Before the Omicron era, the neutralizing antibody targeting the SARS-CoV2 Spike protein Sotrovimab has been shown to reduce the risk of COVID-19-related hospitalization in patients who are at high risk for progression (1, 2). We recently showed that early administration of Sotrovimab in Omicron-infected patients with very high-risk for progression was associated with a low rate of COVID-19-related hospitalization within one month after treatment administration (3%), and with no death (1). However, the dominance of the Omicron sublineage BA.2 led health agencies to suspend Sotrovimab emergency use authorizations because of its lower neutralizing ability in vitro compared to BA.1 sublineage (3, 4). Clinical efficiency of Sotrovimab to prevent COVID-19 related complications in high-risk patients with mild-to-moderate COVID-19 Omicron BA.2 remains unknown. Our aim was to compare the clinical and virological outcomes of Omicron BA.1 and BA.2-infected patients with mild-to-moderate COVID-19 who received 500 mg of Sotrovimab IV to prevent COVID-19-related complications.
Our study is based on the ANRS 0003S CoCoPrev study (NCT04885452 (1)), an ongoing multicentric prospective cohort study that includes patients considered to be at high-risk for progression to severe COVID-19, having PCR-proven mild-to-moderate COVID-19 in the first five days of symptoms, and who are treated under an emergency use authorization (EUA) in one of the 32 participating centers. Treatment initiation, based on French Health Authorities recommendation, was left at the treating physician discretion. In this study we have included Omicron-infected patients with either the BA.1 or the BA.2 sublineages that have received 500 mg of Sotrovimab IV. The primary outcome was the proportion of patients with COVID-19-related hospitalization or death within one month of treatment administration. Secondary outcome was the slope of the change over time in the cycle threshold (Ct) value assessed by nasopharyngeal PCR, predictive factors related to the virological response (viral genotypes, emergence of resistant strains), and genotypic and phenotypic characterization of resistance variants (supplementary methods). Mixed effect models were used to estimate the temporal dynamics of the Ct value. Written informed consent was obtained from each patient before enrolment. The protocol has been approved by the "CPP Sud-Est IV" Ethics Committee (Paris, France) and the French Regulatory Authority (ANSM).
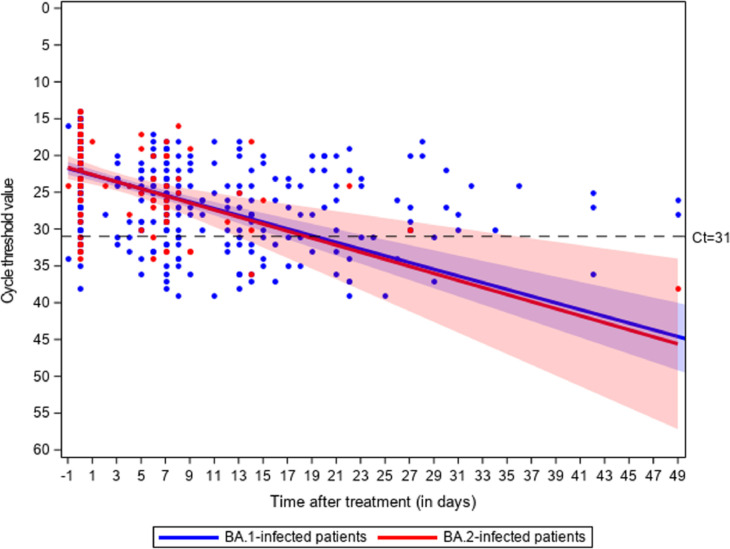
Among 190 consecutive patients who received Sotrovimab a median of 3 days (Q1-Q3 2–4) after first symptoms, 47 (25%) were BA.2-infected, 136 (72%) were immunocompromised, 143 (77%) received ≥ 3 vaccines doses (Table 1 ). There was no significant difference between BA.1 and BA.2 groups with respect to comorbidities and anti-Spike IgG positivity. At the 28th day visit after treatment administration, respectively 3/125 (2.4% - 95% Confidence Interval (CI): 1–7%) and 1/42 (2.4% - 95% CI: 0–13%) BA.1 and BA.2-infected patients were hospitalized due to COVID-19, and none died. All of them were immunocompromised. The slope of Ct values did not differ between groups (p = 0.87, Fig. 1 ). Among the 86 patients who had extended nasopharyngeal virological follow-up due to persistent PCR positivity, 15/68 BA.1-infected patients (22%, 95%CI 13–34%) developed mutations in the Spike protein vs none of the 18 BA.2 infected patients (0%, 95%CI 0–19%, P = 0.033) (Supplementary Table 1). Emergence of these mutations was not associated with baseline characteristics, did not occur among patients who experienced COVID-19-related hospitalization, and did not significantly affect the slope of Ct values (Supplementary Tables 2 and 3 and supplementary figure 1). Plasma collected at day 7 from 60 patients with negative IgG anti-Spike serology at Sotrovimab administration showed a four-fold reduction of neutralizing titers on BA.1 compared to BA.2 (Supplementary Table 4). No major side effects have been reported.
