Abstract
Targeted delivery of chemotherapeutic drugs can improve their therapeutic efficiency by localizing their toxic effects at the diseased site. This is often achieved either by direct conjugation of drugs to antibodies targeting overexpressed receptors on cancer cells (antibody-drug conjugates/ADCs) or by conjugating antibodies to nanoparticles bearing drugs (antibody-nanoparticle conjugates/ANCs). Here, we report a platform for utilizing hinge cysteines on antigen-binding fragment (Fab’) of an anti-CD4 antibody for site-specific conjugation to nanoparticles giving rise to anti-CD4 Fab’- nanoparticle conjugates (Fab’-NCs). We demonstrate a convenient route for obtaining functional anti-CD4 Fab’ from full-length antibody and examine the targeted delivery efficiencies of anti-CD4 Fab’-NCs vs ANCs for selective delivery to CD4high mT-ALL cells. Our results indicate that higher avidity of full-length anti-CD4 antibody i.e., protein alone translated to higher binding ability to CD4high mT-ALL cells in comparison with anti-CD4 Fab’ alone. However, the targeted delivery efficiency of anti-CD4 Fab’-NCs were comparable to ANCs indicating that the avidity of Fab’s is restored in a nanoparticle-conjugate format. Our results indicate that Fab’-NCs are equally capable of achieving targeted drug delivery to CD4high T-cells as ANCs and are a versatile alternative to ANCs by offering site-selective modification strategy while retaining their advantages.
Keywords: Antigen-binding Fragment (Fab’), Antibody Nanoparticle Conjugates, Targeted Delivery, Polymeric Nanoparticles/Nanogels, T-ALL
Graphical Abstract

INTRODUCTION
The field of antibody-drug conjugates (ADCs) as therapeutics for cancer treatment has seen tremendous growth in the past decade.1 With ~11 FDA approved ADCs on the market and multiple ongoing clinical trials, the field is only expected to grow more.2 ADCs improve the cytotoxicity of chemotherapeutic drugs by localizing their effects on cancer cells. Therefore, ADCs enhance the therapeutic window of anti-cancer drugs by minimizing their systemic toxicities by lowering the burden of chemotherapeutics on healthy cells.3,4 Even though ADCs have been successful, they suffer from an inherently low drug-to-antibody ratio (DAR) imposing restrictions on the potency of drug as well as on linker requirements for conjugation of drugs to the targeting antibody. Antibody-nanoparticle conjugates (ANCs) can address the challenges associated with ADC development while retaining their advantages. For instance, several nanoparticle formulations have been approved by FDA5,6 since nanoparticles enhance the pharmacokinetics and pharmacodynamics of chemotherapeutics by offering stability in blood circulation and stimuli-responsive release in the tumor microenvironment.7–11 Therefore, tunability in nanoparticle design for high drug loading and a universal handle for antibody conjugation can be combined with the targetability of antibodies to generate next-generation nanomedicines, viz. ANCs.12,13,14
Attachment of targeting antibody to nanoparticles is commonly achieved either by reacting lysines with activated groups on nanoparticles using EDC (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide) chemistry or by utilizing lysines to install chemical handles such as thiol, azide, and tetrazine for bio-conjugation to nanoparticles.15 Since lysine modifications result in a heterogenous modifications, more site-selective methods for conjugation have evolved such as engineering proteins to introduce cysteines (Thiomabs16–18), using interchain disulfide bonds, introducing unnatural amino acids and via engineered tags.19,20 The enhanced efficacy of homogeneity obtained as a result of site-specific conjugation of drugs to antibodies has been demonstrated in ADC format with Thiomab technology developed by Genentech.16
Inspired by the success of site-selective modification strategies, we developed a methodology for achieving site-specific conjugation of targeting antibody to the nanoparticles. We envisaged an approach to combine the advantages of ANC system with the benefits of site-selective modification of targeting antibody. This was carried out in three steps: (a) obtaining the antigen-binding domain with an intact thiol (Fab’) from the full-length antibody in two steps, (b) formulating a nanoparticle system with a chemical handle for bioconjugation and (c) installing the targeting antibody on the nanoparticle using a strain-promoted alkyne-azide cycloaddition (SPAAC) reaction. The smaller size of Fab’ confers additional advantages over full-length antibody21 such as ease of production in microbial systems22 (libraries generated via phage display23), higher penetration depths in solid tumors24, and minimized off-target interaction with Fc-receptor bearing immune cells.25 Consequently, many antibody fragments have entered the clinical trials with three FDA-approved Fabs in the market today.26
Due to the presence of unique receptors on immune cells such as with CD19/CD22 on B-cells and CD30 on T-cells, there are currently three FDA approved antibody-drug conjugates that are successful in the treatment of B/T-cell malignancies.27 T-cells have been shown to sustain anti-tumor immunity in addition to giving rise to T-cell lymphomas/leukemias. Therefore, targeting CD4 receptors on T-cells hold great therapeutic value as indicated by improved T-cell activity and tumor remodeling upon targeted delivery of TGF-β blocker using anti-CD4, TGF-β bispecific antibodies.28–29 The anti-CD4 antibody/Fab’ – nanoparticle conjugates can also be used for targeting CD4high mT-ALL cells30 for achieving targeted delivery of cytotoxic drugs (DM1 here) or immunomodulatory drugs for efficient cancer therapy. In this work, we investigate the targeting ability of Fab’-nanoparticle conjugates (anti-CD4 Fab’-NCs) and compare their performance with full-length anti-CD4 nanoparticle conjugates (anti-CD4 ANCs). We have developed a platform to obtain functional Fab’s from full length anti-CD4 antibody, utilized the hinge thiols to perform site-selective conjugation to nanoparticles and showed that smaller sized Fab’s conjugated nanoparticles, i.e., Fab’-NCs resulted in enhanced uptake in CD4high mT-ALL cells compared with their counterparts ANCs (Scheme 1).
