Abstract
Healthy horses and other animals have large numbers of resident leukocytes in the intestinal wall, but there is scant information regarding which and how many leukocytes are normally present in the equine intestinal wall. Our aim was to provide a reference range of leukocytes in the intestinal mucosal and submucosal propria of normal horses. We included in our study intestinal tissues from 22 Thoroughbred racehorses with no clinical intestinal disease, which had been euthanized because of catastrophic musculoskeletal injuries. Neutrophils, lymphocytes, eosinophils, macrophages, and plasma cells were counted in 5 random 17,600-µm2 areas of villus lamina propria of the duodenum, jejunum, and ileum, and deep lamina propria of the duodenum, jejunum, ileum, right ventral colon, left ventral colon, left dorsal colon, right dorsal colon, and small colon. Other features investigated in the same intestinal segments included villus height and width (small intestine), presence of ciliated protozoa, Paneth cells number, subcryptal leukocyte layers (number of leukocyte layers between the bottom of the crypts and the muscularis mucosae), and submucosal leukocytes. Lymphocytes were the most numerous cells in all segments analyzed, followed by plasma cells, eosinophils, macrophages, and neutrophils. Eosinophil numbers were significantly higher in both lamina propria and submucosa of the large intestine than in the small intestine. The duodenum had shorter and thinner villi than either jejunum or ileum. The data provided from our study will be useful for diagnosticians examining inflammatory processes in the intestinal tract of horses.
Keywords: anatomy, horses, intestine, lamina propria, leukocytes, villi
The gastrointestinal (GI) tract is one of the most studied systems in horses. As in most domestic species, the equine small intestine is divided into duodenum, jejunum, and ileum. The large intestine is divided into cecum, large colon, which has 4 segments (right ventral colon, RVC; left ventral colon, LVC; left dorsal colon, LDC; right dorsal colon, RDC), small colon (SC), and rectum. 12
The equine GI system can be affected by a broad spectrum of inflammatory conditions characterized, among other lesions, by infiltration of leukocytes in one or more intestinal layers. Interpretation of those lesions may be challenging because the mucosa of clinically healthy horses has an abundance of resident leukocytes, whose function is to protect the intestine from exogenous and endogenous insults. Despite the plethora of literature describing the changes occurring in the inflamed equine intestine,7,8,11 information about the leukocyte population in the intestinal mucosa of healthy horses is scant. In particular, there is a lack of consensus regarding which and how many leukocytes are considered normal in the intestinal lamina propria and submucosa. These parameters would be very helpful for diagnostic purposes, given that increased numbers of leukocytes are considered among the main criteria for the diagnosis of most inflammatory bowel diseases. 8 Other factors, such as age, diet, and physiology, may also play a role in the inflammatory response of the equine intestine, as has been reported in dogs. 10 Analyzing these factors was, however, beyond the scope of our study.
Normal proprial leukocyte populations have been studied, mostly in small companion animals, and the major findings have been reviewed in numerous articles,2,3 especially regarding intestinal biopsies. To our knowledge, however, only 2 articles have focused on the intestinal leukocyte population of clinically healthy horses. In one study, 9 eosinophils were counted in the lamina propria in different parts of the equine GI tract. Another study 6 evaluated the leukocyte population of the jejunum.
Our aim was to provide information on the number and type of proprial and submucosal leukocytes and other histologic features (i.e., morphology of small intestinal villi) in the various intestinal segments of racehorses with no gastrointestinal disease.
Materials and methods
We included 22 Thoroughbred racehorses in our study (Table 1). All horses were clinically healthy until they suffered acute catastrophic musculoskeletal injuries and were euthanized. The postmortem interval was 6–24 h. None of the animals had gross or microscopic evidence of gastrointestinal disease, and fecal flotations with saline solution were negative for parasites or eggs.
Table 1.
