Abstract
A nested-PCR method was used to detect the occurrence of human adenovirus in coastal waters of Southern California. Twenty- to forty-liter water samples were collected from 12 beach locations from Malibu to the border of Mexico between February and March 1999. All sampling sites were located at mouths of major rivers and creeks. Two ultrafiltration concentration methods, tangential flow filtration (TFF) and vortex flow filtration (VFF), were compared using six environmental samples. Human adenoviruses were detected in 4 of the 12 samples tested after nucleic acid extraction of VFF concentrates. The most probable number of adenoviral genomes ranged from 880 to 7,500 per liter of water. Coliphages were detected at all sites, with the concentration varying from 5.3 to 3332 PFU/liter of water. F-specific coliphages were found at 5 of the 12 sites, with the concentration ranging from 5.5 to 300 PFU/liter. The presence of human adenovirus was not significantly correlated with the concentration of coliphage (r = 0.32) but was significantly correlated (r = 0.99) with F-specific coliphage. The bacterial indicators (total coliforms, fecal coliforms, and enterococci) were found to exceed California recreational water quality daily limits at 5 of the 12 sites. However, this excess of bacterial indicators did not correlate with the presence of human adenoviruses in coastal waters. The results of this study call for both a reevaluation of our current recreational water quality standards to reflect the viral quality of recreational waters and monitoring of recreational waters for human viruses on a regular basis.
Southern California and its beaches are unique recreational and economic resources due to the long coastline and year-round temperate climate. More than 100 million people worldwide visit Southern California beaches annually to surf, swim, sunbathe, snorkel, and scuba dive (State of California Economic Resources data, 1993). Therefore, the safety of beach waters is of concern to both the state and county health departments and beachgoers. For example, the closure of Huntington Beach in Orange County, Calif., due to elevated levels of bacterial indicators during the summer of 1999, caused a tremendous economic loss to the county and state (www.ocsd.com/Environment/Monitoring The Environment) and raised concerns among local residents regarding the safety of coastal recreational waters. Southern California is one of the most densely populated coastal regions in the country. Population growth results in conversion of open land into nonpermeable surfaces, which increases the rate of runoff and can impact water quality through addition of sediment, toxic chemicals, microbial pathogens, and nutrients to the coastal ocean.
To govern the public health and safety of recreational beaches, the State of California has instated beach-monitoring programs in accordance with U.S. Environmental Protection Agency (EPA) policy. Bacterial indicators, such as total coliforms, fecal coliforms, and enterococci, are routinely monitored by more than 20 agencies at 510 stations along the Southern California coast (Southern California Coastal Water Research Project, 1998 [http://www.sccwrp.org/regional/98bight/micbio/]). Routine bacteriological monitoring has reduced the incidence of waterborne diseases related to recreational water contact, but water-related illnesses, particularly those caused by viral pathogens, are still of concern (3).
More than 100 different types of viruses are found in human waste and are potentially transmitted by water (3). These viruses are more resistant to environmental conditions and sewage treatment processes, including chlorination and UV radiation, than many of the sewage-associated bacteria (11). In laboratory studies, enteric viruses have been reported to survive for 2 to 130 days in seawater. These survival periods surpass those reported for coliform bacteria in similar environments (23). Therefore, the use of bacterial indicators to predict the virological quality of water is questionable.
The human enteric virus group, which includes Norwalk virus, rotavirus, hepatitis A virus, adenovirus, and enterovirus, is one of the leading causes of human illness. Adenoviruses are the only human enteric viruses that contain DNA rather than RNA. Specifically, adenoviruses 40 and 41 have been recognized as important etiological agents of gastroenteritis in children (10, 32), second in importance only to rotaviruses. Respiratory adenoviruses are capable of causing large, persistent epidemics (22), and it is suspected that they may be more important than enteric viruses as a contagion during recreational water activities because of their potential transmission in aerosols. Studies conducted in Europe have suggested using adenovirus as an index of human viral pollution since this virus was most often detected in aquatic samples (27). Adenoviruses have been consistently found in greater numbers than enteroviruses in raw sewage around the world (18, 20, 21) and are substantially more stable than either poliovirus or hepatitis A virus in tap water and seawater (13). For example, adenovirus survives three to five times longer in seawater than poliovirus, taking 99 days for a 2-log reduction of infectivity (13). The presence of adenovirus in U.S. coastal water has not been investigated. Here we report the detection of human adenovirus in Southern California coastal waters by nested PCR and a preliminary examination of their relationship to coliphage, F-specific coliphage, and bacterial indicators.
