Abstract
BACKGROUND:
Hispanic/Latinx smokers living in the United States face unique challenges in quitting smoking. This study evaluated the efficacy of a culturally relevant, Spanish-language, extended self-help smoking cessation intervention among Hispanic smokers.
METHODS:
A 2-arm parallel randomized controlled trial was conducted with Hispanic/Latinx smokers living in the United States who preferred health information in Spanish and smoked 5 or more cigarettes per week. Participants were randomly allocated to receive Libre del Cigarrillo (LDC), which consisted of 11 booklets and 9 pamphlets mailed monthly over 18 months, or the usual care (UC), which was a single Spanish-language self-help booklet from the National Cancer Institute. The primary outcome was self-reported 7-day point prevalence smoking abstinence assessed 6, 12, 18, and 24 months after the baseline. Eight prespecified moderators of the intervention were evaluated. Cost-effectiveness was also evaluated. All statistical tests were 2-sided.
RESULTS:
Data from all participants randomized to LDC (n = 714) or UC (n = 703) were used for analyses after multiple imputation to manage missing data. Generalized estimating equation analyses indicated that LDC abstinence rates were higher (P < .001) across all assessments. Logistic regression analyses revealed that at 24 months, the abstinence rate was greater for LDC (33.1%) than UC (24.3%; odds ratio, 1.54; 95% confidence interval, 1.18–2.02; P = .002). Men exhibited a strong intervention effect at all assessments (P values < .001), whereas the intervention effect for women was observed only at 6 and 12 months (P values < .018). In comparison with UC, the incremental cost per quitter in the LDC arm was $648.43 at 18 months and $683.93 at 24 months.
CONCLUSIONS:
A culturally relevant, Spanish-language intervention was efficacious and cost-effective for smoking cessation.
Keywords: Hispanic, Latino, Latinx, randomized controlled trial, self-help, smoking cessation
LAY SUMMARY:
• Research is needed to develop interventions for ethnic minority smokers.
• The aim of the current study was to test a Spanish-language adaptation of a validated and easily implemented self-help smoking cessation intervention in a nationwide randomized controlled trial.
• The findings demonstrated that the intervention produced greater smoking abstinence in comparison with a standard self-help booklet.
• Participants also were more satisfied with the intervention, and it was cost-effective.
• Efforts aimed at promoting tobacco abstinence in this underserved population could have significant public health implications, including potential reductions in cancer health disparities associated with tobacco smoking.
INTRODUCTION
Hispanics/Latinxs living in the United States have a lower smoking prevalence (9%) than non-Hispanic Whites (16%).1 Hispanic smokers have also been consistently found to smoke fewer cigarettes per day and to have shorter smoking histories than non-Hispanic White smokers.2–4 However, smoking prevalence rates vary greatly by subgroups, with rates reaching as high as 28.5% (ie, Puerto Ricans).5–8 Cigarette smoking is the primary risk factor for lung cancer, which is the leading cause of cancer death among Hispanic men and is second only to breast cancer among Hispanic women.8
The burden of smoking-related diseases may be explained by smoking cessation disparities observed in Hispanic smokers due to less successful smoking cessation attempts in comparison with non-Hispanic Whites.9–11 Poor cessation outcomes may be attributed to limited health care access, financial strains, and language barriers.8,12–16 Hispanics are also less likely to receive tobacco screening, be advised to quit by health professionals, and to use evidence-based cessation treatment (ie, behavioral counseling or pharmacotherapy).2,17–20 Two decades ago, Hispanics were recognized as an important group with unique smoking cessation needs, and this led to recommendations for culturally targeted interventions.21 However, few such interventions have been developed or evaluated.22,23
Minimal self-help interventions (ie, written materials) have considerable public health potential: they are low-cost, highly accessible, and easy to disseminate and implement in health care and community settings. Previous research has demonstrated the efficacy and cost-effectiveness of an English-language self-help intervention for smoking cessation called “Forever Free®: Stop Smoking for Good” (SSFG), which comprises booklets and pamphlets mailed monthly over 18 months. Abstinence rates for the SSFG intervention 6 and 12 months after intervention were 30.0% and 33.4%, respectively, versus 18.9% and 23.3% for the comparison condition of a single self-help booklet from the National Cancer Institute (NCI).24,25
To increase the reach of this validated intervention and address the gap in the availability of Spanish-language interventions, SSFG was transcreated (ie, translated and culturally adapted) for Hispanic smokers who prefer health information in Spanish.26 Previous research has shown that health interventions tailored to Hispanics’ culture and preferred language have higher acceptability and efficacy.27,28 Our multistep transcreation process led to the development of a Spanish-language smoking cessation intervention called “Libre del Cigarrillo, por Mi Familia y por Mí: Guía Para Dejar de Fumar” (“Free From Cigarettes, for My Family and for Me: Guide to Quitting Smoking”). We hypothesized that this series of booklets and pamphlets would produce higher smoking abstinence rates than usual care (UC)—a Spanish-language self-help booklet developed by the NCI—through the 24 months of assessment as well as favorable cost-effectiveness.
MATERIALS AND METHODS
Study Design
This 2-arm parallel randomized controlled trial (NCT02945787 at ClinicalTrials.gov) compared Libre del Cigarrillo (LDC) with UC. Intervention materials were distributed by postal mail with the option of receiving additional electronic versions via email links. Assessments for both arms were conducted every 6 months for 2 years. The Advarra institutional review board approved this study. Participants provided verbal informed consent. During telephone screening, participants were told that they would receive educational smoking cessation materials. Because all participants received self-help cessation materials, the existence of study arms or group allocation was not disclosed. The study design, procedures, and recruitment were previously published.29,30 Data collection in Florida was funded by a state grant, whereas data collection elsewhere in the United States, including Puerto Rico, was funded by the NCI.
Participants
Study recruitment occurred from October 2016 to June 2018 through paid advertising on television, radio, social media, and public buses as well as television interviews and local distribution of flyers.
The inclusion criteria were an age ≥ 18 years, smoking 5 or more tobacco cigarettes per week over the past year, not currently being enrolled in a face-to-face smoking cessation program, and a preference for educational health materials in Spanish. The exclusion criteria were an inability to provide a valid US mailing address and previous enrollment of another household member.
Procedures
Figure 1 displays the study recruitment and flow. Participants were screened by telephone. Eligible participants were sent a baseline questionnaire. Participants who returned the baseline were randomized to 1 of 2 intervention arms via computer-generated randomization with balanced permuted blocks (block size, 4), and they were stratified by sex, smoking status (daily vs nondaily), and household income (<$10,000 vs ≥$10,000 vs refused to answer). Participant eligibility was confirmed by a review of baseline assessment responses, and individuals were enrolled if they continued to meet the inclusion criteria. The study statistician created sequences a priori that were applied by a database software system. Research staff were not masked to group assignment.
Figure 1.
