Abstract
Objective
We investigated whether a positive thyroid peroxidase antibody (TPO Ab) status before radioactive iodine (RAI) therapy in patients with Graves’ hyperthyroidism is a predictive factor for developing hypothyroidism post RAI.
Methods
We performed a retrospective study of patients with Graves’ hyperthyroidism with known TPO Ab status, receiving the first administration of RAI. Patients from four thyroid outpatient centres in Belgium receiving their first RAI therapy between the years 2011 and 2019 were studied. Clinical, laboratory, imaging, and treatment data were recorded from medical charts. Hypothyroidism and cure (defined as combined hypo- and euthyroidism) were evaluated in period 1 (≥2 and ≤9 months, closest to 6 months post RAI) and period 2 (>9 months and ≤24 months post RAI, closest to 12 months post RAI).
Results
A total of 152 patients were included of which 105 (69%) were TPO Ab-positive. Compared to TPO Ab-negative patients, TPO Ab-positive patients were younger, had a larger thyroid gland, and had more previous episodes of hyperthyroidism. In period 1, 89% of the TPO Ab-positive group developed hypothyroidism and 72% in the TPO Ab-negative group (P = 0.007). In period 2, the observation was similar: 88% vs 72% (P = 0.019). In the multivariate logistic regression analysis, a positive TPO Ab status was associated with hypothyroidism in period 2 (adjusted OR: 4.78; 95% CI: 1.27–20.18; P = 0.024). In period 1, the aOR was 4.16 (95% CI: 1.0–18.83; P = 0.052).
Conclusion
A positive TPO Ab status in patients with Graves’ hyperthyroidism receiving the first administration of RAI is associated with a higher risk of early hypothyroidism.
Keywords: thyroid peroxidase antibodies, Graves’ disease, radioactive iodine therapy, hypothyroidism
Introduction
Radioactive iodine (RAI) has been used for the treatment of patients with Graves’ hyperthyroidism since the 1950s. After a single RAI administration, patients ideally become euthyroid but frequently develop hypothyroidism. On the other hand, hyperthyroidism can persist or relapse necessitating either a second RAI administration or other treatment modalities.
The optimal method for determining the activity of RAI and predicting the thyroid functional outcome remains challenging (1). Several studies identified pre-treatment parameters that could help in the prediction of the thyroid functional outcome such as age, gender, thyroid gland volume, free thyroxine (fT4) and free triiodothyronine (fT3) at diagnosis, and thyroid-stimulating hormone receptor antibody (TSH-R Ab) titer or radiation absorbed dose (2, 3, 4, 5, 6, 7, 8).
Thyroid peroxidase antibodies (TPO Abs) are associated with autoimmune thyroid disease, and their measurement is helpful in the diagnostic workup of hypothyroidism (9). Besides, in euthyroid patients, a positive TPO Ab status is associated with an increased risk for the development of hypothyroidism (10), and patients with silent or post-partum thyroiditis typically have elevated TPO Ab levels (11). In patients with Graves’ hyperthyroidism, TPO Ab status is neither routinely evaluated during the diagnostic workup nor measured to guide management. Treatment with RAI is known to elicit an increase in thyroid antibodies for approximately 1 year, including TPO Ab, considered an immune response secondary to the release of thyroid antigens (12, 13, 14).
To date, the role of the TPO Ab status in patients with hyperthyroidism due to Graves’ disease prior to RAI administration has not been well studied as a predictive factor for thyroid functional outcome.
In this study, we hypothesize that a positive TPO Ab status prior to the first administration of RAI in patients with Graves’ disease increases the incidence of post RAI hypothyroidism.
