Abstract
Objective
Klinefelter syndrome (KS) is the second-most prevalent chromosomal disorder in men, though late diagnosis is very common and 50–75% of men remain undiagnosed. Evidence suggests that men with KS have impaired quality of life (QoL) but research on how the diagnosis of KS is associated with different QoL domains and what factors influence patients’ QoL is limited. This study aimed to provide a systematic review of the published evidence on factors that influence QoL in men with KS.
Design
Systematic review and meta-analysis with narrative synthesis.
Methods
Medline, Cochrane, Embase, Psychinfo, CINAHL, BASE and relevant publication reference lists were searched in January 2021. Eligible studies included randomised control trials, cohort studies, cross-sectional studies and epidemiology studies on KS and its effect on QoL and all domains of World Health Organisation (WHO) Quality of Life 100 (WHOQOL-100). Clinical studies with no date restriction published in English were included.
Results
Thematic analysis was completed on 13 studies, with a meta-analysis of intelligence quotient completed on 7 studies. Twelve out of the 13 studies suggested that KS negatively affected the QoL outcomes and KS was associated with impairments in physical, psychological, level independence and social relationship domains of WHOQOL-100. Meta-analysis suggested that men with KS have significantly lower full-scale Intelligence Quotient vs controls (P < 0.00001).
Conclusions
This is the first evidence synthesis of QoL in men with KS. Current evidence suggests that combined physical and psychological impairments affect men with KS who also experience impairments in relationships and independence in society. Further research is needed to identify factors that influence the QoL in men with KS.
Introduction
Rationale
Klinefelter Syndrome (KS) first described by Harry Klinefelter in 1942 (1) is a common aneuploidy in men clinically characterised by small testes, gonadal failure (hypergonadotropic hypogonadism), disrupted spermatogenesis (infertility), gynaecomastia and eunuchoid proportions (arm span exceeds height by ≥7 cm) (2, 3). It affects 1 in 600 men, but 50–75% of men with KS go undiagnosed in their lifetime (2, 4, 5). Almost 90% of men with KS have an XXY karyotype and the remaining 10% have mosaicism (46, XY/47, XXY), higher-grade aneuploidy (48, XXXY; 49, XXXXY), or structurally abnormal X chromosomes (2).
The extent of mosaicism in KS causes an array of cognitive, psychosocial, and physical symptoms which can affect men at varied degrees of severity. These include hypogonadism, gynecomastia, tall stature, small phallus, reduced level of intelligence, depression, autism traits, schizotypal traits and social anxiety which lead to impaired quality of life (QoL) (2, 6, 7, 8, 9). Milder phenotype and lack of distinct dysmorphic features present a real challenge for early diagnosis (3). Testosterone replacement therapy is recommended for patients with KS once serum gonadotrophins begin to rise in early puberty or when serum testosterone levels become hypogonadal (3, 10, 11).
Evidence suggests that patients with KS have more impaired QoL compared to healthy controls; however, research on how the diagnosis of KS affects a patient’s QoL is limited (2, 12, 13).
There is limited knowledge of the various symptoms, outcomes and patient experiences, which may result in health and social inequalities for patients with KS. A greater understanding of the associations between KS and the domains of QoL can better support clinical decision-making and meet the condition-specific needs of patients with KS.
Objectives
The objective of this study was to conduct a systematic review with meta-analysis to provide new insights and further understanding of QoL in patients with KS and to answer the following research question:
What is the association between Klinefelter syndrome and the WHOQOL-100 domains/facets of QoL?
Underpinning framework WHOQOL-100
Due to the many factors influencing QoL in patients with KS, the World Health Organisation (WHO) Quality of Life 100 (WHOQOL-100) was adopted as the overarching framework to underpin this systematic review. The WHOQOL-100 is a validated psychometric scale which can be used to measure QoL as an overall construct and across its six QoL domains: ‘overall QoL’, ‘physical health’, ‘psychological health’, ‘level of independence’, ‘social relations’ and ‘environment’ (14). The subsections were developed by the WHO, by incorporating the important aspects of QoL defined by a range of patients and health professionals from various diseases, specialisms and cultural backgrounds. The WHOQOL-100 is a universal Patient-Reported Outcome Measure (PROM) that can measure individual QoL domains to provide evidence of unmet needs and impaired aspects of QoL.
Methods
Protocol and registration
The systematic review followed the Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for quantitative systematic reviews (15), and the study protocol was registered with PROSPERO (CRD42020173435). The Systematic Review Without Meta-analysis (SWiM) guidelines (16) were adopted for narrative synthesis which was guided by the overarching WHOQOL-100 and its six QoL domains (17). Meta-analysis was conducted where possible by grouping studies measuring overall QoL or outcome measures relating to the domains and facets of the WHOQOL-100 structure. Review manager 5.4 (18) was utilised for this analysis.
Eligibility criteria and participants
All empirical studies involving male children and/or adults diagnosed with KS and measuring a quantifiable factor of QoL that could be defined within the WHOQOL-100 were reviewed for eligibility. No publication date restrictions were imposed (see Supplementary material, see section on supplementary materials given at the end of this article).
Search
The search was completed on 21 January 2020 using the following databases: MEDLINE (1946 to present), APA Psychinfo (1956 to present), Embase (1974 to present), CINAHL (1963 to present), Cochrane (2005 to present) and grey search via Bielefeld Academic Search Engine (BASE). A secondary search, using the same strategy, was run covering the period between 21 January 2020 and 20 April 2021 to ensure no recent studies were missed; no new studies were included.
Each database was searched individually, the search keywords included ‘Klinefelter Syndrome’ and MeSH terms ‘48, XXYY Syndrome’, ‘49 XXXXY Syndrome’, ‘XXXY Males’, ‘XXY Syndrome’, ‘XXY Trisomy’ and ‘XXYY Syndrome’ all combined with ‘OR’. Keywords for QoL factors, combined with ‘OR’ included: ‘physical health’, ‘psychological health’, ‘level of independence’, ‘social relations’, ‘environment’, ‘spirituality’, ‘faith’ and ‘personal beliefs’; both groups were then combined with ‘AND’. A full search from CINAHL and Medline is included in the Supplementary material.
Study selection
The eligibility assessment was performed in a blind independent review by two authors (BM and SL); all disagreements were resolved by consensus and did not require a third reviewer. The blind review for abstracts and full text articles was done in Rayyan QCRI systematic review manager (https://rayyan.ai/) using pre-specified inclusion/exclusion criteria (Table 1). The PRISMA flow diagram in Fig. 1 annotates the study selection process. A detailed inclusion/exclusion review of all full text articles is included in Supplementary material.
Table 1.
