Abstract
Background
The cause of ankle osteoarthritis (OA) is usually trauma. Patients are relatively young, since ankle trauma occurs at a relatively young age. Several conservative treatment options are available, evidence of the benefits and harms of these options are lacking.
Objectives
To assess the benefits and harms of any conservative treatment for ankle OA in adults in order to provide a synthesis of the evidence as a base for future treatment guidelines.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, issue 9), MEDLINE (Ovid) (1946 up to 11 September 2014), EMBASE (1947 to September 2014), PsycINFO (1806 to September 2014), CINAHL (1985 to September 2014), PEDro (all years till September 2014), AMED until September 2014, ClinicalTrials.gov, Current Controlled Trials, The Dutch Register. To identify potentially relevant studies we screened reference lists in retrieved review articles and trials.
Selection criteria
We considered randomised or controlled clinical trials investigating any non‐surgical intervention for ankle OA for inclusion.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
No other RCT concerning any other conservative treatment besides the use of hyaluronic acid (HA) for ankle OA was identified. Six randomised controlled trials (RCTs) were included.
A total of 240 participants diagnosed with ankle OA were included in this review. The primary analysis included three RCTs (109 participants) which compared HA to placebo. One study compared HA to exercise therapy, one compared HA combined with exercise therapy to an intra‐articular injection of botulinum toxin and one compared four different dosages of HA.
Primary analysis: a pooled analysis of two trials (45 participants) found that the Ankle Osteoarthritis Scale (AOS) total score (measuring pain and physical function) was reduced by 12% (95% CI −24% to −1%) at six months (mean difference (MD) −12.53 (95% CI −23.84 to −1.22) on a scale of 0 to 100; number needed to treat for an additional beneficial outcome (NNTB) = 4 (95% CI 2 to 205); this evidence was graded as low quality, due to limitations in study design (unclear risk of selection bias for two studies and unclear risk for attrition bias for one study) and imprecision of results: a small population size (45 participants). It is not known if a mean difference of 12.53 points on a 100 point scale is clinically relevant. No minimal important clinical difference is known for this score. Pain and function outcomes were not reported separately. Radiographic joint structure changes were not investigated. For the mean quality of life at six months (two trials; 45 participants) no meta‐analysis could be performed due to missing data. No serious adverse events (SAEs) were noted and no participants withdrew because of an adverse event. There were a few adverse events (AEs) 5/63 (8%) in the HA group and 2/46 (4%) in the placebo group. The Peto odds ratio (Peto OR) to have an adverse event was 2.34 higher compared to the control group (95% CI 0.45 to 12.11). This evidence is inconclusive because of a wide CI and a small number of events.
For comparing HA to exercise therapy (30 participants) the results for pain on a Visual Analogue Scale (VAS 0 to 10) at 12 months are inconclusive (MD 0.70, 95% CI −2.54 to 1.14). The American Orthopedic Foot and Ankle Society score (AOFAS score) was 13.10 points (MD) higher in favour of HA (95% CI 2.97 to 23.23) on a scale of 0 to 100. The evidence was graded as low. No adverse events were found. Radiographic structure changes were not measured; no participants withdrew due to AEs; no SAEs were found.
For the comparison of HA injection combined with exercise therapy to an intra‐articular injection of botulinum toxin A (BoNT‐A) (75 participants), the outcome of the AOS pain score of the affected joint at six months is inconclusive (MD 0.10, 95% CI −0.42 to 0.62). The physical function (the AOS disability score) at six months is inconclusive (MD 0.20, 95% CI −0.34 to 0.74). The same number of AEs were found in both groups; HA 2/37 (5.9%), BoNT‐A 2/38 (5.8%) (risk ratio (RR) 1.03, 95% CI 0.15 to 6.91). Radiographic changes were not examined, no SAEs were found and no participants withdrew because of an AE. The evidence was graded as low.
The RCT comparing four different dosing schedules for HA (26 participants) showed the best median decrease in pain on walking VAS (on a scale of 0 to 100) for 3 x 1 ml at 27 weeks with a median decrease of 30. Physical function, radiographic changes and quality of life were not measured.Twenty‐seven percent of all participants had AEs, most of them in the 2ml group (57% in this group). No participants withdrew due to an AE and no SAEs were noted.
Overall the quality of the evidence showed some serious limitations. The evidence was graded low for the primary analysis comparing HA to placebo. This was based on a limitation in design and implementation: sample sizes were small (45 to 92 participants) and and imprecision in results: there was an unclear risk of bias for several items concerning the three studies used in the meta analysis.
Authors' conclusions
Currently, there is insufficient data to create a synthesis of the evidence as a base for future guidelines for ankle OA. Since the aetiology of ankle OA is different, guidelines that are currently used for hip and knee OA may not be applicable for ankle OA. Simple analgesics as recommended for hip and knee OA seem however a reasonable first step to treat ankle OA. It is unclear if there is a benefit or harm for HA as treatment for ankle OA compared to placebo at six months based on a low quality of evidence. Inconclusive results were found comparing HA to other treatments. HA can be conditionally recommended if patients have an inadequate response to simple analgesics. It remains unclear which patients (age, grade of ankle OA) benefit the most from HA injections and which dosage schedule should be used.
Plain language summary
Hyaluronic acid and other non‐surgical treatment options for ankle osteoarthritis
Cochrane researchers conducted a review of the effect of non‐surgical treatment for people older than 18 with ankle osteoarthritis in order to provide a synthesis of the evidence as a base for future treatment guidelines. After searching for all relevant studies up to September 2014, no study using any other non‐surgical treatment besides the use of hyaluronic acid for ankle osteoarthritis was identified. They found six studies evaluating hyaluronic acid with a total of 240 people. Their findings are summarised below:
Five studies showed the results of the use of hyaluronic acid for ankle osteoarthritis compared to other treatment (exercise (30 people) or botulinum toxin A injections (75 people) or to placebo (fake injection) (3 studies, 109 people). One study was a dose‐finding study (26 people). Follow‐up was three to six months. The quality of the evidence was graded as low, due to an unclear risk of bias and a low number of participants.
In people with ankle osteoarthritis:
‐ No studies were identified to support the use of any other non‐surgical treatment.
‐ We are uncertain if there is a benefit of hyaluronic acid for the treatment of ankle osteoarthritis compared to placebo.
‐ Results comparing hyaluronic acid to other treatment are inconclusive.
‐ Results about the best dosing schedule for hyaluronic acid are inconclusive.
‐ Possible side effects of hyaluronic acid might include swelling and pain of the joint which subsides within a couple of days.
‐ Hyaluronic acid injections might be conditionally recommended when simple analgesics have failed.
What is osteoarthritis, what is hyaluronic acid and what other non‐surgical treatment options are there?
Osteoarthritis (OA) is a disease of the joints. When the joint loses cartilage, the bone grows to try to repair the damage. Instead of making things better, however, the bone grows abnormally and makes things worse. For example, the bone can become misshapen and make the joint painful and unstable. This can affect your physical function or ability to use your ankle.
Hyaluronic acid is a natural component of synovial fluid. Hyaluronic acid injections (also called 'viscosupplementation') are gel‐like fluid injections which help to lubricate the joint and act as a shock absorber for joint loads. These injections are used in a hospital environment when simple analgesics have failed.
Other non‐surgical options for ankle OA are, for instance, the use of different types of analgesics and the use of non‐pharmacological therapy like shoe adjustments, braces, weight loss and exercises or a combination of any of those.
What happens to people with ankle osteoarthritis who get injections with hyaluronic acid compared to placebo?
After six months (45 people) pain and physical function were measured using a combined score (scale of 0 to 100; 0 is the best score and 100 the worst):
‐ People who got injections with hyaluronic acid rated their pain and physical function 12.3 points lower compared to placebo (12% absolute improvement).
‐ People who got injections with hyaluronic acid rated their pain and physical function 24.4 points lower.
‐ People who got injections with placebo rated their pain and physical function 12.1 points lower.
Radiographic joint structure changes:
‐ No studies were found that looked at this outcome.
Quality of life:
‐ No data is available to make a statement about quality of life.
Number of people experiencing any serious adverse events (109 people):
‐ No patient in either group experienced a serious adverse event.
Number of people experiencing any adverse event (109 people):
‐ 35 more people per 1000 who are treated with hyaluronic acid will experience an adverse event compared to placebo (3.5% absolute increase).
‐ 78 people per 1000 who are treated with hyaluronic acid will experience an adverse event.
‐ 43 people per 1000 who are treated with placebo will experience an adverse event.
People who withdraw because of an adverse event (109 people):
‐ No participants withdrew in either group.
Summary of findings
Summary of findings for the main comparison. Hyaluronic acid for osteoarthritis of the ankle.
| Hyaluronic acid for osteoarthritis of the ankle | ||||||
| Patient or population: patients with osteoarthritis of the ankle Settings: Rehabilitation centre / hospital Intervention: hyaluronic acid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Hyaluronic acid | |||||
| AOS total (Pain & Physical function) AOS total score. Scale from: 0 to 100 (0 = being no pain/disability, 100 = worst imaginable pain/disability). Follow‐up: 6 months | The mean pain/physical function change ranged across the control groups from 6.8 to 20.9 points lower with a weighted mean of 12.14 lower | The mean pain/physical function in the hyaluronic acid group was 12.53 points lower (23.84 lower to 1.22 lower) compared to placebo at 6 months. | 45 (2 studies) | ⊕⊕⊝⊝ Low1 | A lower score indicates less pain and a better physical function. It is not known if a change of 12 points is clinically relevant. NNT = 4 (95% CI 2 to 205) (using a SMD = 0.5 as a minimum important difference). Absolute risk difference is −12.53% (95% CI −23.84 to −1.22). Relative percentage change is 1.85% (95% CI 0.18 to 3.58%). |
|
| Radiographic Joint Structure Changes | See comment | See comment | Not estimable | 0 (0) | See comment | Radiographic joint structure changes were not investigated. |
|
Quality of Life SF12. Scale from: 0 to 100. Follow‐up: mean 6 months. |
See comment | See Comment | Not estimable | 45 (2 studies) | See comment | Cohen 2008 only described that there was no significant difference between placebo and intervention for the SF12 outcome, no exact data was provided. Salk 2006 could not provide us with the standard deviations, so no estimate of the SF12 could be made. He demonstrated a statistically significant difference in his paper favouring hyaluronic acid at 6 months. |
| Number of participants experiencing any serious adverse events Follow‐up: 3 to 6 months | See comment | See comment | Not estimable | 109 (3 studies) | See comment | No serious adverse events (SAEs) were noted |
| Number of participants experiencing any adverse event Follow‐up: 3 to 6 months | 43 per 1000 | 35 per 1000 higher (26 fewer to 241 more) compared to placebo. | RR 1.66 (0.47 to 5.88) | 109 (3 studies) | ⊕⊕⊝⊝ low1 | Peto Odds Ratio is 2.34 (95% CI 0.45 to 12.11) Absolute risk difference is 5.00% (−5 to 14), relative percentage change is 66% (−53% to 488%). Adverse events for all 3 studies were reported, even though DeGroot had a follow up of 3 months. All adverse events resolved within a week after injection, so a shorter follow up has no effect on the estimate of effect. |
| Participants who withdraw because of an adverse event or any other reason Follow‐up: 3 to 6 months | See comment | See comment | Not estimable | 109 (3 studies) | See comment | No participants withdrew because of an adverse event |
| AOS: Ankle Osteoarthritis ScaleCI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SF12: short form 12 | ||||||
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. Grade criteria: study limitation, indirectness, inconsistency, imprecision, publication bias. | ||||||
* The assumed risk was based on the weighted mean of the scores in the control groups across the 2 studies. The range was based on the mean change in pain on a visual analogue scale (100 mm) of the control group in each separate study.The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
1 Evidence was downgraded based on limitations in study design and imprecision of results. Limitation in study design: there was a unclear risk of selection bias for Salk and Cohen, unclear risk for attrition bias for Salk. Imprecision of results: the population size is small (45 participants). No indirectness of evidence was found, no inconsistency and no publication bias.
2 Evidence was downgraded based on limitations in study design and imprecision of results. Limitation in study design: there was a unclear risk of selection bias for Cohen, an unclear risk for reporting bias for DeGroot. Imprecision of results: the total population size is small (92 participants). No indirectness of evidence was found, no inconsistency and no publication bias.
Background
Description of the condition
Osteoarthritis (OA) is a chronic and degenerative disorder associated with joint pain and loss of joint function. OA can affect any synovial joint but is found most frequently in the hip, knee and hand; the majority of these patients present with primary OA (idiopathic disease) (Buckwalter 2004; Kalunian 2012; Witteveen 2008). Reliable figures on the prevalence of OA in other joints are not readily available but estimates suggest that the incidence of symptomatic ankle OA is 1% to 4% in the adult population (Cushnaghan 1991; Peyron 1984). In contrast to knee and hip OA, 70% to 78% of people with ankle OA present with secondary, post‐traumatic disease (sequelae after ankle fracture, ankle instability or fracture of the lower leg); the remainder is primary OA as well as inflammatory diseases, such as rheumatoid arthritis and gout (Saltzman 2005; Valderrabano 2009). Ankle trauma occurs in many people at a relatively young age (Agel 2005; Saltzman 2005). Consequently, the expected life span of many people with ankle OA is significantly longer than the life span of hip or knee OA patients; this affects their quality of life for a substantial length of time. Saltzman 2006 demonstrated that the self reported physical function in people with symptomatic ankle OA quantified using the Short Form‐36 (SF‐36) questionnaire was equivalent to or worse than that of patients with end‐stage kidney disease or congestive heart failure suggesting that these people are seriously impaired.
