Graphical abstract
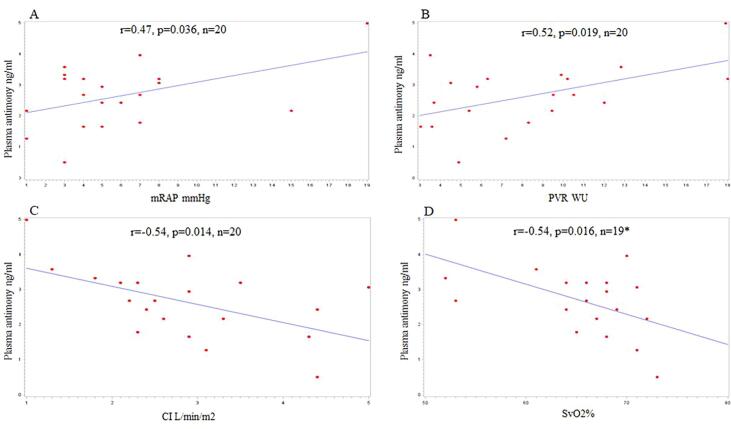
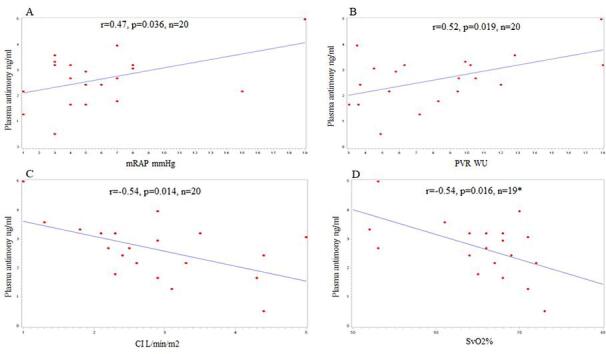
Scatter plots of plasma antimony (Sb) and mRAP (A), PVR (B), CI (C), and SvO2% (D). *Svo2% was missing in one patient.
Keywords: PAH, RV dysfunction, Metal toxicity, Antimony, Non-essential metals
Highlights
-
•
Antimony blood and plasma levels were significantly higher in PAH in comparison to controls.
-
•
Significant correlation between plasma antimony level and prognostic hemodynamic parameters of PAH.
Abstract
It is unknown if environmental antimony exposure influences pulmonary arterial hypertension (PAH) and right ventricular function. We performed a pilot study to evaluate antimony levels in 20 PAH patients and 10 controls. Also, we explored the correlation of antimony level with PAH prognostic hemodynamic markers. Antimony blood and plasma levels were significantly higher in PAH patients when compared to controls [blood: PAH mean ± SD (95%CI) 1.3 ± 0.6 (1.0–1.5) ng/ml vs. control mean ± SD (95%) 0.7 ± 0.5 (0.4–1.0) ng/ml, p = 0.017] [plasma: PH mean ± SD (95%CI) 2.6 ± 1 (2.2–3.1) ng/ml vs. control mean ± SD (95%CI) 1.5 ± 0.8 (1.0–2.0) ng/ml, p = 0.004]. Also, antimony blood and plasma levels were significantly higher in idiopathic-PAH patients and non-idiopathic PAH when compared to controls. There was a trend for higher blood and plasma antimony levels in idiopathic PAH [blood1.6 ± 0.6 (1.1–2.1) ng/ml and plasma 3.1 ± 1.2 (2.2–4.1) ng/ml] when compared to non-idiopathic PAH [blood 1.1 ± 0.6(0.8–1.4) ng/ml and plasma 2.5 ± 0.9(2–2.9) ng/ml], but it did not reach statistical significance. There was a significant correlation between plasma antimony level and all the prognostic hemodynamic parameters of PAH including mRAP (r = 0.47, p = 0.036), CO (r = −0.50, p = 0.026), CI (r = −0.54, p = 0.014), PVR (r = 0.52, p = 0.019), and SvO2 (r = -0.54, p = 0.016).
1. Introduction:
Pulmonary arterial hypertension (PAH) group 1 is a rare disease with an estimated annual incidence of about 2 to 5 cases per million and a prevalence of 10.6 cases per million adults with a higher prevalence among women. (Ruopp & Cockrill, 2022) It is characterized by the precapillary hemodynamic profile that includes mean pulmonary artery pressure > 20 mm Hg, pulmonary vascular resistance ≥ 3 Wood Units (WU), and pulmonary artery wedge pressure ≤ 15 mmHg as measured by right heart catheterization (RHC) during rest with exclusion of other causes of precapillary pulmonary hypertension.(Simonneau et al., 2019) PAH can be idiopathic, heritable, drug- and toxin-induced, or associated with HIV infection,connective tissue disease, congenital heart disease, portal hypertension, or Schistosomiasis.(Simonneau et al., 2019).
