Abstract
BACKGROUND
Anti-N-methyl-D-aspartate receptor encephalitis (NMDARe) is capable of presenting a relapsing course and coexisting with myelin oligodendrocyte glycoprotein antibody disease, whereas it has been relatively rare. We describe a man with no history of tumor who successively developed anti-NMDARe and anti-myelin oligodendrocyte glycoprotein antibody disease.
CASE SUMMARY
A 29-year-old man was initially admitted with headache, fever, intermittent abnormal behavior, decreased intelligence, limb twitching and loss of consciousness on July 16, 2018. On admission, examination reported no abnormality. During his presentation, he experienced aggravated symptoms, and the re-examination of cranial magnetic resonance imaging (MRI) indicated punctate abnormal signals in the left parietal lobe. External examination of cerebrospinal fluid and serum results revealed serum NMDAR antibody (Ab) (-), cerebrospinal fluid NMDAR-Ab (+) 1:10 and Epstein-Barr virus capsid antigen antibody IgG (+). Due to the imaging findings, anti-NMDARe was our primary consideration. The patient was treated with methylprednisolone and gamma globulin pulse therapy, mannitol injection dehydration to reduce intracranial pressure, sodium valproate sustained-release tablets for anti-epilepsy and olanzapine and risperidone to mitigate psychiatric symptoms. The patient was admitted to the hospital for the second time for “abnormal mental behavior and increased limb movements” on December 14, 2018. Re-examination of electroencephalography and cranial MRI showed no abnormality. The results of autoimmune encephalitis antibody revealed that serum NMDAR-Ab was weakly positive and cerebrospinal fluid NMDAR-Ab was positive. Considering comprehensive recurrent anti-NMDARe, the patient was treated with propylene-hormone pulse combined with immunosuppressive agents (mycophenolate mofetil), and the symptoms were relieved. The patient was admitted for “hoarseness and double vision” for the third time on August 23, 2019. Re-examination of cranial MRI showed abnormal signals in the medulla oblongata and right frontal lobe, and synoptophore examination indicated concomitant esotropia. The patient’s visual acuity further decreased, and the re-examination of cranial MRI + enhancement reported multiple scattered speckled and patchy abnormal signals in the medulla oblongata, left pons arm, left cerebellum and right midbrain, thalamus. The patient was diagnosed with an accompanying demyelinating disease. Serum anti-myelin oligodendrocyte glycoprotein 1:10 and NMDAR antibody 1:10 were both positive. The patient was diagnosed with myelin oligodendrocyte glycoprotein antibody-related inflammatory demyelinating disease of the central nervous system complicated with anti-NMDARe overlap syndrome. The patient was successfully treated with methylprednisolone, gamma globulin pulse therapy and rituximab treatment. The patient remained asymptomatic and follow-up MRI scan 6 mo later showed complete removal of the lesion.
CONCLUSION
We emphasize the rarity of this antibody combination and suggest that these patients may require longer follow-up due to the risk of recurrence of two autoimmune disorders.
Keywords: Autoimmune encephalitis, Recurrent anti-N-methyl-D-aspartate receptor encephalitis, Myelin oligodendrocyte glycoprotein, Psoriasis, Case report
Core Tip: Here we present a man with autoimmune encephalitis in whom antibodies against N-methyl-D-aspartate receptor and myelin oligodendrocyte glycoprotein were sequentially detected. This is the first recurrent N-methyl-D-aspartate receptor encephalitis case in the literature for which antibodies of N-methyl-D-aspartate receptor and myelin oligodendrocyte glycoprotein were positive simultaneously and both supratentorial and infratentorial cranial magnetic resonance imaging were involved. Also, the patient responded very well with the optic nerve injury and encephalitis completely recovering. Psoriasis detected at the 6-mo follow-up may also be an immune-related disease, but the mechanism is unknown.
INTRODUCTION
In several individuals, anti-N-methyl-D-aspartate receptor encephalitis (anti-NMDARe) may occur with myelin oligodendrocyte glycoprotein (MOG) antibody disease sequentially or simultaneously[1-3]. However, there have been few reports of recurrent anti-NMDARe with MOG antibody disease overlap syndrome worldwide. We present a case of a young man initially admitted with headache, fever, behavioral abnormalities and intellectual decline, followed by hoarseness, blurred vision, disturbance of consciousness as well as seizures. Magnetic resonance imaging (MRI) involved multiple regions (e.g., the parietal lobe, frontal lobe, midbrain, thalamus, cerebellum and medulla oblongata). From this case, we recommend the simultaneous detection of viruses, autoimmune encephalitis-associated antibodies and central nervous system demyelination-associated antibodies for patients suspected of having central nervous system demyelinating disease or anti-NMDARe. The aim is to increase the understanding of autoimmune encephalitis overlap syndrome as their clinical and prognostic features may differ from those of single-antibody disease.
