Abstract
Pre-eclampsia is a severe pregnant complication, mainly characterized by insufficient trophoblast invasion, impaired uterine spiral artery remodeling, placental hypoxia and ischemia, and endothelial dysfunction. However, the potential mechanisms of pre-eclampsia remain unclear. SIRT1 is a NAD+-dependent deacetylase, involving in multiple biological processes, including energy metabolism, oxidative stress, inflammatory response, and cellular autophagy. Several studies showed that SIRT1 might play a vital role in the pathogenesis of pre-eclampsia. In this review, we aim to integrate the latest research on SIRT1 and pre-eclampsia to explore the comprehensive mechanisms of SIRT1 in pre-eclampsia. More specifically, SIRT1 might affect placental development and trophoblast invasion through autophagy and senescence in pre-eclampsia, and SIRT1 protects vascular endothelial cells from oxidative stress, inflammatory response, autophagy, and senescence. Furthermore, SIRT1 deficiency mice showed typical pre-eclampsia-like performances, which can be reversed via direct SIRT1 supplement or SIRT1 agonist treatment. Additionally, resveratrol, a SIRT1 agonist, attenuates vascular endothelial injury and placental dysfunction, and exerts protective effect on decreasing blood pressure. In this review, we provide new insights into the development of pre-eclampsia, which can establish a theoretical basis for prevention and treatment for pre-eclampsia. Besides, we also propose questions that still need to be further addressed in order to elucidate the comprehensive molecular mechanisms of pre-eclampsia in the future.
Keywords: pre-eclampsia (PE), SIRT1, trophoblasts, endothelial cells (ECs), resveratrol
Introduction
Pre-eclampsia (PE) is a hypertensive disorder of pregnancy (HDP), characterized by new-onset hypertension and proteinuria at 20-week of pregnancy. It affects 2%-8% pregnancy women worldwide, causing severe fetal and maternal morbidity and mortality 1-3. Although the comprehensive mechanisms of pre-eclampsia remain unknown, the current mainstream view is the two-stage model of disease 4-6. Stage1 mainly manifests as impaired placentation due to inadequate trophoblastic invasion of maternal spiral arteries, which leads to reduced placental perfusion and release of numerous secreted factors causing vascular endothelial dysfunction and multiorgan failure, which is called stage2. Recently, the effects of SIRT1 on the biological functions of trophoblasts and endothelial cells have gradually emerged, and the expression of SIRT1 is lower in serum samples and placental tissues of pre-eclampsia patients. Therefore, we inferred that SIRT1 might play a significant role in the pathogenesis of pre-eclampsia.
SIRT1, a NAD+-dependent deacetylase, mediates various biological functions including oxidative stress, aging, inflammatory response and autophagy via deacetylating multiple substrates, such as NF-κB (nuclear factor-kappaB), FOXOs (forkhead box O), and PPARγ (peroxisome proliferator-activated receptor γ) 7-11. For example, it is reported that SIRT1 promotes the deacetylation of Nrf2 (nuclear factor-erythroid 2 (NF-E2)-related factor2), and increases its transcriptional activity, thereby promoting the expression of downstream two-phase detoxification NQO1 (NADPH quinone oxidoreductase 1) and HO-1 (heme oxygenase-1), and exerting anti-oxidative stress effect in vascular endothelial cells 12-15. In addition, SIRT1 deacetylates and activates eNOs (neuronal nitricoxide synthase) to produce more nitric oxide (NO), which can dilate blood vessels 16. In recent years, the research of SIRT1 in pre-eclampsia has progressed. SIRT1 deficiency attenuates the invasion, migration and proliferation of trophoblasts, thereby participating in the development of pre-eclampsia. Our recent study showed that SIRT1 knockdown mice exhibited significantly pre-eclampsia-like symptoms, suggesting that SIRT1 might be a novel protective biomarker in pre-eclampsia 17.
In this review, we mainly explored the role of SIRT1 in pre-eclampsia from the following four aspects. 1) SIRT1 affects the biological functions of trophoblasts; 2) SIRT1 protects vascular endothelial cells; 3) SIRT1 attenuates the performances of pre-eclampsia in animal models; 4) the effect of SIRT1 agonist resveratrol in pre-eclampsia.
2. SIRT1 affects the biological functions of trophoblasts
2.1. SIRT1 affects placental development and differentiation
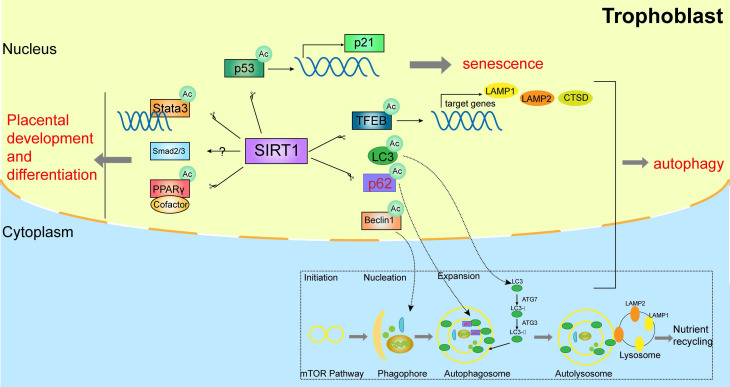
Trophoblastic dysfunction is a typical feature of pre-eclampsia, resulting in uterine spiral artery remodeling disorder. It is reported that SIRT1 is critical in trophoblast differentiation and placental development 18-21. SIRT1 is lower in placentas and serum samples of pre-eclampsia patients, and is mainly expressed in the nuclei of trophoblasts including syncytiotrophoblasts and cytotrophoblasts in placental tissues 22, 23. SIRT1 possibly involves in trophoblastic maintenance and differentiation by mediating SMAD2/3, STAT3 or PPARγ pathways 24-27. Arul Nambi Rajan et al. 22 found that placentas of SIRT1-null mice were small and showed abnormalities in both labyrinthine layer and junctional zone, and SIRT1-null trophoblast stem cell (TSC) showed blunted differentiation. Specifically, the RNA levels of PPARγ were decreased, and the protein levels of SMAD2, SMAD3 and STAT3 were downregulated in differentiated SIRT1-null TSC. Studies reported that STAT3 was associated with the differentiation of trophoblast giant cells and syncytiotrophoblasts and might be deacetylated and inhibited by SIRT1 25, 28, 29. Additionally, the potential role of PPARγ in trophoblast differentiation and placental development is also highlighted 24, 30, and the activity of PPARγ can be deacetylated and regulated by SIRT1 through recruiting cofactors, such as NCoR1 (nuclear receptor corepressor 1), SMRT (silencing mediator of retinoic acid and thyroid hormone) and Prdm16 (PR domain-containing protein 16) 31, 32. The above-mentioned pathways are shown in Figure 1. Furthermore, our previous research also demonstrated that the placental labyrinthine layer was significantly narrow in SIRT1+/- mice and the invasive ability was relatively lower in SIRT1 knockdown trophoblasts 17. This evidence indicated that SIRT1 plays a significant role in placental development and differentiation.
