various senescence-related markers, including p53, p21, p16, SA-β-gal, and IL-6, were downregulated in IL-1β-stimulated tenocytes and rat tendinopathic tendons receiving CD44 gene transfer. Anti-inflammation (phospho-NF-κB and COX-2), cell proliferation (BrdU), and ECM homeostasis (MMP-1 and -3, COL1A1, and tnmd) were also observed in the present experimental settings (Figure 6).
Figure 6.
Working model of CD44 gene therapy in tendinopathy
In vitro CD44 gene transfer by lentiviral vectors in IL-1β-stimulated tendinopathic tenocytes resulted in CD44 overexpression that mediated the downstream effects, including the blocking of NF-κB activation and gene expression levels of senescence markers (p53, p21, P16, COX-2, and SA-β-gal) and SASPs (IL-6, and MMP-1 and -3), whereas the expression levels of tendon healing molecules (tnmd and COL1A1) were increased under the IL-1β-stimulated condition. In vivo gene therapy via intratendinous injection of LVCD44 in rat collagenase-treated tendinopathic tendons reduced the ultrasound and histological scores as well as senescence and SASPs and then facilitated tendon healing.
Altered cell fate in senescent tendon-derived stem cells (TDSCs) has been well documented before.18,19 TDSCs isolated from collagenase-treated rat patellar tendons have lower proliferation potential and higher cellular senescence compared with those from healthy tendons, which might also contribute to pathological chondro-ossification and failed tendon healing.18 We correlated these results with our findings that chondrocyte-like cells presented in the severe histological grade expressed a higher proportion of senescent markers in the tendinopathic LHB tendons (Figures 1A and 1B) and that the T tenocytes had a higher SA-β-gal-positive cell ratio and expression levels of p53, p16, and p21 than the Un tenocytes (Figure 2). In aged rat TDSCs, the expression of tendon lineage markers was decreased, whereas adipocytic differentiation and CD44 expression were increased,19 indicating a critical role of CD44 in the regulation of altered cell fate during the aging process. Aged TDSCs altered their cell fate toward chondrocyte or adipocytic differentiation,18,19 and we showed that lentiviral transduction of CD44 ameliorated senescence in tenocytes, implying that cell fate might be altered toward tenocyte differentiation in TDSCs transduced with LVCD44 from a tendinopathic environment. Interestingly, increased CD44 expression levels were positively correlated with the histopathological grade of tendinopathy, whereas various SASP-associated markers, including IL-6 and MMP-1 and -3, also increased in primary tenocytes after CD44 inhibition, as shown in our previous study.16 Furthermore, tnmd knockout (KO) TDSCs exhibit senescence properties and reduced proliferative potential, and KO mice can also exhibit significant heterotopic ossification during Achilles tendon repair.20,21 These findings raise the possibility that CD44 might protect tendinopathic tendons from cellular senescence and decrease their disease activity by increasing tnmd expression, as demonstrated in our findings (Figures 3 and 5).
NF-κB is a critical regulator of cellular senescence. For example, reactive oxygen species (ROS) can cause cell senescence by activating the NF-κB signaling, which results in pro-inflammatory SASPs in fibroblasts that secrete cytokines IL-6 and IL-8.22 Indoxyl sulfate-induced cellular senescence in proximal tubular cells can also occur through the NF-κB signaling and induce p53 and p21 expression, as well as increase SA-β-gal activity.23 Blocking COX-2 activity by a selective inhibitor in stress-induced senescent fibroblasts provided an NF-κB-dependent manner.24 In aged skin fibroblasts, COL1A1 gene expression is downregulated by NF-κB-mediated transcriptional repression.25 According to our findings, IL-1β upregulated the expression of matrix-degrading enzymes (MMP-1 and -3), inflammatory mediators (phospho-NF-κB, IL-6, and COX-2), and senescent markers (p53, p21, and p16), and these effects were abrogated in LVCD44-transduced tendinopathic tenocytes (Figure 3A). In contrast, tenocytes in which CD44 was overexpressed had higher expression of COL1A1 than the control vector-treated cells in response to IL-1β stimulation (Figure 3A), indicating that the CD44 signaling pathway targeting NF-κB-mediated cell senescence is closely related to tendon healing.
