Summary
Background
Treating memory and cognitive deficits requires knowledge about anatomical sites and neural activities to be targeted with particular therapies. Emerging technologies for local brain stimulation offer attractive therapeutic options but need to be applied to target specific neural activities, at distinct times, and in specific brain regions that are critical for memory formation.
Methods
The areas that are critical for successful encoding of verbal memory as well as the underlying neural activities were determined directly in the human brain with intracranial electrophysiological recordings in epilepsy patients. We recorded a broad range of spectral activities across the cortex of 135 patients as they memorised word lists for subsequent free recall.
Findings
The greatest differences in the spectral power between encoding subsequently recalled and forgotten words were found in low theta frequency (3–5 Hz) activities of the left anterior prefrontal cortex. This subsequent memory effect was proportionally greater in the lower frequency bands and in the more anterior cortical regions. We found the peak of this memory signal in a distinct part of the prefrontal cortex at the junction between the Broca's area and the frontal pole. The memory effect in this confined area was significantly higher (Tukey–Kramer test, p<0.05) than in other anatomically distinct areas.
Interpretation
Our results suggest a focal hotspot of human verbal memory encoding located in the higher-order processing region of the prefrontal cortex, which presents a prospective target for modulating cognitive functions in the human patients. The memory effect provides an electrophysiological biomarker of low frequency neural activities, at distinct times of memory encoding, and in one hotspot location in the human brain.
Funding
Open-access datasets were originally collected as part of a BRAIN Initiative project called Restoring Active Memory (RAM) funded by the Defence Advanced Research Project Agency (DARPA). CT, ML, MTK and this research were supported from the First Team grant of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund.
Keywords: Memory encoding, Human verbal memory, Frontal pole, Anterior prefrontal cortex, Intracranial recordings
Research in context.
Evidence before this study
Memory and cognitive functions were historically mapped using lesion and cortical stimulation studies in a clinical context. With the rise of electrophysiological and neuroimaging techniques, processing of specific memory functions has been localised to discrete brain areas. The original research papers that associated various brain regions with verbal and non-verbal memory encoding were reviewed, starting from the classic study by Wagner et al. 1998. Subsequent memory effect, i.e. the difference between trials with successful and failed memory encoding, was used in particular to find evidence for a hotspot of memory processing in the human brain. Key review articles (Paller and Wagner 2002, Kim 2011) and book chapters (Rugg and Coles 1996) that summarise the neuroimaging and electrophysiological studies served as sources of references and opinions for the context of this study. The resultant evidence from the neuroimaging studies delineated wider anatomical areas with a significant memory effect, whereas the electrophysiological data determined the timing and the sequence of the effect across the spectrum of multiple frequency bands. Combination of the precise anatomical localization with the temporal dynamics of the memory signal requires large datasets from intracranial recordings that were not available until recently. A number of recent studies looked at the memory effect but were missing a comprehensive investigation of a hypothetical hotspot of memory encoding in distinct anatomical areas, frequency ranges, and times of memory processing.
Added value of this study
Our study provides a comprehensive view (over 150 patients) of the memory effect in the spatial, temporal and spectral dimensions of a range of brain activities. This enabled a direct head-to-head comparison of the effect across the human cortex to determine exact localization, timing and the neural activity underlying successful memory encoding. Intracranial recordings acquired directly from the human brain offered uniquely high spatiotemporal resolution and access to the very sources of the brain's activity.
Implications of all the available evidence
Our results suggest an anatomically confined area and specific neural activities that are critical for successful formation of declarative memories in humans. The proposed hotspot of verbal memory encoding presents a discrete target for modulating cognitive functions with invasive and noninvasive brain stimulation techniques in human patients suffering from memory deficits. Localization of the hotspot area is congruent with the cortical mapping studies of the language and executive functions and with previous studies of the memory effect in a wider area of the lateral prefrontal cortex. The neural activities were identified in the theta frequency range, which were associated with memory processing in numerous animals and human studies. In summary, the results validate an electrophysiological biomarker of memory processing in the human brain that can be used to develop new targeted therapies and brain stimulation devices for treating memory and cognition.
Alt-text: Unlabelled box
Introduction
To find and localise the neural activities critical for memory formation has been one of the main limiting factors for applying brain stimulation therapies. It is a major challenge to identify a target neural activity, timing, and location for stimulation that would be effective for treating memory deficits in general. Processing various types of verbal and non-verbal information is known to engage multiple brain regions and electrophysiological activities for the purposes of perceiving, maintaining and storing particular information. Determining localization of the activities that are critical for successful formation of new memories for later recall would accelerate the development of new treatments based on brain stimulation and shed light on the organisation of memory in the human brain. The available technologies are missing suitable targets and biomarkers of neural activity to test and validate new therapeutic approaches.
Mapping the brain areas and the neural activities that are critical for encoding of human memory has been investigated in the advent of neuroimaging and electrophysiological techniques. Scalp EEG was first used to quantify the difference between evoked response potentials during presentation of stimuli that were subsequently remembered and those that were forgotten, providing a neural activity measure of memory processing.1 This subsequent memory effect (SME) was then localised to specific anatomical regions using various neuroimaging techniques.2, 3, 4 Encoding words and photographs that were successfully remembered induced a greater hemodynamic response in the same prefrontal and temporal cortical areas of the left and right hemisphere, respectively.5,6 The neural activities behind these localised SME signals in the brain were then confined to the theta and gamma frequency ranges of scalp and intracranial EEG (iEEG) electrodes implanted over or in the frontal and temporal cortex.7, 8, 9, 10, 11 More recent iEEG studies showed that these SME signals have both positive and negative polarity, i.e. more or less spectral power induced during successful memory encoding, depending on the frequency band, brain region, and phase of stimulus presentation.10, 11, 12 A comprehensive study of the frequency band, magnitude, and polarity of SME across both anatomical space and time of memory processing has so far not been possible due to spectral and temporal limitations of the fMRI studies and limited anatomical coverage of the iEEG studies.
Large multi-centre iEEG studies can now provide datasets from more than one hundred patients with thousands of electrodes to record neural activities from the entire cortex. Spectral power in the high gamma range of frequencies (60–120 Hz), which is known to correlate with the hemodynamic signals of the neuroimaging techniques13,14 and with the neuronal spiking activity,15, 16, 17 has been proposed to map and investigate cognitive functions in the iEEG studies.18 Our previous study used the high gamma power to map and compare SME magnitude and temporal dynamics across the human cortex, showing a widespread distribution with relatively equal magnitude in multiple temporal and prefrontal areas.11 The lower frequency range of the spectrum has either been unexplored, studied separately, or not compared ‘head-to-head’ with the high gamma activities, despite the original report of SME in both theta and gamma frequencies.7 Both theta and gamma activities play important roles in memory functions19, 20, 21, 22 and can be used to predict states for subsequent recall and its modulation.23 Still, the contribution of the lower iEEG spectrum to SME relative to the high gamma power remains to be determined. The goal of this study was not only to identify the anatomical location of a hypothetical hotspot but also the spectral activity in a particular frequency band and phase of memory encoding. Since spectral power in the lower frequency bands is less correlated with the hemodynamic responses,13,14 any memory effects in these low ranges would only be reliably detected across the cortex in the iEEG studies with adequate electrode coverage.
