Abstract
Context
Obesity seems to decrease levels of testosterone. It is still unknown what role inflammation plays in the secretion of testosterone in men.
Objective
The objective is to study the association between levels of C-reactive protein and testosterone and its role in predicting biochemical hypogonadism in men.
Design
This was a longitudinal observational study between 2002 and 2014 in Sweden.
Patients or other participants
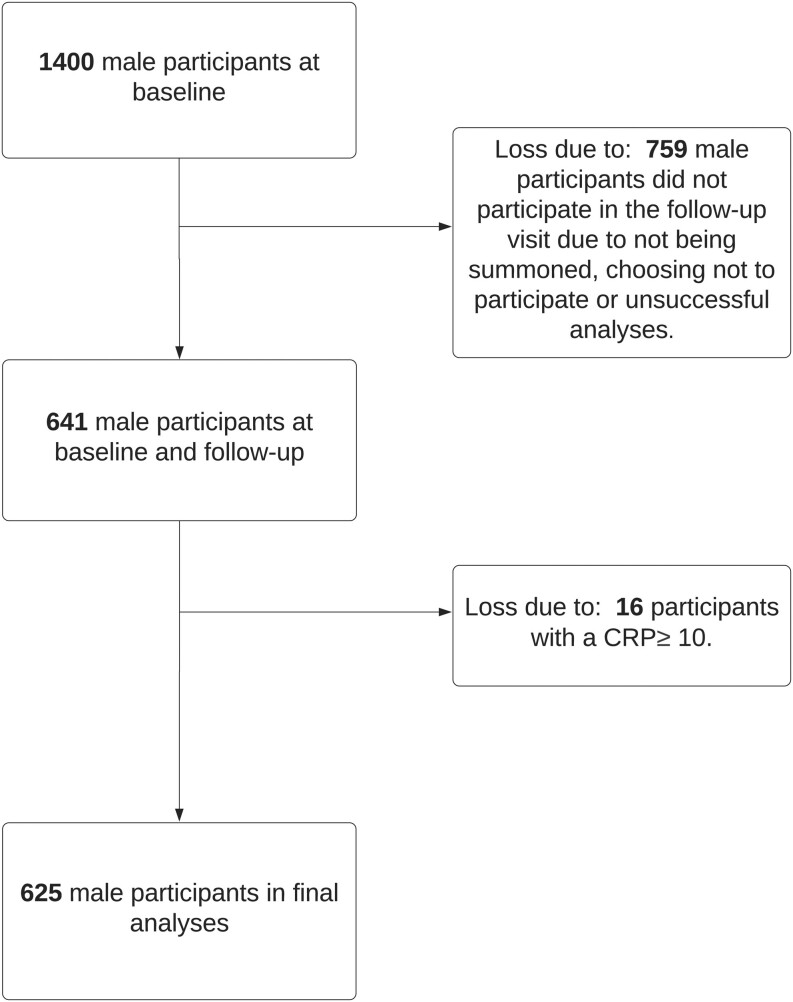
At the first visit, a random population sample of 1400 men was included, and 645 men fulfilled a similar protocol at a 10-year follow-up visit. After exclusion, 625 men remained to be included in the final analyses.
Main outcome measure(s)
Serum concentrations of testosterone and C-reactive protein (CRP) were measured at both visits. Bioavailable testosterone was calculated. Biochemical hypogonadism was defined as total testosterone levels <8 nmol/L.
Results
At the first visit and in the longitudinal analyses, a strong association was found between high levels of CRP and low levels of calculated bioavailable testosterone even after adjustments for age, waist–hip ratio, hypertension, smoking, type 2 diabetes, and leisuretime physical activity (B = −0.31, 95% CI −0.49 to −0.13, P = 0.001, B = −0.26, 95% CI −0.41 to −0.11, P = 0.001). Similarly, increase with one s. d. in CRP was associated with increased risk of having hypogonadism after adjustment in the final model (odds ratio (OR) 1.76, 95% CI 1.12–2.78, P = 0.015, OR 1.80, 95% CI 1.16–2.78, P =0.008).
Conclusions
In this representative cohort of men in southwestern Sweden, high levels of CRP were longitudinally associated with low concentrations of calculated bioavailable testosterone and increased risk of biochemical hypogonadism.
Keywords: CRP, testosterone, biochemical hypogonadism, bioavailable testosterone
Introduction
Low testosterone as well as high C-reactive protein (CRP) levels have been associated with increased risk for all-cause mortality in men (1, 2, 3, 4). Testosterone concentration decreases with aging, and if reaching hypogonadic levels in adulthood, this is defined as late-onset hypogonadism (LOH), according to the European Association of Urology (EAU) (5). EAU guidelines from 2019/2021 and Swedish National Guidelines consider male hypogonadism to be defined as an integration between clinical hypogonadism, a triad of symptoms (erectile dysfunction, low libido, and loss of morning erections), and biochemical hypogonadism with the strongest predicting value of total testosterone (TT) level <8 nmol/L or a TT range between 8 and 11 nmol/L and free testosterone <220 pmol/L (5, 6). In men with LOH, symptoms may be mild and are often confused with both aging and other comorbidities (5). It has previously been observed that testosterone, through its androgen receptors, regulates the expression of cytokines, providing a modulating role in the inflammatory response (2, 7). In addition, recent clinical studies have suggested a bidirectional association between concentrations of cytokines stimulated by obesity and testosterone (8). Studies have proposed that obesity, comorbidities, and aging play a central role in this association, promoting androgen deficiency by the secretion of adipocytokines and CRP (5, 7).
