Abstract
Background
Epidermal function is associated with diabetes and renal disease. Whether obesity can reflect the changes in epidermal function is not clear yet.
Objective
We assessed here the correlation of epidermal functions with body mass index (BMI) in a large Chinese cohort.
Methods and Subjects
A total of 1,405 Chinese aged 21–98 years old were enrolled in this study. Epidermal functions, including transepidermal water loss (TEWL), stratum corneum hydration, and skin surface pH, were measured on the flexor forearm and the shin. Subjects' height and body weight were also measured.
Results
Age positively correlated with both TEWL and skin surface pH, while it negatively correlated with stratum corneum hydration on both the forearm and the shin of females. Similarly, age positively correlated with skin surface pH, while negatively correlating with stratum corneum hydration on both the forearm and the shin of males. In females, BMI positively correlated with skin surface pH, while it negatively correlated with stratum corneum hydration on both the forearm and the shin. However, BMI correlated neither with skin surface pH on both the forearm and the shin nor with stratum corneum hydration on the shin of males.
Conclusion
These results demonstrate that correlations of BMI with age and epidermal functions vary with gender.
Keywords: Body mass index, pH, Hydration, Barrier, Transepidermal water loss, Gender
Introduction
A number of endogenous and external factors regulate the epidermal functions, including epidermal permeability barrier function, stratum corneum hydration, and skin surface pH. Alteration in either cutaneous or extracutaneous condition can reflect the changes in epidermal functions. Previous studies have demonstrated elevations in skin surface pH and transepidermal epidermal water loss (TEWL), and a reduction in stratum corneum hydration in inflammatory dermatoses such as eczematous dermatitis, contact dermatitis, and psoriasis [1, 2, 3, 4, 5]. Similarly, individuals with rosacea also display higher TEWL rates and lower stratum corneum hydration than normal controls [6, 7, 8]. Moreover, skin pigmentation also influences epidermal function. For example, stratum corneum hydration levels were lower in the vitiligo-involved sites than in the uninvolved sites [9]. Although baseline TEWL rates were comparable between vitiligo-involved and uninvolved sites, barrier recovery was markedly delayed in the vitiligo-involved sites [9]. In contrast, highly pigmented skin displays lower skin surface pH, enhanced stratum corneum integrity, and accelerated permeability barrier recovery in comparison to lightly pigmented skin in both murine and humans [10, 11]. This line of evidence indicates that cutaneous conditions can affect epidermal functions, reflecting in the changes of epidermal biophysical properties.
In addition to cutaneous condition, systemic condition can also regulate epidermal functions. In both humans and mice, psychological stress delays epidermal permeability barrier recovery and decreases stratum corneum integrity, in part, attributable to the reductions in epidermal lipid synthesis, lamellar body formation, and keratinocyte differentiation [12, 13, 14]. Likewise, patients with either type 1 or type 2 diabetes exhibit reduced stratum corneum hydration levels and delayed permeability barrier recovery in both murine models and humans [15, 16, 17, 18]. Studies have also demonstrated that chronologically aged skin exhibits multiple alterations in epidermal functions, including elevated skin surface pH, reduced stratum corneum hydration, and delayed permeability barrier recovery [19]. Additionally, studies showed that obesity, a metabolic disorder, displays altered epidermal permeability barrier function and stratum corneum hydration although the results are controversial, possibly due to the inconsistence of methodology and small sample size [20, 21, 22, 23]. Nonetheless, these results demonstrate a link between epidermal functions and systemic condition.
In the present study, we assessed the correlations of body mass index (BMI) with epidermal permeability barrier function, stratum corneum hydration, and skin surface pH in a large Chinese cohort. Moreover, aging-associated changes in epidermal function and BMI were also assessed.
Participants and Methods
A total of 1,405 subjects, including 601 males and 804 females aged 21–98 years (44.97 ± 0.53; 95% CI of median, 38–42), were enrolled in this study (detailed in Table 1). All participants had no skin disorders that are known to influence TEWL, skin surface pH, or stratum corneum hydration. Except regular shower and wash, no moisturizer lotion or cream was applied to measurement site 12 h prior to measurements taken. Epidermal biophysical properties, including TEWL rates and stratum corneum hydration, were measured with GPskin Barrier® (GPower Inc., Seoul, South Korea) [24], while skin surface pH was measured with a portable skin pH meter (Nate Instrument, Suzhou, China) on the flexor of the left forearm 10 cm above the wrist and the right shin 10 cm below the knee. BMI was calculated using the formula: BMI = body weight in kilograms ÷ square of height in meters. This study was approved by the institutional review board of the Dermatology Hospital of Southern Medical University (#2021025) and performed in accordance with the Declaration of Helsinki. Verbal informed consents were obtained from all subjects prior to the study. This study was carried out in Guangdong, China, from October to December.
Table 1.
