Highlights
-
•
Effect of multi-frequency ultrasonic treatment on infrared drying of pineapple is studied.
-
•
Nature of immersion medium (distilled water, sucrose, and ethanol) played a major role.
-
•
Ultrasound modified the microstructure and enhanced moisture elimination during drying.
-
•
Pretreatments improved the overall quality but degraded color and bioactive compounds.
-
•
Tri-frequency ultrasound maximized both drying efficiency and quality.
Keywords: Bromelain, Ultrasound-assisted ethanol solution pretreatment, Microstructure, Flavor, Nutrients
Abstract
This study evaluated the effect of mono-frequency ultrasound (MFU, 20 kHz), dual-frequency ultrasound (DFU, 20/40 kHz), and tri-frequency ultrasound (TFU, 20/40/60 kHz) on mass transfer, drying kinetics, and quality properties of infrared-dried pineapple slices. Pretreatments were conducted in distilled water (US), 35 °Brix sucrose solution (US-OD), and 75% (v/v) ethanol solution (US-ET). Results indicated that ultrasound pretreatments modified the microstructure of slices and shortened drying times. Compared to the control group, ultrasound application reduced drying time by 19.01–28.8% for US, 15.33–24.41% for US-OD, and 38.88–42.76% for US-ET. Tri-frequency ultrasound provoked the largest reductions, which exhibited time reductions of 6.36–11.20% and better product quality compared to MFU. Pretreatments increased color changes and loss of bioactive compounds compared to the control but improved the flavor profile and enzyme inactivation. Among pretreated sample groups, US-OD slices had lower browning and rehydration abilities, higher hardness values, and better retention of nutrients and bioactive compounds. Therefore, the combination of TFU and osmotic dehydration could simultaneously improve ultrasound efficacy, reduce drying time, and produce quality products.
1. Introduction
Pineapple, first discovered in South America, is nowadays cultivated in tropical and subtropical regions across the globe due to its nutritional and health-enhancing properties. It is known for its unique taste and aroma, making it an excellent ingredient for several preparations, including desserts, fruit salads, pies, puddings, cakes, garnish, ice creams, compotes, and candies [1]. In addition, due to high production rates and the likelihood of crop degradation, pineapples are processed into different products such as juice, nectar, syrup, vinegar, wine, canned slices, jam, powder, and dried pieces [2]. Among different food processing technologies, drying remains one of the ancient and most preferred techniques for preservation. It has gained prominence for seasonal crops like pineapples, where it was imperative to guarantee product availability all year round [3].
Generally, pineapples are dried using natural or conventional hot-air drying, but these methods are time-consuming and result in quality deterioration and higher energy consumption [4]. To overcome these impediments, advanced drying technologies or pretreatment of fresh foods before drying could be employed as enhancement approaches [5], [6].
Infrared drying is a modern drying method that is recognized as one of the most effective dehydration techniques for food materials [7], [8]. When exposed to infrared rays, samples absorb the radiation, leading to uniform molecular heating without impacting the temperature of the air in the drying chamber. As a result, drying rates are improved, foods are dried faster, energy consumption is decreased, and product quality is greatly preserved [9]. The research on infrared drying of pineapple is scarce. The existent works investigated the drying efficiency and color properties of dried products [10], [11], [12]. Although it was revealed that infrared radiative drying improved heat transfer and decreased drying time, there is still room for improvement in an attempt to minimize drying time while retaining the quality of dried products. In this perspective, the application of pretreatments such as ultrasound could enhance the infrared drying process of pineapple slices.
Many preliminary operations, classified as physical and chemical pretreatments, and their combinations have been used for drying enhancement. Physical pretreatments include thermal blanching methods (steam, hot water, infrared, microwave, and ohmic heat) and nonthermal strategies (ultrasound, high hydrostatic pressure, cold-plasma, and pulsed-electric field) while chemical pretreatments involve soaking foods in a liquid (osmotic solutions or liquors) or a gaseous phase (carbon dioxide and ozone) [13], [14]. Some examples of hybrid pretreatments include osmosonication, thermosonication, and osmovacuum [15]. Pretreatments are often carried out to deactivate enzymes, fasten the drying process, minimize energy expenses, and preserve food nutrients [16], [17]. However, compared to heat-based pretreatments, nonthermal pretreatments can limit the harmful impact of heat on the nutritional and sensorial properties of foods [18].
Recently, power ultrasound applied as a nonthermal pretreatment before drying has become a fundamental topic of experimental investigations due to its effectiveness over traditional methods [14]. Principal mechanisms taking place during US pretreatment are the cavitation of liquid bubbles, the compression and rarefaction of samples known as the “sponge effect”, moisture microstreaming, and the generation of shear stress. These physical effects of ultrasound result in the weakening of cell walls, the extraction of bound water molecules, and the creation of a porous structure in food tissues which enhances osmotic transfers during pretreatment and moisture diffusion during drying [19], [20], [21].
The application of ultrasound in different processing domains resides primarily on the cavitation effect. Ultrasound frequency plays a critical role in sonochemical reactions as it determines the intensity of the main physical phenomena such as cavitation and the sponge effect [22]. In drying processes, lower frequencies are preferred to reduce acoustic energy losses and minimize the production of free radicals [23], [24]. Mono-frequency ultrasound is the conventional frequency mode applied during ultrasound pretreatment of fruits and vegetables. However, under lower frequencies, the number of the most active cavitation bubbles (transient cavitation bubbles) is reduced [24]. Hence, recently, the combination of two or more frequencies (multi-frequency ultrasound) has gained momentum. This strategy aims to improve sound resonance, increase the number and the radius of cavitation bubbles, uniformize bubble dispersion, and intensify their implosion strength [25], [26], [27]. Under such high sonochemical energy, violent microjets and shock waves resulting from cavitation maximize cell distortion and improve moisture diffusion during the subsequent drying process compared to the single frequency mode. For example, Ma et al.[28] found that dual-frequency osmosonication (40/80 kHz) improved water loss and solid gain and led to higher preservation of phenolic compounds and antioxidant activity than the mono-frequency treatments (40 kHz and 80 kHz). Besides, in previous studies, the multi-frequency power ultrasound has been proven to have great effects in food processing, such as extraction [29], freezing [30], drying [25], [31], thawing [32], enzymolysis of protein [33], [34], modification of starch [35], inactivation of enzymes [36], [37], as well as cleaning [38], [39].
Ultrasound transmission medium influences the outcome of the pretreatment [23]. Popular sonication liquids are distilled water [40], hypertonic solutions [15], [25], and ethanol [41], [42]. However, each liquid group can lead to a different trend in osmotic movements, ultrasound wave transmission, cavitation induction, and the preservation of sensorial and nutritional quality of end products [16]. Thus, also investigating the impact of all these liquid media on a single sample is paramount.
Ultrasound pretreatment has been applied before pineapple drying. For example, Fernandes et al. [43] showed that osmosonication pretreatment can reduce up to 31% of the drying time compared to untreated pineapple samples, which in another study [44] was explained by the generation of micropores in the internal structure of slices. Ultrasound pre-steps of pineapple slices in distilled water and pineapple juice increased the coefficients of moisture diffusivity and resulted in better retention of bioactive compounds [45]. Freitas et al. [46] reported that the combined effects of ultrasound and ethanol pretreatments improved pineapple drying rates and provoked excellent preservation of carotenoids and ascorbic acid. However, the color of dried products was darker. In distilled water, Rani & Tripathy [47] found time reductions of 14.3–19% compared to the control group and final products had brighter color and lower firmness. Notwithstanding, no study has investigated the effect of different frequency modes (mono-, dual-, and tri-frequency), applied in the three most popular US transmission media (distilled water, sucrose solution, and ethanol solution) on drying kinetics and physicochemical properties of dried products.
Therefore, this paper aims to: (1) understand the effects of mono-, dual-, and tri-frequency ultrasound applied in distilled water, sucrose, and ethanol on mass transfers and the internal structure of pineapple slices; (2) examine the effect of pretreatments on the behavior of the infrared drying process; (3) evaluate the quality properties of dried samples such as rehydration, color, hardness, enzyme activities, antioxidant compounds (total phenolic compounds, total flavonoid content, vitamin C), and antioxidant activities.
2. Material and methods
2.1. Fresh pineapple samples
Matured pineapples (13.18 ± 0.53 °Brix and pH 3.20 ± 0.08) having an approximate weight were purchased from an online platform based in Zhenjiang, China, and stored at 4 °C in a refrigerator. The fruits were cleaned with tap water to remove dust and surface impurities. Afterward, pineapple cylinders (80-mm outer diameter and 30-mm inner diameter) were obtained using a pineapple coring and peeling tool. A stainless-steel knife was then used to manually cut rings of 5 ± 1-mm thickness (Fig. 1). The moisture content on a dry basis of fresh fruits at 105 °C was estimated to be 6.27 ± 0.11 g H2O/g according to the method of Xu et al. [25].
Fig. 1.
Flowchart of pineapple pretreatment and drying experiments.