Description of the intervention
In clinical practice, patients diagnosed with end‐stage ankle OA (Kellgren Lawrence 3 or 4 and van Dijk 3) are offered operative treatment if they have significant clinical symptoms (Harada 2011; van Dijk 1997). These people are treated by arthrodesis, ankle replacement or osteotomy. Surgical treatment is specifically reserved for end‐stage arthritis. It is considered to be controversial due to short‐ and long‐term complications. Complications consist of wound healing problems, infectious disease, non‐ or delayed union and OA of adjacent joints due to overloading (Chang 2013; Deorio 2008; Jung 2007; Krause 2012; Rippstein 2012; Suckel 2012). Complication rates vary up to 44% depending on the type of surgery; and the type of complication — short‐ or long‐term. OA of adjacent joints after ankle arthrodesis occurs for instance in 44% to 50% of cases after 20 years (Morrey 1980; Pagenstert 2008; Takakura 1995). Operative treatment is therefore not considered in an early phase of OA and it remains a challenge to treat people that are diagnosed with a low grade OA of the ankle (Kellgren Lawrence 1, 2, or 3 and Van Dijk 1 or 2) (Harada 2011; van Dijk 1997). They are young and they experience serious disabilities which prevent them from participating in more heavily physical work as well as sports activities. Several conservative treatment options are available; however evidence of the benefits and harms of these options are lacking.
The conservative treatment of symptomatic ankle OA, like general OA, consists mainly of treating symptoms like pain and stiffness. Since no cure is available at this point another treatment goal is preventing deterioration of the joint (Towheed 2006). Non‐pharmacological therapy is to be considered the foundation for the successful medical management of general OA (Hochberg 2012; Zhang 2008; Zhang 2010). There are systematic reviews published for knee and hip OA and include weight reduction (BMI > 25), physiotherapy and occupational therapy (Brosseau 2011; Brouwer 2005; Rutjes 2009; Rutjes 2010). For ankle OA, offloading the joint by brace, cane, rocker sole or inlay is commonly used in clinical practice to reduce pain; however no evidence is available to support this treatment (Bartels 2007; Brosseau 2003; Fransen 2009; Janisse 1998; Kempson 1991; Messier 2005; McGuire 2003; Wu 2004). If this non‐pharmacological treatment is not successful a painkiller can be added. Several pain relief options are available, e.g. painkillers like acetaminophen, opioids and non‐steroidal anti‐inflammatory drugs (NSAIDs) (Cepeda 2006; Garner 2005; Nuesch 2010; Towheed 2006). Hyaluronic acid (HA) for ankle OA has been shown to reduce pain as well. HA is currently used in clinical practice when simple analgesics have failed (Chang 2013; Cohen 2008; Pleimann 2002; Salk 2006; Sun 2006; Witteveen 2008; Witteveen 2010). The benefit of glucosamine/chondroitin for pain reduction in general OA was not shown (Towheed 2005).
How the intervention might work
Ankle OA pain might be reduced by offloading the joint through rest, wearing a brace or using a cane. A cane can reduce the amount of bodyweight going through the ankle joint by 25% (Kempson 1991). Rocker soles are thought to offload the ankle joint by decreasing the ankle motion at heel strike to push off during walking (Wu 2004). Weight loss by dietary adjustments or exercises are thought to offload a joint as well (Bartels 2007; Brosseau 2003; Fransen 2009). In Messier 2005, each pound of weight loss created a 4‐fold reduction in the load exerted by step at the knee during daily activities. Shoe adjustment like inlays can correct alignment issues and in this way offload a part of the joint thus creating pain reduction (Janisse 1998; McGuire 2003). It is possible that in this way the joint can be preserved from further deterioration. Several analgesics are available like acetaminophen, opioids and NSAIDs. They either act as a simple analgesic, have anti‐inflammatory effects, a sedative effect or a combination of these. Recommendations for hip, knee or hand OA are well described (Hochberg 2012). Hyaluronic acid (viscosupplementation) is thought to restore rheologic properties of the joint by creating a more viscoelastic synovial fluid which improves mobility and restores the natural protective function of the joint, like shock absorption during gait (Balazs 1993; Bellamy 2006). Several studies have suggested pain reduction as well (Chang 2013; Cohen 2008; Pleimann 2002; Salk 2006; Sun 2006; Witteveen 2008; Witteveen 2010). Glucosamine/chondroitin may be potentially chondro‐protective and may modify the progression and course of general OA, though improvement in pain and function are not conclusive (Singh 2015; Towheed 2005).
Why it is important to do this review
Lots of treatment modalities are offered, however no clear‐cut treatment algorithm for ankle OA is used in clinical practice. The choice of treatment depends on the severity of the disease; the person's age, medical and social history; and the level of physical activity expected to be demanded of the joint. For knee and hip OA several treatment algorithms are advocated (Kalunian 2012; Pendleton 2000; Tannenbaum 2000; Towheed 2005; Towheed 2006; Zhang 2008; Zhang 2010). However, since ankle OA may be caused by a different mechanism, it is not unthinkable that these patients need a different treatment.
At this point there is no evidence‐based treatment algorithm for ankle OA. Several papers have been published concerning the cause of ankle OA and the possible conservative and operative treatment strategies. The conservative section mainly sums up the possibilities, however no algorithm is suggested (Demetriades 1998; Katcherian 1998; Martin 2007; Rao 2010; Thomas 2003). We conducted this review to find evidence for the benefits and harms of non‐pharmacological and pharmacological treatment of ankle OA in general or by stage of the disease. We will try to provide a synthesis of the evidence as a base for future treatment guidelines.
Objectives
To assess the benefits and harms of any conservative treatment for ankle OA in adults in order to provide a synthesis of the evidence as a base for future treatment guidelines.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and controlled clinical trials (CCTs) were included in this review.
Types of participants
Adults with the diagnosis of symptomatic ankle osteoarthritis (OA) (primary or secondary) were included in this review. The diagnosis was based on well‐described clinical criteria e.g. the American College of Rheumatology (ACR) criteria (Hochberg 2012), or based on a previously taken X‐ray, which was classified using either the Kellgren Lawrence or the Van Dijk scale (Harada 2011; van Dijk 1997).
Types of interventions
Trials investigating any non‐surgical intervention were eligible.
Trials investigating the following interventions were included:
pharmacologic therapy — analgesics: acetaminophen, opioid analgesics like codeine, oxycodone or tramadol; NSAIDs like ibuprofen or celecoxib, intra‐articular glucocorticoids, intra‐articular hyaluronan, glucosamine and chondroitin;
non‐pharmacologic therapy such as weight loss, rest, physical therapy and orthoses; braces, taping, insoles, exercise (strengthening, mobility, endurance and joint stability), manual therapy, diet, self management, psychosocial interventions (Kalunian 2012).
Other methods including traditional medicine (e.g. herbs, acupuncture) and naturopathies were excluded.
We tried to identify two special types of RCTs or CCTs:
RCTs or CCTs that compared a treatment/therapy alone to placebo; and
RCTs or CCTs that compared one treatment to the other.
Types of outcome measures
Benefits
Pain with a hierarchy of seven levels (Ghogomu 2014):
pain of the affected joint;
pain on walking;
pain on activities other than walking;
rest pain or pain during the night;
other algofunctional scale (e.g. AOS pain or AOS total, Domsic 1998);
patient's global assessment;
physician's global assessment.
When more than one was reported, the highest on the list was taken.
Physical function with a hierarchy of eight levels (Ghogomu 2014):
global disability score;
walking disability;
disability other than walking;
American Orthopedic Foot and Ankle Society score (AOFAS score, Kitaoka 1994);
Foot and Ankle Outcome Score (FAOS, Roos 2001);
Foot Function Index (FFI, Budiman‐Mak 1991) ;
Function (Range of Motion (ROM));
other algofunctional scale (e.g. AOS disability or AOS total, Domsic 1998).
When more than one was reported the highest on the list was taken.
-
Radiographic joint structure changes according to the given hierarchy:
Kellgren Lawrence score (Harada 2011);
van Dijk score (van Dijk 1997).
Quality of Life:
Short Form‐36 (SF‐36,Ware 1992)
EuroQoL‐5 Dimensions (EQ‐5D, Salén 1994).
Harms
Participants experiencing any serious adverse events (SAEs); a serious adverse event is defined as any adverse event, irrespective of a possible relationship to the administered treatment which leads to e.g. death, a life‐threatening event or requires hospitalisation.
Number of participants experiencing any adverse event (AE); an adverse event is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product, which does not necessarily have a causal relationship with the treatment.
Participants who withdraw because of an adverse event or any other reason
If pain or function outcomes were reported at several time‐points, the end of treatment was taken as primary time‐point for pharmacologic treatment such as acetaminophen, opioids or NSAIDs, with the three‐months interval as an additional time‐point.
In case of hyaluronan, glucocorticoids, glucosamine and chondroitine and nonpharmacologic therapy, six months was considered as primary time‐point and the three‐month interval as an additional time‐point.
Search methods for identification of studies
Electronic searches
A sensitive search strategy was designed to retrieve trials from electronic bibliographic databases, not limited to any intervention. The search strategy was devised for the Ovid MEDLINE interface The sensitivity maximizing filter for retrieving RCTs from MEDLINE and EMBASE was used as recommended in the Cochrane Handbook for for Systematic Reviews of Interventions (Higgins 2011). No language restriction was applied. 11 to 18 September 2014, we searched the following electronic databases, unrestricted by date (from database inception) or language:
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 9, 2014) (Appendix 1);
MEDLINE (Ovid) 1946 to present (Appendix 2);
EMBASE (Ovid) 1947 to present (Appendix 3);
PsycINFO (American Psychological Association) 1806 to present (Appendix 4);
CINAHL (Cumalitive Index to Nursing and Allied Health Literature) (EBSCO)1985 to present (Appendix 5);
PEDro (Physiotherapy Evidence Database) (all years (Appendix 6));
AMED (Allied and Alternative Medicine) (Ovid) 1985 to present (Appendix 7).
Searching other resources
We searched the following clinical trial registries to identify ongoing trials:
ClinicalTrials.gov (http://clinicaltrials.gov/);
Current Controlled Trials (http://www.controlled‐trials.com/);
The Dutch Register (http://www.trialregister.nl/trialreg/index.asp).
We also screened reference lists in retrieved review articles and trials to identify potentially relevant studies.
Data collection and analysis
Selection of studies
Two authors (AW, CH) independently screened records identified from database searches for possible inclusion. Full‐text articles were retrieved for further assessment when the initial information appeared to align with the review criteria. Trials not fulfilling the outlined selection criteria were excluded. Reasons for exclusion were documented. A third author (GK) moderated any disagreement.
Data extraction and management
Two authors (AW, GK) completed data extraction of the included studies and recorded this on a data extraction form. Disagreements were resolved by discussion.
We collected data on study design characteristics, descriptive characteristics of the participants, interventions, outcome measures, and length of follow‐up. Trialists were contacted for clarification when necessary.
The data extraction included the following:
Generic publication characteristics:
type of publication;
title;
authors;
year of publication.
Research design:
randomised controlled study/controlled clinical trial;
blinding of outcome assessors;
allocation concealment.
-
Descriptive characteristics of participants:
number of participants;
age;
sex;
duration of ankle OA;
grade of ankle OA;
baseline measures;
diagnoses; inclusion and exclusion criteria;
if applicable, randomisation outcomes such as numbers allocated to each group at baseline, withdrawals, intention‐to‐treat numbers, and losses to follow‐up.
Intervention characteristics:
non‐surgical intervention: analgesics — acetaminophen, opioid analgesics like codeine, oxycodone or tramadol, NSAIDs such as ibuprofen or celecoxib, intra‐articular glucocorticoids, intra‐articular hyaluronan, glucosamine and chondroitin;
non‐pharmacologic therapy: weight loss, rest, physical therapy and orthoses: braces, taping, insoles, exercise (strengthening, mobility, endurance and joint stability), manual therapy, diet, self management, psychosocial interventions;
comparative intervention;
duration of the intervention (duration (weeks/months) and frequency);
follow‐up.
Outcomes (benefits and harms):
pain;
safety;
quality of life;
physical function.
Disagreements in data extraction were resolved via discussion and further scrutiny of the original data.
Assessment of risk of bias in included studies
The Cochrane's tool for assessing risk of bias was used in the selected studies (Higgins 2011). Two authors (AW, GK) independently assessed generation of allocation sequence, allocation concealment, blinding, incomplete outcome data, selective outcome reporting (reporting bias), and other sources of bias (baseline imbalance in factors which are strongly related to outcome measures e.g. grade of ankle OA; intervention characteristics e.g. dosage of medication, frequency of therapy).