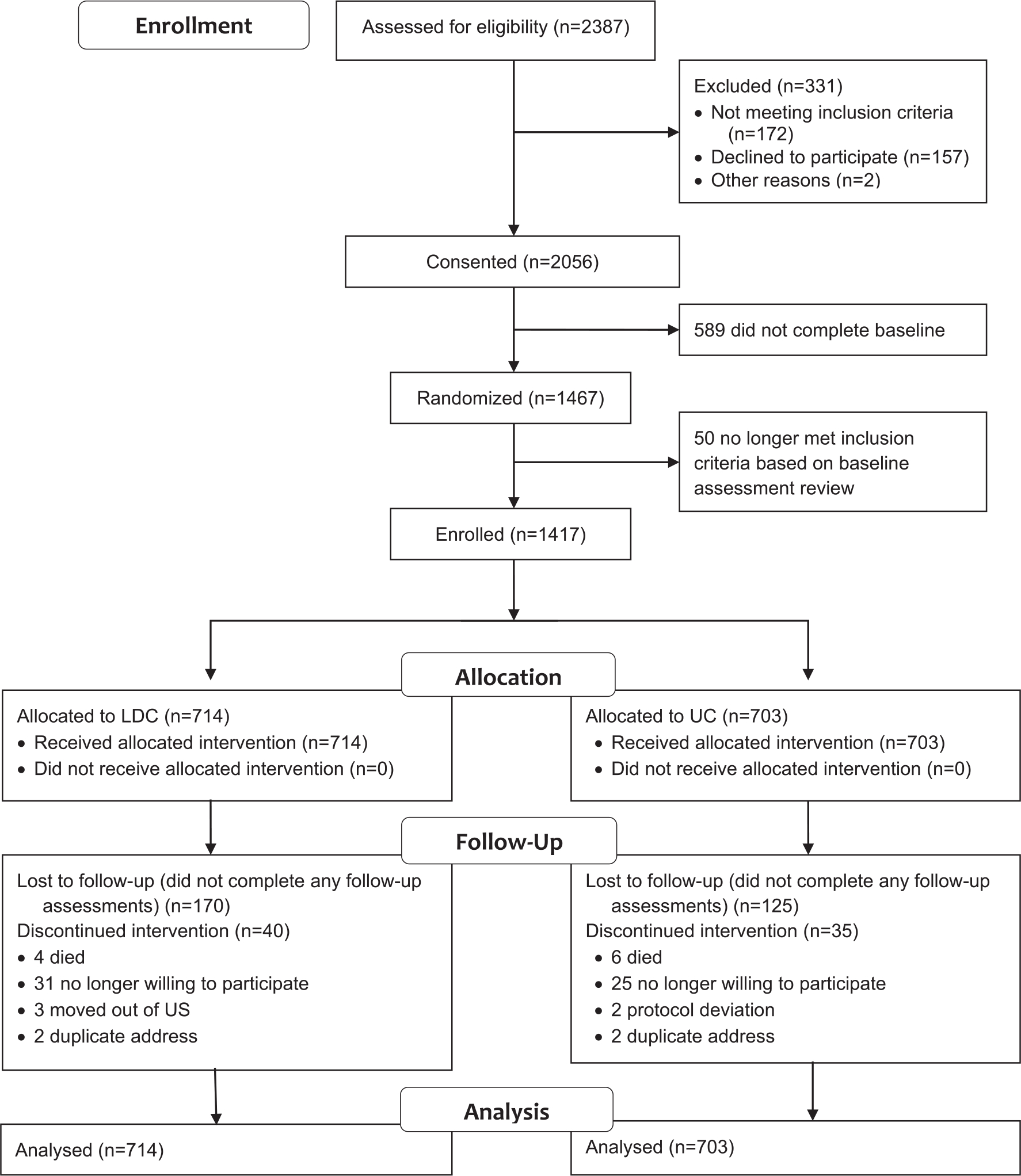
Consolidated Standards of Reporting Trials diagram. LDC indicates Libre del Cigarrillo; UC, usual care.
Participants in UC received the NCI booklet entitled “Guia: Viva de Forma Más Saludable Para Usted y Su Familia, Deje de Fumar Hoy Mismo” (“Live Healthier for You and Your Family, Quit Smoking Today”).31 This comprehensive 40-page Spanish-language booklet with high-quality content and visual presentation was mailed upon randomization. A credible UC comparison condition was selected rather than a no-treatment control to fulfill an ethical obligation to provide high-quality UC when a recruitment strategy publicizing smoking cessation assistance was being used.
LDC was based on the validated English-language SSFG.32 The first of the original 10 booklets provides a general summary of the process of quitting smoking, preparing to quit, pharmacotherapies, and potential challenges. The remaining 9 booklets provide information on cessation and relapse prevention (eg, stress management and coping strategies) as well as the benefits of long-term tobacco abstinence. The first booklet was sent at randomization, and the remainder were sent at 1, 2, 3, 5, 7, 9, 12, 15, and 18 months. During nonbooklet months, participants received trifold color pamphlets to complement and reinforce key messages from the booklets.24 Each pamphlet shares a former smoker’s story from a first-person perspective to provide a socially supportive role model.
As described in a prior publication,26 our intervention development was informed by a multiphase transcreation (translation and cultural adaptation) process to ensure cultural relevance for a diverse Spanish-speaking population. A prominent recurring theme during the development of LDC was the Hispanic cultural value of familism, which emphasizes the importance of family support during smoking cessation. Therefore, we added a booklet called “Para Mis Familiares y Amigos” (“For My Family and Friends”) to help family and friends to understand, support, and assist in the cessation attempt. This booklet was sent with the first booklet along with instructions to share. Participants could request additional copies.
Another recurring theme was the desire for personal contact.26 In response, we included a brief telephone call within the LDC arm. The call, which occurred 1 week after randomization, aimed to build rapport and garner trustworthiness and intervention credibility. During the approximately 10-minute call, bilingual staff introduced the intervention, suggested how to share the family booklet, asked about reasons for quitting, and encouraged setting a quit date.
Assessments
Baseline
Participants completed assessments by mail or online. Self-report measures in Spanish assessed sociodemographic characteristics as well as the smoking history, including nicotine dependence, via the Spanish Fagerström Test for Nicotine Dependence.33 Measures also assessed quitting motivation (Contemplation Ladder),34 smoking cessation self-efficacy,35 abstinence-related motivational engagement,36 acculturation (Short Acculturation Scale for Hispanics),37 and familism (Attitudinal Familism Scale).38 Measurement specifics are reported elsewhere.29
Follow-Up
At the 4 assessment points, smoking behavior was collected to derive the primary outcome of 7-day point prevalence abstinence (ie, no smoking in previous 7 days). To assess more sustained smoking abstinence, we also report 30- and 90-day point prevalence abstinence as secondary smoking outcomes. The evaluation of the intervention materials was assessed via the 8-item Client Satisfaction Questionnaire.39
Statistical Analysis
All analyses were performed with SAS v 9.4 (SAS Institute, Cary, North Carolina). An intent-to-treat approach was used for the evaluation of the intervention. Multiple imputation using the multivariate normal approach managed missing data.40,41 Twenty data sets were created. Preliminary analyses identified auxiliary variables (ie, baseline measures predicting subsequent smoking or unreturned surveys) for the imputation model to increase the credibility of the missing-at-random assumption. A post hoc adjustment was applied to imputed smoking status values to reflect a small to medium missing-implies-smoking effect (ie, Cohen’s d = 0.35).40 Final imputed values were dichotomized by adaptive rounding. See Appendix 1 in the supporting information for details.
