Abstract
Aims
The aim was to investigate sex- and age-stratified risks of cause-specific death and life expectancy in individuals with post-pancreatitis diabetes mellitus (PPDM).
Methods
Nationwide data on mortality in New Zealand were obtained. For two head-to-head comparisons (PPDM versus type 2 diabetes mellitus [T2DM]; PPDM versus type 1 diabetes mellitus [T1DM]), the groups were matched on age, sex, and calendar year of diabetes diagnosis. Multivariable Cox regression analyses were conducted to estimate risks of vascular, cancer, and non-vascular non-cancer mortality. Remaining life expectancy at age of diabetes diagnosis was estimated using the Chiang II method.
Results
A total of 15,848 individuals (1,132 PPDM, 3,396 T1DM, and 11,320 T2DM) were included. The risks of vascular mortality and non-vascular non-cancer mortality did not differ significantly between PPDM and T2DM or T1DM. PPDM was associated with a significantly higher risk of cancer mortality compared with T2DM (adjusted hazard ratio, 1.32; 95% confidence interval, 1.08–1.63) or T1DM (adjusted hazard ratio, 1.65; 95% confidence interval, 1.27–2.13). The risk of cancer mortality associated with PPDM (versus T2DM) was significantly higher in women than in men (p for interaction = 0.003). This sex difference in cancer mortality risk was also significant in the comparison between PPDM and T1DM (p for interaction = 0.006). Adults of both sexes with PPDM had the lowest remaining life expectancy (in comparison with T2DM or T1DM) up to 64 years of age.
Conclusions
People with PPDM have a higher risk of cancer mortality compared with those with T2DM or T1DM. This is especially pronounced in women. Young and middle-aged adults with PPDM have a lower life expectancy compared with their counterparts with T2DM or T1DM.
Introduction
Pancreatitis is the most common disease of the exocrine pancreas, with the global incidence of 43 cases per 100,000 person-years [1]. Individuals with pancreatitis frequently develop metabolic abnormalities after hospital discharge. Specifically, it is known that 20–30% of individuals with pancreatitis develop diabetes mellitus [2]. Post-pancreatitis diabetes mellitus (PPDM) is the largest contributor to diabetes of the exocrine pancreas, which is more common in adults than type 1 diabetes mellitus (T1DM) [3, 4]. Moreover, its incidence is projected to increase by more than 2% per year in the 2020s [5]. Population-based studies have shown that PPDM yields 14.8 excess all-cause mortality per 1,000 person-years (which increases up to 68 if not medicated) and is associated with a 13% higher risk of all-cause mortality, as compared with type 2 diabetes mellitus (T2DM) [6, 7].
Mounting evidence suggests sex difference in the mortality risks associated with T2DM and T1DM. A meta-analysis of 35 prospective cohort studies demonstrated a significant sex difference in the risk of all-cause mortality associated with T2DM versus those without diabetes (2.3-times higher in women and 1.9-times higher in men) [8]. This difference was mainly attributable to vascular mortality (3.8-times higher in women and 2.1-times higher in men) [8]. A meta-analysis of 26 studies demonstrated a significant sex difference in the risk of all-cause mortality associated with T1DM versus those without diabetes (5.8-times higher in women and 3.8-times higher in men) [9]. This difference was mainly attributable to vascular mortality (11.3-times higher in women and 5.7-times higher in men) [9]. Age is another characteristic that exhibits differences in the risks of all-cause and cause-specific death associated with T2DM and T1DM (versus general populations). A nationwide study from Australia found that a younger age at diagnosis of T2DM was associated with higher risks of all-cause and vascular mortality but a lower risk of cancer mortality [10]. Nationwide data from Sweden showed that individuals with young-onset T2DM (age < 40 years) had a 2.1-times higher risk of all-cause mortality [11], as well as that a younger age at diagnosis of T1DM was associated with a higher risk of all-cause mortality [12]. The above-mentioned studies from Sweden also demonstrated that younger individuals with T2DM or T1DM had a greater loss of remaining life expectancy—a common measure of premature death [11, 12]. To date, there has been a dearth of data on sex and age differences in the risk of death associated with PPDM. Given the well-documented poor health outcomes in people with PPDM [5–7, 13], it is important to identify high-risk groups for mortality among them with a view to curbing the burden of PPDM.
The primary aim was to examine age- and sex-stratified risks of cause-specific death associated with PPDM versus the other common types of diabetes (i.e., T1DM and T2DM). The secondary aim was to estimate remaining life expectancy at age of diabetes diagnosis in PPDM versus T1DM and PPDM versus T2DM.
Methods
Data source
The data extraction was performed by the Ministry of Health Analytical Services (National Health Board, New Zealand). From the nationwide hospital discharge data (covering all the 20 District Health Board in the entire country) between January 1, 1995, and December 31, 2016, all records of individuals who were diagnosed with pancreatitis and diabetes mellitus based on the International Classification of Diseases (ICD) codes were extracted. The data included information on age, sex, ethnicity, area of residence, ICD codes (both primary and up to 20 secondary), and date of admission. The hospital discharge database was linked to the mortality database containing date of death and cause of death.
Study cohort
Individuals who were first diagnosed with pancreatitis (ICD-10, K85; K86.0; K86.1) or diabetes mellitus (E10; E11; E13) were identified during the study period from January 1, 1998, to December 31, 2016 (Supplementary Fig. 1). To ensure that these individuals were newly diagnosed, a 3-year washout period from 1995 to 1997 was used. The PPDM group was assembled first and, then, its matched groups of T2DM and T1DM were established using the frequency matching method with a view to comparing head-to-head PPDM versus T2DM and PPDM versus T1DM. The PPDM group included individuals who were diagnosed with diabetes (ICD-10, E11; E13) in more than 90 days after first diagnosis of acute pancreatitis (K85) or chronic pancreatitis (K86.0; K86.1). The 90-day lag period was used to preclude the inclusion of patients with preexisting diabetes or stress-induced hyperglycemia [4, 14, 15]. Exclusion criteria were as follows: individuals who were diagnosed with type 1 diabetes (ICD-10, E10) from 1998 to 2016, those who were diagnosed with diabetes mellitus during and/or prior to the first pancreatitis diagnosis, and those who were diagnosed with diabetes mellitus during ≤ 90 days after the first pancreatitis diagnosis. Finally, a total of 1,132 individuals were included in the PPDM group. The T2DM group included individuals who were diagnosed with T2DM (ICD-10, E11) and never with T1DM (E10) or pancreatitis (K85; K86.0; K86.1) from 1998 to 2016 were first identified (n = 207,863). Of these, based on age (< 30, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥ 80 years), sex, and calendar year of diabetes diagnosis, ten matched individuals with T2DM for each individual with PPDM were randomly selected (n = 11,320). The T1DM group included individuals who were diagnosed with T1DM (ICD-10, E10) and never with pancreatitis (K85; K86.0; K86.1) from 1998 to 2016 were first identified (n = 25,838). Of these, based on age (< 30 and ≥ 30 years), sex, and calendar year of diabetes diagnosis, three matched individuals with T1DM for each individual with PPDM were randomly selected (n = 3,396).
Endpoints
Date of first diagnosis of diabetes was set as index date (i.e., follow-up start date). All individuals were observed until the end of the study period (December 31, 2016) or date of death, whichever came first. The primary endpoint was cause-specific death, categorized as vascular, cancer, and non-vascular non-cancer causes (based on the relevant ICD codes and in line with the previous literature [16]). Cancer death was subcategorized based on cancer sites: pancreas, colon, liver, lung, prostate (men only), breast (women only), and others. The secondary endpoint was remaining life expectancy (years) at diabetes diagnosis (in line with the previous literature [17]).