Scheme 1:
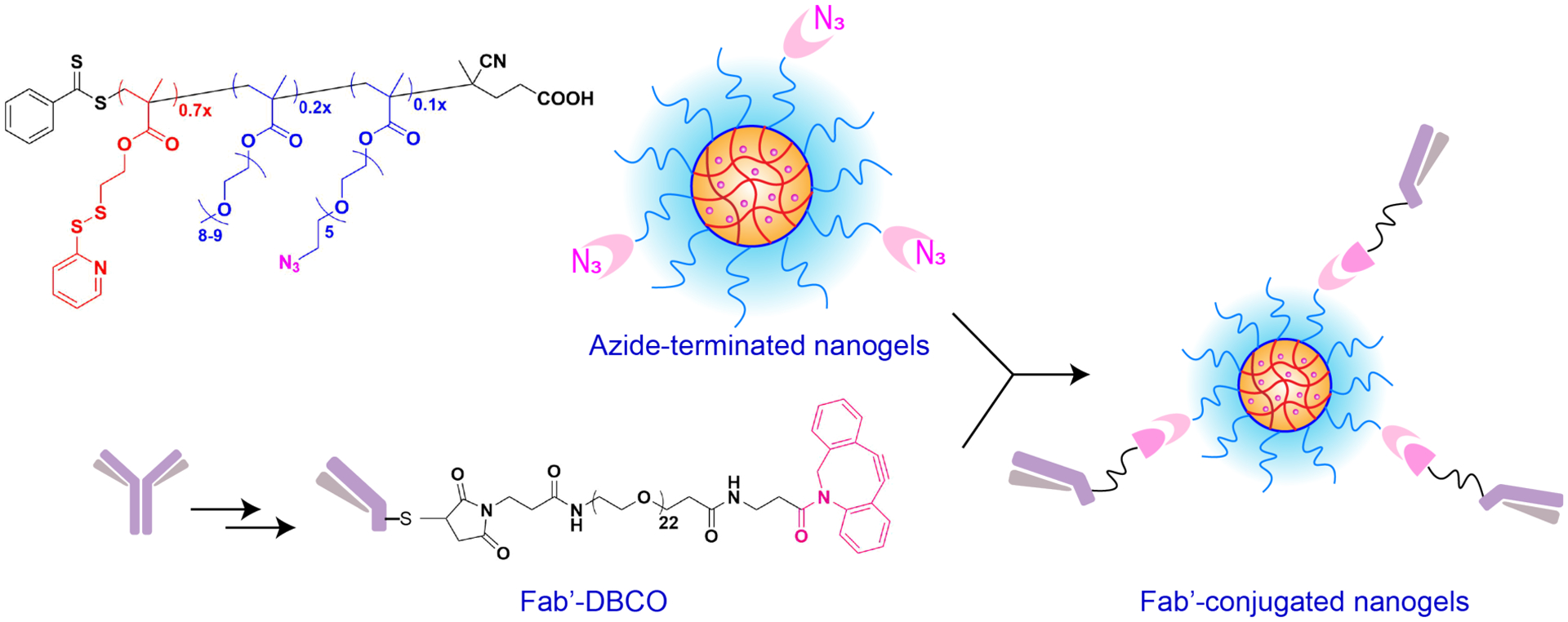
Formulation of antigen-binding fragment (Fab’) conjugated nanogels.
RESULTS AND DISCUSSION
Smaller antibody fragments such as Fab’ offer the opportunity to achieve site-specific conjugation distal from the binding domain of the antibody without the requirement for engineering proteins. Fab’ for any targeting antibody can be made from full-length antibody in two steps. In this work, anti-CD4 Fab’ with intact thiols for conjugation to nanogels were obtained from full-length anti-CD4 antibody (Figure 1a). In the first step, we treated anti-CD4 antibody with immobilized pepsin for various time intervals.32–33 Pepsin digestion of an antibody results in degradation of crystallizable fragment (Fc) of an antibody into multiple small fragments thereby yielding a dimer of Fab’ (Fab’)2. Since pepsin is a non-specific endopeptidase, we first optimized the time of incubation for digestion of anti-CD4 antibody for a recommended ratio of 4:1 IgG: immobilized pepsin resin. The time of incubation was varied from 15 minutes to 24 hours and the conversion percentage from full-length IgG to (Fab’)2 was monitored using size-exclusion chromatography (SEC) (Figure 1b) as well as SDS-PAGE gel electrophoresis (Figure S13). Complete conversion of anti-CD4 to (Fab’)2 was obtained in 24 hours at 37 °C as seen in Figure 1c. The (Fab’)2 was purified from the reaction mixture using size-exclusion chromatography. The hinge thiols on purified (Fab’)2 were selectively reduced using tris-(2-carboxyethyl)phosphine hydrochloride (TCEP) as the reducing agent. The conversion of (Fab’)2 to Fab’ was monitored using SEC (Figure 1d).
Figure 1:

(a) Schematic illustration for obtaining anti-CD4 Fab’ from full length antibody. (b) Time dependent pepsin digestion of full-length antibody followed by purification via size-exclusion chromatography (SEC) to obtain (Fab’)2; degraded products from Fc fragments can be observed at higher retention times i.e., after 10 minutes. (c) Inset of graph – b from 5 minutes to 10 minutes indicating decreasing concentrations of antibody (elution time ~6 min) and increasing concentrations of (Fab’)2 (elution time ~7 min). (d) Reduction of (Fab’)2 (elution time ~ 27 min) using TCEP at 24 °C for 35 minutes followed by purification using SEC to obtain Fab’ (elution time ~ 31 min).
After obtaining the anti-CD4 Fab’ from full-length antibody in two steps, we demonstrated that the CD4 recognition of anti-CD4 Fab’ were not lost during the reactions. Therefore, we next evaluated the binding ability of anti-CD4 Fab’ compared to the full-length antibody. For this assay, we treated CD4high mouse T-acute lymphoblastic leukemia (mT-ALL) cells with varying concentrations of Fab’/full-length antibody and quantified the amount of full-length antibody/Fab’ bound to the CD4 receptors on T-cells using a BV650 fluorophore tagged anti-k-light chain secondary antibody. As shown in Figure 2a, anti-CD4 Fab’ showed a concentration dependent binding to CD4high mT-ALL cells. Similarly, we observed that full-length anti-CD4 antibody demonstrated a concentration dependent binding (Figure 2b). However, the binding ability of anti-CD4 Fab’ towards CD4 receptor was lower in comparison to that of full-length antibody. To ascertain that the difference in binding ability does not originate from a difference in binding affinity, we performed a surface plasmon resonance experiment using soluble mouse CD4 protein and anti-CD4 antibody/Fab’. As shown in Figure S14, the binding affinities of the anti-CD4 full-length antibody as well as Fab’ are comparable suggesting that the difference in binding activity to CD4high mT-ALL cells could be attributed to higher avidity arising from the dimeric binding of the full-length antibody. (Figure 2b).
Figure 2:

(a) Evaluating the binding ability of the anti- CD4 Fab’ on CD4high mT-ALL cells using flow cytometry analysis. (b) Comparison of the binding ability of Fab’ in comparison with full-length antibody. (c) The availability of thiols on the Fab’ was investigated using western blot. The Fab’ with intact thiol was reacted with a maleimide-PEG-biotin linker and probed using streptavidin-HRP.
