Summary
Background
Although the association between short-term antipsychotics exposure and triglycerides (TG) levels has been confirmed, the effects of long-term antipsychotics exposure on TG trajectories and its implications in cardiovascular disease (CVD) remains largely unknown.
Methods
A total of 39,988 participants with at least 3 TG measurements between January 2014 and February 2021 were included in this longitudinal study, with a median follow-up was 4.48 years. A latent class growth mixed model (LCGMM) was used to identify TG trajectories. Based on the LCGMM parameters, we calculated the area under the curve (AUC) and estimated the effect of antipsychotics on AUC and TG trajectory slopes. The primary outcome was CVD events. We also investigated and compared the association between antipsychotics and CVD in subgroups stratified by TG trajectory and TG levels.
Findings
A total of 11,543 CVD events were documented and the incidence density was 64.64 per 1000 person-years. We identified two TG trajectories labeled as inverse-U shape (30.77%, n=12306) and low-decreasing (69.23%, n=27682). The antipsychotic exposure increased total AUC by 13% and increased the slopes of TG trajectories before age 48 years. In the inverse-U and low-decreasing group, the adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for antipsychotics associated with CVD were 1.40 (1.21-1.62) and 1.29 (1.14-1.45), respectively, and the difference between the two trajectory groups become larger with the increase of the antipsychotic exposure. The association of antipsychotics with CVD (HR=1.72, 95%CI: 1.36-2.19) in inverse-U trajectory and high TG group was stronger than that in other subgroups.
Interpretation
Long-term antipsychotic exposure increased the TG burden and TG increase rate early in life. The strength of the association between antipsychotics and CVD risk in the inverse-U group was stronger than that in the low-decreasing group.
Funding
The National Key Research and Development Program of China, Shandong Province Major Science and Technology Innovation Project, and National Natural Science Foundation of China.
Keywords: Antipsychotics, Triglyceride, Trajectory, CVD
Research in context.
Evidence before this study
We searched PubMed for articles published up to December 1, 2021, with the search terms “antipsychotics”, “triglycerides”, “TG”, “lipid”, “trajectory”, and “CVD”. Antipsychotic medications may cause lipid disorders, particularly elevated triglyceride levels, thereby contributing to the development of CVD. Nevertheless, most of the studies were conducted using observational data over several weeks, which are usually collected at specific time points or several time points in a short period, and could only reflect the short-term effects of antipsychotics on TG. The clinical significance of antipsychotic drugs on TG might be more evident after long-term antipsychotic treatment. Different lipid trajectories in the population are associated with CVD risk. At present, no studies have explored the effect of antipsychotics on TG trajectory and its implication in CVD risk.
Added-value of this study
Collectively, we identified two trajectories of TG using LCGMM. It is estimated that the whole life-course antipsychotics exposure can increase total AUC by 13%; In the early life course (before age 48 years), antipsychotics increased the TG increase rate in the inverse-U group and decreased the TG decline rate in the low-decreasing group. We also found that the adjusted HRs for antipsychotics associated with CVD in the inverse-U group were higher than those in the low-decreasing group, and the difference between the two trajectories groups become larger with the increase of the cumulative antipsychotics’ exposure. Among the four subgroups stratified by TG trajectory and TG level, individuals exposed to antipsychotics in the inverse-U trajectory and high TG level group had the highest risk of CVD.
Implications of all the available evidence
Those findings provided new insights into the effects of antipsychotics on TG on a whole life course scale, and suggested new strategies for primary public health services and clinicians to screen patients at high risk of CVD treated with antipsychotics.
Alt-text: Unlabelled box
Introduction
Major mental illness (SMI) includes several serious neuropsychiatric disorders such as schizophrenia, bipolar disorder, major depression, and their related spectrum disorders, most patients with SMI need long-term antipsychotic treatment.1, 2, 3, 4 While most antipsychotics, especially second-generation antipsychotics (SGAs), are related to weight gain, lipid disturbance, and glucose dysregulation, thereby leading to the development of metabolic syndrome.5 A meta-analysis showed that the overall rate of MetS was 32.5% in patients with schizophrenia and related disorders,6 much higher than that in general populations.7,8 In terms of lipid disturbance, although the effects of antipsychotics on total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) are still controversial,5,9, 10, 11, 12, 13 most studies have confirmed that antipsychotics can increase triglyceride (TG) levels.14 These studies were mostly conducted using cross-sectional data or short-term observational data, which are usually collected at specific time points or several time points in a short period and can only reflect the short-term effects of antipsychotics on TG. Whereas, the clinical implications of the effect of antipsychotics on TG are even more evident after mid-and long-term antipsychotic treatments.15,16
Compared with the general population, patients with SMI have a 10-17.5-year shorter life expectancy,17 with CVD contributing 17.4% and 22.0% to the reduction in life expectancy in men and women with SMI, respectively.18 The gap in mortality and life expectancy between patients with schizophrenia and the general population appears to be widening,19 suggesting a need for further understanding of underlying CVD factors in this population. A large portion of this excess mortality is due to cardiometabolic disorders caused by antipsychotics.20 At present, most studies focus on exploring the short-term effects of antipsychotics on metabolic indicators levels and their relationship with CVD risk. Whereas, many studies have shown that there are different trajectories of body mass index (BMI), fasting plasma glucose (FPG), and lipids with age in the population, and trajectory categories are level-independent risk factors for CVD.21, 22, 23, 24 Additionally, Alimu et al. further found that the lipid change rate in early life is a better predictor of CVD than the lipid level in early life.21 Therefore, it is necessary to investigate the effects of antipsychotics on TG trajectories and their implications in CVD.
