Abstract
Evaluation of gastrointestinal (GI) biopsies is a multistep process that includes reviewing an appropriate history, determining sample quality, and evaluating histologic sections. Selected diagnostic parameters that, in combination with intestinal histopathology, can be useful to localize disease to the intestinal tract in the horse include hypoproteinemia and hypoalbuminemia, ultrasound evidence of increased thickness of the small intestinal wall, and alterations in glucose or D-xylose absorption tests. Biopsies may be acquired either endoscopically, or via laparoscopy or standing flank incisional approaches. GI sections should be evaluated using a systematic approach that includes both architectural changes and inflammatory cell infiltrates. Although strategies have been developed for assessment of GI biopsies from the dog and cat, a standardized approach to interpretation of the equine GI biopsy has yet to be developed. GI biopsies pose several challenges to the pathologist, especially for endoscopic biopsies in which the quality of the specimen and its orientation may vary greatly. Architectural changes are arguably the most critical changes to evaluate. In a horse with chronic GI inflammation, such as occurs in idiopathic inflammatory bowel disease (IBD), the cell types encountered frequently are macrophages, eosinophils, lymphocytes, and plasma cells. Increased numbers of these cell types are categorized loosely as mild, moderate, and severe. Specific forms of idiopathic IBD have been further classified by this infiltrate as granulomatous enteritis, eosinophilic enteritis, and lymphoplasmacytic enteritis; there is limited information on microscopic changes with each. Unfortunately, microscopic GI lesions are usually nonspecific, and determination of etiology requires further investigation.
Keywords: biopsy, gastrointestinal, horses, inflammatory bowel disease
Gastrointestinal (GI) biopsy is an essential tool in the diagnosis of chronic alimentary diseases. In veterinary medicine, guidelines for interpretation of GI biopsies are best described in the canine and feline literature. The information available regarding GI biopsies in the horse is more limited, despite the use of this technique in the diagnosis of equine GI disease.
Our goal in this review is to consolidate relevant information from the canine, feline, and where available, the equine, literature to 1) present some of the special circumstances and strategies for GI biopsy collection in the horse, 2) provide the pathologist with a review of general concepts for interpretation of GI biopsies, and where possible, 3) provide specific information on the histopathology of chronic intestinal inflammation, including idiopathic inflammatory bowel disease (IBD) in the horse. A limitation of our review is the information gaps on accepted histopathologic changes in the equine GI tract that are associated with chronic GI inflammation, including idiopathic IBD, and other chronic conditions of the equine GI tract. To address this limitation, correlations from the appropriate small animal literature are applied. Correlation must be done with caution, with consideration of the likely species differences.
Special considerations for GI biopsy
GI biopsy is a valuable tool in the diagnosis of alimentary disease given that biopsy can lead to characterization of inflammatory infiltrates, identification of major mucosal architectural changes, detection of neoplastic processes, and potentially the presence of pathogens. However, there are unique aspects in the interpretation of GI biopsies that the pathologist should recognize to avoid overinterpretation.11,40 First, the quality of the biopsies can vary widely, which greatly impacts the ability to accurately interpret histopathologic changes. Second, a distinctive histologic feature of the GI tract is the variation in the proportion of lymphocytes, plasma cells, and to a lesser extent eosinophils and macrophages, in the resident mucosal cell population of various segments of the GI tract. Differentiating the resident cell population from true inflammatory infiltrates is difficult and, in some cases, can be subjective, given that limited information is available about the normal leukocytic population in the equine GI tract. 33 Third, diagnosis of neoplasia relies heavily on the quality of the biopsy and the location of the neoplasm in the intestinal wall; superficial endoscopic biopsies will not reach a neoplasm in the deep submucosa or muscularis. Moreover, there can be significant inflammation in segments of bowel adjacent to a neoplasm. Thus, biopsy of a region adjacent to a neoplasm may lead to a diagnosis of inflammation, while the underlying neoplasm goes undetected. Finally, the surface area of the GI tract is vast and, unless a lesion is visible grossly or the disease process is distributed widely, the probability of obtaining samples that are truly representative of the underlying disease process is not high.
Although obtaining GI biopsies is routine in canine and feline patients, there are additional challenges associated with collection of equine GI biopsies related to the size of the animal, and the associated anesthetic, surgical, and post-surgical risks.
Clinical information for the equine patient
As with any biopsy submission, evaluation of an appropriate case history is important for evaluation of equine GI biopsies. This assessment will assist in understanding the chronicity and severity of the disease, potential differentials based on the clinical history, and factors that will influence the histopathology such as prior or current therapies (e.g., glucocorticoid administration). Key information will vary with each case, but should include the clinical signs and their duration, current and prior therapies, rectal palpation findings, and alterations in CBC, urinalysis, and serum chemistry results. Results of fecal examination and/or prior deworming are important in addressing potential parasitic disease; underlying parasitic disease can lead to intestinal inflammatory changes that share features with idiopathic IBD.13,21 Weight loss and diarrhea are commonly associated with GI disease. Less commonly, extraintestinal factors, including diet as well as hepatic, renal, or cardiac disease, can cause similar clinical signs. 22 The diagnostic approach for diarrhea and weight loss in the horse typically involves various noninvasive procedures that may be used in combination with GI biopsy. This approach can help to eliminate extraintestinal factors and potentially begin to localize disease within the GI tract.
A multi-pronged approach is used in the diagnosis of GI disease in the horse, and a single assay that will lead to a diagnosis has not been identified. In addition to GI biopsy, findings that have been correlated with GI disease in the horse include: 1) hypoproteinemia and hypoalbuminemia, 2) ultrasound evidence of increased thickness of the small intestinal wall, and 3) alterations in glucose or D-xylose absorption tests. 26
Hypoproteinemia can suggest protein-losing enteropathy once other forms of protein loss have been ruled out, as well as intestinal malabsorption. 37 Protein loss can be associated with various changes, including mucosal inflammatory infiltrates, villus atrophy, altered epithelial permeability, and lymphangiectasia. 5 Hypoproteinemia has been associated with chronic intestinal inflammation and the different forms of idiopathic IBD, including lymphoplasmacytic, eosinophilic, and granulomatous enteritis.
