Abstract
We considered 351 patients affected by neuroendocrine tumors (NETs), followed at the University Hospital of Padua and at the Veneto Oncological Institute. Of these, 72 (20.5%) suffered from bone metastases. The sample was divided according to the timing of presentation of bone metastases into synchronous (within 6 months of diagnosis of primary tumor) and metachronous (after 6 months). We collected data on the type and grading of the primary tumor and on the features of bone metastases. Our analysis shows that the group of synchronous metastases generally presents primary tumors with a higher degree of malignancy rather than the ones of the metachronous group. This is supported by the finding of a Ki-67 level in GEP-NETs, at the diagnosis of bone metastases, significantly higher in the synchronous group. Moreover, in low-grade NETs, chromogranin A values are higher in the patients with synchronous metastases, indicating a more burden of disease. The parameters of phospho-calcium metabolism are within the normal range, and we do not find significant differences between the groups. Serious bone complications are not frequent and are not correlated with the site of origin of the primary tumor. From the analysis of the survival curves of the total sample, a cumulative survival rate of 33% at 10 years emerges. The average survival is 80 months, higher than what is reported in the literature, while the median is 84 months. In our observation period, synchronous patients tend to have a worse prognosis than metachronous ones with 52-months survival rates of 58 and 86%.
Keywords: bone metastases, neuroendocrine tumors, NET, carcinoid, chromogranin A
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous family of neoplasms that originate from cells belonging to the widespread neuroendocrine system. These cells appear to be ubiquitous in the human body, but the main sites are the gastro-entero-pancreatic and the bronchopulmonary tract (1). NETs may exhibit a wide range of biological behaviors, from slow-progressing to highly aggressive tumors (2), and are considered rare, in terms of incidence, when compared to the corresponding non-neuroendocrine neoplasms. However, data from SEER registries in the United States demonstrate an increased incidence, in recent decades, from one case per 100,000 inhabitants in 1973 to about seven cases per 100,000 in 2012 with an increasing prevalence due to the more favorable prognosis (3). In Europe, the number of patients affected by NET in 2016 was estimated to be 292,971 (4).
The skeletal system is a common site of metastasis for many solid tumors, but bone involvement by NETs has always been considered a rare and late event. To date, thanks to the improvement of therapies, which has lengthened the life expectancy of NET patients, and imaging techniques, in particular PET with 68Ga-SSA, the amount of diagnosis of bone metastases from NET is increasing. It is currently believed that bone is the third most frequent site of NET metastasis after liver and lung (2). The primary sites of NET most frequently associated with bone metastases are the small intestine (32%) and the lung (24%). Furthermore, it appears that NETs, compared to their corresponding adenocarcinomas, have a slightly increased rate of bone metastasis: 15% vs 13%, respectively (5). The primary sites most frequently involved were the small intestine and pancreas, followed at a distance by the lung and rectum (6). Bone metastases can have an important impact on the patient’s quality of life and are considered negative prognostic factors of NETs (7, 8). The sites most frequently affected are the truncal skeleton, mostly in the vertebral region, followed by the pelvic region and ribs. Involvement of the limbs, however, is rarer (6). In total, 59–77% of patients with bone metastases from NET are symptomatic (2). Characteristic symptoms are pain, spinal compression, pathologic fractures and hypercalcemia (7, 9). In recent years, a growing interest has been directed to the prevention, diagnosis and treatment of them, and despite this, the data in this regard are still scarce.
Our aim was to investigate, both in clinical and laboratory settings, bone metastases in NETs as well as to understand if there were differences between synchronous and metachronous bone metastases. The terms synchronous and metachronous are here used to distinguish the patients based on bone metastases time onset. By ‘synchronous’, we mean bone metastases detected by morpho-functional analysis within 6 months from the diagnosis of primary NET, while with ‘metachronous’, we identify the metastases absent at primary NET diagnosis (evaluated by the same morpho-functional analysis), that appeared after 6 months or later.
By defining these two groups we analyzed the possible differences in terms of tumor aggressiveness and patient survival. Moreover, we tried to investigate in both these groups the relationship between bone metastases, primary type/site of NET, laboratory parameters (chromogranin A (CgA), calcium, phosphate, parathyroid hormone (PTH) and vitamin D) and proliferation markers (Ki-67 and mitotic index).
Materials and methods
Patient identification
The patients included in the study were selected based on two inclusion criteria: (i) diagnosis of gastrointestinal-NET or bronchopulmonary-NET, lung neoplasms small or large cell carcinoma were not included and (ii) presence of related bone metastases. For the diagnosis of primary NET, we referred to the pathological and immunohistochemical analysis of the biopsy of the primary site. Regarding the diagnosis of bone metastases, radiological and nuclear medicine reports were mainly taken into consideration. All patients underwent total-body PET/CT imaging with 68Ga-SSA, MRI and fluorodeoxyglucose-PET/CT focused on bone lesions. Patients who presented reports of ambiguous interpretation were discarded due to the frequent difficulties in differential diagnosis with traumatic, osteoporotic or PTH-related outcomes.
