Abstract
Endocrine therapies are the main treatment strategies for the clinical management of hormone-dependent breast cancer. Despite prolonged time to recurrence in the adjuvant setting and the initial clinical responses in the metastatic setting, many patients eventually encounter tumour relapse due to acquired resistance to these agents. Other patients experience a lack of tumour regression at the beginning of treatment indicating de novo resistance that significantly limits its efficacy in the clinic. There is compelling evidence that human epidermal growth factor receptor-2 (HER2) overexpression contributes to resistance to endocrine therapies in oestrogen receptor-positive (ER+) breast cancer. ER+/HER2+ tumours comprise about 10% of all breast cancer cases and about 60% of the whole set of HER2+ tumours. Most patients with primary ER+/HER2+ disease will receive antibody-based HER2-targeted therapy, but this is generally for no more than one year while endocrine treatment is usually for at least 5 years. A number of HER2-kinase inhibitors are also now in clinical use or in clinical trials, and the interaction of these with endocrine treatment may differ from that of antibody treatment. In this review article, we aim to summarise knowledge on molecular mechanisms of breast cancer resistance to endocrine therapies attributable to the impact of HER2 signalling on endocrine sensitivity, to discuss data from clinical trials addressing the role of HER2 in the development of endocrine resistance in the metastatic, neoadjuvant and adjuvant settings and to explore rational new therapeutic strategies.
Keywords: breast cancer, oestrogen receptor, human epidermal growth factor receptor-2, resistance, endocrine therapies
Introduction
Breast cancer development and progression are significantly affected by signalling pathways involving oestrogen receptor (ER) and growth factor receptors (Arpino et al. 2008). Over 80% of all breast cancer cases are deemed ER-positive and/or progesterone receptor (PgR)-positive (Dodson et al. 2018), and oestrogen is the primary growth stimulant of these tumours (Dixon 2014). Hormone receptor (HR) status is measured in all primary breast cancers and is used as a predictive marker for selecting patients, who are more likely to benefit from hormonal therapy strategies whether this will be in the early or the metastatic context (Ring & Dowsett 2003). Among these therapies are the selective ER modulator, tamoxifen, which inhibits tumour growth by binding and blocking ER (EBCTCG 1998) and aromatase inhibitors (AIs) that inhibit the enzyme that converts androgens into oestrogen (Gradishar 2004). When given as adjuvant therapy to postmenopausal women, 5 years’ tamoxifen or AI reduce the risk of patients dying from their breast cancer by about 30 or 40%, respectively (EBCTCG 2015).
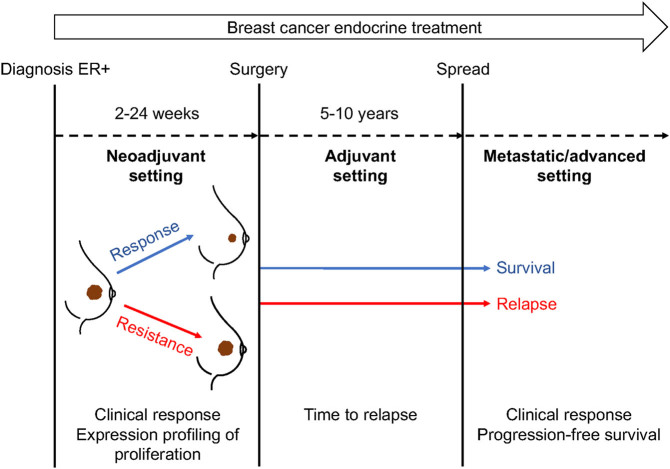
Despite the benefits from hormonal therapy, de novo and acquired resistance to treatment occur in a large number of patients and this significantly limits its optimal use in the clinic (Larionov & Miller 2009). Resistance is exhibited in different ways according to the disease/treatment setting (Fig. 1): (i) as disease recurrence during or after post-surgical adjuvant therapy; (ii) as a lack of tumour regression during neoadjuvant treatment, suggesting intrinsic resistance, or regrowth after an initial response, indicating acquired resistance; (iii) as progression of metastatic disease as (re-)growth of existing metastases or development of new metastases (Gonzalez-Angulo et al. 2007). Besides clinical response, the proliferation marker Ki67 has been used as a measure of response/resistance to neoadjuvant endocrine therapy (Selli & Sims 2019). Evidence from clinical trials of adjuvant endocrine therapy in the past suggested that disease recurred in up to 40–50% of ER-positive patients (Ring & Dowsett 2003, Dixon 2014), but contemporary rates are lower with more modern therapy used in patients with the earlier disease. In the neoadjuvant setting, clinical response rates range from 50 to 70% of patients (Colleoni & Montagna 2012). Almost all patients with advanced or metastatic ER+ breast cancer will relapse if treated with endocrine therapy alone during the first few years of treatment, and eventually die from the disease (D’Souza et al. 2018). The response can occur sequentially with different endocrine agents. The duration of response is increased by combination with other agents, such as cyclin-dependent kinase 4/6 (CDK4/6) inhibitors (Portman et al. 2019).
Figure 1.
Main events and response endpoints over the course of endocrine treatment settings in breast cancer. Endocrine resistance is manifested clinically as an increase in tumour volume, relapse and progression in the neoadjuvant, adjuvant, and metastatic treatment setting, respectively. Biological endpoints have, also, been considered as an indication of endocrine treatment resistance. For instance, Ki67 is often measured either as a static marker of proliferation, or as a dynamic surrogate marker of drug response, when the expression levels are measured at multiple times during neoadjuvant treatment.
At diagnosis, around 15% of ER+ breast cancers exhibit a concurrent human epidermal growth factor receptor 2 (HER2) gene-amplification, such that approximately 10% of all breast cancers are ER+/HER2+ (Dodson et al. 2018). In most studies, ER+/HER2+ tumours have a more aggressive phenotype, as indicated by the patients’ poor prognosis and higher levels of tumour proliferation than those that do not demonstrate HER2-overexpression or gene amplification (Dowsett et al. 2001). Within HR+/HER2+ tumours, about 30% are considered HER2-enriched (HER2-E), the intrinsic subtype that is associated with high activation of the HER2 signalling pathway, enhanced proliferation, and increased numbers of tumour-infiltrating lymphocytes in the surrounding stroma and is characterised by a worse prognosis. Many tumours classified as HER2-E are not HER2+ by immunohistochemistry (IHC) or fluorescent in situ hybridisation (FISH) (Pascual et al. 2021). According to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) updated guidelines on HER2 testing, HER2 IHC may be considered a screening test, and FISH can act as a confirmatory test for HER2 IHC equivocal cases. Thus, any IHC 3+ staining result indicates a HER2 positive diagnosis, and 0/1+ staining is considered negative. IHC 2+ results are considered positive if the FISH analysis indicates amplification with the updated criteria considering several scenarios based on HER2/CEP17 ratio and HER copy number (Wolff et al. 2018). Assigning HER2 status can also be complicated by the presence of intratumoural heterogeneity in HER2 overexpression, increase in chromosome enumeration probe 17 signals, alteration of HER2 status following neoadjuvant chemotherapy, or during metastatic progression (Ahn et al. 2020). These major nuances in HER2 status have rarely been addressed in studies of resistance to endocrine therapy and most likely contribute to different findings in different studies.
The degree of overexpression of HER2 is inversely correlated with ER expression (Ring & Dowsett 2003). This may relate to the repression of HER2 by ER through PAX2 and the ER coregulator AIB1/SRC3 competing for HER2 transcription (Hurtado et al. 2008). Increased HER2 protein expression due to expression loss of the oestrogen-regulated microRNA cluster comprising let-7c, miR99a, and miR125b, is another explanation of the inverse correlation between ER and HER2 (Bailey et al. 2015). This inverse correlation results in there being a greater proportion of HER2 immunohistochemistry (IHC) 2+ cases among ER+ than among ER− tumours.
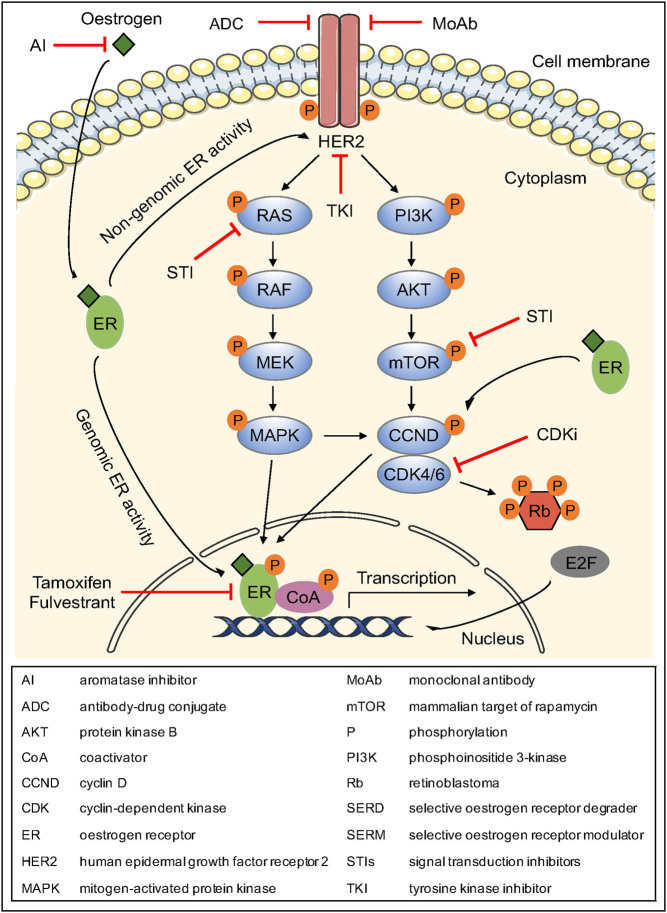
Compelling evidence suggests that breast cancer growth in at least some ER+ and HER2-overexpressing tumours is regulated by bi-directional crosstalk between ER and HER2 signalling pathways that can drive the development of resistance to endocrine therapy (Arpino et al. 2008, Goutsouliak et al. 2020) (Fig. 2). Several in vitro studies suggested that HER2 overexpression can facilitate both the genomic and non-genomic action of ER and its coactivator AIB1 in breast cancer cells, leading to tamoxifen resistance (Chung et al. 2002, Shou et al. 2004). Interestingly, upregulation of downstream signalling molecules, such as p42/44 mitogen-activated protein kinase (MAPK) and protein kinase B (AKT), is also an indication of endocrine resistance of breast cancer cells (Knowlden et al. 2003). It is plausible that HER2 overexpression might generate alternative signals of proliferation and survival to circumvent ER inhibition that subsequently result in endocrine therapy resistance.