Figure 1.
SIRT1 affects the biological functions of trophoblasts.
2.2. SIRT1 affects trophoblast autophagy
Autophagy is a cellular homeostasis pathway targeted aggregated proteins and damaged organelles for lysosomal degradation 33-37. Importantly, autophagy protects the placentas against pathogens and stress. There are impaired trophoblast autophagy and increased protein accumulation in the placentas of patients with pre-eclampsia 38. Studies showed that SIRT1 prevents H2O2-induced oxidative stress and apoptosis by mediating autophagy in trophoblasts 39. Mechanistically, some evidence on the autophagic machinery demonstrated that SIRT1 participates in autophagy via deacetylating TFEB (transcription factor EB), LC3 (microtubule associated protein 1 light chain 3), Beclin-1, p62, ATG5 (autophagy-related gene 5), ATG7 (autophagy-related gene 7), and ATG8 (autophagy-related gene 8) in a NAD+-dependent manner 40, 41.
Autophagy-lysosomal biogenesis is tightly regulated by TFEB, which can be deacetylated by SIRT1 and activate the expression of several downstream autophagy-associated genes, such as LAMP1 (lysosomal associated membrane protein 1), LAMP2 (lysosomal associated membrane protein 2) and CTSD (cathepsin D) 42, 43. Furthermore, the initial stage markers of autophagy activation in pre-eclampsia, such as LC3-II, Beclin-1, and SQSTM1 (sequestosome 1) 44-46, were also significantly altered and could be regulated by SIRT147-49. The above-mentioned pathways are shown in Figure 1. This evidence demonstrated that SIRT1 might exert a potential role in trophoblastic autophagy by deacetylating multiple substrates.
2.3. SIRT1 affects placental senescence
Premature placental senescence is a critical characteristic of pre-eclampsia, with senescence-associated secretory phenotype and increased expression of p53 and p21, which are markers of cellular senescence. SIRT1 is also a specific marker of senescence, and SIRT1 deficiency leads to premature senescence of placentas during placentation 50-53. Interestingly, Xiong et al. found that SIRT1 deficiency promotes the acetylation of P53, elevates the expression level of P21, and impairs trophoblast invasion and migration in advanced maternal age (AMA) pregnancy women, indicating that SIRT1 might involve in the pathogenesis of pre-eclampsia by inducing placental senescence 50.
2.4. The functions of other sirtuins proteins in trophoblasts
There are seven orthologs (SIRT1-7) of sirtuins family in mammals 54. All sirtuins deacetylate multiple target proteins using NAD+ as co-substrate and participate in cellular oxidative stress, energy metabolism, and inflammatory response and so on 55. Several reports revealed that sirtuins play a significant role in the development and differentiation of trophoblasts, as shown in Table 1. SIRT2, one member of the sirtuins family, localizes in placental syncytiotrophoblasts and is downregulated in the placentas of patients with pre-eclampsia. It could inhibit proliferation, migration and invasion, and induce necrosis of placental trophoblast cells 56, 57. Additionally, it is reported that SIRT3 affects the migration, invasion, tube formation and necroptosis of trophoblasts and is implicated in the pathogenesis of pre-eclampsia 58. Furthermore, studies showed that SIRT4 might trigger senescence of trophoblasts 59-61. This evidence further confirms our hypothesis that SIRT1 might participate in the pathogenesis of pre-eclampsia by regulating trophoblastic invasion, migration and proliferation.
Table 1.
The effect of sirtuins family in trophoblasts
| Source | Sirtuins | Expression | Location | Effect in trophoblasts | Mechanisms |
|---|---|---|---|---|---|
| Arul Nambi Rajan et al. Lappas et al. Barak et al. Borg et al. Erlebacher et al. Tang et al. |
SIRT1 22-27 |
Downregulated in placentas and serum samples from PE, significantly lower after adjusting for gestational age (WB, qPCR, IHC) | Placental syncytiotrophoblasts and cytotrophoblasts (IHC) | SIRT1promotes development, differentiation, migration, invasion, and angiogenesis, while inhibits apoptosis, and senescence of trophoblasts. Furthermore, SIRT1 exerts anti-inflammatory effects and anti-oxidative stress in trophoblasts | SMAD2/3, STAT3 or PPARγ pathways; triggering p53 deacetylation; medicating autophagy |
| Yu et al. Hannan et al. |
SIRT2 56, 57 |
Downregulated in placentas from PE, but no significance after adjusting for gestational age (microarray, WB, qPCR, IHC) | Placental syncytiotrophoblasts, scattered interstitial cells, the endothelial cells lining, and the vessel walls of the placental villi (IHC) | SIRT2 deficiency inhibits proliferation, migration and invasion, while promotes apoptosis and necroptosis of trophoblasts | Triggering p65 deacetylation |
| Yu et al. | SIRT3 58 |
Downregulated in placentas from PE, but no significance after adjusting for gestational age (WB, qPCR, IHC) | Placental syncytiotrophoblasts and cytotrophoblasts (IHC) | SIRT3 deficiency inhibits proliferation, migration, invasion and tube formation, while promotes cell death and necroptosis of trophoblasts | —— |
| Castex et al. Sandvoß et al. Bartho et al. |
SIRT4 59-61 |
Upregulated in HUVECs from HELLP, but no difference in placentas of FGR | —— | SIRT4 triggers senescence of trophoblasts | Induced by inactivation of LSD1 |
| Lim et al. | SIRT6 62 |
Downregulated in fetal membranes from preterm labor | Placental chorionic trophoblasts and decidua tissues, fetal membranes, and amnion epithelium | —— | —— |
3. SIRT1 protects vascular endothelial cells
The dysfunction of endothelial cells is one of the typical features in pre-eclampsia, causing by multiple factors, including oxidative stress, inflammatory response and autophagy and so on. SIRT1, a member of sirtuins family, exerts anti-oxidant, anti-inflammatory, and anti-aging effect. Some research showed that SIRT1 expression is lower in serum samples of pre-eclampsia women, and also decreased in human umbilical vein endothelial cells (HUVECs) incubated with pre-eclamptic serum 63. It is reported that SIRT1 can protect HUVECs from death in pre-eclampsia patients, therefore blocking the development of pre-eclampsia 64. Mechanistically, SIRT1 might protect endothelial cells from oxidative stress, inflammatory response, senescence and autophagy by various pathways, as shown in Figure 2.
Figure 2.
SIRT1 protects vascular endothelial cells.