Characteristics of included studies.
| Reference | n | Country | Setting | Study design | Methodology | Primary outcome | Measure | Comparator | JB quality score |
|---|---|---|---|---|---|---|---|---|---|
| Ferlin et al. (31) | 62 | Italy | Hospital clinic | Cross-sectional analysis/non-RCT | Interview, self-reported questionnaires | Type of sexual dysfunctions within KS | IIEF-15 | 60 aged-matched | 6/8 |
| Fisher et al. (32) | 46 | Italy | Hospital nits | Cross-sectional analysis | Clinical interviews, psychometric analysis | Prevalence of sexual disorders (GD, paraphilia) in KS | AQ, RME, GIDYQ-AA, SAST, SCL-90-R, IEEF, | 43 male controls | 7/8 |
| Herlihy et al. (21) | 87 | Australia | Participant home | Cross-sectional analysis | DNA self-admin test, self-administered questionnaires | Psychosocial impact of KS on QoL | PWI, MBSRQ-AS, RSE, K10, short form-1, SIS | General population normative means | 7/8 |
| Sorensen (27) | 14 | Denmark | Schools and clinical setting | Cross-sectional analysis | Clinical examination/ psychological assessment | Physical and mental development in KS | WAIS, school attainment, behaviour rating schedules | 19 male controls | 6/8 |
| Rapp et al. (23) | 219 | Germany | 14 recruitment centres. | Cross-sectional analysis | Medical examination/interviews, (PRO) questionnaires | Measuring QoL in patients with (DSD) | WHOQOL-bref | Healthy European populations | 8/8 |
| van Rijn (35) | 20 | Netherlands | Not specified | Cross-sectional analysis | Self-reported questionnaires, salivary T | Effect of T levels on ‘social anxiety, social cognition’ in KS | FSIQ, KDEF, SCST, SAS | 25 male controls | 6/8 |
| Skakkebaek et al. (33) | 132 | Denmark | University Hospital | Cross-sectional analysis | Questionnaires, salivary T | Determinants of anxiety and depression | WHOQOL-bref, SF-36, IIEF-15, demographics | 313 matched controls | 7/8 |
| Skakkebaek et al. (34) | 69 | Denmark | Clinical | Cross-sectional analysis | Questionnaires, cognitive assessments | Cognitive performance in KS | NEO PI-R, AQ, FSIQ | 69 matched controls | 6/8 |
| Van Rijn et al. (7) | 34 | Netherlands/Belgium | Academic medical clinics /support groups | Cross-sectional analysis | Patient- and parent-reported questionnaires | Social behavioural phenotype in children with KS | ADI-R, SRS, SAS, SSRS | 46 male Controls | 7/8 |
| Van Rijn et al. (26) | 31 | Netherlands | Not Specified | Cross-sectional analysis | Self-administered questionnaires and tests | Social difficulties in adult men with Klinefelter syndrome | NART, WAIS-R IQ, SIB, Short form, AQ | 2 male control groups (n = 20, n = 24) | 6/8 |
| Liberato et al., (22) | 58 | Italy | Clinical | Cross-sectional analysis | Blood sampling, self-reported questionnaire, and clinical interview | Investigate fluid intelligence, personality traits, personality disorders (PD) in adult KS | SCIDII, MMPI2, SPM | Community samples | 5/8 |
| Nielsen & Pelsen (24) | 34 | Denmark | Clinical | Cohort longitudinal study | Telephone interview | Differences between KS and hypogonadal males with a normal karyotype 46, XY | Job status, Martial status and adoption, Criminality, illness ‘physical or mental’ | 16 hypogonadal males | 6/11 |
| Fabrazzo et al. (30) | 23 | Italy | Academic and medical clinics | Cross-sectional analysis | Questionnaires including pre-existing scales and interviews | Impact of 1 year on TRT on psychopathological recovery and QoL in KS | Q-LES-Q, SCL-90-R, MMSE, TCI-R. | 23 matched healthy subjects. | 7/8 |
AQ, Autism Spectrum Quotient (62); ADI-R, Autism diagnostic interview – Revised (73); GSIS, General symptomatic index score; GIDYQ-AA, Gender Identity/Dysphoria Questionnaires for Adults and Adolescents (63); IIEF, International Index of Erectile function (75); K10, Kessler Psychological Distress Scale (66); MMPI-2, Minnesota Multiphasic Personality Inventory 2 (70); MMSE, Minimental State Examination; MBSRQ-AS, Multidimensional body-self relations questionnaire (64); NEO PI-R, Revised NEO personality inventory (67); NART, National adult reading test (81); PWI, Personal Wellbeing index (42); QRI, Qualitative reading inventory (67); Q-LES-Q, Italian Quality of Life Enjoyment and Satisfaction Questionnaire (38); RSE, Rosenberg self-esteem test (65); SI, Social interaction; SAS, Social anxiety scale (78); SF1, short form 1 (61); SF-36, 36 Item short form survey (37); SPM, Standard Progressive Matrices (74); SRS, The Social Responsiveness Scale (80); SAST, Short Anxiety Screening test (76); SCID-II, Structured Clinical Interview for Axis II Disorders (71); SCST, Social cognitive skills test (77); SRSS, Social skills rating scale (79); SCL-90-R, Symptom Checklist-90-R; SLC-ANX/DEP, Subscales of Symptoms checklist 90 Anxiety/Depression (72); TCI-R, Temperament and Character Inventory-Revise; WHOQOL100, World Health Organisation Quality of Life 100 bref (14).
Figure 1.
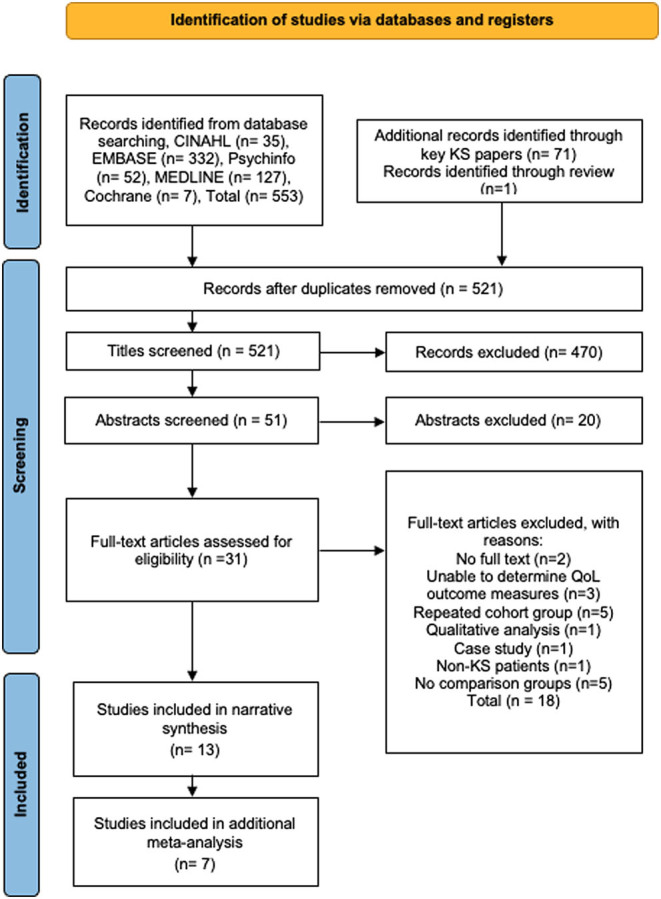
PRISMA flowchart of study search, screening, and selection. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1239.
Data items
Information was extracted from each study on the following: (i) number of participants; (ii) study settings, study country and study design; (iii) outcome measures related to the WHOQOL-100 framework (domains/facets) of QoL such as ‘physical health’, ‘social relations’, ‘psychological’, ‘environment’, ‘level of independence’ or ‘religion/personal beliefs/spirituality’; (iv) comparison groups where possible (Table 1).
Risk of bias of individual studies
The Joanna Briggs quality appraisal tool for cross-sectional studies/cohort studies (19) was used on all 13 studies included in the systematic review to ensure validity and to examine the reliability with a quantifiable score on each included study. Each question was dichotomised to either YES (1 point) or NO (0 points) producing a scale ranging from 0 (poor quality) to 8 or 10 or 11 (high quality) depending on the appraisal tool. Studies were given an appraisal score depending on how many categories of the appraisal they met: ‘inclusion criteria’, ‘study settings and subjects’, ‘exposure’, ‘confounding factors’, ‘outcomes’ and ‘statistics’ (see Supplementary material).