Figure 2.
Crosstalk between ER and HER2 signalling pathways and its role in endocrine resistance and therapeutic agents that block specific molecules. A more detailed description is presented in the main text.
In addition to HER2 protein overexpression or gene amplification, other predictive biomarkers have been reported for patients with HR+/HER2+ breast cancer, including gene expression scores, DNA mutations, proliferation, and the immune microenvironment (Dieci et al. 2020). Subtype classification of patients with metastatic HR+/HER2+ tumours using the PAM50 gene signature could be used as a useful tool for identifying patients with Luminal A tumours that exhibit longer progression-free survival (PFS) among other subtypes (Prat et al. 2016). In addition, PIK3CA and ERBB2 mutations are associated with reduced pathological complete response rates and endocrine resistance (Loibl et al. 2016, Hyman et al. 2017). Another important predictor of resistance to endocrine therapies in HR+/HER2+ disease is the tumour immune microenvironment. Dunbier et al. have previously observed that an immune-gene signature was the strongest signature associated with poor antiproliferative response to an AI in a set of patients with ER+ tumours that were either HER2+ or HER2- (Dunbier et al. 2013).
Accumulating knowledge of the biology of this breast cancer subtype and understanding the mechanisms by which cells become resistant to endocrine therapies may provide useful information to refine the current treatment approach and enhance patients’ outcome. While there was much attention to the ER+/HER2+ subgroup of breast cancer in the previous decade, less attention has been paid in the recent past, most probably because of the advent of trastuzumab treatment and many other antibody-based or kinase-based anti-HER2 treatments. However, it is important to note that these anti-HER2 therapies are generally administered for a limited period; in the primary disease setting this is most often 12 months yet endocrine treatment is generally for at least 5 years and often for 10 years. Thus, any residual micro-metastatic ER+/HER2+ disease after surgery is not targeted by anti-HER2 therapy for the majority of the duration of endocrine treatment. In that case, recurrence may occur from persistent micro-metastatic disease that includes any remaining HER2+ clones.
The aim of this review is therefore to summarise the current knowledge on the response of patients with ER+ and HER2-overexpressing tumours to endocrine therapies comparing the different clinical treatment settings. This information is of paramount clinical importance, as it can provide a rational basis for the use of emerging combination therapies that may potentially evade endocrine resistance and eventually lead to complete tumour eradication.
Role of HER2 in the development of endocrine resistance: in vitro and preclinical studies
Several preclinical studies suggested that growth factor signalling induces both de novo and acquired resistance of breast cancer cells to endocrine therapy. Overexpression of HER2 is associated with the development of de novo resistance of breast cancer cells to tamoxifen. For example, direct interaction between HER2 and ER in the BT474 HER2-overexpressing breast cancer model promoted resistance to tamoxifen by inhibiting its apoptotic effects (Chung et al. 2002). Benz et al. also demonstrated that xenograft tumours that were formed by inoculation of MCF7/HER2-18 cells stably transfected with HER2 were not sensitive to tamoxifen treatment (Benz et al. 1992). More recently, MCF7/HER2-18 cells were shown to be growth stimulated by tamoxifen in a low oestrogen environment, suggesting that tamoxifen can act as a potent agonist on tumour growth in this model (Shou et al. 2004).
In the above breast cancer cell lines, the presence of either oestrogen or tamoxifen instigates HER2 overexpression, which further enhances molecular crosstalk with the ER pathway (Shou et al. 2004). This leads to the activation of molecules involved in AKT and MAPK signalling pathways that, in turn, phosphorylate and augment the functional activity of ER and the coactivator AIB1, rendering breast cancer cells resistant to endocrine therapy (Shou et al. 2004, Arpino et al. 2008) (Fig. 2). Interestingly, although tamoxifen induces both genomic and non-genomic ER activation in HER2-overexpressing MCR7 cells in vitro (Chung et al. 2002, Shou et al. 2004), xenografts generated by the same cells in vivo are mainly characterised by non-genomic ER function as a mechanism of de novo resistance to tamoxifen (Massarweh et al. 2008). Moreover, tamoxifen induces the expression of oestrogen-regulated genes by facilitating the recruitment of coactivators, such as AIB1, rather than corepressor complexes in these HER2-overexpressing cells (Shou et al. 2004). The above events can be inhibited by treatment with anti-HER inhibitors, such as the EGF receptor (EGFR)/HER2 inhibitor, gefitinib, or the monoclonal anti-HER2 antibody, trastuzumab, which have the ability to eliminate the ER and HER2 crosstalk via blocking of EGFR/HER2 heterodimers or HER2, respectively, indicating that they are directly implicated in the growth-inducing role of tamoxifen in cells overexpressing HER2. In this regard, gefitinib or trastuzumab could restore tamoxifen’s anti-tumour effects in MCR7/HER2-18 cells, whereas it had only a modest effect on the inhibition of oestrogen-stimulated growth (Shou et al. 2004).
Experimental evidence suggested that acquired resistance to fulvestrant and tamoxifen can occur in continuous culture of breast cancer cells to these agents. Long-term culture of MCF7 cells with anti-oestrogens generated sublines that were insensitive and proliferated at rates equivalent to those of untreated WT cells (McClelland et al. 2001). The resistant MCF7 cells exhibited higher levels of EGFR and HER2 expression, enhanced activation of EGFR/HER2 heterodimers, and elevated levels of phosphorylation of MAPK, AKT, and nuclear ER (Knowlden et al. 2003). Similar to the de novo resistant models, gefitinib or trastuzumab, could act by hindering cell proliferation after acquiring resistance to tamoxifen (Knowlden et al. 2003). Taken together, the above in vitro and in vivo studies indicated that induced growth factor signalling and as a result, increased non-classic genomic or non-genomic ER activities, play an important role in the mechanism of both de novo and acquired resistance to anti-oestrogens. These mechanisms that sustain altered ER signalling in endocrine-resistant tumours can result in further unbalanced activity of ER co-regulator, ligand-independent ER activation, and altered ER-dependent transcriptional reprogramming to further support endocrine resistance (Massarweh et al. 2008, Evans et al. 2010, Lupien et al. 2010, Nardone et al. 2015).
Role of HER2 in the development of endocrine resistance: clinical studies
Endocrine therapy for metastatic disease
Conflicting results have been reported by several studies that assessed the effect of HER2 overexpression on endocrine resistance in the metastatic setting (Table 1) (Wright et al. 1992, Archer et al. 1995, Leitzel et al. 1995, Willsher et al. 1996, Yamauchi et al. 1997, Elledge et al. 1998, Houston et al. 1999, Hayes et al. 2001, Lipton et al. 2002, 2003). Some studies suggested that HER2 overexpression is associated with high levels of failure and poor response to endocrine treatment (Wright et al. 1992, Leitzel et al. 1995, Yamauchi et al. 1997, Houston et al. 1999, Hayes et al. 2001, Lipton et al. 2002, 2003), whilst others have not found enough evidence to verify this association (Archer et al. 1995, Willsher et al. 1996, Elledge et al. 1998).
Table 1.
Summary of trials assessing HER2 in predicting responses to endocrine therapy for metastatic breast cancer.
