Abstract
Hypertension (HTN) is associated with gonadal dysfunction and impaired reproductive health in both men and women. An imbalance in the systemic and renal proinflammatory (M1)/anti-inflammatory (M2) macrophage ratio, increased inflammation, and inflammation-associated lymphangiogenesis have been observed in animals with HTN. However, the impact of HTN on gonadal macrophages, inflammation, and lymphatics remains obscure. We hypothesized that salt-sensitive HTN (SSHTN) and HTN alters gonadal macrophage polarization, which is associated with inflammation, inflammation-associated lymphangiogenesis, and reproductive dysfunction. Flow cytometry analyses revealed a significant increase in M1 macrophages in the testes of SSHTN and nitro-L-arginine methyl ester hydrochloride (L-NAME)-induced HTN (LHTN) mice, with a concurrent decrease in M2 macrophages in SSHTN mice yet an increase in M2 macrophages in LHTN mice. Ovaries from SSHTN mice exhibited an increase in M1 and a decrease in M2 macrophages, while ovaries from LHTN mice had a significant increase in M2 and a decrease in M1 macrophages. Gene expression patterns of proinflammatory cytokines revealed gonadal inflammation in all hypertensive mice. Increased lymphatic vessel density in the gonads of both male and female hypertensive mice was confirmed by immunofluorescence staining for lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1). HTN adversely affected the expression pattern of steroidogenic enzymes, hormone receptors, and secretory proteins in both the testes and ovaries. In line with these results, male hypertensive mice also presented with decreased sperm concentration, and increased percentage of sperm with abnormal morphology, damaged acrosome, and nonfunctional mitochondrial activity. These data demonstrate that HTN alters gonadal macrophage polarization, which is associated with gonadal inflammation, inflammation-associated lymphangiogenesis, and dysfunction.
Introduction
Hypertension (HTN), affecting nearly 50% of the population, is the primary risk factor contributing to cardiovascular disease, accounting for over half a million deaths in the United States each year [1,2]. Individuals with HTN are not only susceptible to serious health issues like stroke, kidney disease, and heart failure but also experience impaired sexual health [3,4]. Erectile dysfunction, reduction in semen volume, serum testosterone, sperm count and motility, and an increase in abnormal sperm morphology are conditions described in hypertensive men [4–7]. Similarly, hypertensive females exhibit decreased vaginal lubrication, reduced orgasm, and several complications during pregnancy leading to fetal and maternal morbidity and mortality [8–10]. These impairments in reproductive health have been ascribed primarily to attenuated levels of hormones and altered vasculature of reproductive organs [11–15]. However, the detailed molecular mechanisms underlying the reproductive dysfunction associated with HTN remain obscure.
Studies in humans and animals have established a strong link between systemic inflammation and HTN [16–20]. Macrophages are one of the most abundant immune cells that are implicated in the chronic, low-grade inflammation associated with HTN [21]. It is shown that genetic deletion of monocytes/macrophages prevents HTN [17,22,23]. Our lab and others have demonstrated previously increased macrophages, inflammation, and inflammation-associated lymphangiogenesis in the kidneys of mice with HTN and salt-sensitive HTN (SSHTN) [24–29]. The heterogeneity and plasticity of macrophages permit them to perform various tissue-specific functions by switching between proinflammatory (M1) and anti-inflammatory (M2) phenotypes [30]. In HTN and SSHTN, circulating monocytes and resident macrophages have been reported to possess a tendency to polarize toward M1 phenotype in kidneys and other organs [31–36]. Nevertheless, it is unknown whether HTN alters gonadal M1/M2 macrophage ratios and if this is associated with inflammation, inflammation-associated lymphangiogenesis, and gonadal dysfunction.
Based on the limited studies available, we hypothesized that SSHTN and HTN increases M1 macrophages in the gonads, which is associated with inflammation, inflammation-associated lymphangiogenesis, and gonadal dysfunction. To test the above hypothesis, the study employed two models of HTN that simulate humans with SSHTN and hypertensive patients with elevated levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. Here, we report the effect of SSHTN and HTN on male and female gonadal macrophage polarization, inflammation, lymphatics, and function.
Methods
Animal models
All the experimental protocols performed at College of Medicine, Texas A&M University were approved by the Texas A&M University (IACUC: 2022-0083) in accordance with the NIH Guide for the Care and Use and Care of Laboratory Animals. Wild-type C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Male and female mice 10–14 weeks of age were made hypertensive by either providing nitro-L-arginine methyl ester hydrochloride (L-NAME) (0.5 mg/ml; Sigma, St. Louis, MO), a nitric oxide synthase inhibitor in the drinking water for 2 weeks, followed by a 2-week washout period and a subsequent 3-week 4% high-salt diet (Teklad Envigo, Huntingdon, U.K.) (SSHTN) or by providing L-NAME in their drinking water for 3 weeks (LHTN) [26,28]. Control mice received a normal diet and tap water ad libitum. Mice were euthanized after the completion of treatment period by exsanguination under 5% inhalational isoflurane anesthesia with death confirmed by cervical dislocation before tissue collection.
Blood pressure measurements
Systolic blood pressure (SBP) was measured weekly using the IITC Life Science (IITC Inc, Woodland Hills, CA, U.S.A.) noninvasive tail-cuff blood pressure acquisition system. The entire procedure was performed in a designated quiet area, and mice were acclimatized for 30 min. The restrainers and warming chambers were preheated to 34°C. Mice were gently placed into a restrainer of appropriate size and acclimatized for 5 min in the warming chamber prior to SBP recordings. SBP values were determined from the blood pressure traces by blinded investigators.
Flow cytometry
Testes/ovaries were collected, minced with scissors, and digested in buffer with Collagenase II (1 mg/ml) (Worthington Biochemicals, Lakewood, NJ, U.S.A.), DNAase I (0.15 mg/ml) (Sigma) and Dispase II (1 mg/ml) (Sigma, St. Louis, MO, U.S.A.) at 37°C for 30 min in gentle MACS™ Octo dissociator with heaters (Miltenyi Biotec, San Diego, CA, U.S.A.). Samples were filtered and rinsed with Dulbecco’s phosphate-buffered saline (DPBS; Thermo Fisher Scientific, 14190250) through sterile 100 and 40 μm strainers. The cellular components were isolated by centrifugation, and red blood cells were lysed in ACK lysis buffer (Thermo Fisher Scientific, Waltham, MA, U.S.A.). Cells were resuspended in RPMI 1640 (Roswell Park Memorial Institute) media (containing 10% fetal bovine serum v/v, 100 IU/ml penicillin/streptomycin) to stop lysis, centrifuged and rinsed twice with DPBS. For immunophenotyping of macrophages in testes/ovaries, single-cell suspensions were stained for 30 min with Ghost red 710 viability dye (Tonbo Bioscience, San Diego, CA). Following washing with DPBS, cells were subsequently Fc blocked by using an antimouse CD16/CD32 antibody (BD Pharmingen, San Jose, CA) for 10 min on ice. Next, cells were stained using fluorescent-conjugated antibodies against CD45, CD11b, F4/80, CD11c, and CD206 (1:50 and 1:100 dilutions of antibodies were used for testes and ovaries, respectively). All antibodies were purchased from either Biolegend (San Diego, CA) or BD Biosciences (San Jose, CA). See Supplementary Table S1 for descriptive panel. Populations of up to 1 × 106 total cells were analyzed. Acquisition was performed on a BD LSR Fortessa X-20 flow cytometer using FACS DIVA software (BD Biosciences, San Jose, CA). Analysis was performed using Flow Jo v 10.7.1 (FlowJo, LLC, Ashland, OR). Macrophage populations were quantified within the CD45+CD11b+F4/80+ gate and identified as M1 (CD45+CD11b+F4/80+CD11c+CD206low) or M2 (CD45+CD11b+F4/80+CD11c−CD206+) cells in testes as described previously [37]; M1 (CD45+CD11b+F4/80+CD11c+CD206−) or M2 (CD45+CD11b+F4/80+CD11c−CD206+) cells in ovaries as described previously [38]. Results are expressed as a percentage of CD11b+F4/80+ cells per animal. Unstained specimen and compensation control were used for all relevant samples to justify gating strategy. Fluorescence minus one (FMO) control was used wherever needed.
Real-time quantitative PCR
Testes/ovaries were homogenized, and total RNA was isolated using the Quick-RNA mini prep kit (Zymo Research, Irvine, California, U.S.A.) following the manufacturer’s instructions. cDNA synthesis was performed using 0.5 μg total RNA by following the manufacturer’s instructions provided by the Qiagen RT2 First Strand kit (Germantown, Maryland, U.S.A.). Ten microliter of qRT-PCR reactions was carried out using SYBR Green ROX qPCR Mastermix (Qiagen), nuclease-free water (Invitrogen, Carlsbad, California, U.S.A.), and primers (10 μM) (Sigma-Aldrich, St. Louis, Missouri, U.S.A.) along with cDNA from testes/Ovaries. Reactions were run in duplicate using the QuantStudio6 Flex Real-Time PCR system (Applied Biosystems, Foster City, California, U.S.A.). All the data were normalized to the expression levels of Ubiquitin (Ubc) and fold-changes were calculated using the 2−ΔΔCT method. The primers used in the current study are shown in Supplementary Table S2.