In order to create our sample, we drew from both records of patients followed in Medical Clinic III – University Hospital and from a database of the Oncology Department I – Veneto Oncological Institute of Padua. Among the 351 patients considered, we were able to extract 72 that met our criteria of inclusion. We carried out a retrospective study that did not provide for a specific time window for patients’ election but considered for each patient any precedent oncological documentation and our follow-up until death or last clinical evaluation. Consent has been obtained from each patient after a full explanation of the purpose and nature of all procedures used.
Data collection
We created a database collecting all of the relevant information about the patients included in the study. The database consisted of the following four cards: (i) personal data: date of birth, gender, age at diagnosis of primary NET and at diagnosis of bone metastases and age of death; (ii) data of the primary NET: site, genetics (e.g. multiple endocrine neoplasia syndrome (MEN-1)), grading (the World Health Organization (WHO) 2010 or WHO 2019) and pathological parameters, presence/absence of specific endocrine syndrome, medical and/or surgical treatments; (iii) data on bone metastases: site, timing (synchronous or metachronous), morphology, symptoms, diagnostic methods and specific treatments; (iv) laboratory parameters: CgA, calcium, phosphate, PTH and vitamin D. About these, the values closest to the diagnosis of bone metastasis were selected for each patient, when present.
The study was approved by the Ethics Committee for the clinical trial in Padua. All data were drawn from medical records present in the ‘Galileo’ programs, as regards the University Hospital of Padua, and ‘Oncosys’, as regards the Veneto Oncological Institute.
Statistical analysis
The data obtained from the two groups of patients were analyzed according to descriptive and inferential statistical methods. Quantitative variables were processed through the Student’s t-test. Qualitative variables, such as the grading or the location of NET, have been evaluated according to the chi-squared test. The survival curves of the patients were drawn following the Kaplan–Meier technique. Statistical significance was calculated with the log-rank test (or Mantel-Cox test). The calculations and graphical representations were obtained with ‘Microsoft Excel’ and ‘R’. The results were considered statistically significant for P values <0.05.
Results
Table 1 summarizes the clinical features of patients affected by synchronous and metachronous bone metastases of NET with reference to anthropological data, type and location of primary tumor and metastases, tumor grading at diagnosis and tumor treatments.
Table 1.
Clinical features of patients with NETs bone metastases.
| Synchronous | Metachronous | P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Number of patients | 47 | 65 | 25 | 35 | |
| Mean age at NET diagnosis | 63.91 | 61 | |||
| Mean age at BM diagnosis | 65.78 | ||||
| Gender | 0.276 | ||||
| F | 20 | 43 | 12 | 48 | |
| M | 27 | 57 | 13 | 52 | |
| MEN-1 | 1 | 2 | 1 | 4 | |
| NET Primary site | |||||
| Bronchopulmonar | 6 | 13 | 7 | 28 | |
| Gastric | 4 | 9 | |||
| Pancreatic | 10 | 21 | 5 | 20 | |
| Small bowel (ileus) | 10 | 21 | 11 | 44 | |
| Colon, rectum, appendix | 5 | 11 | 1 | 4 | |
| Multiple sites | 1 | 2 | 1 | 4 | |
| Unknown | 11 | 23 | |||
| Grading | <0.001 | ||||
| Atypical carcinoid | 3 | 6 | 4 | 16 | |
| Typical carcinoid | 1 | 2 | 3 | 12 | |
| G1 | 8 | 17 | 9 | 36 | |
| G2 | 16 | 34 | 7 | 28 | |
| G3 | 5 | 11 | |||
| NEC | 5 | 11 | |||
| MiNEN | 1 | 2 | |||
| Unknown | 8 | 17 | 2 | 8 | |
| Functional | 0.006 | ||||
| Yes | 9 | 19 | 8 | 32 | |
| No | 38 | 81 | 17 | 68 | |
| Metastases | 0.896 | ||||
| Just bone | 2 | 4 | 1 | 4 | |
| Other sites | 45 | 96 | 24 | 96 | |
| Surgery | <0.001 | ||||
| Yes | 21 | 45 | 21 | 84 | |
| No | 26 | 55 | 4 | 16 | |
| Treatments | |||||
| SSA | 33 | 70 | 23 | 92 | |
| Chemotherapy | 31 | 66 | 13 | 52 | |
| PRRT | 10 | 21 | 10 | 40 | |
| Everolimus | 8 | 17 | 9 | 36 | |
| Sunitinib | 1 | 2 | 1 | 4 | |
MiNEN, mixed neuroendocrine non-neuroendocrine neoplasms; NEC, neuroendocrine carcinoma; PRRT, peptide receptor radionuclide therapy; SSA, somatostatin analogs.