3.1. SIRT1 protects vascular endothelial cells from oxidative stress and inflammatory response
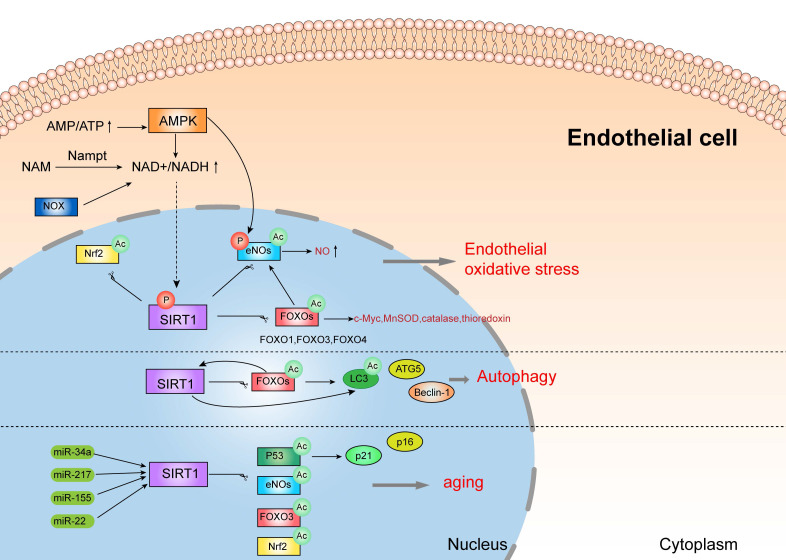
Oxidative stress and inflammation are closely related pathophysiological process and are both involved in the pathogenesis of pre-eclampsia. Oxidative stress is manifested as an overload of reactive oxygen species (ROS), which always result in inflammatory response and endothelial dysfunction. In pre-eclampsia, mitochondrial function is destroyed and reactive oxygen species (ROS, mainly superoxide anions) are excessively produced, triggering oxidative stress and systemic inflammation 65-68. In vitro model of PE, inhibition of SIRT1 decreases antioxidant activity, and lowers the level of intracellular NO and supernatant nitrite 69, 70. Additionally, SIRT1 also acts as a necessary role in antagonizing oxidative stress and inflammation in the pathogenesis of diabetic vasculopathy 71-73, which is also a critical etiological factor for pre-eclampsia. For instance, the downregulation of SIRT1 induced by hyperglycemia causes vascular dysfunction, while upregulation of SIRT1 attenuates oxidative stress-induced endothelial senescence in diabetic mice 74, 75.
Notably, SIRT1 attenuates oxidative stress and inflammation to regulate vascular endothelial functions through several important signal mediators, such as AMPK, NOXs, eNOs, and FOXOs 76. There is a complex crosstalk network between AMPK and SIRT1. Studies showed that SIRT1 can stimulate AMPK via the modulation of upstream AMPK kinase such as liver kinase B1(LKB1) 76, 77, suppressing the production of ROS and inflammation response in HUVECs, while AMPK influences SIRT1 deacetylation activity by increasing cellular NAD+ levels or directly phosphorylating SIRT1. Furthermore, increased activity of NOX (NADPH oxidase) may also enhance NAD+ content to elevate SIRT1 levels in endothelial cells 78. In addition, SIRT1 deacetylates FOXOs and stimulates FOXO-dependent antioxidant [such as catalase (CAT), manganese superoxide dismutase (MnSOD) and thioredoxin] expression to eliminate ROS in endothelial cells, and prevent endothelial dysfunction 78-80. It is documented that the activation of SIRT1 stimulates the expression of c-Myc by promoting the degradation of FOXO1 to prevent endothelial cell dysfunction and angiogenesis induced by hyperglycemia 81. eNOs, a member of NOS families, is expressed in vascular smooth muscle 82. eNOS plays a crucial role in the pathogenesis of pre-eclampsia, since it makes a great contribution to fight against oxidative stress by producing NO and inhibiting the generation of ROS 83. SIRT1 can directly deacetylate or phosphorylate eNOs, or indirectly stimulate eNOs activity by FOXOs and AMPK pathway 84, which might participate in the pathogenesis of pre-eclampsia. This evidence demonstrated that SIRT1 might protect endothelial cells from oxidative stress and inflammation by interacting with various substrates, which might be associated with pre-eclampsia.
3.2. SIRT1 can also protect endothelial cells by autophagy
In endothelial cells, autophagy is mainly regulated by SIRT1/FOXO1 pathway, which might play a crucial role in the pathogenesis of pre-eclampsia 85. Studies showed that SIRT1 actives FOXO1 to protect vascular endothelial cells by regulating autophagy 86. More specifically, SIRT1 deacetylates and activates FOXO1, while FOXO1 can also positively regulate the expression of SIRT1 after activation 87. FOXO1 is closely related to autophagy, since FOXO1 modulates the expression of many autophagy related proteins such as LC3, ATG5 and Beclin-188. These results suggested that SIRT1 protects vascular endothelial cells by regulating autophagy via many pathways.
3.3. SIRT1 can also protect endothelial cells from senescence
Vascular endothelial senescence is a major risk factor for cardiovascular disease and a leading cause of death in patients 89, 90. Interestingly, patients with pre-eclampsia exhibit senescence and dysfunction of endothelial progenitor cells 91, 92. And SIRT1 protects endothelial cells from senescence by various pathways, such as p53, eNOs, Nrf2, FOXO3, and p21/p16, which can be regulated by several miRNA, including miR-217, miR-34a, miR-155, and miR-22 93-99. However, more evidence is needed to further verify the functions of SIRT1 in endothelial aging.
This evidence demonstrated that SIRT1 can protect endothelial cells from oxidative stress, inflammatory response, senescence and autophagy by deacetylating various substrates, which might be involved in the pathogenesis of pre-eclampsia.
4. SIRT1 attenuates the performances of pre-eclampsia in animal models
4.1. SIRT1 knockdown drives the development of pre-eclampsia
Studies reported that SIRT1 is decreased in placentas and serum samples of pre-eclampsia patients, as well as in placentas of pre-eclampsia mice model 100. Importantly, in our previous research, we found that SIRT1 knockdown mice (SIRT1+/- mice) exhibits significant pre-eclampsia-like performances, such as hypertension, proteinuria, fetal growth restriction, kidney injury, and narrow labyrinthine layer, while the manifestations could be reversed after intraperitoneally injecting SRT2104, which is a highly selective agonist of SIRT117. Similarly, Arul Nambi Rajan et al. 22 also found that embryos and placentas were smaller in SIRT1 absence mice, with placentas showing abnormalities in both the labyrinthine layer and junctional zone. Additionally, SIRT1 deficiency mice show multiple developmental defects, ranging from embryonic lethality to postnatal lethality during embryogenesis, with embryo growth restriction 1, 101-103. Furthermore, placentas of SIRT1-KO mice exhibit senescence markers and morphological disruption 50, which is closely associated with the development of pre-eclampsia.