Material and methods
Patient selection and data recorded
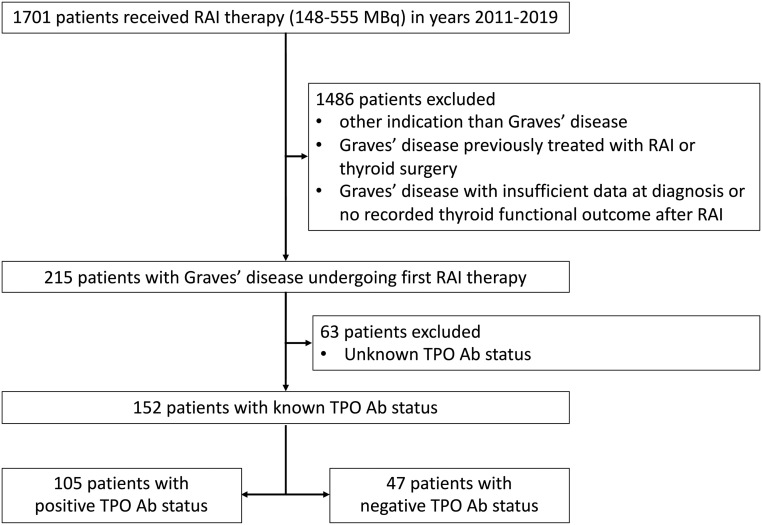
We performed a retrospective study of medical records from patients with Graves’ disease treated with RAI between 1 January 2011 and 31 December 2019 at four hospitals in Belgium, one academic hospital (University Hospitals Leuven) and three large non-academic hospitals (Imelda Hospital Bonheiden, OLV Hospital Aalst-Asse-Ninove, General Hospital Sint Jan Brugge). The patients were identified by registers of administered RAI kept by the departments of nuclear medicine. The search was limited to patients who were administered RAI in the activity range from 148 to 555 MBq, as lower activities are only used for diagnostic purposes and higher activities for selective ablation post-thyroidectomy in patients with differentiated thyroid cancer. The medical records were screened for eligibility. Only patients with Graves’ disease receiving the first administration of RAI were included. Graves’ disease was defined as a combination of biochemical hyperthyroidism (suppressed TSH with high fT4 and/or fT3) and a positive TSH-R Ab titer or a homogeneous normal to increased technetium (Tc)-99m uptake on thyroid scintigraphy. Patients with a history of thyroid surgery, central hypothyroidism, insufficient data at diagnosis, no recorded thyroid functional outcome after RAI, and patients without TPO Ab assessment prior to RAI were excluded (Fig. 1).
Figure 1.
Patient selection.
The following data were extracted at the diagnosis of Graves’ disease or at the most recent episode prior to first RAI in case of relapse hyperthyroidism: gender, age, body weight, number of previous episodes of Graves’ hyperthyroidism, history of spontaneous hypothyroidism (in case of female patients), history of post-partum thyroiditis, smoking status, presence of active Graves’ orbitopathy, uptake (%) of technetium on thyroid scintigraphy. Thyroid gland dimensions and presence of nodules >1 cm (maximum diameter) on ultrasonography (US) were recorded based on the US report. The thyroid volume of each lobe was calculated using the formula: width (cm) × length (cm) × depth (cm) × 0.479 for each lobe (15). The thyroid volume was the sum of the volume of both lobes.
The following data were extracted between diagnosis and administration of first RAI: treatment with anti-thyroid drugs (ATD) with or without levothyroxine (L-T4) and treatment with corticosteroids. During and after RAI administration, the following data were collected: administered activity, time of initiation and dosage of L-T4, use of ATDs, execution of thyroid surgery, and second RAI administration.
The following laboratory measurements were recorded at diagnosis: TSH, fT4, TSH-R Abs, TPO Abs. The TPO Ab level closest to the date of diagnosis of Graves’ hyperthyroidism and always prior to RAI administration was recorded, with a limit of 3 months around the diagnosis of last episode of Graves’ hyperthyroidism and before RAI. The TPO Ab status was expressed as positive or negative compared to the manufacturer’s cut-off value. For period 1 (defined as the period between ≥2 and ≤9 months and closest to 6 months post RAI) and period 2 (defined as the period between >9 months and ≤24 months post RAI and closest to 12 months post RAI), TSH and fT4 were recorded. In one centre (University Hospitals Leuven), instead of assessing fT4 directly by immunoassay, total T4 was measured and a free T4 index (FTI) was calculated as total T4 (μg/dL) divided by T3 uptake (%). Therefore, in this study, fT4 is a term encompassing direct measurements of fT4 and calculated FTI. As different laboratory assays for fT4 and TSH-R Abs were used between the centres and during the time period studied (years 2011–2019), results were expressed relative to the upper limit of normal (ULN) and the cut-off value of the assay used, respectively.