Note that the reduction of (Fab’)2 with TCEP results in Fab’ with free thiol groups. To demonstrate that the thiols on Fab’ were available for reaction with a maleimide linker, we incubated Fab’ with maleimide-PEG-biotin linker (Figure 2c). The linker modified Fab’ was purified using ultracentrifugation and excess linker was removed prior to gel electrophoresis. Biotin terminated Fab’ was then subjected to Western blot analysis using streptavidin-horseradish peroxidase (HRP) for visualization. As shown in Figure 2c, the Western blot analysis contained a bright band at ~55 kDa corresponding to Fab’-PEG-biotin. We also reacted the full-length antibody with maleimide-containing linker and analyzed the physical mixture using western blot (Figure S15a) and UV-Vis (Figure S15b). The blot probed with streptavidin-HRP (Figure S15a) shows that only Fab’ reacts with a maleimide linker whereas the antibody does not react significantly. Similarly, the DBCO peak at 310 nm are only observed with Fab’ (Figure S15b). Furthermore, the obtained anti-CD4 Fab’- linker when analyzed via SEC reveals that the Fab’ does not dimerize back to (Fab’)2 during this process (Figure S16). These data combined demonstrate that we are indeed able to obtain anti-CD4 Fab’ from full-length antibody, while retaining their binding ability and thiol reactivity.34–35
To facilitate conjugation of full-length antibody as well as the antigen-binding fragment (Fab’) of an antibody to the nanoparticle, we utilized a methacrylate based random copolymer. The antibody-nanoparticle conjugates (ANCs) were formulated by utilizing a random copolymer comprising of three monomer units. Polymer P1 was synthesized by reversible addition-fragmentation chain transfer polymerization (RAFT) using three methacrylate monomers, the hydrophobic component - pyridyl disulfide (PDS), the hydrophilic component comprising polyethylene glycol (PEG), and the azide-terminated polyethylene glycol (PEG-az). The details of the PDS and PEG-Azide monomer syntheses can be found in supplementary information (Figure S1–S8) and Figure S9–S11 describe in detail the polymer P1 synthesis/characterization. We have previously demonstrated that an amphiphilic polymer with a ratio of 3:7 between the hydrophilic components (PEG) and hydrophobic component (PDS) self-assembled to form nanoaggregates.36 The PDS units in these nanoaggregates can be cross-linked to obtain stable nanogels (NG) and these crosslinks are essential in stable encapsulation of guest molecules such as chemotherapeutic drugs for delivery to cancer cells. Therefore, to ensure that we maintain this PEG:PDS ratio at 3:7, we synthesized P1 with the monomer ratios of 0.7x PDS, 0.2x PEG and 0.1x PEG-az [see SI for synthesis and characterization details of monomers and polymer S1–S12]. As seen from the 1H-NMR integration of the methoxy peak from PEG units at 3.37 ppm and the aromatic peaks from PDS at 8.45 ppm, the ratio of 3x PEG and 7x PDS was confirmed (Figure S9 in SI). To validate that the polymer also contained PEG-az monomer, we showed that P1 contained a peak at 2100 cm−1 corresponding to the azide in the PEG-az monomer (Figure S12 in SI). In contrast, this peak was absent in a control PEG-PDS polymer – P0 (Figure S12).
We have previously shown that the nanoparticle formulated from PEG-PDS copolymer can further be conjugated to an antibody by carrying out a disulfide exchange reaction between the thiol terminated linker on the antibody and PDS groups on the nanogel.31 However, we anticipated that utilizing a dithiol reactive linker for conjugating the hinge-thiols on the antigen-binding domain (Fab’) of an antibody to PDS of the nanogel could result in undesired crosslinking between the Fab’ themselves as well as between the nanoparticles (Figure S17).
Therefore, we reasoned that an azide-containing PEG monomer would be a better alternative for facilitating conjugation of antibody/Fab’ to the nanoparticles. Moreover, we show that P1 PEG-PDS-PEG-az formed nanoparticles of similar sizes (Figure S18 for dynamic light scattering data) as compared with nanoparticles formulated from P0 PEG-PDS (Figure S19)31 The nanoparticles formed from P1 presented azide groups on the surface for conjugation of targeting units such as anti-CD4 antibody/Fab’.
Next, we formulated anti-CD4 Fab’-nanoparticle conjugates (Fab’-NCs) and compared the targeting ability of Fab’-NCs to ANCs. For conjugation of Fab’ to nanoparticles, we first reacted Fab’ with a maleimide-PEG-DBCO linker for 12 hours at 4 °C. The Fab’-maleimide-PEG-DBCO analyzed using MALDI-TOF MS (Figure S20) exhibits band broadening in comparison to Fab’ alone. After purification, Fab’-PEG-DBCO was incubated with nanogels (made from pol P1) to facilitate a bio-orthogonal strain-promoted azide-alkyne click (SPAAC) reaction between Fab’-PEG-DBCO and azide on the polymer to give anti-CD4 Fab’-NCs. The incorporation of PEG-DBCO on the Fab’ was measured using UV-Vis spectroscopy (Figure S 21a). In our previous report31, we first modified a full-length -antibody with an amine reactive NHS-PEG-PDS linker, deprotected the PDS unit to reveal the thiol followed by conjugation to the PDS of the nanoparticles via a disulfide reaction. Note that this conjugation strategy, however, would be inefficient in conjugation of Fab’ with an intact thiol to PDS of the nanogel (Figure S17). Therefore, in this study we installed an azide on the nanoparticle to design a universal strategy for conjugation of targeting units. The formulation and purification of anti-CD4 Fab’-nanoparticle conjugates (Fab’-NC) was studied by gel-shift assay and western blots (Figure S22). As shown in Figure S22(a), a PAGE gel shows that the crosslinked nanogel alone is unable to enter the gel and stains near the entrance of the gel lane. The PAGE gel indicates successful conjugation and purification of Fab’-NCs as seen by the absence of Fab’ in the Fab’-NC lanes (compared with a physical mixture of Fab’ with nanogel). To further confirm that the Fab’ was incorporated in the ANC format, a western blot was carried out using anti-rat light chain antibody. As shown in Figure S22b, only conjugates show a band compared with nanogel alone confirming the incorporation of Fab’ in the nanoparticle system.
To investigate the targetability and selectivity of anti-CD4 Fab’-NC for CD4high mT-ALL cells, we treated CD4high mT-ALL, CD4low mT-ALL and DO11.10 clone 2 cells (CD4−ve cells) with Fab’-NCs for 30 minutes at 4 °C and compared with the anti-CD4 ANC (Figure 3a and 3b). The CD4+ mT-ALL cells were first obtained from mice. However, since we have shown that mT-ALL cells lose CD4 expression in culture, we obtained two population from this culture (a) an inherently low CD4 expressing population (CD4low) and (b) transfected CD4low cells with a CD4 expressing construct to obtain CD4high cells.31 The nanogel utilized in this study were formulated to contain a lipophilic dye, DiO, to allow for targeting studies using fluorescence-activated cell sorting (FACS). The results comparing Fab’-NCs and ANCs, henceforth, refer to anti-CD4 Fab’s and full-length antibody conjugated to polymeric nanoparticles. As indicated in Figure 3b, Fab’-NCs selectively targeted CD4high cells over CD4low T-cells and DO11.10 clone 2 cells, whereas the unconjugated nanoparticles exhibited minimal staining. Note that the uptake of both - DiO containing nanoparticle (NG) as well as anti-CD4 antibody/Fab’ conjugated nanoparticles (NGAb / NGFab) is lower in CD4low mT-ALL cells compared to CD4 negative DO11.10 cells for the same concentration of nanoparticle feed. This difference in uptake could be due to an inherent difference in the rate of nanoparticle endocytosis between the two cells.37 In a similar study, we incubated these cells with anti-CD4 Fab’-NCs for 3 hours at 37 °C to evaluate targeted uptake of these conjugates. The data in Figure 3d shows that Fab’-NCs exhibited minimal uptake in CD4low mT-ALL whereas the uptake in CD4high mT-ALL cells were much higher than the bare nanoparticles alone. These data confirm that the selectivity of Fab’-NCs for CD4high mT-ALLs is indeed due to anti-CD4 Fab’ interaction with CD4 receptors on T-cells.