Immunofluorescence
Testes/ovaries were dissected and fixed in 4% PFA (Sigma) for 24 h. Tissues were then rinsed with phosphate-buffered saline (PBS) and stored in 70% ethanol until they were embedded in paraffin. Five-micrometer tissue sections were deparaffinized, rehydrated, and permeabilized with 0.1% Triton solution. The tissue sections were then blocked with 10% AquaBlock (EastCoastBio, North Berwick, Maine, U.S.A.) for 1 h at room temperature and were incubated with antibodies against lymphatic endothelial cell marker, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) (Goat polyclonal, R&D Systems, Minneapolis, MN, U.S.A.) and endothelial cell marker, platelet endothelial cell adhesion molecule 1 (PECAM-1), also known as CD31 (Rabbit polyclonal, Abcam, Cambridge, U.K.) at 4°C overnight. Alexa Fluor 488 or 594 secondary antibodies (Life Technologies, Carlsbad, California, U.S.A.) were used for visualization by incubating the sections at room temperature for 1 h. Sections incubated with only secondary antibody served as negative controls. Labeled slides were mounted with Prolong Gold antifade reagent with DAPI (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.A.) and imaged using an Olympus BX51 fluorescence microscope with Olympus Q5 camera. Images were captured at 4× and 10× magnification using the Olympus CellSens software (Olympus, Shinjuku, Tokyo, Japan). For quantification of LYVE-1+ lymphatic vessels, four images from predetermined areas at outer tunica albuginea area in testes sections were captured at 10× magnification, by two independent, blinded investigators. In ovaries, images were captured at 4× magnification. The area values measuring total number of LYVE-1+ pixels were determined using ImageJ software (NIH, Rockville, MD) after setting the threshold for positive endothelium.
Tissue clearing and 3-D imaging
Tissue clearing was carried out using the previously described method [39]. Briefly, mice were anesthetized under 5% isoflurane and perfused with 20 ml of PBS and 30 ml of 4% PFA in PBS through the left ventricle of the heart. Testes/ovaries were dissected out and fixed in 4% PFA at 4°C for 24 h, then washed with PBS for 2 h, thrice at room temperature. Then, the fixed organs were immersed in 50% (v/v) CUBIC-L (1:1 mixture of water and CUBIC-L) for 6 h and then switched to 100% CUBIC-L with shaking at 37°C for 5 days, refreshed daily. Testes/ovaries were washed with PBS for 2 h, thrice after the delipidation process. The samples were then stained with primary antibody against the lymphatic endothelial cell marker, LYVE-1 (Goat polyclonal, R&D Systems, Minneapolis, MN, U.S.A.), in the staining buffer containing 0.5% Triton X-100, 0.5% casein (Thermo Fisher Scientific, Waltham, MA, U.S.A.) and 0.05% sodium azide (Sigma) for 7 days at 37°C with shaking. Testes/ovaries were then washed with 0.5% triton X-100 in PBS (PBST) for 1 day, and then stained with Alexa Flour 488 secondary antibody (Life Technologies, Carlsbad, California, U.S.A.) in the staining buffer consisting of 0.5% Triton X-100, 0.1% casein, and 0.05% sodium azide for 7 days at 37°C with gentle shaking. After staining the samples were washed with PBST for 1 day and fixed in 1% formaldehyde solution for 3 h, followed by PBS wash for 2 h, thrice. Finally, the refractive index was matched by immersing the samples in 50% (v/v) CUBIC-R+ (1:1 mixture of water and CUBIC-R+) for 1 day and in 100% CUBIC-R+ with gentle shaking for 2 days. The confocal images were acquired using Olympus Fluoview 3000 laser scanning confocal microscope (Olympus, Tokyo, Japan) at 10× magnifications with excitation wavelengths of 488 nm for imaging lymphatic vessels. All raw image data were collected in a 16-bit TIFF format. 3D-rendered images were visualized, captured, and analyzed with Imaris software (Bitplane, Switzerland).
Sperm concentration, motility, and morphology
Caudal epididymides from each animal were snipped and placed in 2 ml of preheated (37°C) Biggers-Whitten-Whittingham (BWW) media. The caudal portions were punctured three- to four-times with a 27-gauge needle and incubated for 10 min to allow the sperm to swim out. For determining sperm concentration, 10 μl of the diluted spermatozoa (1:20) was added to each side of a Neubauer chamber and counted under light microscope (400×) and the results were expressed as million sperm/ml. Sperm motility was assessed immediately by placing 10 μl of diluted sperm suspension on prewarmed microscope slide and covered with a cover slip. Two hundred spermatozoa per animal were evaluated under light microscope (400× magnification) for motility and expressed as the % motile cells. Sperms that moved forward were considered and those with in situ movement were excluded. For the assessment of sperm morphology smears were stained with hematoxylin-eosin. A total of 200 spermatozoa per mouse were evaluated under light microscope, using 400× magnification and sperm with abnormalities, such as lack of a hook, having amorphous, banana-shaped heads, folded heads, being twin tailed, or having twin heads, were recorded.
Sperm acrosome integrity and mitochondrial activity
Sperm acrosome integrity was assessed using a fluorescent-labeled peanut agglutinin (FITC-PNA) (Sigma-Aldrich) as described previously [15]. Briefly, smears of sperm suspension were fixed in methanol for 15 min, air-dried, and stained with FITC-PNA (60 μg/ml in PBS) in the dark for 30 min. Slides were then washed with Milli-Q water to remove excessive staining. Two hundred spermatozoa were evaluated under Olympus Fluoview 3000 confocal microscope at 600× magnification and classified as intact or damaged acrosome based on the staining since FITC-PNA binds exclusively to the outer acrosomal membrane.
Sperm mitochondrial activity was assessed using a fluorescent dye Rhodamine 123 that is rapidly taken up by functional mitochondria. In brief, 5 μl of Rhodamine 123 (Sigma, U.S.A.) solution (1 mg/ml in DMSO) was added to 1 ml of diluted sperm suspension and incubated for 10 min at 25°C in the dark. Following incubation, the supernatant was removed by centrifugation (300× g, 10 min) and the sperm pellets were resuspended in 1 ml PBS. A drop of 10 μl of the suspension was placed on microscopic slides, covered with coverslips, and examined at 400× magnification under Olympus BX51 fluorescence microscope with Olympus Q5 camera. A total of 200 spermatozoa were examined per animal and expressed as percentage of mitochondrial activity.
Statistical analysis
Statistical analysis was performed using GraphPad Prism, version 8.4.3. Data are presented as bar graphs/dot plots representing the mean ± SEM. Two-tailed Student’s t test was used to assess data between the groups and the significance level was set at P<0.05. Statistical test and number of animals in each group are indicated in each figure panel or legend.
Results
SSHTN increases M1 macrophages and is associated with inflammation, and increased lymphatics in both testes and ovaries
To evaluate the direct impact of SSHTN on the gonads, mice were administered L-NAME in their drinking water for 2 weeks, followed by a washout period of 2 weeks, and then provided a 4% high-salt diet for 3 weeks as described previously [26]. As expected, both male (Figure 1A) and female (Figure 1B) mice developed SSHTN. There were no differences in the body weights of male and female mice and relative weight of ovaries, but there was a significant increase in the relative weight of testes in SSHTN mice (Supplementary Table S3). To investigate gonadal macrophage changes in SSHTN, we immunophenotyped gonadal cell populations by flow cytometry. The data revealed a significant increase in M1 macrophages and a significant decrease in M2 macrophages in both the testes (Figure 1C) and ovaries (Figure 1D) of mice with SSHTN when compared with control mice. Since SSHTN is well associated with inflammation, we then examined whether SSHTN promotes inflammation in the gonads, for which we measured the gene expression of proinflammatory mediators. We found a significant increase in the mRNA expression of the proinflammatory mediators Il1b, Il6, Il17, Tnfa, Ifng, and Nos2 in the testes of mice with SSHTN (Figure 1E). Ovaries from SSHTN mice demonstrated significantly increased expression of Il6, Il17, and Nos2 (Figure 1F).
Figure 1. SSHTN mice had increased M1 macrophages, decreased M2 macrophages, and inflammation in the gonads.
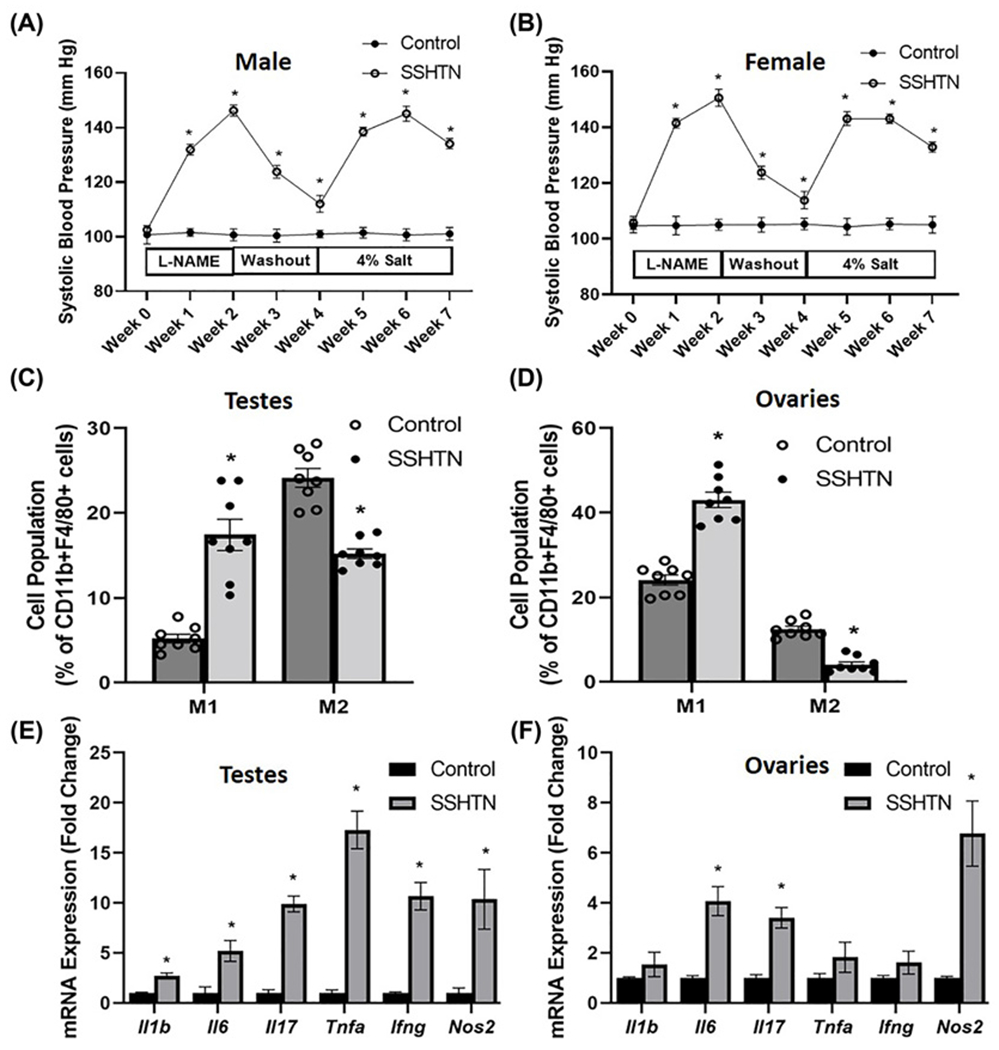
SBP measures in (A) male and (B) female control and mice administered L-NAME in the drinking water for 2 weeks, then 2 weeks of tap water washout, and then 3 weeks of 4% salt diet (SSHTN) (n=6 in both males and females). Macrophage (M1 and M2) populations expressed as percentage of CD11b+F4/80+ cells in (C) testes and (D) ovaries from control and SSHTN mice, as determined by flow cytometry (n=8 in both males and females). Gene expression of proinflammatory mediators in (E) testes and (F) ovaries from control and SSHTN mice (n=6 in both males and females). Results are expressed as mean ± SEM, and statistical analysis consisted of a Student’s t test. *P<0.05 vs control.
