Abstract
The prevalence of urolithiasis in humans is increasing worldwide; however, nonsurgical treatment and prevention options remain limited despite decades of investigation. Most existing laboratory animal models for urolithiasis rely on highly artificial methods of stone induction and, as a result, might not be fully applicable to the study of natural stone initiation and growth. Animal models that naturally and spontaneously form uroliths are an underused resource in the study of human stone disease and offer many potential opportunities for improving insight into stone pathogenesis. These models include domestic dogs and cats, as well as a variety of other captive and wild species, such as otters, dolphins, and ferrets, that form calcium oxalate, struvite, uric acid, cystine, and other stone types. Improved collaboration between urologists, basic scientists, and veterinarians is warranted to further our understanding of how stones form and to consider possible new preventative and therapeutic treatment options.
Introduction
Urolithiasis is a debilitating and painful disease that affects an increasing proportion of the global population. Prevalence rates range from 7–14% in North America, 5–9% in Europe, and 1–5% in Asia1. The proportion of Americans affected by stone disease has more than doubled over the past 40 years, a rise in prevalence that has also been observed in other countries around the globe2,3. The increase is thought to be due to calculogenic changes in diet and altered lifestyle factors, such as decreased physical activity2. Recurrences occur at an estimated rate of 10–23% per year, with men having up to 1.5 times the recurrence rates of women4. The increasing prevalence and high recurrence rate of urinary stones make the study of the pathogenesis, treatment, and prevention of urolithiasis a priority for the health-care community.
In the past, induced animal models have been used to investigate the pathological process of stone formation. However, many of the methods to study stones in such models rely on artificial mechanisms that are not comparable to the pathophysiology of naturally occurring stone disease in humans. For example, induction of a hyperoxaluric state in rodents via ingestion of ethylene glycol and vitamin D3 causes intratubular calcium oxalate crystallization and — in the setting of renal injury — can lead to discrete crystal formation5,6. However, in natural human stone formation, no evidence exists to show that stones form secondary to oxalate-induced renal injury7,8.
An animal model of naturally occurring stones would be preferable for both scientific and ethical reasons; however, to date, few comprehensive articles have described the homology of natural stone formation between humans and animals5,9-11. The study of stone disease in companion animals has particular benefits for modelling human disease. For example, animals living with humans can serve as sentinel species to detect environmental hazards and aid in the identification of lifestyle factors affecting stone risk. Additional benefits of companion animals include a larger size than common laboratory animals and heterogeneous genetics that might mimic the variable presentations of stone disease seen in humans.
This Review will describe the characteristics and treatment of spontaneous stone disease in a variety of animal species and discuss the potential of each species as a model for different stone types in humans. Although humans form many types of urinary tract stones, this Review will focus on the four most common types: calcium, struvite, uric acid and cystine. Calcium oxalate (CaOx) and struvite are the predominant stone types in companion dogs and cats; these species also naturally form uric acid, cystine and other rare hereditary and drug-induced stone types12. Stones from other animal species comprise <1% of uroliths submitted for compositional analysis13, and only a select subset of species are discussed, which have been selected based on a strong predisposition for one of the top four stone types and their potential role for use in translational research.
Calcium stones
Calcium urolithiasis is a common and complex disease in humans and companion animal species (Figs 1,2). Inherited and environmental factors contribute to risk, and the aetiology is incompletely understood.