Covariates
Alcohol abuse (ICD-10, F10) and ever smoking (ICD-10, Z720; Z8643; Z87891) were defined based on the relevant ICD codes during the entire study period [18]. Ethnicity was classified as European, Māori or Pacific Islander, Asian, and others. Social deprivation index (based on area of residence) was classified into quartiles; individuals with missing values were categorized as an additional category [19]. The Charlson comorbidity index was calculated in line with the previous literature [20] and treated as a categorical variable (1, 2, 3, and ≥ 4).
Statistical analysis
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Two-sided p < 0.05 was deemed to be statistically significant. Mortality rate with corresponding standard error (SE) was calculated as the number of deaths per 1,000 person-years. To estimate mortality risk associated with PPDM in each head-to-head comparison (i.e., PPDM versus T2DM; PPDM versus T1DM), crude and multivariable Cox regression analyses were performed. The multivariable model included age (as a continuous variable), ethnicity, social deprivation index, alcohol abuse, ever smoking, and the Charlson comorbidity index as covariates. The survival curves were created after adjustment for the above covariates. The Cox regression analyses were performed after stratification by sex (women and men) or age (< 45 years, 45–64 years, and ≥ 65 years of age). Significance of sex or age difference in mortality risk was tested using the Altman–Bland method [21] and expressed as p for interaction. The resulting p values for interaction were corrected for multiple testing using the false discovery rate (FDR) method. In addition, these analyses were repeated after categorizing PPDM as post-acute pancreatitis diabetes mellitus (PPDM-A) and post-chronic pancreatitis diabetes mellitus (PPDM-C) [13, 14]. Individuals who had diagnostic codes of both acute pancreatitis and chronic pancreatitis were classified as PPDM-C. We also estimated the risk of site-specific cancer death in each head-to-head comparison. The risk of death was expressed as hazard ratio (HR) with 95% confidence interval (95% CI). The assumption of proportionality was graphically evaluated and found fulfilled.
Remaining life expectancy (with 95% CI) at age of diabetes diagnosis by 5-year age intervals was estimated using the Chiang II method [22–24] in each of the three study groups (T1DM, T2DM, and PPDM). This method enabled us to account for age intervals with zero deaths and small populations. Given a very limited number of individuals aged < 20 years in the T2DM and PPDM groups, we constrained the analysis to those aged ≥ 20 years. In each study group, the numbers of population and deaths by sex and 5-year age intervals were calculated. Using these aggregated data, abridged period life tables were constructed by 5-year age intervals from 20 years up to age 75 + years, stratified by sex. The resulting remaining life expectancy (years) by 5-year age intervals was plotted by fitting a smooth curve using locally estimated scatterplot smoothing regression [25].
A post hoc analysis was conducted to investigate sex difference in the risk of mortality from pancreatic cancer (the most common site-specific cancer in PPDM) associated with PPDM, as compared with T2DM or T1DM. After stratification by sex, the above multivariable Cox regression analysis was performed in each head-to-head comparison.
Results
Characteristics of the study groups
A total of 15,848 individuals with diabetes were observed for a mean ± standard deviation (SD) period of 4.1 ± 3.6 years. The PPDM group had the highest all-cause mortality rate (80.8 per 1,000 person-years), as well as the lowest survival probability during follow-up after adjustment for covariates (Fig. 1). Other characteristics are presented in Table 1.
Fig. 1.
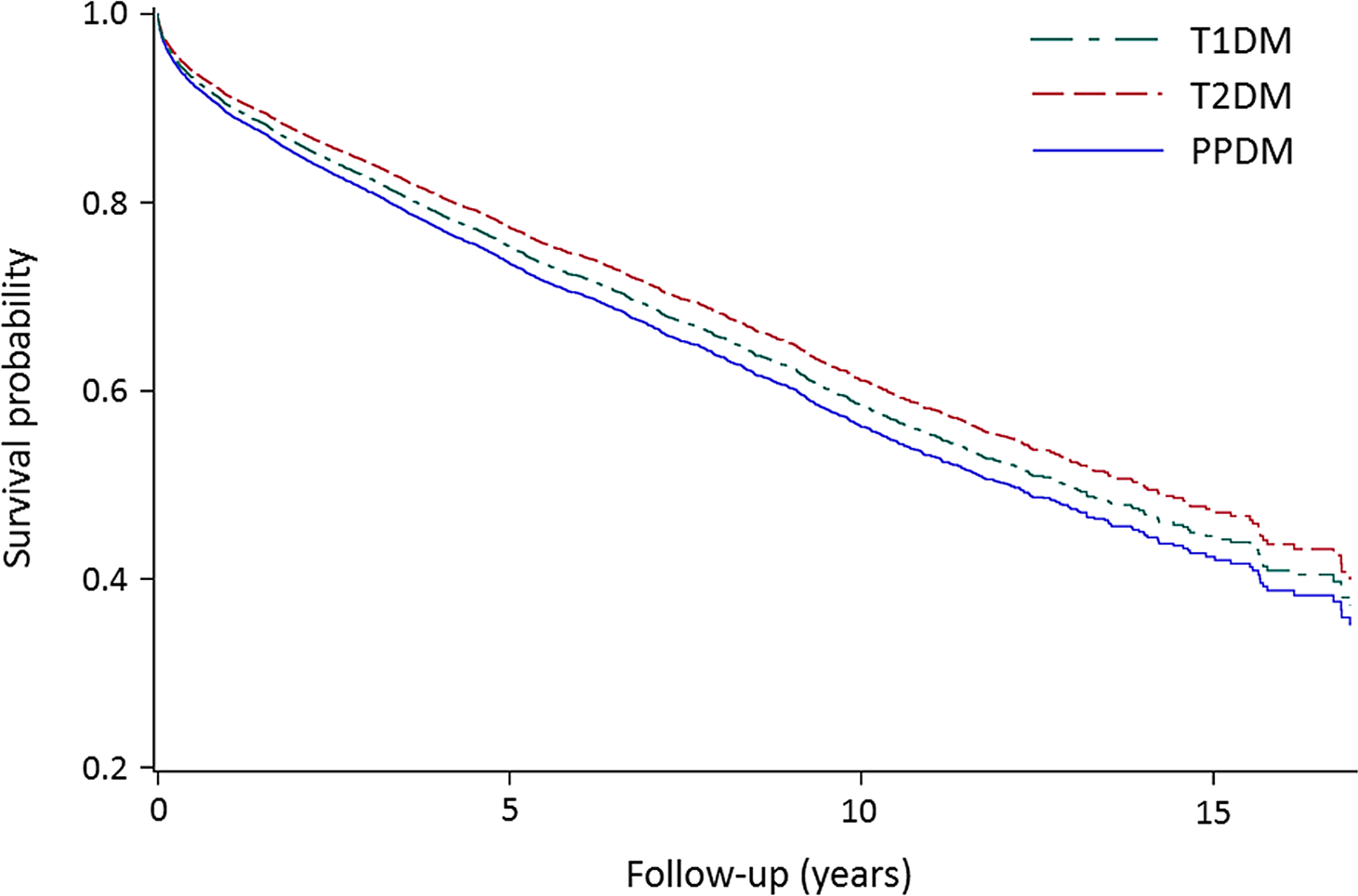
Survival probability in type 1, type 2, and post-pancreatitis diabetes mellitus. T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, PPDM post-pancreatitis diabetes mellitus. The analyses were adjusted for age, ethnicity, social deprivation index, alcohol abuse, ever smoking, and the Charlson comorbidity index
Table 1.