Demographic characteristics of subjects
| Age group, years | Gender | N (%) | Age ($$ ± SEM) | BMI ($$ ± SEM, IQR) | |
|---|---|---|---|---|---|
| 21–30 (N = 500) | Males | 183 (36.60) | 25.10±0.24 | 23.29±0.43c, 5.29 | |
| Females | 317 (63.40) | 25.32±0.15 | 21.15±0.21, 3.93 | ||
|
| |||||
| 31–40 (N = 207) | Males | 70 (33.82) | 35.20±0.33 | 23.86±0.53a, 5.07 | |
| Females | 137 (66.18) | 34.96±0.27 | 22.4±0.30, 4.05 | ||
|
| |||||
| 41–50 (N = 175) | Males | 70 (40) | 45.86±0.32 | 24.78±0.43, 4.22 | |
| Females | 105 (60) | 45.34±0.30 | 23.87±0.29, 4.02 | ||
|
| |||||
| 51–60 (N = 132) | Males | 57 (43.18) | 55.09±0.40 | 24.14±0.50, 3.58 | |
| Females | 75 (56.82) | 55.59±0.32 | 24.51±0.44, 4.56 | ||
|
| |||||
| 61–70 (N = 207) | Males | 106 (51.21) | 65.83±0.53 | 24.11±0.44, 4.34 | |
| Females | 101 (48.79) | 65.39±0.30 | 24.20±0.45, 5.62 | ||
|
| |||||
| 71–80 (N = 117) | Males | 75 (64.10) | 74.47±0.31 | 24.10±0.54, 3.87 | |
| Females | 42 (35.90) | 74.79±0.40 | 24.73±0.66, 5.18 | ||
|
| |||||
| >80 (N = 67) | Males | 40 (59.70) | 85.55±0.64 | 23.17±0.48b, 4.98 | |
| Females | 27 (40.30) | 85.33±0.83 | 21.49±0.70, 3.11 | ||
|
| |||||
| Total (N = 1,405) | Males | 601 (42.78) | 48.91±0.85c | 23.85±0.19c, 4.74 | |
| Females | 804 (57.22) | 42.03±0.64 | 22.61±0.14, 5.00 | ||
IQR, interquartile range. Data are expressed as mean ± SEM.
p < 0.05.
p < 0.01.
p < 0.0001 versus females.
Statistical Analysis
GraphPad Prism 5 software was used for all statistical analyses. A two-sided unpaired test with post Mann-Whitney test was used to determine significance between two groups. Pearson r analysis was used to determine the significance of correlation. Data are expressed as mean ± SEM.
Results
Aging-Associated Changes in Epidermal Functions
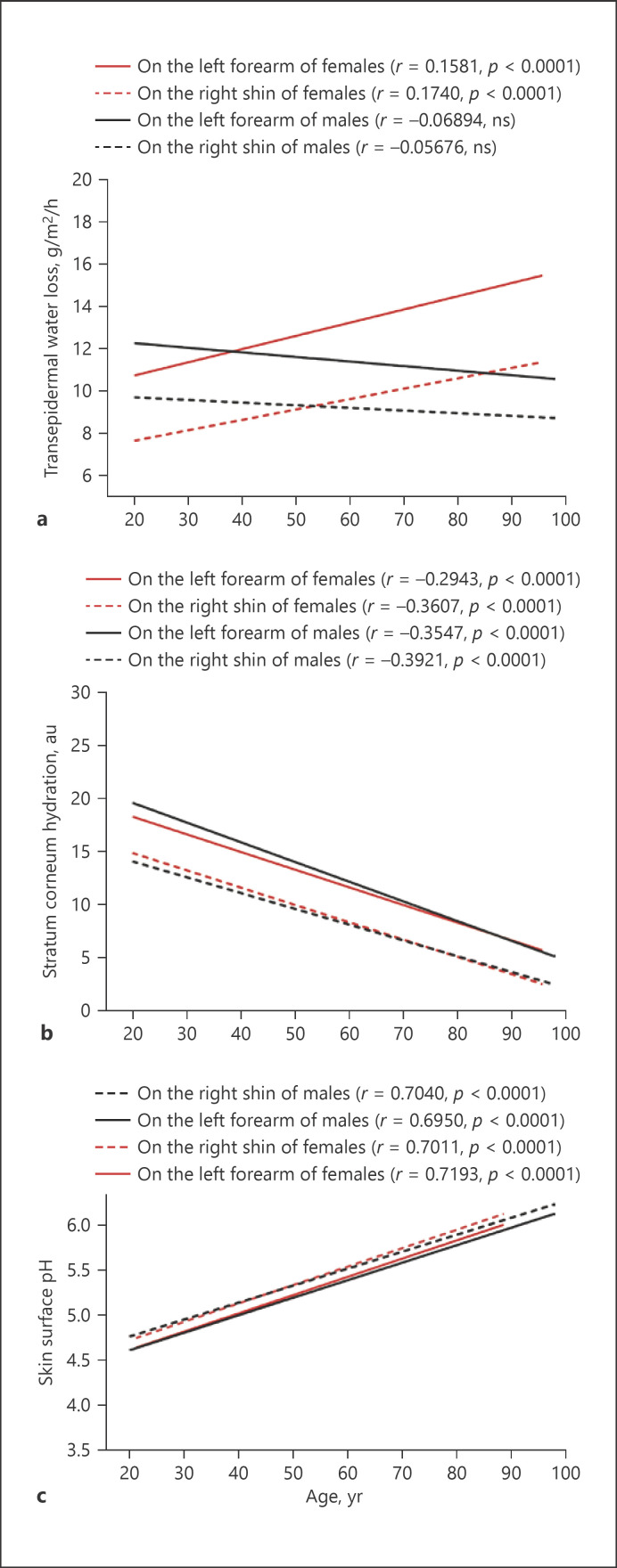
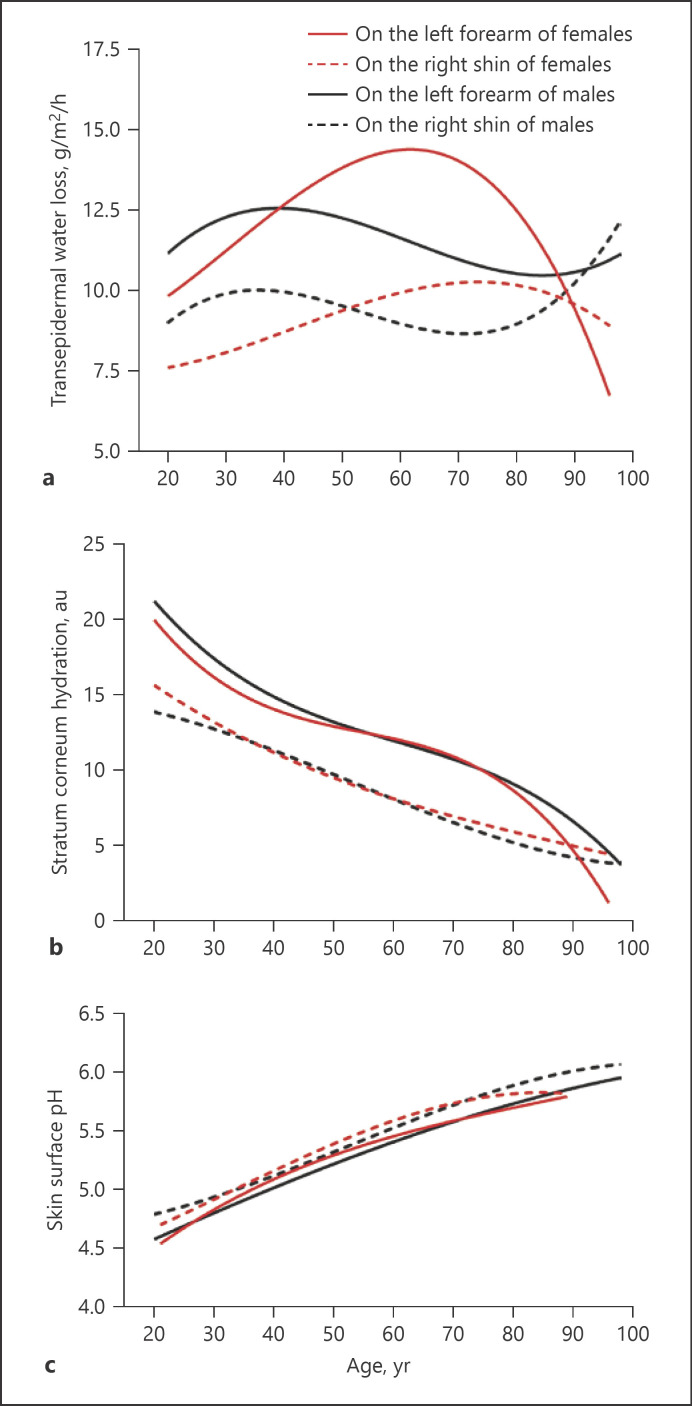
Although previous studies showed some alterations in epidermal functions in the chronologically aged skin [19], aging-associated changes in baseline epidermal permeability barrier function are still inconclusive. Therefore, we first assessed correlations of age with baseline epidermal functions. Surprisingly, a significant correlation of baseline TEWL on both the forearm and the shin with age was observed only in females, but not in males (Fig. 1a). Notably, TEWL rates on the right shin were significantly higher in males than in females aged 21–50 years old (Table 2. p < 0.01 to p < 0.0001), consistent with previous studies [25]. However, stratum corneum hydration levels correlated negatively with age in both females and males (Fig. 1b). In contrast, skin surface pH correlated positively with age in both females and males (Fig. 1c). We analyzed next