Summary
Background
Prior studies have revealed remarkable phenotypic heterogeneity in KCNQ2-related disorders, correlated with effects on biophysical features of heterologously expressed channels. Here, we assessed phenotypes and functional properties associated with KCNQ2 missense variants R144W, R144Q, and R144G. We also explored in vitro blockade of channels carrying R144Q mutant subunits by amitriptyline.
Methods
Patients were identified using the RIKEE database and through clinical collaborators. Phenotypes were collected by a standardized questionnaire. Functional and pharmacological properties of variant subunits were analyzed by whole-cell patch-clamp recordings.
Findings
Detailed clinical information on fifteen patients (14 novel and 1 previously published) was analyzed. All patients had developmental delay with prominent language impairment. R144Q patients were more severely affected than R144W patients. Infantile to childhood onset epilepsy occurred in 40%, while 67% of sleep-EEGs showed sleep-activated epileptiform activity. Ten patients (67%) showed autistic features. Activation gating of homomeric Kv7.2 R144W/Q/G channels was left-shifted, suggesting gain-of-function effects. Amitriptyline blocked channels containing Kv7.2 and Kv7.2 R144Q subunits.
Interpretation
Patients carrying KCNQ2 R144 gain-of-function variants have developmental delay with prominent language impairment, autistic features, often accompanied by infantile- to childhood-onset epilepsy and EEG sleep-activated epileptiform activity. The absence of neonatal seizures is a robust and important clinical differentiator between KCNQ2 gain-of-function and loss-of-function variants. The Kv7.2/7.3 channel blocker amitriptyline might represent a targeted treatment.
Funding
Supported by FWO, GSKE, KCNQ2-Cure, Jack Pribaz Foundation, European Joint Programme on Rare Disease 2020, the Italian Ministry for University and Research, the Italian Ministry of Health, the European Commission, the University of Antwerp, NINDS, and Chalk Family Foundation.
Keywords: KCNQ2, Gain-of-function, Amitriptyline, Autism, Developmental and epileptic encephalopathy
Research in context.
Evidence before this study
Pathogenic variants in KCNQ2, encoding for Kv7.2 voltage-gated potassium channel subunits, represent one of the most common causes of childhood-onset genetic epilepsies, with an incidence in young population of 1 per 17,000 live births. Most disease-causing KCNQ2 variants cause loss-of-function (LoF) when studied in vitro, and have been associated with a broad spectrum of neonatal-onset epilepsy phenotypes. In addition to LoF, few gain-of-function (GoF) variants have been described; these affect residues within the voltage-sensing domain (VSD) and are associated with a distinct phenotype, as evidenced by the absence of neonatal seizures. GoF and LoF variants may need different therapeutic approaches, but, unlike for KCNQ2 LoF, targeted treatment for KCNQ2/3 GoF patients have not emerged to date.
Added value of this study
Twenty patients (14 novel and 6 published), all carrying pathogenic variants at position R144 in the VSD, were identified. Detailed clinical information was collected on 9 R144W and 6 R144Q individuals for analysis of clinical features. All patients had developmental delay with prominent language impairment. R144Q patients were generally more severely affected than R144W patients; in fact, patients carrying the R144W variant showed a mild to severe degree of intellectual disability (ID), whereas all patients with the R144Q variant had severe ID. Infantile to childhood onset epilepsy was seen in 40%, while 67% of sleep-EEGs showed sleep-activated epileptiform activity. None of the patients had neonatal-onset epilepsy. Ten patients (67%) presented autistic features. In vitro, Kv7.2 channels incorporating R144 pathogenic variants displayed a leftward shift in activation gating, suggesting GoF effects; the extent of this shift was larger for R144G (∼25 mV) and R144Q (∼18 mV) than for R144W (∼4 mV) channels, and correlated with severity of neurodevelopmental problems, highlighting novel genotype-phenotype correlations and supporting a role for in vitro functional assays among predictors of neurodevelopmental trajectories in these children. Amitriptyline (1–10 μM) dose-dependently blocked channels containing both Kv7.2 and Kv7.2 R144Q subunits.
Implications of all the available evidence
This study confirms the existence of a distinct phenotype associated with KCNQ2 GoF variants characterized by developmental delay (DD) with prominent language impairment and autistic features in the absence of neonatal seizures. In some (but not all), DD is accompanied by infantile- to childhood-onset epilepsy and EEG sleep-activated epileptiform activity. These phenotypic characteristics are clearly distinct from those of patients carrying KCNQ2 loss-of-function variants, where early neonatal-onset epilepsy is the unifying clinical feature across the severity spectrum. The present results provide novel genotype-phenotype correlations in KCNQ2-related disorders, and underline the importance of including the “epilepsy gene” KCNQ2 in diagnostic intellectual disability (ID) gene panels. The absence of neonatal seizures has emerged as a robust differentiator between the (rarer) KCNQ2 GoF and (more common) LoF variants. This distinction is clinically very important, as the entities present in very different clinical settings and require development of different therapeutic approaches. Raising awareness about these distinct phenotypes has become especially important now that an international randomized clinical trial with a pediatric formulation of the potassium channel opener retigabine (ClinicalTrials.gov Identifier: NCT04639310; https://clinicaltrials.gov/ct2/show/NCT04639310) for patients with KCNQ2-encephalopathy has recently been initiated. In this trial, only patients with clear LoF variants can be included, and the “presence of a known GoF variant in the KCNQ2 gene, or clinical characteristics consistent with previously reported pathogenic GoF variants in the KCNQ2 gene” are explicitly listed among exclusion criteria for enrolment in the trial. Finally, the Kv7.2/7.3 channel blocking effect of amitriptyline holds promise for its use as a potential targeted treatment for patients with KCNQ2 GoF variants and deserves further study.
Alt-text: Unlabelled box
Introduction
KCNQ2 and KCNQ3 genes encoding Kv7.2 and Kv7.3 voltage-gated potassium (K+) channel subunits are widely expressed in the nervous system.1 In neurons, Kv7.2 subunits form homotetrameric or heterotetrameric channels with Kv7.3 subunits to generate the M-current (IKM), a non-inactivating K+ current with slow activation and deactivation kinetics that regulates the resting membrane potential and suppresses repetitive neuronal firing.1 Each Kv7 subunit is formed by six transmembrane segments (S1-S6); the voltage-sensing domain (VSD) is encompassed by S1-S4, whereas S5, S6 and the S5-S6 intervening linker form the pore region.2 In addition to membrane voltage, Kv7 channel opening requires phosphatidylinositol 4,5-bisphosphate (PIP2), a membrane lipid which binds to distinct channel regions at each VSD gating state.3
KCNQ2/3 pathogenic variants exerting loss-of-function (LoF) effects are associated with a spectrum of neonatal-onset phenotypes ranging from self-limited familial neonatal epilepsy (SLFNE, MIM:121200) to KCNQ2 developmental and epileptic encephalopathy (KCNQ2-DEE, MIM:613720) with therapy-resistant seizures and intellectual disability (ID).4 Most KCNQ2-DEE pathogenic variants exert dominant-negative LoF effects when studied in vitro.5 Seizures in these children tend to respond well to sodium channel blockers, now recommended as first-line treatment,6 and a randomized, double-blind, placebo-controlled trial with the potassium channel opener retigabine has recently been initiated (ClinicalTrials.gov Identifier:NCT04639310).7
Few heterozygous KCNQ2 missense variants enhance channel function (gain-of-function, GoF) by causing a hyperpolarizing shift in voltage-dependent activation.8, 9, 10, 11 Phenotypes associated with GoF variants differ from the classical KCNQ2-DEE phenotype. They range from profound ID without neonatal seizures but with a characteristic nonepileptic myoclonus and poor prognosis for the R201C variant with strong GoF effect,8 to epileptic spasms with hypsarrhythmia and severe developmental delay (DD) for the R198Q variant with milder GoF effect.10 Interestingly, KCNQ3 variants at R230, the homologous residue of KCNQ2 R201, also have strong GoF in vitro effects; patients carrying these variants display global developmental delay within the first 2 years of life, sleep-activated near-continuous multifocal spikes, and autistic features, but no neonatal seizures.12 Prognosis is often poor since most patients with KCNQ2/3 GoF variants do not respond well to anti-seizure medications.8 GoF and LoF variants may need different therapeutic approaches, but, unlike for KCNQ2 LoF, targeted treatment for KCNQ2/3 GoF patients have not emerged to date.