Characteristics of the study cohort
| Overall (n = 15,848) | PPDM (n = 1,132) | T1DM (n = 3,396) | T2DM (n = 11,320) | |
|---|---|---|---|---|
|
| ||||
| Age (years), mean (SD) | 62.0 (16.4) | 63.8 (16.1) | 55.7 (16.7) | 63.6 (15.9) |
| Women, n(%) | 6,426 (40.5) | 459 (40.5) | 1,377 (40.5) | 4,590 (40.5) |
| Ethnicity, n(%) | ||||
| European | 9,805 (61.9) | 761 (67.2) | 2,552 (75.2) | 6,492 (57.4) |
| Māori or Pacific | 4,087 (25.8) | 297 (26.2) | 576 (17.0) | 3,214 (28.4) |
| Asian | 1,304 (8.2) | 56 (5.0) | 153 (4.5) | 1,095 (9.7) |
| Others | 652 (4.1) | 18 (1.6) | 115 (3.4) | 519 (4.6) |
| Social deprivation index, n(%) | ||||
| Quartile 1 | 3,778 (23.8) | 231 (20.4) | 978 (28.8) | 2,569 (22.7) |
| Quartile 2 | 4,388 (27.7) | 331 (29.2) | 970 (28.6) | 3,087 (27.3) |
| Quartile 3 | 4,326 (27.3) | 338 (29.9) | 860 (25.3) | 3,128 (27.6) |
| Quartile 4 | 2,483 (15.7) | 190 (16.8) | 380 (11.2) | 1,913 (16.9) |
| Missing | 873 (5.5) | 42 (3.7) | 208 (6.1) | 623 (5.5) |
| Alcohol abuse, n(%) | 532 (3.4) | 179 (15.8) | 120 (3.5) | 233 (2.1) |
| Ever smoking, n(%) | 8,376 (52.9) | 764 (67.5) | 1,763 (51.9) | 5,849 (51.7) |
| Charlson comorbidity index, n (%) | ||||
| 1 | 8,201 (51.8) | 585 (51.7) | 1,716 (50.5) | 5,900 (52.1) |
| 2 | 1,662 (10.5) | 143 (12.6) | 190 (5.6) | 1,329 (11.7) |
| 3 | 3,012 (19.0) | 179 (15.8) | 746 (22.0) | 2,087 (18.4) |
| ≥ 4 | 2,973 (18.8) | 225 (19.9) | 744 (21.9) | 2,004 (17.7) |
| Mortality, n (%) | ||||
| Vascular | 1,321 (8.3) | 112 (9.9) | 200 (5.9) | 1,009 (8.9) |
| Cancer | 1,106 (7.0) | 107 (9.5) | 179 (5.3) | 820 (7.2) |
| Non-vascular non-cancer | 2,335 (14.7) | 207 (18.3) | 503 (14.8) | 1,625 (14.4) |
| All-cause | 3,686 (23.3) | 336 (29.7) | 668 (19.7) | 2,682 (23.7) |
| Mortality rate per 1,000 person-years (standard error) | ||||
| Vascular | 20.3 (0.6) | 26.9 (2.6) | 13.8 (1.0) | 21.8 (0.7) |
| Cancer | 17.0 (0.5) | 25.7 (2.6) | 12.4 (0.9) | 17.7 (0.6) |
| Non-vascular non-cancer | 36.0 (0.8) | 49.8 (3.5) | 34.8 (1.6) | 35.1 (0.9) |
| All-cause | 56.8 (1.0) | 80.8 (4.7) | 46.2 (1.8) | 57.9 (1.2) |
PPDM post-pancreatitis diabetes mellitus, T2DM type 2 diabetes mellitus, T1DM type 1 diabetes mellitus, SD standard deviation, 95% CI 95% confidence interval
Risk of cause-specific death in the study groups
The PPDM group had the highest rates of death from vascular (26.9 per 1,000 person-years), cancer (25.7 per 1,000 person-years), and non-vascular non-cancer (49.8 per 1,000 person-years) causes (Table 1). In the head-to-head comparison between PPDM and T2DM, the PPDM group had significantly higher risks of all-cause mortality (adjusted HR, 1.26; 95% CI, 1.12–1.41), cancer mortality (adjusted HR, 1.32; 95% CI, 1.08–1.63), and non-vascular non-cancer mortality (adjusted HR, 1.27; 95% CI, 1.09–1.47) compared with the T2DM group. The risk of vascular mortality associated with PPDM was not statistically significant in the adjusted model (HR, 1.14; 95% CI, 0.94–1.39). In the analysis of site-specific cancer mortality, the PPDM group had significantly higher risks of pancreatic cancer (adjusted HR, 3.83; 95% CI, 2.38–6.17) and colon cancer mortality (adjusted HR, 2.13; 95% CI, 1.13–4.03) (Fig. 2).
Fig. 2.
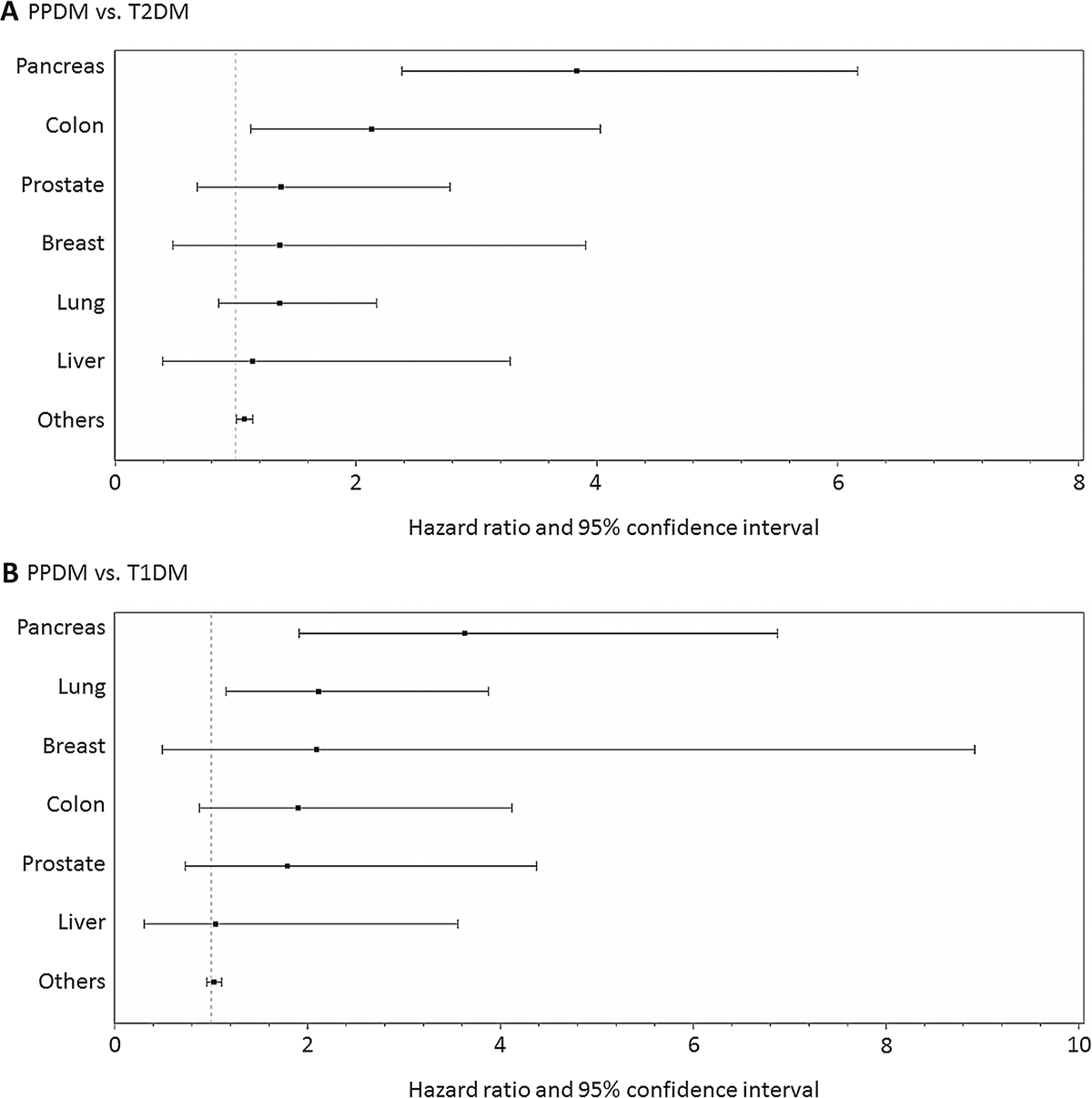
Risk of site-specific cancer mortality in type 1, type 2, and post-pancreatitis diabetes mellitus. T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, PPDM post-pancreatitis diabetes mellitus. Hazard ratios were adjusted for age, ethnicity, social deprivation index, alcohol abuse, ever smoking, and the Charlson comorbidity index
In the head-to-head comparison between PPDM and T1DM, the PPDM group had a significantly higher risk of cancer mortality (adjusted HR, 1.65; 95% CI, 1.27–2.13). The risks of all-cause (HR, 1.11; 95% CI, 0.97–1.28), vascular (HR, 1.09; 95% CI, 0.85–1.40), and non-vascular non-cancer mortality (HR, 0.89; 95% CI, 0.75–1.06) associated with PPDM were not statistically significant in the adjusted model. In the analysis of site-specific cancer mortality, the PPDM group had significantly higher risks of pancreatic cancer (adjusted HR, 3.63; 95% CI, 1.92–6.88) and lung cancer mortality (adjusted HR, 2.11; 95% CI, 1.15–3.88) (Fig. 2).
Sex-stratified risk of cause-specific death