The study was approved by the Ethical Committee of the University Hospitals Leuven and the Ethical Committee of each participating centre.
Definitions of outcomes
Hypothyroidism was defined as a TSH above the ULN, or fT4 below the lower limit of normal, or use of L-T4 excluding patients with concomitant use of ATD as block-replacement therapy.
Cure was defined as the sum of hypothyroidism (as defined above) and euthyroidism, with euthyroidism defined as TSH and fT4 within the normal reference range in a patient not synchronously treated with ATDs or L-T4.
Hyperthyroidism was defined as either not meeting the criteria of cure or having undergone thyroid surgery or having received a second administration of RAI prior to evaluation in period 1 or 2.
Statistics
The Kolmogorov–Smirnov test was used to verify the assumption of symmetrical distribution of all continuous variables. Symmetrically distributed variables and descriptive data are presented as mean ± s.d., whereas asymmetrical variables are presented as median with first and third quartiles (Q1:Q3). For the analysis of symmetrically distributed variables, unpaired t-tests were performed to detect differences between the groups. Non-symmetrically distributed variables were analysed by Mann–Whitney tests. For categorical data, presented as % (n), and Pearson’s χ2-test was used. In the case of patient numbers ≤5, the χ2-test with Yates’s correction for continuity was used.
A multivariate logistic regression was performed with hypothyroidism as a dependent outcome and TPO Ab status as a predictor, adjusted for the following variables: age at diagnosis, fT4 at diagnosis, TSH-R Ab at diagnosis, thyroid volume at diagnosis, ATD preceding RAI (ATD alone or ATD+LT4), and RAI activity. Results are expressed as crude and adjusted odds ratios with 95% CI. A P value of <0.05 was considered statistically significant. Analyses were performed with IBM SPSS statistics version 28.0.0 and R Statistical Software (v4.1.3; R Core Team 2021).
Results
Baseline patient characteristics
During the study period, out of 1701 patients who received activity of RAI between 148 and 555 MBq, 152 patients with Graves’ hyperthyroidism and a known TPO Ab status were included (Fig. 1). The baseline patient characteristics according to the TPO Ab status are summarized in Table 1. Sixty-nine percent of patients (n = 105) are TPO Ab-positive, and in both groups, most patients are female. Compared to patients with a negative TPO Ab status, patients with a positive TPO Ab status are younger, had more previous episodes of hyperthyroidism, have a higher thyroid gland volume, and more frequently received ATD before administration of RAI.
Table 1.
Baseline patient characteristics prior to first radioiodine therapy.
| TPO Ab-positive (n = 105) | TPO Ab-negative (n = 47) | P value | |
|---|---|---|---|
| Female | 77.1% (81) | 70.2% (33) | 0.362 |
| Age (years) | 52.8 ± 13.5 | 61.3 ± 14.4 | 0.001 |
| Body weight (kg) | 69.2 ± 12.4 | 68.6 ± 12.4 | 0.768 |
| Active orbitopathy | 6.8% (5/74) | 7.1% (2/28) | 1.000 |
| Active smoker | 12.1% (11/91) | 5.3% (2/38) | 0.394 |
| History of HYPO or PPT | 2.9% (3) | 4.3% (2) | 1.000 |
| First episode of hyperthyroidism | 51.4% (54) | 76.6% (36) | 0.035 |
| Laboratory values at diagnosis | |||
| Free T4a | 1.6 (1.1:2.4) | 1.8 (1.3:2.5) | 0.293 |
| TSH-R Abb | 6.3 (2.7:10.3) | 5.4 (2.8:9.0) | 0.500 |
| Imaging | |||
| Tc uptake (%) | 5.6 (3.6:8.6) | 4.4 (2.6:7.5) | 0.066 |
| US thyroid volume (mL) | 17.3 (12.1:26.6) | 13.0 (9.6:21.9) | 0.037 |
| US ≥ 1 nodulec | 15.9% (13/82) | 32.4% (11/34) | 0.081 |
| Therapy preceding RAI | |||
| ATD + L-T4 | 28.6% (30) | 23.4% (11) | 0.507 |
| ATD alone | 68.6% (72) | 63.8% (30) | 0.565 |
| CS | 0.0% (0) | 2.1% (1) | 0.679 |
| None | 2.9% (3) | 12.8% (6) | 0.043 |
| Interval diagnosis – RAI (days) | 77 (46:157) | 80 (45:201) | 0.951 |
| RAI activity (MBq) | 370 (370:518) | 370 (296:518) | 0.823 |
Data are presented as % (n) for categorical data. Continuous data are presented as mean ± s.d. or median (Q1:Q3) for symmetrically and non-symmetrically distributed variables, respectively.