We have previously shown that the KCNQ2 R144Q variant, identified in a single patient, results in a GoF effect.9 Here, we investigated the phenotypic spectrum associated with KCNQ2 variants at the R144 position by describing detailed clinical features of 15 patients, and summarizing published clinical data on 5 additional cases. To highlight potential genotype-phenotype correlations, we compared the functional properties of Kv7.2 R144W and R144G mutant subunits with those of the previously described R144Q.9 At last, we demonstrate that amitriptyline inhibits channels carrying both wild-type and mutant Kv7.2 R144Q subunits, suggesting a potential targeted treatment for patients with KCNQ2 R144 variants.
Methods
Patient series collection
We collected detailed clinical information of patients carrying a KCNQ2 R144W (NM_172107.3:c.430C>T), R144G (NM_172107.3:c.430C>G), or R144Q (NM_172107.3:c.431G>A) variant. Patients were recruited through the Rational Intervention of KCNQ2 Epileptic Encephalopathy (RIKEE) database (http://www.RIKEE.org), a curated database aggregating (un)published patient data provided by physicians or families after parental informed consent. The site is hosted at Baylor College of Medicine under an institutional review board– approved research protocol. Additional patients were directly referred for inclusion in the study by collaborating paediatric neurologists and medical geneticists. A standardized phenotyping sheet, including data fields on technical exams as EEG and MRI of the brain, was sent to the referring physician of all unpublished patients (n=14) and of Patient 11, who was previously reported with minimal clinical details as part of an Epi4K epileptic encephalopathy cohort.13 Degree of ID was defined based on the level of adaptive functioning across multiple domains (cognitive, social, practical), based on local multidisciplinary clinical assessment, as proposed by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).14
Mutagenesis and heterologous expression of KCNQ2 and KCNQ3 cDNAs
Variants were engineered in KCNQ2 human cDNA cloned into pcDNA3.1 by QuikChange site-directed mutagenesis (Agilent Technologies, Milan, Italy), as described.5 Channel subunits were expressed in Chinese Hamster Ovary (CHO) cells by transient transfection using Lipofectamine 2000 (Invitrogen, Milan, Italy). Total cDNA in the transfection mixture was kept constant at 4 μg.
Whole-cell electrophysiology
Currents from CHO cells were recorded at room temperature (20–22 °C) 1-2 days after transfection, using the whole-cell configuration of the patch-clamp technique with glass micropipettes of 3–5 MΩ resistance. The extracellular solution contained (in millimolar): 138 NaCl, 2 CaCl2, 5.4 KCl, 1 MgCl2, 10 glucose and 10 HEPES, pH 7.4 with NaOH. The pipette (intracellular) solution contained (in millimolar): 140 KCl, 2 MgCl2, 10 EGTA, 10 HEPES, 5 Mg-ATP, pH 7.3–7.4 with KOH. The pCLAMP software (version 10.0.2) was used for data acquisition and analysis. Current densities, conductance-voltage curves, and current activation and deactivation kinetics were obtained and analysed as previously described.15
A stock solution (10 mM) of amitriptyline (Sigma-Aldrich, Germany) was prepared in dimethyl sulfoxide (DMSO); extracellular solution was used for subsequent drug dilutions. In the experiments with amitriptyline, currents were activated by 3s voltage ramps from −80 to +20 mV applied every 15 s. Currents at +20 mV were measured before and after drug application, and drug's effects expressed as % of blockade.
Structural modelling
Three-dimensional models of Kv7.2 subunits in activated and resting gating states were generated by multistate modelling, as previously described.9
Statistics
Values are expressed as the mean±SEM of cells recorded in at least three independent experimental sessions. Statistically significant differences between experimental groups were evaluated with the Student's t-test (p<0.05).
Ethics
Written informed consent for participation/publication was obtained from all parents or legal guardians of recruited patients. This study was approved by the Human Research Ethics Committees of the University Hospital of Antwerp, Belgium (Ref. 19/20/257).
Data availability
All data that support the findings of the study are available from the corresponding author, with the exception of primary patient sequencing data, as they are derived from patient samples with unique variants that are impossible to guarantee anonymity for.
Role of the funding source
The funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Cohort description and genotypes
Fourteen previously unpublished KCNQ2-DEE patients were included in our study: 9 carrying the p.R144W variant, and 5 the p.R144Q variant (eTable 1 and 2). We also collected clinical data on a sixth p.R144Q patient (patient 11) who was previously published with limited clinical information.13 We defined this cohort of 15 well-phenotyped patients as our “study cohort”. Eight of the 15 were male. Age at last follow-up of R144W patients and R144Q patients ranged from 3.5 to 27 years (median 7.5 years), and from 3 years 9 months to 26 years (median 9 years), respectively. In 14 patients, the pathogenic variant occurred de novo. For patient 13, the variant was shown to be absent in mother, but paternal DNA was not available.
We also summarized published phenotypical data of 5 patients: 3 carrying the p.R144W,16,17 1 the p.R144Q16 and 1 the p.R144G17 variant (eTable 3). Due to the limited clinical data available, these previously published patients were not included in the analysis of specific clinical features. R144W, R144Q, and R144G are all considered pathogenic according to ACMG/AMP criteria (PS2, PS3, PM1, PM2, PM5, PP2, PP3).18
Neurodevelopmental and behavioural phenotype of patients in the study cohort
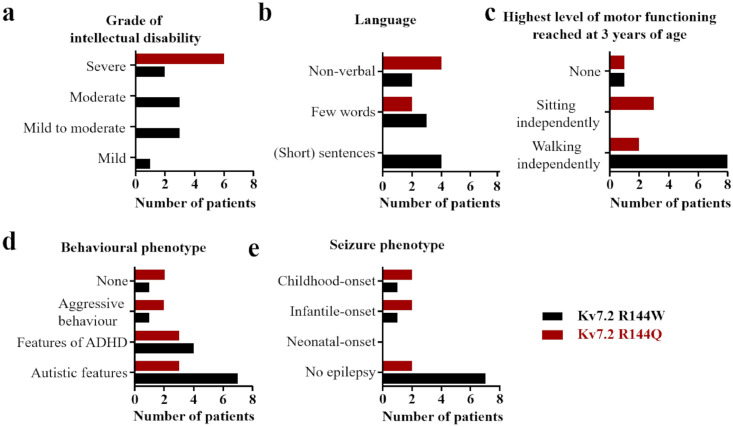
Detailed clinical information can be found in eTable 1, a summary is provided in Table 1. Figure 1 gives a graphical overview of the main clinical features shared by patients with the R144W and R144Q variant.
Table 1.
Overview of clinical features of patients with KCNQ2 R144 gain-of-function variants.
| Patient | Age at last FU | KCNQ2 variant |
Degree of ID (DSM-V) | Behavioural phenotype |
Current level of functioning | Onset-age of epilepsy | Episodes with reduced responsiveness of unclear origin | EEG sleep-activated epileptiform activity |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.5y | p.R144W | Mild | Features of ASD and ADHD | Speaks words, walks independently | / | Yes | |
| 2 | 6y | p.R144W | Mild to moderate | Features of ADHD | Speaks sentences, walks independently | / | No | |
| 3 | 4.5y | p.R144W | Mild to moderate | Features of ASD and ADHD | Speaks words, walks independently | / | No sleep-EEG | |
| 4 | 11y | p.R144W | Mild to moderate | Features of ASD | Speaks short sentences, walks independently | / | Yes | |
| 5 | 21y | p.R144W | Moderate | Features of ASD and aggressive behavior | Speaks short sentences, walks independently | / | Yes (3-6y) | No sleep-EEG |
| 6 | 8y | p.R144W | Moderate | Features of ASD | Speaks words, walks independently | / | Yes | |
| 7 | 7.5y | p.R144W | Moderate | Features of ASD | Speaks short sentences, walks independently | / | No | |
| 8 | 27y | p.R144W | Severe | Features of ASD and ADHD | Nonverbal, walks independently | 5y | Yes | |
| 9 | 3.5y | p.R144W | Severe | Normal | Nonverbal, cannot sit or walk independently | 4m | Yes | |
| 10 | 4y | p.R144Q | Severe | Aggressive behavior | Nonverbal, cannot sit or walk independently | / | Yes (12m) | No |
| 11 | 14y | p.R144Q | Severe | Features of ASD and ADHD | Nonverbal, walks independently | 6m | Yes | Yes |
| 12 | 14y | p.R144Q | Severe | Normal | Nonverbal, sits independently | 18m | Yes | No sleep-EEG |
| 13 | 26y | p.R144Q | Severe | Features of ASD and ADHD | Speaks words, walks independently | 8m | No | |
| 14 | 4y | p.R144Q | Severe | Features of ASD and ADHD, aggressive behavior | Speaks words, sits independently | / | Yes | |
| 15 | 3y9m | p.R144Q | Severe | Normal | Nonverbal, sits independently | 2y | Yes | Yes |
Abbreviations: ADHD = attention deficit hyperactivity disorder; ASD = autism spectrum disorder; EEG = electroencephalogram; FU = follow-up; ID = intellectual disability; M = month; Y= year.