In the PPDM group, women had a higher rate of cancer mortality than men (28.6 versus 23.7 per 1,000 person-years), whereas men had a higher rate of vascular mortality than women (30.6 versus 21.6 per 1,000 person-years) (Supplementary Table 1). In the head-to-head comparison between PPDM and T2DM, men with PPDM had a significantly higher risk of vascular mortality (adjusted HR, 1.29; 95% CI, 1.01–1.65), whereas the risk was not significant in women with PPDM (adjusted HR, 0.96; 95% CI, 0.68–1.34) (Table 2). This difference was statistically significant (p = 0.036) but did not remain significant after FDR correction (p = 0.096). Women with PPDM had a significantly higher risk of cancer mortality (adjusted HR, 1.82; 95% CI, 1.34–2.48) than men with PPDM (adjusted HR, 1.09; 95% CI, 0.83–1.44), and this difference was statistically significant (p = 0.003; FDR-corrected p = 0.024). In the post hoc analysis, the risk of pancreatic cancer mortality associated with PPDM was significantly higher in both men (adjusted HR, 3.37; 95% CI, 1.74–6.55) and women (adjusted HR, 4.53; 95% CI, 2.28–8.99). After stratification by sex and age, the highest risk of cancer mortality associated with PPDM was observed in younger women (adjusted HR, 4.13; 95% CI, 0.87–19.68) (Supplementary Table 2). When categorizing PPDM into PPDM-A and PPDM-C, both PPDM-A (adjusted HR, 1.60; 95% CI, 1.11–2.30) and PPDM-C (adjusted HR, 2.62; 95% CI, 1.56–4.40) were significantly associated with a higher risk of cancer mortality in women, whereas the risks were not significant in men. Other findings are presented in Supplementary Table 3.
Table 2.
Sex-stratified risk of cause-specific death in post-pancreatitis diabetes mellitus versus type 2 and type 1 diabetes mellitus
| Mortality | PPDM vs. T2DM |
PPDM vs. T1DM |
||||
|---|---|---|---|---|---|---|
| Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | p for interaction† | Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | p for interaction† | |
|
| ||||||
| Vascular | ||||||
| Women | 1.00 (0.72–1.40) | 0.96 (0.68–1.34) | 0.036 | 1.63 (1.11–2.41) | 0.74 (0.48–1.13) | 0.30 |
| Men | 1.36 (1.07–1.73) | 1.29 (1.01–1.65) | 2.11 (1.58–2.82) | 1.30 (0.95–1.78) | ||
| Cancer | ||||||
| Women | 1.92 (1.42–2.60) | 1.82 (1.34–2.48) | 0.003* | 3.03 (2.06–4.47) | 2.78 (1.83–4.22) | 0.006* |
| Men | 1.13 (0.86–1.49) | 1.09 (0.83–1.44) | 1.51 (1.10–2.06) | 1.22 (0.88–1.71) | ||
| Non-vascular non-cancer | ||||||
| Women | 1.48 (1.19–1.86) | 1.37 (1.09–1.72) | 0.68 | 1.62 (1.26–2.10) | 0.94 (0.72–1.24) | 0.17 |
| Men | 1.35 (1.12–1.63) | 1.21 (1.00–1.47) | 1.29 (1.05–1.59) | 0.87 (0.70–1.09) | ||
| All-cause | ||||||
| Women | 1.50 (1.26–1.79) | 1.41 (1.18–1.69) | 0.31 | 2.00 (1.63–2.46) | 1.23 (0.98–1.53) | 0.061 |
| Men | 1.28 (1.11–1.49) | 1.19 (1.02–1.38) | 1.55 (1.31–1.83) | 1.06 (0.88–1.27) | ||
PPDM post-pancreatitis diabetes mellitus, T2DM type 2 diabetes mellitus, T1DM type 1 diabetes mellitus, 95% CI 95% confidence interval
Adjusted hazard ratios were estimated from multivariable Cox regression models including age, ethnicity, alcohol abuse, ever smoking, social deprivation index, and Charlson comorbidity index as covariates
Significance of difference in adjusted hazard ratios between women and men, determined using the Altman and Bland method
Statistically significant after false discovery rate correction for multiple testing
In the head-to-head comparison between PPDM and T1DM, women with PPDM had a significantly higher risk of cancer mortality (adjusted HR, 2.78; 95% CI, 1.83–4.22) than men with PPDM (adjusted HR, 1.22; 95% CI, 0.88–1.71), and this difference was statistically significant (p=0.006; FDR-corrected p=0.024). Other findings are presented in Table 2. In the post hoc analysis, the risk of pancreatic cancer mortality associated with PPDM was significantly higher in both men (adjusted HR, 2.46; 95% CI, 1.07–5.67) and women (adjusted HR, 7.25; 95% CI, 2.56–20.58). After stratification by sex and age, the highest risk of cancer mortality associated with PPDM was observed in younger women (adjusted HR, 41.22; 95% CI, 2.70–629.73) (Supplementary Table 2). When categorizing PPDM as PPDM-A and PPDM-C, both PPDM-A (adjusted HR, 2.41; 95% CI, 1.52–3.84) and PPDM-C (adjusted HR, 3.95; 95% CI, 2.20–7.10) were significantly associated with a higher risk of cancer mortality in women, whereas the risks were not significant in men. Other findings are presented in Supplementary Table 3.