At first, we do not observe any statistically significant difference between the two groups regarding gender, mean age at NET and bone metastases (BM) diagnosis, MEN-1 prevalence and NET primary sites. However, it is noteworthy that synchronous metastases represent 65% of the total while the metachronous ones only 35%. The mean age at diagnosis of bone metastases falls in the seventh decade (64 years in the synchronous group vs 66 years in the metachronous group) and no differences have been observed in terms of gender distribution. Only two patients present MEN-1, one with synchronous bone metastases and one with metachronous. Regarding the NET primary site, the pancreatic and ileal sites are cumulatively the most represented in both groups (42% in synchronous vs 64% in metachronous), the gastric tumors are all in the synchronous group, accounting for 9%, the bronchopulmonary tumors seem to be preferentially represented in the metachronous group (28% vs 13%). Little differences can be observed in the distribution of colorectal and appendicular tumors. Finally, in the metachronous group, we have been able to identify the primary site of NET in all patients while in the synchronous group 23% of the patients have an unknown primary site. As regards the medical treatment of these tumors, somatostatin analogs (SSA) are the most used treatment for NETs, followed in order of frequency by chemotherapy, peptide receptor radionuclide therapy (PRRT), everolimus and sunitinib.
On the contrary, a significant difference has been observed in the distribution of grading in the two groups of patients (P < 0.001). In fact, the metachronous tumors whose grade we have been able to identify (92% of total) are invariably low-grade NETs (grading equal to or lower than G2 or typical/atypical carcinoid), on the other hand, in the synchronous group, the low-grade tumors represent only 70% and there is a 13% of high-grade neuroendocrine carcinomas (5 neuroendocrine carcinoma (NEC) and 1 mixed neuroendocrine non-neuroendocrine neoplasms). These differences in grading seem to be reflected in some aspects of tumor histology and immunohistochemistry.
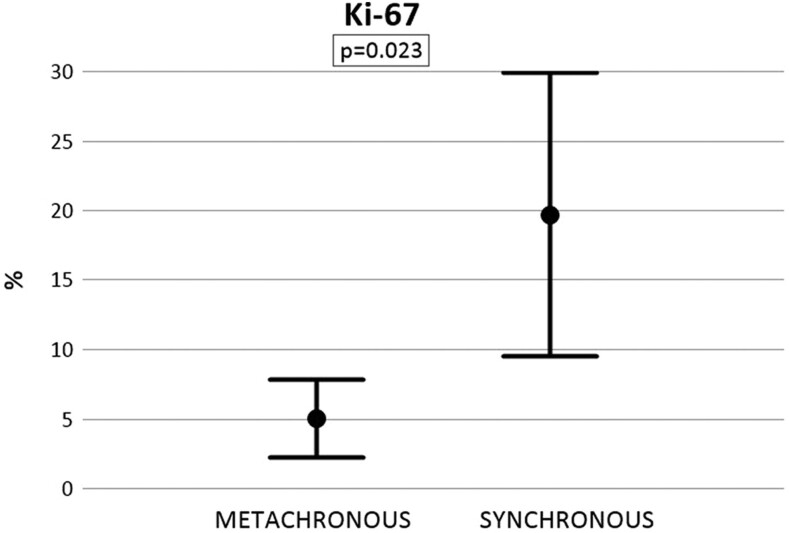
In Fig. 1, we analyze the values of Ki-67 of GEP-NET differentiating between metachronous and synchronous groups. The mean ki-67 in the metachronous group is 5.0% while in the synchronous group it is 19.7%. This difference is statistically significant (P = 0.023). The parameters have been detected in 23 of the 25 metachronous patients and in 41 of the 47 synchronous patients. Missing data are not available.
Figure 1.
Ki-67 index (%) in patients affected by synchronous and metachronous bone metastases of GEP-NETs. Mean ± s.d., P = 0.023.
Table 2 summarizes the clinical features of synchronous and metachronous bone metastases in NET patients with reference to sites, morphology, pain, complications and treatments.
Table 2.
Clinical features and treatment of bone metastases in NET patients.
| Synchronous | Metachronous | P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Bone metastasis site | <0.001 | ||||
| Truncal | 28 | 60 | 20 | 80 | |
| Limbs | 1 | 2 | 0 | 0 | |
| Truncal+limbs | 15 | 32 | 4 | 16 | |
| Unknown | 3 | 6 | 1 | 4 | |
| Lytic lesions | 0.202 | ||||
| Yes | 7 | 15 | 5 | 20 | |
| No | 40 | 85 | 20 | 80 | |
| Pain | |||||
| Yes | 20 | 43 | 14 | 56 | |
| No | 27 | 57 | 11 | 44 | |
| Bone complications | |||||
| Spinal compression | 2 | 4 | 2 | 8 | |
| Fractures or vertebral collapse | 2 | 4 | 3 | 12 | |
| Asymptomatic | 27 | 57 | 10 | 40 | |
| Treatment | 0.151 | ||||
| Biphosphonates (only) | 6 | 13 | 6 | 24 | |
| Radiotherapy (only) | 5 | 10 | |||
| Biphosphonates and radiotherapy | 6 | 13 | 3 | 12 | |
| Untreated | 30 | 64 | 16 | 64 | |
Focusing on bone metastases sites, it shows that the topographical distribution of lesions between the two groups presents a significant difference. In fact, synchronous metastases tend to affect the limbs, mainly together with the truncal localizations, to a greater extent than metachronous (34% vs 16%, P < 0.001), indicating a more diffuse disease at primary tumor diagnosis. The difference in frequency of lytic-type lesions is comparable in both groups (15% vs 20%, P = 0.202); however, it should be emphasized that the morphological characterization of bone lesions is often lacking in the reports in our possession.