MATERIALS AND METHODS
Sampling sites.
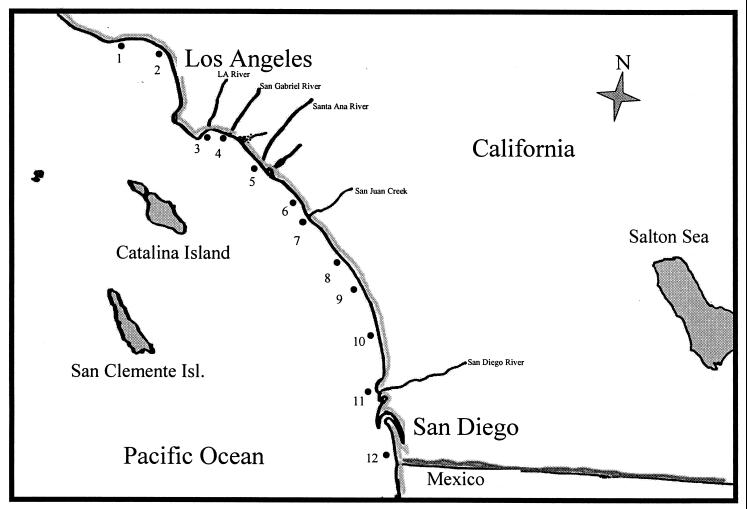
Water samples were taken from the wavewash (point zero location where the freshwater input meets the ocean) of 12 freshwater outlets from Malibu to the Mexican border between 8 February and 1 March 1999 (Fig. 1). The locations sampled were Malibu Lagoon, Santa Monica Canyon storm drain, Los Angeles River, San Gabriel River, Santa Ana River, Aliso Creek, San Juan Creek, San Luis Rey River, Moonlight Creek, Los Penosquitos Lagoon, San Diego River, and Tijuana River. Twenty- to forty-liter water samples were collected with acid-washed and sample-rinsed carboys at each sampling site and transported back to the laboratory for immediate processing. The sample salinity was determined by refractometry.
FIG. 1.
Sampling locations for detection of human adenoviruses, coliphage, and fecal bacterial indicators in coastal waters of Southern California. All sampling sites are located at the mouth of a river or creek: 1, Malibu Lagoon; 2, Santa Monica Canyon Creek; 3, Los Angeles River; 4, San Gabriel River; 5, Santa Ana River; 6, Aliso Creek; 7, San Juan Creek; 8, San Luis Rey River; 9, Moonlight Creek; 10, Los Penosquitos Lagoon; 11, Sand Diego River; 12, Tijuana River.
Concentration of viruses from water samples.
To compare the efficiency of viral recovery, six of the water samples (from Malibu Lagoon, Santa Monica Canyon storm drain, Los Angeles River, San Gabriel River, Santa Ana River, and Aliso Creek) were concentrated both by tangential flow filtration (TFF) with a 30-kDa spiral filter cartridge (29) and by vortex flow filtration (VFF) with a 100-kDa filter cartridge (19). Samples from the other six locations were concentrated using only VFF.
Using TFF, 20 liters of water was first sequentially filtered through a 142-mm-diameter glass fiber filter and a 0.22-μm-pore-size Durapore filter to remove particulates. This filtration step is necessary to prevent clogging of the spiral filter cartridge (Amicon, Inc.). The filtrates were concentrated from 20 liters to ca. 150 ml using the TFF system and then further concentrated using a Centriprep-30 ultraconcentration unit to a final volume of about 1 ml (R. Noble and J. Fuhrman, submitted for publication). Using VFF, 20-liter water samples were concentrated directly to 40 to 80 ml without removal of particulates from the sample (19, 24).
Detection of indicator bacteria and coliphages.
Water samples were collected at the same time from all sites for analysis of total coliforms, fecal coliforms, and enterococci by local agencies following the EPA standard protocols for bacterial indicator analysis (9). Coliphage densities were determined using either freshly VFF-concentrated or unconcentrated water samples. One milliliter and 0.1 ml of sample were mixed with 1 ml of exponential Escherichia coli host in 1% soft agar, then overlaid over bottom nutrient agar. Two E. coli hosts ATCC 15597 and HS (pFamp)R, were used. E. coli ATCC 15597 is a general host for both DNA and RNA coliphage; E. coli HS (pFamp)R contains a plasmid coding for both ampicillin and streptomycin resistance and is a specific host for F-specific coliphage (25). When HS (pFamp)R was employed the bottom agar contained 15 μg each of ampicillin and streptomycin per ml to prevent background growth of indigenous bacteria. On each host, plaques were enumerated after 12 h of incubation at 37°C.