Sex and age of 22 racehorses included in our study of intestinal morphometrics.
| Case | Sex | Age (y) |
|---|---|---|
| 1 | G | 3 |
| 2 | G | 3 |
| 3 | F | 4 |
| 4 | F | 3 |
| 5 | F | 5 |
| 6 | M | 2 |
| 7 | M | 5 |
| 8 | M | 3 |
| 9 | F | 7 |
| 10 | G | 5 |
| 11 | F | 3 |
| 12 | M | 4 |
| 13 | F | 3 |
| 14 | G | 3 |
| 15 | G | 3 |
| 16 | G | 3 |
| 17 | F | 2 |
| 18 | G | 4 |
| 19 | F | 5 |
| 20 | F | 4 |
| 21 | G | 3 |
| 22 | F | 3 |
F = mare; G = gelding; M = stallion.
Samples of duodenum, jejunum, ileum, RVC, LVC, LDC, RDC, and SC were collected from approximately the same anatomical location in each horse, fixed in 10% neutral-buffered formalin (pH 7.4) for ≥ 24 h, and processed routinely to produce 4-µm thick sections to be stained with hematoxylin and eosin. All sections were examined, and counts performed, by one of the authors (G. Rocchigiani). Leukocytes were counted in the following micro-compartments: villus lamina propria (lamina propria within the villus), deep lamina propria (lamina propria between and below intestinal crypts), and submucosa. For each of these areas, lymphocytes (except in the submucosa), plasma cells, eosinophils, macrophages, and neutrophils were counted on 5 random non-overlapping fields of 0.02 mm2 (area of interest, AOI) using a 40× objective (Fig. 1). Neutrophils were identified by lobulated nuclei and absence of eosinophilic granules; eosinophils were recognized by bi-lobed nuclei and characteristic eosinophilic cytoplasmic granules. Plasma cells were identified by an eccentric nucleus with heterochromatin in a characteristic cartwheel arrangement, perinuclear halo, and amphophilic cytoplasm. Lymphocytes were recognized by their round nucleus, small size, and scant cytoplasm. Macrophages were identified by their larger size (~2× the lymphocyte size), large amount of often vacuolated cytoplasm, and round or reniform nucleus.
Figure 1.
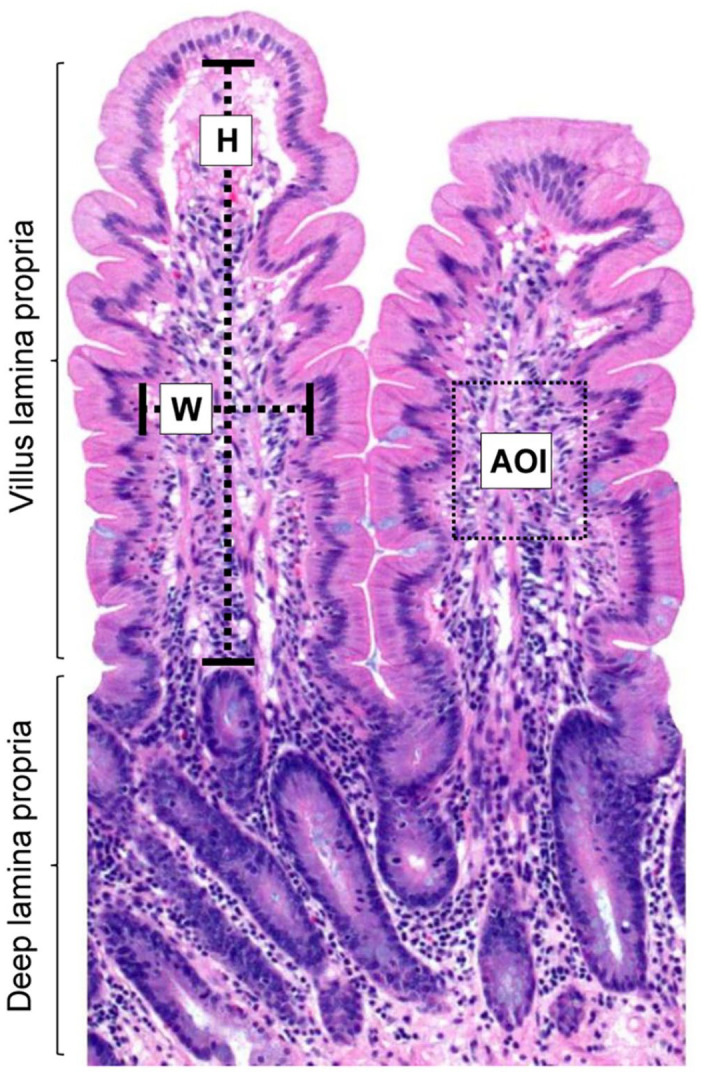
Intestinal mucosal histomorphometric analysis in a normal Thoroughbred horse. The rectangle represents an area of interest (AOI; 17,600 µm2); H = villus height; W = villus width. H&E.