Table 1.
Baseline characteristics of patients and outcomes at the 28th day visit.
| Baseline characteristics | All N = 190 | BA.1-infected patients N = 143 (75%) | BA.2-infected patients N = 47 (25%) | p-value |
|---|---|---|---|---|
| Median age (years, Q1-Q3)* | 59 (45–70) | 59 (44–70) | 55 (50–72) | 0.75 |
| ≥ 80 years old (%) | 17 (9) | 14 (10) | 3 (6) | 0.57 |
| Median BMI (Q1-Q3)* | 25 (22–29) | 24 (22–29) | 26 (22–30) | 0.42 |
| Male sex (%)⁎⁎ | 98 (52) | 71 (50) | 27 (57) | 0.38 |
| Immunocompromised patients (%), including: | 136 (72) | 101 (71) | 35 (75) | 0.61 |
| Solid organ transplantation | 55 (40) | 39 (39) | 16 (46) | 0.46 |
| Immunosuppressive therapy including rituximab | 53 (39) | 44 (44) | 9 (26) | 0.06 |
| Ongoing chemotherapy | 29 (21) | 20 (20) | 9 (26) | 0.46 |
| Corticosteroids >10 mg/day for > 2 weeks | 13 (10) | 10 (10) | 3 (9) | 1 |
| Allogeneic hematopoietic stem cell transplantation | 7 (5) | 6 (6) | 1 (3) | 0.68 |
| Systemic lupus or vasculitis with immunosuppressive medications | 7 (5) | 5 (5) | 2 (6) | 1 |
| Cancer | 3 (2) | 1 (1) | 2 (6) | 0.16 |
| Other risk factors for severe COVID-19 (%), including: | 98 (52) | 75 (53%) | 23 (49%) | 0.68 |
| Diabetes (type 1 and type 2) | 30 (31) | 22 (29) | 8 (35) | 0.62 |
| High blood pressure | 28 (29) | 21 (28) | 7 (30) | 0.82 |
| Obesity BMI>30 | 25 (26) | 19 (25) | 6 (26) | 0.94 |
| Other chronic pathologies | 25 (26) | 20 (27) | 5 (22) | 0.64 |
| Chronic kidney disease | 20 (20) | 16 (21) | 4 (17) | 0.78 |
| Congestive heart failure | 7 (7) | 7 (9) | 0 | 0.19 |
| COPD and chronic respiratory failure | 6 (6) | 3 (4) | 3 (13) | 0.14 |
| Having received ≥ 3 doses of vaccine (%)⁎⁎⁎ | 143 (77) | 102 (73) | 41 (89) | 0.08 |
| Positive IgG anti-Spike serology at d0 (%)⁎⁎⁎⁎ | 118 (63) | 85 (61) | 33 (70) | 0.26 |
| Median IgG anti-spike level at d0 (BAU/mL, Q1-Q3) | 531 (120–2383) | 807 (126–2500) | 395 (91–1574) | 0.26 |
| Day 28 outcome (% of patients with available data) | 167/190 (88) | 125/143 (87) | 42/47 (89) | |
| COVID-19–related hospitalization at d28 (%) | 4 (2) | 3 (2) | 1 (2) | 1 |
| COVID-19-related death (%) | 0 | 0 | 0 |
Age and BMI were missing in 6 BA.1-infected patients and 3 BA.2-infected patients.
Sex was missing in 1 BA.1-infected patients.
Vaccination status was missing in 4 BA.1-infected patients and 1 BA.2-infected patients.
IgG anti-Spike serology was missing in 4 BA.1-infected patients.
Fig. 1.
Change in Ct value of gene N in 143 BA.1 and 47 BA.2-infected patients treated with Sotrovimab. The p-value for the slope difference is 0.87.
In this prospective real-life cohort study that included mostly severely immunocompromised patients, administration of Sotrovimab in BA.2-infected patients was associated with a similarly low rate of COVID-19-related hospitalization, and decline of the nasopharyngeal viral load, as in BA.1-infected patients.
