Summary
The diagnostic criteria for paediatric mastocytosis are largely based on adult studies and bone marrow findings are not well described in children. We evaluated use of the World Health Organization (WHO) criteria for the diagnosis of systemic disease in paediatric mastocytosis. In addition, we identified unique clinico-histopathological features within the biopsies. One hundred and thirteen children with paediatric mastocytosis were evaluated at the National Institutes of Health between 1986 and 2013. Complete bone marrow evaluations were performed in 50 cases. Seven children had repeat procedures. Bone marrows were analysed by histopathology, flow cytometry and for KIT D816V. Bone marrow biopsies displayed mild atypical haematopoietic maturation, increased haematogones and hypocellularity in a sub-set of patients with urticaria pigmentosa, diffuse cutaneous mastocytosis and indolent systemic mastocytosis. Hypocellularity was most pronounced in those with urticaria pigmentosa. Haematogones were highest, on average, in patients with diffuse cutaneous mastocytosis or mastocytomas. There was no evidence of peripheral blood cytopenias, myelodysplastic syndrome, myeloproliferative neoplasm or leukaemia within this cohort. The WHO criteria are applicable for the diagnosis of systemic mastocytosis in paediatrics. Although unsuspected bone marrow findings typically seen in myeloproliferative disorders are frequent in paediatric mastocytosis, patients within this study remained clinically stable without progression to a more aggressive variant.
Keywords: Paediatrics, Mastocytosis, Childhood, Bone marrow, Myeloproliferative
Introduction
Paediatric mastocytosis presents with cutaneous manifestations of mast cell proliferation in about 85% of cases. (Lange, et al 2013, Middelkamp Hup, et al 2002, Valent, et al 2004) Previous studies have focused predominantly on cutaneous and symptomatic manifestations and skin pathology.(Akoglu, et al 2006, Ben-Amitai, et al 2005, Bodemer, et al 2010, Kiszewski, et al 2004, Muller, et al 1990, Wolff, et al 2001) Bone marrow biopsies are not routinely performed. In the only study where a significant number of bone marrow biopsies were obtained (n=41), a complete pathological analysis was not reported.(Azana, et al 1994) In that study, only six of 41 patients who underwent a procedure were reported to have slight abnormalities on the bone marrow aspirate smears. There has been limited information published with regard to marrow characteristics of paediatric mastocytosis across all variants.
The World Health Organization (WHO) criteria for the diagnosis of systemic disease are based largely on data from adult patients with mastocytosis.(Horny, et al 2008) The WHO classifications and other published studies include extensive descriptions of the pathology seen in adult marrows with systemic disease.(Horny, et al 2008) An important issue not previously addressed in the literature is the implication of bone marrow pathology as it relates to overall prognosis of paediatric disease. Adult patients with systemic disease have an abnormal expansion of a mast cell clone typically carrying a point mutation in codon 816 of the KIT gene.(Horny, et al 2008) Although this mutation has also been identified in skin biopsies, there has been no correlative bone marrow data to support the diagnosis of systemic disease from paediatric patients with mastocytosis.(Bodemer, et al 2010) Furthermore, the prognostic impact of this mutation in children with mastocytosis is unknown.
We report in detail on the bone marrow pathology in 50 patients with paediatric-onset mastocytosis. The WHO criteria for the diagnosis of systemic disease are comparable to published data on adult mastocytosis bone marrow pathology (Valent et al 2004). However, previously unrecognized findings of mild atypia, increased haematogones and hypocellarity that may be confused with a myeloproliferative disorder are highlighted. In addition, we have followed a subset of patients clinically for follow-up over an average of 6.9 years (range 1–25 years) to assess for possible progression of bone marrow findings and disease severity.
Methods
Patients
One hundred and thirteen patients aged 6 months to 20 years with a diagnosis of cutaneous mastocytosis were enrolled in institutional Review Board (IRB)-approved protocols (03-I-0041, 80-I-33) at the National Institutes of Health (NIH) following informed consent. The population consisted of 70 males (62%) and 43 females (38%). The diagnostic evaluation included a full blood count (FBC) and differential and serum tryptase. Patients with hepatosplenomegaly on physical examination underwent abdominal ultrasound for verification. Fifty patients with severe mast cell mediator symptoms and/or hepatosplenomegaly underwent a bone marrow biopsy and aspirate based on existing recommendations. (Valent, et al 2007, Valent, et al 2004) The procedures were performed 6 months to 18 years after the onset of cutaneous mastocytosis with a mean time of 3.6, 3.2, 4.6 and 7.1 years for urticaria pigmentosa (UP), diffuse cutaneous mastocytosis (DCM), mastocytoma (MTOMA) and indolent systemic mastocytosis (ISM), respectively. Seven patients had repeat bone marrows for continued severe mediator symptoms or to follow-up as clinically indicated at a mean follow-up time of 7 years (range 2–18 years). Patients were then categorized as to disease variant based on WHO criteria.(Horny, et al 2008)
Serum tryptase
Total serum tryptase was determined on blood samples drawn during routine visits (n=45). None were obtained during general systemic reactions. Samples were assayed using the commercial fluoroenzyme Immunoassay (Pharmacia Immuno CAP, Peapack, NJ; normal reference range 0.0 – 11.5 ng/ml, highest limit of detection 500.0 ng/ml, Mayo Clinic Laboratories, Rochester, MN).