We have previously reported that HTN and SSHTN is well associated with immune cell infiltration, inflammation, and inflammation-associated lymphangiogenesis in kidneys [28,29]. Nonetheless, it is largely unknown whether the same happens in the gonads of hypertensive subjects. We therefore examined gonadal lymphatic vessel density by performing immunofluorescence on testis and ovary sections by staining for the lymphatic vessel marker LYVE-1. Quantification of LYVE-1+ pixels per field confirmed a significant increase in lymphatic density in both testes (Figure 2A) and ovaries (Figure 2B) of SSHTN mice when compared with control mice. In support of these results, we also found significantly increased gene expression of the lymphatic markers Lyve1, Prox1 (a lymphatic endothelial cell transcription factor), Pdpn (podoplanin), Vegfc (vascular endothelial growth factor C), Vegfd (vascular endothelial growth factor D), Vegfr2 and Vegfr3 (the receptors for the lymphangiogenic growth factors VEGF-C and VEGF-D), and adhesion molecules such as Icam and Vcam in the testes of SSHTN mice (Figure 2C). Testes from SSHTN mice also had an increase in the lymphatic-expressed immune cell-trafficking chemokine Ccl21 and its receptor Ccr7 (Figure 2C). Ovaries from SSHTN mice also exhibited increased expression of the lymphatic markers Lyve1, Prox1, Pdpn, Vegfc, Vegfr2, Vegfr3, and the lymphatic-expressed immune cell-trafficking chemokine Ccl19 (Figure 2D). 3-D model and reconstruction of confocal images of LYVE-1+ lymphatic vessels in both testes and ovaries from the control and SSHTN mice can be observed in Figure 2E–H and Supplementary Videos S1–S4, respectively. The quantification of LYVE-1+ lymphatic vessels in CUBIC cleared gonads are given in Supplementary Table S5. Together, these results demonstrate that SSHTN in both males and females induces a significantly increased M1 and decreased M2 macrophages in the gonads, and this is associated with inflammation and inflammation-associated lymphangiogenesis.
Figure 2. SSHTN increased lymphatic vessel density in the gonads.
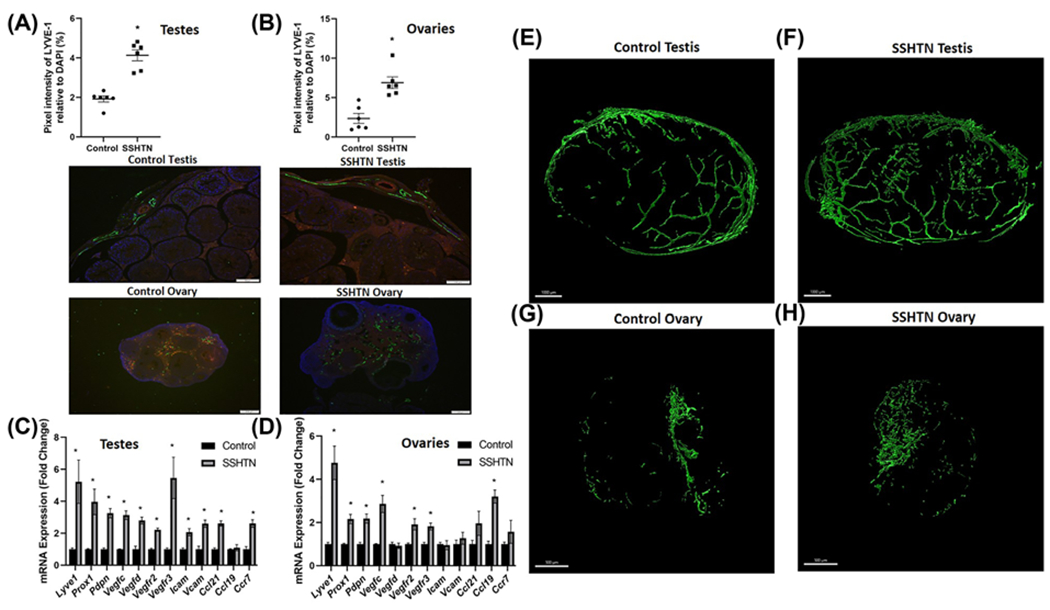
Lymphatic vessel density in (A) testes and (B) ovaries from control and SSHTN mice as determined by LYVE-1+ pixels relative to DAPI per field. Representative images of LYVE-1 immunofluorescence in testis (scale bar = 100 μm) and ovary (scale bar = 200 μm) sections (n=6 in both males and females). Green: LYVE-1; Red: CD31; Blue: DAPI. Gene expression of lymphatic vessel markers in (C) testes and (D) ovaries from the control and SSHTN mice (n=6 in both males and females). Results are expressed as mean ± SEM, and statistical analyses consisted of Student’s t test. *P<0.05 vs control. Representative 3-D model of confocal images of clear, unobstructed brain/body imaging cocktails and computational analysis (CUBIC) cleared testis immunostained with LYVE-1 from (E) control and (F) SSHTN mice, n=3. Representative 3-D model of confocal images of clear, unobstructed brain/body imaging cocktails and computational analysis (CUBIC) cleared ovary immunostained with LYVE-1 from (G) the control and (H) SSHTN mice, n=3. Scale bars = 1000 μm (testis) and 500 μm (ovary).
SSHTN impairs gonadal function
The two critical functions of testes are steroidogenesis [production of testosterone (T)] and spermatogenesis [formation of haploid germ cells (GCs)] [40,41]. These functions are tightly regulated by gonadotropins, luteinizing hormone (LH) that acts on T-producing Leydig cells (LCs) present in the interstitial compartment, and follicle-stimulating hormone (FSH) that acts on Sertoli cells (SCs) in the seminiferous tubule [40,41]. The process of steroidogenesis involves a cascade of complex enzymatic reactions that are catalyzed by two classes of enzymes, cytochrome P450 (CYP) and hydroxysteroid dehydrogenases (HSD), where cholesterol is converted into a biologically active steroid hormone [42]. To uncover the basis of gonadal dysfunction observed in HTN, we analyzed the expression of steroidogenesis pathway genes. The mRNA expression of the steroidogenic acute regulatory protein Star, a rate-limiting protein that controls cholesterol transport, was decreased significantly in the testes of SSHTN mice (Figure 3A). There was also a significant decrease in the expression of Hsd3b1 and Hsd17b1 in the testes of SSHTN mice (Figure 3A). The expression of Cyp17a1 was decreased in the testes of mice with SSHTN, whereas Cyp11a1 remained unchanged (Figure 3A). We next investigated the effect of SSHTN on hormone signaling and analyzed the expression pattern of hormone receptors that regulate steroidogenesis and spermatogenesis. We found that SSHTN caused a significant decrease in the expression of androgen receptor (Ar), estrogen receptor subtype a (Era), and Lhr, whereas Fshr and estrogen receptor b (Erb) were not altered in the testes (Figure 3A).
Figure 3. SSHTN mice exhibited gonadal dysfunction.
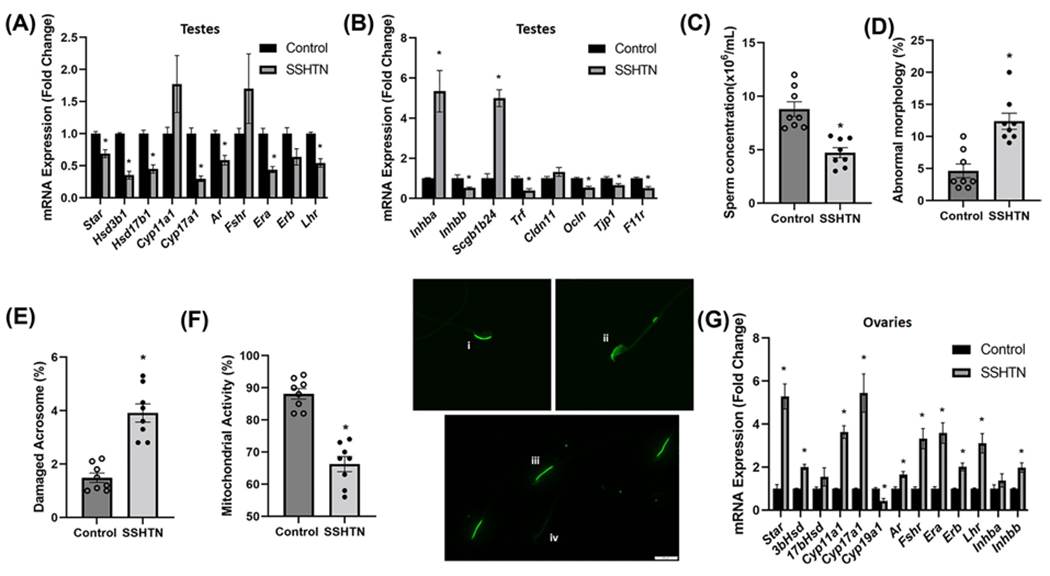
Gene expression of steroidogenic pathway genes and hormone receptors in (A) testes from control and mice-administered L-NAME in the drinking water for 2 weeks, then 2 weeks of washout, and then a subsequent 3 weeks of 4% salt diet (SSHTN), n=6. Gene expression of secretory proteins and tight junction proteins in (B) testes from control and SSHTN mice, n=6. Sperm functional tests in control and SSHTN mice demonstrate (C) decreased sperm concentration, n=8, (D) increased percentage of sperm with abnormal morphology, n=8, (E) increased percentage of sperm with damaged acrosome, n=8, and (F) increased number of sperm with nonfunctional mitochondrial activity n=8. Representative images of (i) intact and (ii) damaged acrosome integrity were assessed using FITC-PNA that binds exclusively to the outer membrane of the acrosome. Scale bars = 10 μm. Florescent dye Rh123 (Rhodamine123) distinguishes (iii) functional and (iv) nonfunctional sperm mitochondria as only live cells can retain the stain after washing. Scale bars = 20 μm. Expression of steroidogenic pathway genes, hormone receptors, and secretory proteins in (G) ovaries from control and SSHTN mice, n=6. Results are expressed as mean ± SEM, and statistical analysis consisted of a Student’s t test. *P<0.05 vs control.