In dogs and cats, abdominal ultrasound is commonly used to detect thickening of the intestinal wall. Although this finding does not identify a specific etiology, it can suggest an inflammatory or neoplastic cellular infiltrate. Abdominal ultrasound, either percutaneous or trans-rectal, is used in the horse to identify thickening of the small intestine and has been correlated with findings of rectal palpation.3,30
The oral glucose tolerance test (OGTT) is a commonly used noninvasive method for the assessment of small intestinal absorptive capacity in the horse. Failure of blood glucose to increase after oral administration of glucose can indicate small intestinal malabsorption, which has been associated with a variety of inflammatory, neoplastic, and idiopathic diseases. A decreased OGTT has been associated with malabsorption and may suggest granulomatous enteritis, lymphoplasmacytic enteritis, and eosinophilic enteritis. A number of factors that influence intestinal glucose absorption need to be considered when interpreting OGTT results, including gastric emptying, intestinal transit time, renal clearance, and intestinal epithelial transport activity.20,26 OGTT results are best interpreted along with other test results, such as ultrasound and blood protein concentrations, in the diagnosis of GI disease. Altered OGTT has also been associated with normal intestinal histology. In one study, low OGTT values were correlated with histologic changes in only 2 of the 5 horses studied. 3 The authors concluded that a larger study was needed and suggested that the lack of correlation may be related to only glucose being measured in this absorption assay and that early malabsorptive changes may not lead to clear histologic changes.3,23
GI biopsies
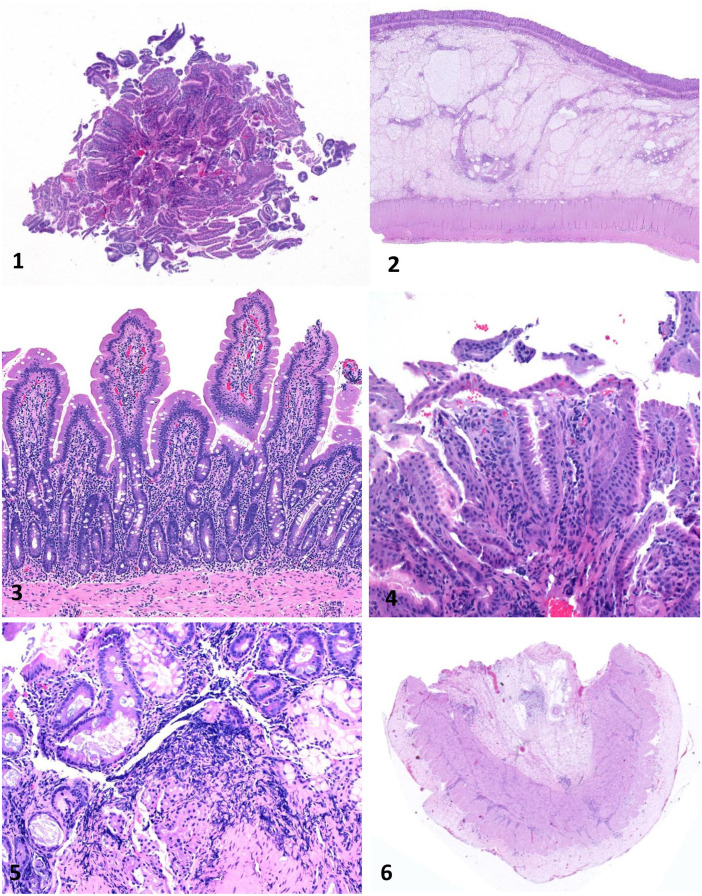
There are 2 general approaches to GI biopsies: endoscopic and surgical. There are advantages and drawbacks to each approach (Table 1). Endoscopic biopsies employ a flexible endoscope to collect pinch biopsies from the mucosal wall of the GI tract; this is one of the most widely used techniques across species. The key advantage of endoscopic biopsies is that they are noninvasive. The endoscope is guided by a fiberoptic camera allowing for evaluation of gross changes along the mucosal surface and targeted collection of the biopsy sample. Moreover, it is possible to take several biopsies from a segment of the GI tract. There are also several disadvantages to endoscopic biopsies. The biopsies are superficial and sample the mucosa and, in some cases, the superficial submucosa (Fig. 1). It is common for the orientation of the section to be distorted, and identification of full crypt-villus units can be difficult. Superficial sections that consist of only villi or sections oriented so that only lamina propria and cross-sections of crypts are visible are common, and these greatly limit the ability to evaluate the section and make an interpretation (Fig. 1). The skill of the endoscope operator is critical to the quality of the sample; truly, there are masters of this technique. The learning curve is fairly steep for the novice, and, early on, the problems mentioned above are commonplace.
Table 1.
Advantages and disadvantages of endoscopic versus surgical biopsies of the equine gastrointestinal tract.
| Type of biopsy | Invasiveness | Areas where it works well | Layers included | Tissue distortion | Processing artifact | Operator expertise required | No. of biopsies |
|---|---|---|---|---|---|---|---|
| Endoscopic | Less invasive | Stomach, duodenum, rectum, distal colon | Mucosa; sometimes superficial submucosa | Frequent | Frequent | Significant | Many |
| Surgical | More invasive | All organs | Full thickness | Infrequent | Infrequent | Routine surgical skills required | Fewer |
Figures 1–6.
Equine gastrointestinal biopsies. Figure 1. Gastric endoscopic biopsy showing only mucosa sectioned tangentially. H&E. Figure 2. Colon surgical biopsy, including full thickness of the colonic wall. H&E. Figure 3. Duodenal surgical biopsy. This biopsy is mostly free of artifact and well oriented, so that numerous villus-crypt units with associated lamina propria can be evaluated. H&E. Figure 4. Gastric endoscopic biopsy that includes only the superficial aspect of the mucosa with artifactually detached mucosal epithelium. H&E. Figure 5. Duodenal endoscopic biopsy with severe crushing artifact. H&E. Figure 6. Duodenal biopsy with muscularis and serosa, but missing mucosa and submucosa. H&E.