Figure 1.
Bar graphs showing the main clinical features shared by 9 patients with Kv7.2 R144W variants (age at last follow up between 3.5 and 27 years) and 6 patients with R144Q variants (age at last follow up between 3 years 9 months and 26 years). Distribution of (a) grade of intellectual disability, (b) language outcome, (c) highest level of motor functioning reached at 3 years of age, (d) behavioral phenotype, and (e) seizure phenotype. Persons presenting only paroxysmal episodes (such as staring) of unclear origin are not included in the epilepsy group.
All patients had DD noted during the first year of life. Patients carrying the R144W variant showed a mild to severe degree of ID; in contrast, all patients with the R144Q variant had severe ID.
Except for patient 2, all patients had marked language impairment. At last follow-up, six patients (40%, aged 3 years 9 months to 27 years) were non-verbal (2/9 R144W, 22%; and 4/6 R144Q, 67%). Five patients (33%, aged 4.5 to 26 years) spoke a few words (3/9 R144W, 33%; and 2/6 R144Q, 33%), and 4 patients (27%, aged 4 to 21 years) spoke (short) sentences (4/9 R144W, 44%).
All patients had delayed motor milestones. 8/9 R144W (89%) patients walked independently at last follow-up, whereas only 2/6 R144Q (33%) patients did so. Age of independent walking in the R144W patients ranged from 16 months to 5.5 years, and all but one walked independently before the end of 2 years (median 1 year 9 months). Age of independent walking in the 2 R144Q patients was 3 years and 3.5 years, respectively (median 3 years 3 months). Age at last follow-up of the patients that could not walk ranged from 3.5 to 14 years.
Developmental regression was present in 3 patients and correlated with time of seizures onset in 2 (patient 11 and 15).
Ten patients (67%; 7/9 R144W and 3/6 R144Q) were reported by their child neurologist to have autistic features (described as difficulties in social interaction or in understanding emotions, appearing “in their own world”, and/or engaging in repetitive, stereotypic movements). Episodes of aggressive behaviour were reported for 3 individuals (20%, 1 R144W and 2 R144Q). Features of attention deficit hyperactivity disorder (ADHD; e.g., hyperactivity, poor attention, and impulsivity) were reported in 7 (47%; 4/9 R144W and 3/6 R144Q).
Neurological exam of patients in the study cohort
Five R144W patients (56%) and 5 R144Q patients (83%) showed signs of hypotonia; 1 R144W and 1 R144Q patient showed axial hypotonia combined with hypertonia in extremities. In 1 R144Q patient only peripheral hypertonia was described. Ataxia was present in 2/9 R144W (22%) and 2/6 R144Q (33%) patients. Head circumference was available for five patients (33%). All had a head circumference smaller than average, two of whom (1 R144W (−2.5 SD) and 1 R144Q (−3.6 SD)) had true microcephaly.
Hyperopia plus strabismus were reported in 3 patients (1 R144W and 2 R144Q). Two R144Q patients showed strabismus or hyperopia alone. Hyperlaxity or marfanoid habitus (moderate hyperlaxity, arachnodactyly, high arche in the palate, scoliosis, tall stature, and dolichostenomelia) was described in 4/6 R144Q cases, but not in R144W cases.
Epilepsy phenotype of patients in the study cohort
In total, epilepsy was diagnosed in 6 of 15 patients (Table 1, Figure 1, eTable 2). At last follow-up, epilepsy had been diagnosed in 2/9 (22%) and was suspected in 1/9 patients with the R144W variant. In patients with the R144Q variant, epilepsy was diagnosed in 4/6 (67%) cases, and suspected in 1 other. Two R144W cases and 1 R144Q patient experienced one or two simple febrile seizures only, and were not included in the group of patients with a diagnosis of epilepsy.
Among those diagnosed with epilepsy, seizure onset ages ranged from 4 months to 5 years (median 13 months). Seizure types varied, and included epileptic spasms (1/9 R144W patients, 2/6 R144Q patients), myoclonic jerks (3/6 R144Q patients) and atonic seizures (1 R144W). In 1 R144W patient, and 4 R144Q patients, episodes with impaired awareness were reported. None of the latter events have been captured on video-EEG, and the distinction between an epileptic or behavioural non-epileptic event was often unclear. Epileptic spasms were controlled with vigabatrin and/or corticosteroids. Where seizure types other than epileptic spasms occurred, valproic acid was the most frequently used agent, and led to seizure freedom in all individuals in whom epileptic seizures were diagnosed.
EEG was performed in 14/15 patients and showed (multi)focal epileptiform abnormalities in 13/14 (92%) patients, most prominent in posterior (7/13) and centro-temporal (4/13) leads in the majority of patients. Hypsarrhythmia was seen in all 3 patients with epileptic spasms. Sleep-EEG was performed in 12 patients, and in 8 patients (67%) activation or augmentation of epileptic activity during sleep was present, without fulfilling criteria of continuous spike and wave during slow wave sleep (CSWS).19 Brain MRI was normal in 10/14 (71%) patients. Of the scans with abnormalities, three (1 R144W and 2 R144Qpatients) showed delayed myelination.
Comparison with previously published cases
Although 5 individuals carrying KCNQ2 R144W,3 R144Q,1 and R144G1 variants have been identified in two earlier studies,16,17 phenotypic details were available for only 4 of the patients (eTable 3). All 5 patients had DD, and all reported (4/4) had language impairment. Focal seizures were reported in 1 patient. EEG was reported for 3 patients and showed multifocal abnormalities in all, with activation of epileptic activity during sleep described in two. Two patients were reported to have behavioural problems (1 with hyperactivity, and 1 with autistic traits).
Functional properties of channels containing Kv7.2 R144W/Q/G subunits
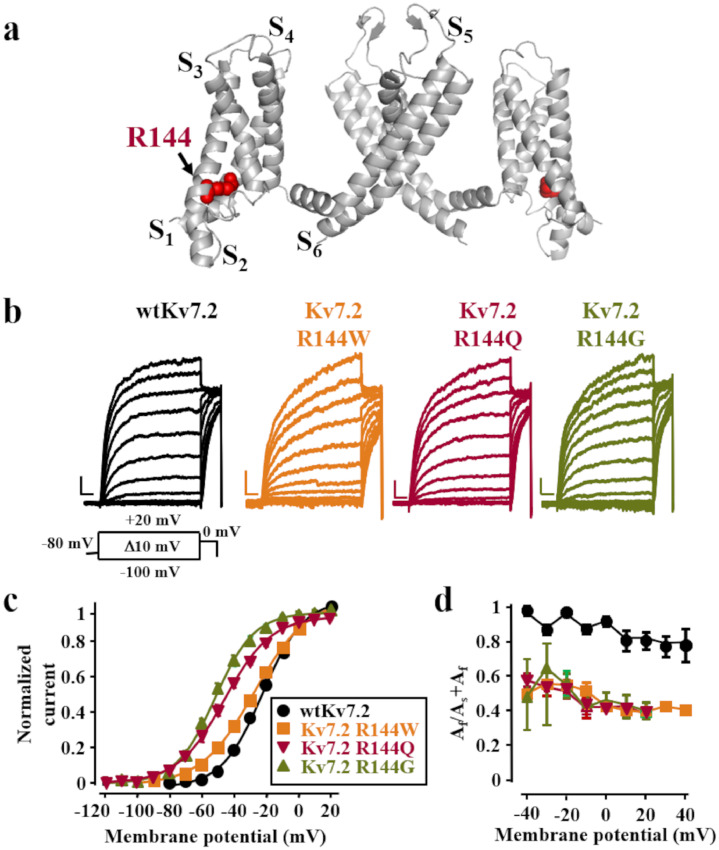
The R144 residue, highly conserved among Kv channels,9 is located at the bottom of the S2 segment within the VSD (Figure 2A). CHO cells expressing homomeric Kv7.2 channels generated voltage-dependent, K+-selective currents characterized by a slow time course of activation/deactivation and an activation threshold around −50 mV (Figure 2B,C). When compared to wild-type (wt) Kv7.2 channels, homomeric Kv7.2 R144W, R144Q or R144G channels displayed similar maximal current densities at +20 mV but a significant hyperpolarizing shift in voltage-dependent activation gating by about 4, 18, and 25 mV, respectively (Figure 2B,C; Table 2). wtKv7.2 channels showed activation kinetics which were adequately fitted by a biexponential function with a fast and a slow time constant (τf and τs, respectively).15 The relative amplitude of the fast component (Af) is dominant over that of the slow component (As); indeed, the Af/Af+As was close to unity at each potential investigated (Figure 2D). By contrast, while no change in activation τf and τs was observed (data not shown), the Af/Af+As ratio for the currents carried by K7.2 R144W/Q/G channels was significantly decreased at all potentials investigated (Figure 2D). In addition, when compared to wtKv7.2 channels, currents carried by Kv7.2 W/Q/G channels all showed slower deactivation kinetics, with Kv7.2 R144G channels having the strongest effect; indeed, the time constant of deactivation was 21.1±2.1 ms, 37.6±4.3 ms, 30.1±3.0 ms and 52.4±3.7 ms for wtKv7.2, Kv7.2 R144Q, Kv7.2 R144W and Kv7.2 R144G channels, respectively (p<0.05 for wtKv7.2 channels vs Kv7.2 R144W/Q/G channels). Despite such changes in gating, selectivity for K+ over Na+ ions as indicated by the current reversal potential in our recording conditions, as well as sensitivity to blockade by tetraethylammonium (TEA) was unaffected in mutant channels (Table 2), confirming that these pathogenic variants did not affect pore function.