The process of spermatogenesis is under the regulation of T and FSH that acts on SCs, which in turn supplies nutrients and growth factors and provides structural support for the developing GCs [40,43]. To investigate the effect of SSHTN on the secretory activity of SCs, we studied the gene expression of a few secretory proteins. Notably, there was a significant increase in the expression of Inhba (Activin A) and Scgb1b24 (androgen-binding protein, ABP) along with a significant decrease in the expression of Inhbb (Inhibin B) and Trf (Transferrin) in the testes of SSHTN mice (Figure 3B). These results highlight the modulating effect of SSHTN on the secretory activity of the testes.
Apart from supplying nutrients and growth factors, SCs also provide a structural barrier called the blood–testis barrier (BTB) that regulates the flow of nutrients and sequesters the developing GCs from the host immune system. The BTB comprises mainly tight junctions (TJs), gap junctions, and adherens junctions [43]. TJs comprises TJ proteins (TJPs) that play a crucial role in maintaining an intact BTB and are under the control of hormones, mainly T and FSH [44,45]. To explore whether SSHTN affects BTB, we examined the expression of key TJPs in the testes. Mice with SSHTN had significantly decreased testes gene expression of the TJPs Ocln (Occludin), Tjp1 (Zona occludens-1), and F11r (Junctional adhesion molecule A), whereas Cldn11 (Claudin11) remained unaltered (Figure 3B).
To validate the gonadal dysfunction associated with HTN, we also examined sperm collected from the caudal portion of the epididymides for sperm concentration, motility, morphology, and various functional traits such as acrosome integrity and mitochondrial activity. Consistent with testes dysfunction, we found a significant decrease in sperm concentration in the SSHTN mice when compared with the control mice (Figure 3C). Sperm progressive motility was unaltered in the SSHTN mice (Supplementary Figure S1). The SSHTN mice also had a significantly increased percentage of sperm with abnormal morphology (Figure 3D) and damaged acrosome (Figure 3E), along with a significant decrease in the percentage of sperm with functional mitochondria (Figure 3F). We found almost identical results in a comparable model of SSHTN in which males were given L-NAME (1.5 mg/ml) for 4 weeks, followed by a tap water washout of 1 week, and then a 4% salt diet for 3 weeks [46]. This comparable method of SSHTN induction was also associated with significantly increased gonadal M1 macrophages (Supplementary Figure S2), significantly decreased M2 macrophages (Supplementary Figure S2), significantly increased gene expression of proinflammatory mediators (Supplementary Figure S3A), significantly increased gene expression of lymphatic markers and chemokines (Supplementary Figure S3B), and testicular dysfunction (Supplementary Figure S3C,D). Collectively, these results indicate that SSHTN leads to poor sperm quality and quantity by adversely affecting the expression of genes involved in the process of steroidogenesis and spermatogenesis.
Like testes, ovarian steroidogenesis is also essential for the maintenance of reproductive tissues, folliculogenesis and ovulation, and establishment of pregnancy, that are under the intricate control of the pituitary gonadotropins FSH and LH [47,48]. We investigated the effect of SSHTN on ovarian steroidogenesis and its regulation by measuring gene expression of cholesterol transport protein, key steroidogenic enzymes, and hormone receptors. Ovaries from SSHTN mice demonstrated a significantly increased expression of Star, Hsd3b1, Cyp11a1, and Cyp17a1, with a simultaneous decrease in the expression of Cyp19a1, the aromatase enzyme responsible for estrogen synthesis from androgen precursors (Figure 3G). The hormone receptors Ar, Fshr, Era, Erb, and Lhr were increased significantly in ovaries from the SSHTN mice (Figure 3G). The expression of Inhbb was increased significantly, whereas Inhba remained unaltered in ovaries from the SSHTN mice (Figure 3G). Taken together, these results indicate that SSHTN increased the expression of steroidogenic enzymes with a simultaneous decrease in aromatase that might lead to reduced availability of estrogen and increased androgen levels in the ovarian tissue.
LHTN is associated with altered macrophages, inflammation, and increased lymphatics in both testes and ovaries
To mimic hypertensive patients with elevated levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine, we administered L-NAME in the drinking water for 3 weeks as described previously [28]. We then sought to determine whether alterations in gonadal macrophages, inflammation, and inflammation-associated lymphangiogenesis also occur in this model of HTN that is independent of salt. We confirmed the development of LHTN in both male (Figure 4A) and female (Figure 4B) mice. There were no differences in body weights of male and female mice, or in the relative weight of ovaries in LHTN mice, but there was a significant increase in the relative weight of testes in LHTN mice compared with control mice (Supplementary Table S4), similar to mice with SSHTN. Using flow cytometry, we found a significant increase in both M1 and M2 macrophages in the testes of LHTN mice (Figure 4C), while ovaries exhibited a significant decrease in M1 macrophages and a significant increase in M2 (Figure 4D). Like mice with SSHTN, the expression of the proinflammatory mediators Il1b, Il6, Il17, Tnfa, Ifng, and Nos2 was increased significantly in the testes of mice with LHTN (Figure 4E). Ovaries from LHTN mice had identical results with significantly increased expression of Il1b, Il6, Il17, Tnfa, Ifng, and Nos2 (Figure 4F). We labeled the lymphatics with LYVE-1 staining in testis (Figure 5A) and ovary (Figure 5B) sections and confirmed a significantly increased lymphatic vessel density in LHTN mice. Gene expression analysis congruently demonstrated a significant increase in lymphatic markers (Lyve1, Prox1, Pdpn, Vegfc, Vegfd, Vegfr2, and Vegfr3), cell adhesion molecule (Icam), and lymphatic endothelial cell-specific chemokines (Ccl21 and Ccl19) and receptor (Ccr7) in the testes of LHTN mice (Figure 5C). There was a significant increase in the expression of Prox1 and Vegfd in ovaries from LHTN mice (Figure 5D). 3-D model and reconstruction of confocal images of testes and ovaries from LHTN mice can be observed in (Figure 5E–H) and Supplementary Videos S5 and S6, respectively. These data suggest that LHTN alters gonadal macrophages and is associated with gonadal inflammation and a compensatory increase in lymphatic density.
Figure 4. LHTN was associated with altered macrophage polarization and inflammation in the gonads.
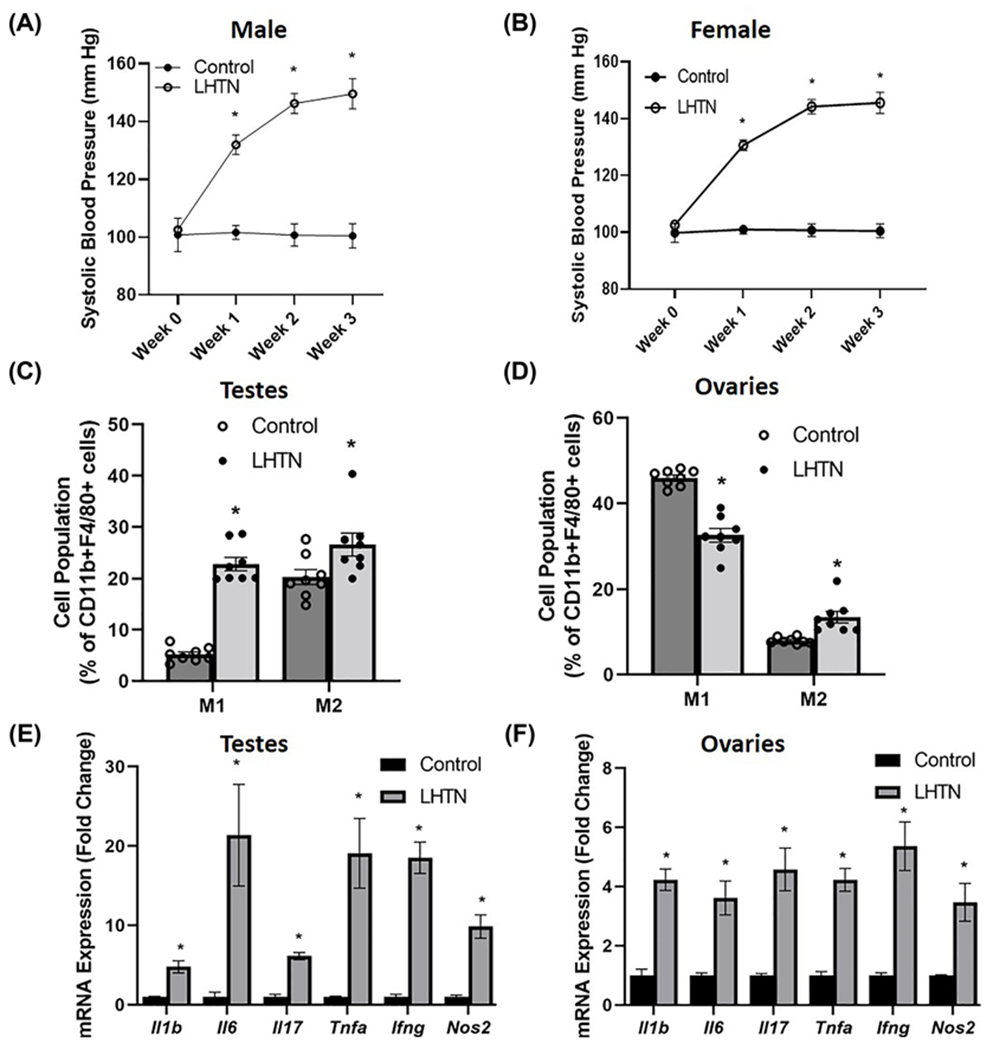
SBP measures in (A) male and (B) female control and mice administered L-NAME in the drinking water for 3 weeks (LHTN) (n=6 in both males and females). Macrophage (M1 and M2) populations expressed as percentage of CD11b+F4/80+ cells in (C) testes and (D) ovaries from control and LHTN mice, as measured by flow cytometry (n=8 in both males and females). Gene expression of proinflammatory mediators in (E) testes and (F) ovaries from control and LHTN mice (n=6 in both males and females). Results are expressed as mean ± SEM, and statistical analyses consisted of Student’s t test. *P<0.05 vs control.