Bianchi et al. found in their systematic review that the vast majority of studies showed an association between low testosterone concentrations and high CRP levels in men (2). Due to the cross-sectional design of studies, it is challenging to understand the direction of these associations. To the best of our knowledge, no studies have examined a longitudinal association between high CRP concentration and the development of hypogonadism.
Therefore, the aim of this study was to investigate the longitudinal association between CRP and testosterone concentrations. More specifically, we investigated whether high levels of CRP are associated with an increased risk of developing biochemical hypogonadism.
Materials and methods
Design
The study was a longitudinal observational study.
Study population
This was a population-based, longitudinal, observational study. The study population has previously been described, but in brief, a random population sample based on the census registry was selected between 2002 and 2005, and a follow-up visit was performed in the same population in 2012–2014 (9). The selected population was based on the census in two municipalities, Vara and Skövde, in southwestern Sweden. In total, 2816 participants completed the study protocol and were included at visit one (baseline). The focus of this cohort study was the detection of early cardiometabolic disorders, and thus the age of participants ranged between 30 and 74, with oversampling of subjects between 30 and 50 years of age. During 2012–2014, a second visit was carried out, and a representative sample was invited to participate with almost identical protocols as at visit one. Participants who did not participate in the second visit or had missing information on anthropometric measures, testosterone levels, sex hormone-binding globulin (SHBG), smoking, hypertension, leisure-time physical activity (LTPA), or diabetes were excluded, leaving a remainder of 641 men. Individuals with CRP >10 mg/dL were excluded in this study, in line with recommendations from the American Heart Association and others indicating that CRP levels >10 mg/dL at these levels signify clinical inflammation, most often caused by an infection (10). Subsequently, male participants who completed the second visit but who were found to have a CRP >10 mg/L at the first visit were also excluded. A total of 625 men were included in the final analyses (Fig. 1). Biochemical hypogonadism was defined as TT <8 nmol/L in accordance with other studies (6, 11, 12).
Figure 1.
Flow chart of the study population.
Data collection
Trained study nurses collected information regarding history of chronic diseases and medication as well as performed anthropometric and blood pressure measurements. Blood pressure was measured in the supine position after 5 min of rest at baseline and follow-up. Similar procedures were performed at both visits (13). Diabetes mellitus type II (T2D) and hypertension were defined according to the World Health Organization and European Society of Cardiology, respectively (14, 15). Validated questionnaires were used to collect information on lifestyle including smoking, alcohol use, and LTPAs (16).
Assessment of androgen hormones and CRP biomarkers
Blood samples were drawn in the morning and frozen at −82°C. Serum concentrations of testosterone were measured using RIA. At baseline, Access Testosterone Assay from Beckman-Coulter (cat no. 2003, 386982A, RRID:AB_2895595) was used (coefficient of variation (CV) = 7–8%), and at follow-up, testosterone concentrations were assessed using Elcsys Testosterone II Assay (Roche cat no. 05200067, RRID:AB_2783736) from Roche Diagnostics (intraassay CV = 1.6–2.6%, interassay CV = 2.3–5.1%) (17). The limit of quantification was 1 nmol/L. As measurement techniques changed during follow-up time, we compared similar age groups at both baseline and follow-up in order to estimate the change in concentration due to method change. We found that the new method gave 11% higher results compared to the method used at baseline, and this increase was similar for every age group. Henceforth, TT at follow-up was multiplied with 0.89 as a computed variable. A strong association between the concentration of testosterone at baseline and at follow-up was observed (Pearson correlation 0.617, P < 0.001). At both baseline and follow-up analyses of the participants, testosterone samples were 95.3% successful. Calculation of bioavailable testosterone was done using the formula according to Vermuelen et al. (18). At both visits, RIA was used to analyze SHBG (19). High-sensitivity CRP (hs-CRP) serum concentrations (intraassay CV = 1.8–6.2%, interassay CV = 2.8–11%) (20) were assessed with RIA at the Department of Clinical Chemistry, Skåne University Hospital.