Detection of human adenovirus by nested PCR.
Concentrated water samples were either used directly for PCR or further purified to remove PCR inhibitors using the method originally developed by Boom et al. (4) with minor modifications. In brief, 50 μl of viral concentrate was lysed by 900 μl of guanidinium thiocyanate (GuSCN) lysis buffer at room temperature for 10 min. Then 40 μl of silica particles (Sigma Chemical Inc.) was added and nucleic acids were adsorbed at room temperature for 10 min with gentle shaking. Silica beads were pelleted, washed, and briefly dried. The nucleic acid was eluted from the beads using 50 μl of Tris-EDTA buffer at a temperature of 56°C.
For samples showing negative results by both the above methods, 10 ml of VFF concentrate was further concentrated by ultracentrifugation at 41,000 rpm (Beckman rotor SW41) for 2 h. The pellets were resuspended in 200 μl of Tris-EDTA buffer and nucleic acid purified by GuSCN extraction as described above.
Nested PCR for human adenovirus was performed following the protocol of Pina et al. (27) with minor modifications. Adenovirus-specific primers were 5′-GCCGCAGTGGTCTTACATGCACATC-3′ and 5′-CAGCACGCCGCGGATGTCAAAGT-3′, yielding an amplicon of 300 bp. The nested primers were 5′-GCCACCGAGACGTACTTCAGCCTG-3′ and 5′-TTGTACGAGTACGCGGTATCCTCGCGGTC-3′, with the resulting amplicon being 143 bp. For each PCR, 1 μl of sample was used in a 25-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1× TaqM (Eppendorf Inc.), 1.3 mM MgCl2, 200 μM deoxynucleoside triphosphosphate and a 0.1 μM concentration of each primer. The PCR cycles were 94°C for 4 min, followed by 30 cycles of 92°C for 1.5 min, 55°C for 1.5 min, and 72°C for 2 min and a final extension at 72°C for 10 min. The second round of PCR was performed with the nested primers using the same conditions as described above. PCR products were resolved on 2% agarose. For PCR-positive samples, multiple analyses (5 to 12 replicates) were performed for each sample to estimate the most probable number using Thomas's formula (9).
Recovery of VNA by GuSCN extraction.
To determine the viral nucleic acid (VNA) recovery using the GuSCN-silica bead extraction method and the presence of PCR inhibition, concentrated seawater samples from Malibu Lagoon, Moonlight Creek, and San Diego River that were negative for adenovirus by nested PCR were seeded with 105 viral genomes of sample per μl. The seeded sample was extracted by the GuSCN-silica bead method, and serial dilutions were made in deionized (DI) water to determine the recovery of viral genome after extraction. In parallel, the seeded sample was also diluted with the concentrated seawater to determine the effect of PCR inhibition among concentrated water samples. Seeding and extraction were performed twice using each sample to determine the variability associated with these procedures.
Statistical analysis.
Pearson linear correlation was used to analyze the relationship between the abundance of human viruses and other parameters tested in this study. The correlation was considered significant at a 95% confidence level. The Microsoft Excel program (Microsoft Inc.) was used for the statistical analysis.
RESULTS
Comparison of TFF and VFF concentration methods.
The use of TFF in combination with Centriprep-30 (Amicon Inc.) concentration was capable of concentrating viruses ca. 105-fold. Among six coastal samples concentrated by this method, only the sample collected from the mouth of the Los Angeles River tested PCR positive for adenovirus (Table 1). PCR inhibition was observed in samples seeded with adenoviruses, while a 1:5 dilution in DI water resulted in an increase of PCR amplification efficiency (data not shown). However, further purification of viral genome by nucleic acid extraction did not yield additional PCR-positive samples (Table 1).
TABLE 1.