Bold indicates statistical significance, P < 0.05.
aValues are expressed relatively to the upper limit of normal of the assay; b Values are expressed relatively to the cut-off of the assay; cNodules with a maximum diameter of >1 cm on ultrasonography.
ATD, antithyroid drug; CS, corticosteroids; HYPO, spontaneous hypothyroidism; L-T4, levothyroxine; PPT, post-partum thyroiditis; RAI, radioactive iodine; Tc, technetium; US, ultrasonography.
Smoking status and the presence of active Graves’ orbitopathy were recorded in a majority of patients, without significant differences between both groups. The maximum number of prior hyperthyroid episodes was four and this was recorded in two TPO Ab-positive patients. No difference was seen in the degree of thyrotoxicosis and in TSH-R Ab level at diagnosis. In four patients, no TSH-R Ab measurement was available at diagnosis, but they all showed diffusely increased Tc uptake on thyroid scintigraphy.
Imaging was performed in the majority of patients and in similar proportions between both groups. In TPO Ab-positive patients, scintigraphy was performed in 90% and an US in 78% of patients, as compared to 91 and 72% in TPO Ab-negative patients. The time interval between the diagnosis of Graves’ hyperthyroidism and the administration of RAI was similar in both groups. No significant difference in administered RAI activity was noted, with a median activity of 370 MBq in both groups.
Incidence of hypothyroidism and cure
The time interval between the administration of RAI and the assessment of thyroid function was comparable between the groups, both for period 1 and period 2 (Table 2).
Table 2.
Thyroid functional outcome in period 1 and period 2.
| TPO Ab-positive | TPO Ab-negative | P value | |
|---|---|---|---|
| Period 1a | n = 103b | n = 46c | |
| Interval RAI – evaluation (days) | 168 ± 40 | 167 ± 43 | 0.878 |
| Cure | 91% (94) | 85% (39) | 0.238 |
| Hypothyroidism | 89% (92) | 72% (33) | 0.007 |
| Euthyroidism | 2% (2) | 13% (6) | 0.017 |
| No cure | 9% (9) | 15% (7) | 0.238 |
| Period 2d | n=94e | n=43f | |
| Interval RAI – evaluation (days) | 367 (349:414) | 366 (345:406) | 0.994 |
| Cure | 94% (88) | 86% (37) | 0.146 |
| Hypothyroidism | 88% (83) | 72% (31) | 0.019 |
| Euthyroidism | 5% (5) | 14% (6) | 0.084 |
| No cure | 6% (6) | 14% (6) | 0.146 |
Data are presented as % (n) for categorical data. Continuous data are presented as mean ± s.d. or median (Q1:Q3) for symmetrically and non-symmetrically distributed variables respectively.
Bold indicates statistical significance, P < 0.05.
aThe thyroid function closest to 6 months after RAI was recorded (with a limit of ≥2 to ≤9 months); bTwo patients (n = 2/105) have an unknown outcome for period 1; cOne patient (n = 1/47) has an unknown outcome for period 1; dThe thyroid function closest to 12 months after RAI was recorded (with a limit of >9 months to ≤24 months); eTen patients have an unknown outcome for period 2 (n= 10/105), one patient died between RAI and the beginning of period 2 (n = 1/105); fFour patients (n = 4/47) have an unknown outcome for period 2.