The intervention’s effect on 7-day point prevalence smoking abstinence was evaluated via 1) generalized estimating equations (GEEs) with condition, time, and their interaction as the primary covariates and 2) logistic regression at the 24-month assessment. Eight prospective moderators of the intervention’s effect (sex, education, employment, income, quitting motivation, nicotine dependence, acculturation, and self-efficacy) were evaluated by the addition of the moderator and its interaction with condition to the GEE and logistic regression models. Results based on each of the 20 data sets were combined for a final test statistic and P value. α was set at .05 (2-sided) for all tests. See Appendix 2 in the supporting information for details.
Power analysis
As previously described,29 an a priori sample size analysis indicated that a sample of 500 (250 per group) for the Florida subsample and a sample of 740 (370 per group) for the non-Florida subsample would provide 80% power with α = .05 to detect 7-day point prevalence abstinence rates linearly increasing from 12% at 6 months to 19% at 24 months for UC and from 16% to 29% for LDC.29 Estimated abstinence rates were based on our prior research.24 Because of a lower than anticipated survey return rate of the 6-month assessment, we increased the target sample to 550 from Florida and 850 from elsewhere.
Cost Assessment
We collected information on all resources needed to conduct the intervention (eg, personnel effort and printing) and assigned appropriate unit prices for each resource type. Research-specific resources (eg, assessments) were excluded. We calculated the total cost per participant in each arm and incremental cost-effectiveness ratios of abstinence (at 18 and 24 months) comparing the LDC intervention with the UC arm. Sensitivity analyses explored the effect of 1) conducting gender subgroup analyses and 2) varying total costs (that may occur with increased levels of automation, differences in local prices and wages, etc).
RESULTS
Of the 2387 individuals screened for eligibility, 2056 (86%) consented, and among those who consented, 1467 (71%) returned the baseline assessment (Fig. 1). After the final review, 1417 participants were enrolled and included in the analyses. Of the enrolled participants, 356 (25%) did not complete any of the 4 follow-up surveys, with higher noncompletion rates for LDC participants (odds ratio, 1.41; 95% confidence interval, 1.10–1.79; P = .006). The percentage of unreturned surveys increased from 36% to 47% from 6 to 24 months.
Most participants were 35 to 65 years old, had a high school diploma or less, were employed, and had an annual household income of less than $20,000. Participants generally reported lower levels of acculturation and higher levels of familism values. Most participants had been smoking for more than 20 years. More than 90% of the participants smoked daily, and more than 80% had smoked 20 or more cigarettes in the past week. Cigarette dependence varied extensively with a moderate average. There were no significant group differences for demographic or smoking-related variables (Table 1).
TABLE 1.
Baseline Sample Characteristics by Treatment Arm
| Variable | UC (n = 703) | LDC (n = 714) |
|---|---|---|
|
| ||
| No follow-ups returned, No. (%) | 154 (22) | 202 (28) |
| Age, mean (SD), y | 49.2 (11.5) | 50.3 (11.9) |
| Women, No. (%) | 339 (48) | 346 (48) |
| Subethnicity, No. (%) | ||
| Puerto Rican | 118 (17) | 117 (16) |
| Central American | 39 (6) | 48 (7) |
| Mexican American | 233 (33) | 244 (34) |
| South American | 61 (9) | 65 (9) |
| Cuban | 154 (22) | 162 (23) |
| Dominican | 21 (3) | 16 (2) |
| Other | 19 (3) | 8 (1) |
| More than 1 | 58 (8) | 54 (8) |
| Marital status, No. (%) | ||
| Single | 154 (22) | 147 (21) |
| Widowed | 35 (5) | 38 (5) |
| Married or living together as married | 316 (45) | 343 (48) |
| Separated | 70 (10) | 77 (11) |
| Divorced | 123 (18) | 104 (15) |
| Education: high school diploma or less, No. (%) | 418 (58) | 430 (59) |
| Employed full- or part-time, No. (%) | 398 (58) | 405 (58) |
| Annual household income, No. (%) | ||
| <$10,000 | 274 (41) | 273 (41) |
| $10,000–$19,999 | 160 (24) | 184 (28) |
| ≥$20,000 | 228 (34) | 211 (32) |
| Familism (18–180), mean (SD) | 148.0 (25.0) | 148.0 (23.7) |
| Acculturation (12–60), mean (SD) | 19.5 (6.5) | 19.8 (6.4) |
| Years as a regular smoker, mean (SD) | 27.6 (12.8) | 28.5 (13.0) |
| Smoking cigarettes daily, No. (%) | 660 (94) | 665 (93) |
| Cigarettes smoked in past week, No. (%) | ||
| 1–9 | 23 (3) | 40 (6) |
| 10–19 | 96 (14) | 104 (15) |
| ≥20 | 583 (83) | 570 (80) |
| Currently using e-cigarettes, No. (%) | 28 (4.0) | 33 (4.6) |
| Contemplation Ladder (0–10), mean (SD) | 6.9 (2.8) | 6.9 (2.8) |
| FTND (0–10), mean (SD) | 4.9 (2.4) | 5.0 (2.4) |
| SSE (9–45), mean (SD) | 19.2 (9.0) | 18.9 (9.0) |
| ARME (5–35), mean (SD) | 28.7 (8.5) | 29.2 (8.2) |
| Used NRT within past 3 mo, No. (%) | 329 (47) | 354 (50) |
| Made a quit attempt in past year, No. (%) | 375 (53) | 354 (50) |
Abbreviations: ARME, Abstinence-Related Motivational Engagement; FTND, Fagerstrom Test for Nicotine Dependence; LDC, Libre del Cigarrillo; NRT, nicotine replacement therapy; SSE, Smoking Cessation Self-Efficacy; UC, usual care.
Percentages are based on those who provided a response. The following categorical variables were missing more than 1% of responses: education (3.7%), employment (2.5%), and income (6.9%).
The multiple imputation model included the treatment arm, the smoking status at each follow-up, the 8 moderators, 3 auxiliary variables that either predicted missing follow-up surveys (survey type and age) or smoking status (abstinence-related motivational engagement), and 11 variables representing the interaction of a moderator or an auxiliary variable with the treatment arm. Smoking status was imputed most frequently on the basis of unreturned surveys. Missingness for moderators and auxiliary variables ranged from 0% (eg, sex) to 6.9% (income). The relative efficiency for tests of the mean differing from 0 was at least 0.97 for all variables.