Synthesis of results
Narrative synthesis
The SWiM reporting items protocol (16) was adopted for the narrative synthesis. Using the WHOQOL-100 framework, subgroup analyses were conducted on each of the six subgroups of the WHOQOL-100 and reported in tables including study, effect size (Cohen’s d) and main findings. A meta-analysis was not possible for all studies due to the large amount of differing and heterogeneous outcome measures and scoring systems for QoL. Therefore, the percentage of significant findings and the strength of the effect sizes are considered within each QoL sub-section.
Data were too heterogeneous for meta-analysis due to the vastly different outcome measures included; therefore, to aid comparability, Cohen’s d was calculated by extracting the mean difference and s.d. from KS and control groups where reported. Accepted categories for Cohen’s d effect sizes as small (0.2), medium (0.5) and large (0.8) were applied (20). Where studies did not report P values, these were calculated using Fisher’s exact tests to show any significant differences (P < 0.05) between patients with KS and controls.
Results
Study characteristics
A total of 665 records were identified from the initial search of which 13 studies, 12 cross-sectional and 1 cohort, met the inclusion criteria and 7 studies were suitable for additional meta-analysis (Fig. 1). The total number of participants across the 13 studies was 829; study sample sizes ranged from 14 to 219 participants. Studies had a mixture of patient-reported, parent-reported, or physician-answered questionnaires. Table 1 presents a full summary of the characteristics extracted from each study.
Quality of studies
The quality of the included studies has an impact on the confidence of findings within the review. First, when assessed many studies did not discuss or include strategies to deal with confounders. Secondly, normative data and population averages were used for controls in three studies which lowers the comparative domain score for those studies (21, 22, 23), Nielsen and Pelsen (24) used hypogonadal males as controls which may also reduce confidence in this study as hypogonadal males QoL outcomes could be reduced due to the symptoms of hypogonadism. Eligible studies were assessed for methodological quality using the Joanna Briggs quality appraisal tools (Table 1).
Sampling methods included non-probability using snowballing (21), self-identification (6, 25, 26), purposive (7, 22, 27, 28, 29, 30) or convenience (12, 24, 31, 32, 33, 34, 35), while one study did not report method of sampling (23).
Narrative synthesis
Overall QoL
Results are reported in Table 2. Three studies measured QoL against controls (21, 30, 33), there was significant difference (P ≤ 0.05) between patients with KS and controls for the outcome measures: Personal Well-being Index (PWI) (36), WHOQOL-100 (17), Short Form Survey (Sf-36) (37) and for all quality of life enjoyment and satisfaction questionnaire (Q-LES-Q) (38) subitems. The PWI measures the subjective well-being as the average levels of satisfaction across eight aspects of personal life: (i) health; (ii) personal relationships; (iii) safety; (iv) standard of living; 5(v achieving in life; (vi) community connectedness; (vii) future security, (viii) religious/spirituality. As such, this was included in the overall QoL subgroup analysis. A medium effect size (d = 0.738, d = 0.706) favouring the control group was recorded for PWI (well-being) and PWI (satisfaction) in Herlihy et al. (21); it was not possible to calculate effect size in Skakkebaek et al. (33). Fabrazzo et al. (30) effect sizes were recorded identifying small, medium and large effect sizes favouring the control group (d = 0.471, d = 0.686, d = 1.185) in Q-LES-Q sub items.
Table 2.
Study results from overall QOL measures, Cohen’s d and findings.
| Reference/outcome measure | Effect size ‘Cohen’s d’ | Main findings |
|---|---|---|
| Herlihy et al. (21) | All measures were significantly different between the two groups (P < 0.001). General population and KS, phenotype severity were shown to affect the results of PWI. | |
| PWI | ||
| Wellbeing | 0.738 | |
| Satisfaction | 0.706 | |
| Rapp et al. (23) | ||
| WHOQOL-100 | Results for WHOQOL-100 for ranges 0–100 and 4–20 were: | |
| Physical health | 0.588 | 66.4 ± 19.4; 14.6 ± 3.1 |
| Psychological | 0.673 | 63.3 ± 17.8; 14.1 ± 2.8. |
| Social relations | 0.659 | 59.4 ± 21.9; 13.5 ± 3.5 |
| Environment | 0.653 | 69.9 ± 14.9; 15.2 ± 2.4 |
| Skakkebaek et al. (33) | All subscales of QoL ‘WHOQOL-100&SF-36’ showed large significant differences between HC and KS (P < 0.001) with the lower scores belonging to KS. | |
| WHOQOL-100 | N/A | |
| SF-36 | – | |
| Fabrazzo et al. (30) | All sub-items showed statistical difference (P <0.05) compared to HC. Subscales ‘physical health/activities, leisure time activities, social relations, and general activities’ (P= ≤ 0.05). No significant differences in subscales ‘Work, household duties, school/class work and subjective feelings.’ | |
| Q-LES-Q sub-items | ||
| General life | 0.686 | |
| Sexual performance | 1.185 | |
| Physical health | 0.471 |
Rapp et al. found significant differences and medium effect sizes in all facets of WHOQOL-100 measured, except for environment, when comparing patients with KS to the reference population (physical health (d = 0.588, P < 0.0001), psychological (d = 0.673, P < .0001), social relations (d = 0.659, P < .0001), environment (d = 0.035, P = 0.635)) (23). Similarly, Skakkebaek et al. found significant differences (P < 0.001) in all domains of WHOQOL-100 between KS patients and healthy controls (33). Fabrazzo et al., when comparing patients with KS post 1-year TRT to healthy controls, found a significant difference of (P < 0.05) in all Q-LES-Q sub items. Q-LES-Q subscales had significant differences favouring controls in scales (physical health/activities (P = 0.038), leisure time activities (P = 0.05), social relations (P = 0.003), general activities (P = 0.045)).
Physical health
Three studies measured outcomes related to physical health against controls (Table 3). Skakkebaek et al. found that patients with KS had significantly worse physical health compared to controls (P < 0.001) for the following parameters: hypogonadism, gynecomastia, undescended testis, osteoporosis, tremor, varicose veins, pulmonary embolism or leg thrombosis, heart valve disease, dental problem, gingiva, chronic headache, fatigue and anxiety (33). On the other hand, Nielsen and Pelsen’s 20-year cohort longitudinal study found no significant differences in physical health between patients with KS and controls (24). Rapp et al. found that patients with KS had significantly lower physical health scores on the WHOQOL-100 (P < 0.001) compared to three other groups of patients with disorders of sexual development (DSD): females with congenital adrenal hyperplasia, females with XY- DSD, males with XY-DSD (23).
Table 3.
Study results from physical health measures, Cohen’s d and findings.
| Reference | Outcome measure | Effect size ‘Cohen’s d’ | Main findings |
|---|---|---|---|
| Skakkebaek et al. (33) | Testicular pain | – | KS experienced significantly more testicular pain than controls, P < 0.001. KS also experience significantly less physical activity and were heavier than controls, P < 0.001. KS had significantly more comorbidities than controls. P < 0.001 |
| Physical activity | – | ||
| Nielsen and Pelsen (24) | Physical health disorders in the last 10 years | – | There were no significant differences between the XXY and XY groups. |
| Herlihy et al. (21) | SF1- Health status (poor/fair) | – | KS = 34%, general population = 15% to answering poor/fair to health status. |
SF1, short form 1 (61).