The predominant symptom is certainly pain which affects 43% of synchronous and 56% of metachronous lesions. Other less frequent symptoms are related to spinal compression and pathological fractures (including vertebral collapses). At a therapeutic level, bisphosphonates are the most widely used drug, both alone and in association with local radiotherapy (23% in synchronous and 24% in metachronous). In our series, few patients are treated with denosumab or orthopedic corset (data not shown).
The skeletal-related events (pathological fractures or spinal compressions) are nine: four in the synchronous group and five in the metachronous group. Table 3 shows that primary tumors in patients with skeletal-related events originate mainly in the pancreas and ileus with a slight, not significant, preference for ileus. All tumors, whose grading has been known, are G2 or G1.
Table 3.
Prevalence of total skeletal-related events regarding primary NET site and grading.
| Skeletal-related events | ||
|---|---|---|
| Total events | 9 | |
| Site of primary NET | n | % |
| Pancreas | 3 | 33 |
| Ileus | 4 | 45 |
| Rectum | 1 | 11 |
| Multiple | 1 | 11 |
| Grading | ||
| G1 | 2 | 22 |
| G2 | 5 | 56 |
| Unknown | 2 | 22 |
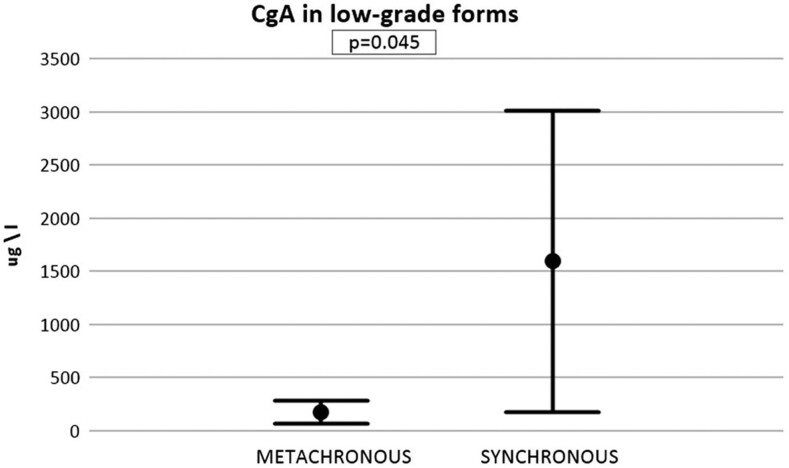
Figure 2 shows a comparison between CgA levels at diagnosis of bone metastases in patients with low-grade NETs with synchronous and metachronous bone metastases (G1, G2, TC and AC). Mean CgA in the metachronous group is 181.7 µg/L, while in the synchronous group is 1597.1 µg/L. This difference is statistically significant (P = 0.045).
Figure 2.
Chromogranin A levels at primary diagnosis of low-grade NETs in patients with metachronous and synchronous bone metastases. Mean ± s.d., P = 0.045.
Figure 3 resumes the main parameters of phospho-calcic metabolism at diagnosis of bone metastases in patients with metachronous and synchronous metastases. Regarding total calcium and phosphate, the values of the metachronous group are slightly higher than the synchronous group; however, no significant differences have been found (P = 0.239 and 0.367, respectively). It should be emphasized that these values result always within the reference range (Ca: 2.10–2.55 mmol/L and P: 0.87–1.45 mmol/L). The same goes for PTH and vitamin D. There are no significant differences in either case. In some subjects with metachronous metastases, PTH levels are over the upper range (Fig. 3C).
Figure 3.
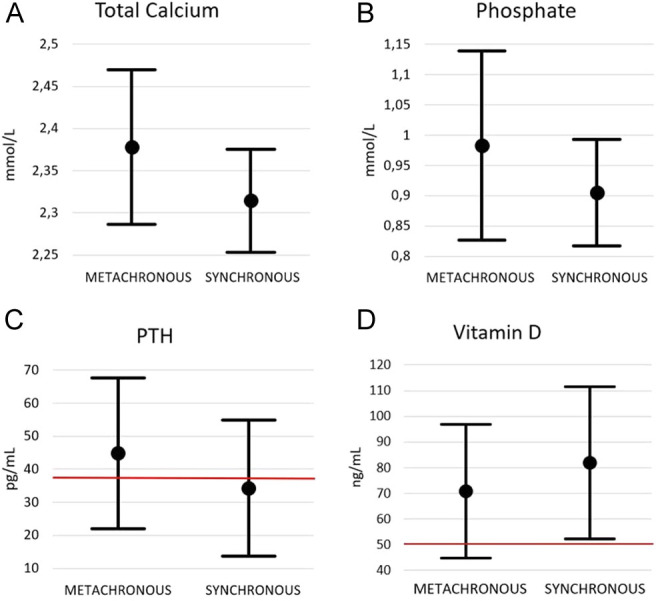
Total calcium (A), phosphate (B), PTH (C) and vitamin D (D) levels at the diagnosis of bone metastases in patients with metachronous and synchronous metastases. Mean ± s.d. The upper limit of PTH reference range and lower limit of vitamin D reference range is marked in red.