Figure 2 and Table 2 present the percentages of 7-day point prevalence abstinence by arm across the 20 data sets. GEE analysis revealed a significant linear increase in abstinence rates (P < .001). Overall, abstinence rates were higher in the LDC group (P < .001). The interaction of time and intervention was not significant (P = .21). Logistic regression revealed that LDC abstinence rates were higher at 24 months (P = .002). Follow-up analyses showed that LDC also produced higher abstinence rates at 6, 12, and 18 months (P values < .001). Table 2 also presents 30- and 90-day abstinence rates at each assessment. The results were highly similar to those for 7-day abstinence rates. For these secondary outcomes, the GEE analysis revealed main effects for time and intervention (P values < .001), and logistic regression revealed higher abstinence rates for LDC at 24 months (P values < .01). With respect to these results, Appendix 3 in the supporting information presents 7-day point prevalence abstinence rates and test statistics 1) for responders only and 2) for missing follow-up smoking status imputed as smoking.
Figure 2.
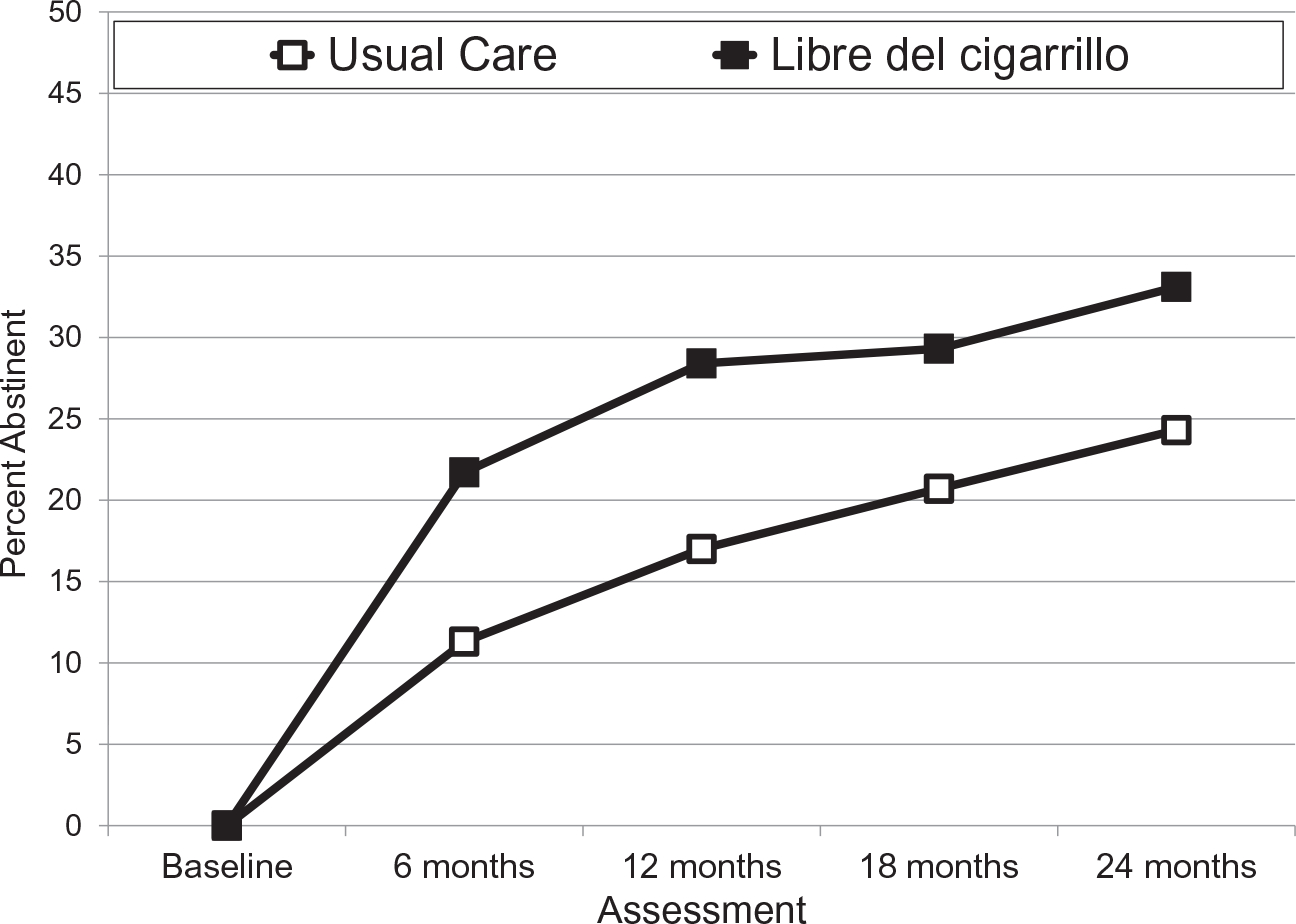
Seven-day point prevalence smoking abstinence rates. The percent abstinent was averaged across 20 multiple imputation data sets. The n value was 1417 for each of these complete data sets. Both treatments began at the baseline. Libre del Cigarrillo ended at 18 months. Abstinence rates at each assessment for both treatments are presented in Table 2 along with intervention odds ratios and P values from logistic regression at each assessment. Generalized estimating equations revealed a main effect for assessment and treatment condition (P values < .001).
TABLE 2.