Comparison of TFF and VFF concentration and nested-PCR detection of adenovirus in coastal waters
| Sampling sitea | Adenovirus detection by nested PCR using:
|
|||
|---|---|---|---|---|
| TFF, Centriprep, and Centricon concentrates | Purified nucleic acid from TFF, Centriprep, and Centricon concentrates | VFF concentrates | Purified nucleic acid from VFF concentrates | |
| Malibu Lagoon | − | − | − | − |
| Santa Monica | − | − | − | − |
| Canyon Creek | ||||
| Los Angeles River | + | + | − | + |
| San Gabriel River | − | − | − | + |
| Santa Ana River | − | − | − | + |
| Aliso Creek | − | − | − | − |
All sites are at the mouth of a river or creek.
VFF coconcentrates viruses along with other plankton and humic material which may interfere or inhibit PCR. When VFF concentrates were used for PCR directly, no positive results were detected in any of the samples (Table 1). To remove PCR-interfering substances, viral nucleic acid was purified from VFF concentrates by the GuSCN-silica bead extraction. After extraction, three of the six coastal samples tested PCR positive for adenovirus (Table 1).
Recovery of VNA using GuSCN and silica beads extraction.
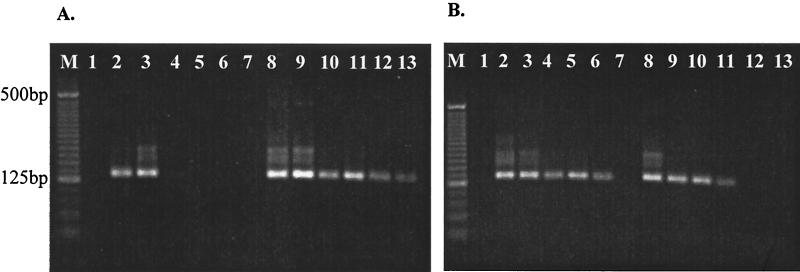
VNA recovery from various seeded samples, shown in Fig. 2, displayed a positive relationship with the presence of PCR-inhibiting substances. For example, samples having less PCR inhibition also had a lower recovery of VNA after GuSCN-silica bead extraction, while a high VNA recovery was seen with samples initially having substantial PCR inhibition. Specifically, little PCR inhibition was detected in samples collected from Moonlight Creek, yet the recovery of VNA after extraction was only 1% of the seeded viral genome (Fig. 2B). In contrast, strong PCR inhibition was experienced in seeded samples from the San Diego River, yet the VNA recovery was nearly 100% (Fig. 2A). Little variation was detected for replicate seeded samples, extracted at different times (data not shown), suggesting the differences in VNA recovery observed were due to the intrinsic character of the original sample.
FIG. 2.
Efficiency of VNA recovery from environmental samples by the GuSCN-silica bead extraction method. Concentrated samples from either San Diego River (A) or Moonlight Creek (B) were seeded with adenoviruses to a final concentration of 105/μl. Lane 1, negative control; lanes 2 to 7, seeded samples were diluted from 100 to 10−5 in seawater concentrates and detected by nested PCR; lanes 8 to 13, seeded environmental samples were extracted by the GuSCN-silica bead method and diluted with sterilized DI water from 100 to 10−5, respectively; lane M, 25-bp molecular weight ladder.
Adenovirus, coliphage, and indicator bacteria in southern California coastal waters.
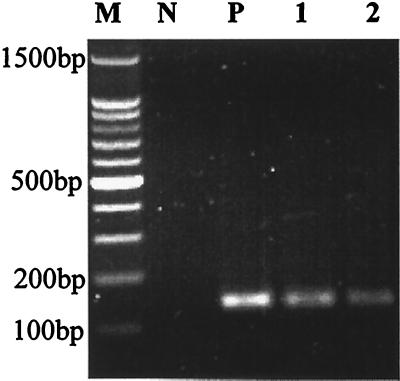
Adenoviruses were detected by PCR in 4 of the 12 samples collected from Southern California beaches (Table 2; Fig. 3). The genomic numbers of adenovirus in each sample were estimated based on the most probable number and then corrected for concentration factors. The concentrations varied from 880 to 7,500 per liter of water. High concentrations of adenovirus were detected at the mouths of the Los Angeles and San Gabriel rivers, while at the mouth of the Tijuana River, which drains northern Mexico, a relatively lower concentration of adenovirus was detected.
TABLE 2.