Paneth cells and leukocyte layers between the bottom of intestinal crypts and the muscularis mucosae (subcryptal leukocyte layers) were evaluated in 5 entire 20× high-power fields (HPF; 1.13 mm2). An AOI larger than in the villus lamina propria was used for this calculation because Paneth cells and subcryptal leukocytes were confined to specific locations (i.e., deep intestinal crypts) and the AOI used for the villus lamina propria would have been too small to assess their real number. Submucosal evaluation was impaired in the duodenum because of the presence of Brunner glands, which obscured the deepest mucosa.
Villus measurements were taken in micrometers in the small intestinal sections, counting 5 villi per location; only intact villi were selected for these measurements. Villus height was measured from the tip of the villus (excluding epithelium) to the junction of the villus lamina propria and the deep lamina propria. The villus width was measured halfway along the height of the villus, also excluding the epithelium (Fig. 1). Presence or absence of ciliated protozoa in the gut lumen and lamina propria was determined in all segments of small and large intestine.
Software (Toupview; ToupTek Photonics) was used to measure and highlight the AOI, and the leukocytes within the AOI were counted manually. The mean with standard deviation for each segment and one-way ANOVA were calculated to determine if a statistical difference (i.e., p ≤ 0.05) was present. If a statistical difference was detected, a Tukey–Kramer test was performed to determine which group was significantly different from the other(s). For presence or absence of ciliates, a Pearson chi square test was performed to determine if a significant frequency distribution was present among intestinal segments.
Results
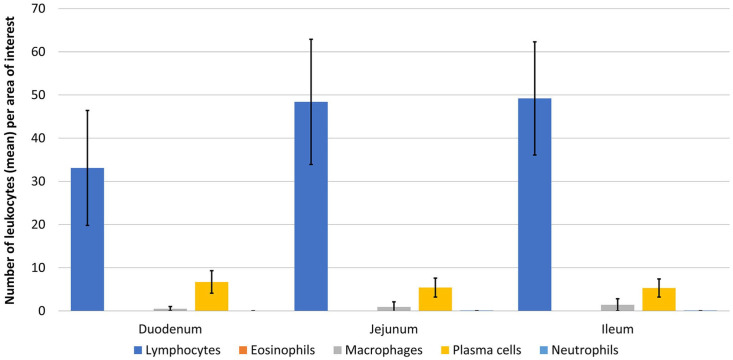
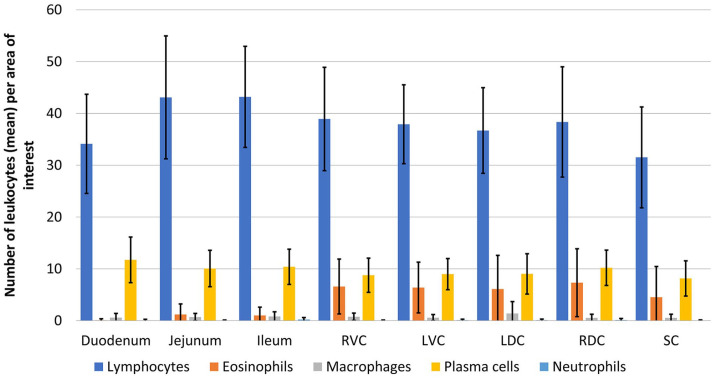
Lymphocytes were, as expected, the most common leukocytes in all intestinal segments (Tables 2, 3; Figs. 2–4). Numbers of plasma cells were significantly lower (p < 0.05) than numbers of lymphocytes in all intestinal segments. Eosinophils were present in variable numbers within the deep lamina propria and were significantly more numerous (p < 0.05) in the large than in the small intestine. No eosinophils were detected within the villus lamina propria. Scattered macrophages were present throughout the villus and deep lamina propria (0–10 within the AOI), regardless of the segment analyzed. Neutrophils were present in the lowest numbers (0–2 per AOI) compared with other leukocytes in the villus and deep lamina propria of all segments.