Abstinence Rates and Paired Comparisons for the 7-, 30-, and 90-Day Point Prevalence
| Point-Prevalence Period | Assessment Point | UC | LDC | P | OR (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| Full sample | |||||
| 7 d | 6 mo | 11.3% | 21.7% | <.001 | 2.18 (1.54–3.08) |
| 12 mo | 17.0% | 28.4% | <.001 | 1.93 (1.45–2.58) | |
| 18 mo | 20.7% | 29.3% | .001 | 1.59 (1.20–2.09) | |
| 24 mo | 24.3% | 33.1% | .002 | 1.54 (1.18–2.02) | |
| 30 d | 6 mo | 6.5% | 15.3% | <.001 | 2.58 (1.67–3.98) |
| 12 mo | 11.1% | 21.3% | <.001 | 2.18 (1.48–3.20) | |
| 18 mo | 14.5% | 20.9% | .009 | 1.57 (1.12–2.19) | |
| 24 mo | 18.6% | 25.8% | .009 | 1.52 (1.11–2.09) | |
| 90 d | 6 mo | 4.1% | 10.2% | <.001 | 2.65 (1.57–4.49) |
| 12 mo | 8.5% | 17.7% | <.001 | 2.31 (1.52–3.53) | |
| 18 mo | 11.0% | 18.3% | .001 | 1.82 (1.28–2.59) | |
| 24 mo | 15.3% | 22.1% | .007 | 1.58 (1.13–2.20) | |
| Men only | |||||
| 7 d | 6 mo | 8.5% | 20.2% | <.001 | 2.72 (1.64–4.52) |
| 12 mo | 15.7% | 29.7% | <.001 | 2.28 (1.50–3.47) | |
| 18 mo | 17.9% | 32.5% | <.001 | 2.20 (1.46–3.32) | |
| 24 mo | 21.8% | 35.9% | <.001 | 2.02 (1.36–2.98) | |
| 30 d | 6 mo | 4.4% | 13.7% | <.001 | 3.45 (1.78–6.69) |
| 12 mo | 10.8% | 23.1% | <.001 | 2.48 (1.47–4.18) | |
| 18 mo | 13.0% | 21.8% | .015 | 1.87 (1.13–3.09) | |
| 24 mo | 16.9% | 28.6% | .005 | 1.97 (1.24–3.14) | |
| 90 d | 6 mo | 2.5% | 8.3% | .007 | 3.58 (1.41–9.06) |
| 12 mo | 8.1% | 19.5% | <.001 | 2.77 (1.55–4.93) | |
| 18 mo | 9.7% | 18.9% | .004 | 2.17 (1.28–3.66) | |
| 24 mo | 13.8% | 22.7% | .012 | 1.84 (1.14–2.95) | |
| Women only | |||||
| 7 d | 6 mo | 14.3% | 23.3% | .015 | 1.83 (1.15–2.91) |
| 12 mo | 18.4% | 26.9% | .017 | 1.63 (1.09–2.44) | |
| 18 mo | 23.7% | 25.9% | .580 | 1.13 (0.74–1.72) | |
| 24 mo | 27.0% | 30.2% | .420 | 1.17 (0.80–1.70) | |
| 30 d | 6 mo | 8.8% | 16.9% | .009 | 2.12 (1.21–3.70) |
| 12 mo | 11.4% | 19.5% | .015 | 1.89 (1.13–3.15) | |
| 18 mo | 16.0% | 19.9% | .243 | 1.30 (0.83–2.04) | |
| 24 mo | 20.3% | 22.8% | .495 | 1.15 (0.76–1.75) | |
| 90 d | 6 mo | 5.8% | 12.1% | .011 | 2.25 (1.20–4.20) |
| 12 mo | 6.1% | 15.8% | .029 | 1.90 (1.07–3.38) | |
| 18 mo | 12.3% | 17.6% | .089 | 1.52 (0.94–2.47) | |
| 24 mo | 16.8% | 21.4% | .169 | 1.35 (0.88–2.08) | |
Abbreviations: CI, confidence interval; LDC, Libre del Cigarrillo; OR, odds ratio; UC, usual care.
OR and P values for each outcome and each assessment were derived from logistic regression for LDC versus UC with the relevant 20 imputed data sets. See Appendix 3 in the supporting information for a comparable table of percent abstinent and test results for 1) responders only and 2) missing follow-up smoking status imputed as smoking.
The only significant moderator of the intervention on 7-day abstinence was sex. GEE analysis revealed a significant sex × intervention interaction (P = .049). Logistic regression at 24 months revealed a marginally significant interaction (P = .0501). Figure 3 and Table 2 present abstinence rates by arm across the 20 imputed data sets separately for men and women. Follow-up GEE analyses within each sex revealed a main effect of treatment arm for both men (P < .001) and women (P = .038). Logistic regression analyses revealed significantly higher LDC abstinence rates for men at all follow-up points, including 24 months (P < .001). In contrast, the LDC arm produced higher abstinence rates for women only at 6 and 12 months.
Figure 3.
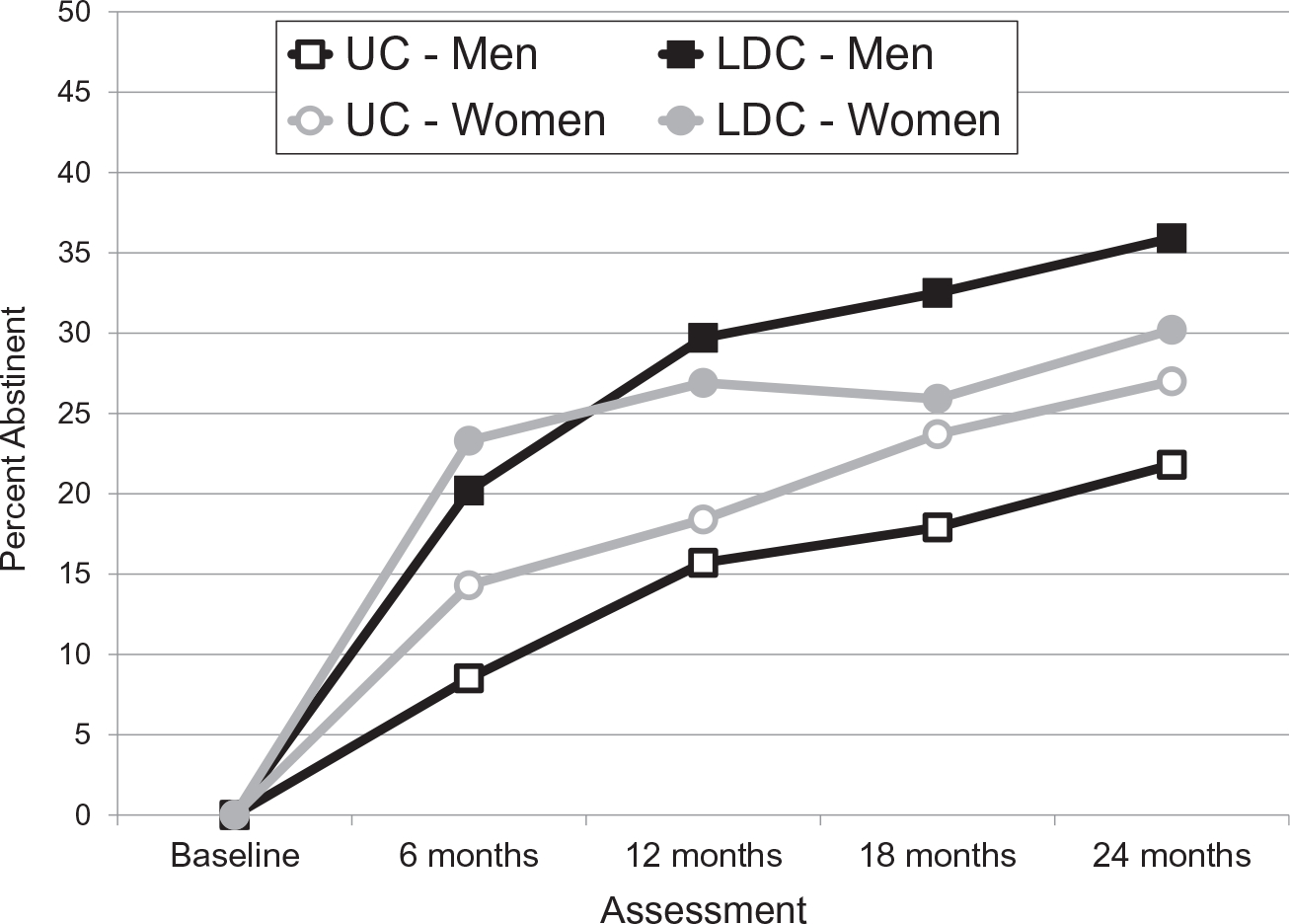
Seven-day point prevalence smoking abstinence rates for men and women receiving the LDC and UC treatments. The percent abstinent was averaged across 20 multiple imputation data sets. The n value was 1417 for each of these complete data sets. Both treatments began at the baseline. LDC ended at 18 months. Abstinence rates at each assessment for both treatments are presented in Table 2 along with intervention odds ratios and P values from logistic regression at each assessment. Generalized estimating equations for each sex revealed a main effect for both assessment and treatment arm for men (P values < .001) and for women (P values < .05). LDC indicates Libre del Cigarrillo; UC, usual care.