Level of independence
There were limited measures on the level of independence in patients with KS. Work capacity was measured by Nielsen and Pelsen, but no significant differences were found regarding skilled/unskilled labour and unemployment between patients with KS and healthy controls (24).
Psychological
A significant difference (P < 0.001) was identified between controls and patients with KS in each of these outcomes: autism spectrum quotient (AQ), gender identity/dysphoria, neocriticism, extraversion, conscientiousness, attention switching, imagination, communication, global severity index (GSI), mini mental state examination (MMSE), positive symptom distress index (PSDI), social skills including social behaviour and negative assertion (6, 30, 32, 34). A significant difference (P < 0.001) between patients with KS and reference population was also reported by Herlihy et al. regarding the psychological measures of body-self relations, self-esteem, sexual identity and psychological distress (21) (Table 4).
Table 4.
Study results from measures of psychological outcomes, effect size and findings
| Reference/outcome measure | Effect size (Cohen’s d) | Main findings |
|---|---|---|
| Fisher et al. (32) | Adjusted P - values between HC and KS were: | |
| AQ | 0.822 | <0.001 |
| GIDYQ-AA | 0.872 | <0.001 |
| SCL 90- R (GSIS) | 0.69 | Positive symptom distress index: 0.03, obsession-compulsive: 0.04, somatization 0.03. |
| Herlihy et al. (21) | Significant difference for all psychosocial outcomes measured, when compared with population normative data (P < 0.001). | |
| MBSRQ-AS | 0.75 | |
| Appearance evaluation | 1.143 | |
| Appearance orientation | ||
| RSE | 2.022 | |
| K10 | – | K10 found 43% of KS had high/very high psychological distress compared to the general population 10%. |
| SIS | – | |
| Sorensen (27) | ||
| Behaviour rating scale | – | P-values between KS and controls were (P < 0.005) in subscales; intelligence, attention, level of activity. (P < 0.05); drive, liveliness. (P < 0.025) endurance and interest. |
| Skakkebaek et al. (34) | KS expressed significantly more neuroticism, less extraversion, conscientiousness, and openness to experience (P - values ≤ 0.01), controls scored higher on attention switching, imagination, communication, and social skills, while the scores of patients with KS were more evenly distributed across these scales. Differences between KS and controls for attention switching, imagination, communication, and social skills (P< 0.01). Attention-to-detail scores were comparably and normally distributed for both patients with KS and controls (P >0 .75) | |
| NEO PI-R | ||
| Neuroticism | 1.15 | |
| Extraversion | 0.73 | |
| Openness | 0.60 | |
| Agreeableness | 0.018 | |
| Conscientiousness | 0.40 | |
| AQ | ||
| Attention to detail | 0.06 | |
| Attention switching | 0.58 | |
| Imagination | 0.65 | |
| Communication | 0.42 | |
| Social skills | 0.52 | |
| Van Rijn et al. (7) | Total ADI-R score for KS participants was (24.3 ± 15.4), showing that the overall range of ASD symptoms was increased in children with KSe. | |
| ADI-R | – | |
| Van Rijn et al. (26) | AQ score and all subscales were significantly different between controls and KS. KS reported to less frequently display negative assertion, significant difference was (P = 0.01). | |
| SIB | ||
| Distress during ‘SI’ | 1.002 | |
| Frequency during ‘SI’ | 0.167 | |
| AQ | 2.111 | |
| Liberato et al. (22) | ||
| SCID-II | – | Detected personality disorders in 31% of the KS sample vs a mean of 10.7% obtained from different community samples. |
| MMPI-2 | – | Showed four altered scales, corresponding to Social Responsibility, Dominance, Ego Strength and Repression, in more than 40% of patients. Twenty-four of 34 MMPI scales were pathological in at least 10% of patients. |
| SPM | – | The mean raw score was 44 ± 10.8 (10–58), with a maximum score of 60. |
| Nielsen & Pelsen (24) | ||
| Mental illness diagnosis | There were no significant differences between controls and KS regarding mental illness. However, at the initial examination 41% of KS participants had a mental illness and which was significantly higher than controls (P < 0.0021). | |
| Fabrazzo et al. (30) | There were statistical differences favouring controls over patients with KS following 1-year TRT in measures of; obsessive-compulsive, anger-hostility, phobias, psychoticism, GSI, PSDI. While MMSE had a much larger statistical difference (P = 0.0001). Measures: interpersonal sensitivity, depression, anxiety, PST and TCI-R showed no significant differences between groups. | |
| SCL-90 subscales | ||
| Somatization | 0.197 | |
| Obsessive-compulsive | 0.870 | |
| Interpersonal sensitivity | 0.209 | |
| Psychoticism | 0.796 | |
| Anxiety | 0.028 | |
| Anger-hostility | 0.709 | |
| Phobias | 0.675 | |
| Paranoid | 0.475 | |
| SCL-90 global- indices | ||
| PST | 0.509 | |
| GSI | 0.724 | |
| PSDI | 1.0 | |
| MMSE | 1.490 | |
| TCI-R | – |
AQ, Autism Spectrum Quotient (62); ADI-R, Autism diagnostic interview – Revised (73); GIDYQ-AA, Gender Identity/Dysphoria Questionnaires for Adults and Adolescents (63); K10, Kessler Psychological Distress Scale (66), MMPI-2, Minnesota Multiphasic Personality Inventory 2 (70); MMSE, Mini-mental State Examination; MBSRQ-AS, Multidimensional body-self relations questionnaire (64); NEO PI-R, Revised NEO personality inventory (67); QRI, Qualitative reading inventory (67); RSE, Rosenberg self-esteem test (65); SIB, Scale for interpersonal behaviour (69); SIS, Sexual Identity scale (68); SPM, Standard Progressive Matrices (74); SCID-II, Structured Clinical Interview for Axis II Disorders (71); SLC-ANX/DEP, Subscales of Symptoms checklist 90 Anxiety/Depression (72); SCL-90-R, Symptom Checklist-90-R; TCI-R, Temperament and Character Inventory-Revised.
Fisher et al. and van Rijn et al. reported significantly greater prevalence of autism symptoms (P < 0.001) as measured by Autism spectrum quotient; both studies and a total of (n = 77) participants were included (26, 32). Furthermore, both studies had large effect sizes (>0.8) suggesting there was an association between KS and autism symptoms in these studies.
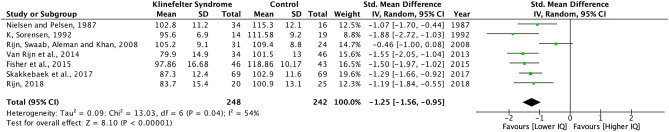
A meta-analysis was possible for Intelligence Quotient (IQ) in seven studies, six cross-sectional and one cohort longitudinal (Fig. 2). This included a total of 490 participants across all ages: 248 patients with KS and 242 controls. To measure full-scale IQ, two studies used the Wechsler Adult Intelligence Scale (WAIS) (39), two studies used Wechsler Adult Intelligence Scale – Revised (WAIS-R) (40), one study used the Wechsler Intelligence Scale for Children-III (41), and two studies reported full-scale IQ scores, participants, SD and control data however the (IQ) test used was not listed. For the meta-analysis the study CI and the overall interval was set at 95%. The meta-analysis suggests an association between lower full-scale IQ and a KS diagnosis. There was a strong significant difference between patients with KS and control suggesting a negative association between full-scale IQ for patients with KS when compared to controls. The I2 result (I2 = 54%) showed moderately high heterogeneity (42), which could be due to the varied ages of participants and differing measure of full-scale IQ.