| Number of patients | HER2+ patients | HER2 assessment | Cut-off | Treatment | Endpoint | Response | Conclusion | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HER2+ patients | HER2− patients | P-value | ||||||||
| 65 | 14 (22%) | IHC | ≥50% of cells | Tamoxifen + ovarian ablation | OR (%) | 20b | 48b | P < 0.01 | HER2 was significantly associated with poorer OR in tamoxifen-treated patients. | a |
| 300a | 58 (19.3%) | Serum ELISA | >30 U/mL | Megestrol acetate/ fadrozole | CR (%) | 20.7 | 40.9 | P = 0.004 | HER2 was significantly associated with poorer response in patients treated with hormone therapies. | b |
| CR (months) | 11.6 | 15.5 | P = 0.04 | |||||||
| Trial survival (months) | 15 | 28 | P < 0.0001 | |||||||
| Duration of cancer survival (months) | 64.5 | 107.7 | P < 0.0001 | |||||||
| 92 | 24 (26%) | IHC | ≥1% stained tumour cell membranes | Tamoxifen, goserelin, goserelin+tamoxifen, megestrol acetate | CR (%) | 29.2 | 42.7 | P = 0.24 | HER2 was not significantly associated with poorer response in patients treated with hormone therapies. | c |
| 52 | 13 (25%) | Serum ELISA | >20 ng/mL | Tamoxifen, tamoxifen+goserelin | CR (%) | 50b | 62.5c | P = 0.84 | HER2 was not significantly associated with poorer response in patients treated with hormone therapies. | d |
| 94 | 32 (34%) | Serum ELISA | ≥5,000 U/mL | Droloxifene | OR (%) | 9 | 56 | P = 0.00001 | HER2 was significantly associated with poorer OR in droloxifene-treated patients. | e |
| 204b | 61 (30%) | IHC | 2+ (≥1% stained cells) | Tamoxifen | CR (%) | 54 | 57 | P = 0.67 | HER2 was not significantly associated with poorer response, shorter TTF and OS in tamoxifen-treated patients. | f |
| TTF (months) | 6 | 8 | P = 0.15 | |||||||
| OS (months) | 29 | 31 | P = 0.36 | |||||||
| 189b | 57 (30.2%) | IHC | ≥1% stained tumour cell membranes | Tamoxifen | Overall response (%) | 24 | 64 | P = 0.05 | HER2 was significantly associated with shorter TTP in tamoxifen-treated patients. | g |
| TTP (months) | 5.5 | 11.2 | P < 0.001 | |||||||
| 103a | 33 (32%) | Serum ELISA | >10.5 ng/mL | Megestrol acetate | OS (months) | 20.2 | 27.8 | P = 0.007 | HER2 was significantly associated with shorter OS in patients treated with megestrol acetate. | h |
| TTP (months) | 6 | 7.4 | P = 0.9 | |||||||
| CR (%) | 37 | 28 | P = 0.41 | |||||||
| CB (%) | 80 | 78 | P = 0.79 | |||||||
| 711a | 219 (30.8%) | Serum ELISA | >15 ng/mL | Fadrazole/letrozole/megestrol acetate | OR (%) | 23 | 45 | P < 0.0001 | HER2 was significantly associated with poorer OS, and shorter TTP, TTF, response and OS in patients treated with hormone therapies. | i |
| TTP (days) | 90 | 180 | P < 0.0001 | |||||||
| TTF (days) | 93 | 175 | P < 0.0001 | |||||||
| CR (months) | 11.7 | 17.4 | P < 0.0001 | |||||||
| OS (months) | 17.2 | 29.6 | P < 0.0001 | |||||||
| 562b | 164 (29%) | Serum ELISA | >15 ng/mL | Tamoxifen/letrozole | OR (%) | 15 | 32 | P < 0.0001 | HER2 was significantly associated with poorer response to tamoxifen and letrozole treatment. | j |
| CB (%) | 30 | 51 | P < 0.0001 | |||||||
| OR (months) | 18.5 | 25.3 | P < 0.014 | |||||||
| CB (months) | 16.3 | 20.9 | P = 0.0067 | |||||||
| TTP (months) | 5.7 | 9.4 | P < 0.0001 | |||||||
| TTF (months) | 4.2 | 9.1 | P < 0.0001 | |||||||
| OS (months) | 20.8 | 36.5 | P < 0.0001 | |||||||
aER-positive/ER-unknown, bER-positive, cER-negative; a (Wright et al. 1992), b (Leitzel et al. 1995), c (Archer et al. 1995), d (Willsher et al. 1996), e (Yamauchi et al. 1997), f (Elledge et al. 1998), g (Houston et al. 1999), h (Hayes et al. 2001), I (Lipton et al. 2002), j (Lipton et al. 2003).
CB, clinical benefit; CR, clinical response; ER, oestrogen receptor; HER2, human EGF receptor-2; IHC, immunohistochemistry; OR, objective response; OS, overall survival; TTF, time to treatment failure; TTP, time to progression.
This could be explained by the use of different techniques to evaluate HER2 status: determination of HER2 protein expression by IHC using a variety of antibodies (Wright et al. 1992, Archer et al. 1995, Elledge et al. 1998, Houston et al. 1999) or the assessment of serum circulating HER2 levels (Leitzel et al. 1995, Willsher et al. 1996, Yamauchi et al. 1997, Hayes et al. 2001, Lipton et al. 2002, 2003). With many different cut-off values being used to distinguish between HER2+ and HER2- status, it is hard to state precisely what percentage of patients overexpressed HER2. This issue was particularly relevant in the metastatic studies, which predated the publication of the ASCO/CAP guidelines for HER2 analysis and reporting (Wolff et al. 2007, 2018). For example, Elledge et al. suggested that inter-observer variability in IHC measurements could generate issues of reproducibility. For that reason, lack of a standardised scoring method for HER2 status could be accounted as a limitation (Elledge et al. 1998). Previous work has shown that only 30% of the HER2-overexpressing invasive human breast tumours were characterised by a phosphorylated form of the receptor, an indication of its active state (DiGiovanna et al. 1996). As the above methods used to define the HER2 status are not functional assays, it is also possible that the measured receptor protein is not activated.
In a number of studies, both ER+ and ER− tumours were included in the analysis (Wright et al. 1992, Archer et al. 1995, Hayes et al. 2001, Lipton et al. 2002, 2003), therefore much of the reported resistance of HER2-positive tumours to endocrine therapy could be due to the ER-negative nature of the tumour rather than HER2-positivity per se (Wright et al. 1992, Dowsett et al. 2001). When analysed separately, the number of tumours co-expressing ER and HER2 was very small in some studies (Wright et al. 1992, Willsher et al. 1996), such that results were underpowered and conclusions should be drawn with caution.
In studies undertaken in the metastatic setting, HER2 status has unavoidably often been assessed on the primary tumour, which differs temporally, topologically, and potentially biologically from the metastatic tumour (Dowsett et al. 2001). This makes it difficult to extrapolate any firm conclusion about the response of tumour in the metastatic sites. It is of particular note that approximately 20% of tumours, which are initially negative for HER2, can become positive over time as they progress, mainly following endocrine treatment (Gutierrez et al. 2005, Priedigkeit et al. 2017, 2021). This upregulation driven by ER blocking can be explained by the ability of ER to downregulate HER2 (Hurtado et al. 2008). In addition, ERBB2 mutations have been acquired in ER+ metastatic breast cancer under the selective pressure of endocrine therapies resulting in treatment resistance (Razavi et al. 2018, Nayar et al. 2019, Bose & Ma 2021). A combination of endocrine therapies with the irreversible pan-HER tyrosine kinase inhibitor, neratinib, could overcome this resistance (Hyman et al. 2017, Nayar et al. 2019).
In an attempt to overcome the inconsistency between a number of the studies, De Laurentiis et al. conducted a meta-analysis of eight clinical trials including more than 1,900 patients with ER+ or ER-unknown disease to get an overall pooled estimate of the correlation between HER2 overexpression and the response to endocrine treatment. Overall, the pooled estimate of relative risk for all studies was 1.45 (95% CI, 1.34–1.57; P < 0.00001), indicating a significant association between HER2 overexpression and treatment failure. The test for heterogeneity (P = 0.27) showed that the differences among individual studies may be explained by chance and that combining data was an appropriate method. Interestingly, the interaction between HER2 overexpression and treatment failure was retained despite the endocrine therapy choice. The relative risk was 1.48 (95% CI, 1.29–1.70; P < 0.00001; test for heterogeneity = 0.09) for studies pertaining to tamoxifen, and 1.43 (95% CI, 1.30–1.58; P < 0.00001; test for heterogeneity = 0.64) for studies involving other endocrine drugs. The results suggested that patients with metastatic breast cancer and HER2 overexpression are relatively less responsive to endocrine therapies than patients in whom HER2 is not overexpressed. Importantly, however, because of the lack of a control arm, in which a therapeutic intervention was not administered, the more rapid progression may, at least to a certain degree, reflect a more aggressive inherent behaviour of the HER2-overexpressing tumours (De Laurentiis et al. 2005).
Neoadjuvant endocrine therapy
The neoadjuvant setting presents the advantage that, unlike the adjuvant setting, the primary tumour is still in place as the treatment continues, and clinical efficacy outcomes can be captured by measurement of changes in tumour volume. Neoadjuvant studies are less time-consuming, and assessment of HER2 and ER status can directly be performed in the lesion being treated and in which response is measured (Ring & Dowsett 2003). Moreover, matched sequential tumour samples can be taken over time, allowing the study of dynamic biological changes during treatment (Dixon 2014). Characterising the molecular response to the treatment multiple times can be an important factor for a more accurate stratification of patients and subsequently for a more effective therapeutic decision making (Selli & Sims 2019). These data provide a unique opportunity to compare molecular and clinical determinants of early response and resistance throughout treatment as well as changes in molecular features that may determine endocrine responsiveness.
Because of the difference in pharmacological activity among types of endocrine therapy, it has been suggested in a neoadjuvant study that the choice of therapeutic agent might affect the responsiveness of HER2-overexpressing tumours (Ellis et al. 2001). Results of studies undertaken in the neoadjuvant setting are less disputable than those in the metastatic setting and offer firm evidence for the role of HER2 in tamoxifen resistance (Table 2). Overall, patients with ER+/HER2+ tumours have a poorer clinical response to tamoxifen than those with ER+/HER2− tumours, while they remain responsive to AIs (Ellis et al. 2001, 2003, 2006, Dixon et al. 2003, Zhu et al. 2004, Smith et al. 2005). Ellis et al. reported a study investigating the association between HER2 and EGFR protein expression by IHC, with response to tamoxifen over AIs. Patients, who were HR+ and overexpressing HER2 and/or EGFR, had a significantly greater clinical response to letrozole than to tamoxifen (88% vs 21%; P = 0.0004), while no significant difference was observed in ER+ and HER2− patients (54% vs 42%; P = 0.078). Moreover, rather than letrozole’s effects being diminished in HER2+ cases, letrozole was significantly more effective in patients overexpressing HER2 and/or EGFR compared to those that were negative for both receptors (88% vs 54%; P = 0.018) (Ellis et al. 2001). An update and expansion of the above study were presented by Ellis et al. in 2006 using FISH analysis to confirm HER2 status (Ellis et al. 2006). In contrast to the earlier report, letrozole was clinically effective in the 202 patients with ER+ tumours, irrespective of the HER2 status (71% in both subsets; P = 0.98), suggesting that they are sensitive to short-term oestrogen deprivation therapy. When ER+ tumours with HER2 gene amplification were treated with tamoxifen, the point estimate for the clinical response was poorer in HER2+ disease than in HER2− tumours, but this difference did not approach statistical significance (33% vs 49%; P = 0.49) (Ellis et al. 2006). Clinical findings of the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) study suggested that HER2 overexpression was associated with poorer response in tamoxifen- but not in anastrozole-treated patients (22% vs 58%; P = 0.18).
Table 2.