In our previous iEEG study, we took advantage of a wide cortical coverage of the implanted electrodes that showed the memory effect in the high gamma power distributed across a widespread network of cortical areas, including Brodmann areas 10 and 11 of the frontal pole.11 This anterior section of the prefrontal cortex, called the frontal pole, is less commonly implanted than the more posterior dorsal and ventral lateral sections and thus commonly ignored in the iEEG studies with epilepsy patients. Even seminal neuroimaging studies of the memory effect excluded the anterior and focused on the lateral areas of the dorsolateral prefrontal cortex in particular.6,24 More recent studies also implicated the ventrolateral prefrontal cortex particularly with predicting memory recall.25,26 The remaining anterior section of the prefrontal cortex was also consistently reported in various cognitive tasks with specific roles proposed for its rostral and lateral subdivisions.27,28 Even though the exact function of this higher-order cortical area remains elusive, we hypothesised that its activity could be critical for predicting stimulus recall. Since the iEEG studies of the cortical activity were predominantly limited to other brain regions and the high gamma frequency range, the relative contribution of the lower frequencies in the prefrontal and other cortical areas to memory encoding8 compared to the high gamma activities remains to be established.
Here, we address the need for a comprehensive study of the memory effect compared across a broad range of anatomical regions, spectral frequencies of neural activities, and times of memory encoding in 135 patients. Given the results of the previous neuroimaging studies5,6,25,26 and the recent iEEG studies with direct electrical brain stimulation that reported memory enhancement,29,30 we expected to observe local areas of greater memory-related activity in the prefrontal and temporal lobes. The key question of this study is whether there are specific subregions of stronger SME within these larger areas and what are the underlying spectral activities. Our goal was to determine the hotspot areas and the neural activities of human verbal memory processing for potential therapeutic targets.
Methods
Subjects
Data come from a large open-access database of 135 patients undergoing intracranial electroencephalographic (iEEG) monitoring for surgical evaluation of drug-resistant epilepsy who were recruited to participate in a multi-centre collaborative study. All of the patients were diagnosed with the drug-resistant epilepsy confirmed in scalp EEG (phase I) monitoring, followed by the iEEG (phase II) monitoring with stereo EEG or electrocorticography (ECoG) surgical procedures. This patient population was well represented by the large sample from different clinical centres in this database. All de-identified raw data may be downloaded at http://memory.psych.upenn.edu/Electrophysiological_Data. Electrophysiological recordings were collected from standard clinical subdural and depth electrodes (AdTech Inc., PMT Inc.) implanted on the cortical surface and into the brain parenchyma respectively. Subdural electrode contacts were arranged either in a grid or a strip configuration with 10 mm separation, and depth electrode contacts were separated by 5 to 10 mm. The placement of electrodes was determined solely by a neurology and neurosurgery team with the goal of determining seizure foci for possible epilepsy resective surgery or implantation of a stimulation device for seizure treatment. The target sites of electrode implantation were not determined based on research purposes, and hence, comprise a heterogeneous and uneven distribution of the implanted brain areas. This was mitigated by the large number of patients to provide adequate electrode coverage across the cortex.
Anatomic localization and brain surface mapping
Cortical surface parcellations were generated for each participant from pre-implant MRI scans (volumetric T1-weighted sequences) using Freesurfer software (RRID:SCR_001847). The hippocampus and surrounding cortical regions were delineated separately based on an additional 2 mm thick coronal T2-weighted scan using the Automatic Segmentation of Hippocampal Subfields (ASHS) multi-atlas segmentation method. Electrode contact coordinates derived from co-registered post-implant CT scans were then mapped to the pre-implant MRI scans to determine their anatomic locations. For subdural strips and grids, the electrode contacts were additionally projected to the cortical surface using an energy minimization algorithm to account for postoperative brain shift. Contact locations were reviewed and confirmed on surfaces and cross-sectional images by a neuroradiologist. The T1-weighted MRI scans were also registered to the MNI152 standard brain to enable comparison of recording sites in a common space across subjects. Anatomic locations of the recording sites, including Brodmann areas, were derived by converting MNI coordinates to Talairach space and querying the Talairach daemon (www.talairach.org).
Electrophysiological recordings
Intracranial data were recorded using one of the following clinical electrophysiological acquisition systems (dependent on the institution for data collection): Nihon Kohden EEG-1200, Natus XLTek EMU 128, or Grass Aura-LTM64. Depending on the acquisition system and the preference of the clinical team, the signals were sampled at either 500, 1000, or 1600 Hz and were referenced to a common contact placed either intracranially, on the scalp, or on the mastoid process. For analysis, all recordings using higher sampling rates were down-sampled to 500 Hz. A bipolar montage was calculated post hoc for each subject by subtracting measured voltage time series on all pairs of spatially adjacent contacts. This resulted in N-1 bipolar signals in case of the penetrating and the strip electrodes, and N = (i-1)*j + (j-1)*i, where i and j are the numbers of contacts in the vertical and horizontal dimensions of the grid. For the data analysis of this study, one “electrode” refers to the bipolar signal from one bipolar pair of contacts. It is important to note that the same contact was to derive at least two ‘electrode’ channels (more in case of the grid of electrode contacts according to the formula above), hence, the derived ‘electrode’ signals are not as independent as the original contact signals (differential). This bipolar montage derivation is a standard signal processing procedure practised in the iEEG and scalp EEG studies.
Free recall task
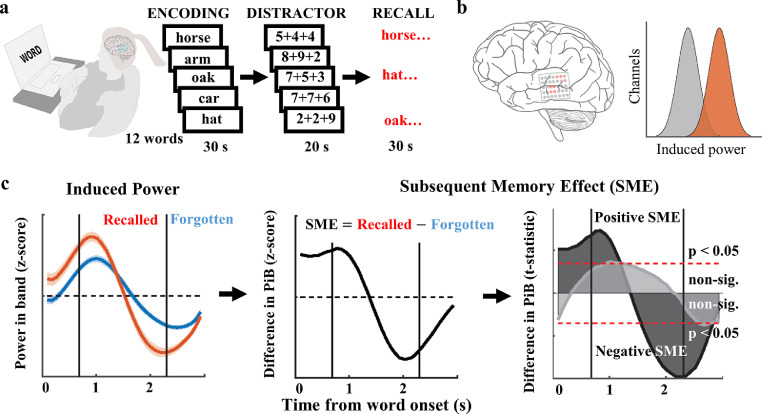
The task used is based on classical paradigms for probing verbal, short-term memory (Kahana, 2012), in which subjects were shown lists of words for subsequent recall. Participants were instructed to study lists of words presented on a laptop computer screen for a delayed test of free recall. Lists were composed of 12 words chosen at random and without replacement from a pool of high-frequency nouns in the subject's native language (either English or Spanish; http://memory.psych.upenn.edu/WordPools). Each word appeared on the screen for 1600 ms, followed by a random jitter of 750 to 1000 ms blank interval between stimuli. At the end of each word list, a distractor task was performed by the subject. This task lasted for 20 s and was composed of a series of simple, arithmetic problems of the format A + B + C, where A, B, and C were random, single-digit integers between 1 and 9. After the distractor task, participants were given 30 s in which to recall as many of the words from the list as possible in any order (Figure 1). Vocal responses were recorded digitally by the laptop and were later scored manually for analysis. Only subjects who recalled at least 15% of words and completed at least 12 trials of the task were included in further analysis. This left 135 of the 164 original subjects for a grand total of 14,219 electrodes for analysis. Electrophysiological recordings were synchronised to stimulus appearance on the screen through an electric pulse generator operated by the task laptop which sent pulses to a designated event channel in the clinical acquisition system. The events were timestamped after the recording session using custom-written MATLAB codes and were used to extract specific epochs of interest around word presentation. Each epoch recorded was 3000 ms long and included 1600 ms of word presentation on the screen with 700 ms of blank screen, interstimulus interval before and after word presentation.