Figure 2.
Forest plot comparing Intelligence Quotient scores from KS and Controls from studies measuring domains of quality of life. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1239.
Social relations
Eight studies included measures relating to social behaviour, sexual function, sexual satisfaction, and sexuality (6, 7, 26, 28, 31, 32, 35) within the social relations subsection of the WHOQOL-100 (Table 5). With the exception of Turiff et al. (6), all studies measuring social relations found that patients with KS have lower scores than their controls.
Table 5.
Study results from measures of social relations, effect size and findings.
| Reference/outcome measure | Effect size (Cohen’s d) | Main findings |
|---|---|---|
| Ferlin et al. (31) | There was significant difference between KS and controls in sexual desire, intercourse satisfaction, overall satisfaction (P < 0.05). Erectile dysfunction (P < 0.0005). | |
| IIEF - 15 | ||
| Erectile dysfunction | 0.385 | |
| Overall satisfaction | 0.675 | |
| Fisher et al. (32) | KS group showed higher risk of developing hypersexuality and voyeuristic fantasies. | |
| SAST | −0.561 | |
| IIEF | ||
| Overall function | 0.706 | |
| Overall satisfaction | 0.375 | |
| Van Rijn (35) | The 47, XXY group lower levels of salivary testosterone were significantly associated with higher levels of social anxiety. Salivary levels of testosterone were uncorrelated to social cognitive skills. | |
| SAS | – | |
| SCST | – | |
| KDEF | – | |
| Van Rijn et al. (7) | The effect size between healthy controls and KS participants was large in all categories measured, there were significant differences (P < 0.05) SRS, SAS. | |
| SAS | 0.793 | |
| SRS | 2.016 | |
| SSRS | −1.369 | |
| Skakkebaek et al. (33) | P value <0.001 in orgasmic function, erectile function 0.003, total sexual function 0.008. Intercourse satisfaction 0.006. Parenthood was significantly lower than controls P < 0.001. | |
| IIEF | ||
| Overall function | – | |
| Overall satisfaction | – | |
| Van Rijn et al. (26) | Overall distress during social interactions was significantly higher in the XXY group as compared to men from the general population. Mean score in the XXY group was 2.2 (s.d. 0.67) and in the control group 1.6 (s.d. 0.49), which was significantly different (F (1,52) = 13.2, P = 0.001). | |
| Social behaviour | – | |
| Overall social distress | 1.002 |
Four studies (21, 26, 32, 34) compared patients with KS against controls which allowed effect size to be calculated (Table 5).
Two studies found that patients with KS had an increased risk in developing negative social traits of anxiety, social responsiveness, and social awareness (28, 29). Tartaglia et al. found that more than 25% (n = 42) of patients with KS scored mild, moderate or severe on all domains of the Social Responsiveness Scale (SRS) except for the social awareness domain (28). Furthermore, Van Rijn et al. identified a strong effect size (d = 2.016) when measuring social responsiveness using SRS in patients with KS when compared to controls (7).
Environment
There were limited measures of ‘environment’, WHOQOL-100 lists the subgroups of ‘environment’ as; financial resources, freedom, physical safety and security, health, and social care: accessibility and quality, home environment, opportunities for acquiring new information and skills, participation in and opportunities for recreation/leisure, physical environment, and transport. Only four studies measured education (21, 24, 25, 33) and two measured financial resources (21, 33).
Acquiring new information and skills can be linked to school attainment and completing education. Herlilhy et al. reported that 34% of patients with KS (n = 87) did not complete high school, 55% completed high school and 10% studied further than high school (21). Results showed significant differences in five categories: lack of interest in schoolwork (P < 0.05), concentration difficulties (P < 0.005), speech difficulties (P < 0.05), lack of self-confidence (P < 0.05), particularly dependent on parents (P < 0.025) (21).
Skakkebaek et al. found that patients with KS (n = 132) were significantly less likely than controls to complete high school (P < 0.001) and at least 1 year of higher education (P < 0.01) (33). School performance was also significantly worse (P < 0.001) for patients with KS when compared to healthy controls (24). Turriff et al. looked at the highest education level obtained by patients with KS (n = 310) and found that 13.6% completed post-graduate education, 23.9% college, 22.6% part of college education, 13.6% technical school, 22.2% high school and 4.1% completed elementary or junior school (6). Herlihy et al. found that 36% of patients with KS (n =87) earned less than AUS$30,000, 27% earned between AUS$30,000 and 69.999 and 30% earned more than AUS$70,000 (21). Similarly, Skakkebæk et al. found that patients with KS (n = 126) had significantly lower (P < 0.001) household income compared to healthy controls (33).
Discussion
This is the first systematic review to provide an in-depth analysis of the associations between KS and QoL. The WHOQOL-100 provided a framework which allowed sufficient synthesis for many parameters and domains of QoL, which highlighted a disparity in the QoL between patients with KS and controls. Furthermore, the meta-analysis from the included studies indicates a lower full-scale IQ is associated with KS diagnosis.
Almost all patients (95.9% of n = 829) across 12 studies included in this systematic review reported that KS had negatively affected the QoL outcome measures. When calculated between patients with KS and controls, a significant effect size (Cohen’s d) was present in most outcomes measured (91.8% of 49). The effect size within this review further quantifies the difference between KS and controls in outcome measures associated with QoL providing hence evidence of the negative impact of the KS diagnosis on patients’ QoL.
Validated measures of QoL, such as PWI (36), WHOQOL-100 (17), Q-LES-Q (38) and SF-36 (37), showed poorer QoL scores for patients with KS compared to controls. This is consistent with previous research which supports overall impaired QoL in patients with KS (13, 43).
Psychological outcomes were the most measured subgroup of QoL and 37 of the 45 outcome measures showed a statistical significance in scores indicating that KS diagnosis is increasing the risk for patients to develop a psychological disorder including cognitive impairment. The results of this systematic review support previous research which found that patients with KS had an increase in psychiatric comorbidities including autism, attention deficit hyperactivity disorder (ADHD), psychosis, personality disorders and developmental disorders (44, 45, 46, 47).
Furthermore, our meta-analysis showed that men with KS have a significantly lower IQ than healthy controls (24, 26, 27, 32, 34, 35) (Fig. 2). Kennedy et al. noted the importance of IQ as a predictor of future success (48). Previous research also shows that lower IQ is associated with negative outcomes such as increased prosocial skill deficits, criminal behaviour, post-traumatic stress disorder and lower academic achievements (48, 49, 50, 51, 52, 53). As these outcomes form subgroups of the QoL construct, it is essential to understand the effect that the diagnosis of KS has on the patient’s IQ, in order to provide the necessary care and support at an early-stage post-diagnosis. Further research is necessary to investigate the effect that lower IQ may have on the QoL outcomes for patients with KS.
This systematic review suggests that men with KS are at higher risk than healthy controls to develop psychiatric disorders associated with autism spectrum symptoms, but these are often not recognised or managed appropriately (7, 26, 28, 30, 32, 34). Previous research found that people with autism have more impaired QoL outcomes compared to healthy controls (54), while two studies suggested improved health-related QoL outcomes in people with less severe autism symptoms (55, 56). Like KS, autism has a broad phenotype with a variety of symptoms ranging from disruptive language to socio-emotional traits. However, unlike KS, the awareness and research conducted on autism are far greater which has led to earlier diagnosis and relevant support for patients diagnosed with autism, with improved QoL outcomes and wider social understanding of autism. Evidence supports the presence of autism symptoms especially social behaviours in patients with KS, yet many patients do not receive appropriate investigations nor are diagnosed with autism spectrum disorders which can have a detrimental impact on their QoL outcomes (26, 32, 34).