Figure 2.
Topological location of the R144 residue and functional characterization of the Kv7.2 R144 W/Q/G variants.a. Homology model of a wtKv7.2 channel, built upon the Kv1.2/2.1 chimera crystal structure (PDB code: 2R9R), showing the localization of the R144 position (side chain colored in red using a space-filling model). For clarity only two of the four subunits are shown. b. Macroscopic currents from wtKv7.2 (Kv7.2), Kv7.2 R144Q, Kv7.2 R144W, and Kv7.2 R144G homomeric channels, in response to the indicated voltage protocol. Current scale, 200 pA; time scale, 0.2 s. c. Conductance/voltage curves for the indicated channels. Continuous lines are Boltzmann fits to the experimental data. Each data point is the Mean±S.E.M. of 13–21 cells recorded in at least 3 separate experimental sessions. d. Relative amplitudes of the fast and slow current activation components (expressed as Af/Af+As), for wtKv7.2, and Kv7.2 R144Q/W/G homomeric channels as a function of membrane voltage.
Table 2.
Biophysical and pharmacological properties of channels containing wild-type and R144 mutant Kv7.2 subunits.
| TEA blockade (% of inhibition) |
||||||||
|---|---|---|---|---|---|---|---|---|
| n | Vmid (mV) | K | Maximal current density (pA/pF) | Current reversal potential (mV) | 0.3 mM | 3 mM | 30 mM | |
| Kv7.2 | 16 | −25.0 ± 1.2 | 14.1 ± 0.7 | 36.5±6.6 | −78±1 | 53.5 ± 11.4 | 87.9 ± 5.1 | 93.0 ± 7.5 |
| Kv7.2+ PIP5K | 14 | −39.5±0.6* | 10.1±0.5 | 107.1±7.3* | – | – | – | – |
| Kv7.2 R144Q | 19 | −43.2 ± 1.6* | 17.2 ± 0.9* | 38.2±5.5 | −79±1 | 57.5 ± 0.4 | 85.5 ± 4.1 | 93.0 ± 4.6 |
| Kv7.2 R144Q + PIP5K | 13 | −81.3±0.7⁎⁎ | 10.5±0.6 | 100.4±17.2⁎⁎ | – | – | – | – |
| Kv7.2 R144W | 15 | −29.1 ± 1.5* | 17.9 ± 1.1* | 25.6 ±3.3 | −79±1 | 60.0 ± 2.8 | 91.7 ± 0.9 | 97.7 ± 0.6 |
| Kv7.2 R144G | 22 | −49.9 ± 1.3* | 12.9 ± 0.4 | 36.0±5.4 | −79±1 | 59.7 ± 3.6 | 93.2 ± 2.1 | 97.2 ± 1.3 |
| Kv7.2 + Kv7.2 R144Q | 12 | −35.7 ± 1.9* | 15.1 ± 0.4 | 39.4 ±4.5 | – | – | – | – |
| Kv7.2 + Kv7.2 R144W | 6 | −27.9±0.9* | 15.8±1.0 | 36.4±10.1 | – | – | – | – |
| Kv7.2 + Kv7.2 R144G | 6 | −36.4 ± 3.4* | 14.8 ± 0.9 | 25.7 ± 6.6 | – | – | – | – |
| Kv7.2 + Kv7.3 | 26 | −29.9 ± 1.1 | 12.7 ± 0.5 | 119.7 ± 12.2 | – | 9.2 ± 2.2 | 42.1 ± 7.4 | 73.1 ± 6.8 |
| Kv7.2 + Kv7.2R144Q + Kv7.3 | 12 | −33.5 ± 1.8 | 11.5 ± 0.4 | 94.8 ± 18.9 | – | 19.0 ±10.0 | 49 ±10.0 | 88 ±2.0 |
| Kv7.2 + Kv7.2R144W + Kv7.3 | 14 | −30.5 ± 1.9 | 11.3 ± 0.5 | 111.9 ± 16.7 | – | 7.8 ± 6.3 | 42.0 ± 8.2 | 78.7 ± 5.9 |
| Kv7.2 + Kv7.2R144G + Kv7.3 | 22 | −37.0 ± 1.4$ | 12.5 ± 0.7 | 87.4 ± 10.3 | – | 8.0 ± 5.3 | 44 .0± 5.0 | 78.5 ± 5.2 |
p<0.05 versus Kv7.2,
p<0.05 versus Kv7.2 R144Q
p<0.05 versus Kv7.2+Kv7.3.
Expression of Kv7.2 precedes that of Kv7.3 at early developmental stages1 whereas in adult superior sympathetic ganglion neurons, IKM is mainly formed by heteromeric assembly of Kv7.2 and Kv7.3 subunits.9,20 Therefore, the effect of all the three variant subunits in heteromeric configuration with wtKv7.2 and/or wtKv7.2/Kv7.3 subunits were also evaluated. Currents from wtKv7.2+Kv7.2R144W-, wtKv7.2+Kv7.2R144Q-, and wtKv7.2+Kv7.2R144G-transfected cells (1:1 cDNA ratio) displayed a significant leftward shift in activation voltage-dependence when compared to Kv7.2 channels, although the magnitude of this effect was smaller than that observed in homomeric configuration. When compared to wtKv7.2/Kv7.3, wtKv7.2+Kv7.2R144G+Kv7.3, but not wtKv7.2++Kv7.2R144Q+Kv7.3 and wtKv7.2+Kv7.2R144W+Kv7.3 heteromeric channels, displayed statistically-significant differences in activation gating (Table 2).