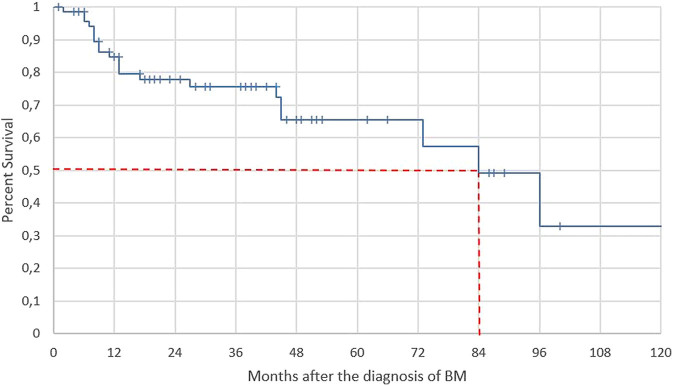
Figure 4 shows the cumulative survival curve of all patients affected by NETs and bone metastases during a 120 months observation, without distinction between synchronous and metachronous, starting from the diagnosis of bone metastases (t = 0). In total, 21 deaths occurred during this time, 18 among synchronous and three among metachronous. The mean survival is 80 months and the median is 84 months. Cumulative survival at 12 months is 85%, at 60 months 66% and at 120 months 33%.
Figure 4.
Kaplan–Meier survival curve of patients (n = 72) affected by NETs, after the diagnosis of bone metastases (t = 0). Coordinates of median survival are given in red.
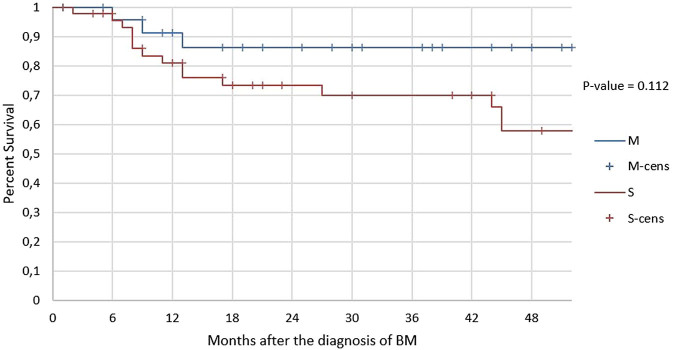
A limitation of the comparison between patients with metachronous metastases and patients with synchronous metastases is that in the metachronous group our information cannot go beyond a censorship time of 52 months after evidence of bone metastases. Thus, the comparison of survival between the two groups (see Fig. 5) has been made considering the first 52 months of follow-up of the synchronous group, though many of these patients have been followed up to 10 years. The average survival of metachronous results is about 43 months while that of synchronous is about 37 months. The deaths are 15 among the synchronous and 3 among the metachronous. Although there is no statistically significant difference (P = 0.112), the trend of the curves suggests a longer survival in the metachronous group. At 12 months, the survival rates of metachronous and synchronous are 91 and 81%, respectively. At 24 months, survival rates are 86% (which is maintained up to 52 months) and 73%, respectively. At 36 months, the survival rate of synchronous drops further to 70%. At 4 years, the survival rate of metachronous is 86%, while the one of the synchronous is 58%. The death risk rate of the synchronous group is approximately 2.59 times the one of the metachronous group, according to the hazard ratio calculated in the reported time interval.
Figure 5.
Kaplan–Meier survival curve of patients after the diagnosis of bone metastases (t = 0). The survival trend of patients with metachronous metastases (n = 25) is given in blue, and the survival trend of patients with synchronous metastases (n = 47) is given in red.
Discussion
In our study, we observe that the incidence of BM and the average survival of patients with NET-related bone metastases is higher than what has been published so far in the literature. Moreover, patients with synchronous BM are affected by more aggressive primary NETs, in terms of grading, than those with metachronous BM. The more aggressiveness of primitive tumors in the synchronous group is also supported by the evidence of a worst survival rate from the diagnosis of BM than the metachronous group. Concerning laboratory parameters, we find, in low-grade NETs, CgA levels are higher in the synchronous group than in the metachronous group. No significant alteration in phospho-calcium metabolism markers has been observed.
The analysis of our cases highlights a total prevalence of bone metastases of 20.5% (72 cases out of 351 patients). It appears to be higher than pooled incidence (18.4%) reported in a recent systematic review published by Garcia-Torralba et al. (10). The growing prevalence of BM from NET through the years could reflect an increasing attention to this topic and a greater diagnostic accuracy related to the more extensive use of MRI and PET/CT with radiolabeled SSA. Indeed, in our department, every patient has been followed up by MR and PET-TC with radiolabeled SSA. This surely helps the early discovery of new bone metastases and might explain why the prevalence in our series is higher than in others. On the other hand, an autoptic series in literature reports a prevalence of skeletal metastases of 42% (11). This supports the hypothesis that, anyway, a major part of bone metastases remains undiagnosed.