CASE PRESENTATION
Chief complaints
A 29-year-old man presented to the Neurology Department of our hospital complaining of headache, fever, intermittent abnormal behavior, decreased intelligence, limb twitching and loss of consciousness. During his presentation, he experienced aggravated symptoms.
The patient was admitted to the hospital for the second time for abnormal mental behavior and increased limb movements.
The patient was admitted for hoarseness and double vision for the third time. During his presentation, the patient’s visual acuity further decreased.
History of present illness
The patient began to experience symptoms of headache, fever, nausea and vomiting 7 d before admission. He experienced limb weakness, intermittent behavioral abnormalities and decreased intelligence 4 d before admission. He experienced limb twitching and loss of consciousness 2 d before admission.
History of past illness
The patient had a history of previous surgery for otitis media.
Personal and family history
The daughter of the uncle in the family suffered from lupus erythematosus.
Physical examination
First admission: Clear consciousness, poor orientation to time, place and personality, poor numeracy and unremarkable physical examination.
Second admission: Intermittent clear consciousness, uncooperative rest of nervous system.
Third admission: Horizontal movement of the eyeball was limited, nystagmus to the left in left vision, nystagmus to the right in right vision, vertical nystagmus in upper and lower visions, decreased lateral acupuncture sensation in bilateral face, weak closure of left eyelid, less sensitive corneal reflex, left central facial paralysis, less powerful elevation of right soft palate, left deviation of uvula, left muscle strength grade 4, less stable finger and nose, decreased tendon reflexes in four extremities and positive Babinski sign on the left side were identified.
Laboratory examinations
First admission: Mycobacterium tuberculosis antibody detection reported no abnormality. Cerebrospinal fluid examination revealed: white blood cells 40 × 106/L; total protein 0.4 g/L; glucose 3.12 mol/L; and chloride 126.9 mmol/L. External examination of cerebrospinal fluid and serum results revealed: serum NMDAR antibody (Ab) (-); cerebrospinal fluid NMDAR-Ab (+) 1:10; cerebrospinal fluid herpes simplex virus antibody (HSVI, II IgG, IgM) (-); rubella virus antibody (RVIgG, IgM) (-); cytomegalovirus (CMVIgG, IgM) (-); Epstein-Barr virus (EBV) early antigen antibody IgG, IgM, IgA (-); EBV virus capsid antigen antibody IgM, IgA (-); and EBV virus capsid antigen antibody IgG (+).
Second admission: The results of autoimmune encephalitis antibody were serum NMDAR-Ab weakly positive and cerebrospinal fluid NMDAR-Ab positive.
Third admission: Serum anti-MOG (+) 1:10 and NMDAR antibody (+) 1:10 were examined.
Imaging examinations
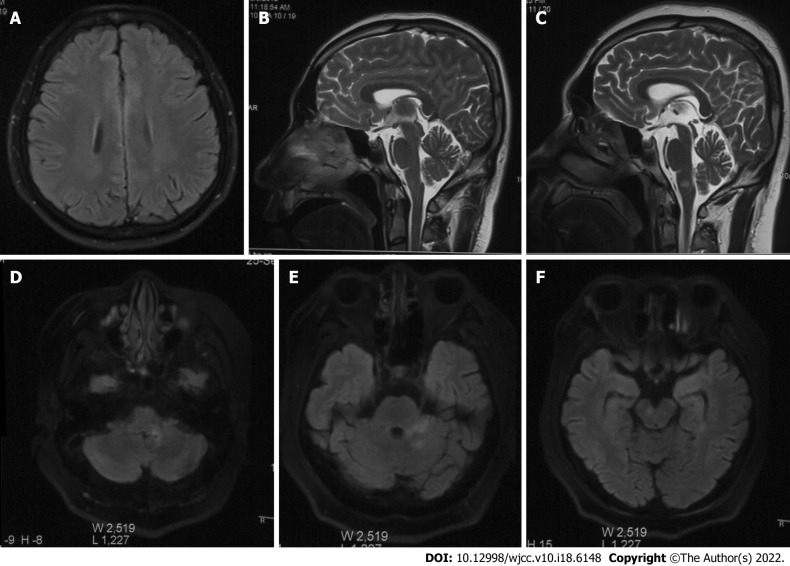
First admission: Head MRI, chest X-ray and electroencephalography were normal. Re-examination of cranial MRI showed punctate abnormal signals in the left parietal lobe (Figure 1A).