Figure 5. LHTN increased lymphatic vessel density in the gonads.
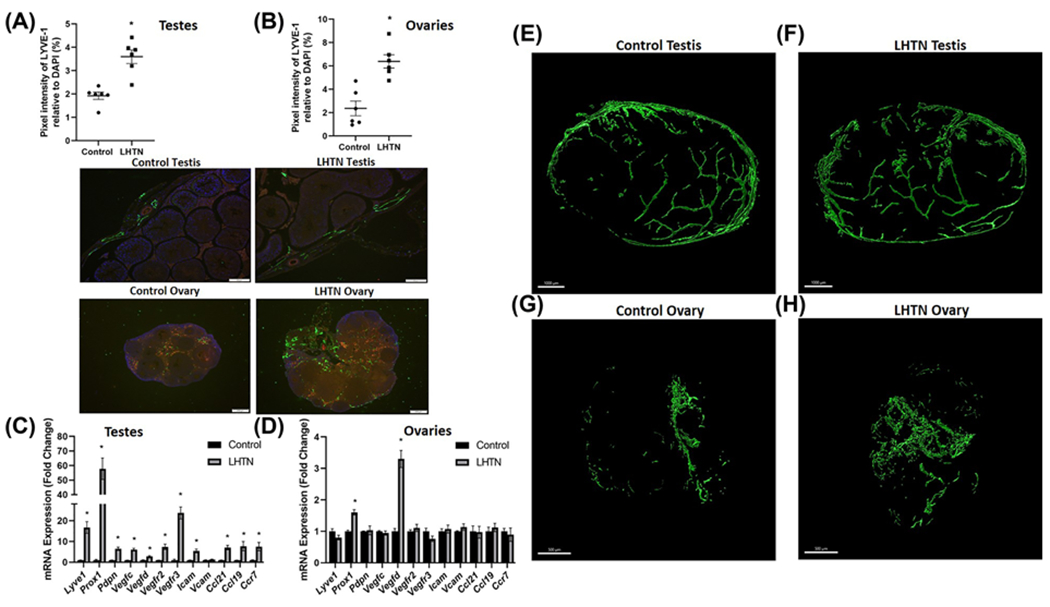
Lymphatic vessel density in (A) testes and (B) ovaries from the control and LHTN mice as determined by LYVE-1+ pixels relative to DAPI per field (n=6 in both males and females). Representative images of LYVE-1 immunofluorescence in testis (scale bar = 100 μm) and ovary (scale bar = 200 μm) sections. Green: LYVE-1; Red: CD31; Blue: DAPI. Gene expression of lymphatic vessel markers in (C) testes and (D) ovaries from the control and LHTN mice (n=6 in both males and females). Results are expressed as mean ± SEM, and statistical analyses consisted of Student’s t test. *P<0.05 vs control. Representative 3-D model of confocal images of clear, unobstructed brain/body imaging cocktails and computational analysis (CUBIC) cleared testis immunostained with LYVE-1 from (E) the control and (F) LHTN mice, n=3. Representative 3-D model of confocal images of clear, unobstructed brain/body imaging cocktails and computational analysis (CUBIC) cleared ovary immunostained with LYVE-1 from (G) the control and (H) LHTN mice, n=3. Scale bars = 1000 μm (testis) and 500 μm (ovary).
LHTN is associated with impaired gonadal function
We next conducted a similar study as that in SSHTN mice to examine whether LHTN is also associated with gonadal dysfunction. Gene expression analysis revealed a significant decrease in Star, Hsd17b1, and Cyp17a1 in the testes of mice with LHTN, whereas Hsd3b1 and Cyp11a1 remained unaltered (Figure 6A). LHTN also significantly decreased the expression of Ar, Era, and Lhr in the testes of LHTN mice (Figure 6A). There was also a significant increase in the expression of Inhba and Scgb1b24, and significantly decreased expression of Inhbb and Trf in the testes of LHTN mice (Figure 6B). We also observed a significantly decreased expression of Cldn11 and Tjp1 in the testes of LHTN mice (Figure 6B). Sperm collected from the caudal portion of the epididymides from LHTN mice revealed a significant decrease in sperm concentration (Figure 6C), whereas sperm motility remained unchanged (Supplementary Figure S4). There was a significantly increased percentage of sperm with abnormal morphology (Figure 6D) and damaged acrosome (Figure 6E), and a simultaneous decrease in the percentage of sperm with functional mitochondria (Figure 6F), which is congruent with that found in the SSHTN mice. Taken together, these results highlight the detrimental effect that LHTN has on the genes regulating steroidogenesis and spermatogenesis in male mice.
Figure 6. LHTN mice exhibited gonadal dysfunction.
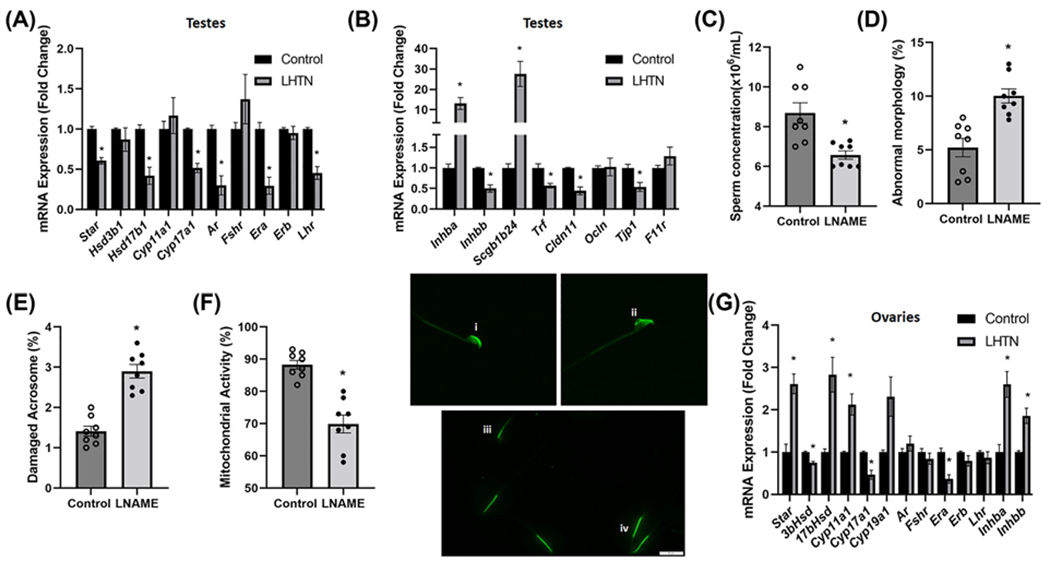
Gene expression of steroidogenic pathway genes and hormone receptors in (A) testes from the control and mice administered L-NAME in the drinking water for 3 weeks (LHTN), n=6. Gene expression of secretory proteins and tight junction proteins in (B) testes from the control and LHTN mice, n=6. Sperm functional tests in the control and LHTN mice demonstrate (C) decreased sperm concentration, n=8, (D) increased percentage of sperm with abnormal morphology, n=8, (E) increased percentage of sperm with damaged acrosome, n=8, and (F) increased number of sperm with nonfunctional mitochondrial activity, n=8. Representative images of (i) intact and (ii) damaged acrosome integrity were assessed using FITC-PNA that binds exclusively to the outer membrane of the acrosome. Scale bars = 10 μm. Florescent dye Rh123 (Rhodamine123) distinguishes (iii) nonfunctional and (iv) functional sperm mitochondria as only live cells can retain the stain after washing. Scale bars = 20 μm Expression of steroidogenic pathway genes, hormone receptors, and secretory proteins in (G) ovaries from control and LHTN mice, n=6. Results are expressed as mean ± SEM, and statistical analysis consisted of a Student’s t test. *P<0.05 vs control.
Ovaries from LHTN mice had significantly increased expression of Star, Hsd17b1, and Cyp11a1, along with a significant decrease in the expression of Hsd3b1 and Cyp17a1, while there was no change in the expression of Cyp19a1 (Figure 6G). There was also a significant decrease in the expression of Erα, whereas Ar, Fshr, Erb, and Lhr were unaltered in the ovaries from LHTN mice (Figure 6G). The gene expression of Inhba and Inhbb were increased significantly in the ovaries from LHTN mice (Figure 6G). Collectively, these results suggest that LHTN modifies the expression of genes involved in the steroidogenic pathway in the ovaries.