Bone marrow preparation and analysis
Bone marrow trephine biopsies were fixed either in B-5 fixative (original specimens) or B-Plus fixative (follow-up specimens), embedded in paraffin and processed for morphological evaluation using standard procedures.(Maric, et al 2007) Immunohistochemical studies with anti-tryptase, CD117 (also termed KIT), CD10 (also termed MME) (Cell Marque, Hot Springs, AR) and CD25 antibodies (Vision BioSystems, Norwell, MA) were performed using immunoperoxidase staining procedures on an automated immunostainer (Ventana Medical System, Tucson, AZ) according to the manufacturer’s instructions. Bone marrow biopsies were evaluated in a blinded fashion by two haematopathologists/haematologists. Bone marrow mast cell involvement and morphology were estimated in core biopsies based on CD117 and on tryptase immunostaining, which provided similar estimates. Images were obtained via digital microscopy using an Olympus BX-51 microscope (Olympus America, Center Valley, PA) equipped with a DPlan 40/0.65numeric aperture objective and captured using an Olympus DP70 digital camera system. Imaging software was Adobe Photoshop CS3 (Adobe Systems, San Jose, CA). Multi-parameter flow cytometry was performed on bone marrow aspirates and mast cells were assessed for expression of CD2 and CD25 (also termed IL2RA), as described.(Maric, et al 2007) Detection of the KIT D816V mutation was performed by reverse transcription polymerase chain reaction (RT-PCR)/restriction fragment length polymorphism (RFLP). The targeted gene area was amplified and the PCR products were subjected to restriction enzymes in order to obtain fragments for analysis as described.(Maric, et al 2007)
Statistical Analysis
The peripheral blood indices and bone marrow pathology were analysed using a Kruskal-Wallis non-parametric analysis of variance (ANOVA) using multiple comparisons between groups. P-values <0.05 were considered statistically significant. GraphPad Prism software (GraphPad Software Inc., La Jolla, CA) was used for data analysis and graphic illustrations.
Results
Fifty patients with paediatric-onset cutaneous mastocytosis were evaluated and subsequently classified based on the WHO criteria for mast cell disease (Horny et al 2008) (Table I). Twenty (40%) children were diagnosed with UP, 7 (14%) had DCM, 2 (4%) had a MTOMA and 21 (42%) had ISM. The onset of disease ranged from birth to 12 years of age, but in the majority of patients (87%), was in the first year of life. Ninety-eight percent of the patients were Caucasian. Most patients, with the exception of MTOMA, had early disease onset (birth-5years) with a slight male predominance (64%).
Table I:
Patient Characteristics
| Variant | Total | Males/Females | Age of Onset | Ethnicity |
|---|---|---|---|---|
| UP | 20 | 13/7 | Birth-5 years | C(19), AA(1) |
| DCM | 7 | 5/2 | Birth-4 months | C(7) |
| MTOMA | 2 | 2/0 | 3 months-12 years | C(2) |
| ISM | 21 | 12/9 | Birth-5 years | C (21) |
UP-urticaria pigmentosa, DCM-diffuse cutaneous mastocytosis, MTOMA-mastocytoma, ISM-indolent systemic mastocytosis, C-Caucasian, AA-African American
Bone Marrow Pathology
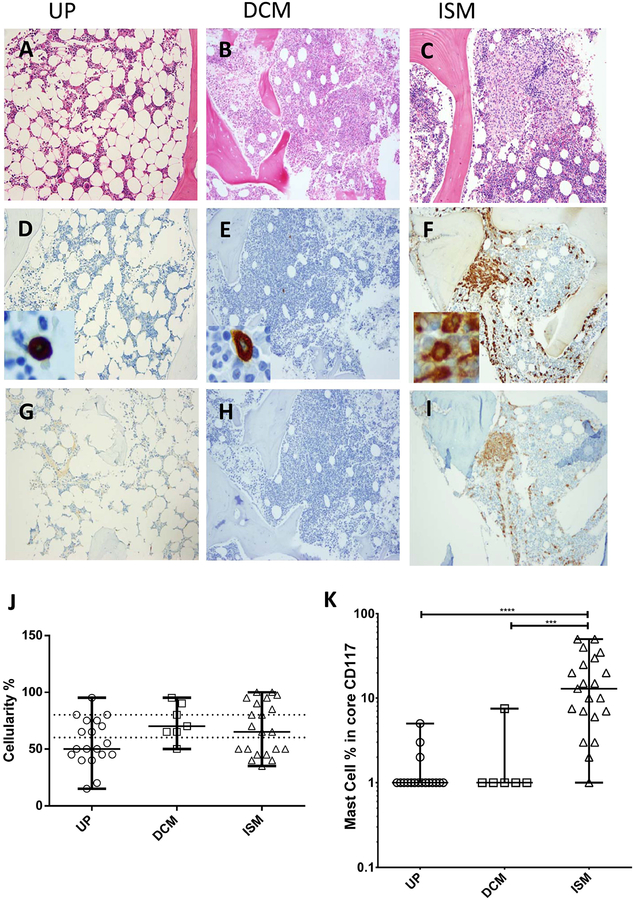