Endoscopy is widely used in horses to examine and biopsy the stomach (glandular and non-glandular), pylorus, proximal duodenum, and rectum. 4 Gastroscopy is particularly useful for diagnosis of gastric ulcerative disease in the horse. 25 Success rates for detection of microscopic lesions in biopsies collected via endoscopy from horses are variable, but have been reported as a 56% detection rate for horses with clinical signs of chronic intestinal inflammation. 3 A relatively new technique, capsule endoscopy, has been used in the horse whereby a wireless camera within a capsule is placed into the stomach via nasogastric tube and the camera transmits images as it travels through the GI tract. 44
Surgical biopsies are collected via laparotomy. The advantage of surgical biopsies is that the biopsy is typically full thickness and consists of mucosa, submucosa, muscularis, and serosa (Fig. 2). Orientation is often better, with multiple full fields containing full crypt-villus units. Because orientation is often accurate and architectural landmarks are preserved, a full-thickness biopsy is more straightforward to evaluate than an endoscopic biopsy. The main disadvantages of full-thickness biopsies are that they require an invasive surgical approach, and generally fewer samples are collected. The risk of anesthesia is another disadvantage of surgical biopsies, and possibly the reason that these biopsies are less common than nonsurgical ones. Rectal biopsy is a safe and relatively low-cost approach that is widely used in horses with chronic GI disease. The benefit of rectal biopsy is that it can be done in the standing horse and does not require excessive restraint or anesthesia. 32 Collection does not require special instruments, although endoscopic exam can be used for biopsy of the proximal rectum and small colon.
Rectal biopsy has been reported to have variable accuracy in detection of GI inflammation. In a 2018 study, 82% of horses with clinical signs consistent with idiopathic IBD had pathologic changes in rectal biopsies. 3 However, in another study, rectal biopsy detected histologic lesions in only 50% of horses with a history of intestinal disease. 18 In California, analysis of 100 cases with clinical history of GI disease in which rectal biopsy was evaluated revealed histologic lesions in ~30% of the cases (F. Uzal, unpublished). Capacity of this approach to recover a biopsy with representative lesions will depend on the distribution of the lesions, and specifically the degree of involvement of the distal colon and rectum. A lack of lesions in a rectal biopsy obviously does not rule out GI disease. 32
As in other species, full-thickness GI biopsies in the horse requires abdominal surgery. Ventral midline celiotomy is a common approach in horses, and an advantage is that there is good access to small and large intestinal segments for examination, palpation, and biopsy. However, laparotomy is invasive and requires general anesthesia and close postoperative monitoring. Post-surgical risks can be high. Debilitated horses may not be good candidates for this approach. 1
Standing laparotomy can be used to obtain full-thickness biopsies in the horse, which can circumvent many of the risks associated with ventral midline celiotomy. Standing laparotomy alone does not require special equipment and is more readily available to the equine surgeon. Addition of laparoscopic equipment brings several advantages to the procedure, including increased access to the intestine for visual examination and biopsy. Repeated biopsy is also an option with laparoscopy. Postoperative complications are reported to be low. 35
Quality of biopsies
A key factor in obtaining an accurate interpretation or diagnosis from both endoscopic and full-thickness GI biopsies is the characteristics of the biopsies, which includes the number of biopsies and their overall quality. 46 Problems arise most frequently with endoscopic samples. The number and quality of the biopsies should be recorded in the biopsy report, which will communicate to the submitter features of the sections that influenced their evaluation and interpretation. 38
Reporting the number of biopsies will alert the submitter to any loss of sections, which is common with endoscopic biopsies. Often the endoscopic sections are loosely secured to a matrix (e.g., sponge or cellulose) before they are placed in a cassette for processing. A common problem is that one or more or the sections will break free from the matrix and float free within the cassette. Hence, some of the tissues will be out of the plane of section during sectioning and will not be included on the slide. If there is a discrepancy in the number of tissues submitted and the number reported, then recutting the block may bring more tissues into the same plane. Reporting the number of full-thickness biopsies is also important to ensure that all submitted sections were processed and were present on the slide.
One of the greatest limiting factors for evaluation of a GI biopsy is the quality of the samples. The gold standard is a biopsy that is sufficiently deep, free of handling artifact, and is oriented so that multiple full villus-crypt units with associated lamina propria can be evaluated (Fig. 3). As will be discussed in the following sections, these anatomical features are critical in evaluating the architectural and cellular components of the tissue. Whereas high-quality samples allow thorough examination, poor-quality samples greatly impede this process when key components of the tissue are lacking or are indistinguishable. 38 The most frequently encountered problem in endoscopic biopsies, including those from the equine stomach and duodenum, is that the biopsy includes only the superficial aspect of the mucosa. These biopsies often consist of portions of detached mucosal epithelium (stomach) or villi (duodenum; Fig. 4). The more superficial the biopsy, the more likely it will be deemed “nondiagnostic” by the pathologist. Having appropriate orientation of the sample is required, and another common problem is that the section consists of cross-sections through the crypts with villi not present (Fig. 1). Artifact is another obstacle to evaluation, and this includes crushing of the tissue during collection and folding of the section during processing (Fig. 5). Even if depth and orientation of the tissue are good, artifact can decrease the tissue available for examination. The main problems encountered with full-thickness biopsies are related to orientation of the tissue. A common situation occurs when only tunica muscularis is present and mucosa and submucosa are not in the section (Fig. 6). In some cases, this can be corrected by reorienting the tissue in the cassette and recutting.
Overview of parameters for evaluation of GI inflammation
Given the density of resident inflammatory cells in the GI tract, the pathologist must not overinterpret their presence and use inflammatory cells alone to support a diagnosis of GI inflammation. Diagnosis of inflammation in the GI tract relies on detection of both architectural and inflammatory cell changes. For dogs and cats, a set of guidelines for the interpretation of histologic lesions has been developed by the World Small Animal Veterinary Association. These guidelines provide standards by which the pathologist can evaluate and score mucosal inflammation.6,46 Similar guidelines have not yet been developed for the equine GI tract. Although there is a need to establish inflammatory parameters specific for the equine GI tract, there are strategies and principles for small animals that also hold true for the equid.
Again, GI sections should be evaluated using a systematic approach that focuses on both architectural changes and inflammatory cell infiltrates. In the following sections, some broad principles established in small animals will be applied to the equid. 46 More specific details on these parameters can be identified in the references. 6 For each parameter, a scoring system developed for canine inflammation reports no change, mild, moderate, and marked change. This may be a reasonable starting point for a scoring system for equine enteric inflammation. 46 A study of histologic parameters in the small intestine of horses without clinical disease has defined villus lengths and typical leukocyte densities in the GI mucosa of adult horses 33 ; this information will be used as a starting point for defining deviations from normal intestine in the horse and a basis for generation of an equine standard of GI histologic evaluation. The values from that reference 33 will be cited where appropriate with the small animal data in the following sections.