Bias was judged as 'high risk' of bias, 'low risk' of bias, or 'unclear risk' of bias. We resolved disagreements by consensus or discussion with a third author (CH).
Measures of treatment effect
Intervention efficacy and safety were assessed by presenting the mean differences (MDs). When data could be pooled to perform a meta‐analysis, standardised mean differences (SMDs) were used when the same outcome was assessed but different scales were used to express this outcome. A 95% confidence interval (CI) was used for continuous outcomes; and risk ratio (RR) and 95% CI for dichotomous outcomes. A Peto odds ratio was used for rare events.
Unit of analysis issues
The unit of analysis was the participant. If RCTs or CCTs were identified that treated both ankles, and the number of ankles was used as the denominator in the analysis without adjustment for the non‐independence between ankles (and thus a potential for unit of analysis error might occur), we attempted to re‐analyse such studies by calculating sample sizes where possible, according to the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If it was stated in the article that more than 10% of the patients suffered from general OA, the treatment effect of any treatment for ankle OA would be very difficult to interpret and therefore these studies were excluded.
Dealing with missing data
Where we could not directly extract data the trialists were contacted, or missing data was imputed with replacement values, and treated as if they were observed (last observation carried forward) (Higgins 2011). If data was imputed, we noted so in the table 'Characteristics of included studies'.
Assessment of heterogeneity
We tested heterogeneity of the data using the Chi² with a P value less than 0.10 indicating significant heterogeneity. The I² statistic was assessed to quantify inconsistency across the results (I² = [Q df / Q] x 100%; where Q is the Chi² statistic and df is the degrees of freedom) (Higgins 2011). A value greater than 50% indicated substantial heterogeneity. Beside this procedure, we also performed a visual assessment of forest plots to assess heterogeneity (Higgins 2011).
Assessment of reporting biases
We investigated selective outcome reporting bias by comparing the study outcomes with those routinely presented for similar studies and also by comparing the Methods section of trial reports with the results reported.
Data synthesis
We pooled results of comparable groups of trials. Initially the fixed‐effect model and 95% CIs was used. A fixed‐effect meta‐analysis provided a result that may be viewed as a 'typical intervention effect' from the studies included in the analysis. A confidence interval for a fixed‐effect meta‐analysis was calculated: in order to do so the assumption was made that the true effect of intervention (in both magnitude and direction) was the same value in every study (that is, fixed across studies). This assumption implied that the observed differences among study results were due solely to the play of chance, i.e. that there was no statistical heterogeneity (Higgins 2011). The random‐effects model was considered, especially where there was unexplained heterogeneity (Higgins 2011). The Cochrane's statistical software for data synthesis, Review Manager 5, was used.
Subgroup analysis and investigation of heterogeneity
Due to the lack of data a subgroup analysis was not performed. If sufficient data had been present, an analysis between the benefits and harms of conservative treatments for each grade of OA of the Kellgren Lawrence score (grade 1, 2, 3) or the van Dijk score (grade 1 or 2) would have been performed.
Sensitivity analysis
Due to the low number of eligible studies no sensitivity analysis was performed.
Summary of findings table
The main findings of the study are presented in a 'Summary of findings' table, produced using GRADEpro software (GRADEprofiler 2008). This table provides key information concerning the quality of the evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. The table includes an overall grading of the evidence related to each of the main outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as indicated in the Cochrane Handbook for Systematic Reviews of Interventions (study limitation, indirectness, inconsistency, imprecision, publication bias) (Higgins 2011). A 'Summary of findings' is made when sufficient data can be pooled (data synthesis) or for any comparison that is deemed clinically important. The important outcomes that were included in the 'Summary of findings' tables are:
pain;
physical function;
combined score of pain and physical function (AOS total)
radiographic joint structures changes;
quality of life;
number of participants experiencing any serious adverse events;
number of participants experiencing any adverse event;
participants who withdraw because of an adverse event or any other reason.
Results
Description of studies
Results of the search
After performing the first search up to 11 September 2014, 2945 references were retrieved; after de‐duplication this resulted in 2257 citations (1126 MEDLINE, 656 EMBASE, 98 CENTRAL, 50 CINAHL, 138 PsycINFO, 14 PEDro, 175 AMED).
No additional studies or ongoing studies were found searching the trial registers.
After screening the titles and abstracts of these references 14 full‐text articles were selected; after de‐duplication 13 remained. Seven were excluded and six were included.
See the study flowchart for further details (Figure 1).
1.
Study flow diagram.
Included studies
The six included studies are listed in the 'Characteristics of included studies' table. Years of publication ranged from 2006 to 2014.
All studies are blinded randomised controlled trials (RCTs), three are double‐blinded RCTs (Cohen 2008; DeGroot 2012; Salk 2006). These three studies compared the intra‐articular injection of hyaluronic acid (HA) to placebo. Authors of these studies were contacted by email to get the exact results of the scores they used in their trials. Cohen 2008 and Salk 2006 were not able to provide us with these data. DeGroot 2012 did send his original database. Two compared two different treatments: HA injection compared to exercise therapy (Karatosun 2008); or HA combined with exercise therapy versus injection of Intra‐articular botulinum toxin A (Sun 2014). Witteveen 2010 compared the efficacy and safety of four different doses of HA. A total of 240 participants were involved. All were clinically diagnosed with ankle osteoarthritis (OA) which was confirmed radiographically. All participants were in generally good health. The Kellgren Lawrence score as well as the van Dijk score was used as classification for the radiographic presence of OA (Kellgren 1957; van Dijk 1997). All studies except Karatosun 2008 investigated people with unilateral ankle pain. The study population sizes at randomisation varied: 17 (Salk 2006), 75 (Sun 2014), 28 (Cohen 2008), 30 (Karatosun 2008), 64 (DeGroot 2012), 26 (Witteveen 2010). Participants were 18 years or older. Sun 2014 included participants between the age of 20 and 85 years and Cohen 2008 participants were 50 years or older.
Follow‐up in all studies ranged from 3 to 12 months. Either the Ankle Osteoarthritis Scale (AOS, Domsic 1998) or American Orthopedic Foot and Ankle Society score (AOFAS, Kitaoka 1994) or the Visual Analogue Scale (VAS, Ohnhaus 1975) were used as primary outcome measure. Different types of HA, dosage or dosing schedules were used in each trial. Salk 2006 used 5 weekly injections of 1 ml hyaluronic acid (Hyalgan®) compared to saline. Cohen 2008 used five weekly injections of 2 ml of hyaluronic acid (Hyalgan®) compared to 5 injections of 2 ml of saline. Sun 2014 used a single injection of 2 ml hyaluronic acid (Hyalgan®). Karatosun 2008 used three weekly injections of 2.5 ml hyaluronic acid (Adant®). DeGroot 2012 used a single 2 ml injection of hyaluronic acid (Supartz®) compared to saline. Witteveen 2010 investigated four different doses; single injections of 1, 2, 3 ml, and 3 weekly injections of 1 ml (3 x 1 ml) of hyaluronic acid (Orthovisc®).
Excluded studies
A total of seven studies were excluded because they were not randomised controlled trials (Huang 2006; Luciani 2008; Mei‐Dan 2010; Sarkin 1974; Sun 2006; Sun 2011; Witteveen 2008). See the table of Characteristics of excluded studies.
Risk of bias in included studies
2.
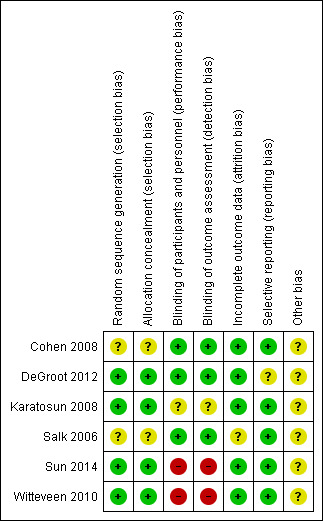
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
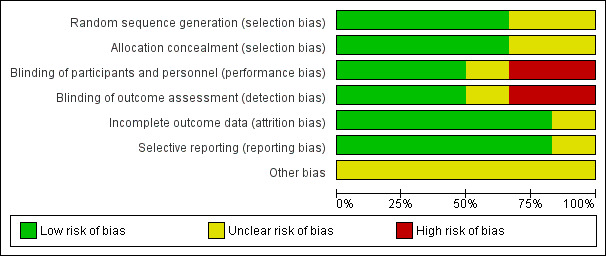
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generally most randomised controlled trials (4/6) described their randomisation process adequately (low risk of bias). Cohen 2008 and Salk 2006 mentioned a randomised component; however the process was not described so it was unclear which process was used to conceal allocation.
Blinding
Three studies were classified as having a low risk for performance bias and detection bias (Cohen 2008; DeGroot 2012; Salk 2006). Karatosun 2008 was classified as unclear for performance bias and detection bias: it is most likely, since the participants were not blinded, that they informed the physical therapist about the treatment they got. Since the outcome was partly participant‐reported, detection bias was considered unclear because these results can be affected by the fact the participant might have a preference for either therapy. For Sun 2014 we assessed a high risk for performance bias, since the participants could not be blinded so most likely this information went to the therapist, which could influence the outcome; the secondary outcomes could be biased by this information so detection bias was considered to be high as well. Witteveen 2010 was classified as high risk for performance and detection bias: participants were not blinded, and it is likely that they judged the fact that they got more injections as better, and therefore performed better, which might have resulted in a better outcome.
Incomplete outcome data
All studies but one were classified as low risk for incomplete data. Salk 2006 described three participants that did not complete the study. However an intention‐to‐treat analysis (ITT) was not described (unclear risk).
Selective reporting
For DeGroot 2012 it was unclear if there was reporting bias: there was a follow‐up of only three months, which can favour placebo and therefore affect the results.
Other potential sources of bias
Cohen 2008 was classified as an unclear risk because there was a difference in participant demographics: a significant difference between the mean age of participants in each group was noted as well as a difference between baseline AOS total scores and Western Ontario and McMasters Universities (WOMAC) pain scores (Bellamy 1988). DeGroot 2012 was also classified as an unclear risk for other bias since the placebo and treatment group were of unequal sizes, 25 compared to 39. Karatosun 2008 was also classified as having an unclear risk because the group that was assigned to exercise therapy had a significantly higher AOFAS score at baseline. Witteveen 2010 was classified unclear since the group that received the 2 ml injections performed unexplainably badly.
Effects of interventions
See: Table 1
Primary analysis: intra‐articular injection of hyaluronic acid compared to placebo (3 studies):
Three studies compared the intra articular injection of hyaluronic acid in the ankle to placebo (saline) (Cohen 2008; DeGroot 2012; Salk 2006). Table 1.
BENEFITS:
PainAnalysis 1.1,
1.1. Analysis.
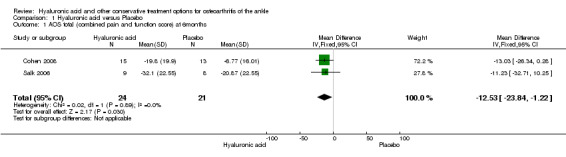
Comparison 1 Hyaluronic acid versus Placebo, Outcome 1 AOS total (combined pain and function score) at 6months.
For the outcome 'pain', the AOS pain (at three months) and the total AOS score (at six months) were used to compare the studies. The total AOS was used to make possible a comparison of the two studies used in the meta‐analysis for the primary outcome at six months. Upon contacting the authors no additional information could be provided to perform a sub‐pooled analysis for AOS pain at six months.
In the meta‐analysis (two studies: Cohen 2008 and Salk 2006; 45 participants) compared to control at six months (primary outcome) the AOS total score was 12.53 points lower mean difference (MD) in favour for HA (95% confidence interval (CI) −23.84 to −1.22; Analysis 1.1). We downgraded the quality of evidence from high to low due to the limitation in study design (unclear risk of bias) and imprecision of result (low number of participants). At three months (two studies: Cohen 2008 and DeGroot 2012; 92 participants) compared to control the total AOS score was 2.26 lower points lower (MD) (95% CI −11.23 to 6.72 Analysis 1.2,) We downgraded the quality of evidence from high to very low due to a serious imprecision of results (low number of participants and studies are on opposite sides of null effect) and limitation in study design (unclear bias). At three months (two studies: Cohen 2008, DeGroot 2012; 92 participants) compared to control the AOS sub score pain was 1.83 points lower (MD) (95% CI −11.33 to 7.68; Analysis 1.3,). We downgraded the quality of evidence from high to very low due to serious imprecision of results (low number of participants and studies are on opposite sides of null effect) and limitation in study design (unclear bias).
1.2. Analysis.
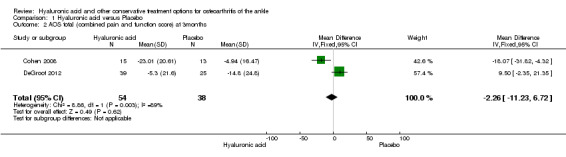
Comparison 1 Hyaluronic acid versus Placebo, Outcome 2 AOS total (combined pain and function score) at 3months.