Adenovirus, coliphage, and bacterial indicators in Southern California beach waters
| Sampling locationa | Date (day/mo/yr) | Salinity (‰) | Indicator exceedanceb | Coliphage (PFU/liter)c | F-specific coliphage (PFU/liter)d | Adenovirus (genomes/liter)e |
|---|---|---|---|---|---|---|
| Malibu Lagoon | 2/8/99 | 10 | Tc, Fc, En | 192 ± 40.7 | <2 | |
| Santa Monica | 2/8/99 | 9 | En | 96 ± 0.2 | 5.5 | |
| Canyon Creek | ||||||
| Los Angeles River | 2/9/99 | 28 | Tc, Fc, En | 472.5 ± 112 | 300 | 7.5 × 103 |
| San Gabriel River | 2/16/99 | 28 | 106.2 ± 30.7 | 38 | 2.3 × 103 | |
| Santa Ana River | 2/16/99 | 33 | 9.5 ± 1.5 | <2 | 9.24 × 102 | |
| Aliso Creek | 2/16/99 | 31 | 23 ± 16 | 4.6 | ||
| San Juan Creek | 3/1/99 | 24 | En | 20.5 ± 6 | <6.5 | |
| San Luis Rey River | 3/1/99 | 27 | 20 ± 13.9 | <3 | ||
| Moonlight Creek | 3/1/99 | 34 | Tc, Fc, En | 37.5 ± 4.4 | <3 | |
| Los Penosquitos Lagoon | 2/22/99 | 32.5 | 5.3 ± 2.5 | <3.53 | ||
| San Diego River | 2/22/99 | 31 | 367.5 ± 22.7 | <3 | ||
| Tijuana River | 2/22/99 | 29.5 | 3,332 ± 80.9 | 22.6 ± 7.8 | 8.8 × 102 |
All sites are near mouths of rivers or creeks.
Bacterial indicators—total coliform (Tc), fecal coliform (Fc), and enterococci (En)—that exceed California recreational water daily limits.
Plaque assay using E. coli ATCC 15597 as a host.
Plaque assay using E. coli HS (pFamp)R as a host.
Determined by nested PCR.
FIG. 3.
Detection of adenoviral genomes from the VFF-concentrated environmental samples by nested PCR. Lane N, negative control; lane P, positive control; lane 1, mouth of Los Angeles River; lane 2, mouth of San Gabriel River; lane M, 100-bp molecular weight ladder.
Fecal bacterial contamination at each sampling site was reported as the exceeding of the California recreational water quality daily limits, where the threshold is set at 10,000, CFU/100 ml for total coliforms, 400 CFU/100 ml for fecal coliforms, and 104 CFU/100 ml for enterococci. Five of the 12 samples tested exceeded at least one daily bacterial indicator threshold (Table 2). The mouths of the Malibu Lagoon, Los Angeles River, and Moonlight Creek exceeded the daily threshold for all three indicators.
The concentration of coliphage ranged from 5.3 to 3,332 PFU/liter (Table 2). The highest concentration was found at the mouth of the Tijuana River, while the lowest concentration was detected at Los Penosquitos Lagoon. F-specific coliphages were detected at five sites (Table 2), but the concentration did not correlate with the concentration of coliphage (r = 0.20). The salinity of samples ranged from 9 to 34 ppt, with most sites above 27 ppt (Table 2), suggesting low levels of freshwater input to the ocean during the time of sampling at most sites.
DISCUSSION
This study demonstrates that ultrafiltration combined with nested PCR is an effective method of detecting human adenoviruses from 20 liters of coastal water. Ultrafiltration concentration requires minimal manipulation of the water sample, avoiding the complicated procedures that cause viral inactivation (i.e., pH adjustment) or PCR inhibition (i.e., beef extract elution and organic flocculation) used in the EPA standard filtration-elution method. Compared with the EPA standard viral concentration method, in which volumes of at least 100 liters of water are typically filtered, the ultrafiltration method has a higher efficiency of viral recovery (24).
In comparing the two ultrafiltration methods used in this study, TFF concentration required preremoval of plankton and other suspended solids; therefore, the final viral concentrates contained fewer potential PCR inhibitors. With TFF, adenovirus was detected directly by PCR without the need for further purification of viral nucleic acid. This method offers a distinct advantage in that viral particles are intact prior to PCR. Protected by the viral protein coat, VNA is not subjected to degradation due to nuclease activity. This is particularly important for detecting RNA viruses such as enterovirus, because RNA is known to be rapidly degraded once exposed to the environment. This method has been successfully used for PCR detection of enterovirus in Santa Monica Bay in a previous study (Noble and Fuhrman, submitted for publication). In contrast, the VFF method is more time-efficient than TFF, by bypassing the filtration of a large volume of water prior to viral concentration, but tends to concentrate more PCR inhibiting substances with the viruses. VNA extraction and purification are necessary to yield PCR-positive results. In the side-by-side comparison of the two methods using environmental water samples, VFF detected adenoviruses more often than TFF (Table 1). Based on a small number (six) of environmental samples collected during this study, it appears that the TFF method has a lower viral recovery than VFF. This may be due to the loss of particle-associated viruses when samples were filtered to remove other plankton and suspended solids prior to TFF concentration. Many viruses are known to attach to particulate material. Previous studies have indicated that a large portion of viruses can be lost during 0.2-μm-pore-size filtration (24).