Statistical analysis
Characteristics of the study population were assessed using descriptive statistics to calculate means and confidence intervals. Continuous variables were reported with means and s.d. Due to right-sided skewness, levels of hsCRP were log10-transformed at baseline (logCRP). Descriptive statistics were used to standardize baseline logCRP variables in all analyses. Outcomes were measured as the change in one standardized unit of CRP. Evaluation of the association between logCRP and testosterone was made through cross-sectional linear regression both at the first visit and in the longitudinal analyses. Testosterone was set as the dependent variable (TT and calculated bioavailable testosterone (cBT)) with logCRP as the independent variable. Data were adjusted in theoretical models for possible confounding variables where Model 1 included age and waist-to-hip ratio (WHR) as continuous variables. Model 2 included the comorbidities hypertension, smoking, T2D, and LTPA assessed as continuous variables. Similar models using BMI instead of WHR were also tested. In the longitudinal analyses, adjustments were also made for baseline (total and calculated bioavailable) testosterone measurement. Logistic regression analyses were used to evaluate the association between logCRP and biochemical hypogonadism in both cross-sectional and longitudinal analysis, using similar models as in the linear regression analyses. Participants with biochemical hypogonadism, that is, TT levels <8 nmol/L at the first visit, were excluded from the longitudinal analyses. General linear models were used to compare the mean changes in stratified age groups in both total and cBT as well as SHBG over time. Data were processed using SPSS Statistics, version 26/27.
Ethical considerations
Written informed consent was given by all participants. The study protocol was approved by the Regional Ethical Review Board in Gothenburg, Sweden (D-nr Ö 199-01 and 036-12).
Results
Levels of sex hormones and aging
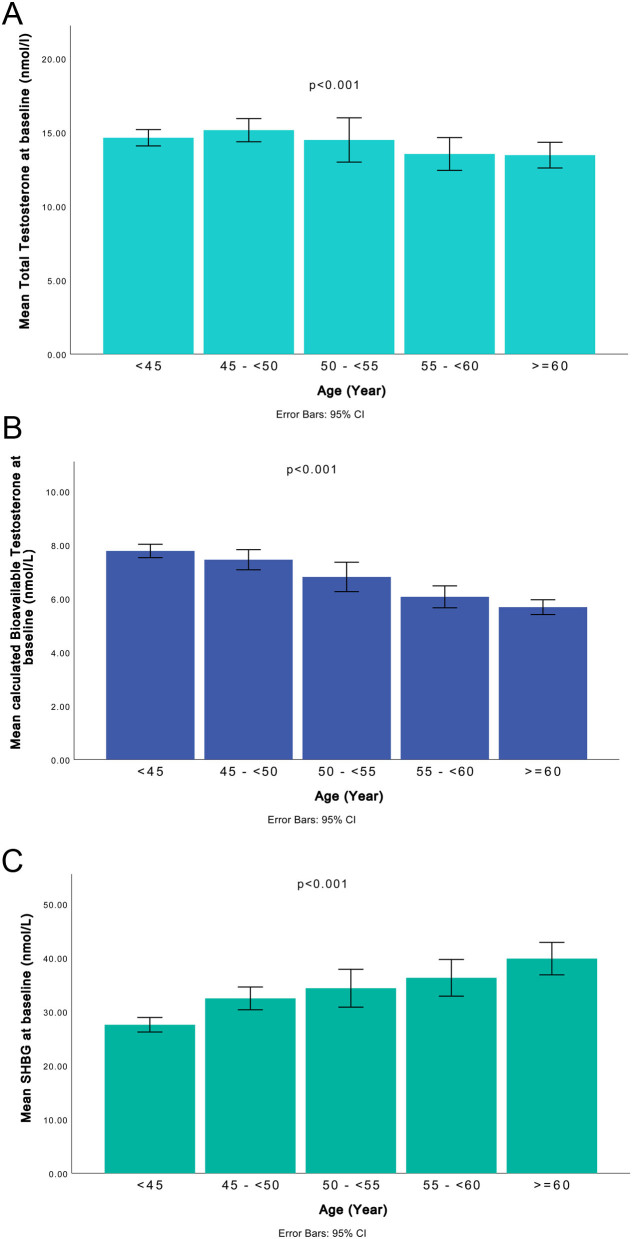
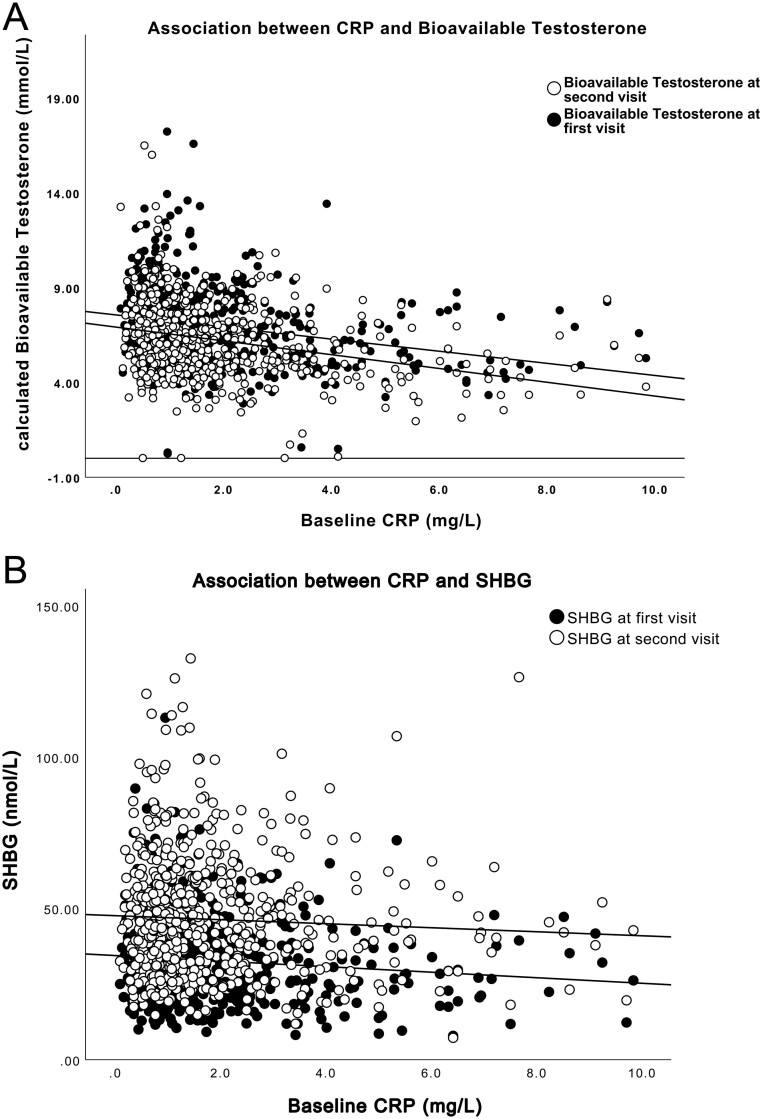
Mean age at the first visit was 49.2 ± 11.6 and 58.9 ± 11.8 years at the second visit (Table 1). Levels of TT remained almost constant until the age of 50, after which they declined somewhat more slowly compared to the decrease in cBT (Fig. 2A and B). Moreover, an increase in levels of SHBG was observed with aging (Fig. 2C). High CRP was associated with low cBT and SHBG levels at both visits (Fig. 3A and B).
Table 1.