Figure 3:

To evaluate receptor-specific surface staining, CD4low mT-ALL, DO11.10 clone 2 and CD4high mT-ALL cells were incubated with anti-CD4 antibody conjugated NG(DiO) and Fab’-conjugated NG(DiO) for 30 minutes at 4 °C. Bar graph plots show DiO fluorescence for surface staining of Ab-NG(DiO) - (a) and Fab’-NG(DiO) - (b). CD4 receptor-mediated uptake of antibody-NG(DiO) – (c) and Fab’-NG(DiO) – (d) was evaluated at 37 °C for 3 hours in CD4low and CD4high mT-ALL cells. CD4low mT-ALLs are shown in red, DO11.10 clone 2 cells are in green and CD4high mT-ALLs are in blue. The concentrations of the nanogels are 100 μg/mL. The error bars are replicative of three measurements.
Since the binding ability of anti-CD4 Fab’ alone is lower when compared to the binding ability of full-length antibody (Figure 2b), we asked if the targeting ability of Fab’-NCs would also be lower when compared to anti-CD4 ANCs. To formulate ANCs using P1, we modified anti-CD4 antibody with an NHS-PEG-DBCO linker for 12 hours at 4 °C, removed excess linker by ultracentrifugation, and quantified the amount of linker incorporated using UV-Vis spectroscopy (Figure S21b). This linker modified antibody was installed on the nanogel using SPAAC reaction, analogous to Fab’-NC, and purified on size-exclusion chromatography (Figure S23). Full-length antibody was also modified with different molar ratio of linker to obtain linker/antibody of 1 and ~6. Analogous to characterization of Fab’-NCs, the anti-CD4 ANCs were subjected to SDS-PAGE (Figure S24a) and western blot analysis (Figure S24b). The results indicate that the purified ANCs do not contain free antibody (Figure S24a) and western blot shows that ANCs indeed are comprised of antibody (Figure S24b). We also investigated the stabilities of Fab’-NCs and ANCs in serum conditions using dynamic light scattering. As shown in Figure S25, the size evolution of both Fab’-NCs as well as ANCs do not change drastically over time and provide an avenue for a fair comparison in cell uptake studies. The ANCs with the linker/Ab ~ 1 were capable of selectively staining CD4high mT-ALL cells at 4 °C and exhibited higher uptake at 37 °C compared to the control cells (Figure 3a and 3c). While the antibody with six linkers resulted in higher uptake in targeted CD4high mT-ALL cells (Figure S26), for the same feed ratio of the antibody/Fab’ to nanoparticle and linker/protein ratio of ~1, we observed that the targeting ability of Fab’-NCs and ANCs were comparable indicating that the targeting ability of Fab’s were restored when presented in a nanoparticle conjugate format (compare Figure 3a/3c with 3b/3d). Additionally, internalization of DiO encapsulated, anti-CD4 ANC and Fab’-NCs in CD4high mT-ALL cells was also demonstrated using confocal microscopy (Figure S27).
Having demonstrated the selectivity of ANCs/Fab’-NCs using a dye encapsulated nanoparticle, we next evaluated the targeted drug delivery ability of the ANCs/Fab’-NCs using mertansine (DM1) as the drug (Figure 4a). We have previously developed and characterized DM1 conjugated nanoparticles that are capable of releasing drug only under the redox conditions of the intracellular environment.31 Before testing the toxicities of DM1-conjugated anti-CD4 ANCs/Fab’-NCs, the effects of the empty nanogel on CD4high mT-ALL cells and CD4low mT-ALL cells were evaluated. As shown in Figure S28, empty nanogel alone is non-toxic to the cells indicating that the small percentage of azide incorporated into the polymer does not lead to cell death. DM1-loaded full-length anti-CD4 antibody nanoparticle conjugates resulted in inhibition of CD4high mT-ALL cell growth with higher efficiency compared to CD4low mT-ALL cells (Figure 4d) and DO11.10 cells (Figure S29a). Similarly, the selective toxicity of anti-CD4 Fab’ nanoparticle conjugates was tested in both CD4high, CD4low mT-ALL cells and DO11.10 cells. Similar to anti-CD4 ANCs, Fab’-NCs displayed a dose-dependent and preferential cell growth inhibition of CD4high mT-ALL cells over CD4low mT-ALL cells (Figure 4e) and DO11.10 cells (Figure S29b). The anti-CD4 Fab’-NCs were more potent compared to the anti-CD4 ANCs resulting in lower inhibitory concentrations (IC50) values for CD4high mT-ALL with IC50 of Fab’-NC being 0.99 μg/mL, compared with 3.18 μg/mL for ANCs. We hypothesized that Fab’-NCs demonstrate higher toxicity in CD4high mT-ALL cells due to a higher number of Fab’s conjugated per nanoparticle as compared to the number of antibodies per nanoparticle. To evaluate the ratio of antibody/Fab’ conjugated to the nanoparticle, we developed a calibration plot of AF647 labelled anti-CD4 antibody and Fab’. The dye labeled targeting moieties were then conjugated to DiO containing nanogel and a ratio of AF647 to DiO was used to provide an estimate for protein conjugated to a constant amount of nanogel (Figure S30). The AF647/DiO ratio is about 1.5 times higher for Fab’-NC than ANC indicating greater amount of Fab’ is conjugated to the nanoparticle surface. To further quantify the amount of functional targeting moieties, we then performed in-cell ELISA and developed a calibration plot for anti-CD4 antibody (Figure S31) and Fab’ (Figure S32) individually. Using the calibration plot, we found that for a fixed concentration of 0.125 mg/mL of polymer nanoparticle seven times more Fab’ was conjugated (132 pmol) as compared to full-length antibody (18.2 pmol). Therefore, anti-CD4 Fab’ have an added advantage due to their small size resulting in higher conjugation efficiency as well as in site-specific conjugation to the nanoparticles for better orientation of the targeting unit. Furthermore, to confirm that this toxicity was a result of targeted delivery of DM1 and did not arise due to antibody/Fab’, we treated the cells with empty nanoparticles conjugated with full-length antibody and Fab’. As shown in Figure 4b and 4c, no obvious toxicity was observed further confirming that both ANCs and Fab’-NCs are indeed capable of achieving targeted delivery of chemotherapeutics.
Figure 4:

To evaluate selective toxicity in CD4high mT-ALL cells over CD4low mT-ALL cells. (a) illustrates the structure of ANCs/Fab-NCs used in this study. (b) evaluates toxicity of empty nanoparticles conjugated with full-length antibody and (c) with Fab’ respectively. Finally, selective toxicity in CD4high mT-ALL over CD4low mT-ALL is demonstrated using DM1 containing ANCs (d) and Fab’-NCs (e). 2-way ANOVA was utilized for analysis in (d) and (e).