Structural basis for the GoF effect of the Kv7.2 R144W/Q/G variants
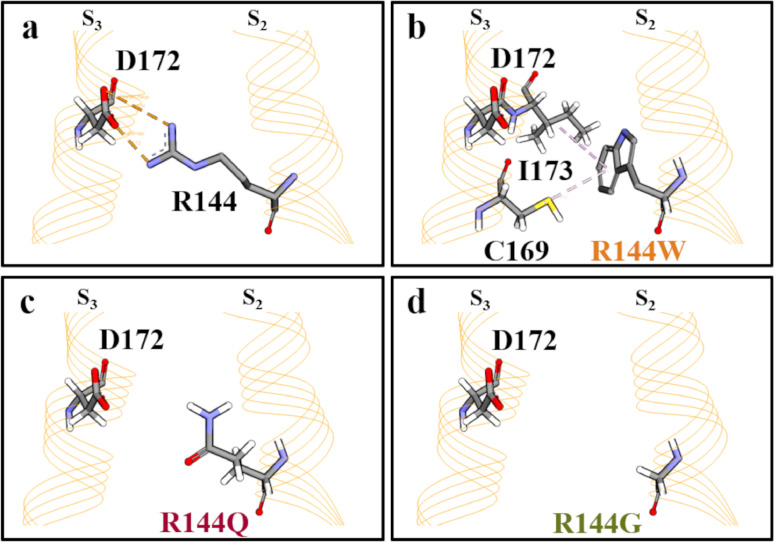
To gain insight into the structural consequences of R144W, Q and G substitutions, we investigated a previously-described homology model of a Kv7.2 subunit in both resting and activated states,9,21 and an atomic structure of Kv7.2 bound to retigabine/ezogabine.22 The latter structure is likely akin to the VSD-activated, conducting state, although bound PIP2 could not be visualized. In the homology model resting state, the R144 side chain in S2 forms an electrostatic interaction with the side chain of D172 in S3 (Figure 3A). In the activated states of both the homology model and the cryoEM structure, this direct interaction is absent, suggesting its prominent role in resting-state VSD stabilization. When the R144 side chain is replaced with W, Q or G, the ionized hydrogen bond between the 144 position and D172 is lost (Figure 3 B,C,D), potentially destabilizing the VSD resting state and favoring transition to the activated state. Notably, substitution of R144 with W, but not Q or G, introduces two novel hydrophobic interactions with the C169 and the I173 residues in the S3 segment (Figure 3B), partially compensating for the variant-induced loss of the electrostatic interaction with D172.
Figure 3.
An enlarged view of the resting state configuration of the region where R144 is located in wtKv7.2 (a), Kv7.2 R144W (b), Kv7.2 R144Q (c) and Kv7.2 R144G (d) channel.
Possible involvement of PIP2 in the gating changes triggered by the Kv7.2 R144Q variant
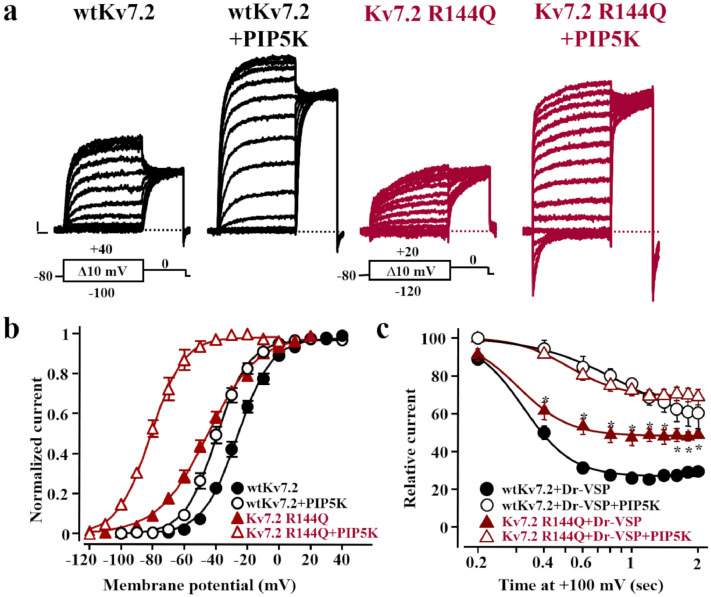
The anionic phospholipid PIP2 acts as a critical Kv7 gating modulator by promoting the electromechanical coupling between VSD movement and pore opening.3 To investigate whether the GoF effects observed in channels carrying R144 neutralizations were due to a facilitation of PIP2-dependent transitions to the open state, resting PIP2 levels were either increased by co-expression of the PIP2-synthesizing enzyme PIP5K, or decreased by co-expressing a voltage-sensitive phosphatase (DrVSP) that activates at very depolarized voltages.23 The R144Q variant was selected for these experiments as this substitution is associated with a lesser degree of α-helical distortion when compared to the R144G, and with stronger functional changes when compared to the R144W.
PIP5K co-expression enhanced wtKv7.2 peak currents, also causing a negative shift in their half-activation potential (ΔV½ = -14.5 mV) (Figure 2A, B; Table 1).23, 24, 25 When PIP5K was co-expressed with Kv7.2 R144Q channels, a similar 3-fold increase in maximal currents was observed, but the leftward shift in activation gating was larger than that observed in wtKv7.2 channels (ΔV½ =-36.2 mV) (Figure 4A,B; Table 2).
Figure 4.
Effect of changes in PIP2 availability on channels incorporating Kv7.2 R144Q mutant subunits. a. Representative current traces from cells expressing wtKv7.2 and Kv7.2 R144Q homomeric channels in the absence or in the presence of PIP5K in response to the voltage protocols shown in each respective panel. Current scale, 100 pA; time scale, 0.2 s. b. Conductance/voltage curves for the indicated channels with or without PIP5K co-expression. Continuous lines are Boltzmann fits to the experimental data. c. Current inhibition upon time-dependent activation of Dr-VSP. Asterisks (*) indicate values significantly different from each respective control (p<0.05).
In CHO cells expressing DrVSP, depolarization to +100 mV time-dependently reduced wtKv7.2 currents to 29.5±2.6% of their starting value after 2 s (n=23; Figure 3C); by contrast, Kv7.2 R144Q currents were reduced to only 49.0±2.0% (n=10; p<0.05 vs Kv7.2). Notably, this functional difference in DrVSP sensitivity between wtKv7.2 and Kv7.2 R144Q channels was abrogated when PIP2 levels were increased upon simultaneous co-expression of both DrVSP and PIP5K enzymes (Figure 3C).
Amitriptyline blockade of Kv7.2- and Kv7.2 R144Q-containing channels
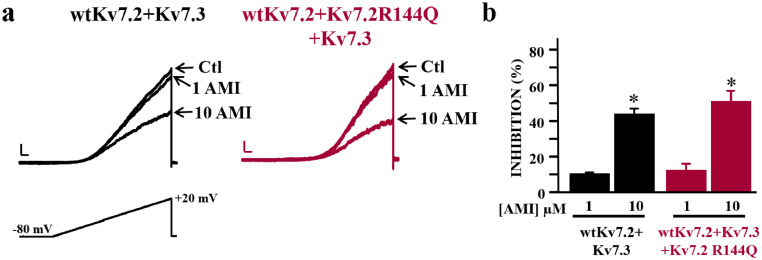
The tricyclic antidepressant drug amitriptyline has been described as a Kv7.2/3 blocker (26); given the GoF effects triggered by the R144 variants, we investigated whether Kv7.2 channels containing R144Q mutant subunits could also be blocked by amitriptyline. Amitriptyline (1 and 10 µM) dose-dependently and reversibly inhibited both heteromeric wtKv7.2+Kv7.3 and wtKv7.2+Kv7.2R144Q+Kv7.3 currents by the same extent (Figure 5A,B).
Figure 5.
Effect of amitriptyline on heteromeric channels incorporating Kv7.2 R144Q mutant subunits. a. Representative current traces recorded from cells transfected with the indicated cDNA combinations in response to the voltage ramp protocol reported, both before and after amitriptyline (AMI) exposure (1 and 10 µM). Current density scale, 20 pA/pF; time scale 0.2 s. b. Percent of current inhibition by amitriptyline. Asterisks (*) indicate values significantly different from each respective control recorded before drug exposure (p<0.05).
Discussion
Pathogenic KCNQ2 variants represent one of the most common causes of childhood-onset genetic epilepsy, with an estimated incidence within the first three years of life of 1 per 17,000.27 Most individual diagnosed with pathogenic KCNQ2 variants experience neonatal-onset epilepsy; the responsible variants include deletions, truncating variants and missense variants exhibiting LoF in vitro.
