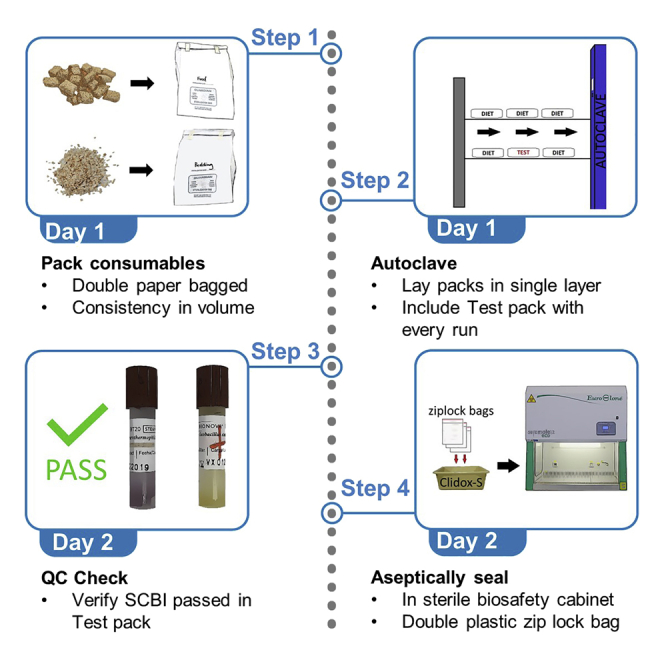
Summary
The cost of supply cylinders can be expensive for smaller gnotobiotic facilities on strict budgets. Here, we present an alternative approach to enter supplies into germ-free isolators that does not require large cylinder drums (supply cylinders). We describe procedures for autoclaving double-bagged packs of supplies, sealing the packs aseptically into zip lock bags in biosafety cabinet, and entering supply packages into isolators. Our gnotobiotic facility has been using this protocol since 2018 and has been effective in maintaining germ-free status.
Subject areas: Microbiology, Model Organisms
Graphical abstract
Highlights
-
•
Useful for basic germ-free facilities that have limited autoclave capacity
-
•
Supplies are double paper bagged to be autoclaved without cylinder drum
-
•
Supply packs are sterile sealed into plastic bags aseptically in biosafety cabinet
-
•
Sterile supply packs are conveniently on hand for quick isolator restock
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The cost of supply cylinders can be expensive for smaller gnotobiotic facilities on strict budgets. Here, we present an alternative approach to enter supplies into germ-free isolators that does not require large cylinder drums (supply cylinders). We describe procedures for autoclaving double-bagged packs of supplies, sealing the packs aseptically into zip lock bags in biosafety cabinet, and entering supply packages into isolators. Our gnotobiotic facility has been using this protocol since 2018 and has been effective in maintaining germ-free status.
Before you begin
This protocol details how our basic gnotobiotic rodent facility has been preparing and supplying sterile diet, bedding and enrichment to germ-free isolators that does not require cylinder drums.
Institutional permissions
The protocol described in this manuscript forms part of the facility’s standard operating procedures. All standard operating procedures within the facility have been submitted to the South Australian Health and Medical Research Institute Animal Ethics Committee.
Autoclave performance
Timing: Not Applicable
-
1.This procedure relies on the performance of a good quality autoclave (see key resources table, Model# GE6610 ER-2) to provide effective steam sterilization. Troubleshooting, problem 1.
-
a.Autoclaves should be serviced and calibrated regularly to ensure consistency in performance.
-
b.Calibration should verify even heat distribution at multiple points through the autoclave.
-
c.The autoclave cycle used at our facility which has proven to be effective for sterilizing rodent diet, bedding and enrichment packs is:
-
i.Pre-vacuum, 15 min.
-
ii.Sterilization, 121 degrees Celsius, 30 min.
-
iii.Drying, 20 min.
-
i.
-
a.
Note: Autoclave cycles can vary between facility protocols depending on the type of diet and bedding utilized at the facility and the autoclave performance/specifications. Therefore, validation of cycles must be performed prior to establishing standard facility protocols.