The efficiency of antibody-drug conjugates as well as antibody-nanoparticle conjugates depend on the choice of targeting receptor both in terms of receptor density and receptor internalization.38–40 Higher receptor expression and higher rate of antibody-receptor internalization are crucial for intracellular drug release in cancer cells. While many ADCs and ANCs are being developed for receptors with higher internalization rate, the portfolio of targeted receptors can be significantly expanded by utilizing multivalent antibody-receptor interactions to improve internalizations of non-internalizing/slow-internalizing receptors.41 Multivalent interactions between ligands and receptors result in intermolecular crosslinking of receptors (i.e., clustering) at the cell surface that improve the rate of receptor internalization.42 Research has indicated that multivalent interactions, either via multimers of ligand or via installation of ligands on the nanoparticle, also alter the mechanism of complex internalization by involving various endocytotic pathways.41,43–44 In our studies, while the Fab’ alone may not bind to the CD4 receptors as strongly as the full-length antibody due to higher avidity of the antibody, when presented in the context of nanoparticle conjugates, anti-CD4 Fab’-NCs as well as anti-CD4 ANCs could possibly engage multiple CD4 receptors on CD4high mT-ALL cells, thereby improving overall drug delivery efficiencies of the nanoparticle conjugates systems.
The data from our studies indicate that both ANCs and Fab’-NCs have their own merits. While anti-CD4 ANCs demonstrated higher selectivity towards CD4high mT-ALL cells over CD4low mT-ALLs, Fab’-NCs demonstrated higher potency than ANCs due to a higher Fab’/nanoparticle ratio. Since the CD4 receptor expression on CD4high mT-ALL cells utilized in our experiments was enhanced by transfecting CD4low mT-ALLs, CD4low mT-ALLs are not truly negative and represent this T-cell population with lower CD4 receptor expression. Our results indicate that the higher potency of anti-CD4 Fab’-NCs can be harnessed in two ways: (a) Fab’-NCs can be translated into achieving targeted delivery of highly potent chemotherapeutic drugs (such as DM1) to cancer cells that moderately overexpress tumor-specific antigens and (b) Fab’-NCs can be utilized to achieve targeted delivery of less potent drugs to cancer cells with high overexpression of tumor receptors – an important avenue where ADCs cannot be utilized for delivery of low potency chemotherapeutics.
CONCLUSIONS
In conclusion, we have demonstrated a platform for obtaining antigen-binding domain of antibody (Fab’) from full length anti-CD4 antibody in two steps. The obtained CD4 Fab’ retains its binding ability to CD4 receptors. Importantly, the process of generating the Fab’ domain naturally places a thiol moiety that offers a convenient handle for conjugation to nanoparticles. The binding capabilities of both Fab’ and full-length antibody was tested in the antibody-nanoparticle conjugates format, as Fab’-NCs and ANCs respectively, on CD4 high and low expressing T-cell lines. The results show that with respect to targeting CD4 receptors on mT-ALL cells, anti-CD4 Fab’-NCs are more potent in targeting and delivering chemotherapeutics as compared to antibody-conjugated nanoparticles whilst maintaining the site-selective conjugation strategy. Our future studies will focus on extending these studies to other antibody/receptor pair as well as utilize these Fab’-NCs/ANCs for the targeted delivery of chemotherapeutics.
MATERIALS AND METHODS
Monomer/Polymer Syntheses
The detailed synthesis and characterization of pyridyl-disulfide monomer, azide containing polyethylene glycol monomer (Figures S1–S8) as well as the polymer P1 (PEG-co-PDS-co-PEGAz) are described in the supplementary information (Figures S9–S12).
Pepsin Digestion
The anti-CD4 antibody (5 mg) was buffer exchanged with digestion buffer using a spin column and concentrated to 10 mg/mL. The composition for digestion buffer is 20 mM sodium acetate trihydrate pH 4. Immobilized pepsin (250 μL) from ThermoFisher was washed with digestion buffer (1 mL) three times. The resin was separated from digestion buffer by centrifugation at 1000 G for 5 minutes. The resin was resuspended in 500 μL digestion buffer and added to 500 μL of the antibody from the step above. The mixture is then incubated at 37 °C for 12 hours, ensuring constant mixing of gel during incubation. The resin is separated by centrifugation and the supernatant decanted into a new tube. To ensure maximal recovery the resin is washed with 10 mM Tris HCl, pH 7.5 and added to the tube containing antibody fragments. The combined fractions were dialyzed overnight in PBS pH 7.2 at 4 °C to remove small Fc fragments, obtaining the anti-CD4 Fab’-dimer.
Reducing Fab’-dimer (Fab’)2 to Fab’ and obtaining Fab’-DBCO
Anti-CD4 Fab’-dimer obtained after pepsin digestion was buffer exchanged with 5 mM Ethyelenediaminetetraacetic acid (EDTA) containing PBS (pH 7.2) buffer using a 0.5 mL (10 kDa) centrifugal filters to obtain a final concentration of 0.75 mg/mL solution of (Fab’)2 in 5 mM EDTA, PBS 7.2 buffer. 4.2 mM Tris(2-carboxyethyl) phosphine (TCEP) (1.2 mg/mL, MW = 286.64 g/mol) in PBS was prepared. 10 μL of this TCEP solution was added to a 100 μL of Fab’-dimer to bring the solution to a final concentration of 0.42 mM TCEP. The solution was incubated at 24 °C for 30 mins and subjected to purification on size-exclusion chromatography. Superdex 200 10/300 GL (purchased from GE) column was used for running the antibody samples. The samples were run in 1X, 5 mM EDTA PBS buffer (pH 7.2) at 0.5 mL/min for 60 minutes each. Anti-CD4 Fab’ were collected after purification, concentrated using a 10 kDa ultra-centrifugation filters and reacted with 10X Maleimide-Peg1k-DBCO linker (PG2-DBML-1k, Nanocs). The reaction was shaken at 4 °C overnight followed by purification using 10-kDa Amicon centrifugal filters. The absorbances at A280 for Fab’ and A310 for dibenzocyclooctyne (DBCO) were used to calculate the concentration of Fab’-DBCO and linker to Fab’ ratio.
Antibody modification with NHS-Peg8-DBCO
Anti-CD4 antibody was buffer exchanged to a reaction buffer composed of 0.2 M Na2HPO4 and 0.1 M NaCl at pH 8.5 using a 50-kDa Amicon ultracentrifugation filter. For 5 mg/mL concentration of antibody (0.5 mg antibody per reaction), 10X of NHS-Peg-8-DBCO (BroadPharm) was added for linker: antibody ratio of 1 (40X for a linker: antibody ratio of 5) and incubated at 4 °C overnight. The unreacted linker was removed from the mixture by centrifugation using 50-kDa filters. The concentration of Ab-DBCO was assessed using UV-Vis spectroscopy reading at A280 and A310.
DiO encapsulated Nanogel (NG) Preparation
The polymer (P1-PEG-PDS-Azide) was dissolved in water at 10 mg/mL concentration to obtain the polymeric micelle. To obtain a DiO encapsulated nanogel, we added 2% w/w DiO dissolved in 5% v/v acetone. The solution was stirred for 6–8 hours at room temperature to allow evaporation of acetone. 20% of pyridyl disulfide (PDS) units in the polymeric micelles were then cross-linked by adding a calculated amount of 0.1 M dithiothreitol (DTT) to yield dye-encapsulated nanogel. The % cross-linking was further verified by monitoring the release of pyridinethione using UV-Vis spectrometer at 343 nm. For calculating the concentration of pyridinethione released, molar extinction coefficient of 8080 M−1cm−1 was used. The sizes of the nanoparticles were measured on Malvern NanoZetasizer- ZS. The samples were filtered through 0.22 μm syringe filter to remove excess unencapsulated dye before using in any other experiment.
