Abstract
Sleep supports healthy cognitive functioning in adults. Over the past decade, research has emerged advancing our understanding of sleep’s role in cognition during development. Infancy and early childhood are marked by unique changes in sleep physiology and sleep patterns as children transition from biphasic to monophasic sleep. Growing evidence suggests that, during development, there are parallel changes in sleep and the brain and that sleep may modulate brain structure and activity and vice versa. In this review, we survey studies of sleep and brain development across childhood. By summarizing these findings, we provide a unique understanding of the importance of healthy sleep for healthy brain and cognitive development. Moreover, we discuss gaps in our understanding, which will inform future research.
Keywords: Sleep, Napping, Early childhood, Brain development, Learning
1. Introduction
It is widely accepted that sleep is important for children. Sleep during the preschool years (~3–5 years) is especially unique in that children transition from a biphasic (naps and overnight sleep) to a monophasic (primarily overnight sleep) sleep pattern (Chokroverty, 1994, Weissbluth, 1995). Naps in young children have been shown to enhance learning through sleep-dependent memory consolidation (i.e., the notion that consolidation processes preferrentially occur during sleep; Cremone et al., 2017; Kurdziel et al., 2013; Lokhandwala and Spencer, 2021; Spanò et al., 2018; Williams and Horst, 2014). Moreover, sleep macrostructure (organization of sleep stages) and microstructure (characteristics of the sleep electroencephalogram (EEG) which measure electrical activity in the brain) change during this period. Brain maturation in particular is suggested to impact the biphasic to monophasic transition and the accompanying changes in EEG.
The purpose of this paper is to review studies on sleep and brain development during childhood. In doing so, we review evidence of sleep and brain relations and argue that a better understanding of this relationship during early development is critical to understanding the nap transition and the consequences of early sleep patterns. Such examination of the relationship between brain maturation and early sleep allows for more informed decisions regarding how sleep opportunities may be structured in school and home settings.
2. Sleep changes across early development
2.1. Sleep architecture
Early in infancy, sleep is divided into 2 main periods- quiet sleep (QS) which is a precursor to non-rapid eye movement (NREM) sleep and encompasses both light and deep sleep. The second period is active sleep (AS) which is a precursor to rapid eye movement (REM) sleep and may be a cycle which involves more vivid dreaming (Carskadon and Dement, 2005). Over early development, QS and AS become NREM and REM as the sleep EEG becomes more pronounced and distinguishable. In adult humans, these two types of sleep alternate throughout the night, roughly every 90 min (McCarley et al., 1995). In young children, this cycle occurs approximately every 60 min (Davis et al., 2004).
Non-REM sleep is further composed of three stages. Non-REM stage 1 is identified by a reduction in alpha (8–13 Hz; characteristic wake activity) activity in the EEG, signaling a transition from wake to sleep (Louis et al., 2016). This stage is often followed by the appearance of nREM stage 2, which is characterized by low-amplitude and mixed frequency waves, primarily in the sigma frequency band (12–16 Hz). This stage is identifiable by unique EEG markers known as sleep spindles and K-complexes. Sleep spindles are short bursts of brain activity in the 11–16 Hz range that last about 0.5–2.5 s. Spindles can be further divided into fast spindles (12–15 Hz) and slow spindles (9–12 Hz) which undergo local development (Mölle et al., 2011). That is, fast spindles are more centrally located while slow spindles show a more frontal distribution (Werth, Achermann, and Borbély, 1997). K-complexes are diphasic (containing a positive and negative component), high in amplitude, and last about 0.5 s. This stage is important for maintaining sleep (Rechtschaffen and Kales, 1968). Non-REM stage 3, also known as slow wave sleep (SWS), is the deepest stage of sleep. This stage is characterized by low frequency (0.4–4 Hz) cortical oscillations. Rapid eye movement sleep, commonly referred to as REM sleep, is identified by theta activity (3–7 Hz), rapid eye movements, and low muscle tone, which is measured via the chin electromyography (EMG) channel. It is in this stage that we may experience more intense and vivid dreams (Siegel, 2003). Regarding sleep architecture, more nREM sleep is present earlier in the night, with REM sleep becoming more prominent towards the end of the night (Carskadon and Dement, 2005). The distinct brain activity during the various stages of sleep underscores that sleep cannot be treated as a homogenous state.
Importantly, sleep architecture and sleep patterns are not homogenous across development. Infants sleep 14–20 h each day, with their sleep distributed across the 24-hr period (polyphasic sleep pattern; Iglowstein et al., 2003). By early childhood (3–5 years), the distribution of sleep across the day undergoes a distinct developmental shift. Children transition from a biphasic sleep pattern, comprised of a midday nap and overnight sleep, to a monophasic sleep pattern, consisting solely of overnight sleep, which is characteristic of adolescent and adult sleep (Galland et al., 2012).
Not only are there changes in sleep patterns (distribution of sleep across the day) during early childhood, but there are also changes in sleep macrostructure and microstructure. For instance, as children age, percent time spent in SWS decreases while nREM 2 increases (Ohayon et al., 2004). Sigma activity (10–15 Hz; spindle range), on the other hand, increases across childhood (McClain et al., 2016, Scholle et al., 2007). EEG characteristics such sleep spindle density (number of sleep spindles/min of sleep) increases until adolescence, while spindle amplitude peaks in the first few years of life and gradually declines across the lifespan (Clawson et al., 2016). Additionally, REM sleep is reduced to 20% of sleep time compared to 50% during infancy (Carskadon and Dement, 2005). Further, slow wave activity (SWA; slow oscillatory neocortical activity between 0.5 and 4 Hz) increases during the first few years of life and begins to decrease around adolescence (Campbell and Feinberg, 2009, Jenni and Carskadon, 2004).
2.2. Sleep timing and regulation
Sleep is suggested to be controlled by two processes- a circadian process (Process C) and a homeostatic process (Process S). Our circadian rhythms are physical, mental, and behavioral changes that follow a 24-hr cycle, including sleep (Borbély et al., 2016). Process C contributes to the timing of sleep and wake through external cues, the most central cue being the light/dark cycle. Sleep homeostasis on the other hand, is the mechanism that drives us to sleep. Homeostatic sleep pressure dissipates when we sleep and slow wave activity (SWA;0.5–4.5 Hz) is considered a marker of sleep pressure, with greater SWA seen following sleep deprivation (Bersagliere and Achermann, 2010).