Figure 1.
Imaging changes in the pathogenesis of overlapping syndrome. A: Punctate abnormality in left parietal lobe (first episode); B: Normal sagittal position; C: High signal intensity was identified in the medulla oblongata in the T2 sagittal view; D: High signal intensity was identified in the left medulla oblongata and cerebellum of Flair; E: Flair showed hyperintensity in the left pontine arm and left cerebellum; F: Flair showed hyperintensity in the right midbrain.
Second admission: Examination of electroencephalography and cranial MRI showed no abnormality (Figure 1B).
Third admission: Examination of cranial MRI showed abnormal signals in the medulla oblongata and right frontal lobe (Figure 1C), and synoptophore examination indicated concomitant esotropia. In such a period, the re-examination of cranial MRI + enhancement reported multiple scattered speckled and patchy abnormal signals in the medulla oblongata, left pons arm, left cerebellum and right midbrain (Figure 1D-F).
MULTIDISCIPLINARY EXPERT CONSULTATION
Lin Wang, MD, Chief Physician, Department of Neurology, Beijing Xuanwu Hospital. The patient confirmed the diagnosis of anti-NMDARe for first admission. The patient should undergo medical treatment with methylprednisolone and gamma globulin pulse therapy and olanzapine to improve sleep. In addition, this patient required regular re-examination of electroencephalography.
Hongzhi Guan, MD, Professor and Chief, Department of Central Nervous System Infection, Beijing Xiehe Hospital. The patient confirmed the diagnosis of recurrent anti-NMDARe for second admission. The patient had psychiatric symptoms, language disorder, autonomic dysfunction and other symptoms in this attack, which were considered to be comprehensive recurrent type. First, the presence of tumors in the patient’s body was assessed, gamma globulin and hormone pulse therapy were standardized in those without tumors, and the hormone dose was reduced to 75 mg, 1 tablet every 2 wk. At the same time, according to the consensus, immunosuppressant (mofetil) 1-2 mg/d, orally for at least 1 year, antiepileptic treatment with sodium valproate and olanzapine increased to 2 mg/time to control psychiatric symptoms.
FINAL DIAGNOSIS
The final diagnosis of the presented case was MOG antibody-related inflammatory demyelinating disease of the central nervous system complicated with anti-NMDARe overlap syndrome.
TREATMENT
The patient underwent medical treatment with methylprednisolone and gamma globulin pulse therapy and olanzapine to improve sleep after the first admission. The patient was assessed to be tumor-free at the second admission and given standard gamma globulin and steroid pulse therapy with a steroid dose reduced to 75 mg, 1 tablet every 2 wk. At the same time, according to the consensus, immunosuppressive agents (mofetil) 1-2 mg/d, orally for at least 1 year and antiepileptic treatment with sodium valproate and olanzapine increased to 2 mg/time to control psychiatric symptoms was prescribed. At the last admission, the patient was successfully treated with methylprednisolone, gamma globulin pulse therapy and rituximab treatment.
OUTCOME AND FOLLOW-UP
The patient had an uneventful clinical course, whilst dexamethasone was decreased progressively until its cessation. At the follow-up visit 1 year after hospital discharge, the patient was asymptomatic. An MRI scan showed complete removal of the lesion. However, we observed scattered red rashes on both upper limbs and trunk. Since dermoscopy showed scattered red spots and plaque changes on the glans penis and ventral surface of the extremities and a few scales, the diagnosis of psoriasis was considered. Halometasone ointment was applied externally.