RAI, radioactive iodine.
In period 1, thyroid function was unknown in two TPO Ab-positive patients and in one TPO Ab-negative patient (2% in both groups). In period 2, ten patients were lost to follow-up in the TPO Ab-positive group and four patients in the TPO-negative group (10% vs 9%). One patient with a positive TPO Ab status died between RAI and the start of period 2, due to an unrelated cause.
The incidence of hypothyroidism was significantly higher in the TPO Ab-positive group compared to the TPO Ab-negative group in period 1 (89% vs 72%, P = 0.007) and period 2 (88% vs 72%; P = 0.019) (Table 2).
In case of hypothyroidism, the time interval between RAI and initiation of L-T4 was similar in TPO Ab-positive patients compared to TPO Ab-negative patients (median, 60 days (Q1, 46 : Q3, 102) vs 76 days (Q1, 47 : Q3, 132); P = 0.193). Also, the dose of L-T4 at evaluation in period 1 was similar in both groups (median dose, 100 μg/day (Q1, 75 : Q3, 125); P = 0.592). The same observation was made in period 2 (median, 100 μg/day (Q1, 75 : Q3, 125) vs 87.5 μg/day (Q1, 75 : Q3, 100); P = 0.994).
The incidence of euthyroidism was significantly lower in the TPO Ab-positive group in period 1 (2% vs 13%; P = 0.017) but not in period 2 (5% vs 14%; P = 0.084). As such, the incidence of cure was not different between the groups in period 1 (91% vs 85%; P = 0.238) nor in period 2 (94% vs 86%; P = 0.146).
Association between a positive TPO Ab status and development of hypothyroidism
In an univariate logistic regression analysis, the OR for hypothyroidism in patients with a positive TPO Ab status was 3.29 (95% CI: 1.35–8.22; P = 0.009) in period 1 and 2.92 (95% CI: 1.17–7.41; P = 0.022) in period 2. In a multivariate logistic regression analysis, adjusting for age at diagnosis, fT4 at diagnosis, TSH-R Ab at diagnosis, thyroid volume at diagnosis, ATD preceding RAI and RAI activity, and the adjusted OR was 4.16 (95% CI: 1.0–18.83; P = 0.052) in period 1 and 4.78 (95% CI: 1.27–20.18; P = 0.024) in period 2 (Table 3). The TPO Ab titre, adjusted for the same variables, was also associated with hypothyroidism in period 2 (Supplementary Table 1, see section on supplementary materials given at the end of this article).
Table 3.
Logistic regression analysis with positive TPO Ab status as independent variable and hypothyroidism as dependent outcome.
| OR | 95% CI | P value | Observations | |
|---|---|---|---|---|
| Period 1 | ||||
| Crude | 3.29 | 1.35–8.22 | 0.009 | 149 |
| Adjusteda | 4.16 | 1.00–18.83 | 0.052 | 112 |
| Period 2 | ||||
| Crude | 2.92 | 1.17–7.41 | 0.022 | 137 |
| Adjusteda | 4.78 | 1.27–20.18 | 0.024 | 103 |
aAdjustments for age at diagnosis, fT4 at diagnosis, TSH-R Ab at diagnosis, thyroid volume at diagnosis, ATD preceding RAI, and RAI activity (all continuous variables except the variable ATD preceding RAI).
Bold indicates statistical significance, P < 0.05.
Discussion
In this multicentric retrospective study, we investigated if the TPO Ab status in patients with Graves’ disease prior to the first administration of RAI plays a role in the incidence of hypothyroidism and cure, defined as combined hypothyroidism and euthyroidism.
Despite the fact that determination of the TPO Ab titer is currently not required for the diagnosis of Graves’ disease, we observed that it was measured at diagnosis in 71% of patients. This is probably explained by the fact that at the initial workup of a patient with thyrotoxicosis, a positive TPO Ab status with absent TSH-R Ab or low uptake at scintigraphy directs the diagnosis towards silent thyroiditis (11). A positive TPO Ab status was found in 69% of patients which is consistent with other studied cohorts of patients with Graves’ disease (3, 16). In both the TPO-positive and -negative groups, most patients are female. This reflects the higher incidence of Graves’ disease in the female population, and the gender proportion is similar to observations by others (2, 7).