Test pack
Timing: 2–5 min
-
2.The Test pack is an identically-prepared pack with a Self-Contained Biological Indicator (SCBI, see key resources table, SKU# NM-SVT-056) buried inside. A test pack is included for each autoclave load of bedding or diet specifically as their dense particle sizes make it difficult for thorough steam penetration. For example, if autoclaving packs of bedding, one of the bedding packs will be clearly labeled as the test pack. If autoclaving packs of diet, one of the diet packs will be clearly labeled as the test pack. Test packs are not required for crinkle nest or nestlet loads as the low particle density of these items do not pose the same level of concern. In these enrichment supply loads, we solely rely on the autoclave read-out to verify that the autoclave has reached sufficient sterilization conditions.
-
a.One Test pack of diet and/or bedding (where appropriate) is included in every autoclave load.
-
b.Placement of the Test pack within the autoclave is random, however this random placement is reliant on the autoclave being calibrated regularly for uniform heat distribution. This may not be the case for other facility autoclaves where there may be cold spots. If this is the case, placement of the Test pack in a cold spot would be ideal.
-
c.The Test pack is opened post-autoclave cycle to remove and incubate the SCBI, then discarded.
-
d.The lack of color change for the SCBI provides assurance that sterilization has occurred, and the batch of autoclaved items is ready for aseptic sealing.
-
e.When incubating SCBIs, it is important to also incubate a control SCBI which has not undergone autoclaving in order to verify that the incubation temperature was adequate for SCBI growth.
-
a.
CRITICAL: Quality Control (QC) verification is critical in successful maintenance of gnotobiotic colonies. Acknowledging the time, energy, cost and welfare considerations (i.e., humanely killing contaminated animals) that go into gnotobiotic research, it is not worth exposing gnotobiotic colonies to items that do not sufficiently pass QC measures. Ensure there are robust and effective QC protocols in place. Quality Control measures include up-to-date autoclave servicing and verification of run success, the use of steam sterilization indicators (such as steam sterilization color change tape or strips), the use of SCBIs as described above and extends to routine microbiological techniques and bacterial 16s rRNA polymerase chain reaction (PCR) screening of animals and isolator units.
Biosafety cabinet modification
Timing: Not Applicable
-
3.We utilize a Class II biosafety cabinet (see key resources table, Product# LDE2822) to provide a sterile environment for manually sealing the autoclaved supplies. There is a high risk of contamination in this setting, but the risks can be mitigated through cleaning, sterilization and excellent aseptic technique.
-
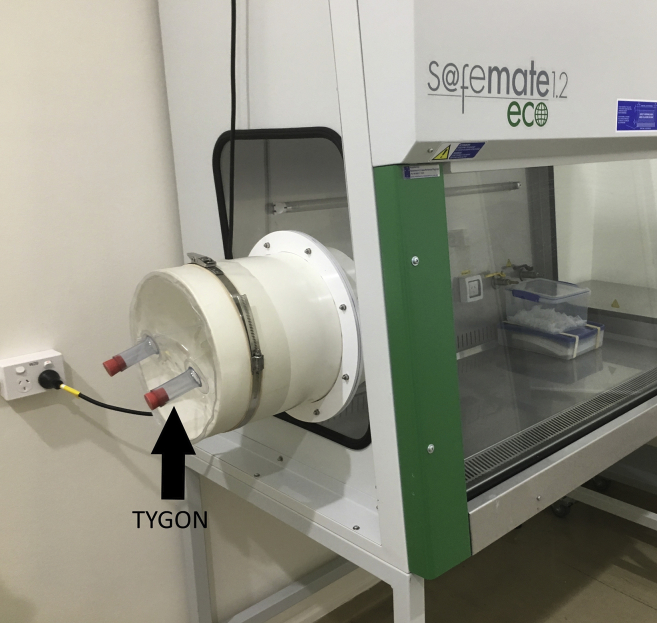
a.The side panels of our Class II biosafety cabinets have been retrofitted with side ports and covers that have tygons. See Figure 1.
-
b.The tygons are sealed with removable rubber bungs.
-
c.These tygons allow us to fog using the Sprayer Less atomizer (see key resources table, Part# PA64-SS, Figure 2.) inside of biosafety cabinets with a non-corrosive disinfectant F10SC (see key resources table, Product# 102-101-100-109) prior to any procedure taking place.
-
d.Our biosafety cabinets are thoroughly wiped with 80% v/v ethanol between use to remove debris and Clidox-S® residue (see key resources table, Product# 95125; Product# 96125).
-
a.
Figure 1.