1.3. Analysis.
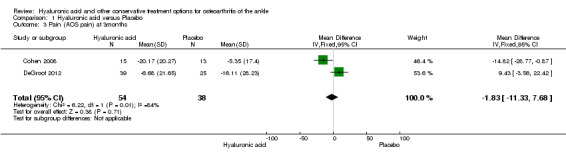
Comparison 1 Hyaluronic acid versus Placebo, Outcome 3 Pain (AOS pain) at 3months.
Physical functionAnalysis 1.1,
To compare physical function between studies, the AOS disability score (at three months) and the AOS total score (at six months) was used. The total AOS was used to make possible a comparison of the two studies used in the meta‐analysis for the primary outcome at six months. Upon contacting the authors no additional information could be provided to perform a sub analysis for AOS disability at six months.
In the meta‐analysis at six months (primary outcome) (two studies: Cohen 2008 and Salk 2006; 45 participants) compared to control the AOS total score was 12.53 points lower (MD) in favour of HA (95% CI −23.84 to −1.22; Analysis 1.1). We downgraded the quality of evidence from high to low due to the limitation in study design (unclear risk of bias) and imprecision of result (low number of participants). At three months (two studies: Cohen 2008 and DeGroot 2012; 92 participants) compared to control the total AOS score was 2.26 points lower (MD) (95% CI −11.23 to 6.72; Analysis 1.2). We downgraded the quality of evidence from high to very low due to serious imprecision of results (low number of participants and studies are on opposite sides of null effect) and limitation in study design (unclear bias). At three months (two studies: Cohen 2008 and DeGroot 2012; 92 participants) compared to control the AOS sub score disability was 0.13 points lower (MD) (95% CI −9.26 to 9.01; Analysis 1.4). We downgraded the quality of evidence from high to very low due to serious imprecision of results (low number of participants and studies are on opposite sides of null effect) and limitation in study design (unclear bias).
1.4. Analysis.
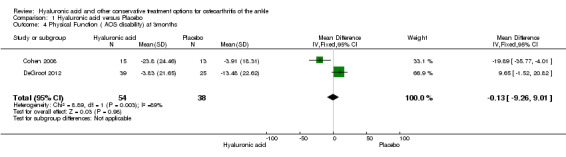
Comparison 1 Hyaluronic acid versus Placebo, Outcome 4 Physical Function ( AOS disability) at 3months.
Radiographic joint structure changes was not examined in either study.
Quality of life as outcome was only described in two studies (Cohen 2008 and Salk 2006); both used the Short‐Form 12 (SF12) (Ware 1996).
Cohen 2008: SF12 demonstrated no significant difference in their paper between either group at six months, no exact scores were mentioned in the study results and could not be provided upon contacting the author.
Salk 2006: SF12 demonstrated a significant difference in their paper favouring hyaluronic acid at six months, no standard deviations were present in the result section of the study, upon contacting the author they could not be provided.
Since the exact scores were not available, no meta‐analysis could be performed for this score.
HARMSAnalysis 1.5; Analysis 1.6; Analysis 1.7
1.5. Analysis.
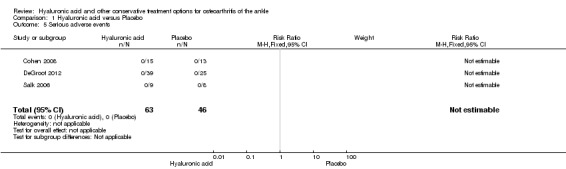
Comparison 1 Hyaluronic acid versus Placebo, Outcome 5 Serious adverse events.
1.6. Analysis.
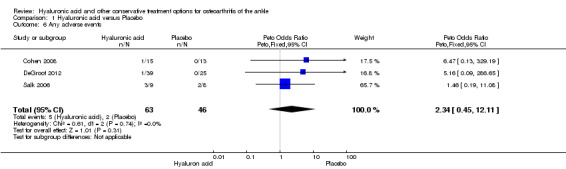
Comparison 1 Hyaluronic acid versus Placebo, Outcome 6 Any adverse events.
1.7. Analysis.
Comparison 1 Hyaluronic acid versus Placebo, Outcome 7 Patients who withdraw because of an adverse event.
A meta‐analysis (three studies: Cohen 2008, DeGroot 2012, Salk 2006; 109 participants) showed a similar amount of AEs in either group (Peto odds ratio (Peto OR) 2.34, 95% CI 0.45 to 12.11; Analysis 1.6). No SAEs were found and no participant withdrew due to an AE (Analysis 1.5; Analysis 1.7).
Heterogeneity and sensitivity analysis:
A substantial heterogeneity of 89% was found for Analysis 1.2 . For Analysis 1.3 84%; and 89% for Analysis 1.4. Due to the fact that each analysis, except the harms analyses, only contained two studies no sensitivity analyses was done.
Intra‐articular injection of hyaluronic acid compared to exercise therapy:
Karatosun 2008 described the comparison of injection HA to exercise therapy (Appendix 8).
BENEFITS:
Pain during activity (VAS 0 to 10) showed a decrease in pain (end point was at 12 months) (MD −0.70, 95% CI −2.54 to 1.14; Analysis 2.1). We downgraded the quality of evidence from high to low due to the unclear risk of bias and small sample size (imprecision of results and limitation of design).
2.1. Analysis.
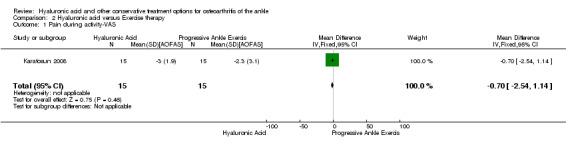
Comparison 2 Hyaluronic acid versus Exercise therapy, Outcome 1 Pain during activity‐VAS.
Physical function : At 12 months compared to exercise the AOFAS score was 13.10 points higher (MD) in favour of hyaluronic acid (95% CI 2.97 to 23.23 Analysis 2.2) on a scale of 0 to 100. We downgraded the quality of evidence from high to low due to the unclear risk of bias (limitation in study design) and small sample size (imprecision of result). At 12 months compared to exercise the walking distance was 0.30 points (MD) better in favour of exercise therapy at 12 months (95% CI −1.27 to 0.67; Analysis 2.3) We downgraded the quality of evidence from high to low due to the unclear risk of bias and small sample size.
2.2. Analysis.
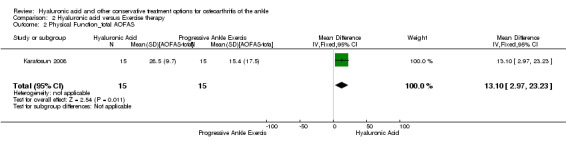
Comparison 2 Hyaluronic acid versus Exercise therapy, Outcome 2 Physical Function_total AOFAS.
2.3. Analysis.
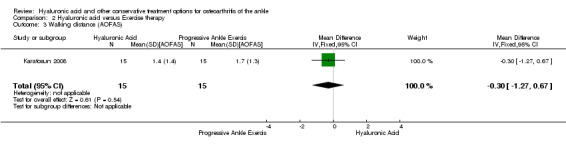
Comparison 2 Hyaluronic acid versus Exercise therapy, Outcome 3 Walking distance (AOFAS).
Radiographic joint structure changes was not measured.
No quality of life score was measured.
HARMS:
No AEs were found for either group.
Intra‐articular injection of hyaluronic acid combined with exercise therapy compared to intra‐articular botulinum toxin A (BoNT‐A) injection:
Sun 2014 described the comparison of HA injection combined with exercise therapy to an intra‐articular injection of botulinum toxin A (Appendix 9).
BENEFITS:
Pain: At six months compared to botulinum toxin A the AOS pain score of the affected joint showed a decrease in pain (MD 0.10, 95% CI −0.42 to 0.62: Analysis 3.1). We downgraded the quality of evidence from high to low due to the high risk of bias and small sample size.
3.1. Analysis.
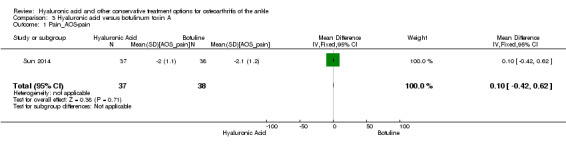
Comparison 3 Hyaluronic acid versus botulinum toxin A, Outcome 1 Pain_AOS‐pain.
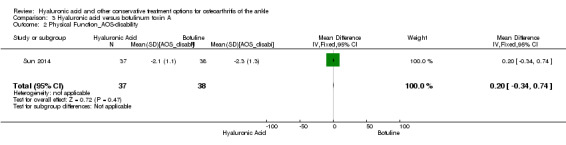
Physical function : At six months compared to botulinum toxin A the AOS disability score showed a decrease in physical function (MD 0.20, 95% CI −0.34 to 0.74; Analysis 3.2). We downgraded the quality of evidence from high to low due to the high risk of bias and small sample size.
3.2. Analysis.
Comparison 3 Hyaluronic acid versus botulinum toxin A, Outcome 2 Physical Function_AOS‐disability.
Radiographic joint structure changes was not measured.
No quality of life score was measured.
HARMS:
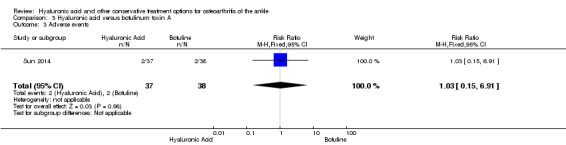
In the HA group 2/37 (5.9%) AEs were found, in the BoNT‐A 2/38 (5.8%) (RR 1.03, 95% CI 0.15 to 6.91; Analysis 3.3). The AEs consisted of transient injection site reaction and were mild/moderately painful and resolved without treatment.
3.3. Analysis.
Comparison 3 Hyaluronic acid versus botulinum toxin A, Outcome 3 Adverse events.
Intra‐articular sodium hyaluronate injections in the osteoarthritic ankle joint: Effects, safety and dose dependency:
Witteveen 2010 randomised trial; four different dosages of intra‐articular injections of HA were randomly allocated; 1 ml, 2 ml, 3 ml and 3 weekly injections of 1 ml were compared for efficacy. Primary endpoint of the study was 15 weeks (Appendix 10).
Benefits:
Pain (during walking (VAS): None of the VAS‐scores for ‘pain during walking activities’ decreased significantly at week 15. The 3 x 1 ml dose group performed best (P = 0.075). The VAS‐scores of the 1, 2, and 3 ml dose groups separately did not change significantly as compared to baseline scores at both secondary endpoints (week 7 and 27) (0.23 < P < 0.74). At week 7, a statistically significant median decrease of the VAS‐score of 29 mm was observed in the 3 x 1 ml dosage group (P = 0.046). The median change in decrease of pain at 27 weeks was best for 3 x 1 (−30), however this was not statistically significant (P = 0.25). We downgraded the quality of evidence from high to moderate due to small sample sizes (imprecision of results).
Physical function: No physical function was measured.
Radiographic joint structure changes was not measured.
Quality of life: No quality of life was measured.
Harms:
Adverse events: AEs happened the most in the 2 ml group (57%), other groups had an adverse event rate of 14% to 17%. The total number of AEs was 7 out of 26 participants (27%). These AEs consisted of increased pain and swelling of the ankle joint. They were mild or moderate in severity and resolved within 3 days. One participant experienced severe pain and swelling for a week.
No serious adverse events were reported.
Discussion
Summary of main results
No other RCT concerning any other conservative treatment was identified except six RCT's, analysing the use of hyaluronic acid (HA) for ankle osteoarthritis (OA).
A total of 240 participants diagnosed with ankle OA were included in this review. The primary analysis concerned three RCTs (109 participants) which compared HA to placebo (Table 1). A meta‐analysis was performed to investigate the benefits and harms: HA showed a lower AOS total score than placebo at six months (primary outcome). The total AOS was used to make a comparison between studies possible, no exact sub‐scores (AOS pain or disability) for the outcome at six months could be provided upon contacting the authors. The difference in score was found to be promising; however it is not known if a mean difference of 12.53 points on a 100 point scale is clinically relevant. No minimal important clinical difference is known for this score. At three months a decrease (1.83 points) was found for the AOS sub‐score pain in favour of HA; however CI are wide and sample sizes are small, which make these results inconclusive. The AOS sub‐score for disability decreased 0.13 points at three months in favour of HA. Since CI were wide and sample sizes are small these results are difficult to interpret and inconclusive.
Quality of life was difficult to judge due to the fact that the exact numbers were missing, Salk 2006 demonstrated a difference in favour of HA in his paper, Cohen 2008 found similar results between both groups.
There were a few adverse events (AEs); 5/63 (8%) in the HA group and 2/46 (4%) in the placebo group. The Peto odds ratio (Peto OR) to have an adverse event was 2.34 higher compared to the control group (95% CI 0.45 to 12.11). This evidence is inconclusive because of a wide CI and a small number of events. Evidence for this pooled analysis was graded as low due to limitation in study design (unclear risk of selection bias for two studies and unclear risk for attrition bias for one study); and imprecision of results based on a small population size (109 participants; the total sample size is lower than the calculated optimal information size of 400 participants for continuous outcomes).