Table 2.
Mean, SD, and range of leukocytes per area of interest counted in villus lamina propria for each small intestinal tract segment.
| Segment | Lymphocytes | Plasma cells | Eosinophils | Macrophages | Neutrophils | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Duodenum | 33 ± 13 | 17–67 | 6 ± 2 | 3–15 | 0 | 0 | 0 | 0–3 | 0 | 0 |
| Jejunum | 48 ± 15 | 23–96 | 5 ± 2 | 1–12 | 0 | 0 | 1 ± 1 | 0–5 | 0 | 0–2 |
| Ileum | 49 ± 13 | 24–82 | 5 ± 2 | 0–12 | 0 | 0 | 1 ± 1 | 0–6 | 0 | 0–1 |
Table 3.
Mean, SD, and ranges of leukocytes per area of interest in the deep lamina propria of each intestinal tract segment.
| Segment | Lymphocytes | Plasma cells | Eosinophils | Macrophages | Neutrophils | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Duodenum | 34 ± 9 | 12–70 | 11 ± 4 | 4–26 | 0 | 0–2 | 0 ± 1 | 0–4 | 0 | 0–1 |
| Jejunum | 43 ± 12 | 28–93 | 10 ± 3 | 5–19 | 1 ± 2 | 0–12 | 0 | 0–3 | 0 | 0–1 |
| Ileum | 43 ± 9 | 29–81 | 10 ± 3 | 3–18 | 1 ± 1 | 0–7 | 0 ± 1 | 0–4 | 0 | 0–2 |
| RVC | 39 ± 10 | 20–77 | 9 ± 3 | 1–20 | 6 ± 5 | 0–21 | 0 | 0–3 | 0 | 0–1 |
| LVC | 38 ± 7 | 17–56 | 9 ± 3 | 4–17 | 6 ± 5 | 0–26 | 0 | 0–2 | 0 | 0–1 |
| LDC | 36 ± 8 | 22–59 | 9 ± 4 | 1–20 | 6 ± 6 | 0–27 | 1 ± 2 | 0–10 | 0 | 0–1 |
| RDC | 38 ± 10 | 15–55 | 10 ± 3 | 5–17 | 7 ± 6 | 0–30 | 0 | 0–2 | 0 | 0–2 |
| SC | 31 ± 9 | 12–54 | 8 ± 3 | 2–18 | 4 ± 6 | 0–41 | 0 | 0–4 | 0 | 0–1 |
LDC = left dorsal colon; LVC = left ventral colon; RDC = right dorsal colon; RVC = right ventral colon; SC = small colon.
Figure 2.
Mean number of leukocytes ±1 SD in the villus lamina propria in small intestinal segments of horses with no clinical alimentary disease.
Figure 3.
Mean number of leukocytes ±1 SD of deep lamina propria of all intestinal segments of horses with no clinical alimentary disease. LDC = left dorsal colon; LVC = left ventral colon; RDC = right dorsal colon; RVC = right ventral colon; SC = small colon.