The PCR detection of viruses in coastal waters offers advantages over current tissue culture methods. PCR is both less laborious and highly sensitive (e.g., see references 2, 7, 16). In addition, the method is capable of detecting viruses that are either difficult to grow in tissue culture or replicate without producing cytopathic effects in cells (6, 26). Adenovirus belongs to this group of viruses and therefore is not easily detected by culture methods. Furthermore, the PCR method is also highly specific and capable of differentiating different types of viruses. The primers used in this study were previously shown to specifically amplify 47 serotypes of human adenoviruses (28). During our experiments, important control measures were included to ensure the quality of our PCR results. Precautions were taken to prevent cross-contamination, i.e., UV sterilization of PCR equipment and the working environment, utilization of aerosol tips, etc. Positive and negative controls were included with each series of reactions.
The application of the nested-PCR protocol in our study further increased the sensitivity and specificity of detection. The nested protocol for adenovirus detection was shown in a previous study (1) to have a sensitivity of detecting a single purified viral particle. Therefore, nested PCR provided the sensitivity level necessary to detect a few viral particles in the sample concentrate. Nested PCR also increased the specificity of detection. Routinely, specific PCR products are often confirmed by probing with an internal oligonucleotide probe (12, 15, 31). In the nested PCR assay, two specific internal primers are used, which increases the specificity of reaction in much the same way.
A major obstacle of PCR detection of viruses in aquatic environmental samples is the presence of organic material that inhibits the PCR. As a result, many studies have focused on developing an efficient method of VNA extraction from such complex samples (e.g., 12, 14, 17, 31). Puig et al. (28) compared a simple GuSCN-silica bead method with several other methods for VNA extraction and found this method to be the most efficient at recovering viral RNA and/or DNA from complex samples. In this study, we have shown that the efficiency of extraction of this method was sample dependent (Fig. 2). Therefore, it was not possible to have a general correction factor to correct for losses during purification procedures.
Of the 12 samples tested in this study, 4 were positive for human adenovirus. The presence of these human viruses did not correlate with the exceedance of daily limits of bacterial indicators in coastal waters. At the three sites where all three indicator bacteria were above daily limits, the mouths of Malibu Lagoon, Los Angeles River, and Moonlight Creek, human viruses were only detected at the Los Angeles River site. In contrast, while no bacterial indicator exceeded the daily threshold at the mouths of the San Gabriel and Santa Ana rivers, viral concentrations of 2,300 and 924/liter were detected at each site, respectively. These results support previous findings by Griffin et al. (15) for the coastal marine environment and suggest that the current coastal recreational water quality standards are inadequate to reflect the viral quality of the water.
The presence of human viruses was also not correlated with the concentration of coliphage. The Tijuana River had the highest concentration of coliphage but a relatively low concentration of adenovirus. However, a correlation exists between the abundance of human adenovirus and F-specific coliphage. The correlation coefficient for samples taken from the mouths of the Los Angeles, San Gabriel, Santa Ana, and Tijuana rivers was 0.99. These results agree with the previous report of a significant association of F-specific phage with human enteric viruses in oysters and their harvest waters (8) yet differ from that reported for Florida coastal waters (15), where high frequencies of human viruses were detected (95%) by PCR but few or no coliphage were found in their samples. Future studies that include a broader geographical area of the marine coast and a larger database are necessary to provide insights into the utility of F-specific coliphage as a human viral indicator.
Human adenoviruses were detected at the mouths of four major urban rivers in Southern California, pointing to urban runoff as a source of coastal viral contamination. This is not surprising, because a wide distribution of adenovirus has been previously reported for urban river waters (30) and a river estuary (5). It is noteworthy that the river flow rate at all four sites was relatively low, as indicated by the salinity, at the time of sample collection. This was due to an unseasonally low level of precipitation during the winter of 1999. A higher level of contamination may be expected during heavy rainfall.