DISCUSSION
The concept of anti-NMDARe was first introduced in 2007 by Dalmau et al[4]. MOG antibodies are related to demyelinating diseases of the central nervous system. Therefore, the concept of MOG antibody-related demyelinating diseases of the central nervous system (MOG antibody disease) was proposed[6,7]. Some patients suffering anti-NMDARe have positive serum MOG antibody, and some patients suffering MOG antibody have positive cerebrospinal fluid anti-NMDAR antibody, which is called MOG antibody disease with anti-NMDARe overlap syndrome (MNOS)[1,8,9]. In several individuals, anti-NMDARe may occur with MOG antibody disease sequentially or simultaneously[1-3]. However, there have been rare reports of recurrent anti-NMDARe with MOG antibody disease overlap syndrome worldwide.
Encephalitis is a neurological disorder caused by diffuse or multiple inflammatory lesions of the brain parenchyma. Among them, autoimmune encephalitis generally refers to a type of encephalitis mediated by autoimmune mechanisms[10]. At present, the proportion of autoimmune encephalitis accounts for 10%-20% of encephalitis cases, of which anti-NMDARe is the most common, accounting for about 80%[11,12]. Autoimmune encephalitis should be differentiated from central nervous system infections caused by herpes simplex encephalitis, epidemic encephalitis B, neurosyphilis, bacteria, fungi, parasites, Creutzfeldt-Jakob disease and the presence or absence of opportunistic infectious diseases associated with immunosuppressive or anti-tumor agents[13,14].
Cerebrospinal fluid antibodies were negative in the acute phase of the above infectious diseases[15]. In this case, relevant examinations such as cerebrospinal fluid cytology, culture, virus, antibody, cranial MRI, electroencephalogram, tumor screening [tumor markers, chest computed tomography, scrotum, both kidneys, hepatobiliary b-ultrasound] and positron emission tomography-computed tomography were perfected for differential significance[9,16]. We report a young man who initially presented with headache, fever and epilepsy as the first symptoms, followed by behavioral abnormalities, intellectual decline, dyskinesia and decreased autonomic function in accordance with the course of “bimodal encephalitis” reported in the literature[17]. Combined with cerebrospinal fluid NMDAR antibody (+) 1:10, EBV viral capsid antigen antibody IgG (+), negative tumor screening program and other examinations, it was considered to be anti-NMDARe secondary to non-tumor viral encephalitis. The disadvantage of this case is that metagenomic next-generation sequencing was not further refined to identify the presence of other bacterial or viral infections.
Five months after improvement of treatment, the patient once again developed psychiatric symptoms and increased limb movements, and the cerebrospinal fluid NMDAR antibody (+) was 1:10. Given the definition of recurrent anti-NMDARe, i.e. new symptoms not able to be explained by other reasons or aggravation of original symptoms were identified 2 mo after the improvement of NMDARe treatment[2,10], the diagnosis of recurrent anti-NMDARe could be confirmed. Subsequently, the patient developed hoarseness and double vision, and the re-examination of cranial MRI + enhancement indicated new lesions. On the whole, anti-NMDARe was not related to optic nerve damage and sensory disturbance in clinical practice, and patients suffering demyelinating diseases of the central nervous system are considered to be combined with MRI and clinical manifestations. The detection of serum MOG antibody indicated MOG (+) 1:10, by complying with the diagnostic criteria of MOG antibody disease[18]. Then diagnosis of anti-NMDARe with MOG antibody disease overlap syndrome was confirmed.
Characteristics of this case include: (1) Etiology: it has been reported in the literature that anti-NMDARe is related to tumors, but the incidence of tumors detected in patients suffering MNOS is small, and the prognosis is good[2,10,19]. The present patient agreed with previous literature reports in which no tumor was detected during a 2-year course; (2) Affected population: MOG antibody disease and anti-NMDARe are usually more common in women, and the incidence of MNOS in children is higher than that in adults[1,5,9]. However, the patient in this case was an adult male, it was relatively rare; (3) Clinical manifestations: the clinical symptoms of recurrent anti-NMDARe are mild, overall manifested as a single symptom, which is mild when recurrent[10,20]. Nevertheless, this patient was inconsistent with existing literature reports, showing psychiatric symptoms, language impairment and autonomic dysfunction. At the time of recurrence, he displayed considerable clinical symptoms, i.e. comprehensive recurrent anti-NMDARe; (4) MRI findings: the cranial MRI of patients suffering anti-NMDARe may be unremarkable, or there may be only scattered cortical and subcortical dot-like abnormalities[4,20]. The first two episodes in this patient were consistent with the findings in previous reports. All patients suffering MNOS will have supratentorial lesions and less infratentorial lesions[1], but both supratentorial and infratentorial cranial MRI were involved in this patient; (5) Prognosis: the optic nerve injury and encephalitis of this patient recovered completely, thereby not complying with the findings of Titulaer et al[2], who found that patients suffering MNOS had a delayed recovery from demyelinating disease and a more pronounced residual deficit; and (6) Concomitant disease: At present, anti-NMDARe secondary to EBV-related viral encephalitis has not been reported worldwide, and psoriasis was reported by dermatoscopy during the 6-mo follow-up of the patient. Psoriasis[21] is an immune-mediated polygenic genodermatosis, which may be the result of a combination of genetic, environmental and immunological factors. To the best of the authors’ knowledge, there have been no reported related cases worldwide.