the trend of changes in epidermal functions over the lifetime starting from 21 years old. Interestingly, aging-associated changes in TEWL displayed a parabolic pattern only in the females, but not in the males (Fig. 2a). TEWL steadily increased from age 20–60 years old and then sharply decreased on the forearm of females, not males. On the contrary, stratum corneum hydration and skin surface pH changed almost linearly with age (Fig. 2b, c). These results demonstrate that stratum corneum hydration negatively and skin surface pH positively correlate with age. Aging-associated changes in TEWL vary with gender.
Fig. 1.
Aging-associated changes in epidermal functions. a TEWL, N = 804 for females and N = 601 for males. b Stratum corneum hydration, N = 804 for females and N = 601 for males. c Skin surface pH, N = 521–523 for females and N = 341 for males. Pearson r analysis is used to determine the significance of correlation of age with epidermal functions. p values are indicated in the Figures.
Table 2.
Epidermal function
| Age group, years | Gender | TEWL on the left forearm | Hydration on the left forearm | pH on the left forearm | TEWL on the right shin | Hydration on the right shin | pH on the right shin |
|---|---|---|---|---|---|---|---|
| 21–30 | Males | 11.60±0.47 | 19.46±0.80 | 4.67±0.04 | 9.67±0.36a | 13.79±0.64 | 4.86±0.04 |
| Females | 10.83±0.37 | 17.85±0.59 | 4.71±0.03 | 7.78±0.21 | 14.04±0.46 | 4.83±0.03 | |
|
| |||||||
| 31–40 | Males | 13.13±0.97 | 15.91±1.40 | 4.96±0.06 | 10.01±0.52b | 11.31±0.90 | 5.04±0.07 |
| Females | 11.00±0.42 | 14.69±0.69 | 4.90±0.04 | 8.41±0.39 | 13.06±0.66 | 5.01±0.04 | |
|
| |||||||
| 41–50 | Males | 14.59±1.01 | 14.43±1.43 | 5.11±0.05 | 10.09±0.67c | 9.43±0.94 | 5.23±0.06 |
| Females | 12.22±0.64 | 12.59±0.92 | 5.21±0.04 | 8.47±0.46 | 9.35±0.75 | 5.28±0.04 | |
|
| |||||||
| 51–60 | Males | 14.04±1.18 | 15.42±1.56d | 5.29±0.04 | 10.54±0.96 | 10.47±1.25 | 5.44±0.05 |
| Females | 13.63±1.16 | 10.43±1.07 | 5.46±0.04 | 9.08±0.84 | 8.92±0.80 | 5.49±0.03 | |
|
| |||||||
| 61–70 | Males | 12.36±0.60 | 11.77±0.95 | 5.53±0.05 | 9.17±0.40 | 7.09±0.60 | 5.67±0.04 |
| Females | 12.75±0.77 | 12.58±1.06 | 5.51±0.06 | 9.83±0.50 | 8.18±0.72 | 5.69±0.04 | |
|
| |||||||
| 71–80 | Males | 10.99±0.61 | 9.89±0.95d | 5.55±0.07 | 9.17±0.49 | 5.65±0.58 | 5.70±0.06d |
| Females | 11.71±0.74 | 6.55±0.84 | 5.76±0.08 | 9.69±0.80 | 5.10±0.70 | 5.89±0.06 | |
|
| |||||||
| >80 | Males | 11.15±1.33 | 6.95±0.98 | 5.78±0.07 | 10.33±1.05 | 4.90±0.73 | 6.11±0.07c |
| Females | 10.78±0.73 | 8.67±1.29 | 5.86±0.06 | 8.37±0.83 | 5.63±0.94 | 5.80±0.06 | |
Data are expressed as mean ± SEM. An unpaired t test was used to determine the significance between two groups.