Characteristics of the study population at both visits. BMI 26.86 ± 3.29.
| Visit 1 (n= 625), mean ± s.d./n (%) | Visit 2 (n= 625), mean ± s.d./n (%) | |
|---|---|---|
| Age (years) | 49.2 ± 11.64 | 58.9 ± 11.8 |
| Systolic blood pressure (mmHg) | 124 ± 15.5 | 127 ± 13.95 |
| Diastolic blood pressure (mmHg) | 72 ± 9.9 | 77 ± 10.52 |
| Waist-to-hip ratio | 0.94 ± 0.06 | 0.97 ± 0.65 |
| BMI | 26.86 ± 3.29 | 27.5 ± 3.63 |
| CRP (mg/L) | 1.76 ± 1.6 | 2.56 ± 5.9 |
| Serum testosterone (nmol/L) | 14.3 ± 4.46 | 15.8 ± 5.74 |
| Smokers | 81 (14.4) | 57 (9.1) |
| Level of leisure-time physical activity | ||
| Sedentary | 52 (8.3) | 71 (11.3) |
| Low level of physical activity | 329 (52.8) | 327(52.3) |
| Moderate level of physical activity | 220 (35.3) | 190 (30.4) |
| Strenuous physical activity | 21 (3.3) | 37 (5.9) |
| Sex hormone-binding globulin (nmol/L) | 32.8 ± 13.5 | 46.5 ± 19.89 |
| Diabetes mellitus tpe 2 | 20 (3.1) | 52 (8.3) |
| Hypertension | 95 (10.4) | 189 (30.2) |
| Biochemical hypogonadis | 35 (5.6) | 56 (8.9) |
CRP, C-reactive protein.
Figure 2.
Mean concentrations of total testosterone (A), calculated bioavailable testosterone (B) and SHBG (C) in different age groups, Vara-Skövde cohort. A constant and significant decrease in bioavailable testosterone was observed with increasing age, as well as increase of SHBG with age.
Figure 3.
A scatterplot presenting the association between baseline CRP and calculated bioavailable testosterone (A) and SHBG (B) at both visits.
Prevalence of biochemical hypogonadism in the study population
At visit one, 35 (5.6%) subjects were identified with biochemical hypogonadism. At the 10-year follow-up, a total of 56 participants (8.7%) presented with testosterone levels below 8 nmol/L, at the second visit. However, when participants with biochemical hypogonadism at baseline were excluded, a total of 38 men (6.4%) were found to develop low testosterone levels during the observation period.
CRP and testosterone cross-sectional analyses
In the cross-sectional analysis at the first visit, a statistically significant association was found between hsCRP and cBT, adjusting for age and WHR in Model 1 (β = −0.32, 95% CI −0.49 to −0.15, P < 0.001) as well as smoking, hypertension, T2D, and LTPA in Model 2 (β = −0.31, 95% CI −0.49 to −0.13, P = 0.001) (Table 2). Similar results were found between hsCRP and TT in Model 1 (β = −0.97, 95% CI −1.37 to 0.57, P < 0.001) and in the fully adjusted model (β = −0.96, 95% CI −1.38 to 0.54, P < 0.001).