Both TPO Ab-positive and TPO Ab-negative groups had comparable disease activity (hyperthyroxinemia, TSH-R Ab titer, uptake at scintigraphy). Patients with positive TPO Abs were younger, which is in line with the recent observation by Khan et al. where higher age was associated with lower odds of having a positive TPO Ab status (17). However, other factors could have interfered since, for example, previous episodes of hyperthyroidism were more frequent in the TPO Ab-positive group, which could have elicited immunization against thyroid antigens, including TPO. The thyroid volume assessed by US, available in 76% of patients, was higher in TPO Ab-positive patients. The high overall incidence of nodularity in both groups is compatible with chronic mild iodine deficiency in Belgium (18).
During the evaluation of thyroid function closest to 6 months post RAI, overall, 89% of patients reached cure, which was similar in both groups and in line with previous observations (7, 19, 20). However, TPO Ab-positive patients were more likely to develop hypothyroidism. This was confirmed in a logistic regression analysis after adjustment for previously established factors associated with thyroid functional outcome after RAI: age, degree of thyrotoxicosis, TSH-R Ab titer, thyroid volume, pre-RAI therapy with ATD, and RAI activity (3, 4, 5, 6, 7, 19, 21, 22).
To our knowledge, there are only two studies that investigated TPO Ab as a predictive parameter for the thyroid functional outcome after RAI (13, 16). In a cohort of 100 patients with Graves’ disease, Catargi et al. observed a higher but not statistically different number of patients with a positive TPO Ab status in the hypothyroid group. Recently, Dong et al. found that patients with Graves’ hyperthyroidism who developed hypothyroidism 12 months after RAI had significantly increased TPO Ab levels prior to RAI.
Thus, based on our and previous observations, the presence of TPO Ab at diagnosis represents a risk factor for the development of early hypothyroidism after administration of RAI. The underlying mechanism remains elusive. If a positive TPO Ab status would suggest a state of concurrent thyroiditis, a contrary point is that hampered thyroid peroxidase functioning would be expected to decrease RAI organification. It could also be speculated that patients with Graves’ disease and a positive TPO Ab status harbour different intrathyroidal lymphocyte subsets and cytokines interfering with thyrocyte survival in case of a second hit, like radiation (23, 24).
Although the increased incidence of hypothyroidism in the TPO Ab-positive group appears to develop early after RAI, the time of onset of hypothyroidism is comparable in both groups. Half of all included patients were already initiated on L-T4 within 2 months after RAI, highlighting the importance of an early assessment of thyroid function within the first weeks of post RAI. Indeed, the presence of early hypothyroidism after RAI is a risk factor for development or deterioration of orbitopathy, which can be prevented by early administration of L-T4 (25, 26).
This study is limited by its retrospective design, patient number, differences in TPO Ab assays, absence of standardized RAI activity, and absence of data on long-term thyroid functional outcome.
In conclusion, to date, the role of the TPO Ab status in patients with Graves’ hyperthyroidism has not been well studied as a predictive parameter for thyroid functional outcome after first administration of RAI. We show that TPO Ab-positive patients were more likely to develop early hypothyroidism after the first administration of RAI, regardless of previously established factors associated with cure or treatment failure after RAI. The underlying mechanism warrants further investigation. Future studies investigating pre-treatment parameters affecting the outcome after RAI in patients with Graves’ disease should incorporate TPO Ab status as a variable.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
S V, B D: conceptualization, collecting data, statistical analysis, writing of the paper, editing of the paper, final approval of the paper. T M, P V C, A V D B: conceptualization, collecting data, reviewing and editing of the paper, final approval of the paper. C M: statistical analysis. F V N: collecting data.
Acknowledgement
The authors thank Anaïs Montenair for helping with the statistical analysis.