Summary of neoadjuvant trials investigating HER2 as a predictor of response to endocrine therapy for early breast cancer.
| Number of patients | HER2+ patients | HER2 assessment | Cut-off | Treatment | Treatment duration | Endpoint | Response (%) | Conclusion | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2+ patients | HER2− patients | P-value | |||||||||||
| HER2+ vs HER2− comparison | Treatment comparison in HER2+ | Treatment comparison in HER2− | |||||||||||
| 237a | 36 (15.2%)b | IHC | 2+/3+ | Tamoxifen | 4 months | CR | 21 | 42 | P = 0.095 | P = 0.0004 | P = 0.078 | HER2 was associated with poorer CR in tamoxifen-treated patients, but not in letrozole-treated patients. | a |
| Letrozole | 88 | 54 | P = 0.018 | ||||||||||
| 22a | 6 (27.3%) | IHC | 3+ | Anastrozole | 4 months | CR | 100 | 94 | P > 0.05 | NR | NR | HER2 was not associated with poorer CR in anastrozole-treated patients | b |
| 185a | 32 (17.3%) | IHC | 2+/3+ | Tamoxifen | 4 months | CR | 17 | 41 | NR | P = 0.0002 | P = 0.0534 | HER2 was associated with poorer CR in tamoxifen-treated patients, but not in letrozole-treated patients. | c |
| Letrozole | 87 | 56 | NR | ||||||||||
| 36a | 16 (44.4%) | FISH | >2 HER2-to-chromosome 17 centromere ratio signals | Exemestane/letrozole | 3 months | CR | 75 | 35 | P = 0.017 | NR | NR | HER2 was associated with higher CR in AI-treated patients. | d |
| 305c | 28 (9.2%) | FISH | >2 HER2-to-chromosome 17 centromere ratio signals | Tamoxifen | 4 months | CR | 33 | 49 | P = 0.49 | P = 0.1 | P = 0.0004 | HER2 was associated with poorer CR in tamoxifen-treated patients, but not in letrozole-treated patients. | e |
| Letrozole | 71 | 71 | P = 0.98 | ||||||||||
Clinical response is referred to a reduction in tumour volume.
aHR-positive, bHER2-positive or EGFR-positive, cER-positive; a (Ellis et al. 2001), b (Dixon et al. 2003), c (Ellis et al. 2003), d (Zhu et al. 2004), e (Ellis et al. 2006).
AI, aromatase inhibitor; CR, clinical response; EGFR, EGF receptor; ER, oestrogen receptor; FISH, fluorescent in situ hybridisation; HER2, human EGF receptor-2; HR, hormone receptor; IHC, immunohistochemistry; NR, not reported.
Besides clinical response, the effect of endocrine therapy on proliferation was used as a biological endpoint in neoadjuvant clinical studies (Table 3) (Dowsett et al. 2001, 2005a , Ellis et al. 2003, 2006, Dixon et al. 2004, Bliss et al. 2017). One of these studies showed that ER+/HER2+ tumours showed much less suppression of proliferation during AI treatment (Ki67 suppression: 45% vs 89.1%; P = 0.0001), even when a clinical response was observed (Ellis et al. 2006). In the IMPACT study, in which biological efficacy was assessed using the nuclear proliferation antigen Ki67, suppression of Ki67 was significantly greater in HER2− tumours compared to those overexpressing HER2 following either tamoxifen or anastrozole, but not the combination treatment (Dowsett et al. 2005a ). The finding that tamoxifen-treated patients showed poorer clinical response could be explained by the reduced levels of apoptosis alongside Ki67 suppression. Interestingly, anastrozole still shrinks the tumour even when the levels of apoptosis are low (Dowsett et al. 2005a ). These data suggest that clinical efficacy following endocrine treatment occurs following both decreased proliferation and maintained rate of apoptosis. Nevertheless, it is hard to study the effect of apoptosis in tumour regression following neoadjuvant endocrine treatment because only a few tumour cells are stained positive for apoptotic markers at baseline and they are not obviously modulated by the treatment (Dowsett et al. 2006). Despite the fact that the number of patients included in this analysis was small, the results agree with the findings of the letrozole study (Ellis et al. 2006). Although HER2 overexpression did not reduce the clinical benefit of neoadjuvant treatment with AIs, it was related to higher tumour proliferation before and during treatment than HER2− tumours.
Table 3.
Clinical neoadjuvant studies of HER2 in predicting Ki67 changes in response to endocrine therapy.
| Number of patients | HER2+ patients | HER2 assessment | Cut-off | Treatment | Endpoint | Response (%) | Conclusion | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HER2+ patients | HER2- patients | P-value | ||||||||
| 115a | 15 (13%) | IHC | 2+/3+ | SERM (tamoxifen/idoxifene) or AI (anastrozole/vorozole) | Ki67B | 27.7 | 11.5 | P = 0.003 | HER2 was associated with high baseline Ki67 and small Ki67 change. | a |
| Ki672w-B | 25 | 62 | P = 0.014 | |||||||
| Ki6712w-B | 29 | 71 | P = 0.047 | |||||||
| 22b | 6 (27.3%) | IHC | 3+ | Anastrozole | Ki67 reduction | 67 | 80 | P > 0.05 | HER2 was not associated with smaller Ki67 changes in anastrozole-treated patients. | b |
| 232a | 34 (14.7%) | IHC | 3+ | Tamoxifen+anastrozole | Ki672w-B | NR | NR | P < 0.05 | HER2 was associated with smaller Ki67 changes in anastrozole- and tamoxifen-treated patients. | c |
| Ki6712w-B | NR | NR | P < 0.05 | |||||||
| 297a | 26 (8.8%) | FISH | >2 HER2-to-chromosome 17 centromere ratio signals | Tamoxifen | Ki67 reduction | 5.8 | 77.7 | P = 0.0925 | HER2 was associated with smaller Ki67 changes in tamoxifen- and letrozole-treated patients. | d |
| Letrozole | 45 | 89.1 | P = 0.0001 | |||||||
| 3861a | 402 (10.4%) | IHC | 3+ | Anastrozole/letrozole | Ki67B | 26.8 | 14.3 | P < 0.001 | HER2 was associated with high baseline Ki67 and small Ki67 change. | e |
| Ki672w-B | 52.9 | 79.2 | P < 0.001 | |||||||
aER-positive, bHR-positive; a (Dowsett et al. 2001), b (Dixon et al. 2004), c (Dowsett et al. 2005), d (Ellis et al. 2006), e (Bliss et al. 2017).
AI, aromatase inhibitor; ER, oestrogen receptor; FISH, fluorescent in situ hybridisation; HER2, human EGF receptor-2; HR, hormone receptor; IHC, immunohistochemistry; Ki67B, Ki67 at baseline; Ki672w-B, Ki67 reduction after 2 week-treatment; Ki6712w-B, Ki67 reduction after 12 week-treatment; NR, not reported; SERM, selective oestrogen receptor modulator.
Endocrine therapy in the adjuvant setting
Several clinical studies have investigated whether HER2 protein overexpression or gene amplification influences the benefit of endocrine therapy in early-stage breast cancer in the adjuvant setting (Table 4) (Borg et al. 1994, Carlomagno et al. 1996, Berry et al. 2000, De Placido et al. 2003, Love et al. 2003, Dowsett et al. 2008, Rasmussen et al. 2008). Most of these studies suggested that early breast cancer patients with HER2-overexpressing tumours get less benefit from adjuvant tamoxifen than those with HER2− tumours and have an increased risk of failing tamoxifen treatment (Borg et al. 1994, Carlomagno et al. 1996, Hu & Mokbel 2001, De Placido et al. 2003, Dowsett et al. 2005b, 2008, Rasmussen et al. 2008). For example, an analysis of the Gruppo Universitario Napoletano 1 study concluded that tamoxifen was effective in reducing the hazard ratio of death among HER2− patients, while in contrast had rather a detrimental effect in ER+ patients with HER2-overexpressing tumours (0.73 vs 1.33, respectively; interaction test: P = 0.038) (De Placido et al. 2003). Nevertheless, only 91 of the 206 tamoxifen-treated patients were ER+ and/or PgR+ and only 58 had HER2 overexpression (De Placido et al. 2003), and the number of patients who were both ER+ and overexpressed HER2 was not reported in the paper, but it is likely to be low as a result of the inverse expression pattern of the two receptors (Ring & Dowsett 2003). As such, these findings should be interpreted critically.
Table 4.
Clinical trials investigating the role of HER2 on the response to adjuvant endocrine therapy.
| Number of patients | HER2+ patients | HER2 assessment | Cut-off | Treatment | Endpoint | Response (%) | Conclusion | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2+ patients | HER2− patients | P-value | ||||||||||
| HER2+ vs HER2- comparison | Treatment comparison in HER2+ | Treatment comparison in HER2- | ||||||||||
| 871 | 175 (20.1%) | Southern blot | 2–30 gene copies | Tamoxifen | OS | 46 | 67 | P < 0.0001 | NR | NR | HER2 was associated with poorer OS in tamoxifen-treated patients. | a |
| Western blot | 100-3440 arbitrary units | Control | 59 | 66 | P = 0.34 | |||||||
| 145 | 43 (29.7%) | IHC | ≥10% of cells | Tamoxifen | OS | 57 | 86 | NR | P = 0.03 | P = 0.09 | HER2 was not associated with poorer DFS and OS in tamoxifen-treated patients. | b |
| Control | 82 | 68 | ||||||||||
| Tamoxifen | DFS | 51 | 82 | NR | P = 0.3 | P = 0.003 | ||||||
| Control | 63 | 54 | ||||||||||
| 651a | 12–24%b | IHC | ≥50% of cells | Tamoxifen (compared to control) | OS (reduction in death) | 30 | 36 | NR | P = 0.18 | P = 0.0037 | HER2 was not associated with poorer DFS and OS in tamoxifen-treated patients. | c |
| PCR | ≥2 gene copies | |||||||||||
| FISH | ≥2 HER2-to-chromosome 17 centromere ratio signals | DFS (reduction in RR) | 32 | 39 | NR | P = 0.12 | P = 0.0001 | |||||
| 3533a | 239 (6.8%) | FISH | ≥2 HER2-to-chromosome 17 centromere ratio signals | Tamoxifen | DFS | 70 | 86 | NR | P < 0.0001 | NR | HER2 was associated with poorer DFS in tamoxifen-treated patients. Letrozole improved DFS compared with tamoxifen regardless of HER2 status. | d |
| Letrozole | 79 | 90 | P = 0.60 | |||||||||
| 1782a | 187 (10.5%) | IHC | 3+ | Tamoxifen | Recurrence rate | 18.8 | 9 | P = 0.0018 | NR | NR | HER2 was associated with shorter TTR in both tamoxifen- and anastrozole-treated patients. | e |
| Anastrozole | 19.8 | 5.9 | P < 0.0001 | |||||||||
aER-positive, bdepending on method used to assess HER2 status; a (Borg et al. 1994), b (Carlomagno et al. 1996), c (Berry et al. 2000), d (Rasmussen et al. 2008), e (Dowsett et al. 2008).