The pathogenesis of tendinopathy resembles osteoarthritis (OA) in many aspects, including IL-1β being a disease initiator that induces the expression of matrix-degrading enzymes and reduces ECM production.10,11 OA chondrocytes from patients who underwent knee arthroplasty revealed increased SA-β-gal activity26 and p16 expression.27 Gao et al. reported that SA-β-gal expression was positively correlated with disease severity in knee OA patients.28 Therefore, cellular senescence may play a significant role in the pathogenesis of OA.29 Furthermore, Jeon et al. found that selective removal of senescent cells in a surgically induced mouse OA model could attenuate the development of post-traumatic OA, reduce pain, and enhance the cartilage repair.30 In tendinopathy, dexamethasone treatment resulted in an increase in SA-β-gal-positive human tenocytes31,32 and activated the p53/p21 rather than the p16 senescence pathway according to the histology of human supraspinatus tendon biopsy.31 In our study, the expression of senescent markers, including p53, p21, p16, and SA-β-gal, was strongly correlated with tendinopathy severity, suggesting that senescence might play a pathological role in this tendon disorder. Overexpression of CD44 in a rat Achilles tendinopathy model reduced the expression of senescent and SASP markers, COX-2, and phospho-NF-κB and significantly lowered ultrasound and histological scores. Our results showed that CD44 overexpression protected tendinopathic tendons against SASPs via anti-inflammation and maintenance of ECM homeostasis. Interestingly, the “senolytic” compounds, such as dasatinib and quercetin, have been shown to selectively kill senescent cells and extend the lifespan of mice.33 We suggest that senolytic compounds might be used for treating tendinopathy in the future, which requires further investigation.
This study had some limitations. First, we used hamstring tendons harvested during anterior cruciate ligament reconstruction surgery rather than age-matched normal tendons as controls because of the limited access to fresh cadavers. Therefore, the mean age of patients with hamstring tendons was much lower than that of patients with tendinopathic LHB tendons, and we might have underestimated the expression of senescent markers in the normal histological grade. However, the expression of senescent markers increased as tendinopathy progressed in human LHB tendons (Figure 1) and rat tenocytes (Figure 2). Second, although lentiviral vector-based CD44 overexpression in the experimental rat model improved tendinopathy severity, tendinopathic features could still be observed by sonographic and histologic examinations in the LVCD44-treated rat tendons (Figures 5A and 5B). Lentiviral vectors are vesicles widely used for gene therapy in preclinical studies and clinical trials with promising safety and efficacy.34 Although vesicular stomatitis virus surface glycoprotein (VSV-G) pseudotype lentiviral vectors confer high stability and wide tropism for gene delivery, complement-mediated vector inactivation limits gene transfer to the target tissue because of the presence of complement-fixing anti-VSV-G antibodies in serum.35 In addition to complement-mediated inactivation, the lentiviral vector RNA genome may act as danger signals by engaging Toll-like receptors 3 and 7 to trigger innate immune responses and subsequent inflammation.36,37 Therefore, lentiviral vectors can efficiently express GFP for 168 days in tendons after intra-articular injection into the knee joints of nude rats.38 However, we observed that the transgene could be expressed in tendinopathic rat tendons for only 14 days after intratendinous injection of the lentiviral vectors. It is critical to decipher the number of vector genomes present in LVCD44-transduced tendons, which might help to understand whether the vector was silenced or lost via immune rejection. Even so, we still observed the upregulation of CD44 levels in LVCD44-injected rat tendons 7 days post-infection (Figure 4B) and the downstream therapeutic effects of CD44 on tendinopathic rats (Figure 5). Third, the repeated dose strategy used in our animal study might have raised the immune response issue. However, we observed that LVCD44-injected tendinopathic tendons still had significantly lower scores than their untreated (collagenase-induced model treated with nothing) and LVSin-treated counterparts in our pilot study, and there were no significant differences in the histological and ultrasound feature scores between the untreated and LVSin groups (supplemental information). Therefore, the untreated group was not included in follow-up experiments. These results indicated that the lentiviral vector-induced immune response might be marginal upon redosing in rat tendinopathic tendons and would not influence our observation of the therapeutic effect of LVCD44.