4.2. Supplement of SIRT1 attenuates the performances of pre-eclampsia
Recently, Huang et al. found that supplement with SIRT1 recombinant protein improved the blood pressure, angiogenic imbalance, inflammation, and pregnancy outcome in RUPP pre-eclampsia rat model 8. Interestingly, in our previous research, the pre-eclampsia-like performances were reversed after intraperitoneally injecting SRT2104 that can elevate SIRT1 protein expression 17. However, more animal experiments and clinical trials are needed to further verify the role of SIRT1 in pre-eclampsia.
5. The effect of SIRT1 agonist resveratrol in pre-eclampsia
Resveratrol (3,5,4´-trihydoxy-trans-stilbene, RESV) is a plant polyphenol found in grape skins and red wine, and mainly functions as SIRT1 agonist. Studies have shown that resveratrol involves in various biological processes, such as anti-oxidation, anti-inflammation, anti-aging and anti-cancer 104. And resveratrol is considered in the treatment of pre-eclampsia according to various pre-clinical experiments and clinical trial.
5.1. The effect of resveratrol on trophoblasts or endothelial cells—in vitro experiments
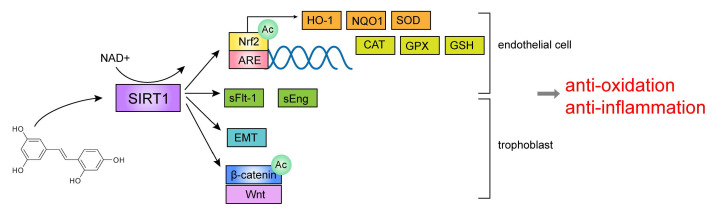
Some studies suggested that resveratrol has an anti-hypertensive effect, which is mainly related to inhibiting the release of sFlt-1 (soluble fms-like tyrosine kinase-1) and sEng (soluble endoglin), reducing the expression of pro-inflammatory molecules, and increasing the expression of anti-oxidant molecules. Resveratrol reduced sFlt-1 and sEng secretion from primary trophoblasts and HUVECs 105, 106, and the elevation of sFlt-1 and sEng is an important feature of pre-eclampsia. Additionally, it is reported that resveratrol could reduce oxidative stress by improving some anti-oxidant markers in endothelial cells of pre-eclampsia, including HO-1, NQO1, Nrf2, GSH (glutathione), SOD (superoxide dismutase) and ARE (antioxidant responsive element) 39, 107-109, which are all crucial molecules regulated by SIRT1. Nrf2, a redox-sensitive transcription factor, can be deacetylated and activated by SIRT1 and promotes the genes transcription of downstream detoxification enzymes and antioxidant enzymes 110, 111, such as SOD and HO-1112-114. In addition, Nrf2 can combine with specific DNA sequence ARE to stimulate the transcription of downstream target genes and antioxidant genes including CAT, SOD, and GPX (glutathione peroxidase) 115. Therefore, resveratrol may play an antioxidant role by upregulating the expression level of SIRT1, thereby activating downstream antioxidant molecules. In addition, some research also reported that resveratrol might promote trophoblast invasion, migration and tube formation by activating epithelial-mesenchymal transition (EMT) and Wnt/β-catenin pathway in pre-eclampsia 116. The above-mentioned pathways are shown in Figure 3. These reports demonstrated that resveratrol, as an agonist of SIRT1, can regulate the functions of trophoblasts and endothelial cells in vitro.
Figure 3.
The effect of SIRT1 agonist resveratrol in pre-eclampsia.
5.2. The effect of resveratrol on blood pressure in animal model of pre-eclampsia—in vivo experiments
Interestingly, it is reported that resveratrol can alleviate the symptoms of pre-eclampsia in animal models. Poudel et al. 117 showed that resveratrol improves artery blood flow and increases fetal weight in COMT-/- mice but not in eNOS-/- mice, which are both animal models of pre-eclampsia. Furthermore, resveratrol reverses the blood pressure and the concentration of urine protein, and inhibits the oxidative stress in L-NAME-induced pre-eclampsia rat model 109, 116. However, Ozlem's research is different from the results of the above-mentioned studies, possibly due to the differentially experimental methodology 5. Therefore, resveratrol reduces blood pressure in pre-eclampsia animal models, indicating that SIRT1 might modulate the progression of pre-eclampsia.
5.3. The effect of resveratrol on blood pressure in pre-eclampsia—clinical trials
Furthermore, several clinical trials also found that resveratrol can decrease blood pressure in hypertensive patients. A randomized clinical trial showed that taking resveratrol can significantly reduce hypertensive symptoms in pre-eclampsia patients, compared with the control group 118. Several meta-analyses and reviews also verify the efficacy of resveratrol in pre-eclampsia 119-122. Moreover, resveratrol also improves flow-mediated dilatation in obese patients and has a controversial anti-hypertensive effect on hypertensive patients 123-127. This evidence suggested that resveratrol might reduce the blood pressure in hypertensive patients, and might play a crucial role in improving the symptoms of pre-eclampsia in a SIRT1 dependent manner. However, resveratrol might also play an anti-hypertensive effect through other pathways, which needs more experiments to verify.
Discussion
In this review, we systematically concluded the role of SIRT1 in pre-eclampsia. SIRT1 can affect the development, differentiation, autophagy and senescence of trophoblasts, thereby regulating their invasion and migration, and participating in the remodeling process of spiral arteries 22, 39, 50. In addition, SIRT1 can also participate in vascular endothelial dysfunction by mediating inflammatory response, oxidative stress, autophagy and aging, and reverse the progression of pre-eclampsia 69, 70, 86. Interestingly, SIRT1 knockout mice exhibited significant pre-eclampsia-like performances, which can be attenuated by SIRT1 supplementation 8, 17. Moreover, the SIRT1 agonist resveratrol also shows a strong anti-hypertensive effect, and might function by increasing the expression level of SIRT1 protein 109, 116-118. However, since resveratrol can also act in other ways, further validation is needed. This evidence suggests that SIRT1 might be an important marker in the pathogenesis of pre-eclampsia.
However, there are still many problems needed further experimental validation. For example, some studies have found that SIRT1 can regulate trophoblast autophagy, but the regulatory mechanisms are not yet completely definite. In addition, SIRT1 is also an important anti-aging molecule involved in a variety of aging-related diseases 8. However, the specific mechanisms of SIRT1 in placental aging need to be further elucidated. Furthermore, it is not clear whether SIRT1 is involved in the progression of pre-eclampsia through other ways, such as abnormal placental metabolism. A typical example is that lipid abnormalities develop in placentas of pre-eclampsia patients. Recent research showed a possible role for LXRβ (liver X receptors beta) as a transcriptional regulator in pre-eclampsia 128. LXRβ is a key regulator of lipid homeostasis, and can be deacetylated by SIRT1129. However, the functions and mechanisms of SIRT1 and LXRβ in pre-eclampsia remain unclear. Moreover, the upstream molecular mechanisms of SIRT1 in pre-eclampsia also needs to be further elucidated. Our previous study found that progesterone can significantly improve the pre-eclampsia-like symptoms in SIRT1 knockdown mice 17, indicating that progesterone might act as an upstream regulator of SIRT1. These issues still need more experiments and clinical trials to further verify, which is also the direction of our future research.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (No. 81971408, 81801469, and 81801468); the National Key R&D Program of China (No. 2016YFC1000403); and the 2020 "Diligence· Excellence" Clinical Innovative Team Project “Study on the comprehensive management of preeclampsia and its pathogenesis” conducted by Obstetrics and Gynecology Hospital of Fudan University (No. 2021fckbc06).