DFS, disease-free survival; ER, oestrogen receptor; FISH, fluorescent in situ hybridisation; HER2, human EGF receptor-2; IHC, immunohistochemistry; NR, not reported; OS, overall survival; PCR, PCR; RR, risk of recurrence; TTR, time to recurrence.
On the other hand, there are a few conflicting studies indicating no significant difference in disease-free and overall survival with adjuvant tamoxifen based on HER2 status (Berry et al. 2000, Love et al. 2003). One of these is the Cancer and Leukaemia Group B 8541 trial, which reported that patients with HER2+ disease, who received tamoxifen, had a 32 and 30% reduction in disease recurrence risk and death, respectively, compared to patients not receiving tamoxifen, a benefit not substantially less than the equivalent 39 and 36% seen in patients with HER2− disease. It should be noted though that the non-randomised nature of the study may have introduced a bias in the selection of patients towards a more advanced stage of tumour in the tamoxifen-treated group. Moreover, all patients received adjuvant chemotherapy, which might itself provide a benefit in the outcome of patients with HER2+ tumours (Berry et al. 2000).
More recent studies have investigated whether the outcome of patients, who received adjuvant AIs, differs according to HER2 status. The Breast International Group (BIG) 1-98 trial assessed the effect of HER2 status on the benefit of tamoxifen and letrozole in early breast cancer patients (Rasmussen et al. 2008). Letrozole improved disease-free survival (DFS) compared to tamoxifen irrespective of HER2 (HER2-negative: HR, 0.72; 95% CI, 0.59–0.87; HER2-positive: HR, 0.62; 95% CI, 0.37–1.03), thus, HER2 status should not be deemed as a selection criterion for letrozole over tamoxifen treatment (Rasmussen et al. 2008). Another study was conducted in order to determine the relationship of HER2 status with time to recurrence (TTR) in postmenopausal women with HR+ primary breast cancer in the large, randomised the Arimidex, Tamoxifen, alone or in combination (ATAC) adjuvant trial. Shorter TTR was observed in patients with HER2+ disease, who were treated with either anastrozole or tamoxifen. For anastrozole, the recurrence rate at 5 years was 19.8 and 5.9% for patients with HER2+ and HER2- tumours, respectively (HR, 2.25; P = 0.0018). For tamoxifen, it was 18.8 and 9% for patients with HER2+ and HER2- tumours, respectively (HR, 3.27; P < 0.0001). The benefit of anastrozole did not differ from that of tamoxifen in the HER2+ cohort, but there were only 44 patients in the HER2+ group (Dowsett et al. 2008). With varying treatment schedules and different techniques being used to score tumours as positive for HER2 expression, it is difficult to compare the two studies. Observations from the ATAC trial are in discordance with data from the IMPACT neoadjuvant trial, in which anastrozole did improve clinical benefit in HER2-overexpressing patients (Dowsett et al. 2008). Therefore, despite the initial effective tumour response to neoadjuvant AIs in the majority of patients with ER+/HER2+ disease, continued proliferation could hint early resistance that may occur at later times in the clinical course of the disease.
To provide higher-level evidence for the suggested association between HER2 status and benefit from endocrine therapy, a meta-analysis was conducted combining data from three randomised trials (ATAC, BIG 1-98, and TEAM). In patients with HER2- tumours, an improved outcome was observed following treatment with upfront AI compared to tamoxifen, while patients with HER2+ tumours showed no difference or slightly worse outcome in the first 2–3 years. Nevertheless, it should be noted that a large degree of heterogeneity in the HER2+ groups across all trials might be attributable to the small number of patients with HER2+ tumours, as well as subtle differences between AIs (Bartlett et al. 2017). Another meta-analysis of recurrence risk reductions combining data from five comparisons of AIs vs tamoxifen demonstrated that AI treatment was advantageous compared to tamoxifen regardless of HER2 status (EBCTCG 2015).
All these studies were limited in terms of assessing the markers and/or determinants of treatment resistance because of the absence of accessible disease after surgery. To produce meaningful and statistically valid results, adjuvant studies need a sufficiently large number of patient samples. The inverse correlation between ER and HER2 expression leads to there being only a minority population, generally around 10% of the total, in studies that recruited ER+ patients irrespective of HER2 status (Ring & Dowsett 2003). Direct comparison and interpretation of the cited studies are further hindered by the use of varying doses and durations of endocrine treatment, various assays and cut-off points for the assessment of ER and HER2 positivity, and confounders such as chemotherapy. Additionally, studies involving adjuvant treatment are made more difficult by the long-term follow-up required to assess disease endpoints and the occurrence or non-occurrence of an event being affected by the intrinsic prognosis of the disease as well as its response or not to therapy (Larionov & Miller 2009).
Therapeutic strategies to combat endocrine resistance
Resistance of HR+/HER2+ breast cancer to endocrine therapy is heterogeneous and complex and may depend on the individual patient’s genetic background, the choice of endocrine therapy, and the type of resistance (Dixon 2014). There is convincing evidence that HER2 overexpression is a significant factor in endocrine resistance. However, stimulation of tumour growth is not solely the result of the crosstalk between ER and HER2, but rather the interaction of a more complex network (Arpino et al. 2008). Conceptually anti-HER2 therapies are given predominantly to target the drive that the tumour cells derive from overexpressed HER2. Such treatment may, however, antagonise HER2-dependent endocrine resistance directly as well as achieving a direct and independent effect on HER2-stimulated tumour growth. Understanding the underlying mechanisms would help to discover novel therapeutic agents and develop new strategies to overcome resistance. Historically, the majority of clinical trials assessing anti-HER2 therapies have not distinguished the ER status in HER2+ disease. Current guidelines recommend combination therapy of anti-HER2 agents including trastuzumab as the first-line treatment for HER2+ advanced breast cancer irrespective of HR status. Endocrine treatment could be limited to patients that are intolerant to chemotherapy or as an empirical maintenance strategy post-chemotherapy (Cardoso et al. 2020). Specifically, the use of tyrosine kinase inhibitors (e.g. lapatinib, tucatinib, neratinib, pyrotinib), monoclonal antibodies (e.g. trastuzumab, pertuzumab, margetuximab), chimeric antibodies with chemotherapeutic drugs, known as antibody-drug conjugates (e.g. trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (DS-8201), trastuzumab duocarmazine (SYD985)) or other signal transduction inhibitors could halt the molecular interaction with the HER2 pathway (Mitsogianni et al. 2021) (Fig. 2). The ExteNET trial showed that 1-year neratinib treatment improved invasive DFS administered after chemotherapy and trastuzumab-based adjuvant therapy to patients with early-stage HER2+ breast cancer. Subgroup analysis demonstrated that neratinib gave greater benefit to patients with HR+ breast cancer than to those with HR− breast cancer, suggesting that there might be a preferential effect of TKIs on targeting the interaction of ER and HER2 than does the continuation of trastuzumab with endocrine treatment (Chan et al. 2016). It is also possible that the effect of TKIs could be dependent on a complete blockade of HER2 signalling and given the average lower expression of HER2 in ER+/HER2+ than ER+/HER2− disease this may be more easily achieved in the former.
Several studies exploring the use of growth factor receptor inhibitors in combination with anti-oestrogen therapy have shown improved outcomes for patients with either metastatic or locally advanced ER+/HER2+ disease (Johnston et al. 2009, Kaufman et al. 2009, Li et al. 2018, Rimawi et al. 2018). For example, data from the randomised open-label TAnDEM phase-III trial suggested that the addition of trastuzumab to anastrozole contributed to significant improvement in PFS in patients with ER+/HER2+ tumours compared to anastrozole treatment alone (5.6 months vs 3.8 months; P < 0.006) (Kaufman et al. 2009). This combination treatment, however, can be accompanied by adverse effects (Kaufman et al. 2009, Li et al. 2018), while in some cases it did not show higher efficacy in a subset of patients (Marcom et al. 2007, Burstein et al. 2014, Loi et al. 2016).
A secondary analysis of the HERA trial showed that a subgroup of patients with ER+/HER2+ breast cancer, with lower HER2 FISH ratios or higher ESR1 mRNA expression, got less benefit from the addition of adjuvant trastuzumab following chemotherapy, with all these patients being given endocrine therapy. This observation indicates that the degree of HER2 amplification and ESR1 expression levels can affect the response to trastuzumab after chemotherapy in the ER+/HER2+ disease and may explain the heterogeneity in response to anti-HER2 agents in this subgroup (Loi et al. 2016). The lower amount of benefit seen in patients with high ER-expressing tumours is consistent with the observation in the recently published overview of trastuzumab trials in which ER+/PgR+ cases appear to receive less benefit than ER+/PgR− cases in which ER levels are lower and HER2 levels are higher (Arpino et al. 2005, EBCTCG 2021). Furthermore, AIB1 overexpression has been associated with tamoxifen resistance in hormone-responsive HER2+ breast cancers, suggesting that AIB1 could be a potential therapeutic target (Osborne et al. 2003, Kirkegaard et al. 2007).