Oxidative and endoplasmic reticulum stress are significant mediators of pulmonary hypertension and right ventricular dysfunction and there is growing evidence of the role of environmental pollution and heavy metals in cardiovascular disease that is mediated through several pathways that are linked to increased oxidative stress and changes in nitric oxide homeostasis. (Bhatnagar, 2006, Lahm et al., 2018) Despite this growing evidence, the knowledge about the effect of environmental heavy metals, including antimony, on PAH and right ventricular dysfunction is sparce. Antimonial compounds are still used in treatment of Leishmaniasis with cardiac toxicity being one of the known potential side effects. (Alvarez et al., 2005) Furthermore, there are data from animal models that show detrimental cardiac effect of antimony exposure. (Alvarez et al., 2005, Jiang et al., 2021). We aimed to explore antimony levels in PAH patients without known previous history of occupational exposure and to evaluate correlation of antimony level with the PAH prognostic hemodynamic parameter.
2. Material and Methods:
This was a prospective, single center pilot study that recruited patients from the University of Louisville PH center over a period of 3 months. Patients with pulmonary arterial hypertension (PAH) who were ≥ 18 years old were eligible to participate. The recruited PAH patients did not have any known prior history of occupational heavy metal exposure. IRB approval (IRB# 20.0947) was obtained, and all the included patients signed an informed consent before recruitment. Blood and urine samples were collected from 20 PAH patients and from 10 volunteer apparently healthy controls to measure antimony levels in blood, plasma, and urine using an X Series II quadrupole inductively coupled plasma mass spectrometry (ICP-MS, Thermo Fisher Scientific). The ICP-MS trace metal assay protocol included several steps with all procedures avoiding metal contamination. The sample digestion tubes (Eppendorf or metal free tubes) were treated with concentrated acid/nitric acid overnight and washed/rinsed by deionized (DI) water 5 times then tubes were dried. A100ul blood samples were transferred to digestion tubes. A 700ul 70% HNO3 (Fisher Scientific cat# A509P500, trace metal grade) were added into sample tubes, and digestion tubes were placed in 65C incubation shaker for 4 h till sample solutions became clear. All digested solution was carefully transferred into 12 ml DI water (∼4% HNO3 concentration required by ICPMS machine). The solution was mixed well and filtered by 45um cell strainer. Sample tubes were standard 15 ml (VWR metal free tube 89049–172). 5 tubes 35 ml 4% HNO3/DI water solutions were made for assay blank and making standards (each tube 33 ml DI water + 2 ml 70% HNO3 and mixed well, VWR 89049–176). At that point samples are ready for assay.
We collected available demographics, hemodynamics, 6-minute walk distance (6MWD), Brain natriuretic peptide (BNP) level, and REVEAL Lite 2 risk score of the recruited PAH patients. The blood, plasma, and urine metal levels between groups were compared using a two-sample t-test (or ANOVA when comparing more than two factors) for continuous variables and a chi-square (χ2)-test (or Fisher’s exact test when the expected frequency within any cell was<5 in a 2 × 2 table) for categorical variables. Pearson's correlation coefficient and p value were used to evaluate the relationship between heavy metals and clinical biomarkers. Statistical analysis was conducted using SAS 9.5 and results with p value < 0.05 were considered significant.
3. Theory
Our hypothesis was that antimony levels would be higher in PAH patients when compared to controls and possibly higher in idiopathic-PAH when compared to non-idiopathic PAH. Also, antimony levels can have significant correlation with prognostic hemodynamic markers of PAH.