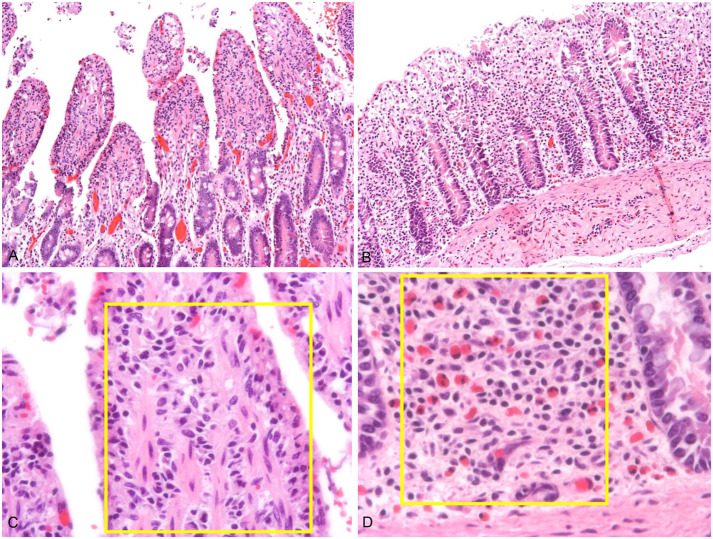
Figure 4.
Representative images of small and large intestinal mucosa, with mild autolysis. A. Low magnification of jejunal mucosa. H&E. B. Low magnification of left ventral colon. H&E. C. High magnification of the jejunal villus lamina propria. The yellow rectangle is an area of interest. H&E. D. High magnification of the deep lamina propria of the left ventral colon. The yellow rectangle is the area of interest. Note the higher number of eosinophils than in the jejunal deep lamina propria. H&E.
The number of leukocyte layers below the crypts (subcryptal leukocyte layers) was 0–3 in most anatomical locations. A subcryptal leukocyte layer count >3 was observed in 24 of 525 AOIs from all GI segments analyzed, with no statistical differences between small and large intestine. Ileum and small colon more frequently had >3 subcryptal leukocyte layers, although these results were not significantly different from other sections. No differences were evident in the number or type of leukocytes between the deep lamina propria and the subcryptal leukocyte populations. Paneth cells were detected only within the small intestinal crypt epithelium and were in significantly lower (p < 0.05) numbers in the duodenum than in the jejunum and ileum.
A variable number of leukocytes was present in the submucosa in all locations (Suppl. Table 1). The numbers of submucosal eosinophils were significantly higher (p < 0.05) in the large than in the small intestine. The duodenum had significantly (p < 0.05) shorter and thinner villi than the jejunum or ileum (Table 4).
Table 4.
Mean, SD, and range of villus height and width, for each small intestinal segment.
| Segment | Villus height | Villus width | ||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Duodenum | 337 ± 116 | 162–637 | 67 ± 13 | 42–100 |
| Jejunum | 479 ± 112 | 255–712 | 109 ± 31 | 52–187 |
| Ileum | 465 ± 112 | 299–845 | 97 ± 27 | 46–167 |
Ciliated protozoa were observed most frequently in the gut lumen, but they were also observed in the lamina propria; they were observed more frequently in the large intestine than the small intestine, although these differences were not statistically significant (p = 0.77). In some horses, ciliated protozoa were observed in multiple intestinal segments; in other horses, the ciliates were seen in just one location.
Discussion
Our study provides a practical reference range for numerous parameters applicable to the microscopic analysis of the intestine in horses. To our knowledge, there are only 2 published papers focusing on this subject.6,9 We normalized the count of those 2 studies with our AOI and compared their results with ours. Our overall eosinophil numbers were slightly lower than those reported elsewhere in all segments, 9 except for ileum, where slightly higher numbers of eosinophils were reported. 9 These minor discrepancies could be the result of a different detection method for eosinophils; we used H&E sections, the other study used Luna-stained sections. 9 Observer bias may have also skewed the results.