Age-stratified risk of cause-specific death
In the PPDM group, the elderly (aged 65 years or other) had the highest rates of vascular (52.1 per 1,000 person-years), cancer (38.9 per 1,000 person-years), and non-vascular non-cancer mortality (81.1 per 1,000 person-years) among the age groups (Supplementary Table 1). In the head-to-head comparison between PPDM and T2DM, none of the age group differences in cause-specific mortality risks were statistically significant (Table 3). When categorizing PPDM into PPDM-A and PPDM-C, the young adults (< 45 years) with PPDM-A were at a significantly higher risk of cancer mortality (adjusted HR, 4.88; 95% CI, 1.36–17.57), whereas the risk was not significant in the elderly. This difference was statistically significant (p = 0.022) but did not remain significant after FDR correction (p = 0.088). Other findings are presented in Supplementary Table 4.
Table 3.
Age-stratified risk of cause-specific death in post-pancreatitis diabetes mellitus versus type 2 and type 1 diabetes mellitus
| Mortality | PPDM vs. T2DM |
PPDM vs. T1DM |
||||
|---|---|---|---|---|---|---|
| Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | p for interaction† | Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | p for interaction† | |
|
| ||||||
| Vascular | ||||||
| < 45 years | 1.61 (0.36–7.12) | 1.16 (0.19–7.29) | p1 = 0.75 | 1.45 (0.31–6.72) | 0.27 (0.03–2.60) | p1 = 0.19 |
| 45–64 years | 1.05 (0.62–1.79) | 0.85 (0.48–1.49) | p2 = 0.98 | 1.37 (0.76–2.47) | 1.31 (0.68–2.53) | p2 = 0.24 |
| ≥ 65 years | 1.28 (1.04–1.59) | 1.19 (0.96–1.48) | 1.25 (0.96–1.61) | 1.05 (0.80–1.39) | ||
| Cancer | ||||||
| < 45 years | 2.88 (0.95–8.77) | 3.28 (0.82–13.07) | p1 = 0.43 | 3.56 (1.04–12.17) | 7.88 (1.59–39.10) | p1 = 0.14 |
| 45–64 years | 1.99 (1.36–2.90) | 1.83 (1.23–2.73) | p2 = 0.15 | 2.01 (1.31–3.09) | 2.26 (1.42–3.58) | p2 = 0.037 |
| ≥ 65 years | 1.23 (0.96–1.57) | 1.17 (0.92–1.50) | 1.21 (0.90–1.63) | 1.39 (1.02–1.90) | ||
| Non-vascular | non-cancer | |||||
| < 45 years | 2.50 (1.25–5.00) | 1.85 (0.82–4.19) | p1 = 0.41 | 2.53 (1.22–5.24) | 1.81 (0.71–4.62) | p1 = 0.36 |
| 45–64 years | 1.76 (1.30–2.37) | 1.28 (0.92–1.77) | p2 = 0.33 | 1.25 (0.91–1.72) | 1.13 (0.79–1.62) | p2 = 0.082 |
| ≥ 65 years | 1.32 (1.11–1.56) | 1.22 (1.03–1.45) | 0.88 (0.73–1.07) | 0.77 (0.63–0.95) | ||
| All-cause | ||||||
| < 45 years | 2.91 (1.64–5.16) | 2.12 (1.06–4.21) | p1 = 0.16 | 3.26 (1.76–6.07) | 2.86 (1.28–6.36) | p1 = 0.057 |
| 45–64 years | 1.63 (1.27–2.09) | 1.24 (0.95–1.63) | p2 = 0.13 | 1.36 (1.04–1.78) | 1.24 (0.92–1.68) | p2 = 0.013 |
| ≥ 65 years | 1.31 (1.15–1.49) | 1.22 (1.07–1.40) | 1.11 (0.95–1.29) | 1.01 (0.86–1.20) | ||
PPDM post-pancreatitis diabetes mellitus, T2DM type 2 diabetes mellitus, T1DM type 1 diabetes mellitus, 95% CI 95% confidence interval
Adjusted hazard ratios were estimated from multivariable Cox regression models including age, ethnicity, alcohol abuse, ever smoking, social deprivation index, and Charlson comorbidity index as covariates
Significance of difference in adjusted hazard ratios between the < 45 years group and the 45–64 years group (p1) or between the < 45 years group and the ≥ 65 years group (p2), determined using the Altman and Bland method
All p values for interaction were not statistically significant after false discovery rate correction for multiple testing
In the head-to-head comparison between PPDM and T1DM, the young adults with PPDM had a significantly higher risk of cancer mortality (adjusted HR, 7.88; 95% CI, 1.59–39.10) than the elderly with PPDM (adjusted HR, 1.39; 95% CI, 1.02–1.90). This difference was statistically significant (p = 0.037) but did not remain significant after FDR correction (p = 0.10). Other findings are presented in Table 3. When categorizing PPDM into PPDM-A and PPDM-C, the young adults with PPDM-A were at a significantly higher risk of cancer mortality (adjusted HR, 13.93; 95% CI, 2.93–66.29), whereas the risk was not significant in the elderly. This difference was statistical significant (p = 0.003; FDR-corrected p = 0.024). Other findings are presented in Supplementary Table 4.
Life expectancy in the study groups
Overall, remaining life expectancies decreased with age in all the study groups (Fig. 3). Of these, the PPDM group had the lowest remaining life expectancy in the up to (and including) 69 years age groups in women and in the up to (and including) 64 age groups in men. The life expectancy differences between the study groups were prominent in the young adults. For example, in women aged 30–34 years, life expectancy was 13.9 years (95% CI, 8.0–19.9) in the PPDM group, which was considerably lower than 21.2 years (95% CI, 17.9–24.4) in the T2DM group and 24.2 years (95% CI, 20.7–27.7) in the T1DM group. In men aged 30–34 years, life expectancy was 11.2 years (95% CI, 0.2–22.7) in the PPDM group, which was considerably lower than 21.0 years (95% CI, 18.2–23.8) in the T2DM group and 17.9 years (95% CI, 15.1–20.6) in the T1DM group. Other findings are presented in Supplementary Table 5.
Fig. 3.