CONCLUSION
In clinical practice, simultaneous detection of viruses, autoimmune encephalitis-related antibodies and central nervous system demyelination-related antibodies is recommended for patients suffering from suspected central nervous system demyelinating disease or anti-NMDARe. First, when the patient has a typical course of “bimodal symptoms,” i.e. the first peak has “fever, psycho-behavioral abnormalities, epilepsy” as the symptoms and the second peak has “psycho-behavioral abnormalities, memory loss, dyskinesia, autonomic dysfunction” as the primary symptoms to consider autoimmune encephalitis secondary to viral encephalitis. Second, when anti-NMDARe patients are identified to develop symptoms involving the optic nerve and spinal cord (e.g., decreased visual acuity, limb motor or sensory impairment), the coexistence of MOG antibody disease should be considered. Third, when patients suffering MOG antibody disease develop encephalitis symptoms (e.g., psycho-behavioral abnormalities or cognitive impairment) and novel lesions are seen on cranial MRI, anti-NMDARe coexistence should be considered.
ACKNOWLEDGEMENTS
We thank the patient for consenting to our reporting of this case.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 26, 2021
First decision: March 7, 2022
Article in press: April 30, 2022
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta SK, India S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Xue-Jing Yin, Department of Neurology, Changzhi Medical College, Changzhi 046000, Shanxi Province, China.
Li-Fang Zhang, Department of Neurology, Changzhi People's Hospital, Changzhi 046000, Shanxi Province, China.
Li-Hua Bao, Department of Neurology, Changzhi People's Hospital, Changzhi 046000, Shanxi Province, China.
Zhi-Chao Feng, Department of Neurology, Changzhi Medical College, Changzhi 046000, Shanxi Province, China.
Jin-Hua Chen, Department of Neurology, Changzhi People's Hospital, Changzhi 046000, Shanxi Province, China. cjhua0355@163.com.
Bing-Xia Li, Department of Neurology, Changzhi People's Hospital, Changzhi 046000, Shanxi Province, China.
Juan Zhang, Department of Neurology, Changzhi People's Hospital, Changzhi 046000, Shanxi Province, China.
References
- 1.Fan S, Xu Y, Ren H, Guan H, Feng F, Gao X, Ding D, Fang F, Shan G, Guan T, Zhang Y, Dai Y, Yao M, Peng B, Zhu Y, Cui L. Comparison of myelin oligodendrocyte glycoprotein (MOG)-antibody disease and AQP4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) when they co-exist with anti-NMDA (N-methyl-D-aspartate) receptor encephalitis. Mult Scler Relat Disord. 2018;20:144–152. doi: 10.1016/j.msard.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Titulaer MJ, Höftberger R, Iizuka T, Leypoldt F, McCracken L, Cellucci T, Benson LA, Shu H, Irioka T, Hirano M, Singh G, Cobo Calvo A, Kaida K, Morales PS, Wirtz PW, Yamamoto T, Reindl M, Rosenfeld MR, Graus F, Saiz A, Dalmau J. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75:411–428. doi: 10.1002/ana.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarigecili E, Cobanogullari MD, Komur M, Okuyaz C. A rare concurrence: Antibodies against Myelin Oligodendrocyte Glycoprotein and N-methyl-d-aspartate receptor in a child. Mult Scler Relat Disord. 2019;28:101–103. doi: 10.1016/j.msard.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez CA, Agyei P, Gogia B, Harrison R, Samudralwar R. Overlapping autoimmune syndrome: A case of concomitant anti-NMDAR encephalitis and myelin oligodendrocyte glycoprotein (MOG) antibody disease. J Neuroimmunol. 2020;339:577124. doi: 10.1016/j.jneuroim.2019.577124. [DOI] [PubMed] [Google Scholar]
- 6.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15:89–102. doi: 10.1038/s41582-018-0112-x. [DOI] [PubMed] [Google Scholar]