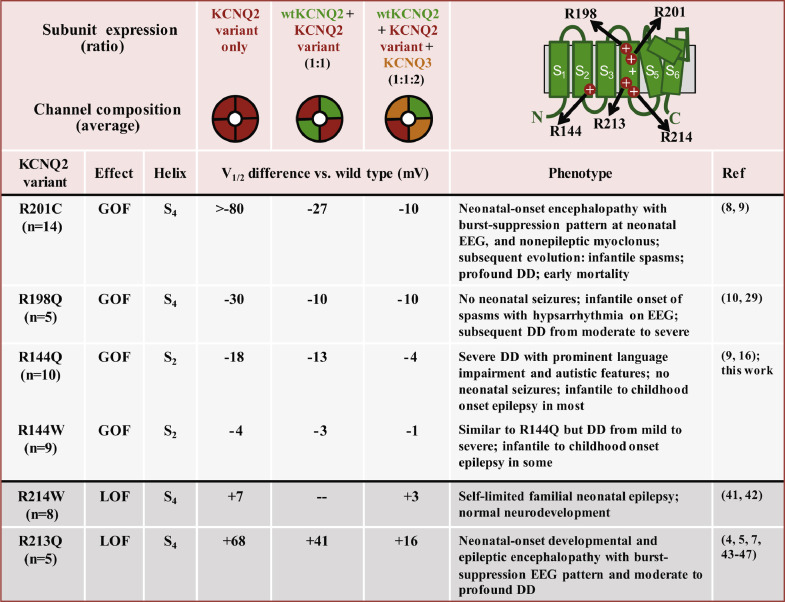
In addition to LoF, a few GoF missense variants have been described; to date these have been located within the VSD and associated with a different phenotypic spectrum, lacking neonatal seizures (Figure 6).8, 9, 10, 11,28 Children carrying KCNQ2 GoF variants at the R201 position displayed a very severe neonatal phenotype characterized by encephalopathy, burst suppression EEG, bouts of non-epileptic myoclonus, hypoventilation, and evidence of hypomyelination on MRI. Evolution to infantile epileptic spasms syndrome is frequent, profound DD persists, and early mortality is frequent.8 Children with the KCNQ2 R198Q variant showed epileptic spasms without preceding neonatal seizures or noted neonatal encephalopathy, suggesting that KCNQ2 is a rare cause of infantile epileptic spasms syndrome.10,29
Figure 6.
Correlation between the shift in voltage-dependent activation midpoint (V1/2) and phenotypes of pathogenic KCNQ2 variants in the VSD. Listed are 6 variants (at 5 codons) found recurrently, the shift observed under three informative and physiologically relevant subunit expression ratios (indicated schematically at top), and the associated phenotypes. The voltage shifts are taken from the publications listed. Remarkably, phenotypic severity appears correlated with the magnitude of the V1/2 shift, but even small shifts (R144W, R214W) can be pathogenic. These correlations are not interpreted as a necessarily complete pathogenic mechanism; and for LoF variants, additional disease pathomechanisms have been described (reviewed in 40).
In the present work, we describe the phenotypes of 20 patients (14 novel and 6 published) carrying KCNQ2 R144 variants (12 R144W, 7 R144Q, and 1 R144G). New clinical information was gathered on 9 R144W and 6 R144Q individuals.
Although little information was available on the one (previously published) R144G patient, the more numerous patients with R144W or R144Q variants showed several phenotypic commonalities. First, none had neonatal seizures. Instead, the first signs of medical concern were neurodevelopmental problems. All were diagnosed with DD. Language delay was particularly prominent. Neuropsychiatric problems were common: autistic features were reported by treating physicians in more than 65%, and aggressive behaviour and/or hyperactivity in more than half of the patients. Seizures ultimately occurred in 40%, always with an age of onset after the neonatal period. Seizure types included epileptic spasms (with hypsarrhythmia on EEG), and myoclonic, atonic and focal seizures. Both myoclonic seizures and epileptic spasms have previously been associated with KCNQ2 GoF variants.8,10,11,28,30, 31, 32, 33 In a third of the children, episodes of reduced responsiveness were reported, but none were captured by video-EEG. Thus, their possible epileptic nature is uncertain. Interestingly, unresponsive staring episodes of unclear origin were also seen in patients with KCNQ3 R230 variants and DD.12 More extensive video-EEG may reveal the exact aetiology of these episodes. Interictal EEGs typically showed (multi)focal epileptiform abnormalities, even in 7 of the 8 individuals who did not have clinical seizures but had EEGs (in 1 patient, EEG was not performed).
Sleep-activated epileptic activity was seen in 8 of the 12 individuals that had sleep-EEGs. Similar findings have been shown for KCNQ3 GoF variants.12 It is currently unclear whether treatment of abundant epileptiform activity during sleep with antiseizure medication can improve developmental outcome. Sands et al. could not consistently demonstrate clinical improvement in patients carrying KCNQ3 GoF variants when sleep-activated spikes were reduced by high-dose oral diazepam or corticosteroids. Nevertheless, the study population was too small to draw conclusions, and prospective studies with standardized sleep-EEG analysis and neuropsychological assessment are warranted to address this question.12
Overall, patients carrying KCNQ2 R144Q variants had a more severe neurodevelopmental outcome compared to R144W patients: first, all R144Q patients had severe ID, whereas the degree of ID of R144W patients ranged from mild to severe; second, 5/6 (83%) R144Q patients were non-verbal and/or non-ambulatory, compared to 2/9 (22%) R144W patients; and third, seizures were more frequently seen in R144Q cases compared to R144W cases (4/6 (67%) versus 2/9 (22%)). No difference in prevalence of behavioral problems was observed.
Patch clamp electrophysiology revealed GoF effects for all three Kv7.2 R144 variants, arising from negative shifts in their activation gating. This effect was largest (∼27 mV) in R144G, intermediate (∼18 mV) in R144Q and smallest (∼4 mV) in R144W channels. Together with the slowing of deactivation observed at negative potentials, these functional changes suggest a destabilization of Kv7.2 VSD resting state. Structural modelling revealed that the R144 residue is involved in an electrostatic interaction with the D172 residue in S3 when the VSD occupies the resting configuration; by contrast, no such interaction occurs when the VSD is in the activated state, consistent with the hypothesis that substitutions neutralizing R144 selectively destabilize the resting VSD configuration, thereby favoring the occupancy of the activated state. Moreover, replacement of R144 with W, but not Q or G residues introduces two novel hydrophobic interactions that could partially compensate for the loss of the electrostatic interaction with D172. This mechanism provides a plausible structural explanation for the gating changes observed under voltage-clamp, and for the smaller shift in V1/2 of Kv7.2 R144W channels compared to Kv7.2 R144Q or R144G channels.
Kv7 currents are characterized by their absolute functional requirement for PIP2.3 Multiple and distinct PIP2 binding sites have been described in each closed and open configuration3,34; in particular, the linker region between S2 and S3 has been proposed as a critical site for VSD coupling to pore opening in Kv7.1,35 Kv7.235,36 and Kv7.337 channels; in particular, PIP2 migration from a peripheral S2-S3 linker site to a more central S4-S5/pre-S6 binding region appears required for pore opening.38 The R144 residue is located in S2, immediately above this PIP2 binding site, suggesting its possible role in PIP2-dependent modulation of Kv7.2 channels gating. When compared to Kv7.2 channels, Kv7.2 R144Q channels display a greater voltage-shift at increased PIP2 levels and decreased sensitivity to PIP2 depletion, suggesting that an increased PIP2 apparent affinity might contribute to the GoF effects triggered by this variant. Given that PIP2 is a negatively charged molecule, and that the Kv7.2 variants herein investigated neutralize a positively-charged arginine residue, the present hypothesis seems consistent with an indirect (allosteric) effect of the variant on PIP2 affinity and, potentially, PIP2-dependent VSD-pore coupling.
While our paper was in preparation, Mary et al. described 2 R144W and 1 R144Q patients among a series of four KCNQ2 patients with DD without epilepsy and considered the potential role of unknown second genetic or environmental factors, or female gender as possible explanations for the absence of seizures.16 Here, through identification of a larger study cohort and inclusion of functional analysis, we provide evidence supportive of a single gene with phenotypic heterogeneity model. The R144W/Q alleles are recurrently associated with a neurodevelopmental disorder without neonatal seizures. This phenotype clearly differs from the phenotype seen in individuals with KCNQ2 variants with LoF effects, and it now strongly predicts a GoF effect of KCNQ2 (and KCNQ3) missense variants (Figure 6).8, 9, 10, 11, 12,28
Recognizing the clinical characteristics of pathogenic KCNQ2 GoF variants, with the absence of neonatal seizures as robust differentiator between KCNQ2 GoF and LoF variants, is of clinical importance since individuals with KCNQ2 GoF variants are likely to need different therapeutic approaches compared to individuals with KCNQ2 LoF variants. Recently, an international randomized clinical trial with a pediatric formulation of the neuronal KCNQ channel opener retigabine (ClinicalTrials.gov Identifier: NCT04639310; https://clinicaltrials.gov/ct2/show/NCT04639310) has been initiated. In this trial, eligibility is restricted to patients with pathogenic KCNQ2 LoF variants. Individuals with known GoF KCNQ2 variant or individuals showing clinical characteristics consistent with previously reported pathogenic GoF KCNQ2 variants, are excluded. This highlights the continued need for description of rare patients such as performed by Mary et al. and in the current study.