Author Contributions
Zhenzhen Liu, Chengjie Wang, and Jiangnan Pei prepared the manuscript. Mingqing Li and Weirong Gu was responsible for overall supervision. All authors reviewed the article critically for intellectual content and agreed to the published version of the manuscript.
Abbreviations
- PE
pre-eclampsia
- ECs
endothelial cells
- SIRT1
sirtuin1
- Ac
acetylation
- P
phosphorylation
- NF-κB
nuclear factor-kappaB
- PPARγ
peroxisome proliferator-activated receptor gamma
- PGC-1α
peroxisome proliferator-activated receptor-gamma co-activator-1alpha
- TFEB
transcription factor EB
- LC3
microtubule associated protein 1 light chain 3
- LAMP1
lysosomal associated membrane protein 1
- LAMP2
lysosomal associated membrane protein 2
- CTSD
cathepsin D
- ATG3
autophagy-related gene 3
- ATG5
autophagy-related gene 5
- ATG7
autophagy-related gene 7
- ATG8
autophagy-related gene 8
- SQSTM1
sequestosome 1
- HUVECs
human umbilical vein endothelial cells
- ROS
reactive oxygen species
- AMPK
AMP-activated protein kinase
- LKB1
liver kinase B1
- NAM
nicotinamide
- Nampt
nicotinamide phosphoribosyltransferase
- NOX
NADPH oxidases
- Nrf2
nuclear factor-erythroid 2 (NF-E2)-related factor2
- eNOs
neuronal nitricoxide synthase
- FOXO
forkhead box O
- MnSOD
manganese superoxide dismutase
- sFlt-1
soluble fms-like tyrosine kinase-1
- sEng
soluble endoglin
- HO-1
heme oxygenase-1
- GSH
glutathione
- SOD
superoxide dismutase
- ARE
antioxidant responsive element
- CAT
catalase
- GPX
glutathione peroxidase
- NQO1
NADPH quinone oxidoreductase 1
- EMT
epithelial-mesenchymal transition
- NCoR1
nuclear receptor corepressor 1
- SMRT
silencing mediator of retinoic acid and thyroid hormone
- Prdm16
PR domain-containing protein 16
- NO
nitric oxide
- STAT3
activating signal transducers and activators of transcription 3
- RUPP
reduced uterine perfusion pressure
- L-NAME
NG-Nitro-L-arginine methyl ester
- LXRβ
liver X receptors beta
- HELLP
hemolysis, elevated liver enzymes, and low platelet count
- FGR
fetal growth restriction
- LSD1
lysine-specific demethylase 1
- WB
western blotting
- qPCR
quantitative real-time PCR
- IHC
immunohistochemistry
References
- 1.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133:1. doi: 10.1097/AOG.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, Maski MR, Bajracharya S, Wenger JB, Zhang D, Salahuddin S. et al. Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation. 2015;132:1726–33. doi: 10.1161/CIRCULATIONAHA.115.015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1690–702. doi: 10.1016/j.jacc.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Eddy AC, Chapman H, George EM. Heparanase regulation of sFLT-1 release in trophoblasts in vitro. Placenta. 2019;85:63–8. doi: 10.1016/j.placenta.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moraloglu O, Engin-Ustun Y, Tonguc E, Var T, Tapisiz OL, Ergun H. et al. The effect of resveratrol on blood pressure in a rat model of preeclampsia. J Matern Fetal Neonatal Med. 2012;25:845–8. doi: 10.3109/14767058.2011.599081. [DOI] [PubMed] [Google Scholar]
- 6.Pankiewicz K, Fijalkowska A, Issat T, Maciejewski TM. Insight into the Key Points of Preeclampsia Pathophysiology: Uterine Artery Remodeling and the Role of MicroRNAs. International journal of molecular sciences. 2021;22:3132. doi: 10.3390/ijms22063132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187:111215. doi: 10.1016/j.mad.2020.111215. [DOI] [PubMed] [Google Scholar]
- 9.Kong P, Yu Y, Wang L, Dou YQ, Zhang XH, Cui Y. et al. circ-Sirt1 controls NF-kappaB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;47:3580–93. doi: 10.1093/nar/gkz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rada P, Pardo V, Mobasher MA, Garcia-Martinez I, Ruiz L, Gonzalez-Rodriguez A. et al. SIRT1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress. Antioxidants & redox signaling. 2018;28:1187–208. doi: 10.1089/ars.2017.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J. et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22:1170–9. doi: 10.1038/s41556-020-00579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole P. [Destructive respiratory infection] Kansenshogaku Zasshi. 1996;70:1133–9. [PubMed] [Google Scholar]
- 13.Huang K, Chen C, Hao J, Huang J, Wang S, Liu P. et al. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol Cell Endocrinol. 2015;399:178–89. doi: 10.1016/j.mce.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Huang K, Huang J, Xie X, Wang S, Chen C, Shen X. et al. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free radical biology & medicine. 2013;65:528–40. doi: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Huang K, Li R, Wei W. Sirt1 activation prevents anti-Thy 1.1 mesangial proliferative glomerulonephritis in the rat through the Nrf2/ARE pathway. Eur J Pharmacol. 2018;832:138–44. doi: 10.1016/j.ejphar.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB. et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei J, Liu Z, Wang C, Chu N, Liu L, Tang Y. et al. Progesterone Attenuates SIRT1-Deficiency-Mediated Pre-Eclampsia. Biomolecules. 2022;12:422. doi: 10.3390/biom12030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213:S115–22. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh J, Dawson D, Roberts D, Bentley-Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta. 2015;36:101–14. doi: 10.1016/j.placenta.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak-Kim J, Bao S, Lee SK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am J Reprod Immunol. 2014;72:129–40. doi: 10.1111/aji.12234. [DOI] [PubMed] [Google Scholar]
- 21.Pham J, Arul Nambi Rajan K, Li P, Parast MM. The role of Sirtuin1-PPARgamma axis in placental development and function. Journal of molecular endocrinology. 2018;60:R201–R12. doi: 10.1530/JME-17-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arul Nambi Rajan K, Khater M, Soncin F, Pizzo D, Moretto-Zita M, Pham J. et al. Sirtuin1 is required for proper trophoblast differentiation and placental development in mice. Placenta. 2018;62:1–8. doi: 10.1016/j.placenta.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84:167–78. doi: 10.1095/biolreprod.110.086983. [DOI] [PubMed] [Google Scholar]
- 24.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR. et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 25.Borg AJ, Yong HE, Lappas M, Degrelle SA, Keogh RJ, Da Silva-Costa F. et al. Decreased STAT3 in human idiopathic fetal growth restriction contributes to trophoblast dysfunction. Reproduction. 2015;149:523–32. doi: 10.1530/REP-14-0622. [DOI] [PubMed] [Google Scholar]