Figure 1.
Subsequent Memory Effect (SME) was estimated to quantify neural processes engaged in encoding of words. (a) iEEG signals were recorded from epilepsy patients during presentation of 12 common English nouns for subsequent free recall, following a short algebraic distractor task. (b) Memory processing was quantified in a subset of electrodes (Saboo et al. 2019) (active electrodes), which showed more task-induced power in a specific frequency band (orange) than the remaining non-active electrodes (grey). (c) For each channel, difference in the mean task-induced power-in-band (PIB) between trials with words that were subsequently recalled and those that were forgotten was used to estimate SME magnitude across the time of word encoding. Note: t-statistic values were used to test times of significantly positive (more power on the recalled trials) or negative (less power on the recalled trials) SME. These were computed for a given frequency band, anatomical brain region, and hemisphere, as in the example of low theta power in the left frontal pole (dark and light grey area plots correspond to the left and right hemisphere, respectively).
Data analysis
We analysed intracranial electrophysiology recordings from drug-resistant epilepsy patients before and after word presentations. Each presentation epoch was filtered (10-order Butterworth) before being spectrally decomposed, normalised, and binned independently into distinct frequency bands between 2 and 120 Hz: low theta (2–4 Hz), high theta (5–9 Hz), alpha (10–15 Hz), beta (16–25 Hz), low gamma (25–55 Hz), and high gamma (65–115 Hz) frequency bands (Figure 2). We used a multi-taper Fast Fourier Transform (Chronux toolbox, RRID:SCR_00554752; with 4Hz bandwidth, 250 ms window and 1 taper to spectrally decompose every signal epoch). Spectrograms of all word epochs were averaged together after a z-score normalisation of each time-frequency point. Power signals obtained from different electrodes, patients, and sessions, and estimated in the six different frequency bands, were normalised using a method based on z-score transformation across the word encoding epochs.11,30 This was used instead of a baseline normalisation approach given that significant power changes related to attention stimulus processing are observed even before the stimulus presentation periods. For each electrode and frequency band, we determined the average spectral power at each time point across all the word presentation epochs. The overall induced power in a given frequency band during the word presentation epochs was quantified as the area under the absolute value of the corresponding time-series curve. Electrodes were then classified into active and inactive clusters using a Gaussian Mixture Model (GMM) based, unsupervised clustering method.31 Active electrodes were classified independently for each subject, so induced power values of active electrodes in a subject may overlap with those of inactive electrodes in other subjects. Encoding power differences of recalled and forgotten words were used to calculate subsequent memory effect (SME), a biomarker for human memory processes in the selected active electrodes. We used t-test statistic normalisation to investigate temporal profiles of SME (Figure 3a). Mean SME values were presented on a brain surface with their interpolated diffusions across brain volumes (per 20 MNI units) from all active electrodes for the low theta and the high gamma frequency bands in four representative time bins (Figure 4).
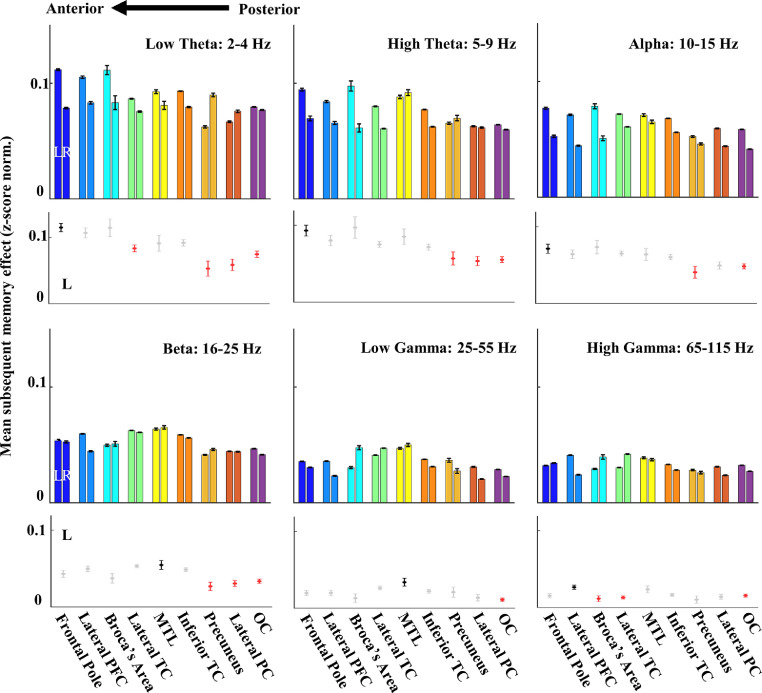
Figure 2.
SME magnitude was found to be the highest in the slow iEEG theta activity of the left prefrontal cortical areas. Mean absolute SME values (positive and negative combined) averaged across the time of word encoding was estimated for all active electrodes (n = 135) in six frequency bands and grouped into nine cortical regions. Bottom subpanels summarise post-hoc comparison of the left hemisphere group means with the three most posterior visual areas of the occipital and parietal cortex showing significantly lower SME (red dots, post hoc Tukey–Kramer test, p<0.05) relative to the top reference area (black dot). Notice proportionally higher magnitude in the slow iEEG activities of the theta bands, which peak in the three most anterior areas of the prefrontal cortex selectively in the left hemisphere. The low theta SME in these three prefrontal cortical areas is significantly greater than in the three occipito-parietal areas, and relatively greater than the three temporal areas.
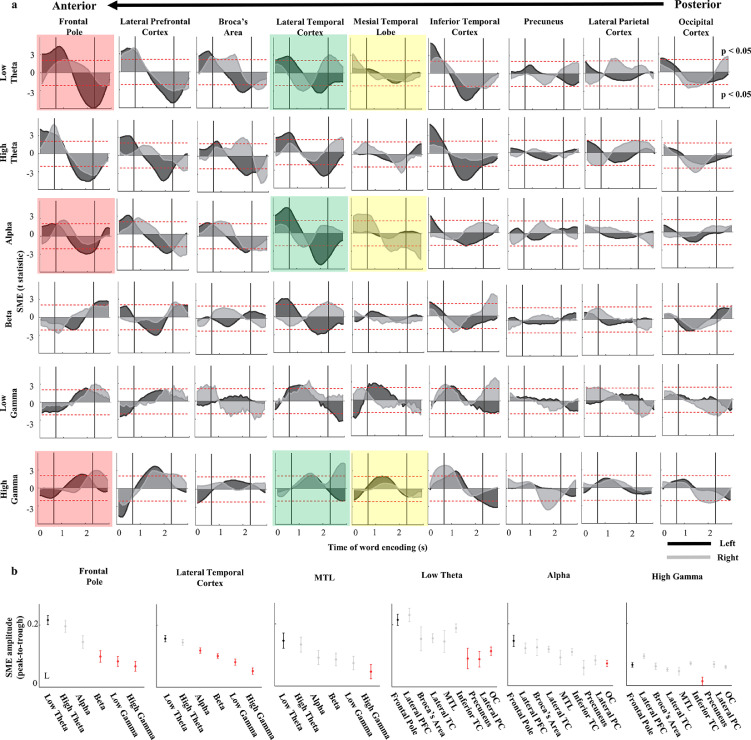
Figure 3.