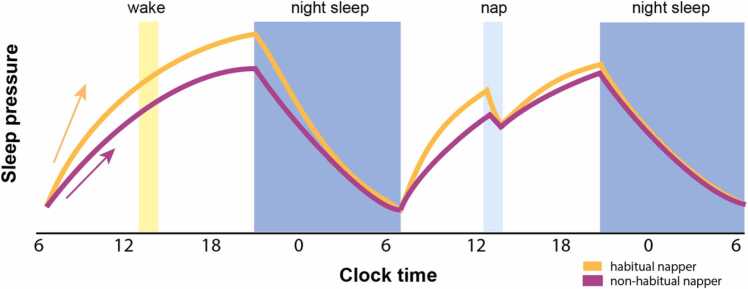
Napping is considered a hallmark of early childhood. The drive to nap in early childhood has largely been associated with Process S. In particular, young children are suggested to have a more rapid accumulation of sleep pressure, with the need to more frequently nap, which may be necessary to dissipate that pressure (Fig. 1; Jenni and LeBourgeois, 2006a; Kurth et al., 2016). While our understanding of the regulatory processes underlying napping is limited, examining naps as they relate to cognition and the factors that may play a role in nap cessation may elucidate the nap transition during this period. Although sleep is dynamic and changes across development, particularly with the transition out of napping in early childhood, there is clear evidence that naps may be necessary for learning and memory early in development. A recent review highlights that naps confer both immediate and delayed benefits on declarative, emotional, and procedural learning across infancy and childhood (see Spencer, 2021 for review). In particular, physiology in these naps (sleep spindles and SWS) has been shown to play a role in supporting these memories (Hahn et al., 2019, Kurdziel et al., 2013, Lokhandwala and Spencer, 2021, Simon et al., 2017, Spanò et al., 2018, Zinke et al., 2017, Züst et al., 2019). These results are in line with the role sleep (i.e., overnight sleep in older children and adults) physiology plays on memory consolidation more broadly (Maingret et al., 2016, Rasch and Born, 2013). Given the beneficial role of early childhood sleep on learning and memory, it is therefore critical to understand the development of early sleep, the nap transition, and the factors that may contribute to the biphasic to monophasic sleep shift during this period in development.
Fig. 1.
The homeostatic sleep process as a function of nap status. Sleep pressure may accumulate more rapidly in habitual nappers (orange) than in non-habitual nappers (magenta). A nap (right) releases this additional pressure. For illustration purposes, sleep pressure is plotted relative to a morning waking baseline (which may in reality differ for these groups).
The rate at which the cessation of napping occurs varies across children (Crosby et al., 2005; S. S. Smith et al., 2019; Touchette et al., 2013a). Current work supports the notion that the transition out of napping may be associated with changes in sleep need, neurocognitive function, and ecological factors (Kurth et al., 2016; Smith et al., 2019). For instance, in an elegant longitudinal design, the developmental trajectories of daytime naps were examined when children were 2, 3, and 5 years of age (Kurth et al., 2016). At each age, naps were taken at different times of the day in order to vary sleep pressure (Borbély et al., 2016). Implementing a strict sleep schedule and sleep EEG, Kurth and colleagues (2016) found that older children did not exhibit an increase in SWA with increased sleep pressure. Further, nap timing differences in sleep were diminished by 5 years of age. These findings indicate that the accumulation of sleep need over the day attenuates across early childhood and may explain the transition out of napping during the preschool years (Jenni and LeBourgeois, 2006b). These results also show there is a maturation of the sleep homeostatic system (i.e., sleep pressure) that may be moderated through changes in sleep depth rather than sleep structure.
Other studies suggest that the cessation of napping may be, at least in part, due to neurocognitive development (Kurdziel et al., 2013; Smith et al., 2019b). In early development, daytime sleep has been associated with multiple measures of behavioral development such as a negative association with gross motor and personal social development (Schoch et al., 2022). In a similar vein, older children (4–6 years-old), daytime napping predicted poorer cognitive functioning (assessed via The Woodcock Johnson III; Smith et al., 2019). Additionally, those who napped frequently had shorter nighttime sleep duration compared to non-habitual nappers and children transitioning out of napping. The finding that napping children performed worse on cognitive measures is consistent with work showing that nappers perform poorly on auditory attention and vocabulary tasks (Lam et al., 2011). One hypothesis as to why napping children may perform more poorly on measures of cognitive functioning is that napping may be a marker of brain maturation (Kurdziel et al., 2013; Kurth et al., 2010; Lam et al., 2011). That is, children who nap may have less developed brains (i.e., hippocampi) and therefore require frequent consolidation of the day’s information in the form of naps. It is possible that as the brain develops, children are more able to tolerate longer durations of continued wakefulness without interference. This in turn (i.e., a more efficient brain) may be why overnight sleep is sufficient for consolidating memories in older children and adults.
Lastly, it is important to consider the role of genetic and ecological factors in relation to sleep EEG and in particular, the cessation of napping. Using high density EEG, a study examining adolescent twin pairs found that in the poster region of the brain, 80–90% of variance in slow oscillations, slow waves, and spindle activity was due to genes. Slow and fast anterior spindle amplitude and sigma power, on the other hand, were largely driven by environmental factors the twins shared (Rusterholz et al., 2018). In line with the notion that neural phenotypes may be influenced by both nature and nurture, a recent study examining adolescent twins found that while spindle activity had almost a 50% genetic contribution, environment may influence sleep EEG coherence across frequencies and sleep states (Markovic et al., 2020).
One twin study examined maternal reports of daytime and nighttime sleep at 6, 18, 30, and 48 months of age revealed that in early childhood, daytime sleep duration may be influenced by environmental settings (Touchette et al., 2013b). In particular, one study found that parental education (used as a proxy for socioeconomic status (SES)) was lower for children considered “problem nappers (Smith et al., 2019).” Here, problem nappers were classified as children who had difficulty lying still during nap time. The authors note that this SES component may be showing itself through various routine components (e.g., having stable home routines, parent work schedules, the role of sleep behaviors in the home).
Other studies have found that Black children were more likely to nap longer into life, spend less time sleeping at night, and take more naps per week than non-Hispanic White children (Crosby et al., 2005, Lavigne et al., 1999). Further, studies have shown that non-Hispanic White children were more likely to have a bedtime routine and have greater 24-hr sleep than minority children (Hale et al., 2009, Schlieber and Han, 2018, Wilson et al., 2014). Racial differences in sleeping behaviors may be, in part, influenced by caregivers’ awareness of children’s sleep needs. It may be that the ability of Black children to meet their total sleep need at night is compromised, thus accounting for the greater amounts of daytime sleep. Together, these studies suggest that the transition out of napping is not homogenous across children and that multiple factors may influence this behavior.
3. Relations between sleep and brain development
There is growing interest in the relations between sleep and brain development. Exploring how brain and sleep physiology modulate one another is important in elucidating the role of early sleep patterns and brain maturation.
3.1. Relations between sleep and structural brain development
Sleep has been shown to modulate brain activity during development. For instance, Cao and colleagues (2020) developed a framework for modeling neural repair, metabolic clearance, and sleep changes during development. Results reveal differences in sleep across phylogeny and during late ontogeny (after 2–3 years of age) are primarily due to sleep functioning for repair or clearance, while changes in sleep during early ontogeny (before 2–3 years of age) primarily support neural reorganization and learning. This process occurs primarily in REM and not NREM (Cao et al., 2020). One cross-sectional study examined the prospective relations between sleep disturbances across childhood and brain morphology at 7 years of age (Kocevska et al., 2017). They found that sleep disturbances from 2 years of age onward were associated with smaller gray matter volumes. In particular, children with sleep disturbances had a thinner dorsolateral prefrontal cortex. While the study did not include any sleep EEG and therefore was unable to examine associations between altered sleep physiology and brain morphology, the results suggest the influence sleep disturbances may have on neurodevelopment. It is important to keep in mind that as the study was cross-sectional and did not include baseline magnetic resonance imaging (MRI) scans, it is possible that rather than being an outcome of sleep disruptions, brain structural development may underlie sleep disturbances in childhood. A study in cats found that sleep augmented the effects of a preceding period of monocular deprivation on visual cortical responses (Frank et al., 2001). This was not the case for wakefulness in complete darkness. This study illustrates that sleep (and the lack thereof) may be critical to modulating experience-dependent plasticity.