In this longitudinal cohort study, we used the latent class growth mixture model (LCGMM) to integrate the repeated measurement data of TG with antipsychotic exposure to evaluate the impact of antipsychotics on TG in the whole life course dimension. Meanwhile, we also explored whether there is a difference in the strength of the association between antipsychotics and CVD risk among the subgroups stratified by TG trajectory and TG level. It is expected to provide clinical and public health personnel with a simple and effective strategy to identify individuals at high CVD risk in antipsychotic-exposed populations.
Methods
Study cohort
The longitudinal cohort of this study was constructed based on the Pingyi Medical big data platform. This platform integrated the electronic medical record data of all hospitals in Pingyi county and the basic public health data of the Center for Disease Control and Prevention, such as the health examination database, which contains annual physical examination data from January 2014 to February 2021. This platform uniformly encrypted the ID number to protect privacy and uses the encrypted ID number as a unique index to link everyone's information on the platform. The inclusion criteria in this study are as follows: (1) without previous history of CVD events; (2) individuals with ages ranging from 20 to 80 years; (3) no records of using lipid-lowering drugs or hyperlipidemia diagnosis; (4) no high lipids (TC ≥ 7.76 mmol/L or LDL ≥ 5.18 mmol/L or TG≥5.65 mmol/L) records; (5) at least 3 lipid measurements. Supplementary Figure S1 presented the flowchart of enrollment. A total of 39,988 participants (48.3% were men) were included in the study, with a mean baseline age of 66.51 ± 8.68 years (ranging from 20 to 80 years). The median follow-up was 4.48 years (25th to 75th, 3.45–5.53 years). Referring to other studies using LCGMM for trajectory analysis,21, 22, 23, 24 the sample size of this study is feasible for LCGMM.
Antipsychotics exposure
The antipsychotics use records were obtained from EMR prescription records of the Pingyi County Psychological Hospital from January 2014 to February 2021, including prescription dates and total dosage of antipsychotics. Antipsychotics are classified into first-generation antipsychotics (FGAs: perphenazine, haloperidol, sulpiride, flupentixol, chlorprothixene, and penfluridol) and second-generation antipsychotics (SGAs: risperidone, quetiapine, ziprasidone, clozapine, olanzapine, amisulpride, and aripiprazole).
When we used the LCGMM to identify different TG trajectories and estimate the effects of antipsychotics on them, we needed to determine whether subjects were on antipsychotic medication at the time of the lipid test. We divided the total amount of the antipsychotics obtained on a specific prescription date by the defined daily dose (DDD) to estimate the number of medication days. Taking into account the possible delayed effects of antipsychotics on lipid, the antipsychotic exposure at the time of lipid test was defined as the lipid test date between 3 days after the prescription date and 5 days after the end date of medication (Supplementary Figure S2). When we calculated the TG burden with 1 year as the smallest unit, we needed to confirm whether participants were exposed to antipsychotics within one year, which was defined as cumulative defined daily doses (cDDDs) exceeding 60 during one year. When we estimated the relationship between antipsychotics and CVD risk, the antipsychotics (categorical) were defined as having antipsychotic use records in the EMR; We also used cDDDs to measure and standardize exposure to different antipsychotics for investigating the dose-response relationship between antipsychotics exposure and CVD.
Examinations
After overnight fasting for at least 12 hours, all participants underwent a health check-up performed by doctors of the community service center, including routine anthropometrics, and clinical and laboratory examinations. The height and weight of the subjects were measured while wearing light clothes and no shoes. Body mass index (BMI) is calculated by dividing body weight (kg) by the square of height (m). After participants rested for 5 min, the right upper arm blood pressure, including systolic blood pressure and diastolic blood pressure, was measured with participants in a sitting position. Smoking was defined as smoking at least 1 cigarette per day on average during the last three months; Drinking was defined as consuming alcohol at least once a week on average during the last three months. Diabetes was defined according to the medical records of the subjects in the hospital EMR. The peripheral blood sample was collected in the morning after 12-hours of fasting to obtain the biochemical measurements. The biochemical tests of blood samples were all completed at the clinical laboratory of the community health service center following standard procedures.