- 7.Rojc B, Podnar B, Graus F. A case of recurrent MOG antibody positive bilateral optic neuritis and anti-NMDAR encephalitis: Different biological evolution of the two associated antibodies. J Neuroimmunol. 2019;328:86–88. doi: 10.1016/j.jneuroim.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Weber MS, Derfuss T, Metz I, Brück W. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther Adv Neurol Disord. 2018;11:1756286418762083. doi: 10.1177/1756286418762083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abboud H, Probasco JC, Irani S, Ances B, Benavides DR, Bradshaw M, Christo PP, Dale RC, Fernandez-Fournier M, Flanagan EP, Gadoth A, George P, Grebenciucova E, Jammoul A, Lee ST, Li Y, Matiello M, Morse AM, Rae-Grant A, Rojas G, Rossman I, Schmitt S, Venkatesan A, Vernino S, Pittock SJ, Titulaer MJ Autoimmune Encephalitis Alliance Clinicians Network. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. 2021;92:757–768. doi: 10.1136/jnnp-2020-325300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, Geis C, Lancaster E, Titulaer MJ, Rosenfeld MR, Graus F. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045–1057. doi: 10.1016/S1474-4422(19)30244-3. [DOI] [PubMed] [Google Scholar]
- 11.Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, Llufriu S, Muchart J, Erro ME, Abraira L, Moris G, Monros-Giménez L, Corral-Corral Í, Montejo C, Toledo M, Bataller L, Secondi G, Ariño H, Martínez-Hernández E, Juan M, Marcos MA, Alsina L, Saiz A, Rosenfeld MR, Graus F, Dalmau J Spanish Herpes Simplex Encephalitis Study Group. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armangue T, Leypoldt F, Málaga I, Raspall-Chaure M, Marti I, Nichter C, Pugh J, Vicente-Rasoamalala M, Lafuente-Hidalgo M, Macaya A, Ke M, Titulaer MJ, Höftberger R, Sheriff H, Glaser C, Dalmau J. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol. 2014;75:317–323. doi: 10.1002/ana.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu CL, Liu L, Zhao WQ, Li JM, Wang RJ, Wang SH, Wang DX, Liu MY, Qiao SS, Wang JW. Anti-N-methyl-D-aspartate receptor encephalitis with serum anti-thyroid antibodies and IgM antibodies against Epstein-Barr virus viral capsid antigen: a case report and one year follow-up. BMC Neurol. 2011;11:149. doi: 10.1186/1471-2377-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger B, Pytlik M, Hottenrott T, Stich O. Absent anti-N-methyl-D-aspartate receptor NR1a antibodies in herpes simplex virus encephalitis and varicella zoster virus infections. Int J Neurosci. 2017;127:109–117. doi: 10.3109/00207454.2016.1147447. [DOI] [PubMed] [Google Scholar]
- 16.Ellul M, Solomon T. Acute encephalitis - diagnosis and management. Clin Med (Lond) 2018;18:155–159. doi: 10.7861/clinmedicine.18-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu Y, Qiu W, Zheng J, Sun X, Yin J, Yang X, Yue X, Chen C, Deng Z, Li S, Yang Y, Peng F, Lu Z, Hu X, Petersen F, Yu X. HLA class II allele DRB1*16:02 is associated with anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2019;90:652–658. doi: 10.1136/jnnp-2018-319714. [DOI] [PubMed] [Google Scholar]
- 18.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, Franciotta D, Fujihara K, Jacob A, Kim HJ, Kleiter I, Kümpfel T, Levy M, Palace J, Ruprecht K, Saiz A, Trebst C, Weinshenker BG, Wildemann B. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15:134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa N, Tajima G, Hyodo S, Takahashi Y, Kobayashi M. Detection of autoantibodies against NMDA-type glutamate receptor in a patient with recurrent optic neuritis and transient cerebral lesions. Neuropediatrics. 2007;38:257–260. doi: 10.1055/s-2007-1004521. [DOI] [PubMed] [Google Scholar]
- 20.Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]