Architectural changes in GI biopsies
Architectural changes are arguably the most critical changes to evaluate in a GI biopsy. This is because, in many cases, alterations in numbers of lymphocytes, plasma cells, and eosinophils can be subjective, especially when there is a suspected mild or moderate increase in the numbers of these cells. Although architectural changes alone do not point to a specific etiology, their presence can be a strong indicator of mucosal inflammation and are less subjective than assessment of numbers of inflammatory cells. 46 Architectural components to be evaluated in the GI biopsy include villi, lacteals, crypts, fibrous tissue, goblet cells, and epithelium. Again, the ability to evaluate these entities depends heavily on the quality of the biopsy. Systematic examination from the tip of the villus to the base of the crypt along with the associated lamina propria, and, if available, submucosa, is essential. In cases of equine chronic intestinal inflammation, including idiopathic IBD, the constellation of these changes will often vary with chronicity and severity, that, combined with inflammatory cell infiltrates, may allow for further characterization of the specific classification or etiology of GI inflammation.
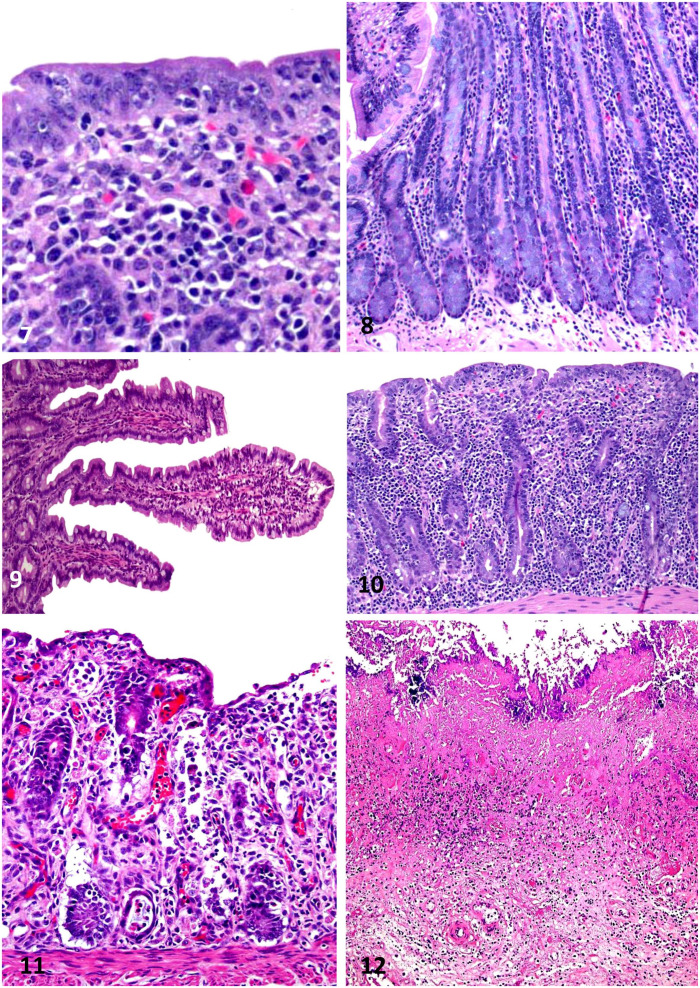
Evaluation of the epithelium includes that covering the surface and proliferative compartments. Key parameters are loss of terminal differentiation, for example, loss of brush border in the small intestine or lack of foveolar epithelium in the fundic stomach. Looking for attenuation of the epithelium from columnar to low cuboidal or flat epithelium is important, and this may progress to areas of epithelial erosion (Fig. 7). 17 A common change in the proliferative compartments, such as the gastric isthmus and crypts, is hyperplasia. In the stomach, this can manifest as mucus cell hyperplasia, and in the small intestine and colon as crypt hyperplasia with crowding of the crypt by immature enterocytes, dilation of the crypt space, and loss of goblet cell differentiation (Fig. 8).
Figures 7–12.
Equine intestinal biopsies. Figure 7. Rectal biopsy from a horse with eosinophilic proctitis of unknown etiology. Observe loss and attenuation of the superficial epithelium and many eosinophils in the superficial lamina propria. H&E. Figure 8. Crypt hyperplasia in the small intestine of a horse, also with loss of goblet cell differentiation. The etiology of this lesion was not determined. H&E. Figure 9. Normal small intestinal mucosa with long, slender villi. H&E. Figure 10. Severe villus blunting in the jejunum of a horse with lymphoplasmacytic enteritis, giving the tissue the appearance of colon. H&E. Figure 11. Crypt dilation and necrosis in the colon; the crypt epithelium is attenuated and/or degenerate, and dead cells are present in the lumen of crypts. The cause of this lesion was undetermined. H&E. Figure 12. Fibrosis in the lamina propria of a horse with severe, chronic necrotizing colitis caused by Clostridioides difficile. H&E.
Villus atrophy is a common reaction pattern to mucosal injury and is a common change in the horse. 37 Histologically, normal villi are long, slender, and uniform across the section (Fig. 9). Villus length in the horse ranges from 479 µm in the jejunum to 337 µm in the duodenum; villi are widest in the jejunum. 33 Villus atrophy manifests as blunting of the villus to a dome shape or even flattening of the intestinal surface to resemble the colon (Fig. 10). In some circumstances, there is fusion of villi, which leads to a loss of the uniformity of the villi along the section. 10 It is common for villus changes to be accompanied by attenuation or erosion of the surface epithelium (Fig. 10).
Crypt lumens are normally seen as thin spaces that are bordered by enterocytes and goblet cells. Crypt changes most frequently involve dilation of the crypt space and may include irregular branching. The crypt lumen may be dilated by mucus, eosinophilic fluid, and cellular infiltrates, which are often degenerate.8,47 The term “crypt abscess” is sometimes applied to this change, especially when leukocytes are present in the lumen (Fig. 11). The severity of the crypt change is related to the degree of crypt distention and to the number of crypts affected in a section. Crypt loss is another change that may result from necrosis of the enterocytes lining the crypts, which may be the result of infectious, ischemic, or toxic injury.