From the multiple lines of evidence shown here, we can conclude that CD44 is a critical protector from tendinopathy via its anti-senescence effects. This study provides new insights into the development of pharmaceutical therapeutics targeting CD44 to prevent cell senescence in tendinopathy.
Materials and methods
Ethics statement
This study complied with the Declaration of Helsinki, and the Institutional Review Board of the National Cheng Kung University Hospital approved the research protocol (nos. A-ER-106-163 and B-ER-106-414). Written informed consent was obtained from all subjects. All animal experiments were performed in strict accordance with the protocols approved by the Institutional Animal Care and Use Committee of National Cheng Kung University (no. 107-165).
Preparation of clinical specimens
Human hamstring tendons were collected from 10 consecutive patients (5 men, 5 women; mean age: 30.1 years; age range: 21–56 years) undergoing anterior cruciate ligament reconstruction using autologous hamstring tendons as the control. LHB tendons were collected during the tenodesis or tenotomy procedure from 12 consecutive patients (3 men, 9 women; mean age: 67.8 years; age range: 52–78 years) who underwent arthroscopic treatment for a rotator cuff tear and LHB tendinopathy at our university hospital. The pathological LHB tendon area and residual hamstring tendons after the surgical procedures were harvested for histological examination.
Collagenase-induced rat tendinopathy model
Male Sprague-Dawley rats (8 weeks old; weight, 250–300 g) were purchased from LASCO, Taiwan, and housed in the Laboratory Animal Center, College of Medicine, National Cheng Kung University, and Taiwan Animal Consortium (AAALAC International Full Accreditation). The healthy conditions of the rats were strictly monitored by administrators at our center. The exclusion criteria were unhealthy (sick, infection, weight loss, and tumor) and uninduced (10%–20% rate) rats. Rats were intratendinously injected with 10 μL (0.015 mg/μL 0.9% saline) bacterial collagenase I (Sigma-Aldrich, St. Louis, MO, USA) into their right Achilles tendons using a 29G needle to induce tendinopathy. The area 7 mm from the calcaneal insertion was the reference site for the intratendinous injection of collagenase39 or lentiviral vectors and hindlimb thickness measurement. On day 3 following collagenase injection, the hindlimb with an increased thickness of less than 30% compared with the contralateral normal side was regarded as an uninduced model. At week 8 after the index procedure, the rat tendinopathy model matured.40,41 All rats underwent ultrasound examination for the evaluation of tendinopathy and were then sacrificed for further analyses. Ultrasound (Vevo 770; VisualSonics, Toronto, ON, Canada) 55-MHz linear transducer was used for high-resolution images, and the three common tendinopathic ultrasound characteristics, including echogenicity, neovascularization, and calcification, under real-time B-mode and color doppler were scored from 0 to 10 as previously described.40,41 Healthy and successfully induced tendinopathic rats were included in the animal study.