Potential additional benefit of the utilisation of other therapeutic approaches is currently undergoing clinical investigation, such as the synergistic combination of endocrine therapy plus dual HER2-targeted therapy and a CDK4/6 inhibitor in patients with ER+/HER2+ metastatic disease (Krause et al. 2019). Recently, data from the prospective, open-label, multicentre phase-II SOLTI-1303 PATRICIA trial showed that palbociclib in combination with trastuzumab is safe and results in longer PFS in trastuzumab pre-treated ER+/HER2+ advanced breast cancer with a PAM50 luminal subtype (12.4 months vs 4.1 months; P = 0.025) (Ciruelos et al. 2020). A number of studies focussed on investigating the treatment of patients with ER+/HER2-low (HER2 IHC 1+/2+, FISH negative) breast cancer, which is not effectively treated with first-generation anti-HER2 agents, such as trastuzumab. The addition of zenocutuzumab (MCLA-128) to endocrine therapy in ER+/HER2-low metastatic breast cancer patients, who had progressed on endocrine therapy and CDK4/6 inhibitors, resulted in a rescue of endocrine sensitivity of 17% (Pistilli et al. 2020). The combination of DS-8201 with anastrozole or fulvestrant is currently being assessed in patients with ER+/HER2-low metastatic breast cancer in the DESTINY-Breast08 phase Ib trial (Jhaveri et al. 2021).
Conclusion
A number of clinical trials have investigated the role of HER2 on the resistance to endocrine therapy over the last decades. In some cases, the results from these studies should be interpreted with caution because of the limited number of patients with ER and HER2 co-expression, the different endocrine therapies administered, and the range of techniques used for the detection of HER2 expression. However, data have given useful prognostic information in the neoadjuvant, adjuvant, and metastatic settings. Metastatic ER+/HER2+ breast cancers appear to be less responsive to both tamoxifen and AIs than ER+/HER2− disease. Evidence from adjuvant and neoadjuvant studies supports the idea that patients with ER+/HER2+ disease may benefit from AIs more than tamoxifen in the same way as ER+/HER2− tumours. Clinical studies suggest that continued proliferation even in the presence of clinical response following neoadjuvant AI treatment could indicate resistance that could be developed at a later stage. The sensitivity of biological endpoints highlights the importance of introducing them into clinical practice, which alongside clinical outcome, could portend a better prognosis. Selection of biomarkers, such as Ki67, to stratify patients into clinically distinctive groups in a move towards personalised therapy will aid to improve poor responsiveness to anti-oestrogen therapies in patients with ER+/HER2+ tumours.
In the future, analyses of circulating tumour DNA may enable the tracking of the response of subclinical disease to anti-HER2 with or without anti-ER treatment, and thereby aid both clinical management of patients and provide improved data for understanding the interaction of ER and HER2 signalling in patients. Other potential biomarkers, such as gene expression signatures, DNA mutations, and the tumour immune microenvironment, could predict the responsiveness of patients with ER+/HER2+ disease to endocrine therapies (Dieci et al. 2020). Determining the most suitable way to monitor response is thus of paramount importance.
The nature of anti-oestrogen therapy resistance in patients with HER2 overexpressing tumours is relative rather than absolute, therefore this therapeutic option should not necessarily be withheld. Ongoing and future clinical trials will evaluate the potential and applicability of combining endocrine therapy with growth factor inhibitors or CDK4/6 inhibitors to overcome intrinsic resistance and prevent or delay acquired resistance in patients with ER+/HER2+ breast cancer.
Declaration of interest
A A has no competing interests. M D served on advisory boards in Radius, G1 therapeutics, AbbVie, Zentalis, H3 Biomedicine; received lecture fees from Nanostring, Myriad, Lilly.
Funding
This work was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London. We also thank Breast Cancer Now for funding this work as part of Programme Funding to the Breast Cancer Now Toby Robins Research Centre. A A was supported by a grant from Le Cure Breast Cancer Research Fund. The funding bodies did not have a role in the design, analysis or interpretation of this review.
Availability of data and materials
Not applicable as no datasets were generated during this study.
Ethics approval and consent to participate
Not applicable as this review does not contain any studies with human patients or animals performed by any of the authors.
Author contribution statement
A A searched and evaluated all relevant literature and wrote the manuscript. M D conceived the topic idea, supervised the search strategy, and reviewed and revised the manuscript drafts. All authors read and approved the final manuscript.
References
- Ahn S, Woo JW, Lee K, Park SY.2020HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. Journal of Pathology and Translational Medicine 5434–44. ( 10.4132/jptm.2019.11.03) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SG, Eliopoulos A, Spandidos D, Barnes D, Ellis IO, Blamey RW, Nicholson RI, Robertson JFR.1995Expression of ras p21, p53 and c-erbB-2 in advanced breast cancer and response to first line hormonal therapy. British Journal of Cancer 721259–1266. ( 10.1038/bjc.1995.497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM.2005Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. Journal of the National Cancer Institute 971254–1261. ( 10.1093/jnci/dji249) [DOI] [PubMed] [Google Scholar]
- Arpino G, Wiechmann L, Osborne CK, Schiff R.2008Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocrine Reviews 29217–233. ( 10.1210/er.2006-0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey ST, Westerling T, Brown M.2015Loss of estrogen-regulated microRNA expression increases HER2 signaling and is prognostic of poor outcome in luminal breast cancer. Cancer Research 75436–445. ( 10.1158/0008-5472.CAN-14-1041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JMS, Ahmed I, Regan MM, Sestak I, Mallon EA, Dell’Orto P, Thürlimann B, Seynaeve C, Putter H, Van de Velde CJHet al. 2017HER2 status predicts for upfront AI benefit: a trans-AIOG meta-analysis of 12,129 patients from ATAC, BIG 1–98 and TEAM with centrally determined HER2. European Journal of Cancer 79129–138. ( 10.1016/j.ejca.2017.03.033) [DOI] [PubMed] [Google Scholar]
- Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK.1992Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Research and Treatment 2485–95. ( 10.1007/BF01961241) [DOI] [PubMed] [Google Scholar]
- Berry DA, Muss HB, Thor AD, Dressler L, Liu ET, Broadwater G, Budman DR, Henderson IC, Barcos M, Hayes Det al. 2000HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. Journal of Clinical Oncology 183471–3479. ( 10.1200/JCO.2000.18.20.3471) [DOI] [PubMed] [Google Scholar]
- Bliss J, Morden J, Evans A, Holcombe C, Horgan K, Mallon E, Raghavan V, Skene A, Dodson A, Hills Met al. 2017Clinico-pathological relationships with Ki67 in POETIC (CRUK/07/015) – critical lessons for assessing Ki67 for prognosis and as a pharmacodynamic marker.Cancer Research 77 (4_Suppl) Abstract P2-05-01. ( 10.1158/1538-7445.SABCS16-P2-05-01) [DOI] [Google Scholar]
- Borg A, Baldetorp B, Ferno M, Killander D, Olsson H, Ryden S, Sigurdsson H.1994ERBB2 amplification is associated with tamoxifen resistance in steroid-receptor positive breast cancer. Cancer Letters 81137–144. ( 10.1016/0304-3835(9490194-5) [DOI] [PubMed] [Google Scholar]
- Bose R, Ma CX.2021Breast cancer, HER2 mutations , and overcoming drug resistance. New England Journal of Medicine 3851241–1243. ( 10.1056/NEJMcibr2110552) [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Cirrincione CT, Barry WT, Chew HK, Tolaney SM, Lake DE, Ma C, Blackwell KL, Winer EP, Hudis CA.2014Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: a randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer-CALGB 40302 (Alliance). Journal of Clinical Oncology 323959–3966. ( 10.1200/JCO.2014.56.7941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli Let al. 20205th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Annals of Oncology 311623–1649. ( 10.1016/j.annonc.2020.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno C, Perrone F, Gallo C, De Laurentiis M, Lauria R, Morabito A, Pettinato G, Panico L, D’Antonio A, Bianco ARet al. 1996c-erbB2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. Journal of Clinical Oncology 142702–2708. ( 10.1200/JCO.1996.14.10.2702) [DOI] [PubMed] [Google Scholar]
- Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, von Minckwitz Get al. 2016Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncology 17367–377. ( 10.1016/S1470-2045(1500551-3) [DOI] [PubMed] [Google Scholar]
- Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH.2002Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. International Journal of Cancer 97306–312. ( 10.1002/ijc.1614) [DOI] [PubMed] [Google Scholar]
- Ciruelos E, Villagrasa P, Pascual T, Oliveira M, Pernas S, Paré L, Escrivá-De-Romaní S, Manso L, Adamo B, Martínez Eet al. 2020Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clinical Cancer Research 265820–5829. ( 10.1158/1078-0432.CCR-20-0844) [DOI] [PubMed] [Google Scholar]
- Colleoni M, Montagna E.2012Neoadjuvant therapy for ER-positive breast cancers. Annals of Oncology 23 (Supplement 10) x243–x248. ( 10.1093/annonc/mds305) [DOI] [PubMed] [Google Scholar]
- D’Souza A, Spicer D, Lu J.2018Overcoming endocrine resistance in metastatic hormone receptor-positive breast Cancer. Journal of Hematology and Oncology 1180. ( 10.1186/s13045-018-0620-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D’Agostino D, Caputo F, Cancello Get al. 2005A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clinical Cancer Research 114741–4748. ( 10.1158/1078-0432.CCR-04-2569) [DOI] [PubMed] [Google Scholar]