Typical bone marrow pathology findings in UP, DCM and ISM, respectively are illustrated in Figures 1A–C. In examining marrow biopsies, our first observation was a relative marrow hypocellularity in a subset of patients compared to the expected marrow cellularity for age.(Horny, et al 2010) When compared to normal paediatric bone marrows,(Foucar et al 2010) the bone marrow cellularity demonstrated a wide range. The patients with UP (Figure 1A & J) had the lowest median bone marrow cellularity and the highest percentage of marrows in the hypocellular range (58%). On average, marrows in patients with DCM and ISM were less hypocellular than in UP patients, however, a significant percentage of patients (42.9 % in ISM and 14.3% in DCM) were still in the hypocellular range, (Figure 1J, Table IIA). On the other hand, hypercellularity was more prevalent in DCM (28.6%) and ISM (33.3%) compared to UP (5%).
Figure 1: Bone marrow histopathology.
Histological analysis was performed using haematoxylin and eosin (H&E), tryptase and CD25 immunohistochemistry stains (100×) for UP (A,D,G), DCM (B,E,H), and ISM (C,F,I). Insets in D-F represent mast cell morphology in each variant (500×). Abnormal mast cells seen in ISM are illustrated by spindle morphology and positive CD25 stain. J & K: Graphical demonstration of bone marrow cellularity and mast cell burden in percentage, numerical and log scales (UP vs ISM P<0.0001 & DCM vs ISM P=0.0002), respectively. The dotted lines represent the normal paediatric range. Horizontal bars represent the median and range.
UP-urticaria pigmentosa DCM-diffuse cutaneous mastocytosis; ISM-indolent systemic mastocytosis
Table IIA:
Bone marrow mast cell findings & World Health Organization mastocytosis diagnostic criteria
| Variant | Total | Cellularity %, median (range) | MCs biopsy %, median (range) | MC aggregate (>15 cells) | Spindle-shaped MCs (>25%) | CD25+ MCs | Serum tryptase ng/ml, median (range) | KIT D816V mutation |
|---|---|---|---|---|---|---|---|---|
| UP | 20 | 50 (15–80) | 1 (1–5) | 0/19* | 0/20 | 0/20 | 6.07 (3.1–99) | 0/8‡ |
| DCM | 7 | 70 (50–95) | 1 (1–7.5) | 0/7 | 0/7 | 0/7 | 61.3 (23.4–167) | 0/7 |
| MTOMA | 2 | 60 60 | 1.5 (1–2) | 0/2 | 0/2 | 0/2 | 12.85 (5.1–20.6) | 0/2 |
| ISM | 21 | 65 (35–100) | 13 (1–50) | 19†/21 | 20≠/21 | 21/21 | 73.6 (15.6–440) | 19‡/21 |
UP-urticaria pigmentosa, DCM-diffuse cutaneous mastocytosis, MTOMA-mastocytoma, ISM-indolent systemic mastocytosis, MC-mast cell
marrow biopsy inadequate
3 minor criteria for the diagnosis of SM
round mast cells,
KIT analysis not available for 2 ISM marrows & 12 UP marrows prior to assay availability
The highest percentage of bone marrow mast cells was observed in patients with ISM compared to UP (p<0.0001) and DCM (p<0.001) (Figures 1F & 1K).
Mast cell aggregates consisting of greater than 15 mast cells (major WHO criterion for the diagnosis of ISM) were seen in all but two patients with ISM. These two exceptions met three minor WHO criteria for the diagnosis of ISM (over 25% spindle-shaped mast cells on the aspirate smear, CD25/CD2 positive mast cells, KIT D816V mutation, or a serum tryptase of ≥ 20 ng/ml). Only one patient with DCM had mild-moderate increased bone marrow mast cells and none had mast cell aggregates (Figure 1E, K). Spindle-shaped mast cells (over 25%) were not observed in any other variants of paediatric mastocytosis; DCM, MTOMA and UP (Figs. 1D, E, F). All but one patient with ISM showed over 25% of spindle shaped mast cells on the marrow biopsy specimen.
CD25-positive mast cells were present only in patients with ISM as assessed by flow cytometry (data not shown) and/or immunohistochemical analysis (Figures 1 G, H, I). All tested ISM patients (19/19) carried the KIT D816V mutation in marrow samples, while this mutation was not detected in the marrows of other patients available for testing (n=17). DCM and ISM patients had the highest serum tryptase values (Table II A). Thus, the current WHO criteria for the diagnosis of systemic mastocytosis were applicable to paediatric disease.