4. Results
A total of 20 PAH patients and 10 controls participated in the study. The mean age of PAH patients was 57.6 ± 11.8 years and 80% of the included patients were females. The control group was younger in age [Mean (95% CI) 37.1 years (29.9–44.3) vs. 57.6 years (52.4–62.7), p < 0.001], and 60% of the control group were females. None of the controls were smokers and only one of the PAH patients was a current smoker. The cause of PAH was idiopathic in 6 patients, connective tissue disease in 5 patients, congenital in 3 patients, porto-pulmonary in 1 patient, drug induced in 2 patients, sarcoidosis in 2 patients, and associated with common variable immunodeficiency in 1 patient. The mean last 6-minute walk distance for PAH patients was 312.7 m ± 96.2 and the mean last BNP level was 118.3 ± 129.6 pg/ml. The right heart catheterization (RHC) hemodynamics showed mean right atrial pressure of (mRAP) 5.9 ± 4.4 mmHg, mean pulmonary artery pressure (mPAP) of mPAP 45.6 ± 13.9 mmHg, mean pulmonary artery wedge pressure (PAWP) of 8 ± 3.5 mmHg, mean cardiac output (CO)of 5.1 ± 1.4 L/min, mean cardiac index (CI) of 2.86 ± 1.1 L/min/m2, and mean pulmonary vascular resistance (PVR) of 8.32 ± 4.4 Wood Units (WU). REVEAL Lite 2 risk score was low risk in 9 patients, intermediate risk in 6 patients, and high risk in 4 patients. All patients were on PAH combination therapies including a prostanoid agent (13 on parenteral treprostinil, 1 on epoprostenol, 5 on inhaled treprostinil, and 1 on selexipag) with a mean parenteral treprostinil dose of 67.8 ± 28.9 ng/kg/min. Blood and plasma antimony levels were significantly higher in PAH patients when compared to controls [blood: PAH mean ± SD (95%CI) 1.3 ± 0.6 (1.0–1.5) ng/ml vs. control mean ± SD (95%) 0.7 ± 0.5 (0.4–1.0) ng/ml, p = 0.017] [plasma: PH mean ± SD (95%CI) 2.6 ± 1 (2.2–3.1) ng/ml vs. control mean ± SD (95%CI) 1.5 ± 0.8 (1.0–2.0) ng/ml, p = 0.004] but no significant differences were noted in urine levels (Table 1). Also, blood and plasma antimony levels were significantly higher in idiopathic-PAH and non-idiopathic PAH patients when compared to controls (Table 2). Blood and plasma antimony levels were higher in idiopathic PAH when compared to non-idiopathic PAH, but they did not reach statistical significance which could be related to the small sample size in each group (Table 3).
Table 1.
Comparing antimony levels in PAH patients vs. controls (n = 30).
| Variables(ng/ml) | Control (N = 10) | PAH (N = 20) | P Value |
|---|---|---|---|
| BLOODSb | 0.017 | ||
| Mean (95%CI) | 0.7 (0.4–1.0) | 1.3 (1.0–1.5) | |
| Standard Deviation | 0.5 | 0.6 | |
| PLASMASb | 0.004 | ||
| Mean (95%CI) | 1.5 (1.0–2.0) | 2.6 (2.2–3.1) | |
| Standard Deviation | 0.8 | 1.0 | |
| UrineSb | 0.512 | ||
| Mean (95%CI) | *0.0 (0.0–0.0) | 0.1 (-0.1–0.3) | |
| Standard Deviation | 0.0 | 0.5 |
*Urine samples were available in 9 controls.
Table 2.
Comparing antimony levels in idiopathic and non-idiopathic PAH patients vs. controls (n = 30).
| Variables(ng/ml) | Control (N = 10) | Idiopathic PAH (N = 6) | Non-idiopathic PAH (N = 14) | P Value |
|---|---|---|---|---|
| BLOODSb | 0.015 | |||
| Mean (95%CI) | 0.7 (0.4–1.0) | 1.6 (1.1–2.1) | 1.1 (0.8–1.4) | |
| Standard Deviation | 0.5 | 0.6 | 0.6 | |
| PLASMASb | 0.006 | |||
| Mean (95%CI) | 1.5 (1.0–2.0) | 3.1 (2.2–4.1) | 2.5 (2.0–2.9) | |
| Standard Deviation | 0.8 | 1.2 | 0.9 | |
| UrineSb | 0.602 | |||
| Mean (95%CI) | *0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.2 (0.0–0.5) | |
| Standard Deviation | 0.0 | 0.0 | 0.6 |
*Urine samples were available in 9 controls.
Table 3.