Karatosun 2008 compared HA and exercise therapy; a decrease in pain (VAS 0 to 10) of 0.7 points was found at 12 months. Since the CI crosses 0 and sample sizes are small (30 participants) these results are inconclusive. For physical function at 12 months the total AOFAS score (0 to 100) was 13.10 higher in favour of hyaluronic acid: this result is considered promising. These results were also graded as low due to limitation in study design; bias of blinding was unclear and other bias was unclear and imprecision of results due to a small population size (30).
Sun 2014 described the comparison of hyaluronic acid injection combined with exercise therapy to an intra‐articular injection of botulinum toxin A. A decrease in pain and physical function were found in both groups. The decrease, however, is small: for pain it was 0.10 and for physical function 0.20 (on a scale of 0 to 100). Since the reduction in pain and physical function is so small, it is probably not clinically relevant. Also sample sizes (75 participants) are small and the CI crosses 0: the results are therefore considered inconclusive. The number of adverse events were comparable in both groups. This evidence was also graded as low due to limitation in study design; a high risk of bias for blinding of outcome and participants; and imprecision of results due to a small population size (75).
Witteveen 2010 compared four different dosing schedules for intra‐articular injections of HA for efficacy and safety (26 participants). The best median decrease in pain on walking VAS (on a scale of 0 to 100) was shown for 3 x 1 ml at 27 weeks with a median decrease of 30. Physical function, radiographic changes and quality of life were not measured. The total number of AEs was 27%; most of them occurred in the 2 ml group (57%). No participants withdrew due to an AE and no SAEs were noted. This evidence was graded as low due to imprecision of results due to a small sample size of participants (26) and a limitation in study design — high risk of bias for blinding of outcome and participants.
Overall completeness and applicability of evidence
The objective of this review was to assess the benefits and harms of any conservative treatment of ankle OA. No randomised or clinical controlled trials were identified besides the six aforementioned RCTs. These trials all concerned the use of HA infiltrations for ankle OA. No trial (RCT/CCT or ongoing trials) were identified concerning any other conservative treatment.
Three trials were pooled; HA was compared to placebo. Different dosage schedules were used between the studies. Cohen 2008 used 5 weekly injections of 2 ml Hyalgan®, Salk 2006 used 5 weekly injections of 1 ml Hyalgan®, whereas DeGroot 2012 used a single injection of 2.5 ml of Supartz®. At this point it is unclear what dosage should be used for each type of hyaluronic acid injections. For instance it was found by Witteveen 2010 that 3 x 1 ml of Orthovisc® performed best for this type of HA. HA restores the rheologic properties of the joint, and is thought to protect the cartilage by improving the viscoelasticity (Balazs 1993; Bellamy 2006).
There is a remarkable difference in results between the primary outcome at six months (AOS total) and the individual scores (AOS pain and AOS disability) and the AOS total at the additional time point of three months. Since the results at three months are difficult to interpret due to a serious imprecision of results this needs further investigation. A possible explanation can be the fact that the placebo effect might wear off at three months, but at this point this is nothing more than speculation. It is not clear which grade of OA responds best to HA infiltrations; however grade 3 van Dijk or grade 4 Kellgren Lawrence are less likely to respond. The three trials included in the meta‐analysis all included grade 2, 3 and 4 of Kellgren Lawrence without making a subgroup analysis.
HA in these studies is thought to improve pain and function; this is mainly the short‐term of effect. The long‐term effect, by improving the rheologic properties, is thought to slow down progression of the osteoarthritis of the joint; however none of these studies investigated this outcome. Karatosun 2008 investigated 3 weekly injections of 2.5 ml Adant® compared to 6 weeks of exercise therapy; these people suffered sometimes from bilateral ankle OA and knee pain as well. Sun 2014 compared one injection of 2ml of Hyalgan® combined with 4 weeks of 3‐weekly sessions of physical therapy to one injection of Botulinum toxin A. Both injections are assumed to improve pain; why exercise therapy was added to hyaluronic acid remains unclear and seems unnecessary. All these differences between studies—the uncertainty about factors like dosage schedule, the ideal grade of ankle OA for this kind of treatment, and the lack of evidence for other types of conservative treatment—make it difficult to assess the applicability of evidence. At this point no valid recommendations can be made.
Quality of the evidence
Overall the quality of the evidence showed some serious limitations. There was a limitation in design and implementation and imprecision of the results for the meta‐analysis. Limitation in study design was based on the risk of bias which was judged to be at low risk or unclear for all the categories concerning the three studies used in the meta‐analysis. Cohen 2008 was marked unclear because this study showed no clear randomisation and allocation process and there was a baseline imbalance between both groups for age. DeGroot 2012 was marked unclear for other bias because the study had an unequal size in number of participants between treatment and placebo group (39/25). The follow‐up of this study was limited to three months, it is possible that if the follow‐up had been longer the treatment group could have performed better due to the diminishing effect of placebo. Salk 2006 was marked unclear because he had no description of the randomisation and allocation process. All three studies concerning the meta‐analysis included Kellgren Lawrence grade IV patients, severe arthritis is known not to respond well to hyaluronic acid treatment, this was also judged as an unclear bias. All these trials had a very low number of participants, the total number of participants used for the pooled analysis was 109, this total sample size is lower than the calculated optimal information size of 400 patients for continuous outcomes. This limitation and imprecision of results led to downgrading the evidence to low for the major outcomes. The limitation in study design and imprecision of results also led to a downgrade of two levels for the primary analysis; comparing HA to placebo, in the SOF table, resulting in a low quality as well.
Karatosun 2008 had a limitation of study design; an unclear risk of performance and detection bias because blinding was unclear for the participant and the evaluator could be biased since the participant was aware which treatment he underwent, other bias was marked unclear because some participants had bilateral involvement of ankle OA which make judgement of efficacy difficult and a small and an imprecision of results due to a small participant size (30), which led to downgrading the evidence to low for all outcomes. Sun 2014 had a limitation study design due to a high risk bias due to the lack of blinding of participants and evaluators and also an imprecision of results due to a small sample size (75). This led to downgrading of the evidence to low for all outcomes. Witteveen 2010 had a limitation in study design due to high risk in performance and detection bias, participants could not be blinded which possibly led to bias in participant‐reported outcome, there was also an imprecision of results due to a small sample size this led to downgrading the evidence to low for all outcomes.
Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. No other reasons for downgrading the evidence were found for any of the included studies (indirectness of evidence, unexplained heterogeneity, high probability of publication bias).
Potential biases in the review process
To minimise the change of bias during the review process, the review was performed according to the published protocol. Due to the fact we did find a low number of eligible studies a sensitive search was added in order to include as much studies as possible and to minimise the chance of publication bias. A sensitive search strategy was designed to retrieve trials from electronic bibliographic databases, not limited to any intervention or language. Our search also included a search for ongoing and recently completed trials. However it is still possible that potentially relevant trials have been missed. In order to get additional data from retrieved trials, trialists were contacted, they were forthcoming, however no further data could be obtained. A meta‐analysis was conducted and data were pooled, it is possible that due to missing data, unclear biases in the pooled trials, pooling of small sample sizes and comparing trials that used different dosing schedules, data were compared that are not truly comparable, in this way potential bias might be introduced.
Agreements and disagreements with other studies or reviews
The number of studies and reviews concerning the use of hyaluronic acid for ankle osteoarthritis are very limited. Three reviews were identified (Abate 2012; Chang 2013; Migliore 2011). One randomised study was included in all these reviews and was not eligible in our review, since HA was administered arthroscopically after arthroscopic debridement (Carpenter 2008). Abate 2012 reviewed four randomised controlled trials—Carpenter 2008, Cohen 2008, Salk 2006, Karatosun 2008—and five case series. No pooled analysis was performed. They concluded that there was no evidence on the efficacy of HA in reducing pain and improving function in ankle OA. Their advice for future research was to look at an adequate dose regimen, a good outcome measure, identify which patients and grade of OA benefit best of hyaluronic acid injections.
Chang 2013 included five randomised controlled trials (Carpenter 2008; Cohen 2008; DeGroot 2012; Karatosun 2008; Salk 2006), one double arm and four single arm prospective studies. All studies were pooled based on improvement scores from baseline. A significant reduction in pain was found for HA injections based on the pooled effect size of improvement scores from baseline at three months, indicating that intra‐articular HA is an effective therapeutic approach for ankle OA. A not statistically significant difference was found in favour for HA comparing HA to placebo at three months.
Migliore 2011 included four randomised trials (Carpenter 2008; Cohen 2008; Karatosun 2008; Salk 2006); and four single arm studies. Due to the heterogeneity of studies, data could not be pooled. Every study and the conclusion was described. The overall conclusion was that viscosupplementation is useful in ankle OA. Future prospective studies need to use standardised outcomes.
The present review was restricted to an analysis of data from randomised controlled trials; only comparable data were pooled. It was found that at six months, which was our primary time point, HA is superior to placebo for the total AOS score (MD −12.53, 95% CI −23.84 to −1.22). However, this is based on low quality of evidence. No individual scores (AOS pain or disability) for this comparison at six months were available. It is not known if a mean difference of 12.53 points on a 100 point scale is clinically relevant. At three months, which we specified as an additional time point, the individual AOS pain and AOS disability score are inconclusive for the pooled analysis (as are the total AOS), due to a serious imprecision of results (studies are on opposite sides of null effect) and limitation in study design; this evidence was downgraded to very low quality.
Authors' conclusions
Implications for practice.
Currently, there is insufficient data to create a synthesis of the evidence as a base for future guidelines for ankle osteoarthritis. Since the aetiology of ankle OA is different, guidelines that are currently used for hip and knee OA may not be applicable for ankle OA. Simple analgesics as recommended for hip and knee OA seem, however, a reasonable first step to treat ankle OA. It is unclear if there is a benefit or harm for HA as treatment for ankle OA compared to placebo at six months based on a low quality of evidence. Inconclusive results were found comparing HA to other treatments. HA can be conditionally recommended if patients have an inadequate response to simple analgesics. It remains unclear which patients (age, grade of ankle OA) benefit the most from HA injections and which dosage schedule should be used.
Implications for research.
To find evidence for conservative treatment of ankle OA current treatment possibilities, as described in the background section, should be tested against placebo in well‐conducted randomised controlled trials. Treatment should be tested for age and grade of osteoarthritis. Dosage schedules for medication should be optimised and tested in RCTs. Validated participant‐ and doctor‐based outcome parameters should be used. Pain and function improvement could be relevant: these parameters can be measured by outcome measures as described in the Method section. Radiographic changes can be important to monitor or to evaluate the radiographic progression of osteoarthritis. Evaluation of evidence from different RCTs in combination with the experience from the different specialists in the field of OA, as well as participants' experiences can lead to a useful guideline for treatment of ankle OA.
Acknowledgements
We would like to thank Elizabeth Ghogomu and Tamara Reader and the editorial team of the Cochrane review group for their contributions.