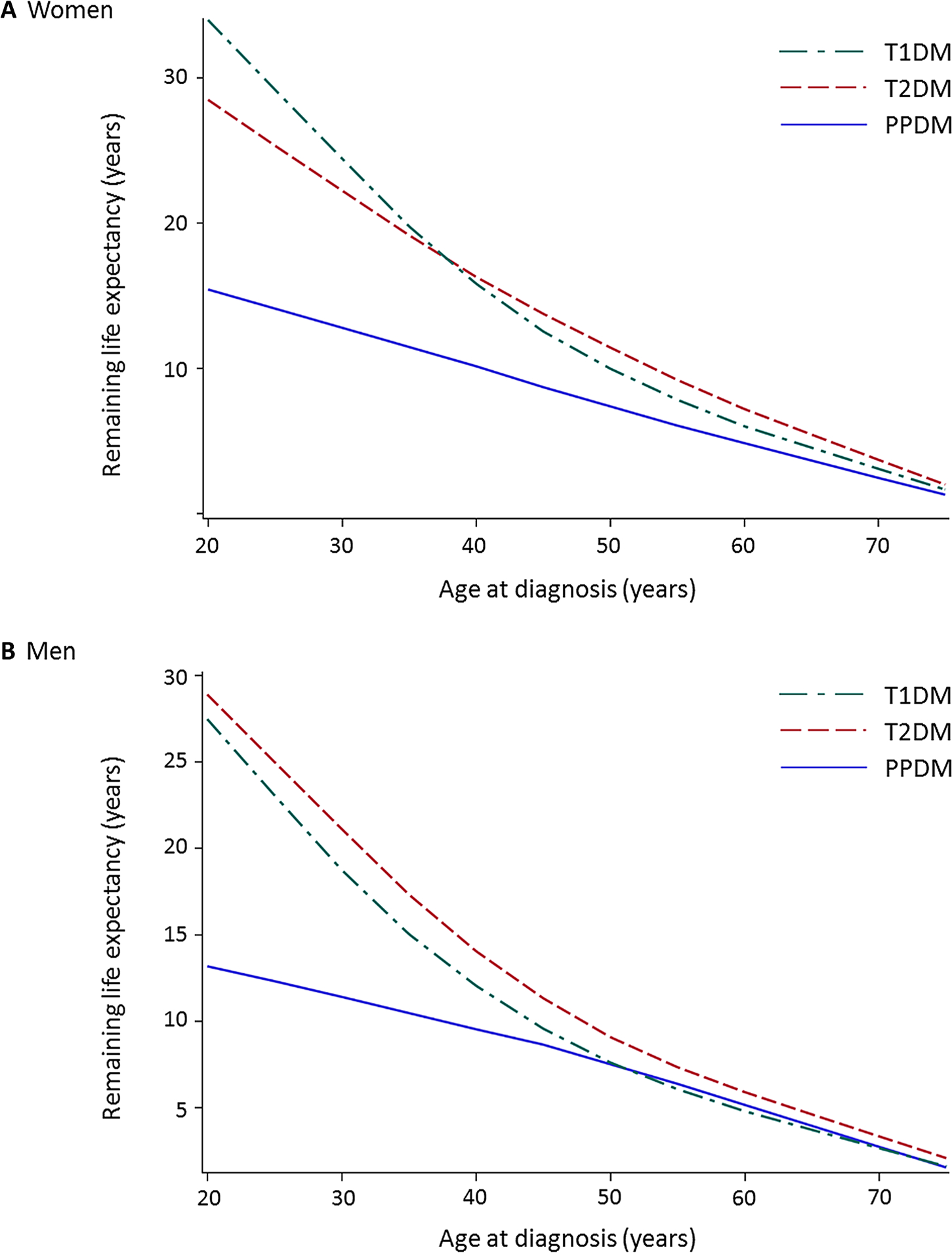
Remaining life expectancy by 5-year age intervals in type 1, type 2, and post-pancreatitis diabetes mellitus in a women and b menT1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, PPDM post-pancreatitis diabetes mellitus.
Discussion
This nationwide population-based study determined age- and sex-stratified risks of cause-specific death in PPDM compared with T2DM or T1DM. One of the most notable findings was that PPDM (versus T2DM) was associated with a 1.8-times significantly higher risk of cancer mortality in women, whereas there was no significant association between PPDM and the risk of cancer mortality in men (p for interaction = 0.003). We also found that PPDM (versus T1DM) was associated with a 2.8-times significantly higher risk of cancer mortality in women, whereas there was no significant association between PPDM and the risk of cancer mortality in men (p for interaction = 0.006). In addition, both women and men with PPDM (versus T2DM or T1DM) had lower life expectancy up to 64 years of age. In particular, young adults tended to exhibit greater life expectancy gaps between PPDM and the other types of diabetes (Fig. 3).
The present study was the first to reveal the sex difference in the risk of cause-specific death associated with PPDM. In the head-to-head comparison between PPDM and T2DM, women with PPDM were at a 70% significantly greater risk of cancer mortality than men with PPDM. Notably, this sex difference was observed in both PPDM-A (a 52% greater risk in women) and PPDM-C (a 115% greater risk in women). In the head-to-head comparison between PPDM and T1DM, women with PPDM were at a 130% significantly greater risk of cancer mortality than men with PPDM. This difference was also observed in both PPDM-A (a 106% greater risk in women) and PPDM-C (a 182% greater risk in women). The above sex differences in the risk of cancer mortality associated with PPDM appear to be driven by higher risks of death from specific cancers in women with PPDM. In both head-to-head comparisons, individuals with PPDM had a higher risk of death from pancreatic cancer, and the risk was higher in women than men (albeit the difference was not statistically significant). The other cancers associated with higher risks of death in individuals with PPDM were colon cancer (compared with T2DM) and lung cancer (compared with T1DM). It is also noteworthy that life expectancy gaps tended to be larger in young women than in young men (albeit wide 95% CIs precluded the determination of statistical significance). The above findings suggest that young women with PPDM may represent a high-risk group for premature cancer mortality among individuals with diabetes mellitus. Although these people comprise a relatively small fraction of individuals with diabetes mellitus, they may yield greater loss of life expectancy and, hence, pose greater disease burden compared with the other age and sex groups. While the most up-to-date guidelines do not recommend screening for some cancers in young adults (e.g., < 50 years old for pancreatic cancer and colon cancer; < 55 years old for lung cancer) [26–28], the results of the present study might trigger a reconsideration of the recommended age for cancer screening, particularly among women.