p < 0.0001, versus females.
p < 0.001.
p < 0.01.
p < 0.05.
Fig. 2.
Trend of aging-associated changes in epidermal functions. a TEWL, N = 804 for females and N = 601 for males. b Stratum corneum hydration, N = 804 for females and N = 601 for males. c Skin surface pH, N = 521–523 for females and N = 341 for males. Nonlinear analysis is used to determine the trend of aging-associated changes in epidermal functions.
Trend of Aging-Associated Changes in BMI
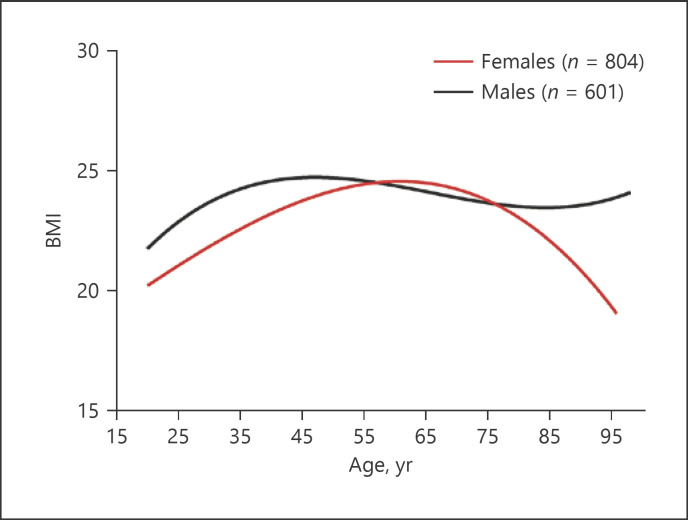
Previous study showed that BMI peaks at ages of 50–59 years old, followed by a decline in northern China [26], where individuals' lifestyle differs greatly from those in Guangdong, South China. Because BMI varies with geographic location and lifestyle [27], we next determined the trend of changes in BMI in this cohort. As shown in Figure 3, BMI gradually increased from age 21 years old to the peak at about 65 years old, followed by an aging-associated decrease in females, similar to the previous findings [26]. In contrast, in males BMI increased from age 21–40 years old. Afterward, BMI did not change dramatically (Fig. 3; Table 1). These results indicate that aging-associated changes in BMI differ between males and females.
Fig. 3.
Trend of aging-associated changes in BMI. Nonlinear analysis is used to determine the trend of aging-associated changes in BMI. Numbers of subjects are indicated in the Figure.
Association of BMI with Epidermal Functions
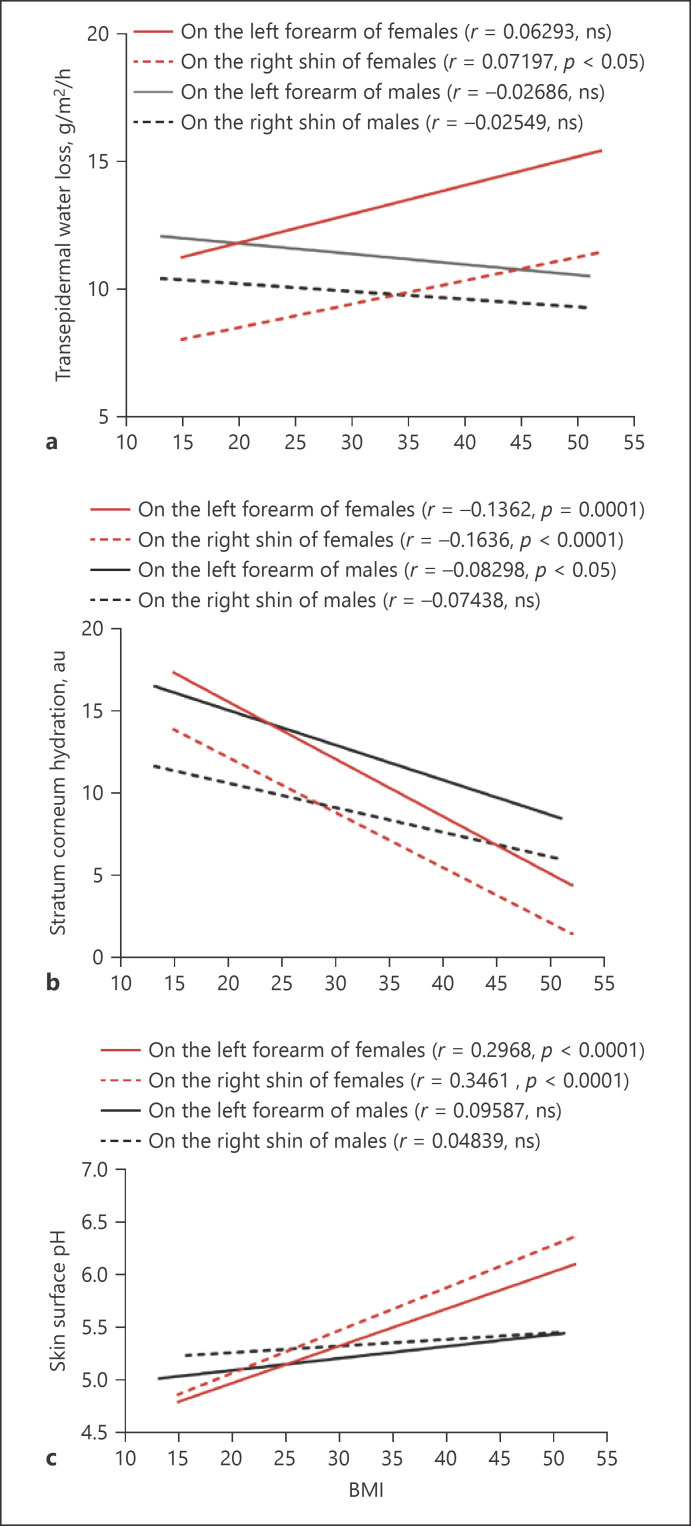
Since both epidermal functions and BMI are associated with aging as shown above, we analyzed next the correlation of BMI with epidermal functions in both males and females. Our results showed that TEWL on the shin of females significantly but weakly correlated with BMI although a tendency of positive correlation was observed on the forearm (Fig. 4a). In contrast, TEWL tended to negatively correlate with BMI on both the shin and the forearm of males (Fig. 4a). Moreover, BMI correlated negatively with stratum corneum hydration levels on both the forearm and the shin of females (Fig. 4b, p < 0.0001), while significantly negative correlation of BMI with stratum corneum hydration was only observed on the forearm, not the shin, of males (Fig. 4b, p < 0.05). Regarding the skin surface pH, only females displayed a positive correlation of pH with BMI on the shin and the forearm (Fig. 4c, p < 0.0001). However, skin surface pH on neither the forearm nor the shin had a significant correlation with BMI in males (Fig. 4c). Taken together, these results show a gender-associated correlation of epidermal functions with BMI.
Fig. 4.
Correlations of BMI with epidermal functions. a Correlation of BMI with TEWL. b Correlation of BMI with stratum corneum hydration. c Correlation of BMI with skin surface pH. Pearson r analysis is used to determine the significance of correlation of BMI with epidermal functions. p values are indicated in the figures. For epidermal function, the number of subjects in each group is the same as in Figure 1. For BMI, N = 804 for females and N = 601 for males.
Discussion