Histopathological and immunohistochemical analyses
The human and rat specimens were fixed in fresh 4% paraformaldehyde for 16–24 h at 4°C, dehydrated, paraffin embedded, and longitudinally sectioned. Sequential 4-μM sections were stained with hematoxylin and eosin (H&E) and examined under a light microscope for changes in tenocyte morphology and collagen-bundle characteristics. For human specimens, a simple semiquantitative scoring system based on tenocyte morphology and collagen-bundle characteristics on a 4-point scale (0–3) was used to classify tendinopathy into normal, mild, moderate, and severe grades (0, ≤2, 3–4, and ≥5 points).16 To evaluate rat Achilles tendinopathy, a 4-point system was scored on the following eight parameters:40,41 fiber structure, fiber arrangement, roundness of the nuclei, regional variations in cellularity, increased vascularity, decreased collagen stainability, fibrosis or hyalinization, and calcification characteristics. The maximum total possible score was 24. Histological grading or score was assessed by three observers blinded to the clinical or experimental settings. If an inconsistency existed, the field was reassessed, and a final score was decided. Some specimens were snap frozen and embedded in optimal cutting temperature (OCT) compound. Four-μM frozen sections were prepared for SA-β-gal activity assay. For immunohistochemical staining, the sections were deparaffinized in xylene, dehydrated in alcohol, epitope unmasked by heating, immersed in H2O2, and stained with antibodies against p53 (#sc-6243, Santa Cruz Biotechnology), p21 (#sc-6246, Santa Cruz Biotechnology), p16 (#sc-6243, Santa Cruz Biotechnology), SA-β-gal (#sc-377257, Santa Cruz Biotechnology), tnmd (#ab203676, Abcam), and BrdU (#GTX128091, GeneTex) in combination with chromogen 3-amino-9-ethylcarbazole (Zymed Laboratories). Cells with immunohistochemical staining for p53, p21, p16, SA-β-gal, and BrdU were identified and counted in five high-power fields (200×) to determine the average percentages of p53-, p21-, p16-, SA-β-gal-, and BrdU-positive cells corresponding to hematoxylin-stained total cells. Tnmd signal intensity was quantitated using ImageJ 1.42q (National Institutes of Health) in five randomly chosen high-power fields (200×).
Primary culture of rat tendinopathic tenocytes
Male Sprague-Dawley rats (4–6 weeks old) were treated with an intratendinous injection of collagenase I (0.015 mg/μL, 10μL injection/rat, Sigma-Aldrich). One week after the index procedure, the Achilles tendons were harvested after the rats were killed with an overdose of isoflurane. The preparation of tendon samples and tenocyte culture methods have been described in our previous study.16,17 Well-characterized second- to fourth-passage cells were used in this experiment as tendinopathic tenocytes.16,17 They showed no phenotypic drift of major tenocyte markers, such as cell shape (elongated and spindle shaped with apposition and tnmd expression identified using anti-tnmd antibody [#ab203676, Abcam]).
Lentiviral vectors and stable tenocyte transfectants in which CD44 is overexpressed
The rat CD44-expressing lentiviral plasmid pLenti-GIII-EF1α-CD44 was purchased from Applied Biological Materials (abm). The pSin-EF2-Puro control plasmid encoding no transgene was constructed from pSin-EF2-Oct4-Puro by digestion with Eco RI and SpeI to delete the Oct4 cDNA, filling the cohesive ends with Klenow enzyme, followed by self-ligation.42 Recombinant lentiviral vectors, LVSin and LVCD44, were produced by transient transfection of 293T cells with pSin-EF2-Puro and pLenti-GIII-EF1α-CD44, along with the packaging plasmid psPAX2 and envelope plasmid pMD2G, as previously described.42 Virus titers were determined using the HIV1 p24 ELISA Kit (#ab218268, Abcam). One nanogram of p24 protein equals 1.25×107 lentiviral particles.
Senescence assay for tenocytes
Tendinopathic tenocytes were transduced with LVSin and LVCD44 for 48 h in the presence of puromycin (2 μg/mL) for approximately 7 days. Human tendon tissues and tenocytes, as well as stable transfectants in which CD44 was overexpressed, were induced with IL-1β (10 ng/mL, R&D Systems) for 5 days, and their senescent status was evaluated using the Senescence β-Galactosidase Staining Kit (#9860, Cell Signaling Technology). SA-β-gal was identified and counted in five high-power fields (200×) to determine the average percentages of SA-β-gal-positive cells corresponding to the total number of cells.
Lentiviral vector-mediated gene transfer in Achilles tendons from rats with tendinopathy
To evaluate in vivo expression duration of lentivirus-borne CD44, rat Achilles tendons were intratendinously injected with LVSin and LVCD44 (n = 4 each). Seven and 14 days after the injection, the rats were sacrificed, and their tendons were isolated to detect CD44 expression. To evaluate the in vivo therapeutic effects of CD44 overexpression, the rat collagenase-induced Achilles tendinopathy model was adopted. All the rats were randomly assigned to the LVSin and LVCD44 groups (n = 8 each). Three days after the index procedure on the right hindlimbs, three consecutive intratendinous injections of LVSin or LVCD44 (4×107 lentiviral particles) into their Achilles tendons in a 2-week interval were given according to the group names (Figure 4A). Eight weeks after collagenase injection, the rats underwent ultrasound examination and were sacrificed for histological examination and immunoblot analysis. Eighteen rats were totally used here. Notably, the researchers who were not aware of the group allocation helped conduct animal experiments, and data were analyzed by the first and corresponding authors.