Class II biosafety cabinet with retrofitted side ports
Tygons are on the port cover as indicated by arrow. The Sprayer Less atomizer nozzle is pointed into the tygon for fogging.
Figure 2.
Sprayer Less atomizer unit which must be coupled by hose to an air compressor to operate
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Self-Contained Biological Indicator, SporView10 Self-Contained Biological Indicator – Steam 10E5 G. stearothermophilus Spores, 8.4 × 45.5 mm | BioNovus Life Sciences https://www.bionovuslifesciences.com.au/ | SKU# NM-SVT-056 |
| Chemicals, peptides, and recombinant proteins | ||
| F10SC Veterinary Disinfectant | Chemical Essentials https://www.chemicalessentials.com.au/ | Product# 102-101-100-109 |
| Clidox-S® Activator 5 Gallons | Pharmacal Research Laboratories, Inc. https://www.pharmacal.com/clidox-s.html |
Product# 95125F |
| Clidox-S® Base 5 Gallons | Pharmacal Research Laboratories, Inc. https://www.pharmacal.com/clidox-s.html |
Product# 96125F |
| Clidox-S® Deactivator 1 Gallon | Pharmacal Research Laboratories, Inc. https://www.pharmacal.com/clidox-s.html |
Product# 97120F |
| Experimental models: Organisms/strains | ||
| Gnotobiotic (axenic, gnotobiotic) mice | University of Queensland, Translational Research Institute, Gnotobiotic Facility | N/A |
| Other | ||
| Autoclave 452 Liter Chamber | Getinge https://www.getinge.com/int/ |
Model# GE6610 ER-2 |
| EuroClone-BioAir S@femate ECO ABC Class II (Type A2) Microbiological Safety Cabinet | Adelab https://adelab.com.au/biological-safety-cabinets/euroclone-bioair-biological-safety-cabinets | Product# LDE2822 |
| Sprayer Less atomizer | Spraying Systems Co. https://www.spray.com/en-au |
Part# 5870A |
| Sprayer Less Drip Free Fluid Cap | Spraying Systems Co. https://www.spray.com/en-au |
Part# PF250DF-SS |
| Sprayer Less Air Cap, ST.STL, 1/4J | Spraying Systems Co. https://www.spray.com/en-au |
Part# PA64-SS |
| Round port caps with tygon | Park Bioservices, https://parkbio.com/product/port-caps/ | N/A |
| Paper satchels, 340 × 165 × 100 mm | Castaway https://www.castawayfoodpackaging.com.au/product/pb-bs08-brown-satchel-paper-bags/ | Item# PB-BS08 |
| Autoclave bags, 57 gm paper satchel with Indicator & labeling No 30 440 × 200 × 98 mm | Adelab http://www.adelab.com.au | Reference# LV-AB30 |
| Autoclave Tape With steam indicator, 25 mm diameter | Adelab https://adelab.com.au/consumables/autoclave-consumables/autoclave-tape/ | Reference# LV-TA25X55 |
| Autoclavable rodent diet, Envigo Teklad global 18% rodent diet | Biological Associates https://www.envigo.com/teklad |
Product# TD2018s.15 |
| Aspen chip bedding, Eco-pure Aspen ab3 Premium Bedding | Allied Group https://allied-group.com.au/product/eco-pure-aspen-ab3-premium-bedding/ | Product# DS-AB3 |
| Crinkle nest, Pura crinkle paper nesting material | Able Scientific https://www.ablescientific.com.au/ | Product code# ASBCN-P |
| Nestlet, 50 mm square cotton nestlets | Able Scientific https://www.ablescientific.com.au/ | Product code# ASNLETS |
| Chux® wipes, 9305B Heavy Duty Superwipes Roll Blue 45 m | OfficeMax https://www.winc.com.au/ | Product code# 86768603 |
| 1 L Plastic Beaker, 1000 mL, polypropylene, low form, with spout | Adelab http://www.adelab.com.au | Reference# TECH-LW0317-01 |
| Plastic zip lock bags, Hercules Resealable Bags, Twin zip, 27 × 33 cm | Woolworths https://www.woolworths.com.au/shop/productdetails/706057 | Product# 706057 |
| Sterile nitrile procedure gloves, 302 mm overlength | Medline https://www.medline.com.au/products/gloves | SKU# MDS2295 |
Materials and equipment
Clidox-S® volume preparation for sealing approximately 30 sterile packs
| Reagent | Final concentration | Amount |
|---|---|---|
| Clidox-S® Activator | 1 volume part | 1 L |
| Water | 3 volume parts | 3 L |
| Clidox-S® Base | 1 volume part | 1 L |
| Total | 5 volume parts | 5 L |
Room Temperature (19°C–23°C), 8 h storage.