3.1.1. The role REM sleep in brain development
Moreover, studies have further underscored the role REM and nREM sleep may be playing in brain development. Studies in human and non-human animals have found that REM sleep may be critical for brain development, particularly the maturation of the visual cortex (de Lima et al., 1985, Shaffery et al., 2006, Dumoulin Bridi et al., 2015) and motor system (Blumberg et al., 2013). With regards to the visual system, Dumoulin Bridi and colleagues (2015) found that withholding REM sleep after monocular deprivation in perinatal cats attenuated ocular dominance plasticity and inhibited the stimulation of the kinase necessary for such plasticity. Additional studies have reported that when rats are deprived of REM sleep (therefore reducing cortical activation during this phase) prior to the end of the critical period, the cessation of this period was delayed (Shaffery et al., 2002) by approximately 3 weeks (Shaffery et al., 2006). The results indicate that REM sleep may play a functional role in the maturational processes that terminate the critical period. Work in human infants found increased retinal activity during REM sleep compared to nREM sleep (Peña et al., 1999). The authors took this to suggest that increased retinal activity during REM may reflect a preferential role of REM in the maturation of the visual system.
Muscle twitches and jerks that are characteristic of early REM sleep may also play a role in the maturation of the motor circuit (Blumberg et al., 2013, Del Rio-Bermudez et al., 2017, Roffwarg et al., 1966, Sokoloff et al., 2020). Sleep in early infancy involves myoclonic twitches during active sleep (the precursor to REM; Kayser and Biron, 2016). These twitches have been shown to generate in the brainstem, which may have implications for experience-dependent development of the sensorimotor system (Blumberg et al., 2013, Khazipov et al., 2004, Tiriac et al., 2015). One study recorded extracellular activity in postnatal day 8 and postnatal day 12 rats as they went about their typical sleep-wake cycles and found that the red nucleus showed prominent theta activity during active sleep (Del Rio-Bermudez et al., 2017). By postnatal day 12, theta oscillations in the hippocampus and red nucleus were interactive. The results demonstrate that active sleep may be critical for functional connectivity early in development.
3.1.2. The role nREM sleep in brain development
With regards to nREM sleep, studies provide support for a functional role of SWS/SWA and sleep spindles. The synaptic homeostasis hypothesis proposes that synaptic downscaling occurs during SWS, a process critical for handling our learning and experiences during the day (Tononi and Cirelli, 2006). The hypothesis not only suggests that SWS may be critical for cortical plasticity but that it may also support cortical processes during early brain development through global synaptic depression (Kurth et al., 2010).
Sleep spindle characteristics have been linked to multiple brain development processes (see Fernandez and Lüthi, 2020 for review). For instance, spindle-like rhythms (~10 Hz) are one of the earliest signs of organized electrical activity in the developing brains of rats and humans (Hanganu-Opatz, 2010) and have been suggested to act as templates for cortical map organization (Lüthi, 2014). Specifically, spindle bursts, which are identified early in development, have been proposed in the configuration of the body map (e.g., sensorimotor system), thus playing a critical role in the development of the somatosensory and motor cortex (Cirelli and Tononi, 2015). Subcortically generated twitches during quiet sleep (QS; precursor to NREM sleep) in human infants have been shown to occur synchronously with sleep spindles (Sokoloff et al., 2021). As these twitches increase with age, so too does the rate of sleep spindles across portions of the motor and somatosensory cortex. This synchrony between twitches and spindles suggests the development of functional connectivity with sensorimotor structures. Collectively, these findings support that sleep in early life may play an instrumental role in structural brain development and that specific physiological components of sleep may be responsible for optimizing neural networks.
The hypothesis that changes in sleep EEG parallel maturational changes in the brain stemmed from a seminal study, which found that the amplitude of delta waves during nREM sleep reached a peak in early childhood and then significantly declined across adolescence (Feinberg et al., 1977). The tremendous post-natal growth in cortical connectivity (Conel, 1941, Conel, 1963) may explain increases in EEG wave amplitude during this time since the size of these waves is reliant on the amount of interconnected cortical neurons (Feinberg Hibi and Carlson, 1977, Feinberg and Campbell, 2010).
Work in non-human animals also demonstrates that aspects of sleep develop simultaneously with cortical plasticity. For instance, a study in rats found that the beginning of the critical period for visual development goes hand-in-hand with nREM sleep homeostasis (Frank et al., 1998). Specifically, sleep deprivation did not increase nREM sleep EEG activity until the fourth postnatal week. The authors took this to suggest that the regulatory relationship between wake and nREM sleep develops alongside periods of heightened cortical plasticity. Collectively, work in non-human animals proposes parallel development of both sleep EEG and brain maturation.
3.1.3. Topography of SWA
With regards to human studies, while many are cross-sectional in nature and thus make it difficult to rule out causality, they provide valuable insight into the possible associations and trajectories of sleep and brain maturation. There appear to be age-dependent changes in SWA topography. For instance, one cross-sectional study examined the topographical distribution of sleep EEG from early childhood to adolescence (Kurth et al., 2010). The results illustrated age-dependent differences in SWA topography. That is, SWA was most concentrated over posterior regions during early childhood and then gradually shifted to more central and then frontal derivations by late adolescence.This trajectory of SWA parallels the course of cortical gray matter maturation (Huttenlocher and Dabholkar, 1997, Shaw et al., 2008). These results are further supported by a study investigating the relationship between sleep SWA and various markers of cortical maturation during adolescence (Buchmann et al., 2011). Using MRI and high-density EEG, the authors found that SWA and cortical gray matter decreases during adolescence (possibly due to synaptic pruning) and that the relationship between SWA and gray matter volume/thickness is heightened in areas showing the greatest maturational changes. Thus, it is possible that the large gray matter volume in children reflects, at least in part, higher synaptic density. As children are learning immensely and rapidly from their environment, frequent consolidation of the days’ learning may be necessary in the form of naps.
3.1.4. Relation between brain development and early sleep patterns
It may be that memory and brain development play a role in nap habituality (the frequency at which a child naps or not). Napping status is of particular interest, as studies have found that when deprived of a midday nap, habitually napping children have greater memory detriment than children who are non-nappers (Kurdziel et al., 2013; Leong et al., 2021). An explanation for this difference is that habitual nappers may have a less developed short-term memory storage (i.e., hippocampus) and thus require frequent consolidation in the form of naps to secure learning from the day. Non-nappers in comparison may have more mature hippocampi and, therefore, staying awake does not confer a similar detriment to memory.
Riggins and Spencer (2020) showed that in younger children (4–6-year-olds), hippocampal subfield volume varied as a function of nap status. That is, children who were “nappers,” had larger CA1 volumes in the body of the hippocampus compared to children who were no longer napping. This supports the hypothesis that hippocampi in nappers may be less developed (i.e., larger prior to synaptic downscaling) compared to non-nappers. While this study did not assess sleep physiology in relation to hippocampal volume, it provides support for the notion that early sleep patterns parallel development in the brain.