Low theta activity in the left frontal pole confirms a consistent pattern of high SME across the time of word encoding. (a) Each panel of the grid summarises the magnitude and polarity of the subsequent memory effect (SME) for all active electrodes in a region as t-statistic values in each 50ms time bin (n = 135). SME is provided separately from the left and the right hemisphere (black and grey area plots, respectively). Notice that the low theta SME in the Frontal Pole is significantly positive (upper dashed line; t-statistic at p=0.05) throughout the initial phase, and significantly negative (lower dashed line; t-statistic at p=0.05) throughout the final phase of the word encoding time, as compared to other frequency bands and brain regions (colour background highlights selected examples in three brain regions and frequency bands). (b) Summary comparison of total SME magnitude (post hoc Tukey–Kramer, p<0.05) between the left Frontal Pole low theta activity (black dot reference; red dot indicates a significant difference) and the other frequency bands (left side) and brain regions (right side). Notice that only the neighbouring left lateral prefrontal cortex has a comparably high SME also in the low theta activity.
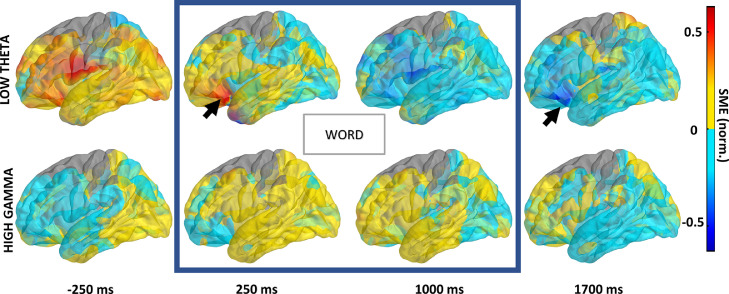
Figure 4.
Cortical mapping of SME across time reveals an anatomically consistent focus in the left prefrontal cortex. Average brain surface plots of mean SME values interpolated from all active electrodes are presented separately for the low theta and the high gamma frequency bands in four representative time bins (50 ms) before, during after word presentation (blue rectangle indicates the time bins with the word presented on the screen). Notice consistent localization of highly positive and highly negative magnitude in the same area of the ventral prefrontal cortex (arrow) for the low theta but not for the high gamma band.
Clustering
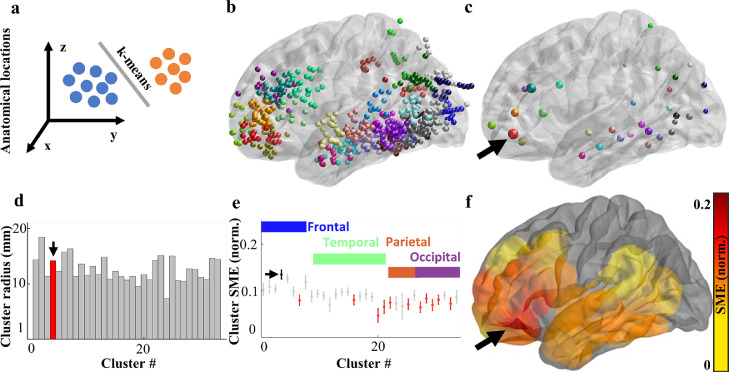
We clustered active iEEG electrodes with their proximity information to automatically group together electrodes according to their anatomical proximity. We used k-means clustering with squared Euclidean distance of the electrode coordinates and repeated clustering 1000 times with new initial cluster centroid positions to avoid local optimums. We divided total number of active electrodes by 20 to get the number of the clusters and to have a reasonable number of active electrodes in each cluster (n ≃ 20). The k-means method created 33 clusters, and we sorted them according to their brain regions from anterior to posterior. Distances between each electrode and their centroid of cluster were calculated and the mean value of these distances, cluster radii were plotted for all extracted clusters in the left hemisphere (see Figure 5d). Mean SME values were illustrated on a brain surface with their interpolated diffusions across brain volumes (per 20 MNI units) for each centroid of the clusters (Figure 5f).
Figure 5.
Hotspot of the memory effect is localised in an anatomically confined area of the left anterior prefrontal cortex. (a) All active electrodes implanted in this study were clustered in a unified brain surface according to their anatomical coordinates. (b) Anatomically distinct clusters of electrodes (sphere colour) reveal greater density and magnitude of SME (sphere size) in the left frontal lobe (n = 81). (c) Centroids of each cluster identify one with the highest mean SME magnitude (arrow) in the left anterior prefrontal cortex. (d) Anatomical spread of each cluster shows a relatively small size of the high SME cluster (arrow) of approx. 14 mm radius. (e) Comparison of mean SME with standard error of the mean across anatomically arranged clusters confirms a significantly greater SME of the prefrontal cluster than the majority of the other clusters (red; post-hoc Tukey-Kramer, p<0.05). Notice that the neighbouring prefrontal clusters also show relatively high mean SME compared to gradually more posterior localizations. (f) Surface colour plot of interpolated SME values confirms an anatomically confined hotspot (arrow) at the junction between the frontal pole and the Broca's area.
Statistics
All statistics were performed in the MATLAB academic version 2018a software package (MathWorks Inc.). ANOVA with Tukey-Kramer post-hoc testing of specific group differences was used to compare magnitude of the memory effect between brain regions (Figures 2 & 3b) frequency bands of neural activity (Figure 3b) and anatomical clusters of active electrodes (Figure 5E). To assess the magnitude of the memory effect in specific time bins of memory encoding, we used t-statistics separately in the left and the right hemisphere (Figure 3a). The ANOVA, the post-hoc testing of the group differences, and the t-statistic assessment of the SME magnitude in time were all performed at the alpha level of 0.05. No testing was performed in case of the t-statistic - the lines were provided as reference points to facilitate interpretation of the SME magnitude. The post-hoc Tukey-Kramer tests were used to account for the multiple comparisons of group combinations. All results are presented as mean+/- S.E.M. unless stated otherwise.
Ethics
Data were collected from the following clinical centres: Thomas Jefferson University Hospital (Philadelphia, PA), University of Texas Southwestern Medical Centre (Dallas, TX), Emory University Hospital (Atlanta, GA), Dartmouth-Hitchcock Medical Centre (Lebanon, NH), Hospital of the University of Pennsylvania (Philadelphia, PA), and Mayo Clinic (Rochester, MN). The research protocol was approved by the respective the Institutional Review Board (IRB) at each clinical centre and written informed consent was obtained from each participant.29,30
Role of funders
The funding sources played no role in the study design, data collection, data analysis, interpretation, writing of the report, or the decision of paper submission.