- De Placido S, De Laurentiis M, Carlomagno C, Gallo C, Perrone F, Pepe S, Ruggiero A, Marinelli A, Pagliarulo C, Panico Let al. 2003Twenty-year results of the Naples GUN randomized trial: predictive factors of adjuvant tamoxifen efficacy in early breast cancer. Clinical Cancer Research 91039–1046. [PubMed] [Google Scholar]
- Dieci MV, Miglietta F, Griguolo G, Guarneri V.2020Biomarkers for HER2-positive metastatic breast cancer: beyond hormone receptors. Cancer Treatment Reviews 88 102064. ( 10.1016/j.ctrv.2020.102064) [DOI] [PubMed] [Google Scholar]
- DiGiovanna MP, Carter D, Flynn SD, Stern DF.1996Functional assay for HER-2/neu demonstrates active signalling in a minority of HER-2/neu-overexpressing invasive human breast tumours. British Journal of Cancer 74802–806. ( 10.1038/bjc.1996.439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JM.2014Endocrine resistance in breast cancer. New Journal of Science 20141–27. ( 10.3109/13697137.2013.864268) [DOI] [Google Scholar]
- Dixon JM, Jackson J, Renshaw L, Miller WR.2003Neoadjuvant tamoxifen and aromatase inhibitors: comparisons and clinical outcomes. Journal of Steroid Biochemistry and Molecular Biology 86295–299. ( 10.1016/S0960-0760(0300370-4) [DOI] [PubMed] [Google Scholar]
- Dixon JM, Jackson J, Hills M, Renshaw L, Cameron DA, Anderson TJ, Miller WR, Dowsett M.2004Anastrozole demonstrates clinical and biological effectiveness in oestrogen receptor-positive breast cancers, irrespective of the erbB2 status. European Journal of Cancer 402742–2747. ( 10.1016/j.ejca.2004.08.025) [DOI] [PubMed] [Google Scholar]
- Dodson A, Parry S, Ibrahim M, Bartlett JM, Pinder S, Dowsett M, Miller K.2018Breast cancer biomarkers in clinical testing: analysis of a UK national external quality assessment scheme for immunocytochemistry and in situ hybridisation database containing results from 199 300 patients. Journal of Pathology: Clinical Research 4262–273. ( 10.1002/cjp2.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Harper-Wynne C, Boeddinghaus I, Salter J, Hills M, Dixon M, Ebbs S, Gui G, Sacks N, Smith I.2001HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Research 618452–8458. [PubMed] [Google Scholar]
- Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley Set al. 2005aBiomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer – a study from the IMPACT trialists. Journal of Clinical Oncology 232477–2492. ( 10.1200/JCO.2005.07.559) [DOI] [PubMed] [Google Scholar]
- Dowsett M, Johnston S, Martin LA, Salter J, Hills M, Detre S, Gutierrez MC, Mohsin SK, Shou J, Allred DCet al. 2005bGrowth factor signalling and response to endocrine therapy: the Royal Marsden experience. Endocrine-Related Cancer 12 (Supplement 1) S113–S117. ( 10.1677/erc.1.01044) [DOI] [PubMed] [Google Scholar]
- Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills Met al. 2006Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clinical Cancer Research 121024s–1030s. ( 10.1158/1078-0432.CCR-05-2127) [DOI] [PubMed] [Google Scholar]
- Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon Eet al. 2008Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the arimidex, tamoxifen, alone or in combination trial. Journal of Clinical Oncology 261059–1065. ( 10.1200/JCO.2007.12.9437) [DOI] [PubMed] [Google Scholar]
- Dunbier AK, Ghazoui Z, Anderson H, Salter J, Nerurkar A, Osin P, A’hern R, Miller WR, Smith IE, Dowsett M.2013Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clinical Cancer Research 192775–2786. ( 10.1158/1078-0432.CCR-12-1000) [DOI] [PubMed] [Google Scholar]
- EBCTCG 1998Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 3511451–1467. ( 10.1016/S0140-6736(9711423-4) [DOI] [PubMed] [Google Scholar]
- EBCTCG 2015Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 3861341–1352. ( 10.1016/S0140-6736(1561074-1) [DOI] [PubMed] [Google Scholar]
- EBCTCG 2021Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet: Oncology 221139–1150. ( 10.1016/S1470-2045(2100288-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge RM, Green S, Ciocca D, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, O’Sullivan J, Martino Set al. 1998HER-2 expression and response to tamoxifen in estrogen receptor-positive breast cancer: a Southwest Oncology Group study. Clinical Cancer Research 47–12. [PubMed] [Google Scholar]
- Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-cussac A, Jänicke F, Miller WR, Evans DB, Dugan M, Brady Cet al. 2001Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. Journal of Clinical Oncology 193808–3816. ( 10.1200/JCO.2001.19.18.3808) [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Jänicke F, Mauriac L, Quebe-Fehling E, Chaudri-Ross HA, Evans DBet al. 2003Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Research 636523–6531. [PubMed] [Google Scholar]
- Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, Renshaw L, Faratian D, Thomas J, Dowsett Met al. 2006Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. Journal of Clinical Oncology 243019–3025. ( 10.1200/JCO.2005.04.3034) [DOI] [PubMed] [Google Scholar]
- Evans AH, Pancholi S, Farmer I, Thornhill A, Evans DB, Johnston SR, Dowsett M, Martin LA.2010EGFR/HER2 inhibitor AEE788 increases ER-mediated transcription in HER2/ER-positive breast cancer cells but functions synergistically with endocrine therapy. British Journal of Cancer 1021235–1243. ( 10.1038/sj.bjc.6605641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN.2007Overview of resistance to systemic therapy in patients with breast cancer. Advances in Experimental Medicine and Biology 6081–22. ( 10.1007/978-0-387-74039-3_1) [DOI] [PubMed] [Google Scholar]
- Goutsouliak K, Veeraraghavan J, Sethunath V, De Angelis C, Osborne CK, Rimawi MF, Schiff R.2020Towards personalized treatment for early stage HER2-positive breast cancer. Nature Reviews: Clinical Oncology 17233–250. ( 10.1038/s41571-019-0299-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradishar WJ.2004Tamoxifen – what next? Oncologist 9378–384. ( 10.1634/theoncologist.9-4-378) [DOI] [PubMed] [Google Scholar]
- Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M.2005Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. Journal of Clinical Oncology 232469–2476. ( 10.1200/JCO.2005.01.172) [DOI] [PubMed] [Google Scholar]
- Hayes DF, Yamauchi H, Broadwater G, Cirrincione CT, Rodrigue SP, Berry DA, Younger J, Panasci LL, Millard F, Duggan DBet al. 2001Circulating HER-2/erbB-2/c-neu (HER-2) extracellular domain as a prognostic factor in patients with metastatic breast cancer: Cancer and Leukemia Group B Study 8662. Clinical Cancer Research 72703–2711. [PubMed] [Google Scholar]
- Houston SJ, Plunkett TA, Barnes DM, Smith P, Rubens RD, Miles DW.1999Overexpression of c-erbB2 is an independent marker of resistance to endocrine therapy in advanced breast cancer. British Journal of Cancer 791220–1226. ( 10.1038/sj.bjc.6690196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JCC, Mokbel K.2001Does c-erbB2/HER2 overexpression predict adjuvant tamoxifen failure in patients with early breast cancer? European Journal of Surgical Oncology 27335–337. ( 10.1053/ejso.2000.1078) [DOI] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS.2008ERBB2 regulation by estrogen receptor-Pax2 determines tamoxifen response. Nature 456663–666. ( 10.1038/nature07483.ERBB2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman D, Piha-Paul S, Saura C, Arteaga C, Mayer I, Shapiro G, Loi S, Lalani A, Xu F, Cutler Ret al. 2017Neratinib + fulvestrant in ERBB2-mutant, HER2–non-amplified, estrogen receptor (ER)-positive, metastatic breast cancer (MBC): preliminary analysis from the phase II SUMMIT trial. Cancer Research 77 (4_Suppl) Abstract PD2-08. ( 10.1158/1538-7445.SABCS16-PD2-08) [DOI] [Google Scholar]
- Jhaveri K, Hamilton E, Loi S, Schmid P, Darilay A, Gao C, Patel G, Wrona M, Andre F.2021Trastuzumab deruxtecan (T-DXd; DS-8201) in combination with other anticancer agents in patients with HER2-low metastatic breast cancer: a phase 1b, open-label, multicenter, dose-finding and dose-expansion study (DESTINY-Breast08). Cancer Research 81 (4_Suppl) Abstract OT-03-05. ( 10.1158/1538-7445.SABCS20-OT-03-05) [DOI] [Google Scholar]
- Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJet al. 2009Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor – positive metastatic breast cancer. Journal of Clinical Oncology 275538–5546. ( 10.1200/JCO.2009.23.3734) [DOI] [PubMed] [Google Scholar]
- Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova Aet al. 2009Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. Journal of Clinical Oncology 275529–5537. ( 10.1200/JCO.2008.20.6847) [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, McGlynn LM, Campbell FM, Müller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JMS.2007Amplified in breast cancer 1 in human epidermal growth factor receptor – positive tumors of tamoxifen-treated breast cancer patients. Clinical Cancer Research 131405–1411. ( 10.1158/1078-0432.CCR-06-1933) [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JMW, Harper ME, Barrow D, Wakeling AE, Nicholson RI.2003Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 1441032–1044. ( 10.1210/en.2002-220620) [DOI] [PubMed] [Google Scholar]
- Krause S, Friedl T, Fehm T, Romashova T, Fasching P, Schneeweiss A, Müller V, Taran F-A, Polasik A, Tzschaschel Met al. 2019DETECT V/CHEVENDO – comparison of dual HER2-targeted therapy with trastuzumab plus pertuzumab in combination with chemo- or endocrine therapy in addition with CDK4/6 inhibition in patients with HER2-positive and hormone-receptor positive metastatic breast. Cancer Research 79 (4_Suppl) Abstract OT2-07-01. ( 10.1158/1538-7445.SABCS18-OT2-07-01) [DOI] [Google Scholar]