Further, there is work to suggest that sleep disturbances in early childhood may be associated with morphologic changes in subcortical structures, particularly in the context of autism spectrum disorder (ASD; MacDuffie et al., 2020). In this study, infants were assessed at 6, 12, and 24 months with MRI scans and parent reported measures of sleep problems. They found that infant sleep problems were related to hippocampal volume trajectories only in high-risk infants who developed ASD. Specifically, sleep onset difficulties were associated with increased hippocampal volume from 6 to 24 months only for high-risk infants who developed ASD. This finding is in line with evidence supporting that early cortical overgrowth is related to later social deficits (Hazlett et al., 2017). While the study does not examine more objective measures of sleep (i.e., actigraphy or polysomnography), the longitudinal reports of sleep problems provide initial evidence that sleep problems in infancy precede ASD diagnosis and are associated with altered hippocampal volume trajectories in infants at high familial risk who go on to develop ASD. Thus, it may be that early sleep disturbances impact typical sleep physiology and, in turn, may compromise typical cortical maturation. It is important to note, however, that early life sleep problems have different origins (e.g., family dynamics) and can often be addressed through interventions (Jenni and O’Connor, 2005, Tikotzky, 2017).
3.1.5. SWA localization
More recent work demonstrates that sleep topography and sleep EEG coherence reflect developing neural networks. However, most of these studies are cross-sectional and/or involve prospective brain measures. While such studies make it difficult to illustrate the development of electrical cortical activity and brain maturation, these works collectively provide support that in early childhood and adolescence, sleep microstructure parallels and changes with brain development. For instance, one longitudinal study examined sleep physiology every 6 months over a period of 6 years in children 6–18 years of age found that delta power decreased earliest at an occipital derivation (O1) and latest at a frontal derivation (Fz; Feinberg et al., 2011). This propagation of delta power is similar to the trajectory of cortical thickness, where occipital regions mature first and frontal regions last (Shaw et al., 2008). A very similar time course of SWA has been found in non-human animals (Olini et al., 2013, Song et al., 1997). The parallel shift in distribution provides support that delta activity may reflect underlying processes of cortical maturation.
Recent advances have identified a sleep EEG marker that reflects processes of brain maturation, that is, the ratio of frontal to occipital SWA (F/O-ratio; Kurth et al., 2010). This ratio allows for the quantification of regional development of SWA topography in nREM sleep. Particular interest surrounds the F/O-ratio since growing evidence suggests SWA distribution reflects developing neural networks (Buchmann et al., 2011a; Kurth et al., 2013; Kurth et al., 2015).
For example, high-density EEG recordings, anatomical MRI, and behavioral assessments in children and adolescents were used to investigate the relation between SWA topography, anatomy, and behavioral skills (Kurth et al., 2012). Maturation of SWA topography was found to predict the maturation of motor skills. That is, the more mature (frontal-oriented) the topography of SWA was, the better subjects performed on the tasks. EEG coherence may also reflect processes of brain connectivity (Kurth et al., 2013; Tarokh et al., 2010). Longitudinal sleep assessments revealed that while coherence decreased intra-hemispherically across the night, there was an inter-hemispheric increase overnight (Kurth et al., 2013). This inter-hemispheric increase demonstrates a transitional stage of sleep-dependent reinforcement of brain connectivity. The authors note that such findings have not been identified in adults, thus, it may be that such connectivity patterns are specific to early neural network maturation.
Other studies support that SWA localization may be a marker of experience-dependent plasticity (Timofeev et al., 2020, Wilhelm et al., 2014). For instance, using a visuomotor adaptation task, high-density EEG recordings, and MRI, one study examined whether local experience-dependent changes in SWA varied as a function of brain maturation in children, adolescents, and adults (Wilhelm et al., 2014). Following learning of a visuomotor task, sleep SWA was heightened over the right parietal cortex relative to a condition without prior learning. This local increase in SWA was most pronounced in children. Moreover, baseline SWA in the parietal area and gray matter volume in the right parietal area were significantly associated with this local increase in SWA. These results demonstrate that markers of brain maturation highlight experience-dependent plasticity and may be important to how specific brain regions respond to learned experiences.
Sleep EEG markers of neurodevelopment may also be helpful in identifying anomalous brain maturation. For instance, one study examined regional maturational changes in SWA topography in children with attention-deficit/hyperactivity disorder (ADHD; Ringli et al., 2013). They found that children with ADHD showed a less mature SWA distribution (more SWA over central versus prefrontal regions) than typically developing controls of the same age and sex. Another study compared nREM sleep in 13-to-30-month-olds with ASD to age-matched typically developing children (Page et al., 2020). Using high density EEG, results showed that there were topographically significant decreases in fast theta oscillations (5–7.25 Hz), fast sigma oscillations (15–16 Hz), and an increase in beta oscillations (20–25 Hz) in children with ASD compared to controls. These frequency bands have been associated with social and cognitive development at this age (Page et al., 2018). Thus, the results from the study suggest that specific topographic features of nREM sleep may provide critical insight into atypical brain development. These studies overall support that sleep EEG markers may be useful for early diagnosis of developmental disorders.
Further implications for typical and atypical development reside in evidence demonstrating that the propagation of slow waves is linked with brain myelin content (Kurth et al., 2017; LeBourgeois et al., 2019). The F/O-ratio in children (2–8 years) strongly predicted brain myelin development at a follow-up examination 3.5 years later (LeBourgeois et al., 2019). This study complements work done in animals, collectively suggesting that neuronal activity specific to sleep plays a role in brain myelin development. Another study in children (2–13 years old) examined the associations between sleep slow oscillatory propagation characteristics (e.g., duration, cortical involvement) and white matter myelin microstructure (Kurth et al., 2017). The authors found that cortical involvement was positively associated with myelin content, while slow oscillation speed was a negative indicator of myelin content. Furthermore, slow oscillation distance was positively associated with whole brain and interhemispheric myelin content. These results demonstrate that slow oscillation features such as distance, cortical involvement, and speed reflect neuronal connectivity. Collectively, these studies provide support for the role sleep EEG features may play in reflecting underlying sleep-dependent plasticity (Muller et al., 2016, Yang et al., 2014a).
3.2. Relations between sleep and brain microstructure
Dendritic spines are projections stemming from dendrites, which form efficient contacts with axons of other neurons, thus playing a critical role in synaptic plasticity (Sala and Segal, 2014, Smith et al., 2014). Likewise, synaptic pruning is essential to brain development. By removing excess synapses, the brain becomes more efficient (Sakai, 2020). This renormalization is important for maintaining proficient brain function.
Sleep in particular has been shown to promote branch-specific formation and maintenance of dendritic spines (Chauvette et al., 2012, Frank et al., 2001, Tononi, 2009, Yang et al., 2014b). For instance, REM sleep in young mice (postnatal day 21–30) was shown to support memory consolidation by maintaining learning-induced spines necessary for motor skill improvement (Li et al., 2017). Sleep has also been associated with increased spine density in hippocampal CA1 and dentate gyrus in mice 2–3 months of age (Havekes et al., 2016, Raven et al., 2019). These studies provide support for spine formation and maintenance being critical support for learned information early in life.
The pruning of spines is equally critical for long-term development, with evidence supporting sleep’s role in the process. A number of studies suggest the role of SWS in particular in synaptic pruning (Diering et al., 2017, Ghilardi et al., 2000, Vyazovskiy et al., 2008). Elegant work done in rat models has shown that there is increased intracortical connectivity during wakefulness and decreased connectivity during sleep (Diering et al., 2017, Vyazovskiy et al., 2008). In adolescent and adult mice, spines were found to be established and lost during both sleep and wake (Maret et al., 2011). In particular, the authors found that in adolescent mice, there was a 2% net loss in spines after sleep and a 1% gain in spines following wakefulness. However, in adult mice, spine loss and gain in the sensorimotor cortex was not impacted by sleep and wake. These results demonstrate that spine turnover in the sensorimotor cortex is affected by behavioral states specifically during early adolescence. Collectively, these studies suggest sleep may modulate synaptic connections important for learning and memory.