Although it is alarming to detect the presence of human viruses in one-third of the beach locations tested in Southern California, little is known about the infectivity of these viruses. The PCR method detects both infectious and damaged viruses. Caution should be made at the interpretation of these results. If none or only a very small portion of viruses are infectious, there is little impact or public health concern. The detection of adenovirus should be viewed as an index for human fecal pollution and the presence of other human viruses. Therefore, this study calls for a reevaluation of our current water quality standards to reflect the viral quality of recreational waters and to possibly include monitoring of recreational waters for human viruses in certain areas.
ACKNOWLEDGMENTS
Funding for this research was provided by a California Sea Grant awarded to S. Jiang (grant NA66RG0477).
Special thanks go to the Southern California Coastal Research Project and local agencies for close collaboration on the sample collection and determination of bacterial indicators in water samples. We also thank J. A. Fuhrman (University of Southern California) for providing working space and equipment for R. T. Noble.
REFERENCES
- 1.Allard A, Albinsson B, Wadell G. Detection of adenoviruses in stools from healthy persons and patients with diarrhea by two-step polymerase chain reaction. J Med Virol. 1992;37:149–157. doi: 10.1002/jmv.1890370214. [DOI] [PubMed] [Google Scholar]
- 2.Atmar R L, Metcalf T G, Neil F H, Esteas M K. Detection of enteric viruses in oysters by using the polymerase chain reaction. Appl Environ Microbiol. 1993;59:631–635. doi: 10.1128/aem.59.2.631-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg G, editor. Viral pollution of the environment. Boca Raton, Fla: CRC Press; 1983. [Google Scholar]
- 4.Boom R, Sol C J A, Salimans M M, Jansen C L, Wertheim-Van Dillen P M E, Van Der Noordaa J. Rapid and simple method for purification of Nucleic Acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castingnolles N, Petit F, Mendel I, Simon L, Cattolico L, Buffet-Janvresse C. Detection of adenovirus in the waters of the Seine river estuary by nested-PCR. Mol Cell Probes. 1998;12:175–180. doi: 10.1006/mcpr.1998.0166. [DOI] [PubMed] [Google Scholar]
- 6.Chapron C D, Ballester N A, Fontaine J H, Frades C N, Margonlin A B. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl Environ Microbiol. 2000;66:2520–2525. doi: 10.1128/aem.66.6.2520-2525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung H, Jaykus L A, Sobsey M D. Detection of human enteric viruses in oysters by in vivo and in vitro amplification of nucleic acids. Appl Environ Microbiol. 1996;62:3772–3778. doi: 10.1128/aem.62.10.3772-3778.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung H, Jaykus L-A, Lovelace G, Sobsey M D. Bacteriophages and bacteria as indicators of enteric viruses in oysters and their harvest waters. Water Sci Technol. 1998;38:37–44. [Google Scholar]
- 9.Clesceri L S, Greenberg A E, Eaton A D. Standard methods for the examination of water and wastewater. 20th ed. Washington, D.C.: American public health association; 1998. [Google Scholar]
- 10.Cruz J R, Caceres P, Cano F, Flores J, Bartlett A, Torun B. Adenovirus types 40 and 41 and rotaviruses associated with diarrhea in children from Guatemala. J Clin Microbiol. 1990;28:1780–1784. doi: 10.1128/jcm.28.8.1780-1784.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Leon R, Jaykus L. Detection of the presence of bacteria and viruses in shellfish. In: Hurst C, Knudsen G R, McInerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: American Society for Microbiology; 1997. [Google Scholar]
- 12.De Leon R, Shieh Y S C, Baric R S, Sobey M D. Proceedings of the Water Quality Conference. Vol. 18. Denver, Colo: American Water Works Association; 1990. Detection of enteroviruses and hepatitis A virus in environmental samples by gene probes and polymerase chain reaction; pp. 833–853. [Google Scholar]
- 13.Enriquez C E, Hurst C J, Gerba C P. Survival of the enteric adenoviruses 40 and 41 in tap, sea, and wastewater. Water Res. 1995;29:2548–2553. [Google Scholar]