In a study that focused only on the jejunum, T and B lymphocytes were identified by immunohistochemistry (IHC), plasma cells by methyl green pyronin, and eosinophils, neutrophils, and macrophages by H&E. 6 The mean leukocyte proprial counts in the previous study were markedly higher for all cells (except neutrophils) than ours, in both villus and deep lamina propria. Markedly more lymphocytes (up to 77 more than our study) and plasma cells (up to 58 more than our study) were counted; this difference was not so marked for eosinophils (up to 16 more than our study) and macrophages (up to 9 more than us). In the same study, 6 just one neutrophil in one horse was reported, whereas we counted a slightly higher number in several animals. The differences observed between lymphocytes and plasma cells are likely the result of the different sensitivity and specificity of IHC and methyl green pyronin staining compared with H&E alone. Nevertheless, the count was also different for macrophages and eosinophils. Several factors, including inter-individual physiologic differences, inter-observer detection sensitivity, and variable degree of autolysis, could have played roles in these differences.
Our results agree with previous studies and confirm the overall predominance of lymphoplasmacytic populations within the intestinal lamina propria compared with other leukocytes. Also, our results agree with results 6 that indicated that in the jejunum, numbers of plasma cells were higher in the deep lamina propria than in the villus lamina propria.
Unlike the previous studies,6,9 we did not detect eosinophils within the villus lamina propria. Eosinophils are among the most easily recognizable leukocytes on H&E sections, given their distinctive morphology. A possible explanation for this difference is that we worked with a group of horses subjected to strict health management (high-profile racehorses), and parasitic infection was unlikely.
Despite the absence of eosinophils within the villus lamina propria in our study, the literature suggests that eosinophils can be found in this micro-compartment, albeit in smaller numbers than in the deep lamina propria. In general, eosinophils are usually present in low numbers within the intestinal lamina propria of horses showing no signs of inflammation. In agreement with one study, 9 we found more eosinophils in the large than in the small intestine. This predilection for the large intestine could be explained by multiple causes, including a higher frequency of parasites within this intestinal segment. It is widely known that eosinophils are the main leukocytes involved in protection against helminths, and it is also known that a large number of equine helminthic endoparasites (e.g., cyathostomes, large strongyles) have tropism for the large colon. 7 The higher number of eosinophils could represent a physiologic adaptation after centuries of coevolution of horses and their common helminthic endoparasites. However, eosinophils have also been observed in the intestine of aborted and newborn foals (authors’ unpublished observation), which were unlikely to have had contact with intestinal parasites. A larger number of eosinophils was also observed in the colonic submucosa. Our findings suggest that a fluctuating number of eosinophils could be detected in healthy horses (Suppl. Table 1). For instance, submucosal AOIs devoid of eosinophils were intermixed with other AOIs having up to 15 or 42 eosinophils in the small or large intestine, respectively. The other leukocytes counted (i.e., plasma cells, neutrophils, macrophages) were much less numerous in this micro-compartment (Suppl. Table 1)
Macrophages in our study were observed in low numbers (0–4 per lamina propria AOI), excluding a single case in which 10 macrophages were seen in a single AOI, with no statistical differences between intestinal segments. These numbers are lower than those reported by one study 6 in the jejunum, with a range of 1–23 per AOI. The reasons for this difference were not determined, but physiologic status of the horses and/or inter-observer variability may have influenced these differences.
Neutrophils were detected very rarely (i.e., 1 or 2 of these cells per AOI were observed in only 46 of 640 AOI examined), including both villus and deep lamina propria, with no statistical differences between intestinal segments. In one study, 6 only one neutrophil was detected in one jejunal section. In small companion animals, the presence of even a single neutrophil within the lamina propria is considered pathologic. 2 Our study cannot confirm or rule out an analogous significance in horses. However, considering that the horses in our study were clinically healthy, it is tempting to conclude that a small number of neutrophils (up to 2 per AOI) could be seen in the intestine of normal horses with no evidence of GI disease.