2.2. Ultrasound pretreatment
A hexagonal ultrasonic bath, self-developed by the School of Food & Biological Engineering of Jiangsu University (Zhenjiang, China) was used for US pretreatments. 8 pineapple slices (200 ± 1 g), contained in a beaker were independently sonicated in distilled water (US) [17], 35 °Brix sucrose solution (US-OD) [22] and 75% (v/v) ethanol solution (US-ET) [48] at a water-to-slices ratio of 4:1(v/w). For each liquid group, three frequency modes were employed: mono-frequency (20 kHz), dual-frequency (20/40 kHz), and tri-frequency (20/40/60 kHz). The dual-frequency and tri-frequency modes suggest that two or three frequencies were simultaneously used during the pretreatment. The ultrasonic power density was 50 W/L, with a pulse-on and off time of 10 s/10 s during a pretreatment time of 30 min [26]. The temperature of the soaking medium was maintained at 25 ± 2 °C by water recirculation. Subsequently to the pretreatment, the excessive solution was gently removed from the surface of slices using adsorbent paper. Samples without pretreatment were considered the control group and both US-treated and control samples were then transferred to the infrared dryer for drying.
2.3. Intermediate-wave infrared drying
The drying process of pineapple slices was performed in an intermediate-wave infrared dryer (Sankoom Co. Ltd., China), which combines infrared radiation with convective drying. The selected parameters were a temperature of 60 °C, an airflow of 2 m/s, infrared power of 675 W, and a radiation distance between infrared lamps and food materials of 6 cm, which were chosen based on our preliminary experiments. The variation of the moisture content during the dehydration process was recorded each 15 min until a final moisture content of 0.15 g H2O/g dry basis. After drying, hot slices were cooled, packaged in a polyethylene bag, and stored in a desiccator for physicochemical analyses.
2.4. Mass transfer during pretreatment
The osmotic exchanges of matter during pretreatments were explained by the percentage of water loss (WL) and solids gain (SG). The moisture contents of the control and pretreated slices were determined at 105 °C and mass transfer parameters were calculated according to Eqs. (1), (2) [25]:
| (1) |
| (2) |
where M0 and Mt are the initial and final mass (after pretreatment) in gram of pineapple slices, respectively; m0 and mt are the dry matter (g) of slices before and after pretreatment, respectively.
2.5. Drying kinetics
2.5.1. Moisture ratio (MR)
The moisture ratio is commonly used to understand the evolution of moisture elimination during drying. The moisture ratio is expressed according to eq. (3) [48], [47]:
| (3) |
Mi, Mt, and Me are the moisture content (g H2O/g dry basis) of pineapple slices before drying, at a random time t during drying, and at the equilibrium, respectively. Me is negligible compared to Mi and Mt, thus it was ignored.
2.5.2. Drying rate (DR)
The drying rate (g/(g.h)) was computed according to eq. (4) [49]:
| (4) |
M1 and M2 are the moisture contents on a dry basis of slices (g/g) at times t1 and t2 in h, respectively.
2.6. Quality attributes
2.6.1. Microstructural images
The microstructure of dried pineapple slices was acquired using a scanning electron microscope (S-3400 N, Hitachi Ltd., Japan). Thin layers of samples were mounted on a conductive disk, covered with a gold film, and photographed at an accelerating voltage of 20 kV.
2.6.2. Rehydration ratio (RR)
A modified method of Xu et al.[25] was used for the rehydration ratio. 1.5 g of pretreated and untreated dried pineapple samples were immersed in 200 mL of 25 °C distilled water for 90 min. Each 10 min, samples were taken out of the water, blotted with absorbent paper, weighed, and returned to the solution. The rehydration ratio is expressed according to eq. (5):
| (5) |
where W1 and W2 are the weight of samples before rehydration and at a time t during rehydration, respectively.
2.6.3. Hardness
A texture analyzer (TA-XT2i Stable Micro Systems Ltd., Vienna 153Court, Surrey, UK) was used to record the hardness of fresh and dried materials. A 25 mm probe double compressed the sample with a trigger force of 5 g, a target distance of 10 mm, a time of 5 s, and a compression strain of 40%. The pre-test, test, and post-test speeds were 3 mm/s, 1 mm/s, and 3 mm/s, respectively. For each sample group, 3 slices were selected, analyzed in triplicate, and maximum responses (g) were recorded.
2.6.4. Color attributes
A handheld colorimeter (Minolta CR-400, Konica Minolta, Tokyo, Japan) was used to evaluate the color characteristics of pineapple slices, namely the redness (a*), brightness (L*), and yellowness (b*). The total color change (ΔE) and browning index (BI) were then calculated following Eqs. (6) and (7) [15], [31]:
| (6) |
“0″ indicates the color attributes of fresh slices.
2.6.5. Ascorbic acid
The ascorbic acid content of pineapple pieces was revealed by the method of Wu et al.[50]. Pineapple (0.1 g) was homogenized in 60 mL of metaphosphoric acid (2% w/v). 6 drops of NaOH were added to half of the solution and left to stand for 40 min while the other portion was analyzed immediately. The absorbance reading was performed at 243 nm and the ascorbic acid content was expressed in mg/g dry weight.
2.6.6. Total phenolic content (TPC) and total flavonoid content (TFC)
Pineapple sample (2 g) was ground in 20 mL of a 70% (v/v) methanol solution. Afterward, the mixture was ultrasonically extracted (40 kHz) for 30 min at 45 °C and centrifuged (10 000 g) at 4 °C for 30 min. The supernatant was considered the extract for the evaluation of TPC and TFC. TPC and TFC assays were performed according to the method of Boateng & Yang[8] with minor adaptations. Results for TPC were presented in mg gallic acid equivalents (GAE)/g dry matter while TFC values were expressed in mg rutin equivalents (RE)/100 g dry matter.
2.6.7. Antioxidant activity (DPPH)
The antioxidant activity of slices was determined as per the procedure of Boateng & Yang [8]. DPPH assay was performed by mixing 1 mL of the extract with 4 mL of DPPH working solution (absorbance value between 0.715 and 0.735 at 520 nm), which resulted from the dissolution of DPPH in methanol (50 mg/L) followed by a 2-h incubation in darkness. The mixture was vortexed, incubated at room temperature for 30 min, and the absorbance was measured at 520 nm. DPPH was expressed in µM Trolox equivalents/g of dry matter.
2.6.8. Polyphenol oxidase (PPO), peroxidase (POD), and bromelain activities
PPO and POD were extracted on an ice cube by homogenizing (400 rpm) 2 g of the sample in 10 mL of an extraction solution, followed by a centrifugation process at (4 °C, 10,000 rpm) for 30 min. The extraction solution is composed of 1% (v/v) Triton X-100, 2% (w/v) polyvinyl polypyrrolidone, and sodium phosphate buffer (0.2 M, pH 6.6) in a 1:1:1 ratio. The method by Osae et al.[51] was employed and enzyme activities (EA) were computed as follows (eq. (8)) [15]:
| (8) |
WS is the mass (g) of the sample, T is the reaction time (min), and ΔA is the absorbance variation.
Bromelain activity was analyzed according to the modified procedure of Soares et al.[52]. The material was previously well mixed with distilled water in a 1:4 (w/v) ratio and the crude extract was collected after centrifugation (10000 rpm, 4 °C). Bromelain activity (U/mL) was expressed as the quantity of bromelain necessary to release 1 µmol of tyrosine at 37 °C per min.
2.6.9. Electronic nose
2 g of slices was placed in a small vial (20 mL) and heated at 40 °C for 30 min to increase the release of the aroma. An electronic nose device (PEN3.5, AIRSENSE Analytics GmbH, Germany) was used to detect the aromatic responses of 10 sensors incorporated in the system. The flushing and analysis times were 2 min and a 400 mL/min injection rate was maintained throughout the detection period.
2.7. Statistical analysis
The computation of means and their statistical comparison were done in SPSS 26.0 (SPSS Inc., USA). Graphs and the principal component analysis (PCA) were performed in OriginPro 9.8 (OriginLab Corporation, MA, USA).
3. Results and discussion
3.1. Mass transfer
Osmotic exchanges between samples and the medium during ultrasound pretreatment were materialized by water loss (WL) and solids gain (SG), as the system samples-solution strived to establish an equilibrium (Fig. 2A). The composition of the immersion liquid strongly influenced the nature of osmotic transfers. In distilled water, the values of WL and SG were negative, meaning that water was incorporated into the fruit tissues while solids were lost in the medium. When sucrose was used, pineapple slices lost water and gained sugars from the solution. However, during ultrasound-assisted ethanol pretreatment, a portion of the initial water of slices was eliminated but solids were drained from samples to the ethanol medium. Analogous results were previously reported for distilled water [17], [40], sucrose solution [15], [25], [44], and ethanol [31], [46], [48]. Compared to distilled water, ethanol and sucrose solutions had higher concentration gradients than the interior of pineapple slices, thus forcing the expel of water and the incorporation of solutes. Nevertheless, sucrose seemed to have exerted a much higher osmotic pressure than ethanol (Fig. 2A).
Fig. 2.
Mass transfer phenomena and drying time of pineapples. A: Water loss and solid gain during ultrasound pretreatment; B: Impact of ultrasound on drying time of pineapple slices. MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US, sonication in distilled water; US-OD (osmosonication), US-ET (ultrasound-assisted ethanol pretreatment). Letters on bars indicate significance (p < 0.05) among groups.