Discussion
HTN is strongly associated with impaired reproductive function in both men and women, however, the mechanisms involved remain obscure. To the best of our knowledge, this is the first study to demonstrate that HTN changes gonadal macrophages, and this is associated with gonadal inflammation, inflammation-associated lymphangiogenesis, and dysfunction. The current study has given a new immunological perspective at the gonadal level in hypertensive mice. We demonstrated an increase in M1 macrophages and decrease in M2 phenotype in both testes and ovaries from the SSHTN mice, which is associated with increased expression of proinflammatory mediators, an increase in lymphatics, and altered gonadal function. Subsequently, we also demonstrated that LHTN significantly increased M2 macrophages in both testes and ovaries along with a concurrent increase in M1 macrophages in testes alone. Identical with SSHTN, LHTN is also associated with gonadal inflammation, inflammation-associated lymphangiogenesis, and impaired gonadal function.
Previous study has reported hypertrophy of testis in Spontaneously Hypertensive Rats [15] that agrees with the increased relative testis weight observed in SSHTN and HTN mice in our present study. There were no significant changes in the relative weight of ovaries in the hypertensive groups.
HTN is characterized by a state of chronic low-grade inflammation with the involvement of activated innate and adaptive immunity. Macrophages are one of the most abundant heterogenous populations of immune cells involved in the inflammatory responses in HTN [21]. Testicular macrophages are involved in the maintenance of the immune privilege state of the testis, steroidogenesis, and differentiation of spermatogonia, in addition to immune surveillance [49]. Similarly, ovarian macrophages are known to stimulate cellular proliferation and follicle growth, steroidogenesis, suppress follicular apoptosis, and regulate vascularization and tissue remodeling during different stages of the ovarian cycle [50]. Under normal conditions, the majority of testicular macrophages exhibit a M2 phenotype [51], consistent with our results in control testes. In contrast, we observed a higher proportion of M1 than M2 macrophages in control ovaries. Unfortunately, little is known about the putative phenotypes of macrophages and their functional polarization in ovaries. Studies have reported that HTN and SSHTN promotes M1 polarization in circulating monocytes and increases accumulation of M1 macrophages in the end organs like the kidney, lung, and liver [31–36]. EAO, an established model of chronic testicular inflammation, was associated with increased M1 macrophages in the testes along with increased levels of monocyte chemoattractant protein-1 (MCP-1), IL-6, and TNF [52]. Lima et al. [53] reported increased M1 macrophages in the ovaries of rats with polycystic ovary syndrome (PCOS) that also associates with low-grade chronic inflammation. Accordingly, one of the prominent observations in our study is increased M1 macrophages in both testes and ovaries from SSHTN mice and in the testes from LHTN mice, which align with results from above studies. However, the exact mechanism behind the increase in M2 macrophages observed in the testes and ovaries from LHTN is unknown. It might be due, in part, to the effect of reduced nitric oxide on macrophage physiology at the gonadal level. Nonetheless, an imbalance in gonadal M1/M2 ratio, as observed in the current study, may be one of the factors contributing to gonadal dysfunction in HTN.
Few studies have demonstrated inflammation and inflammation-associated lymphangiogenesis in reproductive tissues in certain pathological conditions [54–56]. Consistently, in the present study, we found increased gene expression of proinflammatory mediators and a compensatory increase in lymphatics in both testes and ovaries from mice with SSHTN and HTN. Systemic and local inflammation is well known to disrupt the hypothalamic–pituitary–testicular axis, inhibiting LC function and spermatogenesis [57,58]. Resident macrophages, mast cells, and SCs can produce both proinflammatory and anti-inflammatory cytokines including IL-1β, IL-6, TNF-α, IFN-γ, members of the TGF-β family, and IL-10 under physiological conditions [59]. Proinflammatory cytokines are known to promote testicular inflammation and disrupt BTB by down-regulation and delocalization of critical TJPs [60–62]. A study reported down-regulation of SC TJPs such as Claudin-11, Occludin, and TJP-1 in a model of varicocele rat testes along with decreased expression of 3βHsd6 and 17βHsd3 when compared with normal testes [63]. It was also accompanied by increased gene expression of proinflammatory cytokines (Tnfa, Il1a, and Il6), a leukocyte marker (Cd45), and T-cell markers (Cd3g and Cd3d) in the varicocele testes [63]. The inflammatory cytokines TNFα, IL1β, and IL-6 have been reported to diminish steroidogenesis in TM3 LCs [64]. In line with these studies, we also found increased gene expression of proinflammatory mediators and, decreased expression of critical TJPs and steroidogenic pathway genes in testes indicating that HTN might disrupt spermatogenesis and steroidogenesis in mice. This is supported by our results showing poor sperm quantity and quality in the hypertensive mice.
Inhibin is a dimer of a common α subunit and either a βA (inhibin A) or βB (inhibin B) subunit [65]. Inhibin B, predominantly secreted by SCs, suppresses FSH secretion from the pituitary [66]. Activin is a homodimer of two βA (activin A) and two βB subunits (activin B). Activin A stimulates FSH secretion and regulates steroidogenesis and spermatogenesis by influencing the development of SCs and GCs [67]. Besides these functions, activin A plays a critical role in maintaining the immune privileged state within the testis [68]. Elevated expression of activin A in the testis is associated with inflammation and disruption of spermatogenesis in animal models [52,69]. Also, IL-1β is shown to increase activin A and suppress inhibin B levels in SCs in vitro [70]. This agrees with the up-regulated expression of Inhba along with decreased expression of Inhbb observed in the present study in both SSHTN and LHTN models. Transferrin delivers iron to the developing GCs and is a secretory product of SCs [71]. Decreased expression of Trf in the testes of both SSHTN and LHTN mice may be linked to disruption of spermatogenesis, resulting in poor sperm quality and quantity as observed in the current study. ABP (Secretoglobin family 1B, member 24; Scgb1b24), secreted by SCs, is involved in transporting and increasing the bioavailability of androgens [72]. Previous studies have reported an association between excess ABP and increased apoptosis of pachytene spermatocyte, decreased intratesticular levels of androgens, resulting in impaired spermatogenesis in a transgenic mouse model [73,74]. The overexpression of Scgb1b24 in the testes of hypertensive mice in the present study is consistent with these reports. Since ABP-bound androgens are considered biologically inactive [75], the decreased availability of androgens might be one of the reasons for the poor quantity and quality of sperm observed in the current study.
Low-grade, chronic inflammation and impaired folliculogenesis, and ovulation has been documented in females with obesity and PCOS [76,77]. Elevated expression of IL-1, TNF-α, and IFN-γ was observed in autoimmune ovarian disease, a chronic inflammatory disease model [78]. Endometriotic tissue exhibited inflammation-associated lymphangiogenesis in both humans and mice [54,56]. A strong correlation between hyperandrogenism and inflammation has been established in PCOS [79]. Elevated serum inhibin B levels have been reported in women with PCOS [80]. In accordance with the aforementioned studies, the data presented here suggest that in the SSHTN mice, inflammation is associated with increased lymphatics, expression of steroidogenic pathway genes and inhibin B, and a concurrent decrease in the expression of Cyp19a1, which might lead to hyperandrogenism and disturb normal ovarian functions.
Taken together, our explorative study was performed to gain insights into the association between gonadal macrophages and inflammation in the reproductive dysfunction observed in HTN. Although the current study is largely observational, we have established for the first time to our knowledge that altered macrophage polarization occurs in the gonads and is associated with gonadal inflammation, inflammation-associated lymphangiogenesis, and dysfunction in hypertensive animals. Studies are underway to determine if manipulation of M1 and M2 macrophages can reduce gonadal inflammation and improve gonadal function. It is possible that normalizing gonadal macrophage polarization may have beneficial effects on reproductive health in people with HTN.
Supplementary Material
Clinical perspectives.
HTN has been implicated in impaired reproductive health in both men and women. The underlying mechanisms have been largely confined to hormonal imbalance and modified vasculature in reproductive tissues.
We observed an imbalance in macrophage polarization in both testes and ovaries of mice from two different models of HTN, SSHTN, and nitric oxide synthase inhibition-induced HTN. This altered gonadal macrophage polarization was associated with gonadal inflammation, inflammation-associated lymphangiogenesis, and dysfunction.
The current study lays the groundwork for interventional studies to manipulate macrophage polarization, thereby providing a new therapeutic strategy to improve reproductive health in hypertensive patients.
Acknowledgements
We thank Robbie Moore and Dr. Joseph Rutkowski for the technical assistance and advice. We acknowledge the facilities provided by Integrated Microscopy & Imaging Laboratory (IMIL) and College of Medicine-Cell Analysis Facility (COM-CAF) cores at Texas A&M University, Bryan, Texas.
Funding
The present study was supported by an American Heart Association Postdoctoral Fellowship (#916912) to S.N. and National Institutes of Health RO1 (DK120493) to B.M.M.