Results
We investigated the magnitude and localization of encoding word lists in the human brain using a large dataset of human intracranial recordings from 135 epilepsy patients engaged in a free recall memory task (Figure 1a). In this task, lists of 12 words were presented one at a time for subsequent vocal recall in any order. Spectral power of the iEEG signals induced during the presentation epochs with words that were recalled was compared with those that were forgotten to estimate SME - a metric for memory processing.5,7,11 Our study was focused on a subset of automatically selected active electrodes31 that recorded iEEG from brain areas engaged in the task performance, showing a significant change in the power induced during the epochs of memory encoding compared with the remaining electrodes that did not (Figure 1b). These active electrodes were selected independently in six frequency bands (low theta: 2–4 Hz, high theta: 5–9 Hz, alpha: 10–15 Hz, beta: 16-25 Hz, low gamma: 25–55 Hz, high gamma: 65–115 Hz) and comprised of 16.0–24.7% of all 9720 electrodes from all patients pooled in this study (low theta: 1557, high theta: 1582, alpha: 1795, beta: 2405, low gamma:1583, high gamma:1738). SME was estimated from the difference in normalized power estimates between the epochs with subsequently recalled and forgotten words (Figure 1c) independently in each frequency band. Having multiple electrodes localized in 30 Brodmann Areas allowed us to test for significantly higher (positive SME) or lower (negative SME) power on the recalled word epochs at specific times (50 ms bins) of memory encoding.
We first looked at the average SME magnitude from the entire period of memory encoding to compare the total memory signal between brain regions and frequency bands. To account for both positive and negative SME we used absolute values (see Figure 1) and averaged them for all active electrodes localised in nine cortical regions. The electrodes were grouped according to their anatomical distribution in specific Brodmann areas (occipital cortex: BA18 and 19, lateral parietal cortex: BA7 and 39, precuneus: BA30 and 31, inferior temporal cortex: BA20 and 37, mesial temporal lobe: BA28 and hippocampus, lateral temporal cortex: BA21 and 22, Broca's area: BA44 and 45, lateral prefrontal cortex: BA9 and 46, frontal pole: BA10 and 11), which were previously identified in the same task.11,31 We found consistent patterns of the SME magnitude in all six frequency bands. The SME magnitude was relatively greater in the more anterior areas of the higher order association cortex than in the posterior areas of the sensory visual cortex, especially in the low frequency bands (Figure 2). This pattern was specific to the left hemisphere. There was a significant effect of the brain region (ANOVA, 8 df, p<0.001) and of the hemisphere (ANOVA, 1 df, p=0.001) on the SME value in each frequency band (low theta: F=5.78, high theta: F=5.75, alpha: F=4.47, beta: F=7.87, low gamma: F=9.34, high gamma: F=4.61). In general, the SME magnitude in the left hemisphere was significantly greater (post hoc Tukey–Kramer, p<0.05; Supplementary Figure 1). In all frequency bands, SME was significantly greater in one of the higher order areas of the prefrontal or temporal cortex than in the visual areas (Figure 2; black and red dots, respectively). In the low theta band, where the magnitude was significantly greater than in the other bands proportionally to the increasing band frequencies (ANOVA, F=481.64, p<0.001, 5 df; Supplementary Figure 1), SME in the three most anterior regions of the left prefrontal cortex was significantly higher than in the three most posterior regions of the occipital and the parietal cortex (post hoc Tukey–Kramer, p<0.05), but not significantly higher than in the three temporal cortical regions (Figure 2). Overall, we observed the greatest SME magnitude in the low theta frequency band of the left prefrontal cortex.
To explore this general effect in the average SME magnitude with greater temporal resolution we analysed SME dynamics across the encoding period (Figure 3). Pooling all the active electrodes into the selected nine brain regions of the left and right hemispheres (occipital, lateral parietal, precuneus, inferior temporal cortex, mesial temporal lobe, lateral temporal cortex, Broca's area, lateral prefrontal cortex, frontal pole; see Table 1 for the number of electrodes and subjects), we quantified the positive and negative changes in SME magnitude as t-statistic (see Figure 1) in 50ms time bins of the word encoding period. We confirmed a consistent ‘flip-over’ pattern11 of significantly positive and negative SME in distinct time bin ranges before, during, and after the word presentation (Figure 3a). SME polarity was switching between the ranges of positive and negative values in all frequency bands and in every brain region - the timing and magnitude of these changes in polarity were specific to distinct frequencies and anatomical areas. The greatest SME amplitudes were found for the low frequency band activities in the anterior brain regions (Figure 3a; upper left corner) of the higher-order processing areas. These slow activities showed significantly more power before and in the early stages of encoding the subsequently recalled words (positive SME) and significantly less power in the late encoding stages (negative SME), as compared with the words that were forgotten. In contrast, this SME polarity pattern was of reverse order (first negative, then positive) and of relatively lower magnitude in the gamma frequency bands. The highest magnitudes were observed in the anterior (Frontal Pole; n=72 electrodes, N=26 subjects) and the ventrolateral (Lateral Prefrontal Cortex, n=71 electrodes, N=26 subjects) cortical areas and were specific to the left hemisphere. A similar profile of significantly high SME magnitude in the alpha frequency band was observed in the left lateral temporal cortex and in the right mesial temporal lobe. Both of these regions were previously associated with significant effects of electrical stimulation on recall performance in the same task.30,32
Table 1.
Summary of the total number of active electrodes included in the analysis for 6 frequency bands in the left and right brain regions, including the number of patients that these came from (bold).
| FP |
Lat. PFC |
Broca's |
Lat. TC |
MTL |
Inf. TC |
Pre |
Lat. PC |
OC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| High γ | 72 26 |
77 23 |
71 26 |
37 19 |
38 17 |
15 8 |
119 33 |
90 26 |
25 12 |
18 9 |
143 41 |
86 33 |
23 11 |
10 4 |
50 19 |
37 16 |
137 34 |
88 18 |
| Low γ | 73 22 |
58 20 |
61 26 |
30 15 |
24 13 |
14 4 |
126 33 |
149 29 |
28 12 |
20 10 |
124 41 |
78 33 |
17 10 |
8 6 |
44 15 |
37 12 |
130 31 |
82 18 |
| β | 53 17 |
24 13 |
79 24 |
37 19 |
28 15 |
18 9 |
232 55 |
206 43 |
32 19 |
22 15 |
180 49 |
138 44 |
36 14 |
21 10 |
73 24 |
68 19 |
139 32 |
92 21 |
| α | 44 17 |
18 11 |
52 19 |
21 15 |
21 15 |
15 9 |
163 45 |
125 35 |
26 16 |
24 15 |
138 34 |
101 42 |
27 14 |
7 5 |
67 22 |
53 15 |
146 32 |
102 21 |
| High θ | 47 18 |
18 13 |
50 21 |
22 11 |
12 6 |
7 3 |
144 39 |
100 37 |
23 15 |
18 10 |
149 41 |
98 40 |
32 17 |
12 6 |
64 22 |
52 17 |
163 36 |
98 23 |
| Low θ | 84 31 |
50 19 |
58 25 |
38 19 |
18 10 |
9 5 |
125 38 |
60 25 |
22 14 |
13 8 |
145 43 |
74 33 |
25 8 |
13 8 |
44 19 |
32 14 |
146 36 |
85 19 |
We compared selected brain regions from the prefrontal and the temporal cortex (highlighted in Figure 3a): the frontal pole, the lateral temporal cortex, and the mesial temporal lobe, including the hippocampus, associated with the declarative memory functions.33,34 Peak-to-trough magnitude was the highest in the low theta band for all these regions (Figure 3b) and was significantly greater than in the early visual areas (post-hoc Tukey–Kramer, p<0.05). The magnitude in both the frequency and the anatomical space was proportionally higher in the frontal pole than in more posterior areas (Figure 3b). This effect across the anatomical space was gradually decreasing in the higher frequency bands of the alpha and the high gamma activities. In summary, the temporal dynamics of SME confirmed the largest magnitude changes in the low theta activities of the left anterior prefrontal cortex.