Previous studies showed an aging-associated increase in skin surface pH and a decrease in stratum corneum hydration [19], which are consistent with the present findings. However, Luebberding et al. [28, 29] reported that skin surface pH had no significant correlation with age, while stratum corneum hydration levels tended to increase with age in females. In contrast to females, males displayed a positive correlation of skin surface pH with age (p < 0.05), while stratum corneum hydration levels did not significantly correlate with age, but with a tendency of decrease [30]. Another striking difference between our present study and others is the gender difference in skin surface pH. We observed no gender differences in skin surface pH in all age groups except in subjects >81 years old, while others showed that skin surface pH on the cheek was lower in males than in females in all age groups [28]. Although the factors contributing to the discrepancy between our present study and others are unknown, differences in both the race and body sites, which are known to influence epidermal functions [31, 32, 33], can contribute to the discrepant results. We measured epidermal functions on the forearm and the shin of the Chinese, while the others evaluated epidermal functions on the forehead, cheek, hand, and forearm of the Caucasians [28, 29, 30]. Moreover, the difference in geographic locations where the studies were carried out may explain the discrepant results, too because previous studies showed that epidermal biophysical properties vary with geographic region [34]. Our study was carried out in Guangdong, China, while the other studies were in Hamburg, Germany. However, a side-by-side comparison of epidermal functions among different age groups of various races in different regions is needed to elucidate aging- and gender-associated changes in epidermal functions. The underlying mechanisms by which stratum corneum hydration decreases with aging can be attributable to the reductions in epidermal lipid synthesis, aquaporin 3 and filaggrin expression, and stratum corneum glycerol content. Moreover, an aging-associated decline in stratum corneum hydration at least in females can be caused by a reduction in estrogen levels. In comparison to premenopause, postmenopausal women display a smaller corneocyte surface area and lower stratum corneum hydration levels [35, 36]. Supplement of estrogen can improve stratum corneum hydration in postmenopausal women [36]. Thus, reductions in sex hormone can be ascribed to the aging-associated decrease in stratum corneum hydration at least in females. Regarding the skin surface pH, the decreased expression levels of sodium/hydrogen exchanger 1, secretory phospholipase A2, and filaggrin can contribute to the elevated skin surface pH in the elderly [19].
Regarding the epidermal permeability barrier function, previous studies showed no aging-associated changes in TEWL on the forearm of females [29]. But TEWL on the forearm of males aged 20–59 years old remained stable, followed by a significant decline [30]. In contrast, we show here that TEWL rates were relatively consistent in males, whereas changes in TEWL displayed a parabolic shape in the females, which is in agreement with that found on the forehead and the hand of females [29]. Because of changes in TEWL in a parabolic shape only in the females, sex hormones likely contribute to such changes. Menopause usually starts at the age of 52 years old [37], followed by ≈8 years of postmenopausal period [38]. Correspondingly, we observed a decline in TEWL, starting at age of 60 years in females. The underlying mechanisms that contribute the trend of changes in TEWL in females remain to be explored. Nevertheless, the present study clearly demonstrates aging- and gender-associated changes in the epidermal permeability barrier function.