DM1-encpasulated Nanogel – NG (DM1)
A previously reported protocol was followed by making mertansine (DM1) encapsulated nanogel.31 Briefly, for a 10 mg/mL solution of the polymer (P1 – PEG-PDS-Azide) in water, 10% PDS units were replaced by DM1 by adding 20 μL of a 100 mM stock of DM1 in DMSO. The reaction was stirred at room temperature for 1 day followed by cross-linking 20% of remnant PDS units to obtain a stably encapsulated DM1-nanogel solution. Unreacted DM1 and released pyridine-2-thione were removed by dialysis in PBS. The incorporation of DM1 into nanogel was monitored using UV-Vis at 252 nm.
Preparation of Fab’ and Antibody Conjugated Nanogels
NG(DiO) [DiO - 3,3’-Dioctadecyloxacarbocyanine Perchlorate] as well as NG(DM1) (100 μg polymer) were mixed with anti-CD4 antibody-DBCO (100 μg polymer) at a 1:1 weight ratio and shaken at room temperature for 24 hours. The conjugates were purified using SEC to remove unreacted antibody to give anti-CD4 antibody nanoparticle conjugates (ANCs). NG(DiO) and NG(DM1) (100 μg polymer) were reacted with anti-CD4 Fab’-DBCO (100 μg polymer) at 1:1 weight ratio and shaken at room temperature for 24 hours. The conjugates were purified to remove unreacted Fab’ using ultra-centrifugation to obtain anti-CD4 Fab’-nanoparticle conjugates (Fab’-NCs)
Sodium Dodecyl Sulphate – Polyacrylamide Gel Electrophoresis (SDS PAGE) Gel
The gel was run in running buffer comprised of 25 mM Tris base, 0.192 M glycine and 0.1% SDS at a constant voltage of 150 V until the dye from the loading buffer ran out of the gel. The gel was stained in Coomassie stain solution (0.125% w/v Coomassie Blue R250 in 50% v/v methanol and 10% v/v acetic acid) for a few hours followed by de-staining with a solution composed of 45% v/v methanol and 10% v/v acetic acid until appropriately de-stained. The gels were then imaged and processed on ImageJ for analysis.
Western Blots
The SDS-PAGE gels were run according to the protocol stated above. The gel was placed on polyvinylidene difluoride (PVDF) membrane sandwiched between two Whatman filter papers and a sponge on each side. The protein transfer was done at 100 V for an hour. The transfer buffer composition is 25 mM Tris base, 0.192 M glycine and 200 mL methanol. The membrane was blotted in 5% milk, 0.1% Tween-20 in PBS for an hour at room temperature and probed with goat anti-rat light chain primary antibody (Cell signaling technology, 1:1000 dilution in PBS) at 4 °C overnight The membrane is washed with 0.1% Tween-20 in PBS for 15 minutes (three times) and incubated with streptavidin-horseradish peroxidase (HRP) for 30 minutes at room temperature. After another cycle of washing the membrane in 0.1% Tween-20 in PBS, chemiluminescent reagent was used, and the membrane was imaged.
Size-Exclusion Chromatography (SEC)
Superdex 200 10/300 GL (purchased from GE) column was used for running the antibody samples. The samples were run in 1X PBS buffer, 5mM EDTA pH 7.2 at 0.5 mL/min for 60 minutes each.
Flow Cytometry: Calibration Plots for Full-Length Antibody and Fab’
0.3 million CD4high cells in 0.1 mL media were plated in 96-well tissue culture plate. Different dilution of PE-labelled anti-CD4 antibody (BD Biosciences) were added to the wells. The plate was incubated at 4 °C for 30 minutes with 5% CO2 to estimate receptor-specific binding to CD4high cells. For titration with anti-CD4 Fab’ and full-length antibody, we used a dye labeled secondary antibody (BV650 Mouse anti-rat IgG light chain k, BD Biosciences) that recognizes the light chain of the first antibody. The cells were washed with PBS two times and stained with Zombie Voilet dye (Biolegend) as per the protocol provided by the manufacturer. The zombie violet staining helps us in excluding dead cells from data analysis. Zombie dye was quenched with serum containing PBS. The cells were washed with PBS one more time and resuspended in FACS buffer (1% BSA in PBS). The samples were then subjected to flow cytometry on a BD LSRFortessa using FACSDiva Software and the data was analyzed on FlowJo.
General Flow Cytometry Protocol
CD4high, CD4low as well as DO11.10 cells (CD4−ve) are suspension cells. The cells are counted prior to the experiment and 0.4 m cells in 100 μL media are treated with varying concentrations of antibody/Fab’/ANC/Fab’-NC for a specified time duration (nanoparticle concentrations = 100 μg/mL unless otherwise stated). After incubation, the cells are washed with 1X PBS three times, resuspended in FACS buffer, and analyzed by flow cytometry.
Confocal Imaging
0.4 million CD4high T-cells in 0.5 mL media were seeded in 48-well plate and same concentrations of NG(DiO)Ab and NG(DiO)Fab (8 μg/mL) were added to the well. The cells were incubated for 3 hours in 37 °C, 5% CO2 incubator. Following the treatment, cells were collected and transferred to an Eppendorf tube, spun at 300 G, and washed twice with PBS. The cell cytosol was stained using CellMask Deep red plasma membrane stain (ThermoFisher) for 10 minutes at 37 °C. The cells were pelleted and washed with PBS twice followed by fixation with 0.5 mL 4% formaldehyde in PBS at 37 °C for 10 minutes. Finally, the cells were washed with 0.5 mL PBS (three times), resuspended in PBS, added to poly-D-lysine coated confocal dishes and subjected to confocal imaging on Nikon A1R SIMe.
In-cell Enzyme Linked Immunosorbent Assay (ELISA)
100,000 cells in 100 μL PBS were added to a 96-well plate provided as a part of In-Cell ELISA kit from Abcam. The plate was centrifuged at 500 G for 8 min at room temperature (RT), 100 μL of 8% paraformaldehyde was added to each well and immediately centrifuged at 500 G for an additional 8 minutes. The plate was incubated for 15 minutes followed by 3 washes with 300 μL PBS three times. After washes, 200 μL of blocking buffer was added and incubated at RT for 2 hours. Different concentrations of anti-CD4 antibody in incubation buffer was added to the wells and incubated at 4 °C overnight. After this step, the wells were washed with 250 μL wash buffer three times, treated with anti-rat IgG HRP (Abcam) and incubated for 2 hours at RT while being protected from light. The wells were washed three times with wash buffer and ELISA substrates (BD Biosciences) were added to each well. Once the blue color developed, 50 μL Stop solution was added and the plate was read at 450 nm.