Different frequency modes of ultrasound also influenced WL and SG during pretreatment. In general, WL and SG increased with the simultaneous use of more frequencies, which proves that multi-frequency ultrasound enhanced mass transfer already imposed by osmotic solutions. The combination of several frequencies might have increased the sound resonance (combined resonance) and generated homogeneously numerous active cavitation bubbles having a bigger size. The collapse of these bubbles provoked violent microjets, which impinged on slice surfaces and improved porosity by breaking down cellular membranes [28]. In addition, compared to the mono-frequency pretreatment, multi-frequency ultrasound probably imposed a much higher physical stress on samples by alternating cycles of compression and rarefaction, known as the “sponge effect”. This phenomenon resulted in an altered internal structure of slices due to the generation of micropores [24], [29]. These pores were used as pathways to maximize mass transfer during pretreatment [15]. However, with some exceptions in US and US-OD experiments, the values of WL and SG were not statistically different (p > 0.05) between mono-frequency and dual-frequency on one hand and dual-frequency and tri-frequency on the other hand. Xu et al. [25], Feng et al. [53], and Ma et al. [28] found that WL and SG of strawberry, garlic, and apple slices pretreated by multi-frequency ultrasound were noticeably higher than those recorded in the mono-frequency modes, respectively.
In US-ET, although ethanol was incorporated into pineapple tissues, the values of SG were negative. This observation could be linked to 1) the ability of ethanol to rupture or dissolve cellular walls, improve permeability, and enhance the leakage of food compounds [46], [48]; 2) the countercurrent entry and loss of ethanol due to the disruption of cellular structures; 3) the lower molecular weight of ethanol which could not compensate the mass of solid lost.
Besides, we noticed that due to the softer texture of fresh slices, part of the flesh was found in the medium after pretreatment. During US-OD, these losses could be intensified with the elimination of water-soluble solids. However, the incorporation of sucrose which had a heavy molecular weight offset these losses, thereby resulting in positive values of solid gain. Solid losses were the highest during pretreatment in distilled water due to the loss of water-soluble nutrients [47] but values were statistically insignificant (p > 0.05) compared to those observed in ethanol.
3.2. Drying kinetics
The effect of ultrasound pretreatment on drying time is presented in Fig. 2B. The results suggest that sonication fastened the drying process significantly (p > 0.05) compared to untreated samples. This observation could be related to the structural modifications provoked by ultrasound such as the release of bound water molecules, the weakening of cell walls, and the creation of microscopic channels which enhanced water diffusion during infrared drying [17], [25], [43]. US-ET showed the lowest drying times, followed by US, and US-OD, revealing that the type of ultrasound pretreatment and the medium played a major role in drying time reduction. Besides, multi-frequency ultrasound decreased the drying time compared to the single-frequency mode. While there was no statistical difference in drying time among US-ET samples, only the tri-frequency mode showed a difference in relation to mono-frequency ultrasound for US and US-OD samples. Compared to the control group, ultrasound reduced drying time by 19.01–28.8% for US, 15.33–24.41% for US-OD, and 38.88–42.76% for US-ET, with tri-frequency ultrasound provoking the largest reductions. In addition to the contribution of ultrasound, the outcome in US-ET can be linked to the “Marangoni effect”. Due to its higher surface tension over water, ethanol which improves permeability is initially vaporized. This process increases local surface tension, thereby forcing water diffusion out of samples [31], [46], [48]. TFU shortened drying time required for MFU and DFU by 11.2% and 4.86% for US, 10.71% and 6.66% for US-OD, and 6.36% and 3.66% for US-ET, respectively. Likewise, Xu et al. [25] reported for strawberry slices that DFU (20/40 kHz) decreased 50% of the drying time while time reduction at the mono-frequency modes of 20 kHz and 40 kHz were 15.25% and 32.2%, respectively. Besides, TFU (20/28/40 kHz) reduced the infrared drying times of carrots by 15–25% [27].
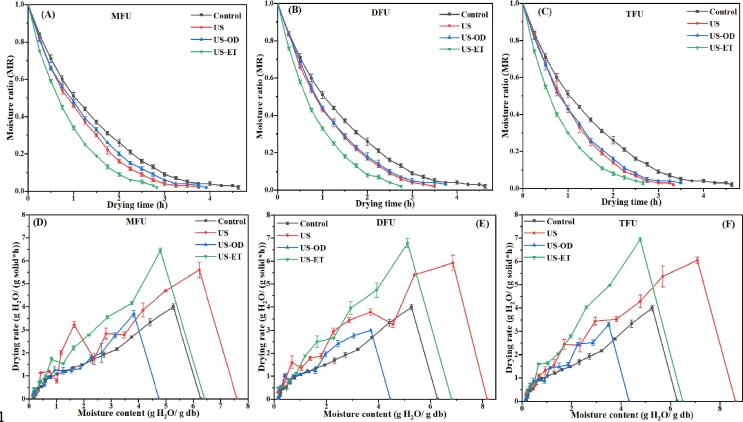
The moisture ratio (MR) curves were plotted to understand the drying behavior of pineapple slices (Fig. 3A–C). After pretreatment, the moisture content (g H2O/g) of fresh slices (6.27 ± 0.11) was modified: 7.6 ± 0.09 to 8.62 ± 0.15 for US, 6.41 ± 0.07 to 6.81 ± 0.10 for US-ET, and 4.32 ± 0.03 to 4.75 ± 0.06 for US-OD. These changes in the initial moisture content of slices may impact drying kinetics. MR of pretreated and non-treated samples decreased stepwise with prolonging the drying period. Drying curves exhibited a continuous falling rate pattern, which corroborates that moisture mobility was governed by diffusion. Similar scenarios were reported during the drying of Ginkgo biloba seeds [49], ginger slices [54], and scallion cylinders [48]. MR curves of pretreated slices were more declined compared to the control group. This is ascribed to the mechanical stress provoked by ultrasound, which created microscopic channels in slices, thus improving moisture movement during drying [16]. In addition, curves were sharper when more frequencies were combined, as the gap compared to the control was increased. This outcome can be related to the increased sonochemical energy, improved bubble burst, and enhanced moisture diffusion when multi-frequency was employed [25], [28]. Considering the type of medium used, the trend in rapid MR decrease was in the order: US-ET > US > US-OD > Control. However, the curves of US and US-OD were quite similar although the initial moisture content of US samples was higher. In fact, the moisture gained was readily eliminated during drying because of its free nature inside pineapple tissues. US-OD led to longer drying times, yet insignificant (p > 0.05) (Fig. 2B) compared to US because the incorporated sucrose formed a layer during drying which decelerated moisture migration. Another explanation could be that the effect of ultrasound was maximized in distilled water than in sucrose solution since cavitation is reduced in viscous solutions [16]. Boateng & Yang [49] and Fernandes et al. [43] reported similar trends between US and US-OD during the dehydration of Ginkgo biloba seeds and pineapple slices, respectively.
Fig. 3.
Drying kinetics of pretreated and non-treated pineapples. A: Moisture ratio curves; B: Drying rate curves; MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US, sonication in distilled water; US-OD (osmosonication), US-ET (ultrasound-assisted ethanol pretreatment).
The effect of pretreatment on drying rate (DR) was also investigated (Fig. 3D–F). DR was maximum at the beginning of the drying process and decreased remarkably at the final period of drying. In essence, water was initially eliminated from larger cavities (free water) while the release of moisture firmly attached to food compounds was challenging at the end of the process. The impact of multi-frequency ultrasound on DR was as follows: TFU > DFU > MFU > Control. In addition, DR curves were in the order: US-ET > US > US-OD > Control, and aligns well with the observation aforementioned for MR and drying time. Zhou et al. [31], Xu et al. [25], and Guo et al. [27] have recently found that multi-frequency ultrasound enhances drying kinetics and reduces the drying time of scallion (54.5%), strawberry (40.68–50%), and carrot slices (13.6–15.8%), respectively.
3.3. Effect of ultrasound pretreatment on the quality of dried pineapples
3.3.1. Microstructure
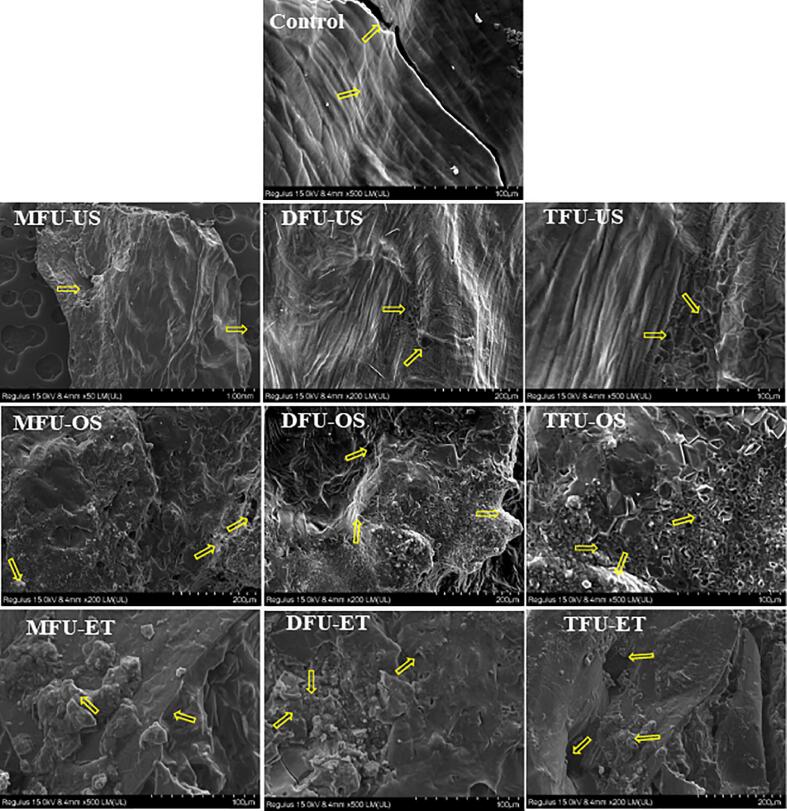
The internal structure of dried pineapple samples is shown in Fig. 4. In the untreated batch, moisture elimination during drying led to a structure marked by higher cell flattening, excessive shrinkage, which suggest a hardening of slices [25]. In addition, some internal cracks were visible due to longer exposure to infrared rays. The pretreatment in distilled water also showed that micropores were generated and were increased in higher multi-frequency modes. In US-OD samples, in addition to the generation of numerous micropores, sucrose was also identified (white spots). The incorporated sucrose created compact zones having no visible channels. The microstructure allows us to predict that sucrose incorporation was lower during MFU than DFU and TFU. The pretreatment in ethanol led to the complete destruction of the cellular structure. This observation is due to the dissolution of cell walls by ethanol. A similar result was reported by Wang et al.[48]. Besides, ultrasound created holes in this disrupted network, with TFU exhibiting the largest pores. A global view of the microstructure shows that the creation of a more porous structure was initiated by multi-frequency ultrasound, especially TFU. These pores originate from the cavitation and the compression and rarefaction of pineapple slices, resulting in tissue distortion [45], [55].