CRITICAL: Clidox-S® causes eye and skin irritation. Always handle with appropriate personal protective equipment (long sleeve gown, nitrile gloves and P2 respirator). Clidox-S® is highly corrosive and must be removed from all bare metal surfaces immediately after use.
F10SC disinfectant
| Reagent | Final concentration | Amount |
|---|---|---|
| F10SC concentrate | 1 volume part | 4 mL |
| Water | 124 volume parts | 496 mL |
| Total | 125 volume parts | 500 mL |
Room Temperature (19°C–23°C), 6 month storage.
Alternatives: There are a range of chemical disinfectants and chlorine-oxide/peroxide based sterilants used in other facilities that are proven to be as effective. F10SC was our choice of disinfectant due to its broad-spectrum antibacterial properties and safety for use around humans and animals. Clidox-S® was our choice of sterilant due to its relatively quick contact time (can be as little as 10 min) and proven effectiveness for gnotobiotic purposes.
Step-by-step method details
Autoclave double-bagged packs of supplies
Timing: 24 h
Rodent chow sterilized by irradiation can be sourced commercially but is costly and generally, irradiation is considered less effective than steam sterilization. Steam sterilization can be easily performed in-house where there is access to an autoclave, thus saving money and providing better oversight of the QC measures used for ensuring sterility. Having ready-made, pre-sterilized, sealed packs of diet/bedding/enrichment is convenient in a gnotobiotic facility for quick entry of supplies into an isolator. These sterile packs can be held in a storeroom and are immediately ready to be cold sterilized into an isolator or into a biosafety cabinet for topping up individual IsoCage type cages. Preparing these packs in bulk will save operational time overall.
-
1.Weigh or scoop equal volumes of diet/bedding inside paper satchels. Seal with autoclave tape.
-
a.Our facility packs 1–1.1 kg of diet, 450–500 g of bedding, a handful of crinkle nest and 12 × nestlet squares per pack.
-
b.Diet and bedding are evenly spread to ensure each pack is no more than 5 cm in thickness when laid flat.
-
a.
-
2.
The supplies are first bagged in brown paper satchels and then the pack is sealed inside autoclave steam sterilization medical paper bags as pictured in Figure 3.
-
3.
Prepare Test packs for diet or bedding as described above under before you begin, test pack section and shown in Figure 4.
-
4.
When loading packs into the autoclave, do not stack them directly on top of each other as this will hinder the penetration of steam. Arrange packs onto autoclave shelves in a single layer.
-
5.
After autoclaving, remove the SCBI from the Test pack and incubate as per manufacturer’s instruction. See troubleshooting, problem 2.
CRITICAL: Keeping pack size/weight consistent for diet and bedding is critical to ensure steam can evenly penetrate through the dense particles for all packs in the load. It is less critical for crinkle nest and nestlets where pack sizes are less dense.
Figure 3.
Order of packing sterile supplies into paper bags
(A–C) Consumables (A) are packed firstly into brown paper bags (B) followed by white autoclave bags (C).
Figure 4.
Example of bedding Test pack
SCBI buried inside as indicated by black arrow. Test packs should also be prepared for autoclave loads of diet.
Prepare biosafety cabinet
Timing: 1 h
Sterilizing the workspace inside the biosafety cabinet is a very critical step and must be done with great thoroughness. Although there are a multitude of different ways to sterilize a biosafety cabinet, this protocol has been working well for our facility at preventing cross-contaminations between use.
-
6.
UV sterilize a clean biosafety cabinet for 30 min.
-
7.Without turning the biosafety cabinet on or opening the sash, fog the biosafety cabinet for 1–3 min(s) through the tygon with F10SC disinfectant.
-
a.Leave for 10 min.
-
b.Keep biosafety cabinet closed and off during this time.
-
a.
-
8.
Once fogging is complete, wipe down the bench surface of the biosafety cabinet with Clidox-S® soaked Chux® wipe (see key resources table, Product code# 86768603) or similar low-lint fabric wipe, along with the guard/armrest area and the bottom of the glass sash.