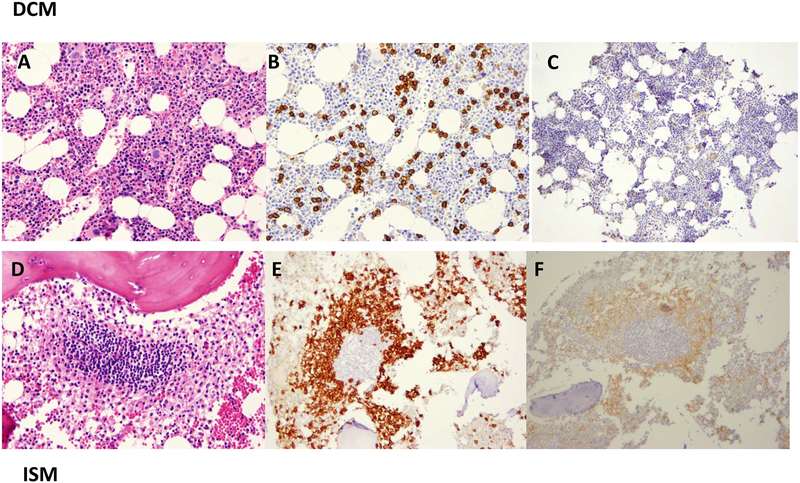
A few cases did not fully conform to a specific variant based on WHO diagnostic criteria. One patient with DCM, no organomegaly and an elevated serum tryptase of 126 ng/ml had an increased number of round mast cells in the bone marrow (7.5%), but no mast cell aggregates, spindle-shaped mast cells or CD25/CD2 positive mast cells (Figures 2A–C). This patient did not meet criteria for systemic disease despite the significant mast cell burden, yet was also distinctly different from the other patients with DCM, all of whom had lower marrow mast cell numbers and a range of tryptase values (Figures 1 B & E; Table IIA). Another patient with ISM had orgnomegaly, exclusively round mast cell morphology, with a tryptase of 184 ng/ml, mast cell aggregates and CD25-positive mast cells on the bone marrow biopsy specimen (Figures 2D–F). Clinically, there were no other apparent differences in comparison to the rest of those with ISM. These two cases illustrate that not all patients exhibit standard diagnostic features and suggest that a more extensive genomic analysis may be useful in paediatric-onset mastocytosis.
Figure 2: Atypical bone marrow histopathology.
DCM is represented in panels A-C using haematoxylin and eosin (H&E) (200×), tryptase (200×) and CD25 immunohistochemistry (100×) staining showing an unusual increase in round mast burden, no aggregates of >15 mast cells, and negativity for CD25. The ISM histopathology is unusual with regard to the mast cells seen using tryptase staining because there are no spindle-shaped mast cells (2E) and CD25 is positive (2F).
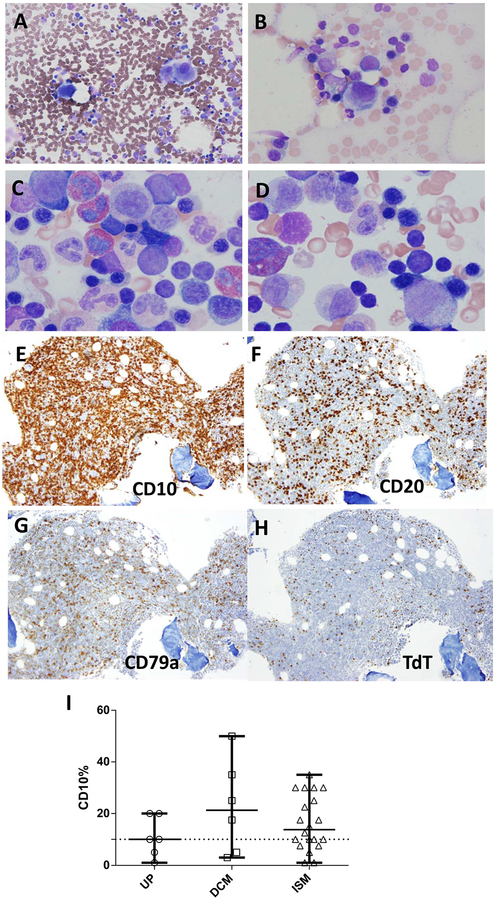
Evaluation of trilineage haematopoiesis in bone marrow aspirates and biopsies revealed atypical maturational features in a subset of patients with both systemic and cutaneous variants of mastocytosis. The aspirate smears showed more than 10% hypolobular megakaryocytes in 5/20 UP, 4/7 DCM, 1/2 MTOMA and 5/19 ISM patients (Table IIB; Fig. 3 A, B). Erythyroid and/or myeloid atypia, particularly megaloblastoid changes, were seen in 8/20 UP, 1/7 DCM and 3/19 ISM patients (Table IIB; Figures 3 C, D). None of these patients had peripheral blood cytopenias at the time of our evaluation or increased marrow blast counts (≥ 5%). Mild marrow eosinophilia (≥ 5%) was seen in 12/20 UP, 5/7 DCM, 1/2 MTOMA and 13/21 ISM patients (Table IIB); however, only two patients had absolute peripheral blood eosinophilia (normal < 0.85 ×109/l). Patients with MTOMA and DCM had higher median absolute eosinophil counts (AEC) compared to patients with UP and ISM (Supplemental Figure). However, there was significant overlap in AEC between all mastocytosis variants and this feature alone did not distinguish cutaneous mastocytosis from systemic disease.