Lacteals are lymphatic vessels in the intestinal mucosa that are often identified centrally within the villi as thin clear spaces lined by flat endothelium. They may also be seen in the deeper lamina propria and submucosa. In many sections they are not visible, which is typical of normal intestine. Lacteal dilation or lymphangiectasia is an architectural change that can be associated with protein-losing enteropathy and has been reported in the horse.24,39 Frequently, lacteal dilation is secondary to chronic mucosal inflammation, with obstruction of lymphatic outflow from the mucosa. Overinterpretation of visible lacteals needs to be avoided; true dilation in the dog is reserved for lacteals that approach ≥50% of the villus width. Although no such information is available in the horse, some degree of dilation can be observed in normal horses. In some cases, lacteal dilation is severe and can fill most of the villus space. 6
Fibrosis indicates chronic mucosal injury, inflammation, or ischemia, and can occur in all segments of the GI tract; it has been reported in the GI tract of horses with gastroenteric disease. 36 Microscopically, fibrosis is identified as increased collagen and fibroblasts, often within the mucosa. This change, which may occur between crypts or gastric glands, increases the separation between these structures in the lamina propria (Fig. 12). In the gastric mucosa, advanced fibrosis can lead to fibrous bands surrounding glands leading to a “nested” appearance of the glands. When severe, fibrosis can replace crypts or gastric glands.
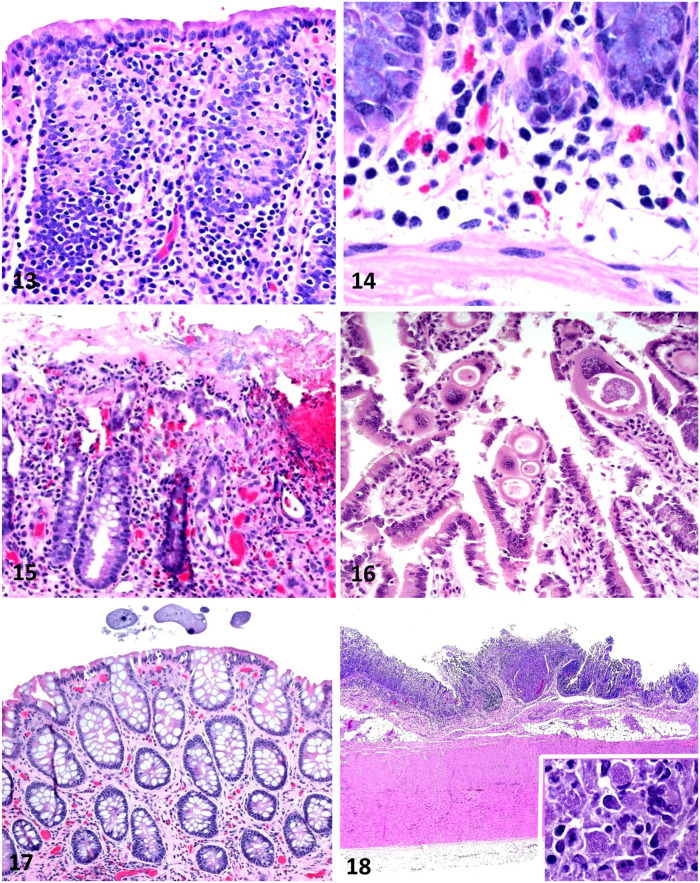
Changes in goblet cell numbers in the large intestine can be an indicator of GI inflammation, and this includes decreases and increases in goblet cell density. In the horse, these changes are often encountered in rectal biopsies. 18 Loss of goblet cells is common in the horse and can be fairly simple to detect (Fig. 13). However, increased goblet cell density (hyperplasia) may also occur and may be associated with alteration in the mucin expression profile in these horses. 27
Figures 13–18.
Equine intestinal biopsies. Figure 13. Loss of goblet cells in the colonic mucosa of a horse with chronic diarrhea. This is a nonspecific lesion in the colon of horses with diarrhea. H&E. Figure 14. Small numbers of eosinophils in the deep lamina propria of the small intestine. This is considered a normal background finding in horses and should not be interpreted as a significant lesion. H&E. Figure 15. Eosinophilic proctitis; large numbers of eosinophils are intermixed with macrophages, lymphocytes, and plasma cells within the lamina propria. The cause of this lesion was not determined. H&E. Figure 16. Eimeria leuckarti in the lamina propria of the small intestine; this parasite is usually considered an incidental finding in horses. H&E. Figure 17. Ciliated protozoa in the lumen of the colon; these parasites are usually considered an incidental finding in horses. H&E. Figure 18. Granulomatous colitis in a horse with Rhodococcus equi infection. Macrophage infiltrates are present. Inset: small coccobacilli are present in the cytoplasm of most macrophages H&E.
Inflammatory cell populations in GI biopsies
True inflammatory cell infiltrates in the GI mucosa can be detected most readily when inflammation is severe. Often in severe inflammation, the cellular changes are accompanied by the constellation of the architectural changes described in the preceding sections. Inflammatory cell infiltrates that are only mild-to-moderate in severity are challenging for the pathologist to discriminate from the normal resident leukocyte population. Again, a thorough search for architectural change will bolster a diagnosis of inflammation that is associated with a suspected increase in leukocytes.
In the normal animal, there is a resident population of leukocytes throughout the digestive tract. This population consists mainly of lymphocytes and plasma cells with fewer widely scattered eosinophils, macrophages, and indistinct mast cells.31,45 Moreover, there are organized collections of lymphoid tissue, termed gut-associated lymphoid tissue (GALT), throughout the tract. The relative proportions of leukocytes in the normal intestinal mucosa provide a reference for identifying increases in cell numbers that suggest a shift towards inflammation. During episodes of intestinal inflammation, all inflammatory cell types may increase in the intestine. In the horse with chronic GI inflammation, such as occurs in idiopathic IBD, the cell types encountered frequently are macrophages, eosinophils, lymphocytes, and plasma cells. 19 Increased numbers of these cell types are loosely categorized as mild, moderate, and marked. Four references, 3 in the horse and 1 in the dog, provide the expected number of leukocytes in different regions of the GI tract of healthy horses (Table 2).6,28,31,46
Table 2.