References
- 1.Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves’ hyperthyroidism. European Thyroid Journal 20187167–186. ( 10.1159/000490384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SCL, Franklyn JA. Age and gender predict the outcome of treatment for Graves’ hyperthyroidism. Journal of Clinical Endocrinology and Metabolism 2000851038–1042. ( 10.1210/jcem.85.3.6430) [DOI] [PubMed] [Google Scholar]
- 3.Chiovato L, Fiore E, Vitti P, Rocchi R, Rago T, Dokic D, Latrofa F, Mammoli C, Lippi F, Ceccarelli C, et al. Outcome of thyroid function in Graves’ patients treated with radioiodine: role of thyroid-stimulating and thyrotropin-blocking antibodies and of radioiodine-induced thyroid damage. Journal of Clinical Endocrinology and Metabolism 19988340–46. ( 10.1210/jcem.83.1.4492) [DOI] [PubMed] [Google Scholar]
- 4.Murakami Y, Takamatsu J, Sakane S, Kuma K, Oshawa N. Changes in thyroid volume in response to radioactive iodine for Graves’ hyperthyroidism correlated with activity of thyroid-stimulating antibody and treatment outcome. Journal of Clinical Endocrinology and Metabolism 1996813257–3260. ( 10.1210/jcem.81.9.8784079) [DOI] [PubMed] [Google Scholar]
- 5.Leslie WD, Ward L, Salamon EA, Ludwig S, Rowe RC, Cowden EA. A randomized comparison of radioiodine doses in Graves’ hyperthyroidism. Journal of Clinical Endocrinology and Metabolism 200388978–983. ( 10.1210/jc.2002-020805) [DOI] [PubMed] [Google Scholar]
- 6.Metso S, Jaatinen P, Huhtala H, Luukkaala T, Oksala H, Salmi J. Long-term follow-up study of radioiodine treatment of hyperthyroidism. Clinical Endocrinology 200461641–648. ( 10.1111/j.1365-2265.2004.02152.x) [DOI] [PubMed] [Google Scholar]
- 7.Aung ET, Zammitt NN, Dover AR, Strachan MWJ, Sackl JR, Gibb FW. Predicting outcomes and complications following radioiodine therapy in Graves’ thyrotoxicosis. Clinical Endocrinology 201990192–199. ( 10.1111/cen.13873) [DOI] [PubMed] [Google Scholar]
- 8.Taprogge J, Gape PMD, Carnegie-Peake L, Murray I, Gear JI, Leek F, Hyer SL, Flux GD. A systematic review and meta-analysis of the relationship between the radiation absorbed dose to the thyroid and response in patients treated with radioiodine for Graves’ disease. Thyroid 2021311829–1838. ( 10.1089/thy.2021.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA. & American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Practice 201218988–1028. ( 10.4158/EP12280.GL) [DOI] [PubMed] [Google Scholar]
- 10.McLeod DSA, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine 201242252–265. ( 10.1007/s12020-012-9703-2) [DOI] [PubMed] [Google Scholar]
- 11.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016261343–1421. ( 10.1089/thy.2016.0229) [DOI] [PubMed] [Google Scholar]
- 12.Lindgren O, Asp P, Sundlöv A, Tennvall J, Shahida B, Planck T, Åsman P, Lantz M. The effect of radioiodine treatment on TRAb, anti-TPO, and anti-TG in Graves’ disease. European Thyroid Journal 2019864–69. ( 10.1159/000495504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Q, Liu X, Wang F, Xu Y, Liang C, Du W, Gao G. Dynamic changes of TRAb and TPOAb after radioiodine therapy in Graves’ disease. Acta Endocrinologica 20171372–76. ( 10.4183/aeb.2017.72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Du WH, Zhang CX, Zhao SX, Song HD, Gao GQ, Dong M. The effect of radioiodine treatment on the characteristics of TRAb in Graves’ disease. BMC Endocrine Disorders 202121 238. ( 10.1186/s12902-021-00905-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. Volumetric analysis of thyroid lobes by real-time ultrasound (author’s transl). Deutsche Medizinische Wochenschrift 19811061338–1340. ( 10.1055/s-2008-1070506) [DOI] [PubMed] [Google Scholar]