School attainment and behaviour were measured by five studies which found that boys with KS had significantly lower (P < 0.05) achievement and worse behaviours than healthy controls at all levels of education (6, 25, 27, 33, 57). Recent research in education and psychology shows that behaviours and attitudes in school have a direct correlation with work status, income earnings and social status later in later life (58). Although there is limited evidence to support that boys with KS have poor school attainment and behaviours, the consequences of this may have severe lifelong implications. Therefore, further research is needed to investigate this area to develop relevant supportive mechanisms at school for young boys with KS.
This systematic review found that the diagnosis of KS has a significant negative impact on the patients’ erectile function and sexual satisfaction, which is most likely secondary to testosterone deficiency and psychological disorders associated with KS (31). Further research is required to address this problem. Similarly, our review suggests that patients with KS have more increased social anxiety and impaired social skills compared to controls (7, 26, 28, 35). This is supported by two earlier studies which provide evidence of the negative effect that low testosterone has on social anxiety (59, 60).
In conclusion, our systematic review with narrative synthesis and meta-analysis, guided by the WHOQOL-100 as an overarching framework, provides evidence that patients with KS have impaired QoL compared to healthy males. Although evidence for overall QoL outcomes was limited, subgroup analysis helped to provide a greater understanding of the WHOQOL-100 subgroups, and the extent to which each of these are affected by patients with KS. Further research is needed to understand the impact the diagnosis of KS has on patients’ QoL. A significant finding from this systematic review was the lack of a condition-specific PROM for patients with KS. Development and validation of a KS-specific PROM that would encompass all domains of QoL for this patient group and would provide a quantifiable and validated measure for QoL is therefore essential.
Supplementary Material
Declaration of interest
Sofia Llahana: Occasional scientific consultant and conference fees for Bayer, Novartis, Pfizer and Ipsen. Unrestricted educational grants from Sandoz and Bayer. Senior Editor for Endocrinology, Diabetes and Metabolism Case Reports. Channa Jayasenna: Investigator- led grant by Logixx Pharma Ltd. The other authors have nothing to disclose.
Funding
No funding has been received for this systematic review. S L is funded by an NIHR Clinical Lectureship. C N J is funded by the Imperial NIHR BRC & NIHR Post-Doctoral Fellowship.
Author contribution statement
B M and S L conceived the presented idea. B M developed the conceptual framework, theory, methods and searching under supervision of S L, B M and S L completed the blind review of studies. B M completed the collection of data and analysis from the included studies. All authors B M, S L, S G, and C J contributed to the discussion of results and provided critical feedback to all aspects of the manuscript which helped shape the final manuscript. B M, C J and S L completed the abstract. B M and S L took the lead on writing the main body of text.
References
- 1.Klinefelter Jr HF, Reifenstein Jr EC, Albright Jr F. Syndrome characterized by gynecomastia, aspermatogenesis without A-Leydigism, and increased excretion of follicle-stimulating hormone. Journal of Clinical Endocrinology and Metabolism 19422615–627. ( 10.1210/jcem-2-11-615) [DOI] [Google Scholar]
- 2.Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebæk A. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocrine Reviews 201839389–423. ( 10.1210/er.2017-00212) [DOI] [PubMed] [Google Scholar]
- 3.Dwyer AA, Quinton R. Classification of hypothalamic-pituitary-gonadal (HPG) axis endocrine disorders. In Advanced Practice in Endocrinology Nursing, pp. 853–870. Eds Llahana S, Follin C, Yedinak C, Grossman A. Cham: Springer International Publishing, 2019. [Google Scholar]
- 4.Herlihy AS, Halliday JL, Cock ML, McLachlan RI. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Medical Journal of Australia 201119424–28. ( 10.5694/j.1326-5377.2011.tb04141.x) [DOI] [PubMed] [Google Scholar]
- 5.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. Journal of Clinical Endocrinology and Metabolism 200388622–626. ( 10.1210/jc.2002-021491) [DOI] [PubMed] [Google Scholar]
- 6.Turriff A, Levy HP, Biesecker B. Prevalence and psychosocial correlates of depressive symptoms among adolescents and adults with Klinefelter syndrome. Genetics in Medicine 201113966–972. ( 10.1097/GIM.0b013e3182227576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rijn S, Stockmann L, Borghgraef M, Bruining H, van Ravenswaaij-Arts C, Govaerts L, Hansson K, Swaab H. The social behavioral phenotype in boys and girls with an extra X chromosome (Klinefelter syndrome and trisomy X): a comparison with autism spectrum disorder. Journal of Autism and Developmental Disorders 201444310–320. ( 10.1007/s10803-013-1860-5) [DOI] [PubMed] [Google Scholar]
- 8.van Rijn S, Swaab H. Vulnerability for psychopathology in Klinefelter syndrome: age-specific and cognitive-specific risk profiles. Acta Paediatrica 2011100908–916. ( 10.1111/j.1651-2227.2011.02289.x) [DOI] [PubMed] [Google Scholar]
- 9.Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A. & Klinefelter ItaliaN Group (KING). Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. Journal of Endocrinological Investigation 201740123–134. ( 10.1007/s40618-016-0541-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llahana S.Testosterone therapy in adult men with hypogonadism. In Advanced Practice in Endocrinology Nursing, pp. 885–902. Eds Llahana S, Follin C, Yedinak C, Grossman A. Cham: Springer International Publishing, 2019. [Google Scholar]
- 11.Jayasena C, Anderson RA, Llahana S, Barth J, MacKenzie F, Wilkes S, Smith N, Sooriakumaran P, Minhas S, Wu FCWet al. Society for Endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clinical Endocrinology 202196200–219. ( 10.1111/cen.14633) [DOI] [PubMed] [Google Scholar]
- 12.Close S, Fennoy I, Smaldone A, Reame N. Phenotype and adverse quality of life in boys with Klinefelter syndrome. Journal of Pediatrics 2015167650–657. ( 10.1016/j.jpeds.2015.06.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Ronde W, de Haan A, Drent ML. Quality of life is reduced in patients with Klinefelter syndrome on androgen replacement therapy. European Journal of Endocrinology 2009160465–468. ( 10.1530/EJE-08-0689) [DOI] [PubMed] [Google Scholar]
- 14.Whoqol G.Development of the World Health Organization WHOQOL-bref quality of life assessment. The WHOQOL Group. Psychological Medicine 199828551–558. ( 10.1017/s0033291798006667) [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 200962e1–e34. ( 10.1016/j.jclinepi.2009.06.006) [DOI] [PubMed] [Google Scholar]