Expected numbers of leukocytes in the gastrointestinal tract of healthy horses and dogs.
| Horse 28 | Horse 33 | Dog 6 | |
|---|---|---|---|
| Stomach | |||
| IELs | NR | NR | 2 IELs/50 enterocytes |
| Lamina propria | NR | NR | Lym, PC <20%, 1–3 Eos/400× field |
| Duodenum | |||
| IELs | NR | NR | 5–10 IELs/50 enterocytes |
| Villus lamina propria | NR | 33 Lym, 6.75 PC, 0 Eos, 0 Neu/0.02 mm2 | Lym, PC <25%, 2 or 3 Eos/400× field |
| Crypt lamina propria | NR | 34 Lym, 11.7 PC, 0.6 Eos, 0.5 Mac, 0 Neu/0.02 mm2 | Lym, PC 1 or 2 between crypts |
| Jejunum | |||
| IELs | NR | NR | 5–10 IELs/50 enterocytes |
| Villus lamina propria | 50 Lym, 18 PC, 4 Eos/9,000 µm2 | 48 Lym, 5 PC, 0 Eos, 1 Mac, 0 Neu/0.02 mm2 | Lym, PC <25%, 2 or 3 Eos/400× field |
| Crypt lamina propria | 35 Lym, 18 PC, 9 Eos, 5 Mac/9,000 µm2 | 43 Lym, 10 PC, 1 Eos, 1 Mac, 0 Neu/0.02 mm2 | Lym, PC 1 or 2 between crypts |
| 2 or 3 Eos/400× field | |||
| Ileum | |||
| IELs | NR | NR | 5–10 IELs/50 enterocytes |
| Villus lamina propria | NR | 49 Lym, 5 PC, 0 Eos, 1 Mac, 0 Neu/0.02 mm2 | Lym, PC <25%, 2 or 3 Eos/400× field |
| Crypt lamina propria | NR | 43 Lym, 10 PC, 1 Mac, 0 Eos, 0 Neu/0.02 mm2 | Lym, PC 1 or 2 between crypts |
| 2 or 3 Eos/400× field | |||
| Colon | |||
| Small: lamina propria | NR | 31 Lym, 8 PC, 4 Eos, 0 Ma, 0 Neu/0.02 mm2 | Lym, PC 4 or 5 between crypts |
| 1 or 2 Eos/400× field | |||
| Large: lamina propria | NR | 38 Lym, 0 PC, 6 Eos, 0 Mac, 0 Neu/0.02 mm2 | NR |
Eos = eosinophil; IEL = intraepithelial lymphocytes; Lym = lymphocyte; Mac = macrophage; Neu = neutrophil; NR = not reported; PC = plasma cell.
Detecting increases in lymphocytes and plasma cells against a background of resident cells can be important, especially in the small intestine and colon. Lymphocytes should be evaluated within the epithelium, the lamina propria, and if available, the submucosa. Lymphocytes organized into GALT should be recognized to avoid interpretation as a lymphocytic inflammatory infiltrate or a neoplastic process. The normal density of lymphocytes and plasma cells must be understood. There are normally low numbers of intraepithelial lymphocytes (IELs) along the mucosal surface from the stomach to the colon (Table 2). Increases in numbers of IELs in the stomach and intestine can be a reliable indicator of inflammation, especially when accompanied by increases in lamina propria inflammatory lymphocytes.6,12
Lymphocytes and plasma cells generally represent the highest proportion of resident leukocytes in the small intestinal and colonic lamina propria, although their numbers vary throughout the GI tract. Although counting cells may not be feasible, the figures provided in Table 2 may help the pathologist to establish a relative range of normal values. In the horse, increases in lymphocytes and plasma cells may occur as the predominant inflammatory cell types or may be mixed with other leukocytes including macrophages and eosinophils. 15
Low numbers of widely scattered eosinophils are normally present throughout the GI tract (Fig. 14), and it is easy to overinterpret resident eosinophils as eosinophilic inflammation (Table 2). Eosinophilic intestinal inflammation in the horse may manifest as a predominant increase in eosinophils, or eosinophils may be intermixed in various proportions with other inflammatory cells, including macrophages, lymphocytes, and plasma cells (Fig. 15). 21
Macrophages are a fairly nondistinct population in normal GI biopsies stained with H&E (Table 2). GI inflammation with a dominance of macrophages is often straightforward to identify. In horses, such inflammation often manifests with numerous macrophages and epithelioid macrophages, with or without lymphocytes, plasma cells, and eosinophils. Macrophage inflammatory infiltrates may be arranged in the lamina propria and submucosa as focal-to-diffuse infiltrates or as distinct granulomas. 16
Protozoa
Coccidia (Eimeria leuckarti; Fig. 16) and several species of ciliated protozoa (Fig. 17) may be observed in the lumen and/or within the small intestinal and colonic mucosa, respectively, of both healthy and sick horses. The latter includes, but is not limited to, animals with diarrhea or other enteric disease. The presence of these parasites in the intestine of horses is usually considered an incidental finding. However, because large numbers of these protozoa have been seen occasionally deep in the mucosa of horses with enteritis or colitis, it has been speculated that these organisms might be responsible for enteric disease.2,7,9 Definitive evidence about the role of these parasites in enteritis or colitis of horses is, however, lacking.
Select chronic gastrointestinal inflammatory diseases of the horse
Our goal in this section is to illustrate briefly how cellular and architectural changes may manifest in selected chronic GI inflammatory diseases in the horse. Granulomatous enteritis, eosinophilic enteritis, and lymphoplasmacytic enteritis are described as forms of equine idiopathic IBD; there is limited information on typical microscopic changes with each. This section draws on the available case reports and descriptions of the forms of chronic intestinal inflammation and specifically idiopathic IBD. Clinical and pathophysiologic information on equine IBD is available in our cited references.3,18,37
Diagnosis of idiopathic IBD in the horse depends on multiple test results in combination with the clinical picture. 3 It is not possible to establish an IBD diagnosis on GI biopsy alone. The underlying cause of idiopathic IBD in horses is not defined. As with other species, various factors, such as mucosal immune responses, 14 diet, microbiota, genetics, and environmental factors, likely contribute. Although idiopathic IBD in general is our focus in this section, differential etiologies for the microscopic changes in the following sections would certainly also include enteric pathogens. For each of these forms of IBD, cellular infiltrates are associated with architectural alterations.