Given that the greatest SME magnitude was found in specific frequency ranges, times, and brain areas, we hypothesised that there was a distinct source of this powerful signal within the anterior prefrontal cortex. For this purpose, the anatomical distribution of SME magnitude was estimated on an average brain surface using all active electrodes identified in the study. A focus of high positive and negative magnitude in the low theta frequency band was localised to a confined area between the frontal pole and the ventral lateral prefrontal cortex in the left hemisphere (Figure 4). The positive and the negative SME was found during the early and the late stages of word encoding, respectively. We did not find an analogous focus of the memory effect in the high gamma band (Figure 4) or in the right hemisphere.
To test for a hypothetical ‘hotspot’ of memory processing with the strongest SME in the identified cortical areas, we clustered all active electrodes by anatomical proximity. Electrodes recording from the same cortical sources in different patients were grouped together in anatomical space to compare their SME values (Figure 5a; SME was not used as a variable for the clustering - only the anatomical coordinates in the anatomical space). This analysis identified 33 clusters with electrodes of various mean SME magnitude in each of the studied cortical regions of the left hemisphere (Figure 5b). The grand average SME from all electrodes in each cluster confirmed a source of relatively greater magnitude, which was localised around the junction of the anterior and the ventrolateral prefrontal cortex (Figure 5c). The cluster was confined to a space of approx. 14 - comparable to the sizes of the other clusters (Figure 5d) - and comprised 23 electrodes from twelve different subjects (Suppl. Figure 2). Variance of the SME magnitude was significantly different across the 33 clusters (ANOVA, F=2.19, p<0.001, 66 df), showing the highest mean in the identified cluster. SME in this one cluster was significantly greater (post-hoc Tukey–Kramer, p<0.05) than in 11 of the remaining 32 clusters (Figure 5e). Clusters with magnitudes that were not significantly lower were localised in anatomical proximity to the ‘hotspot’ cluster in the neighbouring prefrontal areas. The anatomical localization of the clusters confirmed the pattern of SME distribution observed across the larger brain regions (Figure 2) with decreasing mean SME values in gradually more posterior clusters of the temporal, parietal and occipital lobes. Summarising SME magnitude from all electrodes in this cluster analysis confirmed localisation of the hotspot of verbal memory processing in a spatially discrete patch of the left anterior prefrontal cortex (Figure 5F). SME in the right hemisphere did not reveal an analogous change in the cluster variance or a similar hotspot in this or any other cortical region (ANOVA, F=1.07, p=0.371, 36 df, Suppl. fig. 4). Localization and the magnitude of the hotspot memory effect were robust to changing the cluster size (Suppl. Figure 1). The hotspot location at the junction between the anterior and the ventrolateral prefrontal cortex suggests an important node for processing verbal memory, connecting a network of the classic speech-related processing in the Broca's area and the higher-order executive functions of the human frontal pole.
Discussion
Our results revealed a single cortical target with significantly greater memory effect than the other areas classically associated in human memory functions. A distinct hotspot of the memory effect in the low theta frequency range was localised in the ventrolateral portion of the anterior prefrontal cortex. Both the anatomical localization and the spectral range of this activity were obscure in the original neuroimaging studies24 and in the following iEEG studies with limited electrode coverage. Even in our large study, there were only twelve subjects with active electrodes implanted and localised in the hotspot area cluster (electrodes are typically not implanted in these areas for seizure localization). Likewise, theta activities received considerably less attention than the high gamma activity in terms of mapping cognitive functions in the human cortex. Finally, even though the previous neuroimaging and iEEG studies quantified the memory-related activities in multiple cortical regions engaged by various tasks, a comprehensive comparison across the anatomical and the spectral scale was needed to reveal a relatively greater contribution of the slow theta rhythms in one distinct prefrontal area. A single target for potential therapeutic approaches together with a biomarker of specific neural activity is a major translational advance relative to the previous reports of widespread areas in multiple cortical lobes.4, 5, 6,10, 11, 12,25,37
With regard to the anatomical localization of the strongest memory signal for predicting recall, a large portion of the inferior prefrontal cortex has been consistently reported in previous neuroimaging studies. A meta-analysis of 74 studies4 concluded that this area in the left hemisphere together with bilateral premotor, fusiform, posterior parietal cortex and both hippocampi showed a significant memory effect, which was associated more with tasks using verbal as opposed to non-verbal visual stimuli. One of these studies employed a similar free recall task with lists of words that either belonged to a particular semantic category or not.26 The authors associated the categorization effect with the dorsolateral prefrontal cortical activity, whereas the subsequent recall with subregions of the ventrolateral prefrontal cortex. Interestingly, the anterior subregion, at the junction with the frontal pole, including the hotspot area reported here, showed a greater effect during free recall for non-categorized words. Another recent fMRI study with epileptic patients and age-matched healthy subjects confirmed a relatively stronger SME in the anterior division of the inferior frontal gyrus in the same free recall task that we used.25 Therefore, the localization of our reported hotspot activity is confirmed in a larger area where the memory effect was obtained in hemodynamic responses.
Even though the hemodynamic response measured in the fMRI studies was shown to correlate with fast electrophysiological activities in the high gamma frequency range,13,14 our results show the highest magnitude of the memory effect in the low theta band. These slow activities in the left frontal pole and the left lateral prefrontal cortex showed higher SME magnitude than in any other region and frequency band. In the other bands, the magnitudes were relatively lower and without this greater contribution of the prefrontal areas. The memory effect in theta frequency bands was generally reported with both positive and negative polarity in the previous studies,7,9,10,12,35 meaning either more or less spectral power during encoding of the subsequently recalled compared to the forgotten stimuli. According to our results here and in the previous study of the high gamma activities,11 the SME polarity is dependent on the phase of memory encoding and the anatomical location, revealing dynamic profiles of significantly positive and negative alternation at consistent times in specific brain regions. Net positive or negative SME would depend on the particular time-frame of encoding that was selected in a given study (usually the first 1000-1500 ms after stimulus onset). Hence, we decided to analyse the absolute values during an entire epoch, including the phases pre- and post-stimulus presentation. Given the dynamic temporal profiles of the memory effects, they can only be resolved in the iEEG studies. Averaging the effect over an extended period of time would not only compromise the resolution but also decrease the overall magnitude. Still, we were able to find the greatest magnitude of the low theta memory effect within a larger brain region, where the greatest BOLD signal effect was found in the previous neuroimaging studies.4 Compared to the previous neuroimaging studies, our iEEG analysis was limited to unevenly distributed electrodes implanted for seizure monitoring. Even in this large dataset there were cortical areas that remained implanted with few electrodes. The identified hotspot cluster comprised 1–3 electrodes from a given subject. These were localised on the average brain surface with limited insight into individual subject differences. Epilepsy-related processes that could affect the studied cognitive functions present another challenge to interpreting our results. Confirmation of the memory effect in the same frequency band and brain region across multiple electrodes, subjects, and throughout the memory encoding phases is reassuring, as well as the general agreement with the larger cortical region reported in the previous studies.