Our results suggest that, although the neutralizing power of patients' sera seven days after administration of 500 mg of Sotrovimab against the Omicron sublineage BA.2 is reduced in vitro compared to BA.1, Sotrovimab may still be valuable in preventing COVID-19 progression in BA.2-infected patients. A recent modelization study suggested that for current monoclonal antibody regimens, doses between 7- and >1000-fold lower than currently used could still achieve around 90% of the current effectiveness (depending on the variant) (5). In that regard, the dose administered of Sotrovimab might have potentially overcome its decreased neutralizing activity on the BA.2 sublineage. The fragment crystallizable (Fc) of some monoclonal antibodies targeting the SARS-CoV2 Spike protein, such as Casirivimab and Imdevimab, is engineered in order to reduce Fc-dependent activation of immune effector cells and of the complement system, limiting the theoretical risk of antibody dependent-enhancement (6). On the contrary, although modified to enhance its half-life, the preserved ability of Sotrovimab to recruit and engage Fcγ receptor–bearing cells and complement system activator C1q may participate through Antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) to the antiviral effect of Sotrovimab in vivo.
Targeting a single epitope, and used as a monotherapy, the risk of developing resistance mutations of SARS-CoV-2 in Sotrovimab-treated patients is of major concern. Mutations in the spike protein at positions 337 or 340 were shown in patients infected with the Delta (7) and the Omicron lineages (8). A recent report demonstrated across a routine genomic surveillance that these mutations occurred in 24 (0.13%) of 18,882 omicron BA.1 lineages and in one (0.02%) of 4025 omicron BA.2 lineages, affecting mostly immunocompromised patients with persistent SARS-CoV-2 excretion (9). In this work both mutations occurred in a sizeable proportion of BA.1-infected patients but did not occur among patients who experienced COVID-19-related hospitalization, and did not significantly affect the slope of Ct values. None of them were demonstrated among BA.2-infected patients.
Although our work is limited by the relatively small number of BA.2-infected patients, Sotrovimab was associated with a low incidence of COVID-19 related hospitalization or death among very high-risk patients with mild-to-moderate COVID-19 related to the BA.2 sublineage, and with no emergence of mutations.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
Acknowledgments
COCOPREV Study Group collaborators: We thank Pr Yazdan Yazdanpanah and all the ANRS-MIE team for their invaluable support and help.
This study would have not been possible without the teams involved in the COCOPREV Study and designated as the COCOPREV Study Group: Magali Garcia, Valentin Giraud, Agathe Metais, France Cazenave-Roblot, Jean-Philippe Martellosio (CHU de Poitiers) ; Anne-Marie Ronchetti, Thomas Gabas, Naima Hadjadj, Célia Salanoubat, Amélie Chabrol, Pierre Housset, Agathe Pardon, Anne-Laure Faucon, Valérie Caudwell, Latifa Hanafi (CHU Sud Francilien, Corbeil-Essonne) ; Laurent Alric, Grégory Pugnet, Morgane Mourguet, Eva Bories, Delphine Bonnet, Sandrine Charpentier, Pierre Delobel, Alexa Debard, Colleen Beck, Xavier Boumaza, Stella Rousset, Aurore Perrot (CHU de Toulouse) ; Fanny Lanternier, Claire Delage, Elisabete Gomes Pires, Morgane Cheminant, Nathalie Chavarot (Hôpital Necker, Paris) ; Anthony Chauvin, Xavier Eyer ; Véronique Delcey (Hôpital Lariboisière, Paris) ; Simon Bessis, Romain Gueneau (Hôpital du Kremlin Bicêtre) ; Pelagie Thibaut, Marine Nadal, Martin Siguier, Marwa Bachir, Christia Palacios (Hôpital Tenon, Paris) ; Valérie Pourcher, Cléa Melenotte, Antoine Faycal, Vincent Berot, Cécile Brin, Siham Djebara, Karen Zafilaza, Stéphane Marot, Sophie Sayon, Valentin Leducq, Isabelle Malet, Elisa Teyssou, Adélie Gothland (Hôpital de la Pitié Salpétrière, Paris) ; Karine Lacombe, Yasmine Abi Aad, Thibault Chiarabini, Raynald Feliho, Nadia Valin, Fabien Brigant, Julien Boize, Pierre-Clément Thiébaud, Marie Moreau, Charlotte Billard (Hôpital St Antoine, Paris), Nathalie De Castro, Geoffroy Liégeon, Blandine Denis, Jean-Michel Molina, Lucia Etheve (Hôpital Saint Louis, Paris) ; André Cabié, Sylvie Abel, Ornella Cabras, Karine Guitteaud, Sandrine Pierre-François (CHU de Martinique) ; Vincent Dubee, Diama Ndiaye, Jonathan Pehlivan, Michael Phelippeau, Rafael Mahieu (CHU d'Angers) ; Alexandre Duvignaud, Thierry Piston, Arnaud Desclaux, Didier Neau, Charles Cazanave (CHU de