Fig. 4.
Internal structure of dried pineapple slices under different conditions; MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; -US (in distilled water); -OD (in sucrose), -ET (in ethanol).
3.3.2. Rehydration
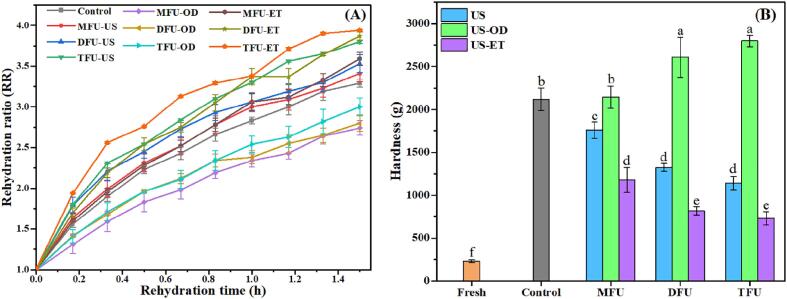
Dried fruits are sometimes rehydrated, for instance, when entering a breakfast preparation. Therefore, rehydration is an important quality parameter of dried products. It is also considered as a metric of the internal modifications that occurred in samples during drying [16], [40]. The evolution of the rehydration ratio (RR) during a soaking period of 90 min is displayed in Fig. 5A. Control samples were rehydrated faster than US-OD samples but slower than US and US-ET samples. The finding in US-OD compared to the control could be related to the formation of a sugary layer at the surface of slices which increased hardness and reduced water uptake during rehydration. A similar outcome was reported in osmosonicated and dried pumpkin [56] and quince slices [57]. On the contrary, osmosonication improved the rehydration ratios of Gingko seeds [49] and strawberry slices [25], [58]. In US and US-ET, ultrasound created microscopic channels which improved the porosity of dried products and reduced the likelihood of shrinkage and product hardening. However, the pretreatment in ethanol provoked more cellular disruptions by dissolving cell walls, resulting in the highest water uptake during the rehydration process. Similar observations were highlighted for pretreated scallion cylinders [31], [48]. The lower RR values of control slices can be explained by the longer dehydration time, resulting in higher shrinkage of pineapple tissues. For each liquid medium, the rehydration ability of TFU was the highest, followed by DFU and MFU. This outcome is possible due to vigorous bubble collapse and greater mechanical stress imposed by ultrasound on materials under multi-frequency ultrasound, thus leading to a plethora of microspores. It is worth noticing that TFU treatment of US samples had water uptake similar to that of DFU in ethanol. Guo et al. [27], Xu et al. [25], and Zhou et al. [31] have also demonstrated the positive effect of multi-frequency ultrasound on the rehydration of agro-products. Generally, ultrasound was found to improve the reconstitution of dried samples due to the rupture of cell walls [40], [49], [58].
Fig. 5.
Impact of multi-frequency ultrasound on rehydration (A) and texture of pineapples slices (B); MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US, sonication in distilled water; US-OD (osmosonication), US-ET (ultrasound-assisted ethanol pretreatment); Significance among groups was recorded at p < 0.05.
3.3.3. Texture
The synergistic effect of the pretreatment and drying process can promote physical and chemical modifications in pineapple tissues and cause texture changes. Hardness, the maximum force needed to deform pineapple slices was recorded and presented in Fig. 5B. Drying provoked a significant increase in the hardness of samples in comparison with fresh slices. In fact, moisture elimination during drying led to the concentration of fibers and sugars, resulting in increased rigidity of slices [49]. Hardness values of untreated samples were higher than those of US and US-ET samples but lower than osmosonicated samples. As supported by the microstructure and the rehydration ratio, the incorporation of sucrose during US-OD might have created a sugar layer at the surface of slices, thus increasing hardness. The process temperature can also caramelize sugars and further contribute to this observation. Besides, hardness increased with the increase in sugar gain during pretreatment. In fact, compared to MFU, values were 21.59% and 30.42% higher respectively for pretreated slices under DFU and TFU. Xu et al. [25] reported similar increments for strawberry slices. In US and US-ET, the amalgamation of more frequencies during pretreatment resulted in a noticeable decrease in hardness compared to MFU but values were not statistically significant. Compared to the control, US-ET and US reduced sample hardness by 44.31–65.43% and 17.05–46.10%, respectively. These results are ascribed to the increased porosity of samples during pretreatment, which decreased the firmness of pineapple slices. Lower hardness for US-ET samples is due to the pronounced collapse of cell walls by ethanol and ultrasound while the higher hardness of untreated samples can be related to intense shrinkage of tissues. Previously, Rani & Tripathy [47] found that 20 min and 30 min of US pretreatment (40 kHz) decreased the hardness of pineapple slices by 28.05 and 39.03%, respectively in comparison with untreated samples due to the generation of micropores which made tissues less dense, thereby producing soft products.
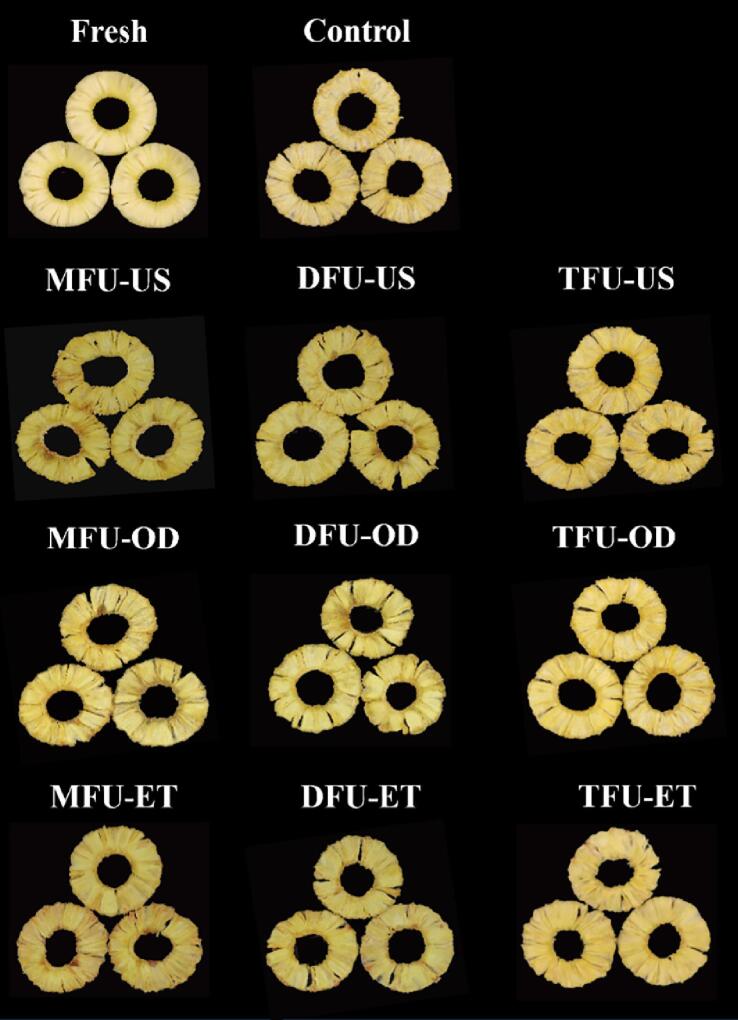
3.3.4. Color attributes
The color of processed food is an essential quality parameter that drives consumers’ vote in the market [16]. Table 1 and Fig. 6 show the color and appearance pictures of fresh and dried pineapple slices, respectively. A visual appreciation of slices reveals that dried samples were in general yellowish with US-ET and control samples exhibiting a redness shade and a pale aspect, respectively. L*, a*, b*, ΔE, and BI were used for better interpretation of the color of slices. Compared to fresh slices, L*, a*, and b* values were increased except in US-ET samples where L* values decreased. The increase in a* and b* values depicts that a certain extent of non-enzymatic browning (Maillard reaction) took place due to sample exposure to hot air [40]. US and US-OD improved the brightness of samples compared to the control and aligns with the findings of Rani & Tripathy [47] and Guo et al. [27]. However, untreated samples had a higher a* value (redness) than US and US-OD groups but smaller than US-ET samples. The rise in a* values and the decrease in L* values suggest that serious browning occurred during US-ET. Similar results were reported during US-ET of scallion [31] and pineapple slices [46]. Ultrasound pretreatment increased the yellowness (b*) more than the control following the order US > US-OD > ET. Adding more frequencies resulted in brighter samples with lower a* values. However, the yellowness was not statistically affected (p > 0.05) with an exception in US samples.