A post hoc analysis of differences between men and women found that women were older, were less likely to be married, were less likely to be employed, were more likely to have an annual household income of less than $10,000, and were less likely to smoke 20 or more cigarettes per week (see Appendix 4 in the supporting information for details). In addition, women had lower average acculturation and familism scores. However, none of these variables were themselves significant moderators of treatment effects, nor did adding the variables negate the moderation of the treatment effect by sex.
Cost Assessment
The total intervention cost per participant was $6.46 in the UC arm and $66.44 in the LDC arm. In comparison with UC, the incremental cost per quitter in the LDC arm was $648.43 at 18 months and $683.93 at 24 months. Table 3 displays results of cost-effectiveness and sensitivity analyses (varying intervention costs at 10% increments).
TABLE 3.
Incremental Cost (in US Dollars) per Quitter for Libre del Cigarrillo Versus the Usual-Care Arm by Assessment Point and Cost Variation
| 6 mo | 12 mo | 18 mo | 24 mo | |
|---|---|---|---|---|
|
| ||||
| Base case | 572.88 | 520.21 | 648.43 | 683.93 |
| Men | 511.78 | 471.18 | 392.03 | 431.20 |
| Women | 717.47 | 740.50 | 2925.88 | 1941.12 |
| 80% costs | 458.30 | 416.17 | 518.75 | 547.14 |
| 90% costs | 515.59 | 468.19 | 583.60 | 615.54 |
| 110% costs | 630.17 | 572.23 | 713.28 | 752.32 |
| 120% costs | 687.46 | 624.26 | 778.13 | 820.72 |
Evaluation of the Intervention
The Client Satisfaction Questionnaire, whose score ranges from 1 to 4 (with higher values indicating greater satisfaction), was completed at 6, 12, and 18 months. Respondents reported higher means in the LDC arm (3.23, 3.28, and 3.29, respectively) than the UC arm (2.94, 3.01, and 3.04, respectively) at each assessment (P values < .001).
DISCUSSION
Hispanic individuals experience a high burden of tobacco-related morbidity and mortality, yet few trials have examined the efficacy of Spanish-language smoking cessation interventions for this population.23 Results from our nationwide RCT demonstrated that a culturally relevant, Spanish-language, extended self-help intervention produced greater smoking abstinence in comparison with a validated NCI-disseminated self-help booklet. Positive intervention outcomes were sustained through 6 months beyond the end of the intervention and were observed for both primary and secondary smoking outcomes. Participants also reported greater satisfaction with the LDC intervention in comparison with UC. Findings extend the demonstrated efficacy of the original English-language SSFG24 to smokers preferring Spanish.
The results support the efficacy of an intervention approach with potential for broad dissemination and reach that is not limited by technology and that overcomes access barriers of in-person treatments. A key advantage of LDC is its relatively low-cost ($66.44 per person) and consequent cost-effectiveness (<$700 per additional quitter); this is significantly lower than the cost of typical smoking cessation interventions.42 In light of costs associated with printing, postage, and labor, electronic distribution would greatly reduce costs, albeit with unknown effects on efficacy.
Prior smoking cessation studies with Hispanic smokers have typically not reported or have not found sex differences.43–47 In a qualitative review of 129 efficacy and effectiveness trials of smokers in general, nearly half found that women were less likely to quit smoking.48 Notably, this sex difference was strongest in studies with longer follow-up periods. This pattern is somewhat consistent with the results of the current study, in which the early efficacy of LDC faded over time among women compared with men. Future studies should investigate how to sustain intervention effects for women.
Although men and women differed on several baseline variables, none of these variables accounted for the observed sex differences in treatment efficacy. However, sociocultural characteristics may contribute to the strong intervention effect found among men. Recent research has shown that Latino males living in the United States seek to embody familism values.49 Men in this study reported higher familism than women; thus, it is plausible that they had a more favorable response to the incorporation of familism values in our intervention. Moreover, men in our sample reported slightly greater acculturation than women. There is some evidence that men with higher levels of acculturation are more likely to quit smoking.50 Future studies may benefit from further exploration of the role of Hispanic sociocultural values in smoking cessation. For example, machismo-related beliefs (an exaggerated sense of masculinity) influence health decision-making and help-seeking behavior of Hispanic men and contribute to a reluctance to seeking psychological help.51,52 Thus, it is possible that our self-help approach to smoking cessation may have been more appealing to men than more traditional in-person counseling or support sessions would have been. This hypothesis requires exploration in future research including measures of this cultural value and comparisons with other cessation approaches.
Our study had several strengths. To our knowledge, this was the first study to test a culturally relevant, Spanish-language, extended self-help intervention for smoking cessation. Additional strengths include the following: a large sample size; use of a randomized design with 2 years of follow-up; nationwide recruitment of Hispanic individuals representing diverse countries of origin, which thereby increased generalizability; and collection of cost data supporting the economic value of the intervention.
A study limitation was the reliance on self-reported smoking status at outcome assessments. This was necessitated by the nationwide recruitment strategy. We attempted to biochemically verify abstinence among local participants, but logistic and technical difficulties prohibited the collection of sufficient data. Although biochemical verification has been judged to be less vital for minimal contact interventions that do not involve face-to-face contact,53 it is likely that the self-reported abstinence rates are somewhat inflated yet unlikely to bias the comparisons between study arms. Additionally, because of the unexpected sex difference, it would have been ideal to have included additional biopsychosocial variables (including cultural factors) to allow for a deeper exploration of its cause and for the identification of future intervention targets. Another limitation related to generalizability is that because study participants responded to recruitment efforts advertising smoking cessation materials, it is unclear how well this intervention would work if it were sent to unmotivated smokers. Finally, because of the imbalance in the number of intervention contacts, future mechanistic studies are needed to disentangle the impacts of intervention content and contact frequency.