Table IIB:
Bone marrow findings
| Variant | Total | Eosinophils increased (>5%) | Megakaryocytes atypia | Blasts elevated (≥5%) | Myeloid atypia | Erythroid atypia | CD10 %, median (range) |
|---|---|---|---|---|---|---|---|
| UP | 20 | 12/20 | 5/20 | 0/20 | 1/20 | 8/20 | 10 (1–20) |
| DCM | 7 | 5/7 | 4/7 | 1/7 | 1/7 | 1/7 | 21.2 (3–50) |
| MTOMA | 2 | 1/2 | 1/2 | 0/2 | 0/2 | 0/2 | 22.5 (15–30) |
| ISM | 21 | 13/21 | 5/19§ | 0/19§ | 2/19§ | 3/19§ | 14 (1–35) |
UP-urticaria pigmentosa, DCM-diffuse cutaneous mastocytosis, MTOMA-mastocytoma, ISM-indolent systemic mastocytosis, MC-mast cell
Not available for 2 outside marrow aspirates
Figure 3: Atypical Haematopoesis and Increased Haematogones.
Panels A-B (500×) demonstrates the megakaryocytic monolobation that was typically seen and shown here in patients with UP and DCM. Panels C-D (1000×) show megaloblastoid changes in erythroid precursors and nuclear/cytoplasmic maturation asynchrony in myeloid precursors using Wright-Giemsa in patients with ISM. Panels E-F illustrate an example of markedly increased CD10 positive B-cell precursor lymphocytes (haematogones) in a 17-month-old child with DCM using immunohistochemical stains for (E) CD10, (F) CD20, (G) CD79a, & (H) TdT (100×). Increased haematogones (above 10% of total marrow cells) were seen in the majority of ISM and DCM patients (Panel I).
Bone marrow aspirates revealed increased numbers of normal haematagones (CD10 positive immature B-cells) in all variants of mastocytosis, but peripheral blood lymphocytosis was observed in only one patient with ISM. Patients with UP had the lowest median percentage of haematogones (10%), followed by ISM (14%), DCM (21%) and MTOMA patients (22%). In some patients with ISM, DCM and MTOMA, the CD10-positive lymphocyte percentage approached 50% of total marrow cells (Table IIB; Fig 3 E), which raised concerns in the differential diagnosis for acute lymphoblastic leukaemia. Flow cytometric analysis, where available, and further immunohistochemical staining confirmed that these immature lymphocytes were normal precursor B-cells (haematogones) (Figure 3 E–H). The increase in haematogones did not correlate with mast cell burden in the marrow, peripheral blood lymphocytosis (data not shown) or the disease variant.
Peripheral Blood Findings
The majority of patients had normal FBCs. Peripheral blood indices, including haemoglobin, neutrophils, lymphocytes, eosinophils and platelets were not significantly different among the mastocytosis variants (supplemental Figure 1 A–E). One patient with UP and two with DCM had low haemoglobin levels that were consistent with iron deficiency anaemia. Thrombocytopenia was not present in any patient. Thrombocytosis was present in a subset of patients with UP, DCM and ISM (Supplemental Figure E), but this finding did not correlate with abnormal bone marrow findings described in Table IIB or other unrelated diagnoses. Follow-up FBCs in patients with longitudinal data, with the exception of one, showed that the platelet elevations normalized over years (Supplemental Figure F).
Patient Follow-Up
Twenty-one patients with ISM and documentation of a bone marrow mast cell clone by aberrant CD25 expression and/or detection of KIT codon 816 mutation, have shown no clinical evidence of progression or second malignancy with a mean follow-up of 6.9 years (range 1–25 years). Repeat marrow biopsies were performed in 4 patients with ISM (Table III) and there was no evidence of histopathological change or disease evolution. Similarly, no progression was noted in follow-up marrow biopsies of 2 patients with UP and one patient with DCM (Table III). As a group, the patients in this study have all improved or disease has remained stable from both a clinical and laboratory perspective. Myelodysplastic syndrome, myeloproliferative neoplasm nor leukaemia was seen in this cohort.