Bordeaux) ; Jean-François Faucher, Benjamin Festou, Magali Dupuy-Grasset, Véronique Loustaud-Ratti, Delphine Chainier (CHU de Limoges) ; Nathan Peiffer-Smadja, Christophe Choquet, Olivia Da Conceicao, Michael Thy, Lio Collas, Cindy Godard, Donia Bouzid, Vittiaroat Ing, Laurent Pereira, Thomas Pavlowsky, Camille Ravaut (Hôpital Bichat, Paris) ; Antoine Asquier-Khati, David Boutoille, Marie Chauveau, Colin Deschanvres, François Raffi (CHU de Nantes) ; Audrey Le Bot, Marine Cailleaux, François Benezit, Anne Maillard, Benoit Hue, Pierre Tattevin (CHU de Rennes) ; François Coustilleres, Claudia Carvalho-Schneider, Simon Jamard, Laetitia Petit, Karl Stefic (CHU de Tours) ; Natacha Mrozek, Clement Theis, Magali Vidal, Leo Sauvat, Delphine Martineau (CHU de Clermond-Ferrand) ; Benjamin Lefèvre, Guillaume Baronnet, Agnès Didier (CHRU de Nancy) ; Florence Ader, Thomas Perpoint, Anne Conrad, Paul Chabert, Pierre Chauvelot (CHU de Lyon) ; Aurélie Martin, Paul Loubet, Julien Mazet, Romaric Larcher, Didier Laureillard (CHU de Nîmes) ; Mathilde Devaux (Hôpital de Poissy) ; Jérôme Frey, Amos Woerlen, Aline Remillon, Laure Absensur-Vuillaume, Pauline Bouquet (CHU de Metz) ; Albert Trinh-Duc, Patrick Rispal (Hôpital d'Agen) ; Philippe Petua, Julien Carillo (Hôpital de Tarbes) ; Aurore Perrot, Karen Delavigne, Pierre Cougoul, Jérémie Dion, Odile Rauzy (Oncopole, Toulouse), Mathieu Blot, Thibault Sixt, Florian Moretto, Carole Charles, Lionel Piroth (CHU de Dijon) ; Sophie Circosta, Lydia Leger, Arulvani Arulananthan, Carine Lascoux, Pascaline Valérie, Léia Becam (Team Biobanque ANRS-INSERM US19,Villejuif) ; Yazdan Yazdanpanah, Ventzislava Petrov-Sanchez, Alpha Diallo, Soizic Le Mestre, Guillaume Le Meut (ANRS-MIE) ; Isabelle Goderel, Frédéric Chau, Brahim Soltana, Jessica Chane Tang (IPLESP), Jeremie Guedj (Université de Paris, IAME, INSERM, Paris), Yvanie Caille (Renaloo)
Contributions
GM (guarantor) was involved in the study conception, data extraction, data analysis, interpretation of results and drafting the manuscript. AGM was involved in the study conception, data extraction, data analysis, interpretation of results and drafting the manuscript. CS was involved in the study conception, data extraction, data analysis, interpretation of results and drafting the manuscript. SK was involved in data extraction, data analysis, interpretation of results and drafting the manuscript. CLN was involved in data extraction, data analysis, interpretation of results and drafting the manuscript. CD was involved in the study conception, data extraction, data analysis, interpretation of results and revising the manuscript. LN was involved in the study conception, data extraction, data analysis, interpretation of results and revising the manuscript. AB was involved in the study conception, data extraction, data analysis, interpretation of results and revising the manuscript. CM was involved in patients inclusion and revising the manuscript. AC was involved in patients inclusion and revising the manuscript. CC was involved in patients inclusion and revising the manuscript. FC was involved in patients inclusion and revising the manuscript. JPM was involved in patients inclusion and revising the manuscript. GG was involved in patients inclusion and revising the manuscript. ATD was involved in patients inclusion and revising the manuscript. AMR was involved in patients inclusion and revising the manuscript. VPM was involved in patients inclusion and revising the manuscript. FR was involved in patients inclusion and revising the manuscript. KL was involved in patients inclusion and revising the manuscript. NPS was involved in patients inclusion and revising the manuscript. pH was involved in patients inclusion and revising the manuscript. AP was involved in patients inclusion and revising the manuscript. GP was involved in patients inclusion and revising the manuscript. AM was involved in patients inclusion and revising the manuscript. VD was involved in patients inclusion and revising the manuscript. MD was involved in patients inclusion and revising the manuscript. JF was involved in patients inclusion and revising the manuscript. CC was involved in patients inclusion and revising the manuscript. RL was involved in the study conception, interpretation of results and revising the manuscript. FC was involved in the study conception, data extraction, data analysis, interpretation of results and drafting the manuscript. YY. (guarantor) was involved in the study conception, data extraction, data analysis, interpretation of results and drafting the manuscript.