Outcome
The primary outcome was CVD events. We used the International Classification of Diseases, 10th Revision (ICD-10) clinical codes to identify CVD events. The ICD-10 codes for CVD included I20, I21, I22, I23, I24, I25, I60, I61, I62, I63, I64, I65, I66, and I67. The health examination data was linked to the death registration data, coronary heart disease registration data, stroke registration data, and hospital electronic medical records (EMR) by using a unique identification number of each participant. If participants had CVD diagnosis records in the above database, we confirmed that they had CVD. The earliest record date of CVD in the above database was defined as the diagnosis date of CVD, and the time from baseline to the diagnosis date of CVD was defined as survival time. Participants without CVD records in the above databases were regarded as free of CVD by February 2021.
Statistical analysis
In this study, we used the LCGMM to identify different trajectory patterns of TG.25 Logarithmic transformation was applied to TG levels because it has a positive skewness (TG all referred to log (TG) hereafter). The trajectory patterns of TG were specified as a function of age (centered to 68 years and divided by 10), with interaction with antipsychotics. The random effects of the models are specified as a linear term of age. We tested multiple trajectory shapes with 3 possible age polynomials: linear, quadratic, and cubic to allow for linear and nonlinear patterns of TG. For each specification of trajectory shape, repeated trajectory analysis was performed by changing the class number from 2 to 4, with the same starting values calculated from the 1-group model. The above trajectory analysis based on LCGMM was performed using the R package lcmm (version 1.7.9). We determined the trajectory shapes and the optimal number of groups based on the following criteria: (1) improvement in the Bayesian information criterion; (2) high mean posterior class membership probabilities (>0.70); (3) no less than 5% membership in any single trajectory group; and (3) high posterior probabilities (>0.70). For more details on LCGMM see Supplementary Text S1.
There are missing values in several variables, including BMI (0.80%), smoker (0.24%), drinker (0.40%), blood pressure (1.35%), LDL (1.51%), TC (1.75%), and HDL (5.42%). The Markov Chain-Monte Carlo (MCMC) algorithm was used to perform multiple imputations. We used Cox proportional hazards model to investigate the relationship between trajectory groups and CVD, including model 1(unadjusted) and model 2 (adjusted for age, sex, smoker, drinker, BMI, diabetes mellitus, SBP, HDL, LDL, TG, and antipsychotics). During the follow-up periods, the integral of the model parameters was used to calculate the total area under the curve (total AUC), which was defined as a measure of the TG burden.26 Baseline AUC is calculated as the product of model-estimated baseline TG levels and follow-up time. Incremental AUC is defined as the value of total AUC minus baseline AUC. AUC values were divided by follow-up time due to different follow-up periods of participants. A logistic regression model adjusted for age, sex, smoker, drinker, BMI, diabetes mellitus, SBP, and antipsychotics, was used to assess the association between total AUC, baseline AUC, and incremental AUC with CVD.
We used LCGMM to estimate the TG level and the AUC in the case of receiving antipsychotic treatment or not receiving antipsychotic treatment, to evaluate the effect of antipsychotics on TG levels at different age points. The first derivative of the LCGMM at each age point was calculated as the slope of the TG trajectory curve to evaluate the effect of antipsychotics on the change rates of TG at different age points. Furthermore, participants were divided into high TG and low TG groups based on median TG levels. In different TG trajectory groups and subgroups combined with different TG levels, we investigated the relationship between antipsychotics and CVD risk using Cox proportional hazards model with adjusted the same covariates as in the previous models. Meanwhile, we also used logistic regression models to explore the dose-response relationship between cumulative exposure to antipsychotics and CVD in different TG trajectory groups. P-value <0.05 was considered statistically significant and had no allowance for multiplicity.
We performed several sensitivity analyses to test the robustness of trajectory analysis. First, we rerun the LCGMM with different random starting values to check the robustness of the trajectory curve. Second, To assess the robustness of the estimates of antipsychotics' effect on TG levels in the trajectory analysis, we defined a negative variable of antipsychotics exposure, that is, we redefined exposure to antipsychotics at the time of blood lipid testing as the date of lipid testing was between 1-3 days after the prescription date and 5-10 days after the end date of the medication (Supplementary Figure S3). Then we re-evaluate the effect of antipsychotics on TG levels in the same trajectory models. Third, we used another different measure, olanzapine equivalents,27 to measure and standardize exposure to different antipsychotics, and repeated the primary analysis involving antipsychotic exposure. Fourth, we introduced the E value () to evaluate the influence of untested confounding on the results.28
Ethics
This study was approved by the ethics committee of the School of Public Health, Shandong University (20190826). Due to the retrospective nature of this study, the requirement for informed consent was waived by the board.
Role of the funding source
The funding sources had no role in the study design, data collection, analysis, interpretation, and writing of the manuscript.