Table III:
Follow-up bone marrow findings & World Health Organization mastocytosis diagnostic criteria
| Variant | Δ years | Cellularity | MC aggregates >15 cells | CD117+ MCs %† | CD25+ MCs | Spindle-shaped MCs >25% | KIT D816V mutation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UP | 6 | 60 | 50 | − | − | 1 | 1 | − | − | − | − | − | − |
| UP | 18 | 45 | 40 | small* | small* | 5 | 5 | N/A | − | − | − | N/A | − |
| DCM | 17 | 100 | 70 | − | − | 15 | 7.5 | N/A | − | − | − | − | − |
| ISM | 2 | 40 | 40 | + | + | 5 | 3 | + | + | + | + | + | + |
| ISM | 4 | 100 | 95 | + | + | 50 | 30 | + | + | + | + | + | + |
| ISM | 7 | 95 | 95 | + | + | 15 | 40 | + | + | + | + | N/A | + |
| ISM | 18 | 45 | 45 | + | + | 25 | 15 | N/A | + | + | + | N/A | + |
UP-urticaria pigmentosa, DCM-diffuse cutaneous mastocytosis, MTOMA-mastocytoma, ISM-indolent systemic mastocytosis, MC-mast cell, NA-not available,
small-<15 MCs per aggregate,
Estimates were comparable to tryptase staining
Discussion
We have reviewed 50 bone marrow biopsies from a cohort of 113 patients with paediatric-onset mastocytosis. Current criteria for the diagnosis of systemic mastocytosis are based on findings in adults. We have described distinct features not previously noted and have shown that the current WHO criteria are applicable for the diagnosis of systemic disease in the paediatric population.
The majority of patients with paediatric mastocytosis have a cutaneous variant, (Lange, et al 2013, Middelkamp Hup, et al 2002, Valent, et al 2004) which is supported by our study, where 81.4% of the total cohort was diagnosed as having UP, DCM, or MTOMA. The ethnic distribution of this series is reflective of what has been previously reported with 98% Caucasian patients (Table I). Patients with UP, DCM and MTOMA variants of cutaneous disease were noted to have a normal to minimally increased bone marrow mast cell burden. Only one patient with DCM was divergent from this pattern, showing a more significant increase in mast cells; however, these mast cells were morphologically and immunophenotypically normal. KIT D816V mutations were not detected in DCM. Other cutaneous variants were also uniformly negative for three of the four minor WHO criteria for the diagnosis of systemic mastocytosis, with the exception of elevated tryptase values.
There has been limited information about bone marrow histopathology in children with cutaneous variants of mastocytosis. Azana et al (1994) reported results of bone marrow aspirates without corresponding biopsies in 41 children with cutaneous mast cell disease. In that study, marrow aspirates demonstrated minor findings in 6 of 41 specimens consisting of an increase in eosinophils in 3 patients, an increase in lymphocytes in 2, a decrease in erythroid elements in 2 and an increase in mast cells in one. Our study includes more detailed analysis of bone marrow and peripheral blood findings in a larger series of children with paediatric mastocytosis variants. In addition, follow-up marrow biopsies were performed when indicated and these remained stable or improved in all 7 cases and clinical improvement in 96% of patients.
Unexpectedly, a substantial subset (44.7 %) of core bone marrow biopsies was hypocellular for age, with UP having the lowest average cellularity. Slight atypia in haematopoietic maturation was noted in the aspirate smears, although no cases fulfilled the criteria for myelodysplasia. Hypolobulated megakaryocytes were particularly prominent, followed by megaloblastoid and erythroid maturation. All patient categories showed significant increase in immature precursor B-lymphocytes (haematogones), (McKenna, et al 2001) in some cases comprising up to 50% of marrow cellular elements. This finding appears to be reactive in nature since it was seen in patients with and without systemic mast cell disease.
In all patients with systemic disease and documentation of a bone marrow mast cell clone by the aberrant expression of CD25 receptor on mast cells and/or detection of a point mutation in codon 816 of KIT, there has been no clinical evidence of progression or second malignancy with a mean follow-up period of 6.9 years (range 1–25 years). In the seven patients who underwent repeat bone marrow biopsies, all remained stable or improved with conservative therapy (follow-up range 2–18 years). Two patients in our series with ISM and tryptase levels of 440 and 328 ng/ml required aggressive therapy, both at age 2 years. The first had cytopenias and severe mast cell mediator symptoms and was treated with interferon-alpha for 5 years. The second had failure to thrive and was treated with oral corticosteroids for 5 years. Both patients had massive hepatosplenomegaly. Repeat marrows, 10 and 7 years after therapy was initiated, reflected an improved mast cell burden without progression of other myeloproliferative markers. In addition, the patient with the initial serum tryptase of 440 ng/ml had a third marrow procedure 4 years later with continued improvement. A recent study in patients > 15 years reported myeloproliferative disorders in th patient population of mainly adults (Cohen, et al 2014). There are, however, paediatric case reports of myeloproliferative diseases associated with systemic mastocytosis (n=7),(Dror, et al 2000, Gadage, et al 2012, Intzes, et al 2011, Kanegane, et al 2009, Lee, et al 2007, Mahadeo, et al 2011) and one child with an apparent transition from cutaneous to systemic disease.(Chantorn and Shwayder 2012)
In our patient cohort included a few rare variants of both cutaneous and systemic disease. Most of the patients with DCM had a low bone marrow mast cell burden, with one exception (noted in figure 3 A–C) showing an increased bone marrow mast cell burden and persistence of disease into adulthood. Another patient with a lesser degree of bone marrow mast cell involvement demonstrated complete resolution by late adolescence. The patient with ISM and round rather than spindle-shaped clonal mast cells on bone marrow biopsy specimen (figure 3 D–F) has had a fairly typical disease course but may fall into a sub-variant of well-differentiated systemic disease noted by Morgado et al.(2013) The patient remains clinically stable but whether the long-term prognosis parallels the patients with typical ISM is unknown.