-
9.Cold sterilize zip lock bags inside and out by completely submerging them (2–5 s) in a tub of Clidox-S® and place them inside the biosafety cabinet.
-
a.For each pack that has been autoclaved, two zip lock bags are required.
-
b.The cabinet will become quite wet. A Chux® wipe and large plastic beaker is also cold sterilized and brought into the biosafety cabinet which can be used to mop up excess Clidox-S® regularly throughout the process.
-
a.
-
10.
Once the cabinet is filled with enough zip lock bags, leaving a small amount of room to work, wait at least 10 min (see Figure 5).
Pause point: The cold sterilized zip lock bags can be left inside the biosafety cabinet for longer than 10 min.
Figure 5.
Plastic zip lock bags, a beaker, and Chux® wipe, all cold sterilized in Clidox-S® and aseptically brought into a sterilized biosafety cabinet by gently tossing them in
Seal the packs aseptically into zip lock bags
Timing: 30 min or more
The timing of this step will depend on the number of parcels that require sealing. This step ideally requires two people but it is possible with one (troubleshooting, problem 3). Person one will maintain sterility and work within the biosafety cabinet. Person two will assist with handling non-sterile items and aseptically pass packages into the biosafety cabinet.
-
11.Person one dons a hair net, an autoclave sterilized, cuffed, long-sleeved, surgical gown (re-usable, cotton, washable and autoclavable laboratory gowns) and disposable, sterile, long-cuffed nitrile gloves (see key resources table, SKU# MDS2295), in similar fashion to a surgeon gowning up for surgery.
-
a.For extra measure, wipe gloved hands in Clidox-S® prior to placing them into the biosafety cabinet.
-
b.Wipe all the way to the end of the cuff.
-
c.Wait for 10 min with sterilized, gloved hands inside the biosafety cabinet before starting the packing process.
-
a.
-
12.
Person two submerges an autoclaved pack (2–3 s) so that it is wet (not completely soaked through) into the tub of Clidox-S® and drain off excess sterilant.
-
13.
Person two tears the outer autoclave tape seal and aseptically unfolds the opening of the package just below the biosafety cabinet sash. The medical sterilization bags are thick enough to resist becoming immediately soggy but steps 12–13 must be done swiftly.
-
14.
Person one aseptically reaches into the package and pulls the inner parcel into the cabinet. See Figure 6.
-
15.
Person two discards the outer package.
-
16.Person one seals the inner parcel into two zip lock bags, pressing out as much air as possible.
-
a.To avoid parcels becoming soaked and contaminated with Clidox-S®, use the Chux® wipe to soak up excess Clidox-S® and wring into the beaker.
-
b.Keeping the outer zip lock bags wet allows longer contact time with Clidox-S® and is therefore ideal.
-
a.
-
17.
Repeat steps 12–16 for all autoclaved packages.
-
18.
Place sealed supply packs in a storeroom.
Note: Person one should regularly wipe hands and surfaces throughout the procedure with Clidox-S®-soaked Chux® wipe to maintain sterility.
Note: The packs can be UV sterilized for extra measure, prior to entry into isolators.
Figure 6.
Person one aseptically pulling sterile, inner parcel from Clidox-S® submerged outer package, into the biosafety cabinet
Enter supply packages into isolators
Timing: 30 min
Whether the isolator is semi-rigid or flexible film, they are typically designed with an entry/exit port where these sterile supply packs can be placed prior to transfer into the isolator. Our facility uses semi-rigid isolators with rectangular ports, the use of which is described below.
-
19.
Prior to opening an isolator, ensure the inner port door is properly closed.
-
20.
Open the outer port door and wipe over the entire port surface with generous volumes of Clidox-S®.
-
21.
Submerge and wipe over the sterile packs in Clidox-S®. Troubleshooting, problem 4.
-
22.
Place the packs into the port.
-
23.
Fog the port with Clidox-S®. A respirator must be worn when doing this.
-
24.
It is recommended to allow the supplies to sit for as long as possible in the port to provide ample Clidox-S® contact time, even overnight if practical. However, in our experience, a minimum of 10 min-contact time in the port was sufficient to avoid contamination.
Note: In line with good gnotobiotic practice, isolators are opened as infrequently as possible to avoid contamination risk. When it is opened, it is ideal to ensure as much waste from the isolator is removed as possible, and as many supplies as allowable are entered in.