Cell Viability Assays
20,000 cells in 100 μL media were added to each well of 96-well plate and treated with serial dilutions of different nanoparticle formulations. After 4 h of incubation, the plate was spun at 300 G for 5 minutes, the old media was removed and 100 μL new media was added. The plate was incubated for an additional of 48 hours. 100 μL of Cell Titer purchased from Promega (G9242) was added to each well, shaken for 2 minutes and incubated for 10 minutes. 100 μL of this solution was transferred to an opaque, white 96-well plate and luminescence was read on SpectraMax® iD5 multiplate reader.
Supplementary Material
ACKNOWLEDGMENTS
Part of this work was supported by the NIGMS of the NIH (GM-136395). This work was supported in part by a Fellowship from the University of Massachusetts to Khushboo Singh as part of the Biotechnology Training Program (National Research Service Award T32 GM108556). We would like to thank Drs. Michelle Kelliher and Justine E. Roderick from University of Massachusetts Medical School (Worcester, MA) for providing the mT-ALL cell line. We thank Dr. James Chambers at the UMass Light Microscopy Center and Dr. Amy Burnside at the UMass Flow Cytometry Center for their help. We also thank Dr. Stephen Eyles, Dr. Eugenia Clerico at UMass Amherst, Dr. Michelle Arkin and Dr. Ziwen Jiang at University of California, SF for helpful discussion with SPR experiments. We thank Dr. Bin Liu at UMass Amherst for helpful discussions and suggestions.
ABBREVIATIONS
- ADC
Antibody Drug Conjugates
- ANC
Antibody Nanoparticle Conjugates
- Fab’-NC
Fab’ Nanoparticle Conjugates
- FDA
Food and Drug Administration
- DAR
Drug to Antibody Ratio
- mT-ALL
mouse T-cell Acute Lymphoblastic Leukemia
- SPAAC
Strain Promoted Azide-Alkyne Cycloaddition
- TCEP
Tris(2-carboxyethyl) phosphine
- DBCO
Dibenzocyclooctyne
- DTT
Dithiothreitol
- NG
Nanogel
- DM1
Mertansine
- SDS-PAGE
Sodium Dodecyl Sulphate – Polyacrylamide Gel Electrophoresis
- PVDF
Polyvinylidene Difluoride
- HRP
Horse Radish Peroxidase
- ELISA
Enzyme Linked Immunosorbent Assay
- RAFT
Reversible Addition-Fragmentation Chain Transfer
- PDS
Pyridyl Disulfide
- PEG
Polyethylene Glycol
- MA
Methacrylate
- DiO
3,3’-Dioctadecyloxacarbocyanine Perchlorate
- PEG
Polyethylene Glycol
- NHS
N-Hydroxysuccinimide
Footnotes
Supporting information contains Materials, detailed synthetic protocols for monomers and polymers; Characterizations of the materials using 1H NMR, 13C NMR and gel permeation chromatography (GPC) analysis of polymers; supporting figures and table.
The authors declare no competing financial interest.
REFERENCES:
- (1).do Pazo C; Nawaz K; Webster RM The Oncology Market for Antibody–Drug Conjugates. Nat. Rev. Drug Discov 2021, No. March, 1–8. [DOI] [PubMed] [Google Scholar]
- (2).Zhao P; Zhang Y; Li W; Jeanty C; Xiang G; Dong Y Recent Advances of Antibody Drug Conjugates for Clinical Applications. Acta Pharm. Sin. B 2020, 10 (9), 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Polakis P Arming Antibodies for Cancer Therapy. Curr. Opin. Pharmacol 2005, 5 (4), 382–387. [DOI] [PubMed] [Google Scholar]
- (4).Wu AM; Senter PD Arming Antibodies: Prospects and Challenges for Immunoconjugates. Nat. Biotechnol 2005, 23 (9), 1137–1146. [DOI] [PubMed] [Google Scholar]
- (5).Anselmo AC; Mitragotri S Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med 2019, 4 (3), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Shi J; Kantoff PW; Wooster R; Farokhzad OC Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17 (1), 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gordon MR; Zhao B; Anson F; Fernandez A; Singh K; Homyak C; Canakci M; Vachet RW; Thayumanavan S Matrix Metalloproteinase-9-Responsive Nanogels for Proximal Surface Conversion and Activated Cellular Uptake. Biomacromolecules 2018, 19 (3), 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Mitchell MJ; Billingsley MM; Haley RM; Wechsler ME; Peppas NA; Langer R Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov 2021, 20 (2), 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zhuang J; Gordon MR; Ventura J; Li L; Thayumanavan S Multi-Stimuli Responsive Macromolecules and Their Assemblies. Chem. Soc. Rev 2013, 42 (17), 7421–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bobo D; Robinson KJ; Islam J; Thurecht KJ; Corrie SR Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res 2016, 33 (10), 2373–2387. [DOI] [PubMed] [Google Scholar]
- (11).Manzari MT; Shamay Y; Kiguchi H; Rosen N; Scaltriti M; Heller DA Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater 2021, 6 (4), 351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Johnston MC; Scott CJ Antibody Conjugated Nanoparticles as a Novel Form of Antibody Drug Conjugate Chemotherapy. Drug Discov. Today Technol 2018, 30, 63–69. [DOI] [PubMed] [Google Scholar]
- (13).Mi P; Cabral H; Kataoka K Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater 2020, 32 (13), 1–29. [DOI] [PubMed] [Google Scholar]
- (14).Arruebo M; Valladares M; González-Fernández Á Antibody-Conjugated Nanoparticles for Biomedical Applications. J. Nanomater 2009, 2009. [Google Scholar]
- (15).Sivaram AJ; Wardiana A; Howard CB; Mahler SM; Thurecht KJ Recent Advances in the Generation of Antibody–Nanomaterial Conjugates. Adv. Healthc. Mater 2018, 7 (1), 1–25. [DOI] [PubMed] [Google Scholar]
- (16).Junutula JR; Raab H; Clark S; Bhakta S; Leipold DD; Weir S; Chen Y; Simpson M; Tsai SP; Dennis MS; et al. Site-Specific Conjugation of a Cytotoxic Drug to an Antibody Improves the Therapeutic Index. Nat. Biotechnol 2008, 26 (8), 925–932. [DOI] [PubMed] [Google Scholar]
- (17).Nunes JPM; Vassileva V; Robinson E; Morais M; Smith MEB; Pedley RB; Caddick S; Baker JR; Chudasama V Use of a next Generation Maleimide in Combination with THIOMAB™ Antibody Technology Delivers a Highly Stable, Potent and near Homogeneous THIOMAB™ Antibody-Drug Conjugate (TDC). RSC Adv 2017, 7 (40), 24828–24832. [Google Scholar]