Immunoblot analysis and enzyme-linked immunosorbent assay (ELISA)
Cell lysates of tenocytes and stable transfectants and tendinopathic tendons receiving various treatments (IL-1β stimulation, LVSin, and LVCD44 infection) were subjected to immunoblot analysis with antibodies against CD44, p53, p21, p16, MMP-1 and -3, COX-2, phospho-NF-κB, total NF-κB, COL1A1, SA-β-gal, and tnmd as described in the histopathological and immunohistochemical analyses section, in combination with a horseradish-peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) and quantitative control anti-β-actin antibodies (Sigma-Aldrich). Protein-protein complexes were visualized using an ECL Plus System (Amersham) and analyzed using a BioSpectrum Imaging System (UVP) for chemiluminescence detection. The signal intensity was further quantified by densitometry, and the relative abundance of each gene was compared with β-actin expression. Supernatant from IL-1β-stimulated LVSin and LVCD44-transduced stable tenocyte transfectants were subjected to ELISA (#DY506, R&D Systems) to detect IL-6 expression levels.
qRT-PCR
Total RNA from rat tenocytes was isolated with TRIzol reagents (#15596018, Invitrogen), and complementary DNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (#4368813, Applied Biosystems) for qRT-PCR by SYBR Green PCR kit (#208054, Qiagen) with primer pairs specific to CD44 (forward, 5′-AAGACATCGATGCCTCAAAC-3′; reverse, 5′-CTCCAGTAGGCTGTGAAGTG-3′); IL-6 (forward, 5′-CGAAAGTCAACTCCATCTGCC-3′; reverse, 5′-GGCAACTGGCTGGAAGTCTCT-3′); MMP-1 (forward, 5′-CATACTGTACTGAGAGGATTCCCCACAGA-3′; reverse, 5′-ACATCATCAACTTTATCGTCAATTCCAGG-3′); COX-2 (forward, 5′-CACGGACTTGCTCACTTTGTT-3′; reverse, 5′-AAGCGTTTGCGGTACTCATT-3′); COL1A1 (forward, 5′-ATCAGCCCAAACCCCAAGGAGA-3′; reverse, 5′-CGCAGGAAGGTCAGCTGGATAG-3′); tnmd (forward, 5′-CGCCACACCAGACAAGCA-3′; reverse, 5′-CCAGCATTGGGTCAAATTCA-3′); p53 (forward, 5′-AGGATTCACAGTCGGATATG-3′; reverse, 5′-GGAGGAAGAAGTTTCCATAAG-3′); p21 (forward, 5′-CCAGCCTAACAGATTTCTATC-3′; reverse, 5′-GGCAGAGTATATACAGGAGAAG-3′); p16 (forward, 5′-CGATACAGGTGATGATGATG-3′; reverse, 5′-GTACTACCAGAGTGTCTAGG-3′); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5′-CCATCTTCCAGGAGCGAGATC-3′; reverse, 5′-GCCTTCTCCATGGTGGTGAA-3′). The comparative Ct method was used to calculate the relative abundance of each gene compared with GAPDH expression.16
Statistical analysis
Data are expressed as the mean ± SEM. Normality was passed in each data point using the Shapiro-Wilk test. Differences between two groups and among groups were analyzed using Student’s t test and one-way analysis of variance, followed by Dunnett’s multiple comparison test, respectively (Prism 5.0). The significance of the correlation between the ratios of senescence marker-positive cells and the histopathological grades of tendinopathy was analyzed using the Spearman correlation rank test. p < 0.05 was considered to be statistically significant.