Our earlier study found that PPDM is associated with a higher risk of pancreatic cancer, as compared with T2DM [29]. This association might be attributed to risk factors for pancreatitis (e.g., tobacco smoking and alcohol abuse), pathophysiology of pancreatitis (e.g., fibrosis and chronic inflammation), and intra-pancreatic fat deposition [30]. The present study adds to the literature a novel finding of the sex differences in the risk of cancer mortality overall (and pancreatic cancer mortality in particular) associated with PPDM (as compared with both T2DM and T1DM). The sex-specific mechanisms behind this finding are a matter of speculation at this stage. First, there might be a sex difference in terms of glycemic control. A population-based study using primary care records in the UK demonstrated that a 5-year incidence of poor glycemic control was significantly higher in individuals with PPDM-A (61.7%) and PPDM-C (64.9%) than those with T2DM (46.3%) in both sexes altogether [4]. A prospective study of 12,792 individuals from the USA found that the risk of cancer mortality associated with hyperglycemia (or poor glycemic control in individuals with diabetes) was significantly higher in women, but not in men [31]. This sex difference was also observed in other large prospective studies [32, 33], and the above studies collectively suggest that women are at a higher risk of cancer incidence and mortality associated with hyperglycemia (or poor glycemic control) compared with men. Hence, it is reasonable to suggest that poor glycemic control disproportionately increases the risk of cancer mortality in women with PPDM. Second, impaired gastrointestinal motility might partly contribute to the higher risk of cancer mortality in women with PPDM. Several previous studies showed that diabetic gastroparesis is more prevalent and severe in women with poor glycemic control [34, 35]. A large population-based study from the USA found that a 5-year survival rate was significantly lower in individuals with gastroparesis than in the general population (67% versus 81%) [36]. A study of individuals after pancreaticoduodenectomy showed that severe postsurgical gastroparesis was an independent risk factor for cancer mortality, which was ascribed to prolonged hospitalization and malnutrition [37]. Taken together, women with PPDM (which confers a higher risk for poor glycemic control compared with T2DM [4]) might be at a higher risk of developing severe gastroparesis, consequently leading to the heightened risk of cancer mortality. Further, the possible impact of estrogen that is known to decrease gastrointestinal motility [38] might, at least in part, support our findings of the considerebly reduced risk of cancer mortality associated with PPDM in aging women (Supplementary Table 2). Third, the poorer cancer-specific survival in women with PPDM might be related to chemotherapy. Preclinical studies suggested that hyperglycemia (or poor glycemic control) can attenuate chemotherapy efficacy via interference with apoptotic signaling and chemotherapy pharmacokinetics (e.g., increasing renal excretion of anticancer drugs) [39]. Moreover, it was reported that women are more likely to develop chemotherapy-induced toxicity than men [40, 41]. The poorer response to chemotherapy and higher likelihood of chemotherapy-induced toxicity might jointly contribute to the poorer cancer-specific survival in women with PPDM. Last, there is a possibility of the effect of sex difference in etiology of pancreatitis on cancer mortality. Although alcohol-related pancreatitis is more common in men than in women [3], women are more likely to develop alcohol-related pancreatitis at young age [42]. Taken together with the observation that young women displayed the highest risk of cancer mortality associated with PPDM (versus T2DM or T1DM) in the present study, young women with PPDM are more likely to have underlying alcohol-related pancreatitis, which might increase the risk for pancreatic cancer and, consequently, cancer mortality.
Several limitations are to be taken into account. First, individuals with diabetes mellitus were identified using hospital discharge data. However, not all included individuals with the diabetes codes were hospitalized for diabetes as both primary and up to 20 secondary codes were considered. Although our approach may have underestimated the number of individuals with diabetes mellitus, it was chosen to improve the comparability between PPDM and the other types of diabetes mellitus as, almost invariably, pancreatitis leads to hospitalization. Moreover, considering that individuals with diabetes mellitus diagnosed and managed in primary care only are more likely to have mild diabetes, our approach likely resulted in conservative risk estimates. Second, data on obesity—a well-established risk factor for cancer mortality [30, 43, 44], were not available. However, a population-based study using primary care records in the UK reported that individuals with T2DM had a higher proportion of obesity (48.2%) than those with PPDM-A (41.8%) and PPDM-C (less than 25%) [4]. Hence, the risks for cancer mortality associated with PPDM versus T2DM observed in the present study were likely conservative. By contrast, given that the above study also showed that the proportion of obesity was lowest in individuals with T1DM (9.9%), the higher risk of cancer mortality associated with PPDM versus T1DM might have been ascribed to the higher proportion of obesity in PPDM. However, the prevalence of obesity in T1DM varies across studies (ranging from 12 to 52%) [45] and, in the above study, 43.5% of the T1DM cases did not have information on body mass index [4]. More investigations are required to understand the role of obesity in the association between PPDM and cancer mortality. Third, alcohol abuse and tobacco smoking were identified using diagnostic codes. Although we used the identical automated methods for all the study groups to identify the confounders, there is a possibility of misclassification. This misclassification may not have differed between the study groups because the identification of alcohol abuse and smoking and that of the study groups were independent of each other. Last, we did not consider duration of diabetes, which may affect mortality risk [46]. However, there is no reason to believe that it would affect the studied associations differentially in women versus men. Further, the impact of diabetes duration on the studied associations may have been minimal in the present study as the study groups were matched based on calendar year of diabetes diagnosis (in addition to age and sex).
In conclusion, the present study unearthed considerable sex differences in the risk of cancer mortality associated with PPDM (compared with the other common types of diabetes). Specifically, women with PPDM had a significantly higher risk of cancer mortality than men with PPDM. Moreover, young women with PPDM had the highest risk of cancer mortality and the largest gap in life expectancy when compared with T1DM and T2DM.
Supplementary Material
Acknowledgement
This study was part of the Clinical and epidemiOlogical inveStigations in Metabolism, nutritiOn, and pancreatic diseaseS (COSMOS) program. COSMOS is supported, in part, by the Royal Society of New Zealand (Rutherford Discovery Fellowship to Professor Max Petrov).
Footnotes
Conflict of Interest The authors have nothing to disclose.
Compliance with ethical standards
Ethical approval Ethics approval was waived as per the New Zealand Ministry of Health guidelines.
Informed consent For this type of study formal consent was not required.
Supplementary Information The online version contains supplementary material available at (https://doi.org/10.1007/s00592-021-01683-0)
References
- 1.Xiao AY, Tan ML, Wu LM et al. (2016) Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 1(1):45–55. 10.1016/S2468-1253(16)30004-8 [DOI] [PubMed] [Google Scholar]
- 2.Bharmal SH, Cho J, Alarcon Ramos GC et al. (2020) Trajectories of glycaemia following acute pancreatitis: a prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol 55(8):775–788. 10.1007/s00535-020-01682-y [DOI] [PubMed] [Google Scholar]
- 3.Petrov MS, Yadav D (2019) Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 16(3):175–184. 10.1038/s41575-018-0087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodmansey C, McGovern AP, McCullough KA et al. (2017) Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care 40(11):1486–1493. 10.2337/dc17-0542 [DOI] [PubMed] [Google Scholar]