- (18).Vollmar BS; Wei B; Ohri R; Zhou J; He J; Yu SF; Leipold D; Cosino E; Yee S; Fourie-O’Donohue A; et al. Attachment Site Cysteine Thiol PKa Is a Key Driver for Site-Dependent Stability of THIOMAB Antibody-Drug Conjugates. Bioconjug. Chem 2017, 28 (10), 2538–2548. [DOI] [PubMed] [Google Scholar]
- (19).Forte N; Chudasama V; Baker JR Homogeneous Antibody-Drug Conjugates via Site-Selective Disulfide Bridging. Drug Discov. Today Technol 2018, 30, 11–20. [DOI] [PubMed] [Google Scholar]
- (20).Yamada K; Ito Y Recent Chemical Approaches for Site-Specific Conjugation of Native Antibodies: Technologies toward Next-Generation Antibody–Drug Conjugates. ChemBioChem 2019, 20 (21), 2729–2737. [DOI] [PubMed] [Google Scholar]
- (21).Richards DA; Maruani A; Chudasama V Antibody Fragments as Nanoparticle Targeting Ligands: A Step in the Right Direction. Chem. Sci 2017, 8 (1), 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hudson PJ; Souriau C Engineered Antibodies. Nat. Med 2003, 9 (1), 129–134. [DOI] [PubMed] [Google Scholar]
- (23).Holliger P; Hudson PJ Engineered Antibody Fragments and the Rise of Single Domains. Nat. Biotechnol 2005, 23 (9), 1126–1136. [DOI] [PubMed] [Google Scholar]
- (24).Yokota T; Milenic DE; Whitlow M; Schlom J Rapid Tumor Penetration of a Single-Chain Fv and Comparison with Other Immunoglobulin Forms. Cancer Res 1992, 52 (12), 3402–3408. [PubMed] [Google Scholar]
- (25).Brouillard A; Deshpande N; Kulkarni AA Engineered Multifunctional Nano- and Biological Materials for Cancer Immunotherapy. Adv. Healthc. Mater 2021, 10 (6), 1–35. [DOI] [PubMed] [Google Scholar]
- (26).Mullard A FDA Approves 100th Monoclonal Antibody Product. Nat. Rev. Drug Discov 2021, No. May, 1–16. [DOI] [PubMed] [Google Scholar]
- (27).Dean AQ; Luo S; Twomey JD; Zhang B Targeting Cancer with Antibody-Drug Conjugates: Promises and Challenges. MAbs 2021, 13 (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Li S; Liu M; Do MH; Chou C; Stamatiades EG; Nixon BG; Shi W; Zhang X; Li P; Gao S; et al. Cancer Immunotherapy via Targeted TGF-β Signalling Blockade in TH Cells. Nature 2020, 587 (7832), 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Liu M; Kuo F; Capistrano KJ; Kang D; Nixon BG; Shi W; Chou C; Do MH; Stamatiades EG; Gao S; Li S; Chen Y; Hsieh JJ; Hakimi AA; Tanuichi I; Chan TA; Li MO TGF-β Suppresses Type 2 Immunity to Cancer. Nature 2020, 587 (7832), 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chiaretti S; Foà R T-Cell Acute Lymphoblastic Leukemia. Haematologica 2009, 94 (2), 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Canakci M; Singh K; Munkhbat O; Shanthalingam S; Mitra A; Gordon M; Osborne BA; Thayumanavan S Targeting CD4+Cells with Anti-CD4 Conjugated Mertansine-Loaded Nanogels. Biomacromolecules 2020, 21 (6), 2473–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Valedkarimi Z; Nasiri H; Aghebati-Maleki L; Abdolalizadeh J; Esparvarinha M; Majidi J Production and Characterization of Anti-Human IgG F(Ab’)2 Antibody Fragment. Hum. Antibodies 2018, 26 (4), 171–176. [DOI] [PubMed] [Google Scholar]
- (33).Jones RGA; Landon J A Protocol for “Enhanced Pepsin Digestion”: A Step by Step Method for Obtaining Pure Antibody Fragments in High Yield from Serum. J. Immunol. Methods 2003, 275 (1–2), 239–250. [DOI] [PubMed] [Google Scholar]
- (34).Merdan T; Callahan J; Petersen H; Kunath K; Bakowsky U; Kopečková P; Kissel T; Kopeček J Pegylated Polyethylenimine-Fab′ Antibody Fragment Conjugates for Targeted Gene Delivery to Human Ovarian Carcinoma Cells. Bioconjug. Chem 2003, 14 (5), 989–996. [DOI] [PubMed] [Google Scholar]
- (35).Licea AF; Becerril B; Possani LD FAB Fragments of the Monoclonal Antibody BCF2 Are Capable of Neutralizing the Whole Soluble Venom from the Scorpion Centruroides Noxius Hoffmann. Toxicon 1996, 34 (8), 843–847. [DOI] [PubMed] [Google Scholar]
- (36).Ryu J-H; Chacko RT; Jiwpanich S; Bickerton S; Babu PR; Thayumanavan S Self-Cross-Linked Polymer Nanogels: A Versatile Nanoscopic Drig Delivery Platform. J. Am. Chem. Soc 2010, 132, 17227–17235. [DOI] [PubMed] [Google Scholar]
- (37).Chen HH; Chien CC; Petibois C; Wang CL; Chu YS; Lai SF; Hua TE; Chen YY; Cai X; Kempson IM; et al. Quantitative Analysis of Nanoparticle Internalization in Mammalian Cells by High Resolution X-Ray Microscopy. J. Nanobiotechnology 2011, 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Damelin M; Zhong W; Myers J; Sapra P Evolving Strategies for Target Selection for Antibody-Drug Conjugates. Pharm. Res 2015, 32 (11), 3494–3507. [DOI] [PubMed] [Google Scholar]
- (39).Sievers EL; Senter PD Antibody-Drug Conjugates in Cancer Therapy. Annu. Rev. Med 2013, 64, 15–29. [DOI] [PubMed] [Google Scholar]
- (40).Ritchie M; Tchistiakova L; Scott N Implications of Receptor-Mediated Endocytosis and Intracellular Trafficking Dynamics in the Development of Antibody Drug Conjugates. MAbs 2013, 5 (1), 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Radford DC; Yang J; Doan MC; Li L; Dixon AS; Owen SC; Kopeček J Multivalent HER2-Binding Polymer Conjugates Facilitate Rapid Endocytosis and Enhance Intracellular Drug Delivery. J. Control. Release 2020, 319, 285–299. [DOI] [PubMed] [Google Scholar]
- (42).York SJ; Arneson LS; Gregory WT; Dahms NM; Kornfeld S The Rate of Internalization of the Mannose 6-Phosphate/Insulin-like Growth Factor II Receptor Is Enhanced by Multivalent Ligand Binding. J. Biol. Chem 1999, 274 (2), 1164–1171. [DOI] [PubMed] [Google Scholar]
- (43).Poźniak M; Porębska N; Krzyścik MA; Sokołowska-Wędzina A; Jastrzębski K; Sochacka M; Szymczyk J; Zakrzewska M; Otlewski J; Opaliński Ł The Cytotoxic Conjugate of Highly Internalizing Tetravalent Antibody for Targeting FGFR1-Overproducing Cancer Cells. Mol. Med 2021, 27 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Pozniak M; Sokolowska-Wedzina A; Jastrzebski K; Szymczyk J; Porebska N; Krzyścik MA; Zakrzewska M; Miaczynska M; Otlewski J; Opaliński Ł FGFR1 Clustering with Engineered Tetravalent Antibod Yimproves the Efficiency and Modifies the Mechanism Of Receptor Internalization. Mol. Oncol 2020, 1998–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