The present data also reveal novel genotype-phenotype correlations in the GoF spectrum of KCNQ2-DEE, as the severity of the phenotype seems to correlate with the electrophysiological effect of the variant in vitro, with larger functional changes of R144Q-carrying channels paralleling the more severe neurodevelopmental outcome (Figure 6). Patients carrying the KCNQ2 R144 GoF variants are also less severely affected than those carrying the strong KCNQ2 GoF variants at R201 in S4.9 The KCNQ2 R198Q variant appears more similar to R144, both in the patch clamp results and phenotypes, namely the emergence of DD after a neonatal period without developmental concerns or seizures.10,29 Interestingly, the phenotype of patients with KCNQ2 R144W is similar to that of patients with KCNQ3 R230C, even though KCNQ3 R230C has much larger effects on voltage-dependence in vitro.12 This discrepancy may potentially be due to the higher expression levels of KCNQ2 compared to KCNQ3 during early neurodevelopment.39 Genotype-phenotype correlations have been also described in the LoF spectrum of KCNQ2-related disorders (reviewed in).40 As illustrated in Figure 6, VSD missense variants responsible for self-limited familial neonatal epilepsy (SLFNE) caused milder effects on activation gating (e.g. R214W),41,42 whereas more severe functional defects are prompted by DEE-causing variants in the same region (e. g. R213Q).4,5, 7,43, 44, 45, 46, 47
Finally, although patients with clinically proven seizures in our cohort responded well to treatment with valproic acid, vigabatrin, or corticosteroids, they all show significant neurodevelopmental problems. This highlights the need for better treatment, ideally reversing the mutational effect of pathogenic GoF variants and improving neurodevelopment. Amitriptyline is a tricyclic antidepressant with analgesic properties due to the serotonin and norepinephrine reuptake inhibition, which also targets some voltage-gated ion channels and receptors.26 It has been widely used for depression, migraine, and neuropathic pain, and has been trialed in children (≥6 years of age) for nocturnal enuresis.48 Based on pharmacokinetic analysis in experimental animals, it has been suggested that clinically-effective steady-state plasma concentrations of amitriptyline in humans (150–250 ng/ml) correspond to brain drug concentrations of 5–7 μM.49,50 These low μM concentrations of amitriptyline similarly block Kv7.2/7.3 and Kv7.2/Kv7.2R144Q/Kv7.3 channels in vitro,26 highlighting the potential ability of this drug to counteract in vivo the GoF effect caused by the R144Q variant.
Limitations
This study has limitations that are intrinsic to our approach: data were collected retrospectively, and as a result not all desired clinical information was available for every patient (e.g., not all had sleep-EEG recordings capturing sleep), and some reports were impossible to further validate (e.g., the etiology of reduced responsiveness episodes). Sampling bias may be arise due to recruitment via a voluntary family registry and referral to specialized centres. In addition, the small size of the two subgroups within our cohort (R144W and R144Q), limits the strength of conclusions drawn about phenotypic differences between these subgroups. Despite these limitations, this study strengthens available evidence that KCNQ2 GoF variants cause DD in the absence of neonatal seizures and that disease severity is correlated with the extent of the V1/2 shift in vitro. Thus, the KCNQ2 GoF spectrum is distinct from that associated with KCNQ2 LoF variants. Owing to rarity, the known set of KCNQ2 missense variants causing GoF and DD likely remains incomplete. To accelerate variant discovery, KCNQ2 should be included in diagnostic ID/DD/ASD multigene panels. Finally, agents withKv7.2/7.3 channel blocking effects, such as amitriptyline, represent candidate as targeted treatments for patients with KCNQ2 GoF variants and deserve further investigation.
Contributors
Conceptualization: E.C., S.W., M.T.; Data curation (access and verification of the data): S.W., M.T., F.M., C.M.; Formal analysis: E.C., S.W., M.T., F.M., C.M.; Writing–original draft: E.C., S.W., M.T., F.M., C.M.; Writing–review & editing: All authors. All authors read and approved the final version of the manuscript.
Data sharing statement
De-identified participant data and study protocol can be requested by contacting one of the corresponding authors by email (sarah.weckhuysen@uantwerpen.vib.be).
Declaration of interests
SW received consultancy and speaker fees from UCB, Biocodex, Xenon, Zogenix, Lundbeck, Knopp Biosciences, Encoded
MT received consultancy fees from Xenon.
ECC received consultancy fees from Xenon, Knopp, these activities have been reviewed and approved by Baylor College of Medicine according to its policy on disclosure of outside interests.
RSM received consultancy and speaker fees from EISAI and UCB. The remaining authors have no conflicts of interest.
Acknowledgments
The authors thank the families and the children for their participation in this study.
SW received funding from FWO (1861419N and G041821N); Queen Elisabeth Medical Foundation for Neurosciences (GSKE); KCNQ2-Cure; Jack Pribaz Foundation; KCNQ2e.v.; European Joint Programme on Rare Disease JTC 2020 (TreatKCNQ).
MT received funding from the Italian Ministry for University and Research (MIUR) (PRIN 2017ALCR7C); the Italian Ministry of Health (Project RF-2019-12370491); the European Commission H2020 (UNICOM – 875299); European Joint Programme on Rare Disease JTC 2020 (TreatKCNQ).
ECC received funding from NINDS NS49119 and NS108874; The Jack Pribaz Foundation; The Chalk Family Foundation and The Miles Family Fund.
FM received funding from the Italian Ministry for University and Research (MIUR) (PRIN 2017YH3SXK).
CM received funding from the University of Antwerp (BOF DOCPRO4– project ID 42329).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104130.
Contributor Information
Maurizio Taglialatela, Email: mtaglial@unina.it.
Sarah Weckhuysen, Email: Sarah.Weckhuysen@uantwerpen.vib.be.