- 16.Catargi B, Leprat F, Guyot M, Valli N, Ducassou D, Tabarin A. Optimized radioiodine therapy of Graves’ disease: analysis of the delivered dose and of other possible facors affecting outcome. European Journal of Endocrinology 1999141117–121. ( 10.1530/eje.0.1410117) [DOI] [PubMed] [Google Scholar]
- 17.Khan SR, Peeters RP, van Hagen PM, Dalm V, Chaker L. Determinants and clinical implications of thyroid peroxidase antibodies in middle-aged and elderly individuals: the Rotterdam study. Thyroid 20223278–89. ( 10.1089/thy.2021.0403) [DOI] [PubMed] [Google Scholar]
- 18.Vandevijvere S, Ruttens A, Wilmet A, Marien C, Hautekiet P, Van Loco J, Moreno-Reyes R, Van Der Heyden J. Urinary sodium and iodine concentrations among Belgian adults: results from the First National Health Examination Survey. European Journal of Clinical Nutrition 202175689–696. ( 10.1038/s41430-020-00766-5) [DOI] [PubMed] [Google Scholar]
- 19.Alexander EK, Larsen PR. High dose 131I therapy for the treatment of hyperthyroidism caused by Graves’ disease. Journal of Clinical Endocrinology and Metabolism 2002871073–1077. ( 10.1210/jcem.87.3.8333) [DOI] [PubMed] [Google Scholar]
- 20.Gibb FW, Zammitt NN, Beckett GJ, Strachan MWJ. Predictors of treatment failure, incipient hypothyroidism, and weight gain following radioiodine therapy for Graves’ thyrotoxicosis. Journal of Endocrinological Investigation 201336764–769. ( 10.3275/8949) [DOI] [PubMed] [Google Scholar]
- 21.Moura-Neto A, Mosci C, Santos AO, Amorim BJ, De Lima MC, Etchebehere EC, Tambascia MA, Ramos CD, Zantut-Wittmann DE. Predictive factors of failure in a fixed 15 mCi 131I-iodide therapy for Graves’ disease. Clinical Nuclear Medicine 201237550–554. ( 10.1097/RLU.0b013e31824851d1) [DOI] [PubMed] [Google Scholar]
- 22.De Jong JA, Verkooijen HM, Valk GD, Zelissen PM, De Keizer B. High failure rates after 131I therapy in Graves hyperthyroidism patients with large thyroid volumes, high iodine uptake, and high iodine turnover. Clinical Nuclear Medicine 201338401–406. ( 10.1097/RLU.0b013e3182817c78) [DOI] [PubMed] [Google Scholar]
- 23.Marique L, Van Regemorter V, Gérard AC, Craps J, Senou M, Marbaix E, Rahier J, Daumerie C, Mourad M, Lengelé B, et al. The expression of dual oxidase, thyroid peroxidase, and caveolin-1 differs according to the type of immune response (TH1/TH2) involved in thyroid autoimmune disorders. Journal of Clinical Endocrinology and Metabolism 2014991722–1732. ( 10.1210/jc.2013-3469) [DOI] [PubMed] [Google Scholar]
- 24.Rapoport B, Mclachlan SM. Graves’ hyperthyroidism is antibody-mediated but is predominantly a Th1-type cytokine disease. Journal of Clinical Endocrinology and Metabolism 2014994060–4061. ( 10.1210/jc.2014-3011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stan MN, Durski JM, Brito JP, Bhagra S, Thapa P, Bahn RS. Cohort study on radioactive iodine-induced hypothyroidism: implications for graves’ ophthalmopathy. Thyroid 201323620–625. ( 10.1089/thy.2012.0258) [DOI] [PubMed] [Google Scholar]
- 26.Perros P, Kendall-Taylor P, Neoh C, Frewin S, Dickinson J. A prospective study of the effects of radioiodine therapy for hyperthyroidism in patients with minimally active Graves’ ophthalmopathy. Journal of Clinical Endocrinology and Metabolism 2005905321–5323. ( 10.1210/jc.2005-0507) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a