Appendices
Appendix 1. CENTRAL search strategy
| 1 MeSH descriptor Osteoarthritis explode all trees 2 MeSH descriptor arthritis explode all trees 3 (osteoarthritis OR arthritis OR arthrosis OR osteoarthrosis OR (degenerative near/3 (arthr* OR disease))) 4 MESH descriptor ankle 5 MESH descriptor ankle joint 6 #4 OR #5 7 ankle 8 (#1 OR #2 OR #3) 9 (#6 OR #7) 10 (#8 AND #9) |
Appendix 2. MEDLINE search strategy
|
Search terms for design 1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 clinical trials as topic.sh. 6 randomly.ab. 7 trial.ti. 8 or/1‐7 9 exp animals/ not humans.sh. 10 8 not 9 |
|
Search terms for population 11 Ankle/ or Ankle Joint/ 12 ankle.af. 13 exp Osteoarthritis/ 14 exp Arthritis/ 15 (osteoarthritis or arthritis or arthrosis or osteoarthrosis or (degenerative adj (arthr$ or disease))).af. 16 11 or 12 17 13 or 14 or 15 18 16 and 17 |
|
Combining terms 19 10 and 18 |
Appendix 3. EMBASE search strategy
|
Search terms for design 1 randomised controlled trial.sh. 2 randomization.sh. 3 exp clinical trials/ 4 (clin$ adj25 trial$).ti.ab 5 random$.ti.ab. 6 or/1‐5 |
|
Search terms for population 7 Ankle/ or Ankle Joint/ 8 ankle.af. 9 exp Osteoarthritis/ 10 exp Arthritis/ 11 (osteoarthritis or arthritis or arthrosis or osteoarthrosis or (degenerative adj3 (arthr$ or disease))).ti.ab. 12 7 or 8 13 9 or 10 or 11 14 12 and 13 |
|
Combining terms 15 6 and 14 |
Appendix 4. PsycINFO search strategy
|
Search terms for design 1 clinical trial.mp or exp Clinical Trials 2 randomised controlled trial.mp. 3 clinical trial*.af. 4 random*.af. 5 placebo.af. 6 (randomised controlled trial or controlled clinical trial) .af. or trial .ti. 7 1 or 2 or 3 or 4 or 5 or 6 or 7 8 limit 7 to human |
|
Search terms for population 9 exp Ankle/ 10 ankle.af. or ankle joint.af. 11 9 or 10 12 exp Arthritis/ 13 (osteoarthritis or arthritis or arthrosis or osteoarthrosis or (degenerative ˜ (arthr* or disease))).af. 14 12 or 13 or 14 15 11 and 15 |
|
Combining terms 16 8 and 15 |
Appendix 5. CINAHL search strategy
|
Search terms for design 1 (MH “Clinical Trials+”) 2 (MH “Random Assignment”) 3 TX (clin$ n25 trial$) 4 TX random$ 5 S1 or S2 or S3 or S4 |
|
Search terms for population 6 Osteoarthritis 7 (MH “Osteoarthritis”) 8 TX osteoarthritis 9 TX arthritis 10 TX osteoarthrosis 11 TX degenerative n3 disease 12 Ankle 13 Ankle joint 14 TX ankle 15 S6 or S7 or S8 or S9 or S10 or S11 16 S12 or S13 or S14 17 S15 and S16 |
|
Combining terms 18 S5 and S17 |
Appendix 6. PEDro search strategy
| 1 Osteoarthritis in title or abstract 2 Method: clinical trial 3 Body part: foot or ankle Combination 1 and 2 and 3 |
Appendix 7. AMED search strategy
| 1 Ankle/ or Ankle Joint/ 2 ankle.af. 3 exp Osteoarthritis/ 4 exp Arthritis/ 5 (osteoarthritis or arthritis or arthrosis or osteoarthrosis or (degenerative adj (arthr$ or disease))).af. 6 1 or 2 7 or/3‐5 8 or/3‐5 9 6 and 7 10 exp Surgery/ 11 Surgery operative/ 12 surg$.tw. 13 surg$.tw. 14 10 or 11 or 12 15 11 or 12 or 13 16 9 not 14 |
Appendix 8. Results included studies: Karatuson 2008
| Karatuson 2008 | ||||||
| follow‐up 12 months | ||||||
| PAIN | DISABILITY | DISABILITY | HARMS | |||
| Pain during activity ‐ VAS SD (mean) | Activity limitation ‐ AOFAS SD (mean) | AOFAS SD (mean) | Adverse Events | |||
| Hyaluronic acid (HA) group | N = 15 | 3 injections of HA at 1‐week intervals, 2.5mg | from 5,4 (2,1) to 1,4 (1,9) | from 6,6 (2,4) to 8,5 (1,8) | from 61.6 (16.8) to 90.1 (9.7) | no complications due to HA injection |
| Progressive Ankle Exercise | N = 15 | 6 weeks exercise (week 1, 2, 3, 6) | from 4,7 (2,8) to 2,4 (3,1) | from 7,2 (2,1) to 8,8 (1,5) | from 72.1 (16.6) to 87.5 (17.5) | |
Appendix 9. Results included studies: Sun 2014
| Sun 2014 | |||||||
| follow‐up 6 months | |||||||
| PAIN | PAIN | DISABILITY | DISABILITY | HARMS | |||
| AOS‐pain SD (mean) | Pain VAS SD (mean) | AOS‐disability SD (mean) | AOFAS SD (mean) | Adverse Events | |||
| Hyaluronate group | N = 37 | 2 ml | from 4,5 (1,1) to 2,5 (1,1) | from 3,9 (1,2) to 1,7 (1,1) | from 5,0 (1,3) to 2,9 (1,1) | from 70,0 (11,7) to 86,4 (12,5) | 2 patients (5.9%) reported mild to moderate pain |
| Botuline | N = 38 | reconstituted in 2 cc normal saline | from 4,5 (1,3) to 2,4 (1,2) | from 4,0 (1,8) to 1,8 (0,9) | from 5,2 (1,9) to 2,9 (1,3) | from 17,3 (11,6) to 88,3 (7,2) | 2 patients (5.6%) reported mild to moderate pain |
Appendix 10. Results included studies: Witteveen 2010
| Baseline | follow‐up 7 weeks | follow‐up 15 weeks | follow‐up 27 weeks | ||||
|
Benefits: PAIN during walking activities |
PAIN during walking activities | PAIN during walking activities | PAIN during walking activities | Harms | |||
| dosage group | VAS pain median (range) | VAS pain median change (range) | VAS pain median change (range) | VAS pain median change (range) | General pain (% improvement at 27 weeks) | Adverse events | |
| 1ml | N = 7 | 43 (7 to 71) | 7 (35 to 21) | 1 (58 to 22) | 6 (22 to 22) | 0% | 14% (1 patient reported mild to moderate pain) |
| 2ml | N = 7 | 81 (46 to 100) | 9 (−65 to 13) | 7 (97 to 19) | 7 (71 to 19) | 0% | 57% (4 patients reported mild to moderate pain) |
| 3ml | N = 6 | 48 (24 to 87) | 6 (39 to 10) | 7 (41 to 2) | 7 (87 to 17) | 0% | 17% (1 patient reported mild to moderate pain) |
| 3x1ml | N = 6 | 61 (16 to 88) | 29 (−78 to 7) | 47 (78 to 26) | 30 (78 to 26) | 67% | 17% (1 patient experienced severe pain) |
Data and analyses
Comparison 1. Hyaluronic acid versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AOS total (combined pain and function score) at 6months | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐23.84, ‐1.22] |
| 2 AOS total (combined pain and function score) at 3months | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐2.26 [‐11.23, 6.72] |
| 3 Pain (AOS pain) at 3months | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐11.33, 7.68] |
| 4 Physical Function ( AOS disability) at 3months | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐9.26, 9.01] |
| 5 Serious adverse events | 3 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Any adverse events | 3 | 109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.34 [0.45, 12.11] |
| 7 Patients who withdraw because of an adverse event | 3 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Hyaluronic acid versus Exercise therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during activity‐VAS | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐2.54, 1.14] |
| 2 Physical Function_total AOFAS | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 13.1 [2.97, 23.23] |
| 3 Walking distance (AOFAS) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.27, 0.67] |
Comparison 3. Hyaluronic acid versus botulinum toxin A.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain_AOS‐pain | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.42, 0.62] |
| 2 Physical Function_AOS‐disability | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.34, 0.74] |
| 3 Adverse events | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 6.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cohen 2008.
| Methods | Randomised controlled trial (RCT), blinded, parallel group. Five weekly injections compared to placebo. | |
| Participants | 28 participants; aged 50 years of older (30 originally at randomisation); intention to treat consisted of 15 in Hyalgan group (mean age 56.2 (SD 15.1), 1 female, 14 male) and 13 in placebo group (mean age 43.4 (SD 14.9), 2 female, 11 male) diagnosed with ankle OA based on pain and osteoarthritis on X‐ray. Kellgren Lawrence stage 2, 3 and 4 were included. | |
| Interventions | Hyalgan 2 ml intra‐articular 5 weekly injections versus Saline 2 ml intra‐articular 5 weekly injections. | |
| Outcomes | Primary outcome: Ankle Osteoarthritis Scale (AOS) (pain on movement and weightbearing) for ITT population at 3 months. Secondary outcome: Western Ontario and McMasters Universities (WOMAC) osteoarthritis (OA) index of pain, Physical function, Short‐Form 12(SF12). Follow‐up at 2 weeks, 6 weeks, 3 months and 6 months. | |
| Notes | After randomisation 2 participants declined an injection in either group. Results show same outcome as method section. Independent investigator‐sponsored trial funded by Sanofi‐Aventis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There is no description how the randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Saline injections were used as comparators to blind participants. While there may be differences in the injection (e.g. difficulty injecting the more viscous hyaluronic acid), we feel it is unlikely that participants would be aware of the difference and that the blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The treating investigator giving the injections did not conduct the evaluations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Adverse effects were not clearly described. However the number was low and there was no preference for either group; so it is considered a low risk. |
| Other bias | Unclear risk | Mean age is statistically different between treatment groups. Stage 4 of Kellgren Lawrence is a severe grade of osteoarthritis (OA) and not likely to respond to treatment with hyaluronic acid, it is unclear if there was any baseline imbalance based on the grade of ankle OA. There is insufficient information to permit judgement of low risk or high risk of bias. |
DeGroot 2012.
| Methods | Randomised controlled trail, double blinded, parallel group trial. | |
| Participants | 64 participants; ankle OA of at least Kellgren Lawrence grade 2. Thirty‐nine participants in hyaluronic acid (HA) group (mean age 54.1 (SD 14.5,2.3), 15 female, 24 male) and 25 in saline group (mean age 61.9 (SD 14.1,2.8), 13 female, 12 male) Diagnosed with ankle OA based on an x ray, grade 2, 3 or 4 of Kellgren Lawrence system | |
| Interventions | Single injection of Supartz®; hyaluronic acid 2.5 ml intra‐articular versus Saline injection | |
| Outcomes | Primary outcome: change in baseline of American Orthopedic Foot and Ankle Society score (AOFAS score) at 6 weeks and 12 weeks. Secondary outcomes: change from baseline AOS score and visual analogue scale (VAS) at 6 and 12 weeks. Safety (recording adverse effects). | |
| Notes | No dose finding, single injection with Supartz®. 87.5 % of participants completed the study (56/64) No external funding was received from any source for this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple non‐block randomisation. |
| Allocation concealment (selection bias) | Low risk | Selection was based on selecting an opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Saline injections were used as comparators to blind participants. While there may be differences in the injection (e.g. difficulty injecting the more viscous hyaluronic acid), we feel it is unlikely that participants would be aware of the difference and that the blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The treating investigator giving the injections did not conduct the evaluations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Unclear risk | AOFAS (not validated) is not a common instrument to report the efficacy of hyaluronic acid compared to literature. Follow‐up is limited to 3 months, it is more common to use 6 months as either primary outcome or additional endpoint. At 3 months the effect of placebo might be higher, thus creating a more positive outcome for placebo (desired outcome) when comparing hyaluronic acid to placebo. |
| Other bias | Unclear risk | Treatment and placebo group are of unequal size (39 vs 25). There is insufficient information to permit judgement of low risk or high risk of bias. |
Karatosun 2008.
| Methods | Randomised controlled trial, blinded, parallel group of 3 weeks. | |
| Participants | 30 participants; 15 in HA group (mean age 52.1 (SD 11.3), 9 female, 6 male) and 15 in exercise group (mean age 58.1 (SD 12.1), 12 female, 3 male) Kellgren Lawrence III Osteoarthritis (OA) Ankle OA could be bilateral. | |
| Interventions | HA intra‐articular 2.5 mg 3 weekly injections versus 6 weeks of daily exercise therapy | |
| Outcomes | Primary outcome AOFAS score. Follow‐up 1, 2, 3 weeks and 2, 3, 6 and 12 months. Safety (recording of adverse effects), Pain and Physical function as described in the AOFAS: and separated in subsections: VAS testing motion, activity limitation, walking distance, walking surface, gait abnormality, sagittal motion. | |
| Notes | Not clear if there was a minimum age required to be eligible for the study. Not described in Method section 43 ankles and 30 participants, some people had bilateral involvement No funding information available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by drawing lots using a computer program |
| Allocation concealment (selection bias) | Low risk | Well described process. participants were randomised by drawing lots using a computer program. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is very possible that participants told the therapist of the treatment they got. There is insufficient information to permit judgement of low risk or high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is possible that participants told the therapist of the treatment they got. There is insufficient information to permit judgement of low risk or high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting. Outcome is consistent with method section. |
| Other bias | Unclear risk | The group that was assigned to exercises had a significantly higher AOFAS score at baseline. Some participants had bilateral involvement of ankle OA which makes judgement of efficacy difficult. There is insufficient information to permit judgement of low risk or high risk of bias. |
Salk 2006.
| Methods | Randomised controlled trial, blinded, parallel group. Follow‐up at week 2, 6, 12 and 26. | |
| Participants | 17 participants (20 originally) 18 years or older, chronic ankle pain for more than 3 months, Baseline Total AOS score of more than 30 and less than 90; 9 in Hyalgan group (mean age 57.8 (SD 14.7), 5 female, 4 male) and 8 in Saline group (mean age 60.0 (SD 13.9), 5 female, 3 male) Kellgren Lawrence score of ,II,III, or IV.AOS score of > 30 and lower than < 90 at baseline. | |
| Interventions | Hyalgan 1 ml (1 mg/ml) intra‐articular 5 weekly injections versus saline 1 ml intra‐articular 5 weekly injections. | |
| Outcomes | Primary outcome total AOS score. Secondary outcome: Pain (AOS, WOMAC, Pain Global Assessment (PGA), 5‐point scale), physical function: (Range of Motion, AOS), Ankle girth, Quality of life (EuroQoL‐5 Dimensions (EQ5D), SF12). Recording of outcome and adverse events at each clinic visit. Safety (number of serious adverse effects, amount of rescue medication). | |
| Notes | Mean and standard deviation (SD) for AOS were only shown in a graph at baseline, 3 months and 6 months follow‐up. F‐values for 6 months follow‐up were provided. We contacted the author, he could not provide us with additional data. The SD at 6 months was obtained from Mean Difference (MD) data and F‐values for differences in means, according to the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 7 (Section 7.7.3.3) (Higgins 2011). Adverse effects: pain at the injection site 29% adverse effects Support for portions of the study were received from Sanofi‐Synthelabo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of process |
| Allocation concealment (selection bias) | Unclear risk | No description of process |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Saline injections were used as comparators to blind participants. While there may be differences in the injection (e.g. difficulty injecting the more viscous hyaluronic acid), we feel it is unlikely that participants would be aware of the difference and that the blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The treating investigator giving the injections did not conduct the evaluations. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three participants did not complete the study, intention‐to‐treat analysis was not described. However the author confirmed by email that a ITT was undertaken. |
| Selective reporting (reporting bias) | Low risk | Results show same outcomes as described in the Method section. |
| Other bias | Unclear risk | Inclusion criteria: Kellgren Lawrence of IV was also included, this is not common, severe arthritis is known not to respond well to hyaluronic acid treatment. There is insufficient information to permit judgement of low risk or high risk of bias. |
Sun 2014.