- 5.Cho J, Petrov MS (2020) Pancreatitis, pancreatic cancer, and their metabolic sequelae: projected burden to 2050. Clin Transl Gastroenterol 11(11):e00251. 10.14309/ctg.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho J, Scragg R, Pandol SJ, Goodarzi MO, Petrov MS (2019) Antidiabetic medications and mortality risk in individuals with pancreatic cancer-related diabetes and postpancreatitis diabetes: a nationwide cohort study. Diabetes Care 42(9):1675–1683. 10.2337/dc19-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho J, Scragg R, Petrov MS (2019) Risk of mortality and hospitalization after post-pancreatitis diabetes mellitus vs type 2 diabetes mellitus: a population-based matched cohort study. Am J Gastroenterol 114(5):804–812. 10.14309/ajg.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 8.Xu G, You D, Wong L et al. (2019) Risk of all-cause and CHD mortality in women versus men with type 2 diabetes: a systematic review and meta-analysis. Eur J Endocrinol 180(4):243–255. 10.1530/eje-18-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huxley RR, Peters SA, Mishra GD, Woodward M (2015) Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endo 3(3):198–206. 10.1016/s2213-8587(14)70248-7 [DOI] [PubMed] [Google Scholar]
- 10.Zoungas S, Woodward M, Li Q et al. (2014) Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 57(12):2465–2474. 10.1007/s00125-014-3369-7 [DOI] [PubMed] [Google Scholar]
- 11.Sattar N, Rawshani A, Franzén Se DK, Eliasson B, Gudbjörnsdottir S (2019) Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 139(19):2228–2237. 10.1161/circulationaha.118.037885 [DOI] [PubMed] [Google Scholar]
- 12.Rawshani A, Sattar N, Franzén S et al. (2018) Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392(10146):477–486. 10.1016/s0140-6736(18)31506-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrov MS (2021) Post-pancreatitis diabetes mellitus: prime time for secondary disease. Eur J Endocrinol. 10.1530/EJE-20-0468 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Petrov MS, Basina M (2021) Diagnosing and classifying diabetes in diseases of the exocrine pancreas. Eur J Endocrinol. 10.1530/EJE-20-0974 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 15.Shen HN, Yang CC, Chang YH, Lu CL, Li CY (2015) Risk of diabetes mellitus after first-attack acute pancreatitis: a national population-based study. Am J Gastroenterol 110(12):1698–1706. 10.1038/ajg.2015.356 [DOI] [PubMed] [Google Scholar]
- 16.The Emerging Risk Factors Collaboration (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364(9):829–841. 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ (2016) Life expectancy of type 1 diabetic patients during 1997–2010: a national Australian registry-based cohort study. Diabetologia 59(6):1177–1185. 10.1007/s00125-015-3857-4 [DOI] [PubMed] [Google Scholar]
- 18.Cho J, Walia M, Scragg R, Petrov MS (2019) Frequency and risk factors for mental disorders following pancreatitis: a nationwide cohort study. Curr Med Res Opin 35(7):1157–1164. 10.1080/03007995.2018.1560748 [DOI] [PubMed] [Google Scholar]
- 19.Cho J, Scragg R, Petrov MS (2020) Use of insulin and the risk of progression of pancreatitis: a population-based cohort study. Clin Pharmacol Ther 107(3):580–587. 10.1002/cpt.1644 [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P et al. (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 21.Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326(7382):219. 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eayres D, Williams E (2004) Evaluation of methodologies for small area life expectancy estimation. J Epidemiol Commun H 58(3):243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schanzer D, Antoniou T, Kwong J, Timmerman K, Yan P (2018) Empirical estimation of life expectancy from a linked health database of adults who entered care for HIV. PLoS ONE 13(4):e0195031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang CL (1968) The life table and its construction. Introduction to stochastic processes in biostatistics. John Wiley & Sons, New York, pp 189–214 [Google Scholar]
- 25.SAS Institute Inc. (2013) The LOESS procedure. In: SAS/STAT® 13.1 user’s guide. SAS Institute Inc., Cary, NC [Google Scholar]
- 26.Humphrey LL, Deffebach M, Pappas M et al. (2013) Screening for lung cancer with low-dose computed tomography: a systematic review to update the US preventive services task force recommendation. Ann Intern Med 159(6):411–420. 10.7326/0003-4819-159-6-201309170-00690 [DOI] [PubMed] [Google Scholar]
- 27.Lin JS, Piper MA, Perdue LA et al. (2016) Screening for colorectal cancer: updated evidence report and systematic review for the US preventive services task force. JAMA 315(23):2576–2594. 10.1001/jama.2016.3332 [DOI] [PubMed] [Google Scholar]
- 28.Aslanian HR, Lee JH, Canto MI (2020) AGA clinical practice update on pancreas cancer screening in high-risk individuals: expert review. Gastroenterology 159(1):358–362. 10.1053/j.gastro.2020.03.088 [DOI] [PubMed] [Google Scholar]
- 29.Cho J, Scragg R, Petrov MS (2020) Postpancreatitis diabetes confers higher risk for pancreatic cancer than type 2 diabetes: results from a nationwide cancer registry. Diabetes Care 43(9):2106–2112. 10.2337/dc20-0207 [DOI] [PubMed] [Google Scholar]
- 30.Sreedhar UL, DeSouza SV, Park B, Petrov MS (2020) A systematic review of intra-pancreatic fat deposition and pancreatic carcinogenesis. J Gastrointest Surg 24(11):2560–2569. 10.1007/s11605-019-04417-4 [DOI] [PubMed] [Google Scholar]
- 31.Joshu CE, Prizment AE, Dluzniewski PJ et al. (2012) Glycated hemoglobin and cancer incidence and mortality in the atherosclerosis in communities (ARIC) study, 1990–2006. Int J Cancer 131(7):1667–1677. 10.1002/ijc.27394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stattin P, Björ O, Ferrari P et al. (2007) Prospective study of hyperglycemia and cancer risk. Diabetes Care 30(3):561–567. 10.2337/dc06-0922 [DOI] [PubMed] [Google Scholar]
- 33.Stocks T, Rapp K, Bjørge T et al. (2009) Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (ME-CAN): analysis of six prospective cohorts. PLOS Medicine 6(12):e1000201. 10.1371/journal.pmed.1000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickman R, Wainstein J, Glezerman M, Niv Y, Boaz M (2014) Gender aspects suggestive of gastroparesis in patients with diabetes mellitus: a cross-sectional survey. BMC Gastroenterol 14(1):34. 10.1186/1471-230X-14-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gold-Smith FD, Chand SK, Petrov MS (2018) Post-pancreatitis diabetes mellitus: towards understanding the role of gastrointestinal motility. Minerva Gastroenterol Dietol 64(4):363–375. 10.23736/s1121-421x.18.02507-2 [DOI] [PubMed] [Google Scholar]
- 36.Jung HK, Choung RS, Locke GR et al. (2009) The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted county, Minnesota, from 1996 to 2006. Gastroenterology 136(4):1225–1233. 10.1053/j.gastro.2008.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Futagawa Y, Kanehira M, Furukawa K et al. (2017) Impact of delayed gastric emptying after pancreaticoduodenectomy on survival. J Hepatobiliary Pancreat Sci 24(8):466–474. 10.1002/jhbp.482 [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Greenwood-Van Meerveld B, Johnson AC, Travagli RA (2019) Role of estrogen and stress on the brain-gut axis. Am J Physiol Gastrointest Liver Physiol 317(2):G203–G209. 10.1152/ajpgi.00144.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerards MC, van der Velden DL, Baars JW et al. (2017) Impact of hyperglycemia on the efficacy of chemotherapy-a systematic review of preclinical studies. Crit Rev Oncol Hemat 113:235–241. 10.1016/j.critrevonc.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 40.Davidson M, Wagner AD, Kouvelakis K et al. (2019) Influence of sex on chemotherapy efficacy and toxicity in oesophagogastric cancer: a pooled analysis of four randomised trials. Eur J Cancer 121:40–47. 10.1016/j.ejca.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 41.Cristina V, Mahachie J, Mauer M et al. (2018) Association of patient sex with chemotherapy-related toxic effects: a retrospective analysis of the PETACC-3 trial conducted by the EORTC gastrointestinal group. JAMA Oncol 4(7):1003–1006. 10.1001/jamaoncol.2018.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masamune A, Kume K, Shimosegawa T (2013) Sex and age differences in alcoholic pancreatitis in Japan: a multicenter nationwide survey. Pancreas 42(4):578–583. 10.1097/MPA.0b013e31827a02bc [DOI] [PubMed] [Google Scholar]
- 43.Parr CL, Batty GD, Lam TH et al. (2010) Body-mass index and cancer mortality in the Asia-Pacific cohort studies collaboration: pooled analyses of 424,519 participants. Lancet Oncol 11(8):741–752. 10.1016/s1470-2045(10)70141-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju S et al. (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388(10046):776–786. 10.1016/s0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbin KD, Driscoll KA, Pratley RE et al. (2018) Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 39(5):629–663. 10.1210/er.2017-00191 [DOI] [PubMed] [Google Scholar]
- 46.Huo L, Magliano DJ, Rancière F et al. (2018) Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997–2011. Diabetologia 61(5):1055–1063. 10.1007/s00125-018-4544-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