Duodenal villi were shorter and thinner than jejunal and ileal villi. The literature 5 indicates that jejunal and ileal villi of horses should be taller and wider than duodenal villi, in agreement with our findings.
Unlike in the 2 papers referred to previously,6,9 the subcryptal leukocyte layer number was consistently determined in our study. It is reported that more than 4 layers composed of lymphocytes, plasma cells, and perhaps eosinophils and neutrophils should be considered abnormal in the small intestine of dog and cats. 12 The majority of sections analyzed in our study had 0–3 subcryptal layers of the same leukocytes described in the upper lamina propria. Only a few AOI (4.5%) exhibited ≥4 subcryptal layers. Only 1 of our sections had 14 layers of lymphocytes below the intestinal crypts. This high value was very localized (only in one 20× HPF) and, considering the restricted area and the anatomical location (i.e., ileum), could represent a lymphoid mucosal cluster not organized in an evident lymphoid follicular structure but which was expanding the lamina propria. When this individual section was removed from our study, the maximum value of subcryptal layers was 7, with an average of 1.38 across all intestinal segments.
Protozoal ciliates are observed commonly within the equine large intestinal lumina. They include numerous species, belonging to multiple genera including Blepharocorys and Bundleia, and they are considered normal intestinal commensals, as in other herbivores. 1 Rarely, it has been reported that such protozoa are also involved in severe colitis. 4 Nevertheless, a clear causative relationship between these protozoa and inflammation has not been fully determined. In our study, ciliates were observed mostly in the large intestine but also in the small intestine. This finding could be explained by a peri- or postmortem migration of ciliates from the large to the small intestine. Supporting this hypothesis, ciliates were detected within the jejunum and ileum but not in the duodenum, which is the segment farthest from the large intestine. Sometimes these protozoa were also observed embedded within the most superficial lamina propria, but the absence of any inflammatory reaction seems indicative of an incidental rather than a pathologic finding.
All of the horses included in our study were young, with only a few >4 y old. Our results should be interpreted cautiously when dealing with animals of ages outside this range. Similarly, all of the horses included were Thoroughbred, and counts could be different in other horse breeds.
Diet could play a role in the inflammatory response of the canine intestine, 10 and it is possible that diet also influences the response of equine GI leukocytes. If this is the case, our counts could have been influenced by diet. However, the diet of the horses examined in our study was not known and we therefore cannot draw conclusions in this regard.
The main limitation of our study was, as commonly occurs in retrospective studies involving intestinal morphology, the incipient autolysis in some cases. Although we selected the freshest cases for our study (postmortem interval ≤24 h), some segments of the intestine had moderate autolysis, which precluded analysis, and these segments were not included in our study. IHC could have been used to overcome this difficulty and give a more detailed and precise number of leukocytes. However, despite these benefits, IHC is time consuming and expensive for routine postmortem examination, especially when multiple segments are evaluated. Our article is meant to give a practical guideline for pathologists dealing with equine postmortem samples, which are frequently in a mild-to-severe autolytic condition. The results of our study should be helpful when interpreting postmortem intestine samples of horses (Suppl. Table 2).
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211031944 for Leukocyte numbers and intestinal mucosal morphometrics in horses with no clinical intestinal disease by Guido Rocchigiani, Emanuele Ricci, Mauricio A. Navarro, Monika A. Samol and Francisco A. Uzal in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the California Horse Racing Board and the California Animal Health and Food Safety Laboratory for their support, Dr. Vladimiro Fettina for his IT assistance, and Ms. Juliann Beingesser for excellent technical assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The travel expenses for one of the authors (G. Rocchigiani) were funded by the Horse Betting Levy Board, as part of a Senior Equine Clinical Scholarship (VET/CS/027).