Table 2.
Association between C-reactive protein and calculated bioavailable testosterone. Linear regression analyses were computed and two theoretical models were built.
| β | 95% CI | P |
|---|---|---|
| Cross-sectional analyses at baseline (n = 625) | ||
| Unadjusted model | ||
| −0.59 | −0.77 to −0.42 | <0.0001 |
| Adjusted for age and WHR at baseline | ||
| −0.32 | −0.49 to −0.15 | <0.001 |
| Adjusted for age, WHR, hypertension, smoking, T2D and LTPA at baseline | ||
| −0.31 | −0.49 to −0.13 | 0.001 |
| Longitudinal analyses (n = 625) | ||
| Unadjusted model | ||
| −0.69 | −0.86 to −0.53 | <0.0001 |
| Adjusted for age and WHR at baseline | ||
| −0.39 | −0.55 to −0.22 | <0.001 |
| Adjusted for age, WHR, and calculated bioavailable testosterone at baseline | ||
| −0.27 | −0.42 to −0.12 | <0.001 |
| Adjusted for age, WHR, calculated bioavailable testosterone in baseline, hypertension, smoking, T2D, and LTPA at baseline | ||
| −0.26 | −0.41 to −0.11 | 0.001 |
LTPA, leisure-time physical activity; P,significance; T2D, type 2 diabetes; WHR, waist-to-hip ratio; β, standardized coefficient of the association. .
CRP and testosterone longitudinal analyses
A significant association was observed between baseline hsCRP and cBT when adjusted for age and WHR in Model 1 (β = −0.39, 95% CI −0.55 to −0.22, P < 0.001), including further adjustment for cBT in baseline in Model 2 (β = −0.27, 95% CI −0.42 to −0.12, P = < 0.001) as well as additional adjustment for smoking, hypertension, T2D, and LTPA in Model 3 (β = −0.26, 95% CI −0.41 to −0.11, P < 0.001) (Table 2). Furthermore, a significant association was found between baseline hsCRP and TT in follow-up when adjusting for age and WHR (β = −0.93, 95% CI −1.39 to −0.47, P < 0.001).
CRP and biochemical hypogonadism cross-sectional and longitudinal analyses
When assessing the relationship between hsCRP levels and biochemical hypogonadism, similar models were used (Table 3). In the cross-sectional analyses, an increase of one s.d. of hsCRP was associated with a higher risk of having biochemical hypogonadism in both models (odds ratio (OR) 1.90, 95% CI 1.22 to 2.94, P = 0.004 and OR 1.76, 95% CI 1.12 to 2.78, P = 0.015). In the longitudinal analyses, an increase of one unit of hsCRP was associated with a significantly increased risk of developing biochemical hypogonadism, independent of confounders (OR 2.03, 95% CI 1.34 to 3.06, P = 0.001 and OR 1.80, 95% CI 1.16–2.78, P = 0.008). The magnitude of the effect was observed to be lower when adjusting for BMI in all fully adjusted models although the same direction in the association was observed (data not shown). Sensitivity analyses at baseline showed that the association between levels of CRP and testosterone in the whole cohort was similar to the association in the subgroup of the participants that were examined at both visits (estimates presented in the fully adjusted model, analyzing total cohort population of 1400 men at visit 1 (β = −0.19, 95% CI −0.315 to −0.064, P = 0.003)).
Table 3.
Association between C-reactive protein and biochemical hypogonadism. Linear regression analyses were computed and two theoretical models were built.
| OR | 95% CI | P |
|---|---|---|
| Cross-sectional analyses at baseline (n = 625) | ||
| Unadjusted model | ||
| 2.61 | 1.74–3.92 | <0.0001 |
| Adjusted for age and WHR at baseline | ||
| 1.90 | 1.22–2.94 | 0.004 |
| Adjusted for age, WHR, hypertension, smoking, T2D, and LTPA at baseline | ||
| 1.76 | 1.12–2.78 | 0.015 |
| Longitudinal analyses (n = 590) | ||
| Unadjusted model | ||
| 2.33 | 1.58–3.43 | <0.0001 |
| Adjusted for age and WHR at baseline | ||
| 2.03 | 1.34–3.06 | 0.001 |
| Adjusted for age, WHR, hypertension, smoking, T2D, and LTPA at baselinea | ||
| 1.80 | 1.16–2.78 | 0.008 |
LTPA, leisure-time physical activity; OR, odds ratio; P, significance; T2D, type 2 diabetes; WHR, waist-to-hip ratio; β, standardized coefficient of the association.
Discussion
In this prospective study, we observed a strong association between high levels of high-sensitive CRP and low concentrations of bioavailable testosterone in men in both cross-sectional and longitudinal analyses, independent of other relevant cardiometabolic and lifestyle factors. Furthermore, high levels of hsCRP were associated with an increased risk of developing biochemical hypogonadism after 10 years. The increment of hsCRP with one s.d. was associated with more than doubled risk of developing biochemical hypogonadism during follow-up, independent of possible confounders (Table 3).