It is worth noting that blood sugar levels can influence epidermal function although no changes in stratum corneum hydration and TEWL were also observed in diabetic patients [39]. Patients with diabetes display significant lower levels of stratum corneum hydration and higher TEWL in comparison to healthy controls, while skin surface pH is comparable between normal and diabetic individuals [40]. Individuals with higher fasting plasma sugar levels (>110 mg/dL) display lower levels of stratum corneum hydration than those with lower fasting plasma sugar levels (<110 mg/dL) [41]. In China, the prevalence of diabetes is sharply increased in individuals after the age of 50 years old. The total prevalence of diabetes and prediabetes is over 60% in subjects >60 years old [42]. However, the contribution of diabetes and/or high blood glucose levels to the aging-associated decline in stratum corneum hydration is unknown.
In agreement with the previous finding that BMI increased from age 20 to 50 years old in females and general population in the USA [43, 44], we also showed that aging-associated changes in BMI were in a parabolic shape in females. However, other studies showed a positive correlation of BMI with age in general population [45, 46]. The correlation of BMI with TEWL is inconclusive. One study showed that TEWL was lower in obese subjects than in normal body weight controls [20], while other studies demonstrated that TEWL on the forearm was higher in obese children [21], and positively correlated with BMI in adults in a small group of subjects [22]. We did not observe a strong correlation of TEWL with BMI in this cohort except a weak, but significantly positive correlation on the shin of females. It has been postulated that the positive correlation of BMI with TEWL is due to high sweat gland activity in obese individuals [22]. However, no correlation was found between TEWL and BMI in males, suggesting additional mechanisms involved in the regulation of TEWL in obese females. We unexpectedly observed the correlations of BMI with stratum corneum hydration levels and skin surface pH, particularly in females. Although it is not clear whether obesity influences stratum corneum hydration levels and skin surface pH or versa vice, evidence points to the potential role of altered stratum corneum hydration levels and skin surface pH in the pathogenesis of obesity. The development of obesity has been linked to inflammatory cytokines [47, 48, 49]. Either decreased stratum corneum hydration levels or increased skin surface pH can provoke or exacerbate cutaneous inflammation [19, 50]. Conversely, improvements in epidermal functions with emollients can lower levels of cutaneous and circulating cytokines [51, 52]. A sustained increase in cutaneous inflammation can eventually induce systemic inflammation, consequently leading to the development of obesity. However, further studies are required to determine whether improvement in stratum corneum hydration and/or pH with topical emollient can mitigate the development of obesity in clinical setting.
In conclusion, aging-associated changes in epidermal functions and BMI vary with gender. BMI correlates negatively with stratum corneum hydration and positively with skin surface pH. Either elevated skin surface pH or reduced stratum corneum hydration levels can provoke cutaneous inflammation, which is linked to the development to obesity. Whether improvements in stratum corneum hydration levels or pH could benefit obesity remains to be elucidated.
Statement of Ethics
This study was approved by the institutional review board of the Dermatology Hospital of Southern Medical University (#2021025) and performed in accordance with the Declaration of Helsinki. Because the measurements were simple and harmless, the institutional review board granted verbal consent.
Conflict of Interest Statement
All authors declare no conflicts of interest.
Funding Sources
This study was supported, in part, by National Natural Science Foundation of China (81903188) and Medical Research Foundation of Guangdong Province, China (A2020249, B2020034). The funders of the study had no role in study design, data collection, and analysis or in writing the report.
Author Contributions
Li Ye, Qingsong Lai, Si Wen, and Xiaohua Wang performed experiments. Mao-Qiang Man originated concept, designed experiments, analyzed data, and wrote draft. Bin Yang supervised the study and critically reviewed the manuscript. All authors approved the final version and the submission.
Data Availability Statement
Data are available from Li Ye or Mao-Qiang Man.
Li Ye and Qingsong Lai contributed equally to this work.