The major microscopic changes in equine granulomatous enteritis are infiltrations of macrophages and epithelioid macrophages into the lamina propria and submucosa. Transmural macrophage infiltrates are reported. Macrophages may be the predominant cell type or may be intermixed with lymphocytes and plasma cells.16,18 Macrophage infiltrates may be diffuse or focal, and may be organized into discrete granulomas (Fig. 18). Organized lymphoid tissue may accompany the macrophage infiltrates. 29 Architectural changes are common with granulomatous enteritis, and, in the small intestine, villi are often markedly blunted and covered by attenuated-to-flattened epithelium. Erosions may be present. Small intestinal crypts may be hyperplastic.16,17 Differentials to consider, in addition to IBD, include mycobacterosis, pythiosis, and intestinal toxins.31,34,43
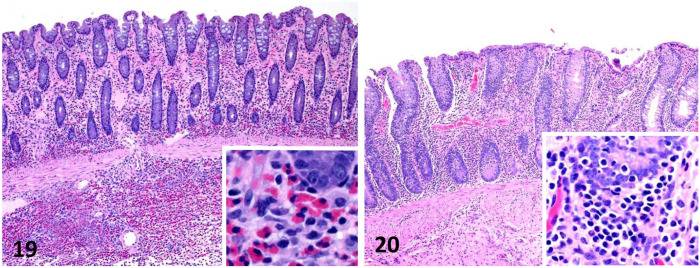
Eosinophilic intestinal inflammation can be associated with intestinal parasites, multisystemic eosinophilic epitheliotropic disease (MEED), and idiopathic eosinophilic enteritis. 37 Moreover, idiopathic eosinophilic enteritis can be focal or diffuse in the intestinal tract. 21 The cause of idiopathic eosinophilic enteritis and MEED have not been determined. Eosinophilic enterocolitis consists of variable infiltrates of eosinophils, lymphocytes, macrophages, and neutrophils in the lamina propria and submucosa (Fig. 19). In some cases, inflammation can be transmural. Architectural changes include villus blunting, epithelial attenuation, and erosions. The focal form of idiopathic eosinophilic enteritis has been suggested to be a localized exacerbation of more diffuse eosinophilic enteritis.21,42 MEED leads to lesions in numerous organs; skin and GI are involved frequently. 41 MEED lesions are often composed of eosinophils intermixed with macrophages, lymphocytes, and plasma cells. Organization into eosinophilic granulomas is also described. Eosinophilic granulomas may be associated with vasculitis in the intestinal mucosa. 18
Figures 19, 20.
Equine intestinal biopsies. Figure 19. Eosinophilic enteritis. Eosinophils and fewer lymphocytes, macrophages, and neutrophils are present in the lamina propria. Inset: higher magnification showing eosinophils in the superficial submucosa. The cause of this lesion was not determined. H&E. Figure 20. Lymphoplasmacytic enteritis consisting of infiltrates of lymphocytes and plasma cells in the lamina propria. Inset: higher magnification of the deep lamina propria showing several layers of lymphocytes and plasma cells. The cause of this lesion was not determined. H&E.
Lymphoid infiltrates—a common microscopic change in a number of chronic inflammatory conditions—often accompany other cell types, such as macrophages and eosinophils in granulomatous and eosinophilic enteritis, cyathostomosis, and lymphoma.18,37 Idiopathic lymphoplasmacytic enterocolitis is an uncommon condition that consists of various degrees of lymphoplasmacytic infiltration into the lamina propria (Fig. 20), which may be accompanied by increased IELs. Architectural changes reported include villus blunting and dilation of lacteals. 15 These changes are based on a small number of cases, and further studies are needed to fully characterize the microscopic changes.
Conclusions
Useful evaluation of GI biopsies is based on an appropriate clinical history, samples of adequate quality, and standardized assessment of histologic sections. Strategies for assessment of GI biopsies that have been developed for the dog and cat provide a good basis for standardized evaluation of equine GI biopsies. The basics themes of GI inflammation likely hold true across species with regard to response to mucosal injury. In the horse, as in all species, closely evaluating both cellular and architectural changes can provide objective evidence of true GI inflammation. The definition of the resident leukocyte population for each region of the equine GI tract will help to differentiate resident cell populations from inflammatory cell infiltrates. Key architectural changes may accompany inflammation, and include villus atrophy, enterocyte alterations, and changes of the proliferative compartment. Chronic inflammatory diseases, including idiopathic IBD, need to continue to be defined more clearly in horses.
Acknowledgments
We thank Ms. Jennifer Kempf for assistance in identifying cases in the histology archives.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jesse M. Hostetter  https://orcid.org/0000-0002-5555-5121
https://orcid.org/0000-0002-5555-5121
Francisco A. Uzal  https://orcid.org/0000-0003-0681-1878
https://orcid.org/0000-0003-0681-1878
Contributor Information
Jesse M. Hostetter, Department of Pathology, College of Veterinary Medicine, University of Georgia, Athens, GA, USA.
Francisco A. Uzal, California Animal Health and Food Safety Laboratory System, University of California–Davis, San Bernardino, CA, USA
References
- 1. Barr BS. Infiltrative intestinal disease. Vet Clin North Am Equine Pract 2006;22:e1–7. [DOI] [PubMed] [Google Scholar]
- 2. Bianchi MV, et al. Fatal parasite-induced enteritis and typhlocolitis in horses in Southern Brazil. Rev Bras Parasitol Vet 2019;28:443–450. [DOI] [PubMed] [Google Scholar]
- 3. Boshuizen B, et al. Inflammatory bowel disease (IBD) in horses: a retrospective study exploring the value of different diagnostic approaches. BMC Vet Res 2018;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camacho-Luna P, et al. Advances in diagnostics and treatments in horses and foals with gastric and duodenal ulcers. Vet Clin North Am Equine Pract 2018;34:97–111. [DOI] [PubMed] [Google Scholar]
- 5. Craven MD, Washabau RJ. Comparative pathophysiology and management of protein-losing enteropathy. J Vet Intern Med 2019;33:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Day MJ, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138(Suppl 1):S1–S43. [DOI] [PubMed] [Google Scholar]
- 7. Dubey JP, Bauer C. A review of Eimeria infections in horses and other equids. Vet Parasitol 2018;256:58–70. [DOI] [PubMed] [Google Scholar]
- 8. Erben U, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 2014;7:4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 9. Headley SA, et al. Balantidium coli-infection in a Finnish horse. Vet Parasitol 2008;158:129–132. [DOI] [PubMed] [Google Scholar]