In our series, patients already affected by bone metastases at the time of primary tumor diagnosis (synchronous) are more frequently affected by aggressive neoplasms, such as G3 NETs or NEC, than patients with metachronous bone metastases. Metachronous patients, in fact, are affected only by G1/G2 NETs and typical/atypical bronchial carcinoids, and therefore low-grade NETs. This would suggest a correlation between the grading of the primary tumor and the precocity of bone metastasis onset. To better evaluate this relationship, we first analyze Ki-67, the main parameter used to determine grading and potential aggressiveness of GEP-NETs. Taking into consideration only the gastrointestinal tumors, the mean value of Ki-67 in the synchronous group is significantly higher than in the metachronous one. For bronchopulmonary NETs, the parameter used for the grading is the mitotic index (MI). Unfortunately, in our study, the anatomopathological diagnosis of typical or atypical carcinoid has been often performed without reporting the exact value of MI. Therefore, synchronous bone metastases seem to be associated with a greater aggressiveness of the primary GEP-tumor. A mean value of ki-67 higher in patients with synchronous bone metastases is reported also in the study published by Alexandraki et al. (12). According to this latter, our study supports that the more frequent sites of primary tumors with BM are the pancreas and small bowel. Our monocentric study, characterized by homogeneous diagnostic and follow-up protocols, substantially confirms the findings of the multicentric study conducted by Alexandraki et al. on the biological behavior of bone metastases in relation to the time of their diagnosis.
Thus, we take into consideration CgA, a marker secreted by neuroendocrine cells and useful in the follow-up of low-grade NETs. It generally correlates positively with tumor burden, treatment response and prognosis (13). It should be noted that cells from higher-grade NETs can lose the ability of secreting CgA due to their loss of differentiation resulting in widely variable values of CgA (14). In order to obtain a more reliable analysis, we decide to consider only the values in low-grade tumors (G1, G2 and bronchial carcinoids). In this comparison, we find a significantly higher mean level of CgA in the synchronous group. This agrees with a greater tumor burden of primary low-grade NETs associated with synchronous bone metastases. However, the role of surgery as a possible bias should also be emphasized. In fact, complete or partial resection of the low-grade primary tumor reduces CgA levels, and in our sample, only 52% of synchronous patients underwent surgery vs 84% of metachronous ones.
Another significant element that emerges from our data is the greater tendency of synchronous bone metastases to affect both truncal skeleton and limbs, compared to the more circumscribed involvement of metachronous metastases. This difference in the topographic distribution of bone metastases is statistically significant, so a greater biological aggression seems to correspond to a greater metastatic spreading already at the time of primary diagnosis.
As regards, the site of origin of the primary tumor in relation to the presence and evolution of bone metastases, we are not able to demonstrate a correlation between the primary sites of NETs and the timing of bone metastases onset. However, the lower average aggressiveness of neoplasms with metachronous metastases in our series could reflect the greater representation in this group of ileal-NETs, on average less aggressive than other types (e.g. pNETs) (15). Similar considerations about NET site of origin and metastases timing in the synchronous group are more difficult, considering the presence of 23% of patients with unknown primary tumor sites. Furthermore, we do not find significant correlations between the occurrence of severe skeletal complications from bone metastases (such as spinal compression and pathological fractures) and the topographic localization of the primary tumor. This agrees with what Van Loon et al. report in their study (7). However, in our study, the NETs that caused skeletal complications are invariably G1 or G2 and this could suggest that bone metastases therapy should therefore be started early also in the less-aggressive types of NET. Preventing skeletal related-events should be one of the primary aims in the treatment process of these patients (16).
Taking into consideration the phospho-calcium metabolism parameters of these patients, no particular differences are highlighted in the levels of calcium, phosphate, parathyroid hormone and vitamin D between the two groups. Except for a slight alteration of PTH in metachronous subjects, all these parameters have been found to be in the laboratory reference range, supporting the hypothesis that the presence of these metastases does not cause significant alterations in phospho-calcium homeostasis. This hypothesis is in agreement with other studies (17, 18). A clarification should be made regarding vitamin D. In the literature, some studies report how patients with bone metastases from NET often present hypovitaminosis D, attributing this deficit to nutritional causes or to the clinical syndromes that are NET related (18, 19). In our research, we do not find this deficiency, but it should be emphasized that the majority of patients are put on early treatment with exogenous cholecalciferol.
The therapies specifically aimed at the treatment of bone metastases are mainly bisphosphonates, which are usually the first choice, and local radiotherapy. The latter is known as a good improver of pain symptoms (20). However, only a relatively small percentage of total cases (36%) are treated with bisphosphonates and local radiotherapy since bone metastases treatment is primarily entrusted to the use of SSA, first-line treatment of NETs. On the contrary, biological therapies and chemotherapy are the main therapies in neuroendocrine carcinomas, in which the attempt to treat bone metastases falls within the scope of these systemic therapies. Newer bone-directed drug therapies are rarely used in our patients. Particularly, we find only two patients who are administered denosumab despite there are studies in the literature that document its greater efficacy compared to bisphosphonates (21, 22). There will probably be greater use of denosumab in the future. The recent introduction of radioreceptor treatment of NETs with SSA conjugated to beta/gamma-emitting isotopes such as yttrium and lutetium could constitute a new resource in the treatment of bone metastases (2). It has recently been recognized that PRRT, reserved in the past only for the most advanced and widespread neoplasm, can determine a greater advantage when used as a second-line treatment, following SSA(23, 24). This could also result in a benefit in patients with bone metastases.