Table 1.
Color parameters of pineapple slices.
| Sample groups | L* | a* | b* | ΔE | BI |
|---|---|---|---|---|---|
| Fresh | 70.69 ± 0.70c | −4.06 ± 0.04 h | 21.94 ± 0.38d | – | – |
| Control | 71.06 ± 0.43c | −2.38 ± 0.14 cd | 27.77 ± 0.44c | 6.32 ± 0.07d | 3.84 ± 0.29e |
| MFU-US | 72.44 ± 0.69b | −3.39 ± 0.50 fg | 30.48 ± 0.63a | 8.90 ± 0.22a | 5.69 ± 0.37c |
| DFU-US | 73.40 ± 0.56ab | −3.64 ± 0.49 g | 30.54 ± 0.44a | 9.02 ± 0.28a | 4.68 ± 0.11de |
| TFU-US | 73.98 ± 0.94a | −3.56 ± 0.29 g | 29.02 ± 0.77b | 8.26 ± 0.60b | 4.13 ± 0.87e |
| MFU-OD | 71.21 ± 1.49c | −2.70 ± 0.43de | 28.83 ± 0.79bc | 7.45 ± 0.88c | 5.27 ± 0.72 cd |
| DFU-OD | 71.15 ± 1.27c | −2.77 ± 0.44de | 29.19 ± 0.67b | 7.87 ± 0.12bc | 5.09 ± 0.84 cd |
| TFU-OD | 72.69 ± 0.78b | −3.04 ± 0.28ef | 28.74 ± 0.71bc | 7.59 ± 0.20c | 4.06 ± 0.68e |
| MFU-ET | 68.84 ± 1.17e | 1.60 ± 0.15a | 28.26 ± 0.40bc | 8.85 ± 0.24ab | 9.75 ± 0.18a |
| DFU-ET | 69.40 ± 1.02de | −0.31 ± 0.09b | 28.32 ± 0.63bc | 7.84 ± 0.27bc | 8.10 ± 0.69b |
| TFU-ET | 70.17 ± 0.98 cd | −2.26 ± 0.32c | 29.06 ± 0.63b | 7.74 ± 0.44bc | 5.79 ± 0.55c |
Note: Results are expressed as mean ± SD. In the same column, statistical differences were determined at p < 0.05. MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US (in distilled water); -OD (in sucrose), -ET (in ethanol).
Fig. 6.
The pictures of fresh and dried pineapple slices; MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US (in distilled water); -OD (in sucrose), -ET (in ethanol).
The increment in b* values in dried products could also be attributed to the concentration of yellow pigments (carotenoids), which were dispersed in fresh tissues. Compared to control, ultrasound might have released and preserved more pigments (lower drying times), thus improving the yellowness. Likewise, Xu et al.[25] found a condensation of anthocyanins (red pigments) in sonicated and dried strawberry slices, which led to the increase in the redness of final products. Besides, the reddish shade was enhanced under intense ultrasound energy (DFU) than in MFU due to high cellular breakage.
The color change (ΔE) was calculated to evaluate the effect of pretreatment and drying on the color variation of dried pineapples in contrast with fresh slices. ΔE of the control was remarkably lower (p < 0.05) than that of pretreated slices. Among pretreated groups, US had the highest color difference followed by US-ET and US-OD. These results are mainly due to the upsurge in b* and L* values in sonicated samples, particularly during US. Overall, according to Table 1, multi-frequency ultrasound showed slight decreases in ΔE (p > 0.05) compared to MFU.
The browning index (BI) was used to assess the responsibility of non-enzymatic browning on the color change of dried pineapples. Alike ΔE, the BI of non-pretreated samples was lower than ultrasound-treated slices. US-ET provoked the highest browning, especially for MFU and DFU, and these results are confirmed with the appearance images of these samples. This indicates that the incorporated ethanol increased the browning of slices [31]. Although the ΔE of US was the highest among pretreated samples, its BI was lower than US-ET and statistically insignificant (p > 0.05) compared to US-OD. It was also found that combining a higher number of frequencies led to a reduction in BI probably due to shorter drying times. Larger values of BI in pretreated slices may spring from enhanced extraction of pigments that were exposed to heat during drying compared to untreated samples [59].
3.3.5. Enzyme activities (EA)
One of the primary purposes of drying is to deactivate enzymes for prolonging shelf life [54]. Enzyme activities of various sample groups are summarized in Table 2. EA of oxidative enzymes (PPO and POD) was significantly reduced after drying compared to fresh samples. Compared to control, PPO activity was decreased by 9.67–38% in US, 41.94–70.96% in US-OD, and 38.71–64.52% in US-ET while POD was reduced by 27.2–58.78% in US, 82.98–95.21% in US-OD, and 98.32–99.16 in US-ET. Therefore, there is a synergistic effect between pretreatment and drying, which led to increased inactivation of enzymes. During pretreatment, ultrasound provoked cavitation of bubbles leading to microjet and temperature increase, which denatured the structure of enzymes. These modifications were higher when multi-frequency ultrasound was applied. TFU exhibited the highest PPO and POD reductions due to increased cavitation and mechanical stress. US-OD and US-ET samples had the lowest EA. Very low PPO and POD activities in US-ET can be ascribed to the high permeability of slices which caused a significant loss of enzymes in the sonication medium. Another reason could be that ethanol extracted more enzymes during pretreatment, which were more exposed to the effect of ultrasound as well as high temperature during drying. In comparison with US, results for US-OD and US-ET could be ascribed to the combined effect of ultrasound and osmotic pressure imposed by these solutions, thereby promoting enzyme structure impairment. A similar explanation was given during the dehydration of Ginkgo seeds where PPO and POD of US-OD samples were 67.87% and 47% higher than that of US group [15].
Table 2.
Enzyme activities of fresh and dried pineapples.
| Samples | PPO (U/g) |
POD (U/g) |
Bromelain (CDU/mL) |
|---|---|---|---|
| Fresh | 1.63 ± 0.12a | 30.91 ± 0.84a | 365.41 ± 4.10a |
| Control | 0.31 ± 0.07b | 16.69 ± 0.35b | 202.20 ± 1.41e |
| MFU-US | 0.28 ± 0.04bc | 12.15 ± 0.43c | 214.39 ± 0.84d |
| DFU-US | 0.23 ± 0.08bc | 9.83 ± 0.18d | 246.10 ± 1.33c |
| TFU-US | 0.19 ± 0.07bc | 6.88 ± 0.14e | 259.02 ± 1.12b |
| MFU-OD | 0.18 ± 0.07bc | 2.84 ± 0.21f | 137.07 ± 0.56 h |
| DFU-OD | 0.13 ± 0.00bc | 2.45 ± 0.12f | 152.44 ± 1.39 g |
| TFU-OD | 0.09 ± 0.04c | 0.80 ± 0.06 g | 159.27 ± 0.28f |
| MFU-ET | 0.19 ± 0.06bc | 0.28 ± 0.03 g | 50.01 ± 0.85 k |
| DFU-ET | 0.16 ± 0.04bc | 0.25 ± 0.01 g | 57.32 ± 0.28j |
| TFU-ET | 0.11 ± 0.03bc | 0.15 ± 0.01 g | 65.88 ± 0.56i |
Note: Results are means ± SD and were statistically analyzed at p < 0.05. PPO, polyphenol oxidase; POD, peroxidase. MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US (in distilled water); -OD (in sucrose), -ET (in ethanol).
Bromelain is an enzyme solely found in pineapple. It improves protein digestion while exhibiting protection against inflammation and cancers [2]. Fresh pineapples had higher bromelain activity (365 CDU/mL) compared to dried samples. US samples showed the best enzyme activity retention among pretreated samples (6.03–28.10 higher than the control). Nevertheless, sonicated samples in sucrose and ethanol led to lower bromelain activities, being 21.23–32.21% and 67.42–75.27% smaller than the control group. Reductions in dried products are explained by the sensitivity of bromelain to high temperatures [52]. While ultrasound reduced drying time, the synergistic effect of osmotic pressure and cavitation led to pronounced destruction of bromelain. In addition, bromelain activity in US-ET samples is extremely low because its activity is usually lost when exposed to organic solvents like ethanol [52]. The trend in different frequency modes indicates that bromelain activity is linked to drying time reduction. The order in enzyme activity preservation was TFU > DFU > MFU, with clear statistic differences (p < 0.05).