Acknowledgments
This study was supported by the Taiwan National Science Council (grants MOST 110-2314-B-006-022, MOST 107-2314-B-006-065-MY3, and MOST 108-2314-B-650-007) and National Cheng Kung University (grants NCKUEDA 10903, NCKUEDA11006, NCKUSCM10705, and NCKUSCM10808). We thank Dr. D. Trono (Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland) for providing the psPAX2 and pMD2G plasmids for the production of lentiviral vectors and the Laboratory Animal Center, College of Medicine, National Cheng Kung University, and Taiwan Animal Consortium (AAALAC International Full Accreditation) for the technical supports in ultrasound and IVIS and animal care. We are grateful to the Biostatistics Consulting Center and the Skeleton Materials and Bio-compatibility Core Lab, Clinical Medicine Research Center, National Cheng Kung University Hospital for assistance with this study. We also wish to thank Ms. Chih-Hui Hsu, Ms. I-Ting Lee and Ms. Yu-Ying Chen for their valuable assistance.
Author contributions
Conceptualization, S.-Y.C., I.-M.J., and P.-T.W.; data curation, S.-Y.C., I.-M.J., P.-Y.K., K.-L.H., W.-R.S., and P.-T.W.; formal analysis, S.-Y.C., I.-M.J., P.-Y.K., L.-C.K., C.-L.W., and P.-T.W.; funding acquisition, P.-T.W.; investigation, S.-Y.C., I.-M.J., P.-Y.K., K.-L.H., W.-R.S., P.-T.W., and P.-Y.L.; methodology, S.-Y.C., I.-M.J., P.-Y.K., and P.-T.W.; project administration, S.-Y.C. and P.-T.W.; resources: S.-Y.C., I.-M.J., P.-Y.K., K.-L.H., W.-R.S., and P.-T.W.; supervision, S.-Y.C., I.-M.J., and P.-T.W.; validation, S.-Y.C., I.-M.J., and P.-T.W.; visualization, S.-Y.C. and P.-T.W.; writing – original draft, S.-Y.C., I.-M.J., L.-C.K., C.-L.W., and P.-T.W.; writing – review & editing, S.-Y.C., I.-M.J., and P.-T.W.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2022.06.006.
Supplemental information
References
- 1.Cook J.L., Purdam C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 2.Dakin S.G., Newton J., Martinez F.O., Hedley R., Gwilym S., Jones N., Reid H.A.B., Wood S., Wells G., Appleton L., et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 2018;52:359–367. doi: 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lui P.P.Y., Wong C.M. Biology of tendon stem cells and tendon in aging. Front. Genet. 2020;10 doi: 10.3389/fgene.2019.01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 5.Hampel B., Fortschegger K., Ressler S., Chang M.W., Unterluggauer H., Breitwieser A., Sommergruber W., Fitzky B., Lepperdinger G., Jansen-Durr P., et al. Increased expression of extracellular proteins as a hallmark of human endothelial cell in vitro senescence. Exp. Gerontol. 2006;41:474–481. doi: 10.1016/j.exger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Millis A.J., Hoyle M., McCue H.M., Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp. Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- 7.Lee B.Y., Han J.A., Im J.S., Morrone A., Johung K., Goodwin E.C., Kleijer W.J., DiMaio D., Hwang E.S. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 8.Abate M., Silbernagel K.G., Siljeholm C., Di Iorio A., De Amicis D., Salini V., Werner R., Paganelli R. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res. Ther. 2009;11:235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Tang H., He G., Shi Y., Kang X., Lyu J., Zhou M., Zhu M., Zhang J., Tang K. High concentration of aspirin induces apoptosis in rat tendon stem cells via inhibition of the Wnt/β-catenin pathway. Cell. Physiol. Biochem. 2018;50:2046–2059. doi: 10.1159/000495050. [DOI] [PubMed] [Google Scholar]