| Methods | Randomised controlled trial, blinded, parallel group. Follow‐up of 6 months, at baseline, 2 weeks, 1 month, 3 months and 6 months. | |
| Participants | 75 participants, unilateral ankle pain for at least 6 months; Age between 20 and 85. 37 Hyalgan group (mean age 50.6 (SD 10.3), 14 female, 23 male) and 38 in Botuline group (mean age 49.5 (SD 10.9), 15 female, 23 male) At baseline a AOS score of > 30 and < 90 was mandatory. Ankle OA based on an X ray within 6 months of baseline and equivalent with Kellgren Lawrence grade II | |
| Interventions | Single injection of 2 ml Hyalgan intra‐articular versus Botuline, combined with 12 session (3 weekly for 4 weeks of physical therapy (PT)). | |
| Outcomes | Primary outcome total AOS. Pain (AOS, AOFAS, VAS, Rescue Medication), Safety (registering amount of adverse events), Physical function (AOS, AOFAS, Single Leg stance test (SLS), Timed up and go Test (TUG)), analgesic consumption, satisfaction. | |
| Notes | Two totally different injections are compared, and one group even had a physical therapy program added. Unclear why they compared these two treatments, as it made more sense to compare without physical therapy. The study was supported by a grant of VGHKS100‐061 (an academic research fund from the hospital's medical research council). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation: groups of 4. |
| Allocation concealment (selection bias) | Low risk | Block randomisation: groups of 4. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Injection compared to injection followed by PT makes it likely that the participants communicated which treatment they underwent. Impossible to blind participants to the therapy they underwent. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The secondary outcomes like SLS might be affected by knowing which therapy the participant underwent. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Results show same outcomes as described in the Method section. |
| Other bias | Unclear risk | Unclear what the effect is of two completely different treatment options where one injection + exercise therapy is compared to another injection. |
Witteveen 2010.
| Methods | Randomised controlled trial, single blinded parallel group trial. Primary endpoint of the study was at 15 weeks. Follow‐up at 7, 15 and 27 weeks. | |
| Participants | 26 participants, 18 years or older, ankle osteoarthritis based on a recent X‐ray, showing grade II ankle OA (van Dijk score), At baseline patients had to score an AOFAS pain score between 20 and 40; 7 patients in 1 ml group (mean age 31 (range 26 to 84), 4 male, 3 female), 7 in 2 ml group (mean age 47 (range 33 to 63), 3 male, 4 female), 6 in 3 ml group (mean age 51 (range 39 to 71), 5 male, 1 female), 6 in 3 x 1 ml group (mean age 40 (range 21 to 63), 6 female). | |
| Interventions | Four different dosages of hyaluronic acid were randomly allocated from the storage at the outpatient clinic and injected in the ankle joint, i.e. 1, 2, 3 ml and 3 weekly injections of 1 ml. The injection was placed in the anteromedial portal of the ankle joint. | |
| Outcomes | Pain during walking activities (measured with a 100 mm visual analogue scale), Pain at night and during the day while at rest (VAS), General pain (4‐points scale), the amount of rescue medication, safety (reports of adverse events (AEs) | |
| Notes | The study was sponsored by ZImmer, hyaluronic acid was supplied without costs. Zimmer had no involvement in developing the protocol or the manuscript, they only supplied the hyaluronic acid. Data is owned by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | participants were not blinded, they were randomly allocated to one of the different dosage groups, no comparison to placebo was performed. Outcome might be affected by the expectation to do better with 3 weekly injections than a single injection. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observer was not aware of dose. The participants' reported outcomes might be affected by the fact that people might think they do better from a higher dose of hyaluronic acid. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a high dropout of participants, however an intention‐to‐treat analysis was undertaken. 9 out of 26 dropouts (35%). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes have been reported in the prespecified way |
| Other bias | Unclear risk | The group that received the 2 ml injections performed unexplainably badly. There is insufficient information to permit judgement of low risk or high risk of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Huang 2006 | Not RCT or CCT |
| Luciani 2008 | Not RCT or CCT, prospective open study |
| Mei‐Dan 2010 | Not RCT or CCT, open prospective study |
| Sarkin 1974 | Unclear diagnosis, unclear outcome |
| Sun 2006 | Not RCT or CCT |
| Sun 2011 | Not RCT or CCT, single arm study |
| Witteveen 2008 | Open label study, not RCT or CCT |
Differences between protocol and review
The title of the protocol was changed from 'Conservative treatment for osteoarthritis of the ankle' to assist the reader to retrieve the relevant information and to increase clarity about the results of the review.
The background section was updated concerning the section description of the intervention, a sentence about the percentage of complications was added including the correct references. The following sentence: "for ankle OA offloading the joint by brace, cane, rocker sole or inlay can reduce the pain as well" has been altered into: "for ankle OA offloading the joint by brace, cane, rocker sole or inlay is commonly used in clinical practice to reduce pain, however no evidence is available to support this treatment". A sentence to the effect that HA is currently used when simple analgesics have failed has been added. The first sentence of 'How the intervention might work' has been changed: "Ankle OA pain might be reduced" instead of "can be".
Types of outcome measures have been altered: our primary time point for the use of hyaluronic acid for ankle osteoarthritis was defined at six months. The only available score to create a pooled analysis was the AOS total (a combined score of pain and function), this was not a prespecified score in the original protocol. Upon contacting the authors of each paper individual exact pain and function scores could not be provided for the outcome at six months. It was then decided to use what was available. The AOS pain and disability and the AOS total were added to the types of outcome measures. The AOS is derived from the FFI it is commonly and very often used in literature describing any treatment for Ankle OA. We feel it is therefore a useful addition since probably more future randomised trials will use this score.
Due to the fact that we found a low number of eligible studies a McMaster sensitive filter was added to the MEDLINE search strategy, to retrieve trials studies from electronic bibliographic databases, not limited to any intervention.
In measures of treatment effect, a Peto odds ratio was added to be used for rare events.
No funnel plots were made to investigate publication bias, since we found a low number of eligible studies,.
Due to a low number of eligible studies a sensitivity analysis was not performed.
In the protocol it was stated that if more than one main comparison was found a separate 'Summary of findings' table for each comparison would be provided; however since we found four comparisons (and, to date, six eligible studies) the number of SOF tables would be overwhelming. We therefore decided to reduce this to describing the main comparison: hyaluronic acid versus placebo. An eighth outcome was added to the 'Summary of findings' table; in order to create a pooled analysis it was decided to use the results of the AOS total.
The following authors that were listed as contributors in the protocol did not take part in either analysing data or carrying out the analysis.
Alfons den Broeder: carry out the analysis, interpret the analysis
Inger N. Sierevelt: carry out the analysis
Contributions of authors
Angelique GH Witteveen: drafted the protocol, developed a search strategy, searched for trials, selected which trials to include, extracted data from trials, entered data into Review Manager 5, interpreted the analysis, drafted the final review, updated the review.
Cheriel J Hofstad: drafted the protocol, developed a search strategy, searched for trials, selected which trials to include, carried out the analysis.
Gino MMJ Kerkhoffs: drafted the protocol, selected which trials to include, interpreted the analysis, extracted data from trials, drafted the final review, updated the review.
Declarations of interest
One study is included in this review in which the main author of this review was involved as the main author (Witteveen 2010).
New
References
References to studies included in this review
Cohen 2008 {published data only}
- Cohen MM, Altman RD, Hollstrom R, Hollstrom C, Sun C, Gipson B. Safety and efficacy of intra‐articular sodium hyaluronate (Hyalgan) in a randomized, double‐blind study for osteoarthritis of the ankle. Foot and Ankle International 2008;29(7):657‐63. [PUBMED: 18785414] [DOI] [PubMed] [Google Scholar]
DeGroot 2012 {published data only}
- DeGroot H, Uzunishvili S, Weir R, Al‐omari A, Gomes B. Intra‐articular injection of hyaluronic acid is not superior to saline solution injection for ankle arthritis: a randomized, double‐blind, placebo‐controlled study. The Journal of Bone and Joint Surgery, American Volume 2012;94(1):2‐8. [PUBMED: 22218376] [DOI] [PubMed] [Google Scholar]
Karatosun 2008 {published data only}
- Karatosun V, Unver B, Ozden A, Ozay Z, Gunal I. Intra‐articular hyaluronic acid compared to exercise therapy in osteoarthritis of the ankle. A prospective randomized trial with long‐term follow‐up. Clinical and Experimental Rheumatology 2008;26(2):288‐294. [PUBMED: 18565251] [PubMed] [Google Scholar]
Salk 2006 {published data only}
- Salk RS, Chang TJ, D'Costa WF, Soomekh DJ, Grogan KA. Sodium hyaluronate in the treatment of osteoarthritis of the ankle: a controlled, randomized, double‐blind pilot study. Journal of Bone and Joint Surgery, American Volume 2006;88(2):295‐302. [PUBMED: 16452740] [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Shu‐Fen Sun, Chien‐Wei Hsu, Huey‐Shyan Lin, Yi‐Jiun Chou, Jun‐Yang Chen, Jue‐Long Wang. Efficacy of intraarticular botulinum toxin A and intraarticular hyaluronate plus rehabilitation exercise in patients with unilateral ankle osteoarthritis: a randomized controlled trial. Journal of Foot and Ankle Research 2014;7(9):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witteveen 2010 {published data only}
- Witteveen AG, Sierevelt IN, Blankevoort L, Kerkhoffs GM, Dijk CN. Intra‐articular sodium hyaluronate injections in the osteoarthritic ankle joint: effects, safety and dose dependency. Foot and Ankle Surgery 2010;16(4):159‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Huang 2006 {published data only}
- Huang YC, Harbst K, Kotajarvi B, Hansen D, Koff MF, Kitaoka HB, et al. Effects of ankle‐foot orthoses on ankle and foot kinematics in patient with ankle osteoarthritis. Archives of Physical Medicine and Rehabilitation 2006;87(0003‐9993 (Print), 0003‐9993 (Linking), 5):710‐6. [DOI] [PubMed] [Google Scholar]
Luciani 2008 {published data only}
- Luciani D, Cadossi M, Tesei F, Chiarello E, Giannini S. Viscosupplementation for grade II osteoarthritis of the ankle: a prospective study at 18 months' follow‐up. La Chirurgia degli Organi di Movimento 2008;92(1973‐2538 (Electronic), 0009‐4749 (Linking), 3):155‐60. [DOI] [PubMed] [Google Scholar]
Mei‐Dan 2010 {published data only}
- Mei‐Dan O, Kish B, Shabat S, Masarawa S, Shteren A, Mann G, et al. Treatment of osteoarthritis of the ankle by intra‐articular injections of hyaluronic acid: a prospective study. Journal of the American Podiatric Medical Association 2010;100(1930‐8264 (Electronic), 1930‐8264 (Linking), 2):93‐100. [DOI] [PubMed] [Google Scholar]
Sarkin 1974 {published data only}
- Sarkin TL. Indications for intra‐articular steroid in osteoarthritis of the ankle and big toe joints. South African Medical Journal 1974;48(0256‐9574 (Print), 0256‐9574 (Linking), 49):2067‐8. [PubMed] [Google Scholar]
Sun 2006 {published data only}
- Sun SF, Chou YJ, Hsu CW, Hwang CW, Hsu PT, Wang JL, et al. Efficacy of intra‐articular hyaluronic acid in patients with osteoarthritis of the ankle: a prospective study. Osteoarthritis and Cartilage 2006;14:867‐74. [DOI] [PubMed] [Google Scholar]
Sun 2011 {published data only}
- Sun SF, Hsu CW, Sun HP, Chou YJ, Li HJ, Wang JL. The effect of three weekly intra‐articular injections of hyaluronate on pain, function, and balance in patients with unilateral ankle arthritis. The Journal of Bone and Joint Surgery, American Volume 2011;93(1535‐1386 (Electronic), 18):1720‐6. [DOI] [PubMed] [Google Scholar]