- 26.Erlebacher A, Price KA, Glimcher LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev Biol. 2004;275:158–69. doi: 10.1016/j.ydbio.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Tang S, Huang G, Fan W, Chen Y, Ward JM, Xu X. et al. SIRT1-mediated deacetylation of CRABPII regulates cellular retinoic acid signaling and modulates embryonic stem cell differentiation. Mol Cell. 2014;55:843–55. doi: 10.1016/j.molcel.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi Y, Takahashi M, Carpino N, Jou ST, Chao JR, Tanaka S. et al. Leukemia inhibitory factor regulates trophoblast giant cell differentiation via Janus kinase 1-signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway. Mol Endocrinol. 2008;22:1673–81. doi: 10.1210/me.2008-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Li F, Xu Y, Wei J, Zhang Y, Yang H. et al. JAK1-mediated Sirt1 phosphorylation functions as a negative feedback of the JAK1-STAT3 pathway. J Biol Chem. 2018;293:11067–75. doi: 10.1074/jbc.RA117.001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tache V, Ciric A, Moretto-Zita M, Li Y, Peng J, Maltepe E. et al. Hypoxia and trophoblast differentiation: a key role for PPARgamma. Stem Cells Dev. 2013;22:2815–24. doi: 10.1089/scd.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y. et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–32. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining "clean" cells. Autophagy. 2005;1:131–40. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 34.Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20:1110–7. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimada Y, Klionsky DJ. Autophagy contributes to lysosomal storage disorders. Autophagy. 2012;8:715–6. doi: 10.4161/auto.19920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimori T. Autophagy: paying Charon's toll. Cell. 2007;128:833–6. doi: 10.1016/j.cell.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Nakashima A, Cheng SB, Ikawa M, Yoshimori T, Huber WJ, Menon R. et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy. 2020;16:1771–85. doi: 10.1080/15548627.2019.1707494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P, Huang CX, Gao JJ, Shi Y, Li H, Yan H. et al. Resveratrol induces SIRT1-Dependent autophagy to prevent H2O2-Induced oxidative stress and apoptosis in HTR8/SVneo cells. Placenta. 2020;91:11–8. doi: 10.1016/j.placenta.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Xia B, Tang L, Wu W, Tang J, Liang Y. et al. Echinacoside protects against MPTP/MPP(+)-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metab Brain Dis. 2019;34:203–12. doi: 10.1007/s11011-018-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Q, Hao R, Wang W, Gao H, Wang C. SIRT1/Atg5/autophagy are involved in the antiatherosclerosis effects of ursolic acid. Molecular and cellular biochemistry. 2016;420:171–84. doi: 10.1007/s11010-016-2787-x. [DOI] [PubMed] [Google Scholar]
- 42.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288–99. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Napolitano G, Esposito A, Choi H, Matarese M, Benedetti V, Di Malta C. et al. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat Commun. 2018;9:1–10. doi: 10.1038/s41467-018-05862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akaishi R, Yamada T, Nakabayashi K, Nishihara H, Furuta I, Kojima T. et al. Autophagy in the placenta of women with hypertensive disorders in pregnancy. Placenta. 2014;35:974–80. doi: 10.1016/j.placenta.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Gao L, Qi HB, Kamana KC, Zhang XM, Zhang H, Baker PN. Excessive autophagy induces the failure of trophoblast invasion and vasculature: possible relevance to the pathogenesis of preeclampsia. J Hypertens. 2015;33:106–17. doi: 10.1097/HJH.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 46.Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15:912–20. doi: 10.1177/1933719108319159. [DOI] [PubMed] [Google Scholar]
- 47.Feng L, Chen M, Li Y, Li M, Hu S, Zhou B. et al. Sirt1 deacetylates and stabilizes p62 to promote hepato-carcinogenesis. Cell death & disease. 2021;12:1–13. doi: 10.1038/s41419-021-03666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang R, Xu Y, Wan W, Shou X, Qian J, You Z. et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell. 2015;57:456–66. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Pi QZ, Wang XW, Jian ZL, Chen D, Zhang C, Wu QC. Melatonin Alleviates Cardiac Dysfunction Via Increasing Sirt1-Mediated Beclin-1 Deacetylation and Autophagy During Sepsis. Inflammation. 2021;44:1184–93. doi: 10.1007/s10753-021-01413-2. [DOI] [PubMed] [Google Scholar]
- 50.Xiong L, Ye X, Chen Z, Fu H, Li S, Xu P. et al. Advanced Maternal Age-associated SIRT1 Deficiency Compromises Trophoblast Epithelial-Mesenchymal Transition through an Increase in Vimentin Acetylation. Aging cell. 2021;20:e13491. doi: 10.1111/acel.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2018;218:S762–S73. doi: 10.1016/j.ajog.2017.11.567. [DOI] [PubMed] [Google Scholar]
- 52.Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD. et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2010;202:381. doi: 10.1016/j.ajog.2010.01.036. e1-7. [DOI] [PubMed] [Google Scholar]
- 53.Tasta O, Swiader A, Grazide MH, Rouahi M, Parant O, Vayssiere C. et al. A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free radical biology & medicine. 2021;164:303–14. doi: 10.1016/j.freeradbiomed.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–8. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 55.Shin-Ichiro Imal, Christopher M.Armstrong, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:796–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 56.Hannan NJ, Beard S, Binder NK, Onda K, Kaitu'u-Lino TJ, Chen Q. et al. Key players of the necroptosis pathway RIPK1 and SIRT2 are altered in placenta from preeclampsia and fetal growth restriction. Placenta. 2017;51:1–9. doi: 10.1016/j.placenta.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Yu Y, An X, Fan D. Histone Deacetylase Sirtuin 2 Enhances Viability of Trophoblasts Through p65-Mediated MicroRNA-146a/ACKR2 Axis. Reprod Sci. 2021;28:1370–81. doi: 10.1007/s43032-020-00398-x. [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Zhang Y, Liu M, Liao L, Wei X, Zhou R. SIRT3 deficiency affects the migration, invasion, tube formation and necroptosis of trophoblast and is implicated in the pathogenesis of preeclampsia. Placenta. 2022;120:1–9. doi: 10.1016/j.placenta.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Castex J, Willmann D, Kanouni T, Arrigoni L, Li Y, Friedrich M. et al. Inactivation of Lsd1 triggers senescence in trophoblast stem cells by induction of Sirt4. Cell death & disease. 2017;8:e2631–e2631. doi: 10.1038/cddis.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandvoss M, Potthast AB, von Versen-Hoynck F, Das AM. HELLP Syndrome. Reprod Sci. 2017;24:568–74. doi: 10.1177/1933719116667216. [DOI] [PubMed] [Google Scholar]
- 61.Bartho LA, O'Callaghan JL, Fisher JJ, Cuffe JSM, Kaitu'u-Lino TJ, Hannan NJ. et al. Analysis of mitochondrial regulatory transcripts in publicly available datasets with validation in placentae from pre-term, post-term and fetal growth restriction pregnancies. Placenta. 2021;112:162–71. doi: 10.1016/j.placenta.2021.07.303. [DOI] [PubMed] [Google Scholar]