Appendix. Supplementary materials
References
- 1.Dirkx N, Miceli F, Taglialatela M, Weckhuysen S. The role of Kv7.2 in neurodevelopment: insights and gaps in our understanding. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.570588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 2011;26(5):365–376. doi: 10.1152/physiol.00009.2011. [DOI] [PubMed] [Google Scholar]
- 3.Zaydman MA, Cui J. PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front Physiol. 2014;5:195. doi: 10.3389/fphys.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71(1):15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- 5.Miceli F, Soldovieri MV, Ambrosino P, et al. Genotype-phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of K(v)7.2 potassium channel subunits. Proc Natl Acad Sci USA. 2013;110(11):4386–4391. doi: 10.1073/pnas.1216867110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56(5):685–691. doi: 10.1111/epi.12984. [DOI] [PubMed] [Google Scholar]
- 7.Millichap JJ, Park KL, Tsuchida T, et al. KCNQ2 encephalopathy: Features, mutational hot spots, and ezogabine treatment of 11 patients. Neurol Genet. 2016;2(5):e96. doi: 10.1212/NXG.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulkey SB, Ben-Zeev B, Nicolai J, et al. Neonatal nonepileptic myoclonus is a prominent clinical feature of KCNQ2 gain-of-function variants R201C and R201H. Epilepsia. 2017;58(3):436–445. doi: 10.1111/epi.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miceli F, Soldovieri MV, Ambrosino P, et al. Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits. J Neurosci. 2015;35(9):3782–3793. doi: 10.1523/JNEUROSCI.4423-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millichap JJ, Miceli F, De Maria M, et al. Infantile spasms and encephalopathy without preceding neonatal seizures caused by KCNQ2 R198Q, a gain-of-function variant. Epilepsia. 2017;58(1):e10–e15. doi: 10.1111/epi.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devaux J, Abidi A, Roubertie A, et al. A Kv7.2 mutation associated with early onset epileptic encephalopathy with suppression-burst enhances Kv7/M channel activity. Epilepsia. 2016;57(5):e87–e93. doi: 10.1111/epi.13366. [DOI] [PubMed] [Google Scholar]
- 12.Sands TT, Miceli F, Lesca G, et al. Autism and developmental disability caused by KCNQ3 gain-of-function variants. Ann Neurol. 2019;86(2):181–192. doi: 10.1002/ana.25522. [DOI] [PubMed] [Google Scholar]
- 13.Allen AS, Berkovic SF, Cossette P, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501(7466):217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association . 5th ed. APA Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [Google Scholar]
- 15.Soldovieri MV, Cilio MR, Miceli F, et al. Atypical gating of M-type potassium channels conferred by mutations in uncharged residues in the S4 region of KCNQ2 causing benign familial neonatal convulsions. J Neurosci. 2007;27(18):4919–4928. doi: 10.1523/JNEUROSCI.0580-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mary L, Nourisson E, Feger C, et al. Pathogenic variants in KCNQ2 cause intellectual deficiency without epilepsy: Broadening the phenotypic spectrum of a potassium channelopathy. Am J Med Genet A. 2021;185(6):1803–1815. doi: 10.1002/ajmg.a.62181. [DOI] [PubMed] [Google Scholar]
- 17.Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542(7642):433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singhal NS, Sullivan JE. Continuous spike-wave during slow wave sleep and related conditions. ISRN Neurol. 2014;2014 doi: 10.1155/2014/619079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HS, Pan Z, Shi W, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282(5395):1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 21.Jensen M, Jogini V, Borhani DW, Leffler AE, Dror RO, Shaw DE. Mechanism of voltage gating in potassium channels. Science. 2012;336(6078):229–233. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Zhang Q, Guo P, et al. Molecular basis for ligand activation of the human KCNQ2 channel. Cell Res. 2021;31(1):52–61. doi: 10.1038/s41422-020-00410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hossain MI, Iwasaki H, Okochi Y, et al. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem. 2008;283(26):18248–18259. doi: 10.1074/jbc.M706184200. [DOI] [PubMed] [Google Scholar]
- 24.Soldovieri MV, Ambrosino P, Mosca I, et al. Early-onset epileptic encephalopathy caused by a reduced sensitivity of Kv7.2 potassium channels to phosphatidylinositol 4,5-bisphosphate. Sci Rep. 2016;6:38167. doi: 10.1038/srep38167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran B, Ji ZG, Xu M, Tsuchida TN, Cooper EC. Two KCNQ2 encephalopathy variants in the calmodulin-binding helix A exhibit dominant-negative effects and altered PIP(2) interaction. Front Physiol. 2020;11:1144. doi: 10.3389/fphys.2020.571813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Punke MA, Friederich P. Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels. Anesth Analg. 2007;104(5):1256–1264. doi: 10.1213/01.ane.0000260310.63117.a2. tables of contents. [DOI] [PubMed] [Google Scholar]
- 27.Symonds JD, Zuberi SM, Stewart K, et al. Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort. Brain. 2019;142(8):2303–2318. doi: 10.1093/brain/awz195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samanta D, Ramakrishnaiah R, Willis E, Frye RE. Myoclonic epilepsy evolved into west syndrome: a patient with a novel de novo KCNQ2 mutation. Acta Neurol Belg. 2015;115(3):475–478. doi: 10.1007/s13760-014-0344-5. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Shen Q, Zheng G, et al. Gene and phenotype expansion of unexplained early infantile epileptic encephalopathy. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.633637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weckhuysen S, Ivanovic V, Hendrickx R, et al. Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology. 2013;81(19):1697–1703. doi: 10.1212/01.wnl.0000435296.72400.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hortigüela M, Fernández-Marmiesse A, Cantarín V, et al. Clinical and genetic features of 13 Spanish patients with KCNQ2 mutations. J Hum Genet. 2017;62(2):185–189. doi: 10.1038/jhg.2016.104. [DOI] [PubMed] [Google Scholar]
- 32.Olson HE, Kelly M, LaCoursiere CM, et al. Genetics and genotype-phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann Neurol. 2017;81(3):419–429. doi: 10.1002/ana.24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima K, Shirai K, Kobayashi M, et al. A patient with early myoclonic encephalopathy (EME) with a de novo KCNQ2 mutation. Brain Dev. 2018;40(1):69–73. doi: 10.1016/j.braindev.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Salzer I, Erdem FA, Chen WQ, et al. Phosphorylation regulates the sensitivity of voltage-gated Kv7.2 channels towards phosphatidylinositol-4,5-bisphosphate. J Physiol. 2017;595(3):759–776. doi: 10.1113/JP273274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaydman MA, Silva JR, Delaloye K, et al. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc Natl Acad Sci USA. 2013;110(32):13180–13185. doi: 10.1073/pnas.1305167110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Zhang Q, Qiu Y, et al. Migration of PIP2 lipids on voltage-gated potassium channel surface influences channel deactivation. Sci Rep. 2015;5:15079. doi: 10.1038/srep15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choveau FS, De la Rosa V, Bierbower SM, Hernandez CC, Shapiro MS. Phosphatidylinositol 4,5-bisphosphate (PIP(2)) regulates KCNQ3 K(+) channels by interacting with four cytoplasmic channel domains. J Biol Chem. 2018;293(50):19411–19428. doi: 10.1074/jbc.RA118.005401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Zhou P, Chen Z, et al. Dynamic PIP2 interactions with voltage sensor elements contribute to KCNQ2 channel gating. Proc Natl Acad Sci USA. 2013;110(50):20093–20098. doi: 10.1073/pnas.1312483110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanaumi T, Takashima S, Iwasaki H, Itoh M, Mitsudome A, Hirose S. Developmental changes in KCNQ2 and KCNQ3 expression in human brain: possible contribution to the age-dependent etiology of benign familial neonatal convulsions. Brain Dev. 2008;30(5):362–369. doi: 10.1016/j.braindev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Nappi P, Miceli F, Soldovieri MV, Ambrosino P, Barrese V, Taglialatela M. Epileptic channelopathies caused by neuronal Kv7 (KCNQ) channel dysfunction. Pflugers Arch. 2020;472(7):881–898. doi: 10.1007/s00424-020-02404-2. [DOI] [PubMed] [Google Scholar]
- 41.Miraglia del Giudice E, Coppola G, Scuccimarra G, Cirillo G, Bellini G, Pascotto A. Benign familial neonatal convulsions (BFNC) resulting from mutation of the KCNQ2 voltage sensor. Eur J Hum Genet. 2000;8(12):994–997. doi: 10.1038/sj.ejhg.5200570. [DOI] [PubMed] [Google Scholar]
- 42.Castaldo P, del Giudice EM, Coppola G, Pascotto A, Annunziato L, Taglialatela M. Benign familial neonatal convulsions caused by altered gating of KCNQ2/KCNQ3 potassium channels. J Neurosci. 2002;22(2):Rc199. doi: 10.1523/JNEUROSCI.22-02-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orhan G, Bock M, Schepers D, et al. Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Ann Neurol. 2014;75(3):382–394. doi: 10.1002/ana.24080. [DOI] [PubMed] [Google Scholar]
- 44.Trump N, McTague A, Brittain H, et al. Improving diagnosis and broadening the phenotypes in early-onset seizure and severe developmental delay disorders through gene panel analysis. J Med Genet. 2016;53(5):310–317. doi: 10.1136/jmedgenet-2015-103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Na JH, Shin S, Yang D, et al. Targeted gene panel sequencing in early infantile onset developmental and epileptic encephalopathy. Brain Dev. 2020;42(6):438–448. doi: 10.1016/j.braindev.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Kim HJ, Yang D, Kim SH, et al. Clinical characteristics of KCNQ2 encephalopathy. Brain Dev. 2021;43(2):244–250. doi: 10.1016/j.braindev.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Dou YL, Chen X, et al. Early initial video-electro-encephalography combined with variant location predict prognosis of KCNQ2-related disorder. BMC Pediatr. 2021;21(1):477. doi: 10.1186/s12887-021-02946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1) doi: 10.1002/14651858.CD002117.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glotzbach RK, Preskorn SH. Brain concentrations of tricyclic antidepressants: single-dose kinetics and relationship to plasma concentrations in chronically dosed rats. Psychopharmacology (Berl) 1982;78(1):25–27. doi: 10.1007/BF00470582. [DOI] [PubMed] [Google Scholar]
- 50.Atkin TA, Maher CM, Gerlach AC, et al. A comprehensive approach to identifying repurposed drugs to treat SCN8A epilepsy. Epilepsia. 2018;59(4):802–813. doi: 10.1111/epi.14037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of the study are available from the corresponding author, with the exception of primary patient sequencing data, as they are derived from patient samples with unique variants that are impossible to guarantee anonymity for.