Abbreviations
- ABP
androgen-binding protein
- Ar
androgen receptor
- BTB
blood–testis barrier
- BWW
Biggers-Whitten-Whittingham
- CYP
cytochrome P450
- DAPI
4’,6-diamidino-2-phenylindole
- DPBS
Dulbecco’s phosphate-buffered saline
- EAO
experimental autoimmune orchitis
- Era
estrogen receptor subtype a
- Erb
estrogen receptor b
- FITC-PNA
fluorescent-labeled peanut agglutinin
- FMO
fluorescence minus one
- FSH
follicle-stimulating hormone
- GC
germ cell
- HTN
hypertension
- HSD
hydroxysteroid dehydrogenases
- Inhbb
Inhibin B
- L-NAME
nitro-l-arginine methyl ester hydrochloride
- LC
Leydig cell
- LH
luteinizing hormone
- LHTN
L-NAME-induced hypertension
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- M1
proinflammatory macrophages
- M2
anti-inflammatory macrophages
- MCP-1
monocyte chemoattractant protein-1
- MIP-1
macrophage inflammatory protein-1
- PBS
phosphate-buffered saline
- PCOS
polycystic ovarian syndrome
- Pdpn
podoplanin
- PFA
paraformaldehyde
- Prox1
a lymphatic endothelial cell transcription factor
- Rh123
rhodamine123
- SBP
systolic blood pressure
- SC
Sertoli cell
- SEM
standard error of the mean
- SSHTN
salt-sensitive hypertension
- StAR
steroidogenic acute regulatory protein
- T
testosterone
- TJ
tight junction
- TJP
tight junction proteins
- Trf
transferrin
- VEGF-C
vascular endothelial growth factor C
- VEGF-D
vascular endothelial growth factor D
- VEGFR-3
vascular endothelial growth factor receptor 3
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
CRediT Author Contribution
Shobana Navaneethabalakrishnan: Conceptualization, Data curation, Software, Formal analysis, Funding acquisition, Investigation, Visualization, Methodology, Writing–original draft, Writing–review & editing. Brooke K. Wilcox: Data curation, Software, Formal analysis, Investigation, Methodology, Writing–review & editing. Bethany L. Goodlett: Data curation, Software, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing–review & editing. Malea M. Murphy: Data curation, Investigation, Visualization, Methodology. Brett M. Mitchell: Conceptualization, Resources, Data curation, Formal analysis, Supervision, Funding acquisition, Investigation, Project administration, Writing–review & editing.
Data Availability
All supporting data are in the paper.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al. (2015) Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–e322 [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, 1269–1324, 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 3.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T et al. (2016) Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 387, 435–443, 10.1016/S0140-6736(15)00805-3 [DOI] [PubMed] [Google Scholar]
- 4.Navaneethabalakrishnan S, Goodlett BL, Lopez AH, Rutkowski JM and Mitchell BM (2020) Hypertension and reproductive dysfunction: a possible role of inflammation and inflammation-associated lymphangiogenesis in gonads. Clin. Sci. (Lond.) 134, 3237–3257, 10.1042/CS20201023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aranda P, Ruilope LM, Calvo C, Luque M, Coca A and Gil de Miguel A (2004) Erectile dysfunction in essential arterial hypertension and effects of sildenafil: results of a Spanish national study. Am. J. Hypertens 17, 139–145, 10.1016/j.amjhyper.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Guo D, Li S, Behr B and Eisenberg ML (2017) Hypertension and male fertility. World J. Mens. Health 35, 59–64, 10.5534/wjmh.2017.35.2.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foy CG, Newman JC, Berlowitz DR, Russell LP, Kimmel PL, Wadley VG et al. (2019) Blood pressure, sexual activity, and erectile function in hypertensive men: baseline findings from the Systolic Blood Pressure Intervention Trial (SPRINT). J. Sex Med 16, 235–247, 10.1016/j.jsxm.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan LE, Lewis C, Jenkins P and Pearson TA (2000) Does hypertension and its pharmacotherapy affect the quality of sexual function in women? Am. J. Hypertens 13, 640–647, 10.1016/S0895-7061(99)00288-5 [DOI] [PubMed] [Google Scholar]
- 9.Doumas M, Tsiodras S, Tsakiris A, Douma S, Chounta A, Papadopoulos A et al. (2006) Female sexual dysfunction in essential hypertension: a common problem being uncovered. J. Hypertens 24, 2387–2392, 10.1097/01.hjh.0000251898.40002.5b [DOI] [PubMed] [Google Scholar]
- 10.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L and Chappell LC (2014) Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 348, g2301, 10.1136/bmj.g2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akagashi K, Itoh N, Kumamoto Y, Tsukamoto T, Suzuki T and Ohta Y (1996) Hypertensive changes in intratesticular arteries impair spermatogenesis of the stroke-prone spontaneously hypertensive rat. J. Androl 17, 367–374 [PubMed] [Google Scholar]
- 12.Breigeiron MK, Lucion AB and Sanvitto GL (2007) Effects of renovascular hypertension on reproductive function in male rats. Life Sci. 80, 1627–1634, 10.1016/j.lfs.2007.01.030 [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro RA, Raineki C, Gonçalves O, Franci CR, Lucion AB and Sanvitto GL (2013) Reproductive dysfunction in female rats with renovascular hypertension. Am. J. Hypertens 26, 104–110, 10.1093/ajh/hps026 [DOI] [PubMed] [Google Scholar]
- 14.Yeasmin N, Akhter QS, Mahmuda S, Banu N, Yeasmin S, Akhter S et al. (2017) Association of hypertension with serum estrogen level in postmenopausal women. Mymensingh Med. J 26, 635–641 [PubMed] [Google Scholar]
- 15.Colli LG, Belardin LB, Echem C, Akamine EH, Antoniassi MP, Andretta RR et al. (2019) Systemic arterial hypertension leads to decreased semen quality and alterations in the testicular microcirculation in rats. Sci. Rep 9, 11047, 10.1038/s41598-019-47157-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae CU, Lee RT, Rifai N and Ridker PM (2001) Blood pressure and inflammation in apparently healthy men. Hypertension 38, 399–403, 10.1161/01.HYP.38.3.399 [DOI] [PubMed] [Google Scholar]
- 17.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM and Schiffrin EL (2005) Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler. Thromb. Vasc. Biol 25, 2106–2113, 10.1161/01.ATV.0000181743.28028.57 [DOI] [PubMed] [Google Scholar]
- 18.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S et al. (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med 204, 2449–2460, 10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY et al. (2013) Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133, 10.1161/HYPERTENSI0NAHA.113.00689 [DOI] [PubMed] [Google Scholar]
- 20.Mian MO, Barhoumi T, Briet M, Paradis P and Schiffrin EL (2016) Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J. Hypertens 34, 97–108, 10.1097/HJH.0000000000000761 [DOI] [PubMed] [Google Scholar]
- 21.Caillon A and Schiffrin EL (2016) Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr. Hypertens. Rep 18, 21, 10.1007/s11906-016-0628-7 [DOI] [PubMed] [Google Scholar]
- 22.Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM et al. (2007) Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am. J. Physiol. Heart Circ. Physiol 292, H1789–H1795, 10.1152/ajpheart.01118.2006 [DOI] [PubMed] [Google Scholar]
- 23.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S et al. (2011) Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124, 1370–1381, 10.1161/CIRCULATI0NAHA.111.034470 [DOI] [PubMed] [Google Scholar]
- 24.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS and Ruiz P (2010) Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol 298, R1089–R1097, 10.1152/ajpregu.00373.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Miguel C, Das S, Lund H and Mattson DL (2010) T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 298, R1136–R1142, 10.1152/ajpregu.00298.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL et al. (2016) CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ. Res 118, 1233–1243, 10.1161/CIRCRESAHA.115.308111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L et al. (2016) Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 68, 167–174, 10.1161/HYPERTENSI0NAHA.116.07493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez Gelston CA, Balasubbramanian D, Abouelkheir GR, Lopez AH, Hudson KR, Johnson ER et al. (2018) Enhancing renal lymphatic expansion prevents hypertension in mice. Circ. Res 122, 1094–1101, 10.1161/CIRCRESAHA.118.312765 [DOI] [PubMed] [Google Scholar]
- 29.Balasubbramanian D, Gelston CAL, Lopez AH, Iskander G, Tate W, Holderness H et al. (2020) Augmenting renal lymphatic density prevents angiotensin II-induced hypertension in male and female mice. Am. J. Hypertens 33, 61–69, 10.1093/ajh/hpz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosser DM and Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol 8, 958–969, 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndisang JF and Mishra M (2013) The heme oxygenase system selectively suppresses the proinflammatory macrophage m1 phenotype and potentiates insulin signaling in spontaneously hypertensive rats. Am. J. Hypertens 26, 1123–1131, 10.1093/ajh/hpt082 [DOI] [PubMed] [Google Scholar]
- 32.Gomolak JR and Didion SP (2014) Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front. Physiol 5, 396, 10.3389/fphys.2014.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harwani SC, Ratcliff J, Sutterwala FS, Ballas ZK, Meyerholz DK, Chapleau MW et al. (2016) Nicotine Mediates CD161a+ Renal Macrophage Infiltration and Premature Hypertension in the Spontaneously Hypertensive Rat. Circ. Res 119, 1101–1115, 10.1161/CIRCRESAHA.116.309402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martín-Fernández B, Rubio-Navarro A, Cortegano I, Ballesteros S, Alía M, Cannata-Ortiz P et al. (2016) Aldosterone induces renal fibrosis and inflammatory M1-macrophage subtype via mineralocorticoid receptor in rats. PloS ONE 11, e0145946, 10.1371/journal.pone.0145946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H and Mattson DL (2019) Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am. J. Physiol. Renal. Physiol 317, F361–F374, 10.1152/ajprenal.00096.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zawia A, Arnold ND, West L, Pickworth JA, Turton H, Iremonger J et al. (2021) Altered macrophage polarization induces experimental pulmonary hypertension and is observed in patients with pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol 41, 430–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsetsarkin KA, Acklin JA, Liu G, Kenney H, Teterina NL, Pletnev AG et al. (2020) Zika virus tropism during early infection of the testicular interstitium and its role in viral pathogenesis in the testes. PLoS Pathog. 16, e1008601, 10.1371/journal.ppat.1008601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ono Y, Nagai M, Yoshino O, Koga K, Nawaz A, Hatta H et al. (2018) CD11c+ M1-like macrophages (MΦs) but not CD206+ M2-like MΦ are involved in folliculogenesis in mice ovary. Sci. Rep 8, 8171, 10.1038/s41598-018-25837-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasegawa S, Susaki EA, Tanaka T, Komaba H, Wada T, Fukagawa M et al. (2019) Comprehensive three-dimensional analysis (CUBIC-kidney) visualizes abnormal renal sympathetic nerves after ischemia/reperfusion injury. Kidney Int. 96, 129–138, 10.1016/j.kint.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 40.O’Donnell L, Stanton P and de Kretser DM (2017) Endocrinology of the male reproductive system and spermatogenesis. In Endocrinology of Male Reproduction (Feingold KR, Anawalt B, Boyce A and McLachlan RI, eds), pp. 1–69 [Google Scholar]