Funding
The ANRS0003S COCOPREV cohort is conducted with the support of ANRS│MIE and funded by French ministries: Ministère des Solidarités et de la Santé and Ministère de l'Enseignement Supérieur, de la Recherche et de l'Innovation»
Ethical approval
This study received the ethical approval of the Comité de Protection des Personnes SUD-EST IV.
Transparency declaration
GM and YY (the manuscript's guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing
Data available upon request for academic researchers.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.06.033.
Appendix. Supplementary materials
References
- 1.Martin-Blondel G., Marcelin A.G., Soulie C., Kaisaridi S., Lusivika-Nzinga C., Dorival C., et al. Outcome of very high-risk patients treated by Sotrovimab for mild-to-moderate COVID-19 Omicron, a prospective cohort study (the ANRS 0003S COCOPREV study) J Infect. 2022 Apr 7 doi: 10.1016/j.jinf.2022.04.010. PubMed PMID: 35398409. Pubmed Central PMCID: PMC8988484. Epub 2022/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Rodrigues Falci D., et al. Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: a Randomized Clinical Trial. JAMA. 2022 Apr 5;327(13):1236–1246. doi: 10.1001/jama.2022.2832. PubMed PMID: 35285853. Pubmed Central PMCID: PMC8922199. Epub 2022/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruel T., Hadjadj J., Maes P., Planas D., Seve A., Staropoli I., et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022 Mar 23 doi: 10.1038/s41591-022-01792-5. PubMed PMID: 35322239. Epub 2022/03/25. [DOI] [PubMed] [Google Scholar]
- 4.Touret F., Baronti C., Bouzidi H.S., de Lamballerie X. In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate. Sci Rep. 2022 Mar 18;12(1):4683. doi: 10.1038/s41598-022-08559-5. PubMed PMID: 35304531. Pubmed Central PMCID: PMC8931583. Epub 2022/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadler ELC K., Schlub T., Cromer D., Polizzotto M., Kent S., Skoetz N., Estcourt L., McQuilten Z., Wood E., Khoury D., Davenport M. Determinants of passive antibody effectiveness in SARS-CoV-2 infection. medRxiv. 2022 [Google Scholar]
- 6.Crowe J.E., Jr Human Antibodies for Viral Infections. Annu Rev Immunol. 2022 Apr 26;40:349–386. doi: 10.1146/annurev-immunol-042718-041309. PubMed PMID: 35113730. Epub 2022/02/04. [DOI] [PubMed] [Google Scholar]
- 7.Rockett R., Basile K., Maddocks S., Fong W., Agius J.E., Johnson-Mackinnon J., et al. Resistance Mutations in SARS-CoV-2 Delta Variant after Sotrovimab Use. N Engl J Med. 2022 Apr 14;386(15):1477–1479. doi: 10.1056/NEJMc2120219. PubMed PMID: 35263515. Pubmed Central PMCID: PMC8929376. Epub 2022/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vellas C., Tremeaux P., Del Bello A., Latour J., Jeanne N., Ranger N., et al. Resistance mutations in SARS-CoV-2 omicron variant in patients treated with sotrovimab. Clin Microbiol Infect. 2022 May 17 doi: 10.1016/j.cmi.2022.05.002. PubMed PMID: 35595125. Pubmed Central PMCID: PMC9112603. Epub 2022/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Destras G., Bal A., Simon B., Lina B., Josset L. Sotrovimab drives SARS-CoV-2 omicron variant evolution in immunocompromised patients. Lancet Microbe. 2022 May 27 doi: 10.1016/S2666-5247(22)00120-3. PubMed PMID: 35636438. Epub 2022/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.