Results
Supplementary Table S1 summarizes the fitting processes of LCGMM. According to the criteria mentioned above, we have chosen a model of quadratic parameters with 2 classes as a trajectory model in our study. The two trajectories were labeled as inverse-U shape (30.77%, n=12306) and low-decreasing (69.23%, n=27682) (Figure 1a). Table 1 summarizes the baseline characteristics and CVD events of the participants across the two TG trajectory classes. All subjects were Han Chinese, and the median age of participants in both trajectories groups was 66 years. The percentage of women in the low-decreasing group (64.5%) is higher than that in the inverse-U group (46.0%). Compared with the inverse-U group, participants in the low-decreasing group had higher BMI, SBP, DBP, HDL-C, rates of diabetes mellitus and antipsychotics exposure, and lower LDL-C, TG, TC, and rates of smoking and drinking. During the follow-up period, a total of 11,543 CVD events were identified and the incidence density was 64.64 per 1000 person-years. The incidence density of CVD in the inverse-U group (69.36 per 1000 person-years) was higher than that in the low-decreasing group (62.58 per 1000 person-years). Kaplan-Meier curve was presented in Supplementary Figure S4.
Figure 1.
The trajectories of triglyceride (TG) over time (a) and the slopes of trajectories (b). In panel a, the red solid line shows the inverse-U shape trajectory of TG for individuals without treatment with antipsychotics (30.77%, n=12306, 45,894 biological replicates), and the orange dashed line shows the inverse-U shape trajectory of TG for a hypothetical individual with treatment with antipsychotics; The blue solid line shows the low-decreasing trajectory of TG for individuals without treatment with antipsychotics (69.23%, n=27682, 103,428 biological replicates), the green dashed line shows the low-decreasing trajectory of TG for a hypothetical individual with treatment with antipsychotics. Lines of different colors in panel b represent the slopes of the corresponding colored lines in panel a.
Table 1.
Baseline characteristics of the study population by triglycerides trajectory classes.
| Variable | Low-decreasing (n=27682) | Inverse-U shape (n=12306) |
|---|---|---|
| Age, years, median (IQR) | 66 (64,71) | 66 (65,72) |
| Women, n (%) | 7943 (64.5) | 12736 (46.0) |
| Ethnic Han n (%) | 27682 (100) | 27682 (100) |
| BMI, kg/m2 | 23.53 (3.00) | 22.48 (2.68) |
| SBP, mmHg | 127.14 (14.27) | 125.09 (13.60) |
| DBP, mmHg | 78.40 (9.21) | 77.20 (9.04) |
| LDL-C, mmol/L | 2.31 (0.30) | 2.42 (0.33) |
| HDL-C, mmol/L | 1.55 (0.34) | 1.47 (0.38) |
| TG, mmol/L | 0.89 (0.46) | 1.55 (0.59) |
| TC, mmol/L | 4.83 (0.26) | 5.14 (0.28) |
| Smoker, n (%) | 2488 (20.2) | 7548 (27.3) |
| Drinker, n (%) | 986 (8.0) | 2915 (10.5) |
| Diabetes mellitus, n (%) | 3066 (24.9) | 4695 (17.0) |
| Antipsychotics | 698 (5.7) | 1297 (4.7) |
| FGAs | 324 (2.6) | 596 (2.2) |
| SGAs | 645 (5.2) | 1202 (4.3) |
| CVD incidence density, per 1000 PYs | 62.58 | 69.36 |
LDL-C, HDL-C, TG, and TG are presented as geometric mean (CV), other variables are presented as mean (SD) or frequency (percentage) or median (IQR). CV, coefficient of variation; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics; CVD, Cardiovascular disease; PYs, person-years.
In LCGMM, antipsychotics and their interaction with polynomial terms of age were statistically significant (P for interaction (LCGMM) < 0.05), indicating a statistically significant effect of antipsychotics on TG trajectories with age. Detailed parameter estimates of the LCGMM are shown in Supplementary Table S2-S4. Individuals treated with antipsychotics had a higher predicted TG level than those not treated with antipsychotics. For both TG trajectories, the effect of antipsychotics on TG levels gradually increased and peaked at age 48. The difference in predicted TG levels between antipsychotic users and non-antipsychotic users increased from 0.07 at age 20 to 0.15 at age 48 (Supplementary Table S5). Overall, individuals with antipsychotic medications got 13% more total AUC between the age of 20 to 80 years. In the Inverse-U group, the incremental AUC for antipsychotics users and non-antipsychotics users were 13.7 (95%CI, 3.5-23.9) mmol/L*year and 8.5 (95%CI, 1.7-15.3) mmol/L*year, respectively; the corresponding values in the low-decreasing group were -4.7 (95%CI, -12.1-2.8) mmol/L*year and -7.3 (95%CI, -3.8 to -10.8) mmol/L*year (Supplementary table S6). Figure 1b presents the slopes of TG trajectories. The slope of the inverse-U trajectory for individuals treated with antipsychotics was higher than that of individuals not treated with antipsychotics before the age of 48, with the mean slopes of 0.08 and 0.05, respectively, and the difference between them became smaller with age; For low-decreasing group, the TG level gradually decreased from age 20 to 80 years. Before the age of 48, the trajectory slopes for non-antipsychotics users were smaller than that of the group with antipsychotics, with the mean slopes of -0.047 and -0.018, respectively (Supplementary Table S7).