Witteveen 2008 {published data only}
- Witteveen AG, Giannini S, Guido G, Jerosch J, Lohrer H, Vannini F, et al. A prospective multi‐centre, open study of the safety and efficacy of hylan G‐F 20 (Synvisc) in patients with symptomatic ankle (talo‐crural) osteoarthritis. Foot and Ankle Surgery 2008;14(3):145‐52. [DOI] [PubMed] [Google Scholar]
Additional references
Abate 2012
- Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle osteoarthritis: why evidence of efficacy is still lacking?. Clinical and Experimental Rheumatology 2012 Mar‐Apr;30(2):277‐81. [PUBMED: 22338615] [PubMed] [Google Scholar]
Agel 2005
- Agel J, Coetzee JC, Sangeorzan BJ, Roberts MM, Hansen Jr ST. Functional limitations of patients with end‐stage ankle arthrosis. Foot and Ankle International 2005;26:537‐9. [DOI] [PubMed] [Google Scholar]
Balazs 1993
- Balazs EA. Viscosupplementation: a new concept in the treatment of osteoarthritis. The Journal of Rheumatology. Supplement 1993;39:3‐9. [PubMed] [Google Scholar]
Bartels 2007
- Bartels EM, Lund H, Hagen KB, Dagfinrud K, Christensen R, Danneskiold‐Samsøe B. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005523.pub2] [DOI] [PubMed] [Google Scholar]
Bellamy 1988
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of Rheumatology 1988;15(12):1833‐40. [PubMed] [Google Scholar]
Bellamy 2006
- Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005321.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brosseau 2003
- Brosseau L, MacLeay L, Welch V, Tugwell P, Wells GA. Intensity of exercise for the treatment of osteoarthritis. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004259.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brosseau 2011
- Brosseau L, Yonge K, Welch V, Marchand S, Judd M, Wells GA, et al. Thermotherapy (heat treatment) for treating osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004522] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouwer 2005
- Brouwer RW, Raaij TM, Jakma TT, Verhagen AP, Verhaar JAN, Bierma‐Zeinstra SMA. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004020.pub2] [DOI] [PubMed] [Google Scholar]
Buckwalter 2004
- Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clinical Orthopaedics and Related Research 2004;427 Suppl:S6‐15. [DOI] [PubMed] [Google Scholar]
Budiman‐Mak 1991
- Budiman‐Mak E, Conrad KJ, Roach KE. The Foot Function Index: a measure of foot pain and disability. Journal of Clinical Epidemiology 1991;44(6):561‐70. [DOI: 10.1016/0895-4356(91)90220-4] [DOI] [PubMed] [Google Scholar]
Carpenter 2008
- Carpenter B, Motley T. The role of viscosupplementation in the ankle using hylan G‐F 20. The Journal of Foot and Ankle Surgery 2008 Sep‐Oct;47(5):377‐84. [DOI: 10.1053/j.jfas.2008.06.013] [DOI] [PubMed] [Google Scholar]
Cepeda 2006
- Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005522.pub2] [DOI] [PubMed] [Google Scholar]
Chang 2013
- Chang KV, Hsiao MY, Chen WS, Wang TG, Chien KL. Effectiveness of intra‐articular hyaluronic acid for ankle osteoarthritis treatment: a systematic review and meta‐analysis. Archives of Physical Medicine and Rehabilitation 2013;94(5):951‐60. [DOI] [PubMed] [Google Scholar]
Cushnaghan 1991
- Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Annals of the Rheumatic Diseases 1991;50(1):8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demetriades 1998
- Demetriades L, Strauss E, Gallina J. Osteoarthritis of the ankle. Clinical Orthopaedics and Related Research 1998;349:28‐42. [DOI] [PubMed] [Google Scholar]
Deorio 2008
- Deorio JK, Easley ME. Total ankle arthroplasty. Instructional Course Lectures 2008;57:383‐413. [PubMed] [Google Scholar]
Domsic 1998
- Domsic RT, Saltzman CL. Ankle osteoarthritis scale. Foot & Ankle International 1998;19(7):466‐71. [DOI] [PubMed] [Google Scholar]
Fransen 2009
- Fransen M, McConnell S, Hernandez‐Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007912] [DOI] [PubMed] [Google Scholar]
Garner 2005
- Garner SE, Fidan D, Frankish RR, Maxwell L. Rofecoxib for osteoarthritis. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD005115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghogomu 2014
- Ghogomu EAT, Maxwell LJ, Buchbinder R, Rader T, Pardo J, et al. the Editorial Board of the Cochrane Musculoskeletal Group. Updated method guidelines for Cochrane Musculoskeletal Group Systematic Reviews and Metaanalyses. The Journal of Rheumatology 2014;41(2):194‐205. [DOI: 10.3899/jrheum.121306] [DOI] [PubMed] [Google Scholar]
GRADEprofiler 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEprofiler (GRADEpro). Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Harada 2011
- Harada Y, Tokuda O, Fukuda K, Shiraishi G, Motomura T, Kimura M, et al. Relationship between cartilage volume using MRI and Kellgren‐Lawrence radiographic score in knee osteoarthritis with and without meniscal tears. American Journal of Roentgenology 2011;196(3):W298‐304. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hochberg 2012
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care & Research 2012;64(4):465‐74. [DOI] [PubMed] [Google Scholar]
Janisse 1998
- Janisse DJ. Prescription footwear for arthritis of the foot and ankle. Clinical Orthopaedics and Related Research 1998;349:100‐7. [DOI] [PubMed] [Google Scholar]
Jung 2007
- Jung HG, Parks BG, Nguyen A, Schon LC. Effect of tibiotalar joint arthrodesis on adjacent tarsal joint pressure in a cadaver model. Foot & Ankle International 2007;28(1):103‐8. [DOI] [PubMed] [Google Scholar]
Kalunian 2012
- Kalunian KC. Nonpharmacologic therapy of osteoarthritis. In: Tugwell P, Romain PL editor(s). UpToDate. Waltham, MA (USA): UpToDate, 2012. [Google Scholar]
Katcherian 1998
- Katcherian DA. Treatment of ankle arthrosis. Clinical Orthopaedics and Related Research 1998;349:48‐57. [DOI] [PubMed] [Google Scholar]
Kellgren 1957
- Kellgren JH, Lawrence JS. Radiological Assessment of Osteo‐Arthrosis. Annals of the Rheumatic Diseases 1957;16(4):494‐502. [DOI: 10.1136/ard.16.4.494] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kempson 1991
- Kempson GE. Age‐related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochimica et Biophysica Acta 1991;1075(3):223‐30. [DOI] [PubMed] [Google Scholar]
Kitaoka 1994
- Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle‐hindfoot, midfoot, hallux, and lesser toes. Foot & Ankle International 1994;15(7):349‐53. [DOI] [PubMed] [Google Scholar]
Krause 2012
- Krause FG, Schmid T. Ankle arthrodesis versus total ankle replacement: how do I decide?. Foot and Ankle Clinics 2012;17(4):529‐43. [DOI] [PubMed] [Google Scholar]
Martin 2007
- Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. Journal of Orthopaedic and Sports Physical Therapy 2007;37(5):253‐9. [DOI] [PubMed] [Google Scholar]
McGuire 2003
- McGuire JB. Arthritis and related diseases of the foot and ankle: rehabilitation and biomechanical considerations. Clinics in Podiatric Medicine and Surgery 2003;20(3):469‐85, ix. [DOI] [PubMed] [Google Scholar]
Messier 2005
- Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee‐joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis and Rheumatism 2005;52(7):2026‐32. [DOI] [PubMed] [Google Scholar]
Migliore 2011
- Migliore A, Giovannangeli F, Bizzi E, Massafra U, Alimonti A, Laganà B, Diamanti Picchianti A, Germano V, Granata M, Piscitelli P. Viscosupplementation in the management of ankle osteoarthritis: a review. Archives of Orthopaedic and Trauma Surgery 2011 Jan;31(1):139‐47. [DOI: 10.1007/s00402-010-1165-5] [DOI] [PubMed] [Google Scholar]
Morrey 1980
- Morrey BF, Wiedeman GP. Complications and long‐term results of ankle arthrodeses following trauma. The Journal of Bone and Joint Surgery, American Volume 1980;62(5):777‐84. [PubMed] [Google Scholar]
Nuesch 2010
- Nüesch E, Rutjes AWS, Husni E, Welch V, Jüni P. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003115.pub3] [DOI] [PubMed] [Google Scholar]
Ohnhaus 1975
- Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain 1975;1(4):379‐84. [DOI] [PubMed] [Google Scholar]
Pagenstert 2008
- Pagenstert G, Leumann A, Hintermann B, Valderrabano V. Sports and Recreation Activity of Varus and Valgus Ankle Osteoarthritis Before and After Realignment Surgery. Foot & Ankle International 2008;29(10):985‐93. [DOI] [PubMed] [Google Scholar]
Pendleton 2000
- Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Annals of the Rheumatic Diseases 2000;59(12):936‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peyron 1984
- Peyron JG. The epidemiology of osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, Mankin HS editor(s). Osteoarthritis: diagnosis and management. Philadelphia and London: Saunders, 1984:9‐27. [Google Scholar]
Pleimann 2002
- Pleimann JH, Davis WH, Cohen BE, Anderson RB. Viscosupplementation for the arthritic ankle. Foot and Ankle Clinics 2002;7(3):489‐94. [DOI] [PubMed] [Google Scholar]
Rao 2010
- Rao S, Ellis SJ, Deland JT, Hillstrom H. Nonmedicinal therapy in the management of ankle arthritis. Current Opinion in Rheumatology 2010;22(2):223‐8. [DOI] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Rippstein 2012
- Rippstein PF, Huber M, Naal FD. Management of specific complications related to total ankle arthroplasty. Foot and Ankle Clinics 2012;17(4):707‐17. [DOI] [PubMed] [Google Scholar]
Roos 2001
- Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot & Ankle International 2001;22(10):788‐94. [DOI] [PubMed] [Google Scholar]
Rutjes 2009
- Rutjes AWS, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2010
- Rutjes AWS, Nüesch E, Sterchi R, Jüni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003132.pub2] [DOI] [PubMed] [Google Scholar]
Saltzman 2005
- Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic centre. The Iowa Orthopaedic Journal 2005;25:44‐6. [PMC free article] [PubMed] [Google Scholar]
Saltzman 2006
- Saltzman CL, Zimmerman MB, O'Rourke M, Brown TD, Buckwalter JA, Johnston R. Impact of comorbidities on the measurement of health in patients with ankle osteoarthritis. Journal of Bone and Joint Surgery. American Volume 2006;88(11):2366‐72. [DOI] [PubMed] [Google Scholar]
Salén 1994
- Salén BA, Spangfort EV, Nygren AL, Nordemar R. The Disability Rating Index: an instrument for the assessment of disability in clinical settings. Journal of Clinical Epidemiology 1994;47(12):1423‐35. [DOI] [PubMed] [Google Scholar]
Singh 2015
- Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database of Systematic Reviews 2015, Issue 1. [CENTRAL: Art. No.: CD005614; DOI: 10.1002/14651858.CD005614.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suckel 2012
- Suckel A, Burger A, Wülker N, Wünschel M. Ankle arthrodesis ‐ clinical, radiological and biomechanical aspects with special regard to the adjacent joints. Zeitschrift für Orthopädie und Unfallchirurgie 2012;150(6):588‐93. [DOI] [PubMed] [Google Scholar]
Takakura 1995
- Takakura Y, Tanaka Y, Sugimoto K, Akiyama K, Tamai S. Long‐term results of arthrodesis for osteoarthritis of the ankle. Clinical Orthopaedics and Related Research 1999;361:178‐85. [DOI] [PubMed] [Google Scholar]
Tannenbaum 2000
- Tannenbaum H, PelosoPM, Russell AS, Marlow B. An evidence based approach to prescribing NSAIDs in the treatment of osteoarthritis and rheumatoid arthritis: the second Canadian Consensus Conference. Canadian Journal of Clinical Pharmacology 2000;7 Suppl A:4A‐16A. [PubMed] [Google Scholar]
Thomas 2003
- Thomas RH, Daniels TR. Ankle arthritis. Journal of Bone and Joint Surgery, American Volume 2003;85(5):923‐36. [DOI] [PubMed] [Google Scholar]
Towheed 2005
- Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Welch V, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002946.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Towheed 2006
- Towheed T, Maxwell L, Judd M, Catton M, Hochberg MC, Wells GA. Acetaminophen for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004257.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valderrabano 2009
- Valderrabano V, Horisberger M, Russell, I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clinical Orthopaedics and Related Research 2009;476(7):1800‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dijk 1997
- Dijk CN, Tol JL, Verheyen CC. A prospective study of prognostic factors concerning the outcome of arthroscopic surgery for anterior ankle impingement. American Journal of Sports Medicine 1997;25(6):737‐45. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220‐33. [DOI] [PubMed] [Google Scholar]
Wu 2004
- Wu WL, Rosenbaum D, SU FC. The effects of rocker sole and SACH heel on kinematics in gait. Medical Engineering and Physics 2004;26:639‐46. [DOI] [PubMed] [Google Scholar]
Zhang 2008
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence‐based, expert consensus guidelines. Osteoarthritis and Cartilage 2008;16(2):137‐62. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis and Cartilage 2010;18(4):476‐99. [DOI] [PubMed] [Google Scholar]