Expected outcomes
The sealed parcels can be stored indefinitely (in accordance with manufacturer’s expiration date) and are readily available for supply into isolators and to top-up food/bedding/enrichment in IsoCages that are opened inside a biosafety cabinet (see Figure 7).
Figure 7.
Sterile packs of diet, bedding, and enrichment, stored and ready to be used
The protocol described here has been used by our facility since 2018 for germ-free experiments and has been successful in maintaining germ-free status in our breeding isolators for over a year. Over this period, upon restocking (on average fortnightly), a swab of the isolator is taken of the mold trap which consists of soiled bedding, diet and other isolator waste and is kept moist with the water supply. We also swab around the surfaces inside the isolator. For IsoCages, we swab through the soiled cage bedding and along cage walls. The swab is immediately submitted to our local microbiology laboratory for inoculation onto select agar plates and monitored for bacterial and fungal growth. Each quarter, we collect fecal samples and submit these to our local diagnostic laboratory for 16s rRNA qPCR testing. The results from these tests indicate that the animals within our isolators have remained germ free, thus validating our protocol. This protocol can be used for other porous supply items that must first be sealed in plastic prior to cold sterilization and entry into isolators.
Limitations
This procedure produces a lot of paper and plastic waste. Plastic zip lock bags can however be re-used as waste bags within isolators. Also, sourcing larger zip lock bags can allow more packs to be sealed into fewer zip lock bags.
It is impossible to quality test every single pack without compromising sterility, which in any case is also true for supply cylinders and irradiated packs. Contamination of isolators can occur from time to time and it is often very difficult to pinpoint when and how it has occurred (troubleshooting, problem 5). Even though batches pass QC checks, this still does not guarantee that all packs are sterile. Facilities will implement other mitigation processes to minimize the impact of potentially losing isolators and colonies due to contaminations.
The entire process is lengthy and perhaps overall, takes longer than using supply cylinders due to the added step of sealing packs into zip lock bags inside a biosafety cabinet. However it is less complicated than preparing cylinders and possibly less expensive as outside of purchasing the cylinder frames, it does not involve using HEPA filter media, nylon tape, high temperature tape, mesh bags, Mylar film, gaskets and rubber bands as described by Vowles et al. (2016) in their illustrated guide to Gnotobiotic Mouse Technology. As we have never used cylinders at our facility, it is difficult to make a direct comparison. Pre-emptively preparing packs is a task that can be done intermittently during non-busy, down times and wait times which is a flexibility that we find favorable using this technique. In comparison to preparing cylinders where the size of the autoclave and cylinder may limit throughput, sterile packs can be autoclaved in bulk which can mean higher throughput of supplies, depending on the size and number of available shelves within the autoclave chamber. Ensuring each autoclave load is completely full before starting the cycle is the most efficient way to save overall time and keep utility bills low.
The process of packing within a biosafety cabinet can become labor-intensive and cause muscle tiredness therefore it is ideal to take regular breaks or swap between person one and person two roles. By comparison, cylinders can become quite heavy and be difficult to maneuver which poses occupational health and safety risks for technicians too. They can also take some time and effort to connect and disconnect to isolators. Hence, there are advantages and disadvantages for both techniques of using cylinders and this protocol.
Having two people perform this protocol means extra human resources are required compared to using supply cylinders whereby one person can perform the task alone. Although having a second person to assist in this technique is preferable for speed and ease, it is not absolutely necessary. One person can perform this entire process alone however there are added logistics to consider. Under troubleshooting, problem 3, we describe how the technique can be performed with just one person.
Overall, this technique allows for the convenience of having sterile supplies in lightweight packs on hand and ready to cold sterilize, which we find makes the end process of supply entry into isolators and for replenishing IsoCages inside a biosafety cabinet, quick and easy.
Troubleshooting
Problem 1
The facility autoclave is sub-optimal therefore the risk of contamination occurrence is higher. Refer to before you begin, autoclave performance, step 1.
Potential solution
Autoclaves are critical for running gnotobiotic facilities. Having a sub-optimal autoclave increases the risk of contamination and can make it nearly impossible to maintain germ free isolators long term. There are measures that can be integrated to this protocol to further mitigate contamination risk. For example, the use of multiple SCBIs/Test packs in different spots per autoclave load will verify uniform heat distribution and provide greater reassurance on the sterility of the batch. Another example is to take swabs for microbiological culture from the supply packs when handling them inside the biosafety cabinet, before sealing them into zip lock bags. This can be done on a large sample size and hence provide greater reassurance on sterility of the batch entirety. Care would need to be taken to avoid Clidox-S® contact with the swab. These extra measures can however increase operational costs and labor and therefore must be weighed up in a risk-benefit analysis.