4. Conclusions: understanding and promoting sleep for healthy cognitive development
In the present review, we have outlined how sleep changes developmentally and, specifically, how these changes may reflect modifications in early brain development in both humans and non-human animals. Broadly speaking, studies support that topographical shifts in maximal SWA, from more posterior regions to more anterior regions, reflect a similar trajectory to cortical maturation (Kurth, Ringli et al., 2010). Work examining both typical development and atypical neurodevelopment (ADHD, ASD) suggests that sleep spectral analyses may play a critical part in elucidating underlying cortical conditions (Page et al., 2020, Ringli et al., 2013). Evidence provided here likewise supports that cortical maturation may in turn reflect changes in early sleep patterns and sleep physiology localization. These results would seem to indicate that sleep and brain development may not be mutually exclusive. That is, it may be that early sleep patterns are indicative of brain maturation. However, whether early sleep characteristics play a causal functional role in brain development or are merely a reflection of cortical maturation is still unclear and requires further investigation.
Work reviewed here highlights the role of sleep early in life on sleep regulation and brain development. Studies in naps in particular and their impact on subsequent overnight physiology (SWS/SWA) show that naps may be important to dissipate the heightened sleep pressure accumulated during the first half of the day, such that missing the midday nap may be detrimental to learning processes (Kurdziel et al., 2013). However, it remains unclear whether SWS in recovery sleep holds similar or different functions on learning and memory depending on early sleep patterns (i.e., habitual and non-habitual nappers). Future work would benefit from examining how memory consolidation over nocturnal sleep changes across this biphasic to monophasic sleep transition. By examining sleep (both naps and overnight sleep) and memory in nappers and non-frequent nappers, studies will be able to show (1) how overnight sleep physiology is impacted by whether one naps or does not nap, and whether one needs to nap or not and (2) how might the changes in sleep EEG impact memory consolidation in children who are nappers and non-frequent nappers. In implementing both behavioral and sleep EEG measures in young children, studies will be able to elucidate the functional role of SWS following nap deprivation in early childhood and speak to how time in classrooms can be organized to accommodate children who still rely on naps and those who do not.
Studies reviewed here also demonstrate that while sleep plays an important role in cognition, sleep and sleep-related cognitive processing may be influenced by brain development (Leong et al., 2021). That is, for habitual nappers, it may be that their hippocampi are not developed enough to hold onto the memories for a full day and therefore the memories need to be offloaded more frequently (via consolidation over a nap). We review preliminary support for this hypothesis where habitual and non-habitual nappers differ in hippocampal subfield volume (Riggins and Spencer, 2020).
Up to this point, studies have examined sleep and brain relations across ages. However, there is a dearth of data exploring the nap transition in early childhood. Considering that it is a unique time in development, with significant changes in both sleep and the brain, it is imperative that future longitudinal work not only examine sleep/brain relations across ages but also across developmental milestones (e.g., the transition out of napping). This will help to better understand early sleep patterns and their implications in early childhood development. Further, a stronger approach to examining the relation between physiological changes in sleep and brain maturation would be to investigate these measures longitudinally and concurrently rather than prospectively. In doing so, we can better understand the developmental trajectory and association between early sleep and brain maturation.
This review highlights a growing interest in childhood sleep and its potential role in brain development. Considering the importance of naps in particular, it remains a puzzle as to why children stop napping anywhere between 3 and 5 years of age. As nap times are being shortened and/or eliminated from the preschool schedule in order to fit more content into the curriculum, awareness of the work reviewed here as well as the directions for future work will help inform how best to utilize napping in the context of classroom curriculum, educational policy, and informing sleep guidelines for parents.
Data Statement
No datasets were generated or analyzed during the current study (review article).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Support for this work is provided by the National Heart, Lung, and Blood Institute (NIH R01 HL111695), USA. The authors are grateful to Dr. Kirby Deater-Deckard and Dr. Sara Whitcomb for their feedback on iterations of the manuscript.
Core concepts
-
1.
Developmental changes in sleep may reflect changes in early brain development.
-
2.
Likewise, cortical maturation may reflect changes in early sleep patterns and topography. Thus, the association between sleep and brain development may not be mutually exclusive.
-
3.
Sleep early in life is critical to sleep regulation (relieving sleep pressure and influencing overnight sleep) and brain development during this time.
Research agenda
-
1.
Examine whether sleep characteristics play a causal functional role in brain development or is merely a reflection of cortical maturation.
-
2.
Investigate the functional role of SWS following nap deprivation in early childhood.
-
3.
Studies of overnight sleep are few particularly in early childhood and necessary for understanding the contribution of REM and interactions of REM with SWS.
-
4.
Conduct longitudinal studies examining sleep/brain relations across developmental milestones and not just across ages.
References
- Bersagliere A., Achermann P. Slow oscillations in human non-rapid eye movement sleep electroencephalogram: Effects of increased sleep pressure. J. Sleep. Res. 2010;19(1 Pt 2):228–237. doi: 10.1111/J.1365-2869.2009.00775.X. [DOI] [PubMed] [Google Scholar]
- Blumberg M.S., Marques H.G., Lida F. Twitching in sensorimotor development from sleeping rats to robots. Curr. Biol. 2013;23(12):R532–R537. doi: 10.1016/J.CUB.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély A.A., Daan S., Wirz-Justice A., Deboer T. The two-process model of sleep regulation: A reappraisal. J. Sleep. Res. 2016;25(2):131–143. doi: 10.1111/JSR.12371. [DOI] [PubMed] [Google Scholar]
- Buchmann A., Ringli M., Kurth S., Schaerer M., Geiger A., Jenni O.G., Huber R. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb. Cortex. 2011;21(3):607–615. doi: 10.1093/CERCOR/BHQ129. [DOI] [PubMed] [Google Scholar]
- Campbell I.G., Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc. Natl. Acad. Sci. 2009;106(13):5177–5180. doi: 10.1073/PNAS.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Herman A.B., West G.B., Poe G., Savage V.M. Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development. Sci. Adv. 2020;6(38):398–416. doi: 10.1126/SCIADV.ABA0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon M.A., Dement W.C. Chapter 2-Normal human sleep: An overview. Princ. Pract. Sleep. Med. 2005;4(1):13–23. [Google Scholar]