In conclusion, a Spanish-language smoking cessation intervention was found to be efficacious and cost-effective with a stronger long-term effect for men versus women. The findings support the tailoring of interventions to Hispanics by identifying and integrating cultural values in addition to literal translation. However, the poorer long-term efficacy observed among women requires research to explain and negate this disparity. Alternative dissemination modalities (eg, mHealth) should also be tested, and they may be particularly beneficial for tailoring interventions by sex. Finally, we encourage additional research to develop and test culturally appropriate materials across the cancer continuum for the largest ethnic minority group in the United States.
Supplementary Material
FUNDING SUPPORT
This work was supported by the National Cancer Institute at the National Institutes of Health (grant R01 CA199143) and by the Florida Department of Heath James and Esther King Biomedical Research Program (grant 5JK03). This work was also supported in part by the Biostatistics and Bioinformatics Shared Resource and the Participant Research, Interventions, and Measures Core at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute–designated comprehensive cancer center (P30CA76292). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the participants and all the research and administrative staff who helped to conduct this trial.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Vani N. Simmons reports payments to her institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program. Steven K. Sutton reports payments to his institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program. Patricia Medina-Ramirez reports payments to her institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program. Ursula Martinez reports payments to her institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program. Karen O. Brandon reports payments to her institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program. Margaret M. Byrne reports payments to her institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program. Cathy D. Meade reports payments to her institution from the National Cancer Institute and the Florida Department of Health. Lauren R. Meltzer reports payments to her institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program. Thomas H. Brandon has received research support from Pfizer, Inc, and is on the advisory board of Hava Health, Inc; he also reports payments to his institution from the National Institutes of Health and the Florida Department of Health James and Esther King Biomedical Research Program, consulting fees from the University at Buffalo, payments or honoraria from Florida International University, the University of Toronto, and the Roswell Park Comprehensive Cancer Center, and participation on a board for Yale University.
This study is registered at ClinicalTrials.gov (NCT02945787).
Additional supporting information may be found in the online version of this article.
DATA AVAILABILITY
Deidentified data will be available with publication through the corresponding author after the approval of a proposal with a signed data access agreement. Only deidentified data that underlie results reported in this article may be shared with investigators who submit an approved proposal. The data may be used only for the aims stated in the approved proposal with investigator support.
REFERENCES
- 1.Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1736–1742. doi: 10.15585/mmwr.mm6946a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babb S, Malarcher A, Asman K, et al. Disparities in cessation behaviors between Hispanic and non-Hispanic White adult cigarette smokers in the United States, 2000–2015. Prev Chronic Dis. 2020;17:E10. doi: 10.5888/pcd17.190279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siahpush M, Singh GK, Jones PR, Timsina LR. Racial/ethnic and socioeconomic variations in duration of smoking: results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. J Public Health (Oxf). 2010;32:210–218. doi: 10.1093/pubmed/fdp104 [DOI] [PubMed] [Google Scholar]
- 4.Trinidad DR, Pérez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res. 2009;11:203–210. doi: 10.1093/ntr/ntn018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caraballo RS, Yee SL, Gfroerer J, Mirza SA. Adult tobacco use among racial and ethnic groups living in the United States, 2002–2005. Prev Chronic Dis. 2008;5:A78. [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan RC, Bangdiwala SI, Barnhart JM, et al. Smoking among U.S. Hispanic/Latino adults: the Hispanic Community Health Study/Study of Latinos. Am J Prev Med. 2014;46:496–506. doi: 10.1016/j.amepre.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martell BN, Garrett BE, Caraballo RS. Disparities in adult cigarette smoking—United States, 2002–2005 and 2010–2013. MMWR Morb Mortal Wkly Rep. 2016;65:753–758. doi: 10.15585/mmwr.mm6530a1 [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2018–2020. American Cancer Society; 2018. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Quitting smoking among adults—United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513–1519. [PubMed] [Google Scholar]
- 10.Babb S. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65:1457–1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 11.Trinidad DR, Perez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101:699–706. doi: 10.2105/ajph.2010.191668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbeau EM, Krieger N, Soobader MJ. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94:269–278. doi: 10.2105/ajph.94.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendzor DE, Businelle MS, Costello TJ, et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health. 2010;100:702–706. doi: 10.2105/ajph.2009.172676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheffer CE, Stitzer M, Landes R, Brackman SL, Munn T, Moore P. Socioeconomic disparities in community-based treatment of tobacco dependence. Am J Public Health. 2012;102:e8–e16. doi: 10.2105/ajph.2011.300519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racial Sohn H. and ethnic disparities in health insurance coverage: dynamics of gaining and losing coverage over the life-course. Popul Res Policy Rev. 2017;36:181–201. doi: 10.1007/s11113-016-9416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargas Bustamante A, Chen J, Rodriguez HP, Rizzo JA, Ortega AN. Use of preventive care services among Latino subgroups. Am J Prev Med. 2010;38:610–619. doi: 10.1016/j.amepre.2010.01.029 [DOI] [PubMed] [Google Scholar]
- 17.Lebrun-Harris LA, Fiore MC, Tomoyasu N, Ngo-Metzger Q. Cigarette smoking, desire to quit, and tobacco-related counseling among patients at adult health centers. Am J Public Health. 2015;105:180–188. doi: 10.2105/ajph.2013.301691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levinson A, Perez-Stable EJ, Espinoza PA, Tanchiva Flores E, Byers TE. Latinos report less use of pharmaceutical aids when trying to quit smoking. Am J Prev Med. 2004;26:105–111. doi: 10.1016/j.amepre.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Quintero C, Crum RM, Neumark YD. Racial/ethnic disparities in report of physician-provided smoking cessation advice: analysis of the 2000 National Health Interview Survey. Am J Public Health. 2006;96:2235–2239. doi: 10.2105/ajph.2005.071035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenfeld N, Schappert SM, Lin SX. Racial and ethnic differences in delivery of tobacco-cessation services. Am J Prev Med. 2009;36:21–28. doi: 10.1016/j.amepre.2008.09.028 [DOI] [PubMed] [Google Scholar]