Problem 2
The doubled-bagged supply packs burst open post-autoclaving. Refer to, step-by-step method details, autoclave double-bagged packs of supplies, step 5.
Potential solution
Excessive steam produced from diet and bedding packs can cause them to burst open at the seams during the autoclave cycle. Packs that open should be discarded or re-autoclaved if possible. Bursting can be due to autoclave bags having weak seams in which case an alternate brand could be the solution or using extra tape to reinforce the seams and openings may be all that is required.
Problem 3
There is only one staff member available to seal supplies in the biosafety cabinet into zip lock bags. Refer to seal the packs aseptically into zip lock bags.
Potential solution
Performing this step can be done with just one person. It would require strategically placing zip lock bags when cold sterilizing them, toward one side of the biosafety cabinet into a mound and allowing the other side to remain relatively dry. With Clidox-S® wet gloves, the autoclaved supply packs can be opened at the sash and the sterile inner parcel gently tossed onto the dry side of the cabinet, discarding the outer package. All the supply packs would be opened and tossed in this way, prior to gowning and stepping into the biosafety cabinet with sterile attire to begin sealing the packs into zip lock bags. This does mean though that space within the biosafety cabinet will be further limiting to allow room to stack the autoclaved supply packs on one side and if there are a lot of packs to zip lock, it may need to be performed over more sessions.
Problem 4
The sealed packs become soaked through with sterilant when entering into an isolator. Refer to enter supply packages into isolators.
Potential solution
Cold sterilant has leaked through the zip lock bags. Care should be taken when handling the sterile packs to avoid puncture. Leaks can be a result of insufficient sealing of the zip lock or there may be tiny holes which are invisible in the seams. If extensive leakage occurs, the pack should be left in the port and discarded. If this is a regular occurrence, an alternative supplier/manufacturer of zip lock bags should be sourced for improved quality.
Problem 5
There is a rising incidence of contamination in the facility that may have come from the supply packs. Refer to, limitations, paragraph 2.
Potential solution
By using a batch numbering system and excellent record keeping, it may be possible to trace contamination to a suspicious batch. This system could be useful where there are many isolators and high throughput of supplies. Batch numbers can include the date of packing/autoclaving and/or expiration dates, even staff initials. Using stamps can make labeling every pack less cumbersome. The batch number can be recorded on an activity log/diary along with the date of supply into specific isolators. Any suspicious batches identified can be immediately discarded from the storeroom and isolators supplied with the same batch can be quarantined for confirmation testing.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Samay Trec (samay.trec@sahmri.com).
Materials availability
This protocol did not generate new materials. All materials used are listed in the key resources table.
Acknowledgments
The work involved in developing this protocol has been funded by the South Australian Health and Medical Research Institute (SAHMRI). We would like to acknowledge the University of Queensland, Translational Research Institute, Gnotobiotic Facility and Walter Eliza Hall Institute, Germ Free Unit for their invaluable guidance and training during the establishment of SAHMRI’s gnotobiotic facility. We’d like to thank the Australian Tecniplast team for their role and support in connecting us to the gnotobiotic community which enabled sharing of knowledge and linking of skills and expertise. We would also like to acknowledge Dr. Miriam Lynn and the Precision Medicine, Computational and System Biology team for their on-going feedback and support in helping us refine our protocols. Lastly, we would like to thank the ComPath diagnostic laboratory for their support and QC testing services.
Author contributions
Procedure development – S.T. and M.D.; Author- S.T.; Graphic creation and review/edit of text- S.T. and M.D.; Review and final edit- C.C.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Samay Trec, Email: samay.trec@sahmri.com.
Mariah De Virgilio, Email: mariah.devirgilio@sahmri.com.
Chris Christou, Email: chris.christou@sahmri.com.
Data and code availability
Quality Control verification data is available from the lead contact upon request.
References
- Vowles C.J., Anderson N.E., Eaton K.A. CRC Press; 2016. Gnotobiotic Mouse Technology; pp. 109–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Quality Control verification data is available from the lead contact upon request.