- 41.Smith LB and Walker WH (2014) The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol 30, 2–13, 10.1016/j.semcdb.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller WL and Auchus RJ (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev 32, 81–151, 10.1210/er.2010-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su W, Mruk DD and Cheng CY (2013) Regulation of actin dynamics and protein trafficking during spermatogenesis–insights into a complex process. Crit. Rev. Biochem. Mol. Biol 48, 153–172, 10.3109/10409238.2012.758084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaitu’u-Lino TJ, Sluka P, Foo CF and Stanton PG (2007) Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction 133, 1169–1179, 10.1530/REP-06-0385 [DOI] [PubMed] [Google Scholar]
- 45.Tarulli GA, Meachem SJ, Schlatt S and Stanton PG (2008) Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction 135, 867–877, 10.1530/REP-07-0572 [DOI] [PubMed] [Google Scholar]
- 46.Giani JF, Bernstein KE, Janjulia T, Han J, Toblli JE, Shen XZ et al. (2015) Salt sensitivity in response to renal injury requires renal angiotensin-converting enzyme. Hypertension 66, 534–542, 10.1161/HYPERTENSIONAHA.115.05320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drummond AE (2006) The role of steroids in follicular growth. Reprod. Biol. Endocrinol 4, 16, 10.1186/1477-7827-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapointe E and Boerboom D (2011) WNT signaling and the regulation of ovarian steroidogenesis. Front. Biosci. (Schol Ed.) 3, 276–285 [DOI] [PubMed] [Google Scholar]
- 49.Bhushan S, Theas MS, Guazzone VA, Jacobo P, Wang M, Fijak M et al. (2020) Immune cell subtypes and their function in the testis. Front. Immunol 11, 583304, 10.3389/fimmu.2020.583304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Huang L and Brayboy L (2021) Macrophages: an indispensable piece of ovarian health. Biol. Reprod 104, 527–538, 10.1093/biolre/ioaa219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhushan S, Tchatalbachev S, Lu Y, Fröhlich S, Fijak M, Vijayan V et al. (2015) Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J. Immunol 194, 5455–5464, 10.4049/jimmunol.1401132 [DOI] [PubMed] [Google Scholar]
- 52.Nicolas N, Michel V, Bhushan S, Wahle E, Hayward S, Ludlow H et al. (2017) Testicular activin and follistatin levels are elevated during the course of experimental autoimmune epididymo-orchitis in mice. Sci. Rep. 7, 42391, 10.1038/srep42391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lima PDA, Nivet AL, Wang Q, Chen YA, Leader A, Cheung A et al. (2018) Polycystic ovary syndrome: possible involvement of androgen-induced, chemerin-mediated ovarian recruitment of monocytes/macrophages. Biol. Reprod 99, 838–852, 10.1093/biolre/ioy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reichelt U, Keichel S, Barcena de Arellano ML, Chiantera V, Schneider A and Mechsner S (2012) High lymph vessel density and expression of lymphatic growth factors in peritoneal endometriosis. Reprod. Sci 19, 876–882, 10.1177/1933719112438440 [DOI] [PubMed] [Google Scholar]
- 55.Hirai S, Naito M, Terayama H, Qu N, Kuerban M, Musha M et al. (2013) Lymphangiogenesis in chronic inflammation in the testis. Andrology 1, 147–154, 10.1111/j.2047-2927.2012.00015.x [DOI] [PubMed] [Google Scholar]
- 56.Hattori K, Ito Y, Honda M, Sekiguchi K, Hosono K, Shibuya M et al. (2020) Lymphangiogenesis induced by vascular endothelial growth factor receptor 1 signaling contributes to the progression of endometriosis in mice. J. Pharmacol. Sci 143, 255–263, 10.1016/j.jphs.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 57.O’Bryan MK, Schlatt S, Phillips DJ, de Kretser DM and Hedger MP (2000) Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology 141, 238–246, 10.1210/endo.141.1.7240 [DOI] [PubMed] [Google Scholar]
- 58.Hales DB (2002) Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol 57, 3–18, 10.1016/S0165-0378(02)00020-7 [DOI] [PubMed] [Google Scholar]
- 59.O’Bryan MK, Gerdprasert O, Nikolic-Paterson DJ, Meinhardt A, Muir JA, Foulds LM et al. (2005) Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses. Am. J. Physiol. Regul. Integr. Comp. Physiol 288, R1744–R1755, 10.1152/ajpregu.00651.2004 [DOI] [PubMed] [Google Scholar]
- 60.Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK et al. (2006) Tumor necrosis factor {alpha} reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J. Endocrinol 190, 313–329, 10.1677/joe.1.06781 [DOI] [PubMed] [Google Scholar]
- 61.Pérez CV, Sobarzo CM, Jacobo PV, Pellizzari EH, Cigorraga SB, Denduchis B et al. (2012) Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on Sertoli cell tight junctions. Biol. Reprod 87, 122, 10.1095/biolreprod.112.101709 [DOI] [PubMed] [Google Scholar]
- 62.Pèrez CV, Pellizzari EH, Cigorraga SB, Galardo MN, Naito M, Lustig L et al. (2014) IL17A impairs blood-testis barrier integrity and induces testicular inflammation. Cell Tissue Res. 358, 885–898, 10.1007/s00441-014-1995-5 [DOI] [PubMed] [Google Scholar]
- 63.Oh YS, Jo NH, Park JK and Gye MC (2016) Changes in inflammatory cytokines accompany deregulation of claudin-11, resulting in inter-sertoli tight junctions in varicocele rat testes. J. Urol 196, 1303–1312, 10.1016/j.juro.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 64.Leisegang K and Henkel R (2018) The in vitro modulation of steroidogenesis by inflammatory cytokines and insulin in TM3 Leydig cells. Reprod. Biol. Endocrinol 16, 26, 10.1186/s12958-018-0341-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayes FJ, Hall JE, Boepple PA and Crowley WF Jr. (1998) Clinical review 96: Differential control of gonadotropin secretion in the human: endocrine role of inhibin. J. Clin. Endocrinol. Metab 83, 1835–1841 [DOI] [PubMed] [Google Scholar]
- 66.O’Connor AE and De Kretser DM (2004) Inhibins in normal male physiology. Semin. Reprod. Med 22, 177–185, 10.1055/s-2004-831893 [DOI] [PubMed] [Google Scholar]
- 67.Mendis SH, Meachem SJ, Sarraj MA and Loveland KL (2011) Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol. Reprod 84, 379–391, 10.1095/biolreprod.110.086231 [DOI] [PubMed] [Google Scholar]
- 68.Hedger MP and Winnall WR (2012) Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol. Cell. Endocrinol 359, 30–42, 10.1016/j.mce.2011.09.031 [DOI] [PubMed] [Google Scholar]
- 69.Tanimoto Y, Tanimoto K, Sugiyama F, Horiguchi H, Murakami K, Yagami K et al. (1999) Male sterility in transgenic mice expressing activin betaA subunit gene in testis. Biochem. Biophys. Res. Commun 259, 699–705, 10.1006/bbrc.1999.0833 [DOI] [PubMed] [Google Scholar]
- 70.Okuma Y, O’Connor AE, Muir JA, Stanton PG, de Kretser DM and Hedger MP (2005) Regulation of activin A and inhibin B secretion by inflammatory mediators in adult rat Sertoli cell cultures. J. Endocrinol 187, 125–134, 10.1677/joe.1.06266 [DOI] [PubMed] [Google Scholar]
- 71.Leichtmann-Bardoogo Y, Cohen LA, Weiss A, Marohn B, Schubert S, Meinhardt A et al. (2012) Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload. Am. J. Physiol. Endocrinol. Metab 302, E1519–E1530, 10.1152/ajpendo.00007.2012 [DOI] [PubMed] [Google Scholar]
- 72.Herbert Z, Weigel S, Sendemir E, Marshall A, Caldwell JD, Petrusz P et al. (2005) Androgen-binding protein is co-expressed with oxytocin in the male reproductive tract. Anat. Histol. Embryol 34, 286–293, 10.1111/j.1439-0264.2005.00605.x [DOI] [PubMed] [Google Scholar]
- 73.Selva DM, Tirado OM, Toràn N, Suárez-Quian CA, Reventós J and Munell F (2000) Meiotic arrest and germ cell apoptosis in androgen-binding protein transgenic mice. Endocrinology 141, 1168–1177, 10.1210/endo.141.3J383 [DOI] [PubMed] [Google Scholar]
- 74.Jeyaraj DA, Grossman G and Petrusz P (2005) Altered bioavailability of testosterone in androgen-binding protein-transgenic mice. Steroids 70, 704–714, 10.1016/j.steroids.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 75.Mendel CM (1989) The free hormone hypothesis: a physiologically based mathematical model. Endocr. Rev 10, 232–274, 10.1210/edrv-10-3-232 [DOI] [PubMed] [Google Scholar]
- 76.Robker RL, Wu LL and Yang X (2011) Inflammatory pathways linking obesity and ovarian dysfunction. J. Reprod. Immunol 88, 142–148, 10.1016/j.jri.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 77.Duleba AJ and Dokras A (2012) Is PCOS an inflammatory process? Fertil. Steril 97, 7–12, 10.1016/j.fertnstert.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bagavant H, Adams S, Terranova P, Chang A, Kraemer FW, Lou Y et al. (1999) Autoimmune ovarian inflammation triggered by proinflammatory (Th1)T cells is compatible with normal ovarian function in mice. Biol. Reprod 61, 635–642, 10.1095/biolreprod61.3.635 [DOI] [PubMed] [Google Scholar]
- 79.Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM et al. (2021) Chronic low grade inflammation in pathogenesis of PCOS. Int. J. Mol. Sci 22, 10.3390/ijms22073789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson RA, Groome NP and Baird DT (1998) Inhibin A and inhibin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono-ovulation. Clin. Endocrinol. (Oxf) 48, 577–584, 10.1046/j.1365-2265.1998.00442.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data are in the paper.