- 10.Tsuzaki M., Guyton G., Garrett W., Archambault J., Herzog W., Almekinders L., Bynum D., Yang X., Banes A.J. IL-1β induces COX2, MMP-1,-3 and-13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. J. Orthop. Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang K., Asai S., Yu B., Enomoto-Iwamoto M. IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem. Biophys. Res. Commun. 2015;463:667–672. doi: 10.1016/j.bbrc.2015.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo C.H., Lee S.Y., Yoon K.S., Shin S. Effects of platelet-rich plasma with concomitant use of a corticosteroid on tenocytes from degenerative rotator cuff tears in interleukin 1beta-induced tendinopathic conditions. Am. J. Sports Med. 2017;45:1141–1150. doi: 10.1177/0363546516681294. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda M., Nakano K., Yasumoto K., Tanaka Y. CD44: functional relevance to inflammation and malignancy. Histol. Histopathol. 2002;17:945–950. doi: 10.14670/HH-17.945. [DOI] [PubMed] [Google Scholar]
- 14.Bourguignon L.Y. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am. J. Pathol. 2014;184:1912–1919. doi: 10.1016/j.ajpath.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lompardía S.L., Papademetrio D.L., Mascaró M., Álvarez E.M.dC., Hajos S.E. Human leukemic cell lines synthesize hyaluronan to avoid senescence and resist chemotherapy. Glycobiology. 2013;23:1463–1476. doi: 10.1093/glycob/cwt074. [DOI] [PubMed] [Google Scholar]
- 16.Wu P.T., Su W.R., Li C.L., Hsieh J.L., Ma C.H., Wu C.L., Kuo L.C., Jou I.M., Chen S.Y. Inhibition of CD44 induces apoptosis, inflammation, and matrix metalloproteinase expression in tendinopathy. J. Biol. Chem. 2019;294:20177–20184. doi: 10.1074/jbc.RA119.009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu P.T., Kuo L.C., Su F.C., Chen S.Y., Hsu T.I., Li C.Y., Tsay K.J., Jou I.M. High-molecular-weight hyaluronic acid attenuated matrix metalloproteinase-1 and-3 expression via CD44 in tendinopathy. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep40840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rui Y.F., Lui P.P., Wong Y.M., Tan Q., Chan K.M. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cell. Dev. 2013;22:1076–1085. doi: 10.1089/scd.2012.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z., Akinbiyi T., Xu L., Ramcharan M., Leong D.J., Ros S.J., Colvin A.C., Schaffler M.B., Majeska R.J., Flatow E.L., et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9:911–915. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caceres M.D., Angerpointner K., Galler M., Lin D., Michel P.A., Brochhausen C., Lu X., Varadarajan A.R., Warfsmann J., Stange R., et al. Tenomodulin knockout mice exhibit worse late healing outcomes with augmented trauma-induced heterotopic ossification of Achilles tendon. Cell Death Dis. 2021;12:1049. doi: 10.1038/s41419-021-04298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberton P., Dex S., Popov C., Shukunami C., Schieker M., Docheva D. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cell. Dev. 2015;24:597–609. doi: 10.1089/scd.2014.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson G., Kucheryavenko O., Wordsworth J., von Zglinicki T. The senescent bystander effect is caused by ROS-activated NF-kappaB signalling. Mech. Ageing Dev. 2018;170:30–36. doi: 10.1016/j.mad.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu H., Bolati D., Adijiang A., Muteliefu G., Enomoto A., Nishijima F., Dateki M., Niwa T. NF-kappaB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am. J. Physiol. Cell Physiol. 2011;301:C1201–C1212. doi: 10.1152/ajpcell.00471.2010. [DOI] [PubMed] [Google Scholar]
- 24.Zdanov S., Bernard D., Debacq-Chainiaux F., Martien S., Gosselin K., Vercamer C., Chelli F., Toussaint O., Abbadie C. Normal or stress-induced fibroblast senescence involves COX-2 activity. Exp. Cell Res. 2007;313:3046–3056. doi: 10.1016/j.yexcr.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Bigot N., Beauchef G., Hervieu M., Oddos T., Demoor M., Boumediene K., Galera P. NF-kappaB accumulation associated with COL1A1 transactivators defects during chronological aging represses type I collagen expression through a -112/-61-bp region of the COL1A1 promoter in human skin fibroblasts. J. Invest. Dermatol. 2012;132:2360–2367. doi: 10.1038/jid.2012.164. [DOI] [PubMed] [Google Scholar]