In our study, it is difficult to draw conclusions about the evolution of bone lesions over time, as well as about the possible response to therapies. In fact, NETs are extremely heterogeneous, and the clinical interest is mainly directed to the most relevant complications such as liver metastases, specific endocrine syndromes or gastrointestinal and/or respiratory occlusive symptoms. Bone involvement is hardly ever studied in specific follow-ups. The difference compared to other cancers (e.g. breast, bladder or prostate cancer) is therefore evident.
The cumulative survival curve of the entire sample, starting from the diagnosis of bone metastases, shows a 10-year survival of 33%. The average overall survival of our sample has been found to be about 80 months, which is higher than the data published before in the literature (10, 16). A possible explanation for this could be that the implementation of MR and PET-TC with radiolabeled SSA allows to obtain at earlier stages the diagnosis, treatment and follow-up of the bone metastases. In addition, in our department, these patients are discussed in a multidisciplinary group in which endocrinologists, oncologists, radiologists and surgeons work together in order to choose the more appropriate therapeutic option for the patient.
The comparison between the survival Kaplan–Meier curves of synchronous and metachronous groups during our interval time of the study (52 months) shows a cumulative mortality of 35%. The analysis of the survival curves highlights a tendential difference between the two groups which, however, does not reach statistical significance. At the end of the period, the survival rate is 58% for the synchronous group and 86% for the metachronous group. This trend is in agreement with other studies (12). During the first year, mortality is particularly high, reaching about 20% in synchronous and 10% in metachronous. The clinical documentation in our possession has not highlighted a direct role of bone metastases as a cause of death. It therefore appears difficult to assess the real impact of bone metastases in the quoad vitam prognosis of patients with NETs since the latter are tumors with very varied complications as well as frequent coexistence of different metastatic sites. In our series, confirming this, we find only 4% of patients with metastases exclusively in the bone. Nevertheless, bone metastases from NET are known as negative prognostic factors and determine a significant decrease in progression-free survival and overall survival (7, 8). In our study, the death risk rate of patients with synchronous BM is approximately 2.59 times the one of patients with metachronous BM.
A qualitative analysis of our cases and scientific literature highlights how there is still no unanimous consensus on the management of bone metastases in NETs and on the usefulness of their early recognition in order to prevent complications and possible disability. However, our study suggests to pay more attention to NET’s bone metastases, both in diagnosis and therapy, especially when the primary NET is more aggressive.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Faggiano A, Ferolla P, Grimaldi F, Campana D, Manzoni M, Davì MV, Bianchi A, Valcavi R, Papini E, Giuffrida D, et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian epidemiological study: the NET management study. Journal of Endocrinological Investigation 201235817–823. ( 10.3275/8102) [DOI] [PubMed] [Google Scholar]
- 2.Altieri B, Di Dato C, Martini C, Sciammarella C, Di Sarno A, Colao A, Faggiano A. & NIKE Group. Bone metastases in neuroendocrine neoplasms: from pathogenesis to clinical management. Cancers 201911 1332. ( 10.3390/CANCERS11091332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncology 201731335–1342. ( 10.1001/JAMAONCOL.2017.0589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergamasco A, Dinet J, Berthon A, Gabriel S, Nayroles G, Moride Y. Prevalence of gastroenteropancreatic and lung neuroendocrine tumours in the European Union. Annals of Oncology 201627 vi139. ( 10.1093/ANNONC/MDW369.09) [DOI] [Google Scholar]
- 5.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. International Journal of Cancer 20161392679–2686. ( 10.1002/IJC.30400) [DOI] [PubMed] [Google Scholar]
- 6.Scharf M, Petry V, Daniel H, Rinke A, Gress TM. Bone metastases in patients with neuroendocrine neoplasm: frequency and clinical, therapeutic, and prognostic relevance. Neuroendocrinology 201810630–37. ( 10.1159/000457954) [DOI] [PubMed] [Google Scholar]
- 7.Van Loon K, Zhang L, Keiser J, Carrasco C, Glass K, Ramirez MT, Bobiak S, Nakakura EK, Venook AP, Shah MH, et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocrine Connections 201549–17. ( 10.1530/EC-14-0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cives M, Pellè E, Rinzivillo M, Prosperi D, Tucci M, Silvestris F, Panzuto F. Bone metastases in neuroendocrine tumors: molecular pathogenesis and implications in clinical practice. Neuroendocrinology 2021111207–216. ( 10.1159/000508633) [DOI] [PubMed] [Google Scholar]