This study confirms earlier cross-sectional observations regarding the inverse association between hsCRP and testosterone concentrations in men (shown in Fig. 3A), including the decline of testosterone levels with aging (shown in Fig. 2A and B) (21, 22, 23, 24). So far, there are only a few prospective studies with repeated measurements of testosterone and defined biochemical hypogonadic levels at different time points. To the best of our knowledge, no study has earlier presented data confirming a longitudinal association between inflammation at baseline and a decrease in both levels of cBT and TT, defined as biochemical hypogonadism. This is the main novel finding in the present study. Moreover, our data indicate that CRP, a marker of inflammation, per se can predict the development of biochemical hypogonadism regardless of anthropometric measures such as WHR and BMI.
There is a great number of studies investigating the association of testosterone levels with inflammatory markers, seemingly to confirm evidence of the association, remaining significant even after adjustments for obesity. In the BACH study (1559 male participants), a significant association was found between CRP and both total and free testosterone levels independent of age, obesity, and comorbidities (25). Similarly, a cross-sectional study by Tsilidis et al., including data from the NHANES population (809 participants), found that men with low testosterone were at higher risk of having high CRP independent of total body weight, age, medication, or other comorbidities (23). In a cross-sectional study on Finnish non-diabetic men (1896 participants), results suggested metabolic syndrome as a great contributor to the high CRP–low testosterone relationship (21). Moreover, Kaplan et al. confirmed an inverse association as men with a higher occurrence of defined metabolic components were found to have higher hsCRP levels (22). In a 5-year long observational study (1344 male participants), Haring et al. reported no significant association between sex hormone concentration and hsCRP, although an association was found with prothrombotic and oxidative markers (26). The study did not report any results on the levels of sex hormones at follow-up and it is unclear whether the hypothesis of inflammatory markers predicting levels of testosterone was investigated.
Other cross-sectional studies suggest that obesity largely could explain in most part the association between inflammatory markers and testosterone levels (27, 28, 29, 30, 31). Furthermore, Zhang et al. (1989 male participants) reported in their cross-sectional study an inverse association between CRP and total and free testosterone as well as SHBG, independent of obesity, insulin resistance, and metabolic syndrome (24). However, the association between CRP and testosterone declined significantly when adjusted for visceral obesity. Similar to their findings, we observed a substantial change in the estimates when WHR or BMI was added to the models. This change in estimates was larger in the cross-sectional analyses than in the longitudinal ones (Table 3).
A partial contributor to the explanation of bidirectionality on the relationship could be reported on obesity-induced cytokines which have been shown to antagonistically act on the reproductive stimulatory axis, impeding the effect on the hypothalamic KISS neuron which regulates the release of luteinizing hormone (LH), causing a decrease in the production of testosterone (5, 32). HsCRP has previously been described to interact with the mediators and further promote an inhibitory effect (33, 34, 35, 36). Additional studies are needed to evaluate the role of CRP as a possible inflammatory mediator in testosterone deficiency.
Strengths and limitations
This is a large cohort-based study of men, representative of the population in Sweden. Although a large part of the cohort could not participate in this study due to loss-to-follow-up, sensitivity analyses showed similar characteristics between participants and non-participants at the second visit. The meticulous sampling of important information using validated instruments permitted adjustments for important variables. However, residual confounding cannot be excluded due to the observational nature of the study. Another strength of the study was the standardized sampling of blood specimens in the morning after fasting according to EAU guidelines, avoiding diurnal changes in the levels of sex hormones (5, 6, 37, 38).
A limitation was the change of the method used for measurements of testosterone during the study. We conducted adjustments based on age group levels of testosterone to overcome this problem; however, we acknowledge that measurements with identical methods would be preferred as according to Travison et al., a temporal decline in testosterone levels has been found in men in recent cohorts when compared to men in the same age group from older cohorts (39). This is a methodological challenge for all long-term observational studies investigating the changes in sex hormones. However, the use of two measurements could also strengthen the study as it would permit the investigation of these associations in a prospective design. RIA technique has been considered a less reliable measurement method of testosterone levels compared to mass spectrometry, especially at lower levels (40). This could affect the precision and therefore increase the risk for type 2 error in the analyses. However, RIAs have been found to be used more often in clinical practice and have shown a justifiable correlation with mass spectrometry (6). According to the European Urology Association guidelines on testosterone measurement, both immuno-assay and mass spectrometry, providing a reference range for normal men, would be applicable with reliable results (6). Another limitation of the study is the possibility of evaluation of body composition with radiological techniques that could have given a more precise estimation of abdominal obesity. It is our opinion that the results should be similar as we did not find large differences when testing BMI and WHR in our models. Furthermore, the incidence of T2D and hypertension increases with age as well as required treatment, further affecting the inflammatory-hypogonadic relationship. Although, in this study, even though the prevalence of T2D and hypertension increased during follow-up time, the association remained statistically significant (data not shown).