- 26.Price J.S., Waters J.G., Darrah C., Pennington C., Edwards D.R., Donell S.T., Clark I.M. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H., Lou S., Zhang K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology. 2004;43:555–568. doi: 10.1093/rheumatology/keh127. [DOI] [PubMed] [Google Scholar]
- 28.Gao S.G., Zeng C., Li L.J., Luo W., Zhang F.J., Tian J., Cheng C., Tu M., Xiong Y.L., Jing W., et al. Correlation between senescence-associated beta-galactosidase expression in articular cartilage and disease severity of patients with knee osteoarthritis. Int. J. Rheum. Dis. 2016;19:226–232. doi: 10.1111/1756-185X.12096. [DOI] [PubMed] [Google Scholar]
- 29.McCulloch K., Litherland G.J., Rai T.S. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16:210–218. doi: 10.1111/acel.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon O.H., Kim C., Laberge R.M., Demaria M., Rathod S., Vasserot A.P., Chung J.W., Kim D.H., Poon Y., David N., et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen R.C., Watts A.C., Murphy R.J., Snelling S.J., Carr A.J., Hulley P.A. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: in vivo and in vitro evidence. Ann. Rheum. Dis. 2014;73:1405–1413. doi: 10.1136/annrheumdis-2012-203146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo C.H., Lee S.Y., Yoon K.S., Shin S. Effects of platelet-rich plasma with concomitant use of a corticosteroid on tenocytes from degenerative rotator cuff tears in interleukin 1β–induced tendinopathic conditions. Am. J. Sports Med. 2017;45:1141–1150. doi: 10.1177/0363546516681294. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., Palmer A.K., Ikeno Y., Hubbard G.B., Lenburg M., et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annoni A., Gregori S., Naldini L., Cantore A. Modulation of immune responses in lentiviral vector-mediated gene transfer. Cell. Immunol. 2019;342:103802. doi: 10.1016/j.cellimm.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DePolo N.J., Reed J.D., Sheridan P.L., Townsend K., Sauter S.L., Jolly D.J., Dubensky T.W., Jr. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2000;2:218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- 36.Agudo J., Ruzo A., Kitur K., Sachidanandam R., Blander J.M., Brown B.D. A TLR and non-TLR mediated innate response to lentiviruses restricts hepatocyte entry and can be ameliorated by pharmacological blockade. Mol. Ther. 2012;20:2257–2267. doi: 10.1038/mt.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown B.D., Sitia G., Annoni A., Hauben E., Sergi L.S., Zingale A., Roncarolo M.G., Guidotti L.G., Naldini L. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109:2797–2805. doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- 38.Gouze E., Gouze J.-N., Palmer G.D., Pilapil C., Evans C.H., Ghivizzani S.C. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol. Ther. 2007;15:1114–1120. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- 39.Oshita T., Tobita M., Tajima S., Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am. J. Sports Med. 2016;44:1983–1989. doi: 10.1177/0363546516640750. [DOI] [PubMed] [Google Scholar]
- 40.Wu P.T., Hsu C.H., Su F.C., Jou I.M., Chen S.Y., Wu C.L., Su W.R., Kuo L.C. Dynamic weight bearing analysis is effective for evaluation of tendinopathy using a customized corridor with multi-directional force sensors in a rat model. Sci. Rep. 2017;7:8708. doi: 10.1038/s41598-017-07812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S.Y., Chieh H.F., Lin C.J., Jou I.M., Sun Y.N., Kuo L.C., Wu P.T., Su F.C. Characteristics of sonography in a rat Achilles tendinopathy model: possible non-invasive predictors of biomechanics. Sci. Rep. 2017;7:5100. doi: 10.1038/s41598-017-05466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S.Y., Shiau A.L., Li Y.T., Lin C.C., Jou I.M., Liu M.F., Wu C.L., Wang C.R. Transcription factor snail regulates tumor necrosis factor alpha-mediated synovial fibroblast activation in the rheumatoid joint. Arthritis Rheumatol. 2015;67:39–50. doi: 10.1002/art.38899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.