- Larionov AA, Miller WR.2009Challenges in defining predictive markers for response to endocrine therapy in breast cancer. Future Oncology 51415–1428. ( 10.2217/fon.09.113) [DOI] [PubMed] [Google Scholar]
- Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, Harvey H, Demers L, Lipton A.1995Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. Journal of Clinical Oncology 131129–1135. ( 10.1200/JCO.1995.13.5.1129) [DOI] [PubMed] [Google Scholar]
- Li H, Zhai Q, Yu B.2018The clinical benefit of epidermal growth factor receptor and human epidermal growth factor receptor 2 targeted agents adding to endocrine therapy in hormone receptor-positive breast cancer. Journal of Cancer Research and Therapeutics 14S218–S223. ( 10.4103/0973-1482.183190) [DOI] [PubMed] [Google Scholar]
- Lipton A, Ali SM, Leitzel K, Demers L, Chinchilli V, Engle L, Harvey HA, Brady C, Nalin CM, Dugan Met al. 2002Elevated serum HER-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. Journal of Clinical Oncology 201467–1472. ( 10.1200/JCO.2002.20.6.1467) [DOI] [PubMed] [Google Scholar]
- Lipton A, Ali SM, Leitzel K, Demers L, Harvey HA, Chaudri-Ross HA, Brady C, Wyld P, Carney W.2003Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. Journal of Clinical Oncology 211967–1972. ( 10.1200/JCO.2003.09.098) [DOI] [PubMed] [Google Scholar]
- Loi S, Dafni U, Karlis D, Polydoropoulou V, Young BM, Willis S, Long B, De Azambuja E, Sotiriou C, Viale Get al. 2016Effects of estrogen receptor and human epidermal growth factor receptor-2 levels on the efficacy of trastuzumab: a secondary analysis of the HERA trial. JAMA Oncology 21040–1047. ( 10.1001/jamaoncol.2016.0339) [DOI] [PubMed] [Google Scholar]
- Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, Denkert C, Schem C, Sotiriou C, Loi Set al. 2016PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Annals of Oncology 271519–1525. ( 10.1093/annonc/mdw197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love RR, Duc NB, Havighurst TC, Mohsin SK, Zhang Q, DeMets DL, Allred DC.2003HER-2/neu overexpression and response to oophorectomy plus tamoxifen adjuvant therapy in estrogen receptor-positive premenopausal women with operable breast cancer. Journal of Clinical Oncology 21453–457. ( 10.1200/JCO.2003.10.133) [DOI] [PubMed] [Google Scholar]
- Lupien M, Meyer CA, Bailey ST, Eeckhoute J, Cook J, Westerling T, Zhang X, Carroll JS, Rhodes DR, Liu XSet al. 2010Growth factor stimulation induces a distinct ERα cistrome underlying breast cancer endocrine resistance. Genes and Development 242219–2227. ( 10.1101/gad.1944810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcom PK, Isaacs C, Harris L, Wong ZW, Kommarreddy A, Novielli N, Mann G, Tao Y, Ellis MJ.2007The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Research and Treatment 10243–49. ( 10.1007/s10549-006-9307-8) [DOI] [PubMed] [Google Scholar]
- Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R.2008Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Research 68826–833. ( 10.1158/0008-5472.CAN-07-2707) [DOI] [PubMed] [Google Scholar]
- McClelland RA, Barrow D, Madden TA, Dutkowski CM, Pamment J, Knowlden JM, Gee JMW, Nicholson RI.2001Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex). Endocrinology 1422776–2788. ( 10.1210/endo.142.7.8259) [DOI] [PubMed] [Google Scholar]
- Mitsogianni M, Trontzas IP, Gomatou G, Ioannou S, Syrigos NK, Kotteas EA.2021The changing treatment of metastatic her2-positive breast cancer (Review). Oncology Letters 21287. ( 10.3892/ol.2021.12548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, De Angelis C, Trivedi MV, Osborne CK, Schiff R.2015The changing role of ER in endocrine resistance. Breast 24 (Supplement 2) S60–S66. ( 10.1016/j.breast.2015.07.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar U, Cohen O, Kapstad C, Cuoco MS, Waks AG, Wander SA, Painter C, Freeman S, Persky NS, Marini Let al. 2019Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nature Genetics 51207–216. ( 10.1038/s41588-018-0287-5) [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SAW, Wong J, Allred DC, Clark GM, Schiff R.2003Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. Journal of the National Cancer Institute 95353–361. ( 10.1093/jnci/95.5.353) [DOI] [PubMed] [Google Scholar]
- Pascual T, Fernandez-Martinez A, Tanioka M, Dieci MV, Pernas S, Gavila J, Guarnieri V, Cortes J, Villagrasa P, Chic Net al. 2021Independent validation of the PAM50-based chemo-endocrine score (CES) in hormone receptor-positive HER2-positive breast cancer treated with neoadjuvant anti-HER2-based therapy. Clinical Cancer Research 273116–3125. ( 10.1158/1078-0432.CCR-20-4102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli B, Wildiers H, Hamilton EP, Ferreira AA, Dalenc F, Vidal M, Gavilá J, Goncalves A, Murias C, Mouret-Reynier M-Aet al. 2020Clinical activity of MCLA-128 (zenocutuzumab) in combination with endocrine therapy (ET) in ER+/HER2-low, non-amplified metastatic breast cancer (MBC) patients (pts) with ET-resistant disease who had progressed on a CDK4/6 inhibitor (CDK4/6i). Journal of Clinical Oncology 381037–1037. ( 10.1200/JCO.2020.38.15_suppl.1037) [DOI] [Google Scholar]
- Portman N, Alexandrou S, Carson E, Wang S, Lim E, Caldon CE.2019Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocrine-Related Cancer 26R15–R30. ( 10.1530/ERC-18-0317) [DOI] [PubMed] [Google Scholar]
- Prat A, Cheang MCU, Galván P, Nuciforo P, Paré L, Adamo B, Muñoz M, Viladot M, Press MF, Gagnon Ret al. 2016Prognostic value of intrinsic subtypes in hormone receptor-positive metastatic breast cancer treated with letrozole with or without lapatinib. JAMA Oncology 21287–1294. ( 10.1001/jamaoncol.2016.0922) [DOI] [PubMed] [Google Scholar]
- Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, Thomas R, Leone JP, Lucas PC, Bhargava Ret al. 2017Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncology 3666–671. ( 10.1001/jamaoncol.2016.5630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priedigkeit N, Ding K, Horne W, Kolls JK, Du T, Lucas PC, Blohmer JU, Denkert C, Machleidt A, Ingold-Heppner Bet al. 2021Acquired mutations and transcriptional remodeling in long-term estrogen-deprived locoregional breast cancer recurrences. Breast Cancer Research 231. ( 10.1186/s13058-020-01379-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BB, Regan MM, Lykkesfeldt AE, Dell’Orto P, Del Curto B, Henriksen KL, Mastropasqua MG, Price KN, Méry E, Lacroix-Triki Met al. 2008Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1–98 randomised trial. Lancet: Oncology 923–28. ( 10.1016/S1470-2045(0770386-8) [DOI] [PubMed] [Google Scholar]
- Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, Cai Y, Bielski CM, Donoghue MTA, Jonsson Pet al. 2018The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34427–438.e6. ( 10.1016/j.ccell.2018.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimawi M, Ferrero JM, De La Haba-Rodriguez J, Poole C, De Placido S, Osborne CK, Hegg R, Easton V, Wohlfarth C, Arpino Get al. 2018First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. Journal of Clinical Oncology 362826–2835. ( 10.1200/JCO.2017.76.7863) [DOI] [PubMed] [Google Scholar]
- Ring A, Dowsett M.2003Human epidermal growth factor receptor-2 and hormonal therapies: clinical implications. Clinical Breast Cancer 4 (Supplement 1) S34–S41. ( 10.3816/CBC.2003.s.013) [DOI] [PubMed] [Google Scholar]
- Selli C, Sims AH.2019Neoadjuvant therapy for breast cancer as a model for translational research. Breast Cancer: Basic and Clinical Research 131178223419829072. ( 10.1177/1178223419829072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R.2004Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Journal of the National Cancer Institute 96926–935. ( 10.1093/jnci/djh166) [DOI] [PubMed] [Google Scholar]
- Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, Ashley SE, Francis S, Boeddinghaus I, Walsh Get al. 2005Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. Journal of Clinical Oncology 235108–5116. ( 10.1200/JCO.2005.04.005) [DOI] [PubMed] [Google Scholar]
- Willsher PC, Beaver J, Pinder S, Bell JA, Ellis IO, Blamey RW, Robertson JFR.1996Prognostic significance of serum c-erbB-2 protein in breast cancer patients. Breast Cancer Research and Treatment 40251–255. ( 10.1007/BF01806813) [DOI] [PubMed] [Google Scholar]
- Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons P, Hanna W, Langer Aet al. 2007American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Archives of Pathology and Laboratory Medicine 13118–43. ( 10.1200/JCO.2018.77.8738) [DOI] [PubMed] [Google Scholar]
- Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna Wet al. 2018Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline focused update. Archives of Pathology and Laboratory Medicine 1421364–1382. ( 10.5858/arpa.2018-0902-SA) [DOI] [PubMed] [Google Scholar]
- Wright C, Nicholson S, Angus B, Sainsbury JRC, Farndon J, Cairns J, Harris AL, Horne CHW.1992Relationship between c-erbb-2 protein product expression and response to endocrine therapy in advanced breast cancer. British Journal of Cancer 65118–121. ( 10.1038/bjc.1992.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, O’Neill A, Gelman R, Carney W, Tenney DY, Hösch S, Hayes DF.1997Prediction of response to antiestrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER-2/c-neu protein. Journal of Clinical Oncology 152518–2525. ( 10.1200/JCO.1997.15.7.2518) [DOI] [PubMed] [Google Scholar]
- Zhu L, Chow LWC, Loo WTY, Guan XY, Toi M.2004Her2/neu expression predicts the response to antiaromatase neoadjuvant therapy in primary breast cancer: subgroup analysis from celecoxib antiaromatase neoadjuvant trial. Clinical Cancer Research 104639–4644. ( 10.1158/1078-0432.CCR-04-0057) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable as no datasets were generated during this study.



 This work is licensed under a
This work is licensed under a