3.3.6. Ascorbic acid (AA), total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity
The content of bioactive compounds of pineapple slices before and after drying is laid out in Table 3. Pineapple is a great reservoir of ascorbic acid, but processing conditions such as exposure to heat, oxygen, and light can affect its preservation [2], [17]. After drying, vitamin C content decremented significantly compared to fresh slices (434.96 µg/g). These losses can be attributed to the oxidation of this thermolabile nutrient during drying. Besides, in pretreated slices, reductions indicate that apart from the effect of the processing temperature, the immersion of slices in different liquids might have engendered a lixiviation of ascorbic acid. A similar observation was highlighted in sonicated pineapples [45], Gingko seeds [15], and pakchoi stems [50]. AA contents of US and US-ET samples were 1.15–6.84% and 1.92–10.04% lower than that of the control group, respectively, proving that ascorbic acid was drained to the solution during pretreatment. However, osmosonication (US-OD) led to the best vitamin C contents, which were slightly higher than the control. Therefore, although the osmotic pressure and ultrasound provoked leaching of AA, these losses were statistically lower (p < 0.05) than when using ethanol and distilled water as soaking media. Likewise, compared to untreated Ginkgo seeds, Boateng et al. [15] reported 21.43% and 43.99% of vitamin C degradations in US-OD and US samples, respectively. On the contrary, Rodríguez et al. [45] found that the average relative ascorbic acid content of pineapples was higher (59.3%) than that of non-treated samples (57.5%). Multi-frequency ultrasound caused improved AA retention in contrast with the single frequency mode, but no statistical difference (p > 0.05) was recorded between DFU and TFU. Improvements in AA contents compared to MFU were 3.67–6.11% during US, 1.17–2.03% in US-OD, and 8.07–9.02% in US-ET. These results are explained by the shortening of drying time, which limited vitamin exposure to thermal degradation.
Table 3.
Bioactive compounds and antioxidant activities of fresh and dried pineapples.
| Samples | Ascorbic acid (µg/g db) |
TPC (mg GAE/g db) |
TFC (mg RE/100 g db) |
DPPH (µM TE/g db) |
|---|---|---|---|---|
| Fresh | 434.96 ± 5.24a | 1.95 ± 0.12a | 25.10 ± 0.54a | 13.15 ± 0.23a |
| Control | 292.90 ± 0.72d | 1.45 ± 0.03b | 16.96 ± 0.65b | 10.39 ± 0.08b |
| MFU-US | 272.88 ± 0.72f | 0.91 ± 0.03e | 6.08 ± 0.27f | 7.03 ± 0.09hi |
| DFU-US | 282.89 ± 2.16e | 1.00 ± 0.02ef | 8.16 ± 0.41e | 7.43 ± 0.08 g |
| TFU-US | 289.54 ± 2.89e | 1.09 ± 0.02de | 13.29 ± 0.18c | 7.95 ± 0.05f |
| MFU-OD | 293.22 ± 3.74d | 1.17 ± 0.02d | 8.25 ± 0.57e | 8.57 ± 0.02e |
| DFU-OD | 296.66 ± 2.16 cd | 1.18 ± 0.02 cd | 10.68 ± 0.62d | 9.23 ± 0.16d |
| TFU-OD | 299.16 ± 5.06bc | 1.27 ± 0.02c | 16.49 ± 0.69b | 9.88 ± 0.03c |
| MFU-ET | 263.50 ± 5.01 g | 0.95 ± 0.01e | 5.14 ± 0.53f | 6.37 ± 0.04j |
| DFU-ET | 284.77 ± 3.07e | 0.91 ± 0.01e | 5.81 ± 0.63f | 6.82 ± 0.06i |
| TFU-ET | 287.27 ± 1.44e | 0.99 ± 0.01e | 8.11 ± 0.31e | 7.17 ± 0.07 h |
Note: Results are expressed as mean ± SD. In the same column, statistical differences were determined at p < 0.05. TPC, total phenolic content; TFC, total flavonoid content; GAE, gallic acid equivalent; RE, Rutin equivalent; TE, Trolox equivalent; MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US (in distilled water); -OD (in sucrose), -ET (in ethanol).
TPC and TFC of pineapple samples are presented in Table 3. Similar to vitamin C, dried products exhibited lower contents compared to fresh slices. The control showed better preservation of TPC and TFC in comparison to ultrasound-treated materials. These results suggest that there is a combined action of bioactive compounds lixiviation during pretreatment and degradation during infrared drying. Among all pretreated samples, US-OD had the lowest degradation of TPC (12.41–19.31%) and TFC (2.77–51.35%), compared to the control. This could be related to the reduced cavitation intensity in viscous solutions (sucrose solution), which limited compound losses compared to pretreatments in distilled water and ethanol [16]. The combination of many frequencies also preserved TPC and TFC better than MFU. However, statistical difference (p < 0.05) was noticed only in TFC values. These preservations can be linked not only to reduced drying times but also to the extraction and release of bound compounds due to strong cavitation, thereby offsetting these losses [45].
Finally, the antioxidant activity of different sample groups was evaluated. According to the results, there is a positive relationship between the content of bioactive compounds (TPC, TFC, and AA) and DPPH values. Others have also observed similar results [8], [25]. The control displayed the highest antioxidant activity among dried samples, which was 20.99% lower than that of fresh materials. At a chosen frequency mode, DPPH values followed the trend: US-OD > US > US-ET. Multi-frequency ultrasound increased significantly the antioxidant activity compared to MFU. In this study, antioxidant activity retention rates from the initial material were 53.46–60.45% for US, 65.17–75.13% for US-OD, and 48.44–54.52% for US-ET.
3.3.7. Flavor
The flavor is an important quality property of pineapple fruits. Maximum responses values from 10 sensors of an electronic nose device are presented in radar plots for different pretreatment conditions in Fig. 7A–C. These plots reveal that the flavor of pineapple samples was dominated by 5 groups of elements: nitrogen oxides (S2), methyl groups (S6), organic sulfides (S7), alcohols, aldehydes, and ketones (S8), and aromatic elements, inorganic sulfides, and organic chemicals (S9). The flavor profile of fresh slices was superior to control, US and US-OD but lower than US-ET. The control recorded the lowest concentrations after drying. Therefore, extended drying periods led to the loss of volatile compounds through evaporation or degradation [19]. Reduced responses during US and US-OD might be due to the loss of compounds in the soaking medium. However, US-OD protected the initial flavor of pineapple more than US. The sonication in ethanol provoked an upsurge in the response of the five aforementioned element groups, probably due to the formation of new compounds during pretreatment. The increment in the response of S8 (alcohol) proved that ethanol residues existed in dried products. In addition, although US-ET improved the flavor profile compared to the fresh material, the increase in elements such as nitrogen (S2) and sulfides (S7) may result in bad odors of products [25]. Tri-frequency ultrasound-assisted ethanol pretreatment also improved the flavor of scallion cylinders more than the pretreatment in distilled water according to Zhou et al. [31]. It can be concluded that the composition of the immersion liquid has an impact on the flavor of final products. A statistical test was needed to elucidate the changes in aroma under various ultrasound frequency modes.
Fig. 7.
Effects of ultrasound on the flavor of final products. A-C: Radar plots of different sample groups: US (A), US-OD (B), US-ET (C); D: Discrimination of flavor profile using the principal component analysis test (PCA); MFU, mono-frequency ultrasound; DFU, dual-frequency ultrasound; TFU, tri-frequency ultrasound; US (in distilled water); -OD (in sucrose), -ET (in ethanol).
Principal component analysis (PCA) is used to discriminate the difference between all samples (Fig. 7D). The sum PC1 + PC2 > 85% and therefore reveal that the PCA analysis was viable for this dataset [19]. As stated before, flavor profile by importance (positive side to negative side) followed the pattern: US-ET > Fresh > US-OD > US > Control. Multi-frequency ultrasound increased the concentration of aromatic compounds compared to MFU. The hypothesis is that a higher combination of frequencies resulted in improved extraction of flavor compounds or faster drying processes limited thermal degradation of flavor. However, apart from US group, flavor profiles of MFU, DFU, and TFU were within a small range. Finally, the flavor of control, US, and US-OD was influenced by the compounds of sensors S9 and S7 while fresh fruits and US-ET were impacted by those of S6, S8, and S2.
4. Conclusions
This research demonstrated that multi-frequency ultrasound applied in distilled water, sucrose, and ethanol influenced mass transfer, drying behavior, and the quality of final infrared dried products. It was found that ultrasound increased the porosity of slices and promoted faster drying periods. In addition, the simultaneous use of multi-frequency ultrasound improved drying kinetics and the quality of products compared to the single frequency mode. Although pretreatment inactivated enzymes, improved rehydration, and preserved the flavor of slices, the immersion in different liquid media engendered higher color changes and loss of food compounds, which were lower than the control. The composition of the liquid medium played also a major role. Pretreatment in distilled water (US) had the lowest color change, browning indexes, enzyme inactivation potentials, and flavor profiles among pretreated slices. When slices were pretreated in ethanol, the permeability of food tissues increased and provoked a significant lixiviation of nutrients and antioxidant compounds (higher than during US), thereby leading to the lowest antioxidant activities. Enzyme inactivation potentials and flavor of dried pineapple were the highest in US-ET samples, but this method led to the greatest color changes and browning indexes. In addition, because ethanol is a volatile and expensive solvent, its use as the pretreatment medium may increase the processing cost compared to distilled water and sucrose solution. Actual findings suggest that applying tri-frequency ultrasound in sucrose solution (TFU-OD) before infrared drying is a promising way to improve the mono-frequency mode, enhance drying kinetics and preserve the overall quality of dried pineapples. However, more investigation is required to limit color and nutrient degradation to promote a possible industrial application.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (31801561), 2020 Provincial Policy Guidance Program (Subei Science and Technology 635 Project, SZ-HZ202002), China Postdoctoral Science Foundation (2019T120401), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX21_1688). The authors are grateful for the support provided by Jinan City Science & Technology Innovation Project of Ten Agricultural Characteristic Industries (JNTSCY001).