Comparing antimony levels in idiopathic PAH and non-idiopathic PAH groups (n = 20).
| Variables(ng/ml) | Idiopathic PAH (N = 6) | Non-Idiopathic PAH (N = 14) | P Value |
|---|---|---|---|
| BLOODSb | 0.123 | ||
| Mean (95%CI) | 1.6 (1.1–2.1) | 1.1 (0.8–1.4) | |
| Standard Deviation | 0.6 | 0.6 | |
| PLASMASb | 0.185 | ||
| Mean (95%CI) | 3.1 (2.2–4.1) | 2.5 (2.0–2.9) | |
| Standard Deviation | 1.2 | 0.9 |
There was a significant correlation between plasma Antimony level and all the prognostic hemodynamic parameters of PAH (Fig. 1) including mean right atrial pressure (mRAP) (r = 0.47, p = 0.036), cardiac output (CO) (r = -0.50, p = 0.026), cardiac index (CI) (r = -0.54, p = 0.014), pulmonary vascular resistance (PVR) (r = 0.52, p = 0.019), and mixed venous oxygen saturation (SvO2) (r = -0.54, p = 0.016) (Figure:1).
Fig. 1.
Scatter plots of plasma antimony (Sb) and mRAP (A), PVR (B), CI (C), and SvO2% (D). *Svo2% was missing in one patient.
5. Discussion
This pilot study showed significantly higher blood and plasma antimony levels in PAH patients when compared to controls. Also, there was a significant correlation between plasma antimony level in PAH patients and all the prognostic hemodynamic markers of PAH including mRAP, CO, CI, PVR, and SvO2. In the current era, patients with group 1 PAH are classified into different risk groups based on the estimated one-year mortality. The risk stratification strategy is a multiparametric assessment that includes some of the RHC hemodynamic parameters. The RHC parameters that are prognostic and currently included in risk assessment include mRAP, CI, SvO2, and PVR.(Benza et al., 2019, Galiè et al., 2015) Our study focused on group 1 PAH and correlated antimony level to the prognostic hemodynamic markers that are currently used in PAH risk assessment which reflect right ventricular function. Given the significant correlation between plasma antimony level and the prognostic hemodynamic markers of PAH that reflects the right ventricular function, it is possible that PAH patients who have higher levels of environmental antimony levels can be predisposed to right ventricular dysfunction which is one of the main determinants of outcomes in PAH. Possible theoretical explanations for the identified higher antimony levels in PAH patients could include the possibility that PAH or some medications involved in treatment may be associated with an alteration in body handling of antimony, leading to higher blood/ plasma but not urine levels, or the possibility of previous co-exposure to antimony and another unidentified toxin that may predispose to PAH in some patients.
The hypothesis of the involved mechanism of right ventricular dysfunction can be extrapolated from previous studies that aimed to better understand antimony cardiac toxicity. Several studies showed cardiac adverse effects from antimony exposure. Guineapigs treated with antimony had several EKG changes and the isolated ventricular myocytes showed impaired contraction responses to changes in stimulus frequency, action potentials with a depressed plateau and prolonged duration with a depressed maximum peak inward calcium current. (Alvarez et al., 2005) L-carnitine had a cardiac protective effect possibly by counteracting antimony induced oxidative stress.(Alvarez et al., 2005) In mice, antimony exposure resulted in cardiac induced apoptosis via endoplasmic reticulum stress that is linked to calcium homeostasis disturbance with cardiac myocytes showing granular degeneration, mitochondrial swelling, and endoplasmic reticulum dilation. (Jiang et al., 2021) Furthermore, cultured cardiac myocytes showed an increased LDH production, increased lipid peroxidation, and glutathione depletion with antimony exposure. (Tirmenstein et al., 1995) Pretreatment with vitamin E was protective to the cardiac myocytes against lipid peroxidation and LDH production when exposed to antimony. (Tirmenstein et al., 1995).
In a large study that included 7781 participants who were selected from the National Health and Nutrition Examination Survey and were followed for an average of 6.04 years, higher level of urinary antimony was linked to increased mortality and an increased risk of self-reported congestive heart failure and heart attacks.(Guo et al., 2016) In another study that included 3022 participants, a positive dose–response relationship was noted between urinary antimony and urinary 8-iso-PGF2α level, which is a common biomarker for lipid peroxidation, as well as negative association of urinary 8-iso-PGF2α with one or more heart rate variability parameters. (Tan et al., 2020) Previous studies showed increased lipid peroxidation in PH patients reflected by increased level of urinary isoprostaglandin F2 α type III which was found to inversely correlates with the PH reversibility. (Cracowski et al., 2001).