- Chauvette S., Seigneur J., Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75(6):1105–1113. doi: 10.1016/J.NEURON.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokroverty S. An overview of sleep. Sleep. Disord. Med. 1994:7–16. doi: 10.1016/B978-0-7506-9002-7.50007-0. [DOI] [Google Scholar]
- Cirelli C., Tononi G. Cortical development, EEG rhythms, and the sleep/wake cycle. Biol. Psychiatry. 2015;77(12):1071. doi: 10.1016/J.BIOPSYCH.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson B.C., Durkin J., Aton S.J. Form and function of sleep spindles across the lifespan. Neural Plast. 2016 doi: 10.1155/2016/6936381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conel J. Harvard University Press,; 1941. The Postnatal Development of the Human Cerebral Cortex: The Cortex of the One-month Infant. [Google Scholar]
- Conel J. Harvard University Press,; 1963. The Postnatal Development of the Human Cerebral Cortex: The Cortex of the Four-year Child. [Google Scholar]
- Cremone A., Kurdziel L.B.F., Fraticelli-Torres A., McDermott J.M., Spencer R.M.C. Napping reduces emotional attention bias during early childhood. Dev. Sci. 2017;20(4) doi: 10.1111/desc.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby B., LeBourgeois M.K., Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics. 2005;115(1 Suppl):225–232. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K.F., Parker K.P., Montgomery G.L. Sleep in infants and young children: Part one: Normal sleep. J. Pedia Health Care. 2004;18:65–71. doi: 10.1016/S0891-5245(03)00149-4. [DOI] [PubMed] [Google Scholar]
- de Lima A., Montero V., Singer W. The cholinergic innervation of the visual thalamus: An EM immunocytochemical study. Exp. Brain Res. 1985;59(1):206–212. doi: 10.1007/BF00237681. [DOI] [PubMed] [Google Scholar]
- Del Rio-Bermudez C., Kim J., Sokoloff G., Blumberg M.S. Theta oscillations during active sleep synchronize the developing rubro-hippocampal sensorimotor network. Curr. Biol. 2017;27(10):1413–1424. doi: 10.1016/J.CUB.2017.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering G.H., Nirujogi R.S., Roth R.H., Worley P.F., Pandey A., Huganir R.L. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 2017;355(6324):511–515. doi: 10.1126/SCIENCE.AAI8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin Bridi M.C., Aton S.J., Seibt J., Renouard L., Coleman T., Frank M.G. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci. Adv. 2015;1(6) doi: 10.1126/sciadv.1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg Hibi S., Carlson V.R. Changes in EEG amplitude during sleep with age. The Aging Brain and Senile Dementia. Advances in Behavioral Biology. 1977;23:85–98. doi: 10.1007/978-1-4684-3093-6_6. [DOI] [Google Scholar]
- Feinberg I., Campbell I.G. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56–65. doi: 10.1016/J.BANDC.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Feinberg I., De Bie E., Davis N.M., Campbell I.G. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. Sleep. 2011;34(3):325. doi: 10.1093/SLEEP/34.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L.M.J., Lüthi A. Sleep spindles: Mechanisms and functions. Physiol. Rev. 2020;100:805–868. doi: 10.1152/physrev.00042.2018. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Issa N.P., Stryker M.P. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30(1):275–287. doi: 10.1016/S0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Frank, M.G., Issa, N.P., Stryker, M.P., & Keck, W.M. (2001). Sleep enhances plasticity in the developing visual cortex. Neuron (Vol. 30). [DOI] [PubMed]
- Frank M.G., Morrissette R., Heller H.C. Effects of sleep deprivation in neonatal rats. Am. J. Physiol. 1998;275(1 44–1) doi: 10.1152/AJPREGU.1998.275.1.R148. [DOI] [PubMed] [Google Scholar]
- Galland B.C., Taylor B.J., Elder D.E., Herbison P. Normal sleep patterns in infants and children: A systematic review of observational studies. Sleep. Med. Rev. 2012 doi: 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Ghilardi M.F., Ghez C., Dhawan V., Moeller J., Mentis M., Nakamura T., Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871(1):127–145. doi: 10.1016/S0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Hahn M., Joechner A.K., Roell J., Schabus M., Heib D.P.J., Gruber G., Hoedlmoser K. Developmental changes of sleep spindles and their impact on sleep-dependent memory consolidation and general cognitive abilities: A longitudinal approach. Dev. Sci. 2019;22(1) doi: 10.1111/DESC.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L., Berger L.M., Lebourgeois M.K., Brooks-Gunn J. Social and demographic predictors of preschoolers’ bedtime routines. J. Dev. Behav. Pediatr. 2009;30(5):394. doi: 10.1097/DBP.0B013E3181BA0E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu-Opatz I.L. Between molecules and experience: Role of early patterns of coordinated activity for the development of cortical maps and sensory abilities. Brain Res. Rev. 2010;64(1):160–176. doi: 10.1016/J.BRAINRESREV.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Havekes R., Park A.J., Tudor J.C., Luczak V.G., Hansen R.T., Ferri S.L., Abel T. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area. ELife. 2016;5:CA1. doi: 10.7554/ELIFE.13424.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett H.C., Gu H., Munsell B.C., Kim S.H., Styner M., Wolff J.J., Piven J. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iglowstein I., Jenni O.G., Molinari L., Largo R.H. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/PEDS.111.2.302. [DOI] [PubMed] [Google Scholar]
- Jenni O.G., Carskadon M.A. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27(4):774–783. doi: 10.1093/SLEEP/27.4.774. [DOI] [PubMed] [Google Scholar]
- Jenni O.G., LeBourgeois M.K. Understanding sleep–wake behavior and sleep disorders in children: the value of a model. Curr. Opin. Psychiatry. 2006;19(3):282. doi: 10.1097/01.YCO.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni O.G., LeBourgeois M.K. Understanding sleep–wake behavior and sleep disorders in children: The value of a model. Curr. Opin. Psychiatry. 2006;19(3):282. doi: 10.1097/01.YCO.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni O.G., O’Connor B.B. Children’s sleep: An interplay between culture and biology. Pediatrics. 2005;115(Supplement_1):204–216. doi: 10.1542/peds.2004-0815B. [DOI] [PubMed] [Google Scholar]
- Kayser M.S., Biron D. Sleep and development in genetically tractable model organisms. Genetics. 2016;203(1):21–33. doi: 10.1534/GENETICS.116.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R., Sirota A., Leinekugel X., Holmes G.L., Ben-Ari Y., Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432(7018):758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kocevska D., Muetzel R.L., Luik A.I., Luijk M.P.C.M., Jaddoe V.W., Verhulst F.C., Tiemeier H. The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: The generation R study. Sleep. 2017;40(1) doi: 10.1093/SLEEP/ZSW022. [DOI] [PubMed] [Google Scholar]