Contributor Information
Baoguo Xu, Email: xbg@ujs.edu.cn.
Cunshan Zhou, Email: cunshanzhou@163.com.
References
- 1.M.F. Hossain, S. Akhtar, M. Anwar. Nutritional Value and Medicinal Benefits of Pineapple. Int. J. Nutr. Food Sci. 4. (2015). 84–88. 10.11648/j.ijnfs.20150401.22.
- 2.Mohd Ali M., Hashim N., Abd Aziz S., Lasekan O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109675. [DOI] [PubMed] [Google Scholar]
- 3.Mohammed S., Fatumah N., Shadia N. Drying performance and economic analysis of novel hybrid passive-mode and active-mode solar dryers for drying fruits in East Africa. J. Stored Prod. Res. 2020;88 doi: 10.1016/j.jspr.2020.101634. [DOI] [Google Scholar]
- 4.A. Chauhan. Mathematical modeling and comparative study of six drying methods based on energy consumption, nutrients retention, and drying time. (2019).
- 5.Boateng I.D., Yang X.M. Process optimization of intermediate-wave infrared drying: Screening by Plackett-Burman; comparison of Box-Behnken and central composite design and evaluation: A case study. Ind. Crops Prod. 2021;162 doi: 10.1016/j.indcrop.2021.113287. [DOI] [Google Scholar]
- 6.Rojas M.L., Augusto P.E.D., Cárcel J.A. Ethanol pre-treatment to ultrasound-assisted convective drying of apple. Innov. Food Sci. Emerg. Technol. 2020;61 doi: 10.1016/j.ifset.2020.102328. [DOI] [Google Scholar]
- 7.Feng Y., Xu B., ElGasim A., Yagoub A., Ma H., Sun Y., Xu X., Yu X., Zhou C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021;343 doi: 10.1016/j.foodchem.2020.128404. [DOI] [PubMed] [Google Scholar]
- 8.Boateng I.D., Yang X. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L.seed. J. Sci. Food Agric. 2021;101:3290–3297. doi: 10.1002/jsfa.10958. [DOI] [PubMed] [Google Scholar]
- 9.I. Doymaz, A.S. Kipcak, S. Piskin. Characteristics of thin-layer infrared drying of green bean. Czech J. Food Sci. 33. (2015). 10.17221/423/2014-CJFS.
- 10.Baptestini F.M., Corrêa P.C., Oliveira G.H.H., Almeida L.F.J., Vargas-Elías G.A. Constant and decreasing periods of pineapple slices dried by infrared. Rev. Bras. Ciências Agrárias – Brazilian J. Agric. Sci. 2016;11:53–59. doi: 10.5039/agraria.v11i1a5160. [DOI] [Google Scholar]
- 11.Tan M., Chua K.J., Mujumdar A.S., Chou S.K. Effect of osmotic pre-treatment and infrared radiation on drying rate and color changes during drying of potato and pineapple. Dry. Technol. 2001;19:2193–2207. doi: 10.1081/DRT-100107494. [DOI] [Google Scholar]
- 12.Ponkham K., Meeso N., Soponronnarit S., Siriamornpun S. Modeling of combined far-infrared radiation and air drying of a ring shaped-pineapple with/without shrinkage. Food Bioprod. Process. 2012;90:155–164. doi: 10.1016/j.fbp.2011.02.008. [DOI] [Google Scholar]
- 13.Deng L., Mujumdar A.S., Zhang Q., Yang X., Wang J., Gao Z., Xiao H. Chemical and physical pretreatments of fruits and vegetables : Effects on drying characteristics and quality attributes – a comprehensive review. Crit. Rev. Food Sci. Nutr. 2017;8398 doi: 10.1080/10408398.2017.1409192. [DOI] [PubMed] [Google Scholar]
- 14.Llavata B., García-Pérez J.V., Simal S., Cárcel J.A. Innovative pre-treatments to enhance food drying: a current review. Curr. Opin. Food Sci. 2020;35:20–26. doi: 10.1016/j.cofs.2019.12.001. [DOI] [Google Scholar]
- 15.Boateng I.D., Zhang W., Li Y., Saalia F.K., Yang X. Non-thermal pretreatment affects Ginkgo biloba L. seed’s product qualities, sensory, and physicochemical properties. J. Food Sci. 2021 doi: 10.1111/1750-3841.15999. [DOI] [PubMed] [Google Scholar]
- 16.Xu B., Sylvain Tiliwa E., Yan W., Roknul Azam S.M., Wei B., Zhou C., Ma H., Bhandari B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2021 doi: 10.1016/j.foodres.2021.110744. [DOI] [PubMed] [Google Scholar]
- 17.Corrêa J.L.G., Rasia M.C., Garcia-Perez J.V., Mulet A., de Jesus Junqueira J.R., Cárcel J.A. In: Dry. Energy Technol. Delgado J.M.P.Q., Gilson B. de L.A., editors. Springer International Publishing; Switzerland: 2016. Use of Ultrasound in the Distilled Water Pretreament and Convective Drying of Pineapple; pp. 71–88. [DOI] [Google Scholar]
- 18.Osae R., Essilfie G., Alolga R.N., Akaba S., Song X., Owusu-Ansah P., Zhou C. Application of non-thermal pretreatment techniques on agricultural products prior to drying: a review. J. Sci. Food Agric. 2020;100:2585–2599. doi: 10.1002/jsfa.10284. [DOI] [PubMed] [Google Scholar]
- 19.Boateng I.D., Yang X.-M., Tahany A.A.A., Li Y.-Y. Yolandani, Drying methods affect organoleptic and physicochemical properties of rehydrated ginkgo seed slices. Ind. Crops Prod. 2021;160 doi: 10.1016/j.indcrop.2020.113166. [DOI] [Google Scholar]
- 20.Prithani R., Dash K.K. Mass transfer modelling in ultrasound assisted osmotic dehydration of kiwi fruit. Innov. Food Sci. Emerg. Technol. 2020;64 doi: 10.1016/j.ifset.2020.102407. [DOI] [Google Scholar]
- 21.Nowacka M., Tappi S., Tylewicz U., Luo W., Rocculi P., Wesoły M., Ciosek-Skibińska P., Dalla Rosa M., Witrowa-Rajchert D. Metabolic and sensory evaluation of ultrasound-assisted osmo-dehydrated kiwifruit. Innov. Food Sci. Emerg. Technol. 2018;50:26–33. doi: 10.1016/j.ifset.2018.08.013. [DOI] [Google Scholar]
- 22.Fernandes F.A.N., Rodrigues S. Application of ultrasound and ultrasound-assisted osmotic dehydration in drying of fruits. Dry. Technol. 2008;26:1509–1516. doi: 10.1080/07373930802412256. [DOI] [Google Scholar]
- 23.Ó. Rodríguez, V. Eim, C. Rosselló, A. Femenia, J.A. Cárcel, S. Simal. Application of power ultrasound on the convective drying of fruits and vegetables: effects on quality. J. Sci. Food Agric. 99. (2019). 966–966. 10.1002/jsfa.9390. [DOI] [PubMed]
- 24.Chemat F., Rombaut N., Sicaire A.-G., Meullemiestre A., Fabiano-Tixier A.-S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Xu B., Chen J., Sylvain Tiliwa E., Yan W., Roknul Azam S.M., Yuan J., Wei B., Zhou C., Ma H. Effect of multi-mode dual-frequency ultrasound pretreatment on the vacuum freeze-drying process and quality attributes of the strawberry slices. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y., Yu X., Yagoub A.E.G.A., Xu B., Wu B., Zhang L., Zhou C. Vacuum pretreatment coupled to ultrasound assisted osmotic dehydration as a novel method for garlic slices dehydration. Ultrason. Sonochem. 2019;50:363–372. doi: 10.1016/j.ultsonch.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y., Wu B., Lu D., Pan Z., Ma H. Tri-frequency ultrasound as pretreatment to infrared drying of carrots: impact on enzyme inactivation, color changes, nutrition quality parameters and microstructures. Int. J. Food Eng. 2021;17:275–284. doi: 10.1515/ijfe-2020-0223. [DOI] [Google Scholar]
- 28.Ma Y., Yi J., Bi J., Zhao Y., Li X., Wu X., Du Q. Effect of ultrasound on mass transfer kinetics and phenolic compounds of apple cubes during osmotic dehydration. LWT. 2021;151 doi: 10.1016/j.lwt.2021.112186. [DOI] [Google Scholar]
- 29.Xu B., Feng M., Tiliwa E.S., Yan W., Wei B., Zhou C., Ma H., Wang B., Chang L. Multi-frequency power ultrasound green extraction of polyphenols from Pingyin rose: Optimization using the response surface methodology and exploration of the underlying mechanism. LWT. 2022;156 doi: 10.1016/j.lwt.2021.113037. [DOI] [Google Scholar]
- 30.Zhu Z., Zhang P., Sun D.-W. Effects of multi-frequency ultrasound on freezing rates and quality attributes of potatoes. Ultrason. Sonochem. 2020;60 doi: 10.1016/j.ultsonch.2019.104733. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C., Cai Z., Wang X., Feng Y., Xu X., Yagoub A.E.A., Wahia H., Ma H., Sun Y. Effects of tri-frequency ultrasonic vacuum-assisted ethanol pretreatment on infrared drying efficiency, qualities and microbial safety of scallion stalk slices. Dry. Technol. 2021:1–16. doi: 10.1080/07373937.2021.1894572. [DOI] [Google Scholar]