Despite these limitations and the highly dynamic nature of the memory effect, the identified low theta activities showed persistently high magnitude in the anterior prefrontal hotspot location. SME was highly positive in the encoding phase preceding and immediately following the stimulus presentation, and highly negative in the post-stimulus presentation phase (see Figure 4). This persistent pattern within a single hotspot area was not observed in the high gamma or any other frequency band. There was only one other area in the left ventral motor cortex that revealed a high positive effect before the word onset and a negative effect toward the end of the stimulus display. We assume that these activations in the motor area for facial movements reflect rehearsal of ‘speaking’ the presented words in the mind (subjects were instructed not to speak the words out loud), which turned out also to predict subsequent recall. The overall magnitude of this motor memory effect in the low theta band of the ventral motor areas, however, was not as high as in the anterior prefrontal hotspot when the entire period of word encoding was summarised (see Figure 5). In addition, the spatial spread of the effect in the motor areas was not as anatomically confined as the prefrontal hotspot. All in all, the low theta hotspot was found to be consistent in space and time, showing a high magnitude of the memory effect in the same confined cortical area and across all, early and late, phases of stimulus encoding (see Suppl. Figure 1&2). A similar pattern of pre-onset memory effect continued in the post-onset phase of stimulus presentation was recently also reported in the single and multi-unit firing of neurons in the human hippocampus, amygdala, and cingulate and prefrontal cortex.36 The authors found this pre-onset memory effect to be related to ‘attention to encoding’ rather than general arousal or attention. The effects that start even before stimulus presentation are key for an early prediction and modulation of brain activities to help in successful encoding of memories that would otherwise be forgotten.
Previous iEEG studies that mapped the memory effect of theta and gamma neural activities in anatomical space and time of memory encoding, reported widespread prefrontal and temporal cortical areas engaged early and late during word encoding in the same task.10,12,37 In one of these studies,12 the memory effect was mapped with fine spatial and temporal resolution on the level of spectral power and coherence in the theta and gamma frequency bands. The authors used a different approach to map the memory effect, which was quantified as the proportion of electrode sites that showed a significant positive or negative SME irrespective of the magnitude. This could explain missing a hotspot of relatively greater magnitude in the previous study. In addition, the other study combined the low and high theta activities. Our approach was also more sensitive to detect a few active electrodes with a high SME magnitude, whereas these could be missed if there were other non-active electrodes with low SME values in close anatomical proximity of the hotspot. Nevertheless, the previous results12 confirmed a hub of high functional connectivity (spectral coherence), which predicted subsequent recall, i.e. showed the memory effect, in the theta frequency bands at the very same location as our reported hotspot.
Local electrical brain stimulation could provide conclusive evidence for whether this or another area of high memory effect is sufficient to enhance performance in a given task. In the previous project with the same patients performing the same free recall task with direct brain stimulation, the effect on memory performance was assessed in multiple target areas, including the hippocampus, entorhinal and parahippocampal cortex, lateral temporal cortex, posterior cingulate cortex, and dorsolateral prefrontal cortex.29,30,38, 39, 40 Only the lateral temporal cortical stimulation was robustly associated with enhanced performance in this task in individual subjects, on a group level, and also across two studies employing different stimulation approaches. None of these studies, however, tested the ventrolateral or the anterior prefrontal cortical areas. The identified hotspot area showed a greater memory effect than the previously successful stimulation targets in the lateral temporal cortex (Figure 5E).
Therefore, the identified hotspot in the anterior prefrontal cortex presents an attractive target for modulating memory functions and developing new therapies. Compared to the other temporal and limbic areas associated with processing declarative memory,33,41 the prefrontal cortex supports higher-order executive roles in effective encoding and retrieval of episodic memory in the left and the right hemisphere, respectively.42 In our task, the anterior prefrontal cortex may be specifically involved in coordinating attention to the remembered stimuli, mentalization of the encoded information, and generating cues for subsequent recall. Evidence from lesion studies suggests involvement of this region in metacognition43 that would be required for successful performance in the free recall task. We localised the hotspot area at the junction between the frontal pole and the ventrolateral prefrontal cortex of the Broca's area. This location is ideally suited for interfacing between the executive functions of the former27,28 and the speech processing functions of the latter.44 On one hand, this top position in the processing hierarchy makes the hotspot a promising target for modulating encoding of verbal memories in general; on the other hand, beneficial effects of modulation in this location may not generalise to encoding non-verbal stimuli. Nevertheless, proximity to the frontal pole areas of the brain, which are evolutionarily more developed in humans than in the nonhuman primates,45,46 opens new avenues for modulating higher cognitive functions beyond memory processing. It has to be stated, however, that the pathway to any clinical translation into a potential treatment approach or a therapy would still require additional safety and feasibility trials and approvals, even though the technology for such targeted brain stimulation for memory enhancement is available.29,30
Besides determining the hotspot target area for therapeutic modulation of memory encoding, we propose a comprehensive clinical biomarker approach, in which normalised measures of the memory effect are compared across the anatomical, spectral and temporal scales of neural activities. This simple biomarker approach can, for instance, delineate brain areas with high memory effect that should be spared in tumour or epilepsy surgeries to prevent post-surgical deficits in verbal memory functions. Other biomarkers of the memory effect based on various measures of functional connectivity would be required to determine multiple anatomical targets to modulate a network of regions beyond its local hubs.12,47 Both these network and local biomarkers of neural activities can be employed in time to predict or detect the states of optimal cognitive performance to trigger therapeutic interventions in a closed-loop design.23,29,48 Our results suggest a new potential target for memory enhancement in the frontal pole and provide a neural biomarker activity with precise timing for control of the intervention like electrical stimulation. How to modulate its local neural activity49,50 and the effect on the connected network of brain regions51 is the outstanding challenge for developing therapies for memory and cognitive deficits. The dynamic biomarkers of neural activities will become increasingly useful for development of novel brain-computer interfaces and therapies for restoring brain functions.
Contributors
ÇT drafted the manuscript, directly accessed and verified the underlying data, conducted data analysis, interpreted the results, revised and approved the manuscript. VSM, KVS, ML, PN, VK, contributed to data analysis, revised and approved the manuscript. GAW designed the study, contributed to data collection, revised and approved the manuscript. MTK designed the study, drafted the manuscript, contributed to data collection, directly accessed and verified the underlying data, contributed to data analysis, interpreted the results, revised and approved the manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
All de-identified raw data may be downloaded at Restoring Active Memory (RAM), RAM Public Data Release. 2018. [Online]. Available: http://memory.psych.upenn.edu/RAM
The following data are included: Electrocorticographic (ECoG) recordings, demographic information (age, gender, race/ethnicity, and handedness), individual electrode contact atlas location and coordinates for localization, experiment design documents.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgements
We thank healthcare workers and technicians assisted in patient recruitment and data collection at Mayo Clinic. This work would not be possible without the dedicated effort and participation of epilepsy patients and their families.