ORCID iDs: Guido Rocchigiani  https://orcid.org/0000-0002-3742-7636
https://orcid.org/0000-0002-3742-7636
Mauricio A. Navarro  https://orcid.org/0000-0002-7744-8052
https://orcid.org/0000-0002-7744-8052
Monika A. Samol  https://orcid.org/0000-0002-5508-4630
https://orcid.org/0000-0002-5508-4630
Francisco A. Uzal  https://orcid.org/0000-0003-0681-1878
https://orcid.org/0000-0003-0681-1878
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Guido Rocchigiani, Department of Veterinary Anatomy, Physiology and Pathology, University of Liverpool, Leahurst Campus, Neston, UK.
Emanuele Ricci, Department of Veterinary Anatomy, Physiology and Pathology, University of Liverpool, Leahurst Campus, Neston, UK.
Mauricio A. Navarro, California Animal Health and Food Safety Laboratory, School of Veterinary Medicine, University of California–Davis, San Bernardino, CA, USA
Monika A. Samol, California Animal Health and Food Safety Laboratory, School of Veterinary Medicine, University of California–Davis, San Bernardino, CA, USA
Francisco A. Uzal, California Animal Health and Food Safety Laboratory, School of Veterinary Medicine, University of California–Davis, San Bernardino, CA, USA.
References
- 1. Belzecki G, et al. Methods for the cultivation of ciliated protozoa from the large intestine of horses. FEMS Microbiol Lett 2016;363:fnv233. [DOI] [PubMed] [Google Scholar]
- 2. Day MJ, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138(Suppl 1):S1–S43. [DOI] [PubMed] [Google Scholar]
- 3. Jergens AE, et al. Maximizing the diagnostic utility of endoscopic biopsy in dogs and cats with gastrointestinal disease. Vet J 2016;214:50–60. [DOI] [PubMed] [Google Scholar]
- 4. Kirkpatrick CE, Saik JE. Ciliated protozoa in the colonic wall of horses. J Comp Pathol 1988;98:205–212. [DOI] [PubMed] [Google Scholar]
- 5. Krunkosky TM, et al. Gross and microscopic anatomy of the equine gastrointestinal tract. In: The Equine Acute Abdomen. 3rd ed. Wiley, 2017:3–18. [Google Scholar]
- 6. Packer M, et al. Quantification of immune cell populations in the lamina propria of equine jejunal biopsy specimens. J Comp Pathol 2005;132:90–95. [DOI] [PubMed] [Google Scholar]
- 7. Pickles KJ, et al. Large intestinal mast cell count and proteinase expression is associated with larval burden in cyathostomin-infected horses. Equine Vet J 2010;42:652–657. [DOI] [PubMed] [Google Scholar]
- 8. Platt H. Chronic inflammatory and lymphoproliferative lesions of the equine small intestine. J Comp Pathol 1986;96:671–684. [DOI] [PubMed] [Google Scholar]
- 9. Rotting AK, et al. Mucosal distribution of eosinophilic granulocytes within the gastrointestinal tract of horses. Am J Vet Res 2008;69:874–879. [DOI] [PubMed] [Google Scholar]
- 10. Swanson KS, et al. Effects of supplemental fructooligosaccharides and mannanoligosaccharides on colonic microbial populations, immune function and fecal odor components in the canine. J Nutr 2002;132(Suppl 2):1717S–1719S. [DOI] [PubMed] [Google Scholar]
- 11. Uzal FA, et al. Clostridium perfringens type C and Clostridium difficile co-infection in foals. Vet Microbiol 2012;156:395–402. [DOI] [PubMed] [Google Scholar]
- 12. Uzal FA, et al. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 2. Elsevier, 2016:91–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211031944 for Leukocyte numbers and intestinal mucosal morphometrics in horses with no clinical intestinal disease by Guido Rocchigiani, Emanuele Ricci, Mauricio A. Navarro, Monika A. Samol and Francisco A. Uzal in Journal of Veterinary Diagnostic Investigation