- 16.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas Jet al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020368 l6890. ( 10.1136/bmj.l6890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group W.Development of the WHOQOL: rationale and current status. International Journal of Mental Health 19942324–56. ( 10.1080/00207411.1994.11449286) [DOI] [Google Scholar]
- 18.The Cochrane C ollaboration. Review Manager (RevMan), 2020. [Google Scholar]
- 19.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis Pet al. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual, pp. 2019, -05. Eds Aromataris E, Munn Z. Joanna Briggs Institute, 2017. [Google Scholar]
- 20.Cohen J.Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum; As; sociates, 1988. [Google Scholar]
- 21.Herlihy AS, McLachlan RI, Gillam L, Cock ML, Collins V, Halliday JL. The psychosocial impact of Klinefelter syndrome and factors influencing quality of life. Genetics in Medicine 201113632–642. ( 10.1097/GIM.0b013e3182136d19) [DOI] [PubMed] [Google Scholar]
- 22.Liberato D, Granato S, Grimaldi D, Rossi FM, Tahani N, Gianfrilli D, Anzuini A, Lenzi A, Cavaggioni G, Radicioni AF. Fluid intelligence, traits of personality and personality disorders in a cohort of adult KS patients with the classic 47, XXY karyotype. Journal of Endocrinological Investigation 2017401191–1199. ( 10.1007/s40618-017-0674-2) [DOI] [PubMed] [Google Scholar]
- 23.Rapp M, Mueller-Godeffroy E, Lee P, Roehle R, Kreukels BPC, Kohler B, Nordenström A, Bouvattier C. & Ute Thyen on behalf of the dsd-LIFE group. Multicentre cross-sectional clinical evaluation study about quality of life in adults with disorders/differences of sex development (DSD) compared to country specific reference populations (dsd-LIFE). Health and Quality of Life Outcomes 201816 54. ( 10.1186/s12955-018-0881-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen J, Pelsen B. Follow-up 20 years later of 34 Klinefelter males with karyotype 47,XXY and 16 hypogonadal males with karyotype 46,XY. Human Genetics 198777188–192. ( 10.1007/BF00272390) [DOI] [PubMed] [Google Scholar]
- 25.Close SM.An Exploratory Study of Physical Phenotype, Biomarkers and Psychosocial Health Parameters in Boys with Klinefelter Syndrome, ProQuest Dissertations Publishing, 2011. [Google Scholar]
- 26.van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. Journal of Autism and Developmental Disorders 2008381634–1641. ( 10.1007/s10803-008-0542-1) [DOI] [PubMed] [Google Scholar]
- 27.Sørensen K.Physical and mental development of adolescent males with Klinefelter syndrome. Hormone Research 199237 (Supplement 3) 55–61. ( 10.1159/000182402) [DOI] [PubMed] [Google Scholar]
- 28.Tartaglia N, Cordeiro L, Howell S, Wilson R, Janusz J. The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome). Pediatric Endocrinology Reviews 20108 (Supplement 1) 151–159. [PMC free article] [PubMed] [Google Scholar]
- 29.van Rijn S, de Sonneville L, Swaab H. The nature of social cognitive deficits in children and adults with Klinefelter syndrome (47,XXY). Genes, Brain, and Behavior 201817e12465. ( 10.1111/gbb.12465) [DOI] [PubMed] [Google Scholar]
- 30.Fabrazzo M, Accardo G, Abbondandolo I, Goglia G, Esposito D, Sampogna G, Catapano F, Giugliano D, Pasquali D. Quality of life in Klinefelter patients on testosterone replacement therapy compared to healthy controls: an observational study on the impact of psychological distress, personality traits, and coping strategies. Journal of Endocrinological Investigation 2021441053–1063. ( 10.1007/s40618-020-01400-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferlin A, Selice R, Angelini S, Di Grazia M, Caretta N, Cavalieri F, Di Mambro A, Foresta C. Endocrine and psychological aspects of sexual dysfunction in Klinefelter patients. Andrology 20186414–419. ( 10.1111/andr.12474) [DOI] [PubMed] [Google Scholar]
- 32.Fisher AD, Castellini G, Casale H, Fanni E, Bandini E, Campone B, Ferruccio N, Maseroli E, Boddi V, Dèttore Det al. Hypersexuality, paraphilic behaviors, and gender dysphoria in individuals with Klinefelter’s syndrome. Journal of Sexual Medicine 2015122413–2424. ( 10.1111/jsm.13048) [DOI] [PubMed] [Google Scholar]
- 33.Skakkebæk A, Moore PJ, Chang S, Fedder J, Gravholt CH. Quality of life in men with Klinefelter syndrome: the impact of genotype, health, socioeconomics, and sexual function. Genetics in Medicine 201820214–222. ( 10.1038/gim.2017.110) [DOI] [PubMed] [Google Scholar]
- 34.Skakkebæk A, Moore PJ, Pedersen AD, Bojesen A, Kristensen MK, Fedder J, Laurberg P, Hertz JM, Østergaard JR, Wallentin Met al. The role of genes, intelligence, personality, and social engagement in cognitive performance in Klinefelter syndrome. Brain and Behavior 20177 e00645. ( 10.1002/brb3.645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rijn S.Salivary testosterone in relation to social cognition and social anxiety in children and adolescents with 47,XXY (Klinefelter syndrome). PLoS ONE 201813 e0200882. ( 10.1371/journal.pone.0200882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Wellbeing Group. Personal Wellbeing Index – Adult, Manuel, 4th ed. Deakin University, 2006. (available at: http://www.deakin.edu.au/research/acqol/instruments/wellbeing_index.htm) [Google Scholar]
- 37.Bjørner JB, Damsgaard MT, Watt T, Bech P, Rasmussen N, Kristensen T. Danish SF-36 Manual – A Health Status Questionnaire. Dansk Manual Til SF-36-Et Spørgeskema Om helbredsstatusKøbenhavn. Denmark: LIF; Lægemiddelindustriforeningen, 2003. [Google Scholar]
- 38.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacology Bulletin 199329321–326. [PubMed] [Google Scholar]
- 39.WAIS. In Encyclopedia of Clinical Neuropsychology, p. 2667. Eds Kreutzer JS, DeLuca J, Caplan B. New York, NY: Springer, 2011. [Google Scholar]
- 40.Wechsler D.WAIS-R: Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological; Corporation, 1981. [Google Scholar]
- 41.Wechsler D.Wechsler Intelligence Scale for Children. San Antonio, TX: Psychological Corporation, 3, 1992. [Google Scholar]
- 42.Deeks JJ, Higgins JPT, Altman DG. Analysing Data and Undertaking Meta-analyses, pp. 241–284. Chichester, UK: Wiley, 2019. [Google Scholar]
- 43.Franik S, Fleischer K, Kortmann B, D’Hauwers K, IntHout J, Bouvattier C, Slowikowska-Hilczer J, Grunenwald S, van de Grift T, Cartault Aet al. The impact of Klinefelter syndrome on quality of life – a multicentre study. Fertility and Sterility 2019112 e64. ( 10.1016/j.fertnstert.2019.07.290) [DOI] [Google Scholar]
- 44.Cederlöf M, Gotby AO, Larsson H, Serlachius E, Boman M, Långström N, Landén M, Lichtenstein P. Klinefelter syndrome and risk of psychosis, autism and ADHD. Journal of Psychiatric Research 201448128–130. ( 10.1016/j.jpsychires.2013.10.001) [DOI] [PubMed] [Google Scholar]