Compared with the low-decreasing group, the unadjusted hazard ratio (HR) of the inverse-U group associated with the risk of CVD was 1.13 (95% CI, 1.09-1.18). Adjusting for baseline age, BMI, SBP, smoke, drink, diabetes mellitus, TG, LDL-C, and HDL-C, the inverse-U trajectory group was still positively associated with CVD risk (HR=1.07, 95%CI, 1.02-1.12). Table 2 presents the odds ratio (OR) of AUC quantiles associated with CVD. Compared with participants in the lowest quantile of total AUC, the ORs of CVD were 1.83 (95%CI, 1.71-1.95), 2.58 (95%CI, 2.42-2.75), and 2.02 (95%CI, 1.89-2.16) for those within higher quantile groups. The corresponding ORs for baseline AUC were 1.58 (95%CI, 1.48-1.69), 2.20 (95%CI, 2.07-2.35), and 1.75 (95%CI, 1.64-1.87). Only the fourth quantile of incremental AUC was positively associated with CVD (OR=2.25, 95%CI, 2.12-2.39).
Table 2.
OR of Log (TG) AUC quantiles on CVD by logistic regression modela.
| Total AUCb |
Baseline AUCb |
Incremental AUCb |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Quantile 1 | Reference | Reference | Reference | |||
| Quantile 2 | 1.83 [1.71;1.95] | <0.001 | 1.58 [1.48;1.69] | <0.001 | 0.71 [0.66;0.76] | <0.001 |
| Quantile 3 | 2.58 [2.42;2.75] | <0.001 | 2.20 [2.07;2.35] | <0.001 | 1.03 [0.97;1.10] | 0.358 |
| Quantile 4 | 2.02 [1.89;2.16] | <0.001 | 1.75 [1.64;1.87] | <0.001 | 2.25 [2.12;2.39] | <0.001 |
When we calculated the TG burden with 1 year as the smallest unit, exposed to antipsychotics within one year was defined as cumulative defined daily doses (cDDDs) exceeds 60 during one year.
Residuals of linear regression adjusted for age and antipsychotics exposure. AUC indicates area under the curve; HRs, hazard ratios; CI, confidence interval; CVD, Cardiovascular disease; CHD, coronary heart disease; CBD, cerebrovascular diseases; TG, triglycerides.
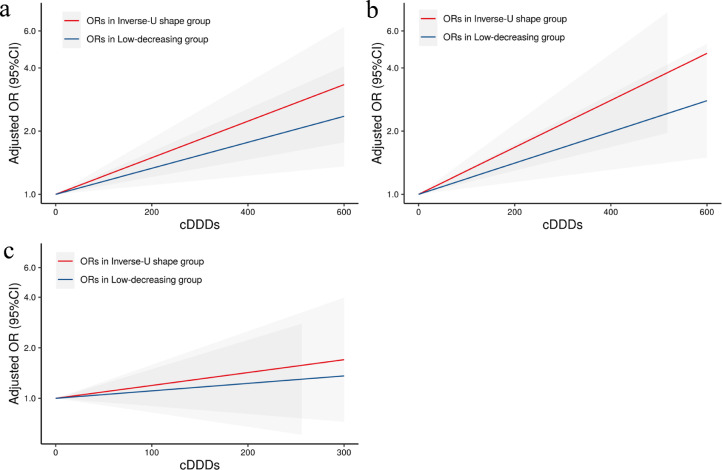
Figure 2 presents the associations between antipsychotics and CVD risk in subgroups stratified by TG trajectories. In the inverse-U group, the adjusted HRs for antipsychotics (HR=1.40, 95%CI: 1.21-1.62), FGAs (HR=1.42, 95%CI: 1.15-1.75), and SGAs (HR=1.35, 95%CI: 1.15-1.58) associated with CVD were all higher than those in the low-decreasing group (antipsychotics, HR=1.29, 95%CI: 1.14-1.45; FGAs, HR=1.29, 95%CI: 1.08-1.53; SGAs, HR=1.24, 95%CI: 1.09-1.41); Further subgroup analysis combined with TG level showed that the HR for antipsychotics associated with CVD (HR=1.72, 95%CI: 1.36-2.19) in inverse-U trajectory and high TG group was higher than that in other subgroups (Figure 3). The same association patterns were also observed for SGAs and FGAs in subgroups analysis. Figure 4 shows the dose-response relationships between CVD with cumulative exposure to antipsychotics, FGAs, and SGAs. The ORs for exposure to antipsychotics associated with CVD in the inverse-U group were higher than those in the low-decreasing group, and the difference of ORs between the two trajectories groups become larger with the increase of the cumulative antipsychotics’ exposure.
Figure 2.
Adjusted HRs (95% CI) for antipsychotics associated with CVD across subgroups stratified by TG trajectories. The HRs were estimated in Cox proportional hazards model adjusted for age, sex, smoker, drinker, BMI, diabetes mellitus, SBP, HDL, LDL, and TG. FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics; CVD, cardiovascular disease; TG, triglycerides.
Figure 3.