References
- 1.Yoshida T, Beck LA, De Benedetto A. Skin barrier defects in atopic dermatitis: from old idea to new opportunity. Allergol Int. 2022;71((1)):3–13. doi: 10.1016/j.alit.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang B, Liu LL, Zhao ZT, Tu P. Impaired skin barrier function and downregulated expression of caspase-14 in moderate to severe chronic hand eczema. Dermatology. 2018;234:180–5. doi: 10.1159/000489701. [DOI] [PubMed] [Google Scholar]
- 3.Laudańska H, Reduta T, Szmitkowska D. Evaluation of skin barrier function in allergic contact dermatitis and atopic dermatitis using method of the continuous TEWL measurement. Rocz Akad Med Bialymst. 2003;48:123–7. [PubMed] [Google Scholar]
- 4.Wang X, Ye L, Lai Q, Wen S, Long Z, Qiu X, et al. Altered epidermal permeability barrier function in the uninvolved skin supports a role of epidermal dysfunction in the pathogenesis of occupational hand eczema. Skin Pharmacol Physiol. 2020;33:94–101. doi: 10.1159/000506425. [DOI] [PubMed] [Google Scholar]
- 5.Ye L, Lv C, Man G, Song S, Elias PM, Man MQ. Abnormal epidermal barrier recovery in uninvolved skin supports the notion of an epidermal pathogenesis of psoriasis. J Invest Dermatol. 2014;134:2843–6. doi: 10.1038/jid.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou M, Xie H, Cheng L, Li J. Clinical characteristics and epidermal barrier function of papulopustular rosacea: a comparison study with acne vulgaris. Pak J Med Sci. 2016;32:1344–8. doi: 10.12669/pjms.326.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan C, Ma Y, Wang Y, Wang X, Qian C, Hocquet D, et al. Rosacea is associated with conjoined interactions between physical barrier of the skin and microorganisms: a pilot study. J Clin Lab Anal. 2020;34:e23363. doi: 10.1002/jcla.23363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie HF, Huang YX, He L, Yang S, Deng YX, Jian D, et al. An observational descriptive survey of rosacea in the Chinese population: clinical features based on the affected locations. PeerJ. 2017;5:e3527. doi: 10.7717/peerj.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Man WY, Lv CZ, Song SP, Shi YJ, Elias PM, et al. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharmacol Physiol. 2010;23:193–200. doi: 10.1159/000288166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunathilake R, Schurer NY, Shoo BA, Celli A, Hachem JP, Crumrine D, et al. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J Invest Dermatol. 2009;129:1719–29. doi: 10.1038/jid.2008.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man MQ, Lin TK, Santiago JL, Celli A, Zhong L, Huang ZM, et al. Basis for enhanced barrier function of pigmented skin. J Invest Dermatol. 2014;134:2399–407. doi: 10.1038/jid.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg A, Chren MM, Sands LP, Matsui MS, Marenus KD, Feingold KR, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53–9. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Denda M, Tsuchiya T, Elias PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R367–72. doi: 10.1152/ajpregu.2000.278.2.R367. [DOI] [PubMed] [Google Scholar]
- 14.Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005;124:587–95. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Yoon NY, Kim DH, Jung M, Jun M, Park HY, et al. Impaired permeability and antimicrobial barriers in type 2 diabetes skin are linked to increased serum levels of advanced glycation end-product. Exp Dermatol. 2018;27:815–23. doi: 10.1111/exd.13466. [DOI] [PubMed] [Google Scholar]
- 16.Okano J, Kojima H, Katagi M, Nakagawa T, Nakae Y, Terashima T, et al. Hyperglycemia induces skin barrier dysfunctions with impairment of epidermal integrity in non-wounded skin of type 1 diabetic mice. PLoS One. 2016;11:e0166215. doi: 10.1371/journal.pone.0166215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekijima H, Goto K, Hiramoto K, Komori R, Ooi K. Characterization of dry skin associating with type 2 diabetes mellitus using a KK-Ay/TaJcl mouse model. Cutan Ocul Toxicol. 2018;37:391–5. doi: 10.1080/15569527.2018.1490746. [DOI] [PubMed] [Google Scholar]
- 18.Horikawa T, Hiramoto K, Goto K, Sekijima H, Ooi K. Differences in the mechanism of type 1 and type 2 diabetes-induced skin dryness by using model mice. Int J Med Sci. 2021;18((2)):474–81. doi: 10.7150/ijms.50764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Man MQ, Li T, Elias PM, Mauro TM. Aging-associated alterations in epidermal function and their clinical significance. Aging. 2020;12:5551–65. doi: 10.18632/aging.102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guida B, Nino M, Perrino NR, Laccetti R, Trio R, Labella S, et al. The impact of obesity on skin disease and epidermal permeability barrier status. J Eur Acad Dermatol Venereol. 2010;24:191–5. doi: 10.1111/j.1468-3083.2009.03503.x. [DOI] [PubMed] [Google Scholar]
- 21.Nino M, Franzese A, Ruggiero Perrino N, Balato N. The effect of obesity on skin disease and epidermal permeability barrier status in children. Pediatr Dermatol. 2012;29:567–70. doi: 10.1111/j.1525-1470.2012.01738.x. [DOI] [PubMed] [Google Scholar]
- 22.Löffler H, Aramaki JU, Effendy I. The influence of body mass index on skin susceptibility to sodium lauryl sulphate. Skin Res Technol. 2002;8:19–22. doi: 10.1046/j.0909-752x. [DOI] [PubMed] [Google Scholar]
- 23.Monteiro Rodrigues LM, Palma L, Santos O, Almeida MA, Bujan J, Tavares L. Excessive weight favours skin physiology − up to a point: another expression of the obesity paradox. Skin Pharmacol Physiol. 2017;30:94–101. doi: 10.1159/000464338. [DOI] [PubMed] [Google Scholar]
- 24.Ye L, Wang Z, Li Z, Lv C, Man MQ. Validation of GPSkin Barrier® for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin Res Technol. 2019;25:25–9. doi: 10.1111/srt.12590. [DOI] [PubMed] [Google Scholar]