- 45.Giagulli VA, Campone B, Castellana M, Salzano C, Fisher AD, de Angelis C, Pivonello R, Colao A, Pasquali D, Maggi Met al. Neuropsychiatric aspects in men with Klinefelter syndrome. Endocrine, Metabolic and Immune Disorders-Drug Targets 201919109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tartaglia NR, Wilson R, Miller JS, Rafalko J, Cordeiro L, Davis S, Hessl D, Ross J. Autism spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. Journal of Developmental and Behavioral Pediatrics 201738197–207. ( 10.1097/DBP.0000000000000429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson AC, King J, Bishop DVM. Autism and social anxiety in children with sex chromosome trisomies: an observational study. Wellcome Open Research 20194 32. ( 10.12688/wellcomeopenres.15095.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy TD, Flach Y, Detullio D, Millen DH, Englebert N, Edmonds WA. Exploring emotional intelligence and IQ as predictors of success of foster care alumni. Journal of Child and Family Studies 2019283286–3295. ( 10.1007/s10826-019-01503-8) [DOI] [Google Scholar]
- 49.Furnham A, Christoforou I. Personality traits, emotional intelligence, and multiple happiness. North American Journal of Psychology 20079 439. [Google Scholar]
- 50.Gallagher EN, Vella-Brodrick DA. Social support and emotional intelligence as predictors of subjective well-being. Personality and Individual Differences 2008441551–1561. ( 10.1016/j.paid.2008.01.011) [DOI] [Google Scholar]
- 51.Koolhof R, Loeber R, Wei EH, Pardini D, D’Escury AC. Inhibition deficits of serious delinquent boys of low intelligence. Criminal Behaviour and Mental Health 200717274–292. ( 10.1002/cbm.661) [DOI] [PubMed] [Google Scholar]
- 52.Lynam D, Moffitt TE, Stouthamer-Loeber M. ‘Explaining the relation between IQ and delinquency: class, race, test motivation, school failure, or self-control?’ Correction. Journal of Abnormal Psychology 1965102552–552. ( 10.1037/0021-843X.102.4.552) [DOI] [PubMed] [Google Scholar]
- 53.Petrides KV, Frederickson N, Furnham A. The role of trait emotional intelligence in academic performance and deviant behavior at school. Personality and Individual Differences 200436277–293. ( 10.1016/S0191-8869(0300084-9) [DOI] [Google Scholar]
- 54.van Heijst BFC, Geurts HM. Quality of life in autism across the lifespan: a meta-analysis. Autism 201519158–167. ( 10.1177/1362361313517053) [DOI] [PubMed] [Google Scholar]
- 55.Eaves LC, Ho HH. Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders 200838739–747. ( 10.1007/s10803-007-0441-x) [DOI] [PubMed] [Google Scholar]
- 56.Kuhlthau K, Orlich F, Hall TA, Sikora D, Kovacs EA, Delahaye J, Clemons TE. Health-related quality of life in children with autism spectrum disorders: results from the autism treatment network. Journal of Autism and Developmental Disorders 201040721–729. ( 10.1007/s10803-009-0921-2) [DOI] [PubMed] [Google Scholar]
- 57.Hanna ES, Cheetham T, Fearon K, Herbrand C, Hudson N, McEleny K, Quinton R, Stevenson E, Wilkes S. The lived experience of Klinefelter syndrome: a narrative review of the literature. Frontiers in Endocrinology 201910 825. ( 10.3389/fendo.2019.00825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spengler M, Damian RI, Roberts BW. How you behave in school predicts life success above and beyond family background, broad traits, and cognitive ability. Journal of Personality and Social Psychology 2018114 620–636. ( 10.1037/pspp0000185) [DOI] [PubMed] [Google Scholar]
- 59.Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Development and Psychopathology 200315431–449. ( 10.1017/S0954579403000233) [DOI] [PubMed] [Google Scholar]
- 60.McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Frontiers in Neuroendocrinology 20143542–57. ( 10.1016/j.yfrne.2013.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological Medicine 200232959–976. ( 10.1017/s0033291702006074) [DOI] [PubMed] [Google Scholar]
- 62.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental Disorders 2001315–17. ( 10.1023/a:1005653411471) [DOI] [PubMed] [Google Scholar]
- 63.Deogracias JJ, Johnson LL, Meyer-Bahlburg HFL, Kessler SJ, Schober JM, Zucker KJ. The gender identity/gender dysphoria questionnaire for adolescents and adults. Journal of Sex Research 200744370–379. ( 10.1080/00224490701586730) [DOI] [PubMed] [Google Scholar]
- 64.Cash TF.The Multidimensional Body-Self Relations Questionnaire. Unpublished Test Manual. Norfolk, VA: Old Dominion University, 1990. [Google Scholar]
- 65.Rosenberg M.Society and the Adolescent Self-Image. Princeton University Press, 2015. [Google Scholar]
- 66.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Howes MJ, Normand SL, Manderscheid RW, Walters EEet al. Screening for serious mental illness in the general population. Archives of General Psychiatry 200360184–189. ( 10.1001/archpsyc.60.2.184) [DOI] [PubMed] [Google Scholar]
- 67.Caldwell J, Leslie L. Qualitative Reading Inventory. 5th edition. Boston, MA: Pearson/Allyn & Bacon, 2011. [Google Scholar]
- 68.Stern BB, Barak B, Gould SJ. Sexual identity scale: a new self-assessment measure. Sex Roles 198717503–519. ( 10.1007/BF00287732) [DOI] [Google Scholar]
- 69.Arrindell WA, Van der Ende J. Cross-sample invariance of the structure of self-reported distress and difficulty in assertiveness. Advances in Behaviour Research and Therapy 19857205–243. ( 10.1016/0146-6402(8590013-X) [DOI] [Google Scholar]
- 70.Framingham J.Minnesota Multiphasic Personality Inventory (MMPI). Psych Central 201828 2019. [Google Scholar]
- 71.First MB, Gibbon M, Spitzer RL, Benjamin LS, Williams JBW. Structured Clinical Interview for DSM-IV® Axis ii Personality Disorders SCID-II. American Psychiatric Publishing, 1997. [Google Scholar]
- 72.Derogatis LR, Unger R. Symptom Checklist-90-Revised, pp. 1–2. Corsini Encyclopedia of; Psychology, 2010. [Google Scholar]
- 73.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised, vol 29, p. 30. Los Angeles,CA: Western Psychological Services, 2003. [Google Scholar]
- 74.Raven JC.SPM Standard Progressive Matrices Standardizzazione Italiana. Firenze: Giunti OS Organizzazioni; Speciali, 2008. [Google Scholar]
- 75.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 199749822–830. ( 10.1016/s0090-4295(9700238-0) [DOI] [PubMed] [Google Scholar]
- 76.Sinoff G, Ore L, Zlotogorsky D, Tamir A. Short anxiety screening test – a brief instrument for detecting anxiety in the elderly. International Journal of Geriatric Psychiatry 1999141062–1071. () [DOI] [PubMed] [Google Scholar]
- 77.Van Manen TG, Prins PJM, Emmelkamp PMG. Manual for the Social Cognitive Skills Test. Houten, The Netherlands: Bohn; Stafleu van Loghum, 2009. [Google Scholar]
- 78.Dekking YM.Handleiding Sociale Angst Schaal Voor Kinderen. Manual Social Anxiety Scale for Children. Lisse, The Netherlands: Swets & Zeitlinger Publishers, 1983. [Google Scholar]
- 79.Gresham FM, Elliott SN. Social Skills Rating System: Manual. American Guidance Service, 1990. [Google Scholar]
- 80.Constantino JN, Gruber CP. Social Responsiveness Scale: SRS-2. CA: Western Psychological Services, 2012. [Google Scholar]
- 81.Nelson HE.National Adult Reading Test (NART): For the Assessment of Premorbid Intelligence in Patients with Dementia: Test Manual. Nfer-Nelson, 1982. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a