- 9.Kos-Kudła B, O'Toole D, Falconi M, Gross D, Klöppel G, Sundin A, Ramage J, Oberg K, Wiedenmann B, Komminoth P, et al. ENETS consensus guidelines for the management of bone and lung metastases from neuroendocrine tumors. Neuroendocrinology 201091341–350. ( 10.1159/000287255) [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Torralba E, Spada F, Lim KHJ, Jacobs T, Barriuso J, Mansoor W, McNamara MG, Hubner RA, Manoharan P, Fazio N, et al. Knowns and unknowns of bone metastases in patients with neuroendocrine neoplasms: a systematic review and meta-analysis. Cancer Treatment Reviews 202194 102168. ( 10.1016/j.ctrv.2021.102168) [DOI] [PubMed] [Google Scholar]
- 11.Ross EM, Roberts WC. The carcinoid syndrome: comparison of 21 necropsy subjects with carcinoid heart disease to 15 necropsy subjects without carcinoid heart disease. American Journal of Medicine 198579339–354. ( 10.1016/0002-9343(8590313-4) [DOI] [PubMed] [Google Scholar]
- 12.Alexandraki KI, Pizanias M, Uri I, Thomas D, Page T, Kolomodi D, Low CS, Adesanya O, Tsoli M, Gross DJ, et al. The prognosis and management of neuroendocrine neoplasms-related metastatic bone disease: lessons from clinical practice. Endocrine 201964690–701. ( 10.1007/s12020-019-01838-8) [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Huang Y, Long J, Yao X, Wang J, Zang S, Qu W, Wang F. Serum chromogranin a for the diagnosis of gastroenteropancreatic neuroendocrine neoplasms and its association with tumour expression. Oncology Letters 2019171497–1504. ( 10.3892/ol.2018.9795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazio N, Gelsomino F, Falconi M, Spada F, Albertelli M, Ambrosini V, Amoroso A, Chiarion Sileni V, Fiore F, Milione M, et al. Linee Guida Neoplasie Neuroendocrine. Milan, Italy: Itanet-AIOM. (available at: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Neuroendocrini.pdf) [Google Scholar]
- 15.Man D, Wu J, Shen Z, Zhu X. Prognosis of patients with neuroendocrine tumor: a SEER database analysis. Cancer Management and Research 201810 5629–5638. ( 10.2147/CMAR.S174907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim KHJ, Raja H, D’Arienzo P, Barriuso J, McNamara MG, Hubner RA, Mansoor W, Valle JW, Lamarca A. Identification of areas for improvement in the management of bone metastases in patients with neuroendocrine neoplasms. Neuroendocrinology 2020110688–696. ( 10.1159/000504256) [DOI] [PubMed] [Google Scholar]
- 17.Milone F, Pivonello C, Cariati F, Sarnataro M, Ramundo V, Marotta V, Jann H, Pape UF, Wiedenmann B, Colao A, et al. Assessment and clinical implications of RANK/RANKL/OPG pathway as markers of bone tumor progression in patients with NET harboring bone metastases. Biomarkers 201318121–125. ( 10.3109/1354750X.2012.745166) [DOI] [PubMed] [Google Scholar]
- 18.Altieri B, Di Dato C, Modica R, Bottiglieri F, Di Sarno A, Pittaway JFH, Martini C, Faggiano A, Colao A. Bone metabolism and vitamin D implication in gastroenteropancreatic neuroendocrine tumors. Nutrients 202012 1021. ( 10.3390/NU12041021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins HL, Symington M, Mosterman B, Goodby J, Davies L, Dimitriadis GK, Kaltsas G, Randeva HS, Weickert MO. Supplementation of vitamin D deficiency in patients with neuroendocrine tumors using over-the-counter vitamin D3 preparations. Nutrition and Cancer 201870748–754. ( 10.1080/01635581.2018.1470650) [DOI] [PubMed] [Google Scholar]
- 20.Guan M, He I, Luu M, David J, Gong J, Placencio-Hickok VR, Reznik RS, Tuli R, Hendifar AE. Palliative radiation therapy for bone metastases in neuroendocrine neoplasms. Advances in Radiation Oncology 20194513–519. ( 10.1016/j.adro.2019.03.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipton A, Fizazi K, Stopeck AT, Henry DH, Smith MR, Shore N, Martin M, Vadhan-Raj S, Brown JE, Richardson GE, et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. European Journal of Cancer 20165375–83. ( 10.1016/j.ejca.2015.09.011) [DOI] [PubMed] [Google Scholar]
- 22.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Journal of Clinical Oncology 2011291125–1132. ( 10.1200/JCO.2010.31.3304) [DOI] [PubMed] [Google Scholar]
- 23.Sabet A, Khalaf F, Haslerud T, Al-Zreiqat A, Sabet A, Simon B, Pöppel TD, Biersack HJ, Ezziddin S. Bone metastases in GEP-NET: response and long-term outcome after PRRT from a follow-up analysis. American Journal of Nuclear Medicine and Molecular Imaging 20133437–445. [PMC free article] [PubMed] [Google Scholar]
- 24.Ezziddin S, Sabet A, Heinemann F, Yong-Hing CJ, Ahmadzadehfar H, Guhlke S, Höller T, Willinek W, Boy C, Biersack HJ. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. Journal of Nuclear Medicine 2011521197–1203. ( 10.2967/jnumed.111.090373) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a