- 32.Wang Y.-Y., Tayyab Rashid M., Yan J.-K., Ma H. Effect of multi-frequency ultrasound thawing on the structure and rheological properties of myofibrillar proteins from small yellow croaker. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu B., Azam S.M.R., Feng M., Wu B., Yan W., Zhou C., Ma H. Application of multi-frequency power ultrasound in selected food processing using large-scale reactors: A review. Ultrason. Sonochem. 2021;81 doi: 10.1016/j.ultsonch.2021.105855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu B., Yuan J., Wang L., Lu F., Wei B., Azam R.S.M., Ren X., Zhou C., Ma H., Bhandari B. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104930. [DOI] [PubMed] [Google Scholar]
- 35.Xu B., Ren A., Chen J., Li H., Wei B., Wang J., Azam M.R., Bhandari B., Zhou C., Ma H. Effect of multi-mode dual-frequency ultrasound irradiation on the degradation of waxy corn starch in a gelatinized state. Food Hydrocoll. 2021;113 doi: 10.1016/j.foodhyd.2020.106440. [DOI] [Google Scholar]
- 36.Xu B., Chen J., Azam S.M.R., Feng M., Wei B., Yan W., Zhou C., Ma H., Bhandari B., Ren G., Duan X. Flat dual-frequency sweeping ultrasound enhances the inactivation of polyphenol oxidase in strawberry juice. J. Food Meas. Charact. 2022;16 doi: 10.1007/s11694-021-01202-3. [DOI] [Google Scholar]
- 37.Xu B., Chen J., Chitrakar B., Li H., Wang J., Wei B., Zhou C., Ma H. Effects of flat sweep frequency and pulsed ultrasound on the activity, conformation and microstructure of mushroom polyphenol oxidase. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2022.105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azam S.M.R., Ma H., Xu B., Devi S., Stanley S.L., Siddique M.A.B., Mujumdar A.S., Zhu J. Multi-frequency multi-mode ultrasound treatment for removing pesticides from lettuce (Lactuca sativa L.) and effects on product quality. LWT. 2021;143 doi: 10.1016/j.lwt.2021.111147. [DOI] [Google Scholar]
- 39.Azam S.M.R., Ma H., Xu B., Devi S., Siddique M.A.B., Stanley S.L., Bhandari B., Zhu J. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020;97:417–432. doi: 10.1016/j.tifs.2020.01.028. [DOI] [Google Scholar]
- 40.Fijalkowska A., Nowacka M., Wiktor A., Sledz M., Witrowa-Rajchert D. Ultrasound as a Pretreatment Method to Improve Drying Kinetics and Sensory Properties of Dried Apple. J. Food Process Eng. 2016;39:256–265. doi: 10.1111/jfpe.12217. [DOI] [Google Scholar]
- 41.Miano A.C., Rojas M.L., Augusto P.E.D. Combining ultrasound, vacuum and/or ethanol as pretreatments to the convective drying of celery slices. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Y., Zhou C., ElGasim A., Yagoub A., Sun Y., Owusu-Ansah P., Yu X., Wang X., Xu X., Zhang J., Ren Z. Improvement of the catalytic infrared drying process and quality characteristics of the dried garlic slices by ultrasound-assisted alcohol pretreatment. LWT. 2019;116 doi: 10.1016/j.lwt.2019.108577. [DOI] [Google Scholar]
- 43.Fernandes F.A.N., Linhares F.E., Rodrigues S. Ultrasound as pre-treatment for drying of pineapple. Ultrason. Sonochem. 2008;15:1049–1054. doi: 10.1016/j.ultsonch.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Fernandes F.A.N., Gallão M.I., Rodrigues S. Effect of osmosis and ultrasound on pineapple cell tissue structure during dehydration. J. Food Eng. 2009;90:186–190. doi: 10.1016/j.jfoodeng.2008.06.021. [DOI] [Google Scholar]
- 45.Rodríguez Ó., Gomes W., Rodrigues S., Fernandes F.A.N. Effect of acoustically assisted treatments on vitamins, antioxidant activity, organic acids and drying kinetics of pineapple. Ultrason. Sonochem. 2017;35:92–102. doi: 10.1016/j.ultsonch.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 46.L.D.C. de Freitas, S.C.R. Brandão, J.H.F. da Silva, O.R.S. da Rocha, P.M. Azoubel, Effect of Ethanol and Ultrasound Pretreatments on Pineapple Convective Drying, Food Technol. Biotechnol. 59 (2021) 209–215. 10.17113/ftb.59.02.21.7045. [DOI] [PMC free article] [PubMed]
- 47.Rani P., Tripathy P.P. Effect of ultrasound and chemical pretreatment on drying characteristics and quality attributes of hot air dried pineapple slices. J. Food Sci. Technol. 2019;56:4911–4924. doi: 10.1007/s13197-019-03961-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Feng Y., Zhou C., Sun Y., Wu B., Yagoub A.E.A., Aboagarib E.A.A. Effect of vacuum and ethanol pretreatment on infrared-hot air drying of scallion (Allium fistulosum) Food Chem. 2019;295:432–440. doi: 10.1016/j.foodchem.2019.05.145. [DOI] [PubMed] [Google Scholar]
- 49.Boateng I.D., Yang X.M. Osmotic, osmovacuum, sonication, and osmosonication pretreatment on the infrared drying of Ginkgo seed slices: Mass transfer, mathematical modeling, drying, and rehydration kinetics and energy consumption. J. Food Sci. 2021;86 doi: 10.1111/1750-3841.15916. [DOI] [PubMed] [Google Scholar]
- 50.Wu X.-F., Zhang M., Mujumdar A.S., Yang C.-H. Effect of ultrasound-assisted osmotic dehydration pretreatment on the infrared drying of Pakchoi Stems. Dry. Technol. 2020;38:2015–2026. doi: 10.1080/07373937.2019.1608232. [DOI] [Google Scholar]
- 51.Osae R., Essilfie G., Alolga R.N., Bonah E., Ma H., Zhou C. Drying of ginger slices—Evaluation of quality attributes, energy consumption, and kinetics study. J. Food Process Eng. 2020;43:e13348. [Google Scholar]
- 52.Soares P.A.G., Vaz A.F.M., Correia M.T.S., Pessoa A., Carneiro-da-Cunha M.G. Purification of bromelain from pineapple wastes by ethanol precipitation. Sep. Purif. Technol. 2012;98:389–395. doi: 10.1016/j.seppur.2012.06.042. [DOI] [Google Scholar]
- 53.Feng Y., Yu X., Yagoub A.E.G.A., Xu B., Wu B., Zhang L., Zhou C. Vacuum pretreatment coupled to ultrasound assisted osmotic dehydration as a novel method for garlic slices dehydration. Ultrason. Sonochem. 2019;50 doi: 10.1016/j.ultsonch.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 54.Osae R., Zhou C., Xu B., Tchabo W., Bonah E., Alenyorege E.A., Ma H. Nonthermal pretreatments enhances drying kinetics and quality properties of dried ginger (Zingiber officinale Roscoe) slices. J. Food Process Eng. 2019;42:1–11. doi: 10.1111/jfpe.13117. [DOI] [PubMed] [Google Scholar]
- 55.Corrêa J.L.G., Rasia M.C., Mulet A., Cárcel J.A. Influence of ultrasound application on both the osmotic pretreatment and subsequent convective drying of pineapple (Ananas comosus) Innov. Food Sci. Emerg. Technol. 2017;41:284–291. doi: 10.1016/j.ifset.2017.04.002. [DOI] [Google Scholar]
- 56.Çağlayan D., Barutçu Mazı I. Effects of ultrasound-assisted osmotic dehydration as a pretreatment and finish drying methods on the quality of pumpkin slices. J. Food Process. Preserv. 2018;42 doi: 10.1111/jfpp.13679. [DOI] [Google Scholar]
- 57.Noshad M., Mohebbi M., Shahidi F., Mortazavi S.A. Kinetic modeling of rehydration in air-dried quinces pretreated with osmotic dehydration and ultrasonic. J. Food Process. Preserv. 2012;36 doi: 10.1111/j.1745-4549.2011.00593.x. [DOI] [Google Scholar]
- 58.Amami E., Khezami W., Mezrigui S., Badwaik L.S., Bejar A.K., Perez C.T., Kechaou N. Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrason. Sonochem. 2017;36:286–300. doi: 10.1016/j.ultsonch.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 59.C.K. Sunil, B. Kamalapreetha, J. Sharathchandra, K.S. Aravind, A. Rawson. Effect of ultrasound pre-treatment on microwave drying of okra. J. Appl. Hortic. 19. (2017). 58–62. 10.37855/jah.2017.v19i01.09.