Male hypogonadism is defined according to both biochemical findings and clinical symptoms, with highest predictive value shown by decreased morning erection, libido, and sexual desire (6). In our study, questionnaires on clinical symptoms were included only at the second visit. Therefore, we were only able to observe biochemical hypogonadism in the longitudinal analyses. Finally, CRP was used as a marker for sub-inflammatory concentrations in this study. However, CRP is known to be unspecific, and the use of other inflammatory markers such as IL-6 might provide a more precise estimate of inflammation (3, 36).
In conclusion, our study confirms an independent association between high levels of hsCRP and low bioavailable testosterone concentrations 10 years later, independent of cardiometabolic and lifestyle factors as well as baseline concentrations. Furthermore, hsCRP was observed to increase the risk of biochemical hypogonadism in men independent of age, obesity, and other confounders. Further studies are needed to confirm the longitudinal association between CRP and androgen levels, adjusting for different confounding cytokines and underlying mechanisms to better understand the possible impact of inflammation on sexual hormonal secretion and male health.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The study was supported by The Local Research and Development Council Göteborg och Södra Bohuslän, the VGR Regional Research and Development Council Grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement.
Acknowledgements
The authors would like to express their gratitude to the participants from Vara and Skövde for making this study possible.
References
- 1.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism 2011963007–3019. ( 10.1210/jc.2011-1137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi VE.The anti-inflammatory effects of testosterone. Journal of the Endocrine Society 2019391–107. ( 10.1210/js.2018-00186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? American Journal of Medicine 2006119166.e17–166.e28. ( 10.1016/j.amjmed.2005.06.057) [DOI] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010375132–140. ( 10.1016/S0140-6736(0961717-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, Cocci A, Corona G, Dimitropoulos K, Gül M, et al. European Association of Urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. European Urology 202180333–357. ( 10.1016/j.eururo.2021.06.007) [DOI] [PubMed] [Google Scholar]
- 6.EAU. EAU Guidelines: edition presented at the EAU Annual Congress Barcelona, Arnhem, The Netherlands: EAU, 2019. [Google Scholar]
- 7.Traish A, Bolanos J, Nair S, Saad F, Morgentaler A. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. Journal of Clinical Medicine 20187 549. ( 10.3390/jcm7120549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez CJ, Chacko EC, Pappachan JM. Male obesity-related secondary hypogonadism – pathophysiology, clinical implications and management. European Endocrinology 20191583–90. ( 10.17925/EE.2019.15.2.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olausson J, Daka B, Hellgren MI, Larsson CA, Petzold M, Lindblad U, Jansson PA. Endothelin-1 as a predictor of impaired glucose tolerance and type 2 diabetes – a longitudinal study in the Vara-Skövde Cohort. Diabetes Research and Clinical Practice 201611333–37. ( 10.1016/j.diabres.2016.01.027) [DOI] [PubMed] [Google Scholar]
- 10.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation 2003107370–371. ( 10.1161/01.cir.0000053731.05365.5a) [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. European Journal of Endocrinology 2008159507–514. ( 10.1530/EJE-08-0601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Frontiers of Hormone Research 2009375–20. ( 10.1159/000175839) [DOI] [PubMed] [Google Scholar]
- 13.Hellgren MI, Larsson CA, Daka B, Petzold M, Jansson PA, Lindblad U. C-reactive protein concentrations and level of physical activity in men and women with normal and impaired glucose tolerance. A cross-sectional population-based study in Sweden. Journal of Physical Activity and Health 201613625–631. ( 10.1123/jpah.2015-0168) [DOI] [PubMed] [Google Scholar]
- 14.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Medicine 199815539–553. () [DOI] [PubMed] [Google Scholar]
- 15.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). European Heart Journal 201941255–323. ( 10.1093/eurheartj/ehz486) [DOI] [PubMed] [Google Scholar]
- 16.Løchen ML, Rasmussen K. The Tromsø study: physical fitness, self reported physical activity, and their relationship to other coronary risk factors. Journal of Epidemiology and Community Health 199246103–107. ( 10.1136/jech.46.2.103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen WE, Rawlins ML, Roberts WL. Selected performance characteristics of the Roche Elecsys testosterone II assay on the modular analytics E 170 analyzer. Clinica Chimica Acta 20104111073–1079. ( 10.1016/j.cca.2010.03.041) [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. Journal of Clinical Endocrinology and Metabolism 1999843666–3672. ( 10.1210/jcem.84.10.6079) [DOI] [PubMed] [Google Scholar]