- 14.Gilgen M, Wegmuller B, Burkhalter P, Buhler H P, Muller U, Luthy J, Candrian U. Reverse transcription PCR to detect enteroviruses in surface water. Appl Environ Microbiol. 1995;61:1226–1231. doi: 10.1128/aem.61.4.1226-1231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin D W, Gibson C J, Lipp E K, Riley K, Paul J H, Rose J B. Detection of viral pathogens by reverse transcriptase PCR and of microbial indicators by standard methods in the canals of the Florida Keys. Appl Environ Microbiol. 1999;65:4118–4125. doi: 10.1128/aem.65.9.4118-4125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hafliger D, Gilgen M, Luthy J, Hubner P. Seminested RT-PCR systems for small round structured viruses and enteric viruses detection in seafood. Int J Food Microbiol. 1997;37:27–36. doi: 10.1016/s0168-1605(97)00041-x. [DOI] [PubMed] [Google Scholar]
- 17.Ijzerman M M, Dahling D R, Fout G S. A method to remove environmental inhibitors prior to the detection of waterborne enteric viruses by reverse transcription-polymerase chain reaction. J Virol Methods. 1997;63:145–153. doi: 10.1016/s0166-0934(96)02123-4. [DOI] [PubMed] [Google Scholar]
- 18.Irving L G, Smith F A. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl Environ Microbiol. 1981;41:51–59. doi: 10.1128/aem.41.1.51-59.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang S C, Thurmond J M, Pichard S L, Paul J H. Concentration of microbial populations from aquatic environments by vortex flow filtration. Mar Ecol Prog Ser. 1992;80:101–107. [Google Scholar]
- 20.Krikelis V, Spyrou N, Markoulatos P, Serie C. Detection of indigenous enteric viruses in raw sewage effluents of the city of Athens, Greece, during a two-year survey. Water Sci Technol. 1985;17:159–164. [Google Scholar]
- 21.Krikelis V, Spyrou N, Markoulatos P, Serie C. Seasonal distribution of enteroviruses in domestic sewage. Can J Microbiol. 1985;31:24–25. doi: 10.1139/m85-006. [DOI] [PubMed] [Google Scholar]
- 22.McNeil K M, Hendrix R M, Lindner J L, Benton R R, Monteith S C, Tuchscherer M A, Gray G C, Gaydos J C. Large, persistent epedemic of adenovirus type 4-associated acute respiratory disease in U.S. army trainess. Emerging Infect Dis. 1999;5:798–801. doi: 10.3201/eid0506.990609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melnick J L, Gerba C P. The ecology of enteroviruses in natural waters. Crit Rev Environ Control. 1989;10:65. [Google Scholar]
- 24.Paul J, Jiang S, Rose J. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol. 1991;67:2197–2204. doi: 10.1128/aem.57.8.2197-2204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul J H, Rose J B, Jiang S C, London P, Zhou X, Kellogg C. Coliphage and indigenous phage in Mamala Bay, Oahu, Hawaii. Appl Environ Microbiol. 1997;63:133–138. doi: 10.1128/aem.63.1.133-138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payment P, Trudel M. Detection and quantitation of human enteric viruses in wastewaters: increased sensitivity using human immune serum globulin-immunoperoxidase assay on MA-104 cells. Can J Microbiol. 1987;33:568–570. doi: 10.1139/m87-097. [DOI] [PubMed] [Google Scholar]
- 27.Pina S, Puig M, Lucina F, Jofre J, Girones R. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl Environ Microbiol. 1998;64:3376–3382. doi: 10.1128/aem.64.9.3376-3382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R. Detection of adenovirus and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol. 1994;60:2963–2970. doi: 10.1128/aem.60.8.2963-2970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suttle C A, Chan A M, Cottrell M T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991;57:721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tani N, Dohi Y, Kurumatani N, Yonemasu K. Seasonal distribution of adenoviruses, enteroviruses and reoviruses in urban river water. Microbiol Immunol. 1995;39:577–580. doi: 10.1111/j.1348-0421.1995.tb02245.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsai Y L, Sobsey M D, Sangermano L R, Palmer C J. Simple method of concentrating enteroviruses and hepatitis A virus from sewage and ocean water for rapid detection by reverse transcriptase-polymerase chain reaction. Appl Environ Microbiol. 1993;59:3488–3491. doi: 10.1128/aem.59.10.3488-3491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhnoo I, Wadell G, Svensson L, Olding-Stenkvist E, Mobby R. Aetiology and epidemiology of acute gastroenteritis in Swedish children. J Infect. 1986;13:73–89. doi: 10.1016/s0163-4453(86)92348-0. [DOI] [PubMed] [Google Scholar]