Adjusted HR (95% CI) for antipsychotics associated with CVD across subgroups stratified by TG trajectories and TG level. The HRs were estimated in Cox proportional hazards model adjusted for age, sex, smoker, drinker, BMI, diabetes mellitus, SBP, HDL, and LDL. “Low” and “High” in the leftmost column of the forest plot refer to high or low TG levels. CVD, cardiovascular disease; TG, triglycerides; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics.
Figure 4.
The dose-response relationships between CVD with exposure to antipsychotics (a), SGAs (b), and FGAs (c), were estimated by the logistic regression model adjusted for age, sex, smoker, drinker, BMI, diabetes mellitus, SBP, HDL, LDL, and TG. The gray sections represent the 95% CIs of OR. CVD, cardiovascular disease; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics; cDDDs, cumulative defined daily doses; CI, confidence interval.
The results of trajectory analysis were robust in the sensitivity analyses. When we redefined a negative variable to represent antipsychotics exposure and evaluated its effect on TG levels in the same trajectory model, no statistically significant effect of antipsychotics on TG levels was observed (Supplementary Table S8). We also observed association findings similar to the main findings of this study when using olanzapine equivalents to standardize exposure to different antipsychotics (Supplementary Table S9, Figure S5). Furthermore, after adjusting for common risk factors of CVD, the E-value for the association between total AUC and CVD risk was 3.45. In the low-decreasing and inverse-U trajectories groups, the E-values for the association between antipsychotics and CVD risk were 1.90 and 2.19, respectively. This indicates that the results of this study are relatively robust.
Discussion
In this large-scale longitudinal study, we identified two trajectories of TG using LCGMM. It is estimated that the whole life-course antipsychotics exposure can increase total AUC by 13%; Before age of 48 years, antipsychotics exposure increased the TG growth rate in the inverse-U group and decreased the TG decline rate in the low-decreasing group. We also found that the adjusted HRs for antipsychotics associated with CVD in the inverse-U group were higher than those in the low-decreasing group, and the difference between the two trajectories groups become larger with the increase of the cumulative antipsychotics’ exposure. Among the four subgroups stratified by TG trajectory and TG level, individuals exposed to antipsychotics in the inverse-U trajectory and high TG level group had the highest risk of CVD.
Previous studies have confirmed that lipid levels at baseline are an important risk factor for CVD.29, 30, 31, 32 We further found that individuals in the inverse-U trajectory group had a higher risk of CVD, even after adjusting for baseline TG and other covariates, indicating the association was independent of baseline TG and other factors. Similar to other studies reported,21,33 our study also found the cumulative effect of TG on CVD risk. The OR of total AUC was higher than that of baseline AUC, which may indicate that the association between the cumulative effect of TG and CVD was stronger than the baseline TG burden. Furthermore, only the fourth quantile of incremental AUC was positively associated with CVD risk, which suggests that a moderate increase in TG incremental AUC with age may be acceptable for CVD risk. A meta-analysis indicated that statin therapy can safely reduce the incidence of CVD events, which was largely irrespective of the initial lipid profile.34 It is reasonable to speculate that statins may reduce the risk of CVD by reducing an individual's incremental lipid burden. More research is needed to investigate the minimum optimal lipid incremental level that has a clinical guidance significance.21
This study indicated that the whole life-course antipsychotic exposure can increase total AUC and the incremental AUC, which were associated with CVD risk. TG burden most likely plays an intermediary role between antipsychotics and CVD. Simultaneously, our study found that antipsychotics increased the slopes of TG trajectories before age 48. Several studies have found that the level-independent change rate of certain exposures in early life has an important impact on the development of outcomes in later life.21, 22, 23, 24 Fan et al. found that the increased rate of BMI levels between 20-30 years was more closely related to hypertension in later life than BMI levels during that period.23 Another study found that although lipid level has been recognized as an important risk factor for CVD, lipid slope, which reflects lipid increasing velocity, was a better predictor up to age 42, and lipid level was a better predictor after age 53.21 In addition to increasing the burden of TG, antipsychotics may also affect the CVD risk of individuals exposed to antipsychotics by increasing the slope of TG changes in early life. This finding may suggest that when antipsychotics are prescribed for patients with mental disorders, especially for young patients in the inverse-U TG trajectory group, antipsychotics with fewer adverse effects on lipids should be selected as far as possible on the premise of meeting the efficacy requirements.
Our study found that the adjusted HRs for antipsychotics associated with CVD in inverse-U group were higher than those of the low-decreasing group, and this difference increased as cumulative antipsychotics exposure increased. Several reasons are contributing to the explanation of this difference. There is a dose-response relationship between total AUC and CVD risk, and the increased rate of CVD risk increases with the increase of total AUC. Although life-course exposure to antipsychotics increased total AUC by 13% in both trajectory groups, the total AUC and the increase of total AUC caused by antipsychotics in the inverse-U group were much more than that of the low-decreasing group; similarly, the incremental AUC and the increase of incremental AUC contributed by antipsychotics in the inverse-U group were both higher than that in the low-decreasing group. On the other hand, Alimu et al. found that the TG increase rate in early life was positively correlated with CVD risk.21 Although antipsychotics increase the slope of the low-decreasing trajectory, its mean was negative; While the TG trajectory growth rate in the inverse-U group was positive and higher than that in the low-decreasing group, and antipsychotics greatly increased the slope of the inverse-U trajectory in early life, which also contribute to the explanation of greater CVD risk caused by antipsychotics in the inverse-U group.