Peripheral blood counts were normal in almost all cases. A slight elevation of lymphocytes or platelets was observed in occasional patients, although there was no evidence of a clonal process and no associated clinical consequences that required intervention. These minor elevations in peripheral blood indices, particularly in the case of platelets, appeared to be reactive events in our patient population. This is demonstrated by the gradual decline to normal range during the follow-up period without targeted therapy in all except one patient with ISM (Supplemental Figure).
This study elucidates bone marrow histopathology not previously reported in paediatric mastocytosis. The observation of hypocellularity and dyspoiesis are of potential concern, although these do not appear to be associated with progressive marrow dysfunction or evolution to more aggressive mast cell disorders. In addition, patients who were followed clinically for up to 25 years have remained stable or have improved. While the diagnosis of systemic disease does not alter therapy in the absence of specific manifestations or clinical compromise, this does impact outlook. Thus, although a bone marrow evaluation will not necessarily lead to a change in therapy, findings may be of prognostic value. Furthermore, bone marrows should be performed if a severe haematological condition, such as myelodysplastic syndrome or malignancy is suspected. In summary, clinically unsuspected bone marrow findings are frequent in paediatric-onset mastocytosis, which merits evaluation and, in selected individuals, follow-up monitoring.
Supplementary Material
Acknowledgements:
We gratefully acknowledge the study participants and their families, referring medical care teams and the faculty and staff of the NIH.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, Center for Cancer Research and Clinical Center, NIH.
Supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, the National Cancer Institute and the Warren Grant Magnuson Clinical Center, National Institutes of Health
Footnotes
Disclosure of Conflicts of Interest:
No conflict of interest.
References
- Akoglu G, Erkin G, Cakir B, Boztepe G, Sahin S, Karaduman A, Atakan N, Akan T & Kolemen F (2006) Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol, 20, 969–973. [DOI] [PubMed] [Google Scholar]
- Azana JM, Torrelo A, Mediero IG & Zambrano A (1994) Urticaria pigmentosa: a review of 67 pediatric cases. Pediatr Dermatol, 11, 102–106. [DOI] [PubMed] [Google Scholar]
- Ben-Amitai D, Metzker A & Cohen HA (2005) Pediatric cutaneous mastocytosis: a review of 180 patients. Isr Med Assoc J, 7, 320–322. [PubMed] [Google Scholar]
- Bodemer C, Hermine O, Palmerini F, Yang Y, Grandpeix-Guyodo C, Leventhal PS, Hadj-Rabia S, Nasca L, Georgin-Lavialle S, Cohen-Akenine A, Launay JM, Barete S, Feger F, Arock M, Catteau B, Sans B, Stalder JF, Skowron F, Thomas L, Lorette G, Plantin P, Bordigoni P, Lortholary O, de Prost Y, Moussy A, Sobol H & Dubreuil P (2010) Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol, 130, 804–815. [DOI] [PubMed] [Google Scholar]
- Chantorn R & Shwayder T (2012) Death from mast cell leukemia: a young patient with longstanding cutaneous mastocytosis evolving into fatal mast cell leukemia. Pediatr Dermatol, 29, 605–609. [DOI] [PubMed] [Google Scholar]
- Cohen SS, Skovbo S, Vestergaard H, Kristensen T, Moller M, Bindslev-Jensen C, Fryzek JP & Broesby-Olsen S (2014) Epidemiology of systemic mastocytosis in Denmark. Br J Haematol, 166, 521–528. [DOI] [PubMed] [Google Scholar]
- Dror Y, Leaker M, Caruana G, Bernstein A & Freedman MH (2000) Mastocytosis cells bearing a c-kit activating point mutation are characterized by hypersensitivity to stem cell factor and increased apoptosis. Br J Haematol, 108, 729–736. [DOI] [PubMed] [Google Scholar]
- Foucar K, Richard K, Czuchlewski D, (2010) Bone Marrow Pathology: ASCP Integrative Hematopathology. ASCP Press, Chicago. [Google Scholar]