- 25.Firooz A, Sadr B, Babakoohi S, Sarraf-Yazdy M, Fanian F, Kazerouni-Timsar A, et al. Variation of biophysical parameters of the skin with age, gender, and body region. ScientificWorldJournal. 2012;2012:386936. doi: 10.1100/2012/386936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W, Zhang H, Paillard-Borg S, Zhu H, Qi X, Rizzuto D. Prevalence of overweight and obesity among Chinese adults: role of adiposity indicators and age. Obes Facts. 2016;9:17–28. doi: 10.1159/000443003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cugnetto ML, Saab PG, Llabre MM, Goldberg R, McCalla JR, Schneiderman N. Lifestyle factors, body mass index, and lipid profile in adolescents. J Pediatr Psychol. 2008;33:761–71. doi: 10.1093/jpepsy/jsm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women: in vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int J Cosmet Sci. 2013;35:477–83. doi: 10.1111/ics.12068. [DOI] [PubMed] [Google Scholar]
- 29.Luebberding S, Krueger N, Kerscher M. Age-related changes in skin barrier function − quantitative evaluation of 150 female subjects. Int J Cosmet Sci. 2013;35:183–90. doi: 10.1111/ics.12024. [DOI] [PubMed] [Google Scholar]
- 30.Luebberding S, Krueger N, Kerscher M. Age-related changes in male skin: quantitative evaluation of one hundred and fifty male subjects. Skin Pharmacol Physiol. 2014;27((1)):9–17. doi: 10.1159/000351349. [DOI] [PubMed] [Google Scholar]
- 31.Mayrovitz HN, Bernal M, Brlit F, Desfor R. Biophysical measures of skin tissue water: variations within and among anatomical sites and correlations between measures. Skin Res Technol. 2013;19:47–54. doi: 10.1111/srt.12000. [DOI] [PubMed] [Google Scholar]
- 32.Berardesca E, Maibach HI. Transepidermal water loss and skin surface hydration in the non invasive assessment of stratum corneum function. Derm Beruf Umwelt. 1990;38:50–3. [PubMed] [Google Scholar]
- 33.Alexis AF, Woolery-Lloyd H, Williams K, Andriessen A, Desai S, Han G, et al. Racial/ethnic variations in skin barrier: implications for skin care recommendations in skin of color. J Drugs Dermatol. 2021;20:932–8. doi: 10.36849/jdd.6312. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Ha J, Shin K, Kim H, Cho S. Different cosmetic habits can affect the biophysical profile of facial skin: a study of korean and chinese women. Ann Dermatol. 2019;31((2)):175–85. doi: 10.5021/ad.2019.31.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fluhr JW, Pelosi A, Lazzerini S, Dikstein S, Berardesca E. Differences in corneocyte surface area in pre- and post-menopausal women. Assessment with the noninvasive videomicroscopic imaging of corneocytes method (VIC) under basal conditions. Skin Pharmacol Appl Skin Physiol. 2001;14((Suppl 1)):10–6. doi: 10.1159/000056384. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson S, Thornton J. Effect of estrogens on skin aging and the potential role of SERMs. Clin Interv Aging. 2007;2((3)):283–97. doi: 10.2147/cia.s798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan S, Gomes A, Singh RS. Is menopause still evolving? Evidence from a longitudinal study of multiethnic populations and its relevance to women's health. BMC Womens Health. 2020;20:74. doi: 10.1186/s12905-020-00932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YW. Depression in post menopausal women. Taehan Kanho Hakhoe Chi. 2003;33:471–7. doi: 10.4040/jkan.2003.33.4.471. [DOI] [PubMed] [Google Scholar]
- 39.Seirafi H, Farsinejad K, Firooz A, Davoudi SM, Robati RM, Hoseini MS, et al. Biophysical characteristics of skin in diabetes: a controlled study. J Eur Acad Dermatol Venereol. 2009 Feb;23((2)):146–9. doi: 10.1111/j.1468-3083.2008.02950.x. [DOI] [PubMed] [Google Scholar]
- 40.Ibuki A, Kuriyama S, Toyosaki Y, Aiba M, Hidaka M, Horie Y, et al. Aging-like physiological changes in the skin of Japanese obese diabetic patients. SAGE Open Med. 2018 Feb 6;6:2050312118756662. doi: 10.1177/2050312118756662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai S, Kikuchi K, Satoh J, Tagami H, Inoue S. Functional properties of the stratum corneum in patients with diabetes mellitus: similarities to senile xerosis. Br J Dermatol. 2005;153((2)):319–23. doi: 10.1111/j.1365-2133.2005.06756.x. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020 Apr 28;369:m997. doi: 10.1136/bmj.m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes A, Gearon E, Backholer K, Bauman A, Peeters A. Age-specific changes in BMI and BMI distribution among Australian adults using cross-sectional surveys from 1980 to 2008. Int J Obes. 2015;39:1209–16. doi: 10.1038/ijo.2015.50. [DOI] [PubMed] [Google Scholar]
- 44.Newport F, Mcgeeney K, Mendes E. In U.S., being middle-aged most linked to having higher BMI. WELLBEING; 2012. Aug 6, In U.S., being middle-aged most linked to having higher BMI (gallup.com) (obtained on January 17, 2022) [Google Scholar]
- 45.Aarestrup J, Bjerregaard LG, Gamborg M, Ängquist L, Tjønneland A, Overvad K, et al. Tracking of body mass index from 7 to 69 years of age. Int J Obes. 2016;40:1376–83. doi: 10.1038/ijo.2016.88. [DOI] [PubMed] [Google Scholar]
- 46.Mungreiphy NK, Kapoor S, Sinha R. Association between BMI, blood pressure, and age: study among tangkhul naga tribal males of northeast India. J Anthropol. 2011;2011:1. Article ID 748147, 6 pages. [Google Scholar]
- 47.Marette A. Pathogenic role of inflammatory cytokines in obesity: from insulin resistance to diabetes mellitus. Nestle Nutr Workshop Ser Clin Perform Programme. 2004;9:141–53. doi: 10.1159/000080650. [DOI] [PubMed] [Google Scholar]
- 48.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Man MQ, Elias PM. Stratum corneum hydration regulates key epidermal function and serves as an indicator and contributor to other conditions. J Eur Acad Dermatol Venereol. 2019;33((1)):15–6. doi: 10.1111/jdv.15374. [DOI] [PubMed] [Google Scholar]
- 51.Ye L, Mauro TM, Dang E, Wang G, Hu LZ, Yu C, et al. Topical applications of an emollient reduce circulating pro-inflammatory cytokine levels in chronically aged humans: a pilot clinical study. J Eur Acad Dermatol Venereol. 2019;33:2197–201. doi: 10.1111/jdv.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu L, Mauro TM, Dang E, Man G, Zhang J, Lee D, et al. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J Invest Dermatol. 2017;137:1277–85. doi: 10.1016/j.jid.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from Li Ye or Mao-Qiang Man.