In this study, we found that the inverse-U TG trajectory was associated with an increased risk of CVD, and the effects of antipsychotics on CVD risk in the inverse-U trajectory and high TG level group were much greater than those in other subgroups. These findings may provide a new perspective for screening the high-risk population of CVD in patients with severe mental illness. In general, we should focus on high-risk groups when resources are limited in basic public health services. Therefore, from the perspective of reducing the risk of CVD in the later life of antipsychotic users, we should strengthen health education and life interventions for groups with high TG levels and inverse-U shaped trajectory groups, such as increasing physical activity. Not only is it protective against hypertension, hyperglycemia, and dyslipidemia, thereby reducing the risk of CVD.35,36 The meta-review conducted by Firth et al.37 also suggests that physical activity has positive effects on the treatment of mental disorders, such as reducing depression and anxiety and improving both positive and negative symptoms of schizophrenia.
The strengths of our research include large-scale longitudinal cohorts, repeated measurement of study variables over time, available antipsychotic exposure information, and the robustness of observed associations. Our study also had a few limitations. First, CVD events were identified based on medical records from hospitals and CVD registration data from CDC, some CVD cases may be missed. Second, we can only obtain the total amount of antipsychotics on each prescription date, but not the frequency and dosage information, thus obtaining the exposure dosages of antipsychotics during the TG test. Third, the lack of medication adherence information may lead to a degree of exposure bias. Logically, when the medicine run out, patients will buy them again. In this study, the vast majority of antipsychotic users had at least two prescription records for antipsychotics. In addition, we mainly focused on the dose-response relationship between cumulative antipsychotic exposure and CVD risk, rather than the specific medication course. Therefore, we cautiously believe that the overall impact of exposure bias due to medication adherence is limited. Fourth, we determined the medication exposure levels based on prescription records, a very small number of individuals may obtain drugs from other sources and we cannot identify them. Fifth, the primary data for this study were only from one county, which limits the representativeness of this study. In addition, the average age of subjects was relatively older, which was mainly due to the requirement of LCGMM that we could only include subjects who had done at least 3 lipid tests. Further studies in younger populations are needed. Finally, due to the relatively small number of participants taking antipsychotics, we did not analyze the effects of individual antipsychotics on TG, so the results of this study provide a general perspective, but cannot be directly applied to specific populations taking specific antipsychotics.
In summary, we have identified two distinct trajectories of TG and suggested that long-term antipsychotic exposure increased the TG burden and TG increase rate in early life. We also found a difference in the strength of the association between antipsychotics and CVD risk in subgroups stratified by TG trajectories, and the difference tends to increase as cumulative antipsychotic exposure increases. Our findings may provide a new perspective on the effects of antipsychotics on TG and its implications in CVD risk. Future research is needed to validate our findings and examine whether our findings can contribute to CVD prevention among individuals taking antipsychotics.
Contributors
LJ, SS, and XF conceived the concept and study design. LJ, WF, and XR obtained the data. LJ, SS, and XF verified all the data in the study. LJ performed the statistical analysis and interpreted the results. LJ drafted the manuscript. SS, WF, XR, and XF revised the manuscript. All authors reviewed and commented on the manuscript, and approved its final submission.
Data sharing statement
The data will be shared at a reasonable request to the corresponding author.
Declaration of interests
None of the authors has any conflict of interest to disclose.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2020YFC2003500), Shandong Province Major Science and Technology Innovation Project (2018CXGC1210), and National Natural Science Foundation of China (71804093). The authors thank the patients included in this study and doctors of the community health service center for their efforts in data collection. We are also very grateful to the engineers of the Healthcare Big Data Research Institute of Shandong University for their help in data collection and processing.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104123.