- Gadage VS, Kadam Amare PS, Galani KS & Mittal N (2012) Systemic mastocytosis with associated acute myeloid leukemia with t (8; 21) (q22; q22). Indian J Pathol Microbiol, 55, 409–412. [DOI] [PubMed] [Google Scholar]
- Horny HP, Akin C, Metcalfe DD, Escribano L, Bennett JM & Valent P (2008) Mastocytosis. In: World Health Organization (WHO) Classification of Tumours. Pathology & Genetics: Tumours of Haematopoietic and Lymphoid Tissues (ed. by Swerdlow S, Campo E Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW), pp. 54–63. IARC Press, Lyon, France. [Google Scholar]
- Horny HP, Sotlar K & Valent P (2010) Differential diagnoses of systemic mastocytosis in routinely processed bone marrow biopsy specimens: a review. Pathobiology, 77, 169–180. [DOI] [PubMed] [Google Scholar]
- Intzes S, Wiersma S & Meyerson HJ (2011) Myelomastocytic Leukemia With t(8;21) in a 3-year-old Child. J Pediatr Hematol Oncol, 33, e372–375. [DOI] [PubMed] [Google Scholar]
- Kanegane H, Nomura K, Abe A, Makino T, Ishizawa S, Shimizu T, Naoe T & Miyawaki T (2009) Spontaneous regression of aleukemic leukemia cutis harboring a NPM/RARA fusion gene in an infant with cutaneous mastocytosis. Int J Hematol, 89, 86–90. [DOI] [PubMed] [Google Scholar]
- Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, Gutierrez-Castrellon P & Ruiz-Maldonado R (2004) Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol, 18, 285–290. [DOI] [PubMed] [Google Scholar]
- Lange M, Niedoszytko M, Renke J, Glen J & Nedoszytko B (2013) Clinical aspects of paediatric mastocytosis: a review of 101 cases. J Eur Acad Dermatol Venereol, 27, 97–102. [DOI] [PubMed] [Google Scholar]
- Lee JW, Yang WS, Chung SY, Kang JH, Cho B, Kim HK, Kim KM & Jeong DC (2007) Aggressive systemic mastocytosis after germ cell tumor of the ovary: C-KIT mutation documentation in both disease states. J Pediatr Hematol Oncol, 29, 412–415. [DOI] [PubMed] [Google Scholar]
- Mahadeo KM, Wolgast L, McMahon C & Cole PD (2011) Systemic mastocytosis in a child with t(8;21) acute myeloid leukemia. Pediatr Blood Cancer, 57, 684–687. [DOI] [PubMed] [Google Scholar]
- Maric I, Robyn J, Metcalfe DD, Fay MP, Carter M, Wilson T, Fu W, Stoddard J, Scott L, Hartsell M, Kirshenbaum A, Akin C, Nutman TB, Noel P & Klion AD (2007) KIT D816V-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRA-associated chronic eosinophilic leukemia are distinct entities. J Allergy Clin Immunol, 120, 680–687. [DOI] [PubMed] [Google Scholar]
- McKenna RW, Washington LT, Aquino DB, Picker LJ & Kroft SH (2001) Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood, 98, 2498–2507. [DOI] [PubMed] [Google Scholar]
- Middelkamp Hup MA, Heide R, Tank B, Mulder PG & Oranje AP (2002) Comparison of mastocytosis with onset in children and adults. J Eur Acad Dermatol Venereol, 16, 115–120. [DOI] [PubMed] [Google Scholar]
- Morgado JM, Perbellini O, Johnson RC, Teodosio C, Matito A, Alvarez-Twose I, Bonadonna P, Zamo A, Jara-Acevedo M, Mayado A, Garcia-Montero A, Mollejo M, George TI, Zanotti R, Orfao A, Escribano L & Sanchez-Munoz L (2013) CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology, 63, 780–787. [DOI] [PubMed] [Google Scholar]
- Muller U, Helbling A, Hunziker T, Wuthrich B, Pecoud A, Gilardi S, Beretta E, Fasel J, Messerli W & Maurer P (1990) Mastocytosis and atopy: a study of 33 patients with urticaria pigmentosa. Allergy, 45, 597–603. [DOI] [PubMed] [Google Scholar]
- Valent P, Sperr WR, Schwartz LB & Horny HP (2004) Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol, 114, 3–11; quiz 12. [DOI] [PubMed] [Google Scholar]
- Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, Castells M, Sperr WR, Kluin-Nelemans HC, Hamdy NA, Lortholary O, Robyn J, van Doormaal J, Sotlar K, Hauswirth AW, Arock M, Hermine O, Hellmann A, Triggiani M, Niedoszytko M, Schwartz LB, Orfao A, Horny HP & Metcalfe DD (2007) Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest, 37, 435–453. [DOI] [PubMed] [Google Scholar]
- Wolff K, Komar M & Petzelbauer P (2001) Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res, 25, 519–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.