- 62.Lim R, Barker G, Lappas M. SIRT6 is decreased with preterm labor and regulates key terminal effector pathways of human labor in fetal membranes. Biol Reprod. 2013;88:1–10. doi: 10.1095/biolreprod.112.105163. [DOI] [PubMed] [Google Scholar]
- 63.Viana-Mattioli S, Nunes P, Cavalli R, Sandrim V. Analysis of SIRT1 Expression in Plasma and in an In Vitro Model of Preeclampsia. Oxid Med Cell Longev. 2020;2020:4561083. doi: 10.1155/2020/4561083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin Y, Feng Y, Zhao H, Zhao Z, Yua H, Xu J. et al. SIRT1 inhibits releases of HMGB1 and HSP70 from human umbilical vein endothelial cells caused by IL-6 and the serum from a preeclampsia patient and protects the cells from death. Biomed Pharmacother. 2017;88:449–58. doi: 10.1016/j.biopha.2017.01.087. [DOI] [PubMed] [Google Scholar]
- 65.Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840:2709–29. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Kaludercic N, Di Lisa F. Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Front Cardiovasc Med. 2020;7:12. doi: 10.3389/fcvm.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keane KN, Cruzat VF, Carlessi R, de Bittencourt PI Jr, Newsholme P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxid Med Cell Longev. 2015;2015:181643. doi: 10.1155/2015/181643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell death & disease. 2018;9:1–9. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caldeira-Dias M, Viana-Mattioli S, de Souza Rangel Machado J, Carlstrom M, de Carvalho Cavalli R, Sandrim VC. Resveratrol and grape juice: Effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. 2021;23:205–10. doi: 10.1016/j.preghy.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Viana-Mattioli S, Cinegaglia N, Bertozzi-Matheus M, Bueno-Pereira TO, Caldeira-Dias M, Cavalli RC. et al. SIRT1-dependent effects of resveratrol and grape juice in an in vitro model of preeclampsia. Biomed Pharmacother. 2020;131:110659. doi: 10.1016/j.biopha.2020.110659. [DOI] [PubMed] [Google Scholar]
- 71.Mishra M, Duraisamy AJ, Kowluru RA. Sirt1: A Guardian of the Development of Diabetic Retinopathy. Diabetes. 2018;67:745–54. doi: 10.2337/db17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic retinopathy. Biomed Pharmacother. 2018;97:190–4. doi: 10.1016/j.biopha.2017.10.075. [DOI] [PubMed] [Google Scholar]
- 73.Kume S, Uzu T, Kashiwagi A, Koya D. SIRT1, a calorie restriction mimetic, in a new therapeutic approach for type 2 diabetes mellitus and diabetic vascular complications. Endocr Metab Immune Disord Drug Targets. 2010;10:16–24. doi: 10.2174/187153010790827957. [DOI] [PubMed] [Google Scholar]
- 74.Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J. et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:889–94. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- 75.Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S. et al. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2205–11. doi: 10.1161/ATVBAHA.110.210500. [DOI] [PubMed] [Google Scholar]
- 76.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. Journal of molecular endocrinology. 2019;63:11–25. doi: 10.1530/JME-19-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–35. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid Med Cell Longev. 2017;2017:7543973. doi: 10.1155/2017/7543973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14:408–12. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K. et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. International journal of molecular medicine. 2005;16:237–43. [PubMed] [Google Scholar]
- 81.Huang X, Sun J, Chen G, Niu C, Wang Y, Zhao C. et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Frontiers in pharmacology. 2019;10:421. doi: 10.3389/fphar.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Frontiers in physiology. 2013;4:347. doi: 10.3389/fphys.2013.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. 37a-37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y. et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circulation research. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cornelius DC, Wallace K. Autophagy in preeclampsia: A new target? EBioMedicine. 2020;57:102864. doi: 10.1016/j.ebiom.2020.102864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Q, Hu Y, Jiang M, Wang F, Gong G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. International journal of molecular sciences. 2019;20:4132. doi: 10.3390/ijms20174132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M. et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10042–7. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ. et al. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell death & disease. 2015;6:e1827–e1827. doi: 10.1038/cddis.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heo JI, Kim KI, Woo SK, Kim JS, Choi KJ, Lee HJ. et al. Stromal Cell-Derived Factor 1 Protects Brain Vascular Endothelial Cells from Radiation-Induced Brain Damage. Cells. 2019;8:1230. doi: 10.3390/cells8101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tian XL, Li Y. Endothelial cell senescence and age-related vascular diseases. J Genet Genomics. 2014;41:485–95. doi: 10.1016/j.jgg.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Hwang HS, Maeng YS, Park YW, Koos BJ, Kwon YG, Kim YH. Increased senescence and reduced functional ability of fetal endothelial progenitor cells in pregnancies complicated by preeclampsia without intrauterine growth restriction. Am J Obstet Gynecol. 2008;199:259. doi: 10.1016/j.ajog.2008.06.060. e1-7. [DOI] [PubMed] [Google Scholar]
- 92.Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H. et al. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005;90:5329–32. doi: 10.1210/jc.2005-0532. [DOI] [PubMed] [Google Scholar]
- 93.Guo Q, Zhang H, Zhang B, Zhang E, Wu Y. Tumor Necrosis Factor-alpha (TNF-alpha) Enhances miR-155-Mediated Endothelial Senescence by Targeting Sirtuin1 (SIRT1) Med Sci Monit. 2019;25:8820–35. doi: 10.12659/MSM.919721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo Y, Chao L, Chao J. Kallistatin attenuates endothelial senescence by modulating Let-7g-mediated miR-34a-SIRT1-eNOS pathway. Journal of cellular and molecular medicine. 2018;22:4387–98. doi: 10.1111/jcmm.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ming GF, Wu K, Hu K, Chen Y, Xiao J. NAMPT regulates senescence, proliferation, and migration of endothelial progenitor cells through the SIRT1 AS lncRNA/miR-22/SIRT1 pathway. Biochem Biophys Res Commun. 2016;478:1382–8. doi: 10.1016/j.bbrc.2016.08.133. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z, Shi D, Zhang N, Yuan T, Tao H. MiR-217 promotes endothelial cell senescence through the SIRT1/p53 signaling pathway. J Mol Histol. 2021;52:257–67. doi: 10.1007/s10735-020-09945-x. [DOI] [PubMed] [Google Scholar]