- Kurdziel L., Duclos K., Spencer R.M.C. Sleep spindles in midday naps enhance learning in preschool children. Proc. Natl. Acad. Sci. USA. 2013;110(43):17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Lassonde J.M., Pierpoint L.A., Rusterholz T., Jenni O.G., McClain I.J., Achermann P, LeBourgeois M.K. Development of nap neurophysiology: preliminary insights into sleep regulation in early childhood. J. Sleep. Res. 2016;25(6):646–654. doi: 10.1111/JSR.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S., Olini N, Huber R., LeBourgeois M. Sleep and early cortical development. Curr. Sleep. Med. Rep. 2015;1(1):64–73. doi: 10.1007/s40675-014-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Achermann P., Rusterholz T., Lebourgeois M.K. Development of brain EEG connectivity across early childhood: Does sleep play a role. Brain Sci. 2013;3(4):1445–1460. doi: 10.3390/BRAINSCI3041445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Riedner B.A., Dean D.C., O’Muircheartaigh J., Huber R., Jenni O.G., Deoni SCL, LeBourgeois M.K. Traveling slow oscillations during sleep: A marker of brain connectivity in childhood. Sleep. 2017;40(9) doi: 10.1093/SLEEP/ZSX121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S., Ringli M., Geiger A., LeBourgeois M., Jenni O., Huber R. Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. J. Neurosci. 2010;30(40):13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth Salome, Ringli M., LeBourgeois M., Geiger A., Buchmann A., Jenni O., Huber R. Mapping the electrophysiological marker of sleep depth reveals skill maturation in children and adolescents. NeuroImage. 2012;63(2):959. doi: 10.1016/J.NEUROIMAGE.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J.C., Mahone E.M., Mason T.B.A., Scharf S.M. The effects of napping on cognitive function in preschoolers. J. Dev. Behav. Pediatr. 2011;32(2):90. doi: 10.1097/DBP.0B013E318207ECC7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne J.V., Arend R., Rosenbaum D., Smith A., Weissbluth M., Binns H.J., Christoffel K.K. Sleep and behavior problems among preschoolers. J. Dev. Behav. Pediatr. 1999;20(3):164–169. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- LeBourgeois M.K., Dean D.C., Deoni S.C.L., Kohler M., Kurth S. A simple sleep EEG marker in childhood predicts brain myelin 3.5 years later. NeuroImage. 2019;199:342–350. doi: 10.1016/J.NEUROIMAGE.2019.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong R.L.F., Yu N., Ong J.L., Ng A.S.C., Jamaluddin S.A., Cousins J.N., Chee M.W.L. Memory performance following napping in habitual and non-habitual nappers. Sleep. 2021;44(6):1–11. doi: 10.1093/SLEEP/ZSAA277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ma L., Yang G., Gan W.B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017;20(3):427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhandwala S., Spencer R.M.C. Slow wave sleep in naps supports episodic memories in early childhood. Dev. Sci. 2021;24(2) doi: 10.1111/DESC.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E.K.S., Frey L.C., Britton J.W., Hopp J., Korb P., Koubeissi M.Z., Pestana-Knight E.M. The normal EEG - Electroencephalography (EEG): An introductory text and atlas of normal and abnormal findings in adults, children, and infants. Am. Epilepsy Soc. 2016 doi: 10.5698/978-0-9979756-0-4. [DOI] [PubMed] [Google Scholar]
- Lüthi A. Sleep spindles: Where they come from, what they do. Neuroscientist. 2014;20(3):243–256. doi: 10.1177/1073858413500854. [DOI] [PubMed] [Google Scholar]
- MacDuffie K.E., Shen M.D., Dager S.R., Styner M.A., Kim S.H., Paterson S., Estes A.M. Sleep onset problems and subcortical development in infants later diagnosed with autism spectrum disorder. Am. J. Psychiatry. 2020;177(6):518–525. doi: 10.1176/appi.ajp.2019.19060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret N., Girardeau G., Todorova R., Goutierre M., Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 2016;19(7):959–964. doi: 10.1038/NN.4304. [DOI] [PubMed] [Google Scholar]
- Maret S., Faraguna U., Nelson A.B., Cirelli C., Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 2011;14(11):1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic A., Kaess M., Tarokh L. Environmental factors shape sleep EEG connectivity during early adolescence. Cereb. Cortex. 2020;30(11):5780–5791. doi: 10.1093/CERCOR/BHAA151. [DOI] [PubMed] [Google Scholar]
- McCarley R.W., Greene R.W., Rainnie D., Portas C.M. Brainstem neuromodulation and REM sleep. Semin. Neurosci. 1995 doi: 10.1006/smns.1995.0037. [DOI] [Google Scholar]
- McClain I.J., Lustenberger C., Achermann P., Lassonde J.M., Kurth S., LeBourgeois M.K. Developmental changes in sleep spindle characteristics and sigma power across early childhood. Neural Plast. 2016;2016 doi: 10.1155/2016/3670951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M., Bergmann T.O., Marshall L., Born J. Fast and slow spindles during the sleep slow oscillation: Disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L., Piantoni G., Koller D., Cash S.S., Halgren E., Sejnowski T.J. Rotating waves during human sleep spindles organize global patterns of activity that repeat precisely through the night. ELife. 2016:5. doi: 10.7554/ELIFE.17267.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M.M., Carskadon M.A., Guilleminault C., Vitiello M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/SLEEP/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Olini N., Kurth S., Huber R. The effects of caffeine on sleep and maturational markers in the rat. PLOS ONE. 2013;8(9) doi: 10.1371/JOURNAL.PONE.0072539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page J., Lustenberger C., Frӧhlich F. Social, motor, and cognitive development through the lens of sleep network dynamics in infants and toddlers between 12 and 30 months of age. Sleep. 2018;41(4) doi: 10.1093/SLEEP/ZSY024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page J., Lustenberger C., Frӧhlich F. Nonrapid eye movement sleep and risk for autism spectrum disorder in early development: A topographical electroencephalogram pilot study. Brain Behav. 2020;10(3) doi: 10.1002/BRB3.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M., Birch D., Uauy R., Peirano P. The effect of sleep state on electroretinographic (ERG) activity during early human development. Early Human Development. 1999;55(1):51–62. doi: 10.1016/S0378-3782(99)00006-7. [DOI] [PubMed] [Google Scholar]
- Rasch B., Born J. About sleep’s role in memory. Physiol. Rev. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven F., Meerlo P., Van der Zee E.A., Abel T., Havekes R. A brief period of sleep deprivation causes spine loss in the dentate gyrus of mice. Neurobiol. Learn. Mem. 2019;160:83–90. doi: 10.1016/J.NLM.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen, A., & Kales, A. (1968). A manual for standardized terminology, techniques and scoring system for sleep stages in human subjects. Brain Information Service. [DOI] [PubMed]
- Riggins T., Spencer R.M.C. Habitual sleep is associated with both source memory and hippocampal subfield volume during early childhood. Sci. Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-72231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli M., Souissi S., Kurth S., Brandeis D., Jenni O.G., Huber R. Topography of sleep slow wave activity in children with attention-deficit/hyperactivity disorder. Cortex. 2013;49(1):340–347. doi: 10.1016/J.CORTEX.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Roffwarg H.P., Muzio J.N., Dement W.C. Ontogenetic development of the human sleep-dream cycle the prime role of “dreaming sleep” in early life may be in the development of the central nervous system. Science. 1966;152(3722):604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Rusterholz T., Hamann C., Markovic A., Schmidt S.J., Achermann P., Tarokh L. Nature and nurture: Brain region-specific inheritance of sleep neurophysiology in adolescence. J. Neurosci. 2018;38(43):9275–9285. doi: 10.1523/JNEUROSCI.0945-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai J. Core concept: How synaptic pruning shapes neural wiring during development and, possibly, in disease. Proc. Natl. Acad. Sci. 2020;117(28):16096–16099. doi: 10.1073/PNAS.2010281117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C., Segal M. Dendritic spines: The locus of structural and functional plasticity. Physiol. Rev. 2014;94(1):141–188. doi: 10.1152/PHYSREV.00012.2013. [DOI] [PubMed] [Google Scholar]