Open-access datasets were originally collected as part of a BRAIN Initiative project called Restoring Active Memory (RAM) funded by the Defense Advanced Research Project Agency (DARPA). CT, ML, MTK, and this research were supported from the First Team grant of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund.
Restoring Active Memory (RAM), RAM Public Data Release. 2018. [Online]. Available: http://memory.psych.upenn.edu/RAM.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104135.
Appendix. Supplementary materials
References
- 1.Rugg MD, Coles MGH. Oxford University Press; New York: 1996. Electrophysiology of Mind. [DOI] [Google Scholar]
- 2.Rugg MD, Otten LJ, Henson RNA. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- 4.Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 6.Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 7.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- 9.Sederberg PB, Schulze-Bonhage A, Madsen JR, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- 10.Long NM, Burke JF, Kahana MJ. Subsequent memory effect in intracranial and scalp EEG. Neuroimage. 2014;84:488–494. doi: 10.1016/j.neuroimage.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucewicz MT, Saboo K, Berry BM, et al. Human verbal memory encoding is hierarchically distributed in a continuous processing stream. eNeuro. 2019;6 doi: 10.1523/ENEURO.0214-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke JF, Zaghloul KA, Jacobs J, et al. Synchronous and asynchronous theta and gamma activity during episodic memory formation. J Neurosci. 2013;33:292–304. doi: 10.1523/JNEUROSCI.2057-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 14.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 15.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clin Neurophysiol. 2008;119:116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson BO, Ding M, Buzsáki G. Temporal coupling of field potentials and action potentials in the neocortex. Eur J Neurosci. 2018;48:2482–2497. doi: 10.1111/ejn.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Düzel E, Penny WD, Burgess N. Brain oscillations and memory. Curr Opin Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Buzsaki G. Oxford University Press; 2006. Rhythms of the Brain. [Google Scholar]
- 21.Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34:1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahana MJ. The cognitive correlates of human brain oscillations. J Neurosci. 2006;26:1669–1672. doi: 10.1523/jneurosci.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezzyat Y, Kragel JE, Burke JF, et al. Direct brain stimulation modulates encoding states and memory performance in humans. Curr Biol. 2017;27:1251–1258. doi: 10.1016/j.cub.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rugg MD. Memories are made of this. Science. 1998;281:1151–1152. doi: 10.1126/science.281.5380.1151. [DOI] [PubMed] [Google Scholar]
- 25.Hill PF, King DR, Lega BC, Rugg MD. Comparison of fMRI correlates of successful episodic memory encoding in temporal lobe epilepsy patients and healthy controls. Neuroimage. 2020;207 doi: 10.1016/j.neuroimage.2019.116397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long NM, Oztekin I, Badre D. Separable prefrontal cortex contributions to free recall. J Neurosci. 2010;30:10967–10976. doi: 10.1523/jneurosci.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362:887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert SJ, Spengler S, Simons JS, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 29.Ezzyat Y, Wanda PA, Levy DF, et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Commun. 2018;9:365. doi: 10.1038/s41467-017-02753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucewicz MT, Berry BM, Miller LR, et al. Evidence for verbal memory enhancement with electrical brain stimulation in the lateral temporal cortex. Brain. 2018;141:971–978. doi: 10.1093/brain/awx373. [DOI] [PubMed] [Google Scholar]
- 31.Saboo KV, Varatharajah Y, Berry BM, et al. Unsupervised machine-learning classification of electrophysiologically active electrodes during human cognitive task performance. Sci Rep. 2019;9:17390. doi: 10.1038/s41598-019-53925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs J, Miller J, Lee SA, et al. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron. 2016;92:983–990. doi: 10.1016/j.neuron.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H. A cortical–hippocampal system for declarative memory. Nature Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 34.Squire L, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 35.Kragel JE, Ezzyat Y, Sperling MR, et al. Similar patterns of neural activity predict memory function during encoding and retrieval. Neuroimage. 2017;155:60–71. doi: 10.1016/j.neuroimage.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urgolites ZJ, Wixted JT, Goldinger SD, et al. Spiking activity in the human hippocampus prior to encoding predicts subsequent memory. Proc Natl Acad Sci USA. 2020;117:13767–13770. doi: 10.1073/pnas.2001338117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, Kahana MJ. Human intracranial high-frequency activity maps episodic memory formation in space and time. Neuroimage. 2014;85 Pt 2:834–843. doi: 10.1016/j.neuroimage.2013.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs J, Miller J, Lee SA, et al. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron. 2016;92:983–990. doi: 10.1016/j.neuron.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 39.Goyal A, Miller J, Watrous AJ, et al. Electrical stimulation in hippocampus and entorhinal cortex impairs spatial and temporal memory. The J Neurosci. 2018;38:4471–4481. doi: 10.1523/jneurosci.3049-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natu VS, Lin J-J, Burks A, Arora A, Rugg MD, Lega B. Stimulation of the posterior cingulate cortex impairs episodic memory encoding. J Neurosci. 2019;39:7173–7182. doi: 10.1523/jneurosci.0698-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squire LR, Zola SM. Amnesia, memory and brain systems. Philos Trans R Soc Lond B Biol Sci. 1997;352:1663–1673. doi: 10.1098/rstb.1997.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher PC, Frith CD, Rugg MD. The functional neuroanatomy of episodic memory. Trends Neurosci. 1997;20:213–218. doi: 10.1016/s0166-2236(96)01013-2. [DOI] [PubMed] [Google Scholar]
- 43.Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83:1002–1018. doi: 10.1016/j.neuron.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flinker A, Korzeniewska A, Shestyuk AY, et al. Redefining the role of Broca's area in speech. Proc Natl Acad Sci USA. 2015;112:2871–2875. doi: 10.1073/pnas.1414491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::aid-ajpa1022>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 46.Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nature Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- 47.Solomon EA, Kragel JE, Sperling MR, et al. Widespread theta synchrony and high-frequency desynchronization underlies enhanced cognition. Nature Commun. 2017;8 doi: 10.1038/s41467-017-01763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burke JF, Merkow MB, Jacobs J, Kahana MJ, Zaghloul KA. Brain computer interface to enhance episodic memory in human participants. Front Hum Neurosci. 2014;8:1055. doi: 10.3389/fnhum.2014.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohan UR, Watrous AJ, Miller JF, et al. The effects of direct brain stimulation in humans depend on frequency, amplitude, and white-matter proximity. Brain Stimul. 2020;13:1183–1195. doi: 10.1016/j.brs.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lech M, Berry BM, Topcu C, et al. Direct electrical stimulation of the human brain has inverse effects on the theta and gamma neural activities. IEEE Trans Biomed Eng. 2021 doi: 10.1109/TBME.2021.3082320. [DOI] [PubMed] [Google Scholar]
- 51.Solomon EA, Kragel JE, Gross R, et al. Medial temporal lobe functional connectivity predicts stimulation-induced theta power. Nat Commun. 2018;9:4437. doi: 10.1038/s41467-018-06876-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: a platform for analyzing neural signals. J Neurosci Methods. 2010;192:146–151. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.