Appendix. Supplementary materials
References
- 1.Freedman R. Schizophrenia. N Engl J Med. 2003;349(18):1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: efficacy, safety and cost outcomes of CATIE and other trials. J Clin Psychiatry. 2007;68(2):e04. doi: 10.4088/jcp.0207e04. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 4.Dennis JA, Gittner LS, Payne JD, Nugent K. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013–2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howes OD, Bhatnagar A, Gaughran FP, Amiel SA, Murray RM, Pilowsky LS. A prospective study of impairment in glucose control caused by clozapine without changes in insulin resistance. Am J Psychiatry. 2004;161(2):361–363. doi: 10.1176/appi.ajp.161.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tillin T, Forouhi N, Johnston DG, McKeigue PM, Chaturvedi N, Godsland IF. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: a UK population-based cross-sectional study. Diabetologia. 2005;48(4):649–656. doi: 10.1007/s00125-005-1689-3. [DOI] [PubMed] [Google Scholar]
- 8.Hwang LC, Bai CH, Sun CA, Chen CJ. Prevalence of metabolically healthy obesity and its impacts on incidences of hypertension, diabetes and the metabolic syndrome in Taiwan. Asia Pac J Clin Nutr. 2012;21(2):227–233. [PubMed] [Google Scholar]
- 9.Huang TL, Chen JF. Serum lipid profiles and schizophrenia: effects of conventional or atypical antipsychotic drugs in Taiwan. Schizophr Res. 2005;80(1):55–59. doi: 10.1016/j.schres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Ou JJ, Xu Y, Chen HH, et al. Comparison of metabolic effects of ziprasidone versus olanzapine treatment in patients with first-episode schizophrenia. Psychopharmacology (Berl) 2013;225(3):627–635. doi: 10.1007/s00213-012-2850-6. [DOI] [PubMed] [Google Scholar]
- 11.Rognoni C, Bertolani A, Jommi C. Second-generation antipsychotic drugs for patients with schizophrenia: systematic literature review and meta-analysis of metabolic and cardiovascular side effects. Clin Drug Investig. 2021;41(4):303–319. doi: 10.1007/s40261-021-01000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Hert M, Yu W, Detraux J, Sweers K, van Winkel R, Correll CU. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733–759. doi: 10.2165/11634500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Salviato Balbão M, Cecílio Hallak JE, Arcoverde Nunes E, et al. Olanzapine, weight change and metabolic effects: a naturalistic 12-month follow up. Ther Adv Psychopharmacol. 2014;4(1):30–36. doi: 10.1177/2045125313507738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi: 10.1016/S2215-0366(19)30416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackin P, Waton T, Watkinson HM, Gallagher P. A four-year naturalistic prospective study of cardiometabolic disease in antipsychotic-treated patients. Eur Psychiatry. 2012;27(1):50–55. doi: 10.1016/j.eurpsy.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Iglesias R, Martínez-García O, Pardo-Garcia G, et al. Course of weight gain and metabolic abnormalities in first treated episode of psychosis: the first year is a critical period for development of cardiovascular risk factors. Int J Neuropsychopharmacol. 2014;17(1):41–51. doi: 10.1017/S1461145713001053. [DOI] [PubMed] [Google Scholar]
- 17.Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kritharides L, Chow V, Lambert TJ. Cardiovascular disease in patients with schizophrenia. Med J Aust. 2017;207(4):179. doi: 10.5694/mja17.00258. [DOI] [PubMed] [Google Scholar]
- 19.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time. Arch Gen Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 20.Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry. 2011;68(6):609–616. doi: 10.1001/archgenpsychiatry.2011.2. [DOI] [PubMed] [Google Scholar]
- 21.Dayimu A, Wang C, Li J, et al. Trajectories of lipids profile and incident cardiovascular disease risk: a longitudinal cohort study. J Am Heart Assoc. 2019;8(21) doi: 10.1161/JAHA.119.013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Z, Yang Y, Wang C, et al. Trajectories of long-term normal fasting plasma glucose and risk of coronary heart disease: a prospective cohort study. J Am Heart Assoc. 2018;7(4):e007607. doi: 10.1161/JAHA.117.007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan B, Yang Y, Dayimu A, et al. Body mass index trajectories during young adulthood and incident hypertension: a longitudinal cohort in Chinese population. J Am Heart Assoc. 2019;8(8) doi: 10.1161/JAHA.119.011937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv J, Fan B, Wei M, et al. Trajectories of early to mid-life adulthood BMI and incident diabetes: the China Health and Nutrition Survey. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2019-000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proust-Lima C PV, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R Package lcmm. J Stat Softw. 2015;78(2):1–56. [Google Scholar]
- 26.Cook NR, Rosner BA, Chen W, Srinivasan SR, Berenson GS. Using the area under the curve to reduce measurement error in predicting young adult blood pressure from childhood measures. Stat Med. 2004;23(22):3421–3435. doi: 10.1002/sim.1921. [DOI] [PubMed] [Google Scholar]
- 27.Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 28.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 29.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger PS, Smith DA, Mehta AE, et al. American association of clinical endocrinologists' guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract. 2012;18(suppl 1):1–78. doi: 10.4158/ep.18.s1.1. [DOI] [PubMed] [Google Scholar]
- 31.Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z. Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Curr Cardiol Rep. 2012;14(6):709–720. doi: 10.1007/s11886-012-0313-7. [DOI] [PubMed] [Google Scholar]
- 32.Boullart AC, de Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochim Biophys Acta. 2012;1821(5):867–875. doi: 10.1016/j.bbalip.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Amarenco P, Goldstein LB, Messig M, et al. Relative and cumulative effects of lipid and blood pressure control in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial. Stroke. 2009;40(7):2486–2492. doi: 10.1161/STROKEAHA.108.546135. [DOI] [PubMed] [Google Scholar]
- 34.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 35.Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
- 36.Tian D, Meng J. Exercise for prevention and relief of cardiovascular disease: prognoses, mechanisms, and approaches. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/3756750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–380. doi: 10.1002/wps.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.