- 19.Ottarsdottir K, Hellgren M, Bock D, Nilsson AG, Daka B. Longitudinal associations between sex hormone-binding globulin and insulin resistance. Endocrine Connections 20209418–425. ( 10.1530/EC-20-0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson WL, Koenig W, Fröhlich M, Sund M, Lowe GD, Pepys MB. Immunoradiometric assay of circulating C-reactive protein: age-related values in the adult general population. Clinical Chemistry 200046934–938. ( 10.1093/clinchem/46.7.934) [DOI] [PubMed] [Google Scholar]
- 21.Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT. Sex hormones, inflammation and the metabolic syndrome: a population-based study. European Journal of Endocrinology 2003149601–608. ( 10.1530/eje.0.1490601) [DOI] [PubMed] [Google Scholar]
- 22.Kaplan SA, Johnson-Levonas AO, Lin J, Shah AK, Meehan AG. Elevated high sensitivity C-reactive protein levels in aging men with low testosterone. Aging Male 201013108–112. ( 10.3109/13685530903440424) [DOI] [PubMed] [Google Scholar]
- 23.Tsilidis KK, Rohrmann S, McGlynn KA, Nyante SJ, Lopez DS, Bradwin G, Feinleib M, Joshu CE, Kanarek N, Nelson WG, et al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology 20131919–928. ( 10.1111/j.2047-2927.2013.00129.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Gao Y, Tan A, Yang X, Zhang H, Zhang S, Wu C, Lu Z, Wang M, Liao M, et al. Endogenous sex hormones and C-reactive protein in healthy Chinese men. Clinical Endocrinology 20137860–66. ( 10.1111/j.1365-2265.2012.04359.x) [DOI] [PubMed] [Google Scholar]
- 25.Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clinical Endocrinology 201072527–533. ( 10.1111/j.1365-2265.2009.03713.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haring R, Baumeister SE, Völzke H, Dörr M, Kocher T, Nauck M, Wallaschofski H. Prospective inverse associations of sex hormone concentrations in men with biomarkers of inflammation and oxidative stress. Journal of Andrology 201233944–950. ( 10.2164/jandrol.111.015065) [DOI] [PubMed] [Google Scholar]
- 27.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. Journal of Clinical Endocrinology and Metabolism 2010951810–1818. ( 10.1210/jc.2009-1796) [DOI] [PubMed] [Google Scholar]
- 28.Pasquali R.Obesity and androgens: facts and perspectives. Fertility and Sterility 2006851319–1340. ( 10.1016/j.fertnstert.2005.10.054) [DOI] [PubMed] [Google Scholar]
- 29.Wajchenberg BL.Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocrine Reviews 200021697–738. ( 10.1210/edrv.21.6.0415) [DOI] [PubMed] [Google Scholar]
- 30.Lemieux I, Pascot A, Prud’homme D, Alméras N, Bogaty P, Nadeau A, Bergeron J, Després JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arteriosclerosis, Thrombosis, and Vascular Biology 200121961–967. ( 10.1161/01.atv.21.6.961) [DOI] [PubMed] [Google Scholar]
- 31.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arteriosclerosis, Thrombosis, and Vascular Biology 199919972–978. ( 10.1161/01.atv.19.4.972) [DOI] [PubMed] [Google Scholar]
- 32.George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 201091302–307. ( 10.1159/000299767) [DOI] [PubMed] [Google Scholar]
- 33.Sudhakar M, Silambanan S, Chandran AS, Prabhakaran AA, Ramakrishnan R. C-reactive protein (CRP) and leptin receptor in obesity: binding of monomeric CRP to leptin receptor. Frontiers in Immunology 20189 1167. ( 10.3389/fimmu.2018.01167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hribal ML, Fiorentino TV, Sesti G. Role of C reactive protein (CRP) in leptin resistance. Current Pharmaceutical Design 201420609–615. ( 10.2174/13816128113199990016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. Journal of Neuroendocrinology 200618298–303. ( 10.1111/j.1365-2826.2006.01417.x) [DOI] [PubMed] [Google Scholar]
- 36.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. Journal of Clinical Endocrinology and Metabolism 200893 (Supplement 1) S64–S73. ( 10.1210/jc.2008-1613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales A.Testosterone deficiency syndrome: an overview with emphasis on the diagnostic conundrum. Clinical Biochemistry 201447960–966. ( 10.1016/j.clinbiochem.2013.11.024) [DOI] [PubMed] [Google Scholar]
- 38.Rastrelli G, Carter EL, Ahern T, Finn JD, Antonio L, O'Neill TW, Bartfai G, Casanueva FF, Forti G, Keevil B, et al. Development of and recovery from secondary hypogonadism in aging men: prospective results from the EMAS. Journal of Clinical Endocrinology and Metabolism 20151003172–3182. ( 10.1210/jc.2015-1571) [DOI] [PubMed] [Google Scholar]
- 39.Travison TG, Araujo AB, Hall SA, McKinlay JB. Temporal trends in testosterone levels and treatment in older men. Current Opinion in Endocrinology, Diabetes, and Obesity 200916211–217. ( 10.1097/med.0b013e32832b6348) [DOI] [PubMed] [Google Scholar]
- 40.Trost LW, Mulhall JP. Challenges in testosterone measurement, data interpretation, and methodological appraisal of interventional trials. Journal of Sexual Medicine 2016131029–1046. ( 10.1016/j.jsxm.2016.04.068) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a