Given all the above findings we hypothesize that the possible mechanism of RV dysfunction with higher antimony level could be involving disturbance in right ventricular calcium homeostasis and/or an increased oxidative/ endoplasmic reticulum stress which can be induced by environmental antimony exposure. The limitations of our study include the small sample size and not investigating potential mechanistic pathways which can be addressed in future studies.
6. Conclusion
In our pilot study we observed significantly higher antimony levels in blood and plasma of PAH patients when compared to controls. Also, we observed significant correlation between plasma antimony level and all the prognostic hemodynamic parameters of PAH. These findings deserve further evaluation and validation in larger studies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported in part by the University of Louisville Executive Vice President for Research and Innovation Internal Grant (JH, LC); University of Louisville School of Medicine Basic Grant (JH, LC); National Institute of Environmental Health Sciences (P30ES030283 to JH, LC, XW, SNR); Gilead Sciences COMMIT COVID-19 RFP Program grant (Gilead IN-US-983-6063 to JH); National Center for Advancing Translational Sciences grant (1U18TR003787-01 to JH).
References
- Alvarez M., Malécot C.O., Gannier F., Lignon J.M. Antimony-induced cardiomyopathy in guinea-pig and protection by L-carnitine. Br. J. Pharmacol. 2005;144(1):17–27. doi: 10.1038/sj.bjp.0706030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benza R.L., Gomberg-Maitland M., Elliott C.G., Farber H.W., Foreman A.J., Frost A.E., McGoon M.D., Pasta D.J., Selej M., Burger C.D., Frantz R.P. Predicting Survival in Patients With Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies. Chest. 2019;156(2):323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ. Res. 2006;99(7):692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- Cracowski J.L., Cracowski C., Bessard G., Pepin J.L., Bessard J., Schwebel C., Stanke-Labesque F., Pison C. Increased lipid peroxidation in patients with pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2001;164(6):1038–1042. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- Galiè N., Humbert M., Vachiery J.-L., Gibbs S., Lang I., Torbicki A., Simonneau G., Peacock A., Vonk Noordegraaf A., Beghetti M., Ghofrani A., Gomez Sanchez M.A., Hansmann G., Klepetko W., Lancellotti P., Matucci M., McDonagh T., Pierard L.A., Trindade P.T., Zompatori M., Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Respir. J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- Guo J., Su L., Zhao X., Xu Z., Chen G. Relationships between urinary antimony levels and both mortalities and prevalence of cancers and heart diseases in general US population, NHANES 1999–2010. Sci. Total Environ. 2016;571:452–460. doi: 10.1016/j.scitotenv.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Jiang X., Yu W., Wu S., Tang L., Zhong G., Wan F., Lan J., Zhang H., Pan J., Tang Z., Zhang X., Hu L., Huang R. Arsenic (III) and/or Antimony (III) induced disruption of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in mice heart. Ecotoxicol. Environ. Saf. 2021;220 doi: 10.1016/j.ecoenv.2021.112394. [DOI] [PubMed] [Google Scholar]
- Lahm T., Douglas I.S., Archer S.L., Bogaard H.J., Chesler N.C., Haddad F., Hemnes A.R., Kawut S.M., Kline J.A., Kolb T.M., Mathai S.C., Mercier O., Michelakis E.D., Naeije R., Tuder R.M., Ventetuolo C.E., Vieillard-Baron A., Voelkel N.F., Vonk-Noordegraaf A., Hassoun P.M. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018;198(4):e15–e43. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruopp N.F., Cockrill B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA. 2022;327(14):1379–1391. doi: 10.1001/jama.2022.4402. [DOI] [PubMed] [Google Scholar]
- Simonneau G., Montani D., Celermajer D.S., Denton C.P., Gatzoulis M.A., Krowka M., Williams P.G., Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q., Ma J., Zhou M., Wang D., Wang B., Nie X., Mu G., Zhang X., Chen W. Heavy metals exposure, lipid peroxidation and heart rate variability alteration: Association and mediation analyses in urban adults. Ecotoxicol. Environ. Saf. 2020;205 doi: 10.1016/j.ecoenv.2020.111149. [DOI] [PubMed] [Google Scholar]
- Tirmenstein M.A., Plews P.I., Walker C.V., Woolery M.D., Wey H.E., Toraason M.A. Antimony-induced oxidative stress and toxicity in cultured cardiac myocytes. Toxicol. Appl. Pharmacol. 1995;130(1):41–47. doi: 10.1006/taap.1995.1006. [DOI] [PubMed] [Google Scholar]