- 21.Fiore MC, Bailey WC, Cohen SJ, et al. A clinical practice guideline for treating tobacco use and dependence—a US public health service report. JAMA. 2000;283:3244–3254. doi: 10.1001/jama.283.24.3244 [DOI] [PubMed] [Google Scholar]
- 22.Doolan DM, Froelicher ES. Efficacy of smoking cessation intervention among special populations: review of the literature from 2000 to 2005. Nurs Res. 2006;55(suppl 4):29–37. doi: 10.1097/00006199-200607001-00005 [DOI] [PubMed] [Google Scholar]
- 23.Webb MS, Rodríguez-Esquivel D, Baker EA. Smoking cessation interventions among Hispanics in the United States: a systematic review and mini meta-analysis. Am J Health Promot. 2010;25:109–118. doi: 10.4278/ajhp.090123-lit-25 [DOI] [PubMed] [Google Scholar]
- 24.Brandon TH, Simmons VN, Sutton SK, et al. Extended self-help for smoking cessation: a randomized controlled trial. Am J Prev Med. 2016;51:54–62. doi: 10.1016/j.amepre.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons VN, Sutton SK, Meltzer LR, Unrod M, Meade CD, Brandon TH. Long-term outcomes from a self-help smoking cessation randomized controlled trial. Psychol Addict Behav. 2018;32:710–714. doi: 10.1037/adb0000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piñeiro B, Díaz DR, Monsalve LM, et al. Systematic transcreation of self-help smoking cessation materials for Hispanic/Latino smokers: improving cultural relevance and acceptability. J Health Commun. 2018;23:350–359. doi: 10.1080/10810730.2018.1448487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender DE, Harbour C, Thorp J, Morris P. Tell me what you mean by “si”: perceptions of quality of prenatal care among immigrant Latina women. Qual Health Res. 2001;11:780–794. doi: 10.1177/104973230101100607 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez Esquivel D, Webb Hooper M, Baker EA, McNutt MD. Culturally specific versus standard smoking cessation messages targeting Hispanics: an experiment. Psychol Addict Behav. 2015;29:283–289. doi: 10.1037/adb0000044 [DOI] [PubMed] [Google Scholar]
- 29.Medina-Ramirez P, Sutton SK, Martinez U, et al. A randomized controlled trial of a smoking cessation self-help intervention for Spanish-speaking Hispanic/Latinx smokers: study design and baseline characteristics. Contemp Clin Trials. 2019;85:105836. doi: 10.1016/j.cct.2019.105836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina-Ramirez P, Calixte-Civil P, Meltzer LR, et al. Comparing methods of recruiting Spanish-preferring smokers in the United States: findings from a randomized controlled trial. J Med Internet Res. 2020;22:e19389. doi: 10.2196/19389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guía: Viva de Forma Más Saludable Para Usted y Su Familia, Deje de Fumar Hoy Mismo. National Cancer Institute. Publication 11–7780. http://smokefree.gov/sites/default/files/pdf/guia-para-dejar-de-fuma-2011.pdf. Accessed October 22, 2020. [Google Scholar]
- 32.Unrod M, Simmons VN, Sutton SK, et al. A randomized clinical trial of self-help intervention for smoking cessation: research design, interventions, and baseline data. Contemp Clin Trials. 2014;38:284–290. doi: 10.1016/j.cct.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becoña E, Vázquez FL. The Fagerström Test for Nicotine Dependence in a Spanish sample. Psychol Rep. 1998;83(suppl 3):1455–1458. doi: 10.2466/pr0.1998.83.3f.1455 [DOI] [PubMed] [Google Scholar]
- 34.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- 35.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e [DOI] [PubMed] [Google Scholar]
- 36.Simmons VN, Heckman BW, Ditre JW, Brandon TH. A measure of smoking abstinence–related motivational engagement: development and initial validation. Nicotine Tob Res. 2010;12:432–437. doi: 10.1093/ntr/ntq020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin G, Sabogal F, Marin BV, Otero-Sabogal R, Perez-Stable EJ. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9:183–205. doi: 10.1177/07399863870092005 [DOI] [Google Scholar]
- 38.Steidel AGL, Contreras JM. A new familism scale for use with Latino populations. Hisp J Behav Sci. 2003;25:312–330. doi: 10.1177/0739986303256912 [DOI] [Google Scholar]
- 39.Roberts RE, Atrkisson CC, Mendias RM. Assessing the Client Satisfaction Questionnaire in English and Spanish. Hisp J Behav Sci. 1984;6:385–396. doi: 10.1177/07399863840064004 [DOI] [Google Scholar]
- 40.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987. [Google Scholar]
- 41.Schafer JL. Analysis of Incomplete Multivariate Data. Chapman & Hall; 1997. Monographs on Statistics and Applied Probability; vol 72. [Google Scholar]
- 42.Ruger JP, Lazar CM. Economic evaluation of pharmaco- and behavioral therapies for smoking cessation: a critical and systematic review of empirical research. Annu Rev Public Health. 2012;33:279–305. doi: 10.1146/annurev-publhealth-031811-124553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nevid JS, Javier RA. Preliminary investigation of a culturally specific smoking cessation intervention for Hispanic smokers. Am J Health Promot. 1997;11:198–207. doi: 10.4278/0890-1171-11.3.198 [DOI] [PubMed] [Google Scholar]
- 44.Wetter DW, Mazas C, Daza P, et al. Reaching and treating Spanish-speaking smokers through the National Cancer Institute’s Cancer Information Service: a randomized controlled trial. Cancer. 2007;109(suppl 2):406–413. doi: 10.1002/cncr.22360 [DOI] [PubMed] [Google Scholar]
- 45.Woodruff SI, Talavera GA, Elder JP. Evaluation of a culturally appropriate smoking cessation intervention for Latinos. Tob Control. 2002;11:361–367. doi: 10.1136/tc.11.4.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muñoz RF, Marín BV, Posner SF, Pérez-Stable EJ. Mood management mail intervention increases abstinence rates for Spanish-speaking Latino smokers. Am J Community Psychol. 1997;25:325–343. doi: 10.1023/a:1024676626955 [DOI] [PubMed] [Google Scholar]
- 47.Leischow SJ, Hill A, Cook G. The effects of transdermal nicotine for the treatment of Hispanic smokers. Am J Health Behav. 1996;20:304–311. [Google Scholar]
- 48.Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA. Sex/gender differences in smoking cessation: a review. Prev Med. 2016;92:135–140. doi: 10.1016/j.ypmed.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffith DM, Jaeger EC, Valdez LA, Schaefer Solle N, Garcia DO, Alexander LR. Developing a “tailor-made” precision lifestyle medicine intervention for weight control among middle-aged Latino men. Ethn Dis. 2020;30(suppl 1):203–210. doi: 10.18865/ed.30.s1.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castro Y, Reitzel LR, Businelle MS, et al. Acculturation differentially predicts smoking cessation among Latino men and women. Cancer Epidemiol Biomarkers Prev. 2009;18:3468–3475. doi: 10.1158/1055-9965.epi-09-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sobralske MC. Health care seeking among Mexican American men. J Transcult Nurs. 2006;17:129–138. doi: 10.1177/1043659606286767 [DOI] [PubMed] [Google Scholar]
- 52.Velásquez RJ, Burton MP. Psychotherapy of Chicano men. In: Velásquez RJ, Arellano LM, McNeill BW, eds. The Handbook of Chicana/o Psychology and Mental Health. Lawrence Erlbaum Associates Publishers; 2004:177–192. [Google Scholar]
- 53.Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. 2019;22:1086–1097. doi: 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data will be available with publication through the corresponding author after the approval of a proposal with a signed data access agreement. Only deidentified data that underlie results reported in this article may be shared with investigators who submit an approved proposal. The data may be used only for the aims stated in the approved proposal with investigator support.