- 10. Jacobs G, et al. Lymphocytic-plasmacytic enteritis in 24 dogs. J Vet Intern Med 1990;4:45–53. [PubMed] [Google Scholar]
- 11. Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci (Elite Ed) 2012;4:1404–1419. [DOI] [PubMed] [Google Scholar]
- 12. Jergens AE, et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol 2014;51:946–950. [DOI] [PubMed] [Google Scholar]
- 13. Kaikkonen R, et al. Diagnostic evaluation and short-term outcome as indicators of long-term prognosis in horses with findings suggestive of inflammatory bowel disease treated with corticosteroids and anthelmintics. Acta Vet Scand 2014;56:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalck KA. Inflammatory bowel disease in horses. Vet Clin North Am Equine Pract 2009;25:303–315. [DOI] [PubMed] [Google Scholar]
- 15. Kemper DL, et al. Equine lymphocytic-plasmacytic enterocolitis: a retrospective study of 14 cases. Equine Vet J Suppl 2000;32:108–112. [DOI] [PubMed] [Google Scholar]
- 16. Lindberg R. Pathology of equine granulomatous enteritis. J Comp Pathol 1984;94:233–247. [DOI] [PubMed] [Google Scholar]
- 17. Lindberg R, Karlsson L. Topography and enterocyte morphology of the small bowel mucosal surface in equine granulomatous enteritis. J Comp Pathol 1985;95:65–78. [DOI] [PubMed] [Google Scholar]
- 18. Lindberg R, et al. Rectal biopsy diagnosis in horses with clinical signs of intestinal disorders: a retrospective study of 116 cases. Equine Vet J 1996;28:275–284. [DOI] [PubMed] [Google Scholar]
- 19. Mair TS, et al. Malabsorption syndromes in horses. Equine Vet Educ 2006;18:299–308. [Google Scholar]
- 20. Mair TS, et al. Small intestinal malabsorption in the horse: an assessment of the specificity of the oral glucose tolerance test. Equine Vet J 1991;23:344–346. [DOI] [PubMed] [Google Scholar]
- 21. Mäkinen PE, et al. Characterisation of the inflammatory reaction in equine idiopathic focal eosinophilic enteritis and diffuse eosinophilic enteritis. Equine Vet J 2008;40:386–392. [DOI] [PubMed] [Google Scholar]
- 22. McGovern K. Approach to the adult horse with chronic diarrhoea. Livestock 2013;18:189–194. [Google Scholar]
- 23. Metcalfe LVA, et al. A retrospective study of horses investigated for weight loss despite a good appetite (2002–2011). Equine Vet J 2013;45:340–345. [DOI] [PubMed] [Google Scholar]
- 24. Milne EM, et al. Intestinal lymphangiectasia as a cause of chronic diarrhoea in a horse. Vet Rec 1994;134:603–604. [DOI] [PubMed] [Google Scholar]
- 25. Nadeau JA, Andrews FM. Equine gastric ulcer syndrome: the continuing conundrum. Equine Vet J 2009;41:611–615. [DOI] [PubMed] [Google Scholar]
- 26. Oliver-Espinosa O. Diagnostics and treatments in chronic diarrhea and weight loss in horses. Vet Clin North Am Equine Pract 2018;34:69–80. [DOI] [PubMed] [Google Scholar]
- 27. Olofsson KM, et al. Expression of T helper type 17 (Th17)-associated cytokines and toll-like receptor 4 and their correlation with Foxp3 positive cells in rectal biopsies of horses with clinical signs of inflammatory bowel disease. Vet J 2015;206:97–104. [DOI] [PubMed] [Google Scholar]
- 28. Packer M, et al. Quantification of immune cell populations in the lamina propria of equine jejunal biopsy specimens. J Comp Pathol 2005;132:90–95. [DOI] [PubMed] [Google Scholar]
- 29. Platt H. Chronic inflammatory and lymphoproliferative lesions of the equine small intestine. J Comp Pathol 1986;96:671–684. [DOI] [PubMed] [Google Scholar]
- 30. Porzuczek A, et al. The use of percutaneous abdominal ultrasound examination in diagnosing equine small intestinal disorders. Pol J Vet Sci 2012;15:759–766. [DOI] [PubMed] [Google Scholar]
- 31. Purcell KL, et al. Jejunal obstruction caused by a Pythium insidiosum granuloma in a mare. J Am Vet Med Assoc 1994;205:337–339. [PubMed] [Google Scholar]
- 32. Ricketts SW. Rectal biopsy—a piece of the diagnostic jigsaw puzzle. Equine Vet J 1996;28:254–255. [DOI] [PubMed] [Google Scholar]
- 33. Rocchigiani G, et al. Leukocyte numbers and intestinal mucosal morphometrics in horses with no clinical intestinal disease. J Vet Diagn Invest 2021. Epub ahead of print 23 July 2021. doi: 10.1177/10406387211031944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarradell JE, et al. Mycobacterium bovis infection in a horse with granulomatous enterocolitis. J Vet Diagn Invest 2015;27:203–205. [DOI] [PubMed] [Google Scholar]
- 35. Schambourg MM, Marcoux M. Laparoscopic intestinal exploration and full-thickness intestinal biopsy in standing horses: a pilot study. Vet Surg 2006;35:689–696. [DOI] [PubMed] [Google Scholar]
- 36. Schultheiss PC, et al. Intestinal fibrosis and vascular remodeling in ten horses and two ponies. J Vet Diagn Invest 1995;7:575–578. [DOI] [PubMed] [Google Scholar]
- 37. Schumacher J, et al. Chronic idiopathic inflammatory bowel diseases of the horse. J Vet Intern Med 2000;14:258–265. [DOI] [PubMed] [Google Scholar]
- 38. Serra S, Jani PA. An approach to duodenal biopsies. J Clin Pathol 2006;59:1133–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silva FS, et al. Mycobacterium branderi infection in a horse with granulomatous mesenteric lymphadenitis. J Comp Pathol 2019;168:30–34. [DOI] [PubMed] [Google Scholar]
- 40. Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract 2011;41:381–398. [DOI] [PubMed] [Google Scholar]
- 41. Singh K, et al. Severe pulmonary disease due to multisystemic eosinophilic epitheliotropic disease in a horse. Vet Pathol 2006;43:189–193. [DOI] [PubMed] [Google Scholar]
- 42. Southwood LL, et al. Idiopathic focal eosinophilic enteritis associated with small intestinal obstruction in 6 horses. Vet Surg 2000;29:415–419. [DOI] [PubMed] [Google Scholar]
- 43. Stegelmeier BL, Davis TZ. Toxic causes of intestinal disease in horses. Vet Clin North Am Equine Pract 2018;34:127–139. [DOI] [PubMed] [Google Scholar]
- 44. Steinmann M, et al. A wireless endoscopy capsule suitable for imaging of the equine stomach and small intestine. J Vet Intern Med 2020;34:1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uzal FA, et al. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol. 2. 6th ed. Elsevier, 2015:1–257. [Google Scholar]
- 46. Washabau RJ, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 47. Willard MD, et al. Protein-losing enteropathy associated with cystic mucoid changes in the intestinal crypts of two dogs. J Am Anim Hosp Assoc 2003;39:187–191. [DOI] [PubMed] [Google Scholar]