- 97.Chen L, Holder R, Porter C, Shah Z. Vitamin D3 attenuates doxorubicin-induced senescence of human aortic endothelial cells by upregulation of IL-10 via the pAMPKalpha/Sirt1/Foxo3a signaling pathway. PLoS One. 2021;16:e0252816. doi: 10.1371/journal.pone.0252816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Z, Yu J, Fu M, Dong R, Yang Y, Luo J. et al. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem Pharmacol. 2020;177:113951. doi: 10.1016/j.bcp.2020.113951. [DOI] [PubMed] [Google Scholar]
- 99.Duan JL, Ruan B, Song P, Fang ZQ, Yue ZS, Liu JJ. et al. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology. 2022;75:584–99. doi: 10.1002/hep.32209. [DOI] [PubMed] [Google Scholar]
- 100.Huang Y, Zheng XD, Li H. Protective role of SIRT1-mediated Sonic Hedgehog signaling pathway in the preeclampsia rat models. J Assist Reprod Genet. 2021;38:1843–51. doi: 10.1007/s10815-021-02158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P. et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10794–9. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR. et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C. et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–23. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Breuss JM, Atanasov AG, Uhrin P. Resveratrol and Its Effects on the Vascular System. International journal of molecular sciences. 2019;20:1523. doi: 10.3390/ijms20071523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Al-Ani B. Resveratrol inhibits proteinase-activated receptor-2-induced release of soluble vascular endothelial growth factor receptor-1 from human endothelial cells. EXCLI J. 2013;12:598–604. [PMC free article] [PubMed] [Google Scholar]
- 106.Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B. et al. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012;206:253. doi: 10.1016/j.ajog.2011.11.010. e10-5. [DOI] [PubMed] [Google Scholar]
- 107.Caldeira-Dias M, Montenegro MF, Bettiol H, Barbieri MA, Cardoso VC, Cavalli RC. et al. Resveratrol improves endothelial cell markers impaired by plasma incubation from women who subsequently develop preeclampsia. Hypertens Res. 2019;42:1166–74. doi: 10.1038/s41440-019-0243-5. [DOI] [PubMed] [Google Scholar]
- 108.Gurusinghe S, Cox AG, Rahman R, Chan ST, Muljadi R, Singh H. et al. Resveratrol mitigates trophoblast and endothelial dysfunction partly via activation of nuclear factor erythroid 2-related factor-2. Placenta. 2017;60:74–85. doi: 10.1016/j.placenta.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 109.Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou S. et al. Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules. 2014;19:20570–9. doi: 10.3390/molecules191220570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev. 2018;98:1169–203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu JJ, Cui J, Lin Q, Chen XY, Zhang J, Gao EH. et al. Protection of the enhanced Nrf2 deacetylation and its downstream transcriptional activity by SIRT1 in myocardial ischemia/reperfusion injury. International journal of cardiology. 2021;342:82–93. doi: 10.1016/j.ijcard.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 112.Dinkova-Kostova AT. The Role of Sulfhydryl Reactivity of Small Molecules for the Activation of the KEAP1/NRF2 Pathway and the Heat Shock Response. Scientifica (Cairo) 2012;2012:606104. doi: 10.6064/2012/606104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- 114.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Nagappan A, Kim JH, Jung DY, Jung MH. Cryptotanshinone from the Salvia miltiorrhiza Bunge Attenuates Ethanol-Induced Liver Injury by Activation of AMPK/SIRT1 and Nrf2 Signaling Pathways. International journal of molecular sciences. 2019;21:265. doi: 10.3390/ijms21010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zou Y, Li S, Wu D, Xu Y, Wang S, Jiang Y. et al. Resveratrol promotes trophoblast invasion in pre-eclampsia by inducing epithelial-mesenchymal transition. Journal of cellular and molecular medicine. 2019;23:2702–10. doi: 10.1111/jcmm.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poudel R, Stanley JL, Rueda-Clausen CF, Andersson IJ, Sibley CP, Davidge ST. et al. Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS One. 2013;8:e64401. doi: 10.1371/journal.pone.0064401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ding J, Kang Y, Fan Y, Chen Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr Connect. 2017;6:595–600. doi: 10.1530/EC-17-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cicero AFG, Fogacci F, Colletti A. Food and plant bioactives for reducing cardiometabolic disease risk: an evidence based approach. Food & function. 2017;8:2076–88. doi: 10.1039/c7fo00178a. [DOI] [PubMed] [Google Scholar]
- 120.de Alwis N, Binder NK, Beard S, Kaitu'u-Lino TJ, Tong S, Brownfoot F. et al. Novel approaches to combat preeclampsia: from new drugs to innovative delivery. Placenta. 2020;102:10–6. doi: 10.1016/j.placenta.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 121.Ozarowski M, Karpinski TM, Szulc M, Wielgus K, Kujawski R, Wolski H. et al. Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials-Review of Perspectives for Novel Therapies. Pharmaceuticals (Basel) 2021;14:269. doi: 10.3390/ph14030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tenorio MB, Ferreira RC, Moura FA, Bueno NB, Goulart MOF, Oliveira ACM. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2018;28:865–76. doi: 10.1016/j.numecd.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Biesinger S, Michaels HA, Quadros AS, Qian Y, Rabovsky AB, Badger RS. et al. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur J Clin Nutr. 2016;70:10–6. doi: 10.1038/ejcn.2015.88. [DOI] [PubMed] [Google Scholar]
- 124.Marques B, Trindade M, Aquino JCF, Cunha AR, Gismondi RO, Neves MF. et al. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin Exp Hypertens. 2018;40:218–23. doi: 10.1080/10641963.2017.1288741. [DOI] [PubMed] [Google Scholar]
- 125.Movahed A, Ostovar A, Iranpour D, Thandapilly SJ, Raj P, Louis XL. et al. The efficacy of resveratrol in controlling hypertension: study protocol for a randomized, crossover, double-blinded, placebo-controlled trial. Trials. 2016;17:296. doi: 10.1186/s13063-016-1426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I. et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819–27. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- 127.Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–6. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 128.Weedon-Fekjaer MS, Johnsen GM, Anthonisen EH, Sugulle M, Nebb HI, Duttaroy AK. et al. Expression of liver X receptors in pregnancies complicated by preeclampsia. Placenta. 2010;31:818–24. doi: 10.1016/j.placenta.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 129.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]