- Schlieber M., Han J. The sleeping patterns of Head Start children and the influence on developmental outcomes. Child.: Care, Health Dev. 2018;44(3):462–469. doi: 10.1111/CCH.12522. [DOI] [PubMed] [Google Scholar]
- Schoch S.F., Castro-Mejía J.L., Krych L., Leng B., Kot W., Kohler M., Kurth S. From Alpha Diversity to Zzz: Interactions among sleep, the brain, and gut microbiota in the first year of life. Prog. Neurobiol. 2022;209 doi: 10.1016/J.PNEUROBIO.2021.102208. [DOI] [PubMed] [Google Scholar]
- Scholle S., Zwacka G., Scholle H.C. Sleep spindle evolution from infancy to adolescence. Clin. Neurophysiol. 2007;118(7):1525–1531. doi: 10.1016/J.CLINPH.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Shaffery J.P., Lopez J., Bissette G., Roffwarg H.P. Rapid eye movement sleep deprivation in post-critical period, adolescent rats alters the balance between inhibitory and excitatory mechanisms in visual cortex. Neurosci. Lett. 2006;393(2–3):131–135. doi: 10.1016/J.NEULET.2005.09.051. [DOI] [PubMed] [Google Scholar]
- Shaffery J.P., Sinton C.M., Bissette G., Roffwarg H.P., Marks G.A. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110(3):431–443. doi: 10.1016/S0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.M. Why we sleep. Sci. Am. 2003;289(5):92–97. doi: 10.1038/SCIENTIFICAMERICAN1103-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K.N.S., Werchan D., Goldstein M.R., Sweeney L., Bootzin R.R., Nadel L., Gómez R.L. Sleep confers a benefit for retention of statistical language learning in 6.5 month old infants. Brain Lang. 2017;167:3–12. doi: 10.1016/J.BANDL.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Smith K., Kopeikina K.J., Fawcett-Patel J.M., Leaderbrand K., Gao R., Schürmann B., Penzes P. Psychiatric risk factor ANK3/Ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron. 2014;84(2):399–415. doi: 10.1016/J.NEURON.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.S., Edmed S.L., Staton S.L., Pattinson C.L., Thorpe K.J. Correlates of naptime behaviors in preschool aged children. Nat. Sci. Sleep. 2019;11:27. doi: 10.2147/NSS.S193115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff G., Dooley J.C., Glanz R.M., Wen R.Y., Hickerson M.M., Evans L.G., Blumberg M.S. Twitches emerge postnatally during quiet sleep in human infants and are synchronized with sleep spindles. Curr. Biol.: CB. 2021;31(15):3426–3432. doi: 10.1016/J.CUB.2021.05.038. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff G., Hickerson M.M., Wen R.Y., Tobias M.E., McMurray B., Blumberg M.S. Spatiotemporal organization of myoclonic twitching in sleeping human infants. Dev. Psychobiol. 2020;62(6):697–710. doi: 10.1002/DEV.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.J., Ming G.L., Fon E., Bellocchio E., Edwards R.H., Poo M.M. Expression of a putative vesicular acetylcholine transporter facilitates quantal transmitter packaging. Neuron. 1997;18(5):815–826. doi: 10.1016/S0896-6273(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Spanò G., Gómez R.L., Demara B.I., Alt M., Cowen S.L., Edgin J.O. REM sleep in naps differentially relates to memory consolidation in typical preschoolers and children with Down syndrome. Proc. Natl. Acad. Sci. 2018;115(46):11844–11849. doi: 10.1073/PNAS.1811488115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R.M.C. The role of naps in memory and executive functioning in early childhood. Adv. Child Dev. Behav. 2021;60:139–158. doi: 10.1016/BS.ACDB.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Tarokh L., Carskadon M.A., Achermann P. Developmental changes in brain connectivity assessed using the sleep EEG. Neuroscience. 2010;171(2):622–634. doi: 10.1016/J.NEUROSCIENCE.2010.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikotzky L. Parenting and sleep in early childhood. Curr. Opin. Psychol. 2017;15:118–124. doi: 10.1016/J.COPSYC.2017.02.016. [DOI] [PubMed] [Google Scholar]
- Timofeev I., Schoch S.F., LeBourgeois M.K., Huber R., Riedner B.A., Kurth S. Spatio-temporal properties of sleep slow waves and implications for development. Curr. Opin. Physiol. 2020;15:172–182. doi: 10.1016/J.COPHYS.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A., Sokoloff G., Blumberg M.S. Myoclonic twitching and sleep-dependent plasticity in the developing sensorimotor system. Curr. Sleep. Med. Rep. 2015;1(1):74–79. doi: 10.1007/s40675-015-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. Slow wave homeostasis and synaptic plasticity. J. Clin. Sleep. Med. 2009;5(2 SUPPL) doi: 10.5664/JCSM.5.2S.S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep function and synaptic homeostasis. Sleep. Med. Rev. 2006;10(1):49–62. doi: 10.1016/J.SMRV.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Touchette E., Dionne G., Forget-Dubois N., Petit D., Pérusse D., Falissard B., Montplaisir J.Y. Genetic and environmental influences on daytime and nighttime sleep duration in early childhood. Pediatrics. 2013;131(6):e1874–e1880. doi: 10.1542/PEDS.2012-2284. [DOI] [PubMed] [Google Scholar]
- Touchette E., Dionne G., Forget-Dubois N., Petit D., Pérusse D., Falissard B., Montplaisir J.Y. Genetic and environmental influences on daytime and nighttime sleep duration in early childhood. Pediatrics. 2013;131(6):e1874–e1880. doi: 10.1542/PEDS.2012-2284. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V., Cirelli C., Pfister-Genskow M., Faraguna U., Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 2008;11(2):200–208. doi: 10.1038/NN2035. [DOI] [PubMed] [Google Scholar]
- Weissbluth M. Naps in children: 6 months-7 years. Sleep. 1995;18(2):82–87. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- Werth E., Achermann P., Borbély A.A. Fronto-occipital EEG power gradients in human sleep. J. Sleep. Res. 1997;6(2):102–112. doi: 10.1046/J.1365-2869.1997.D01-36.X. [DOI] [PubMed] [Google Scholar]
- Wilhelm I., Kurth S., Ringli M., Mouthon A.L., Buchmann A., Geiger A., Huber R. Sleep slow-wave activity reveals developmental changes in experience-dependent plasticity. J. Neurosci. 2014;34(37):12568–12575. doi: 10.1523/JNEUROSCI.0962-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.E., Horst J.S. Goodnight book: sleep consolidation improves word learning via storybooks. Front. Psychol. 2014;5:184. doi: 10.3389/fpsyg.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K.E., Miller A.L., Lumeng J.C., Chervin R.D. Sleep environments and sleep durations in a sample of low-income preschool children. J. Clin. Sleep. Med. 2014;10(3):299–305. doi: 10.5664/JCSM.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Lai C.S.W., Cichon J., Ma L., Li W., Gan W.-B. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344(6188):1173–1178. doi: 10.1126/SCIENCE.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Lai C.S.W., Cichon J., Ma L., Li W., Gan W.-B. Sleep promotes branch-specific formation of dendritic spines after learning. Sci. (N. Y., N. Y. ) 2014;344(6188):1173. doi: 10.1126/SCIENCE.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke K., Wilhelm I., Bayramoglu M., Klein S., Born J. Children’s initial sleep-associated changes in motor skill are unrelated to long-term skill levels. Dev. Sci. 2017;20(6) doi: 10.1111/DESC.12463. [DOI] [PubMed] [Google Scholar]
- Züst M.A., Ruch S., Wiest R., Henke K. Implicit vocabulary learning during sleep is bound to slow-wave peaks. Curr. Biol. 2019;29(4):541–553. doi: 10.1016/j.cub.2018.12.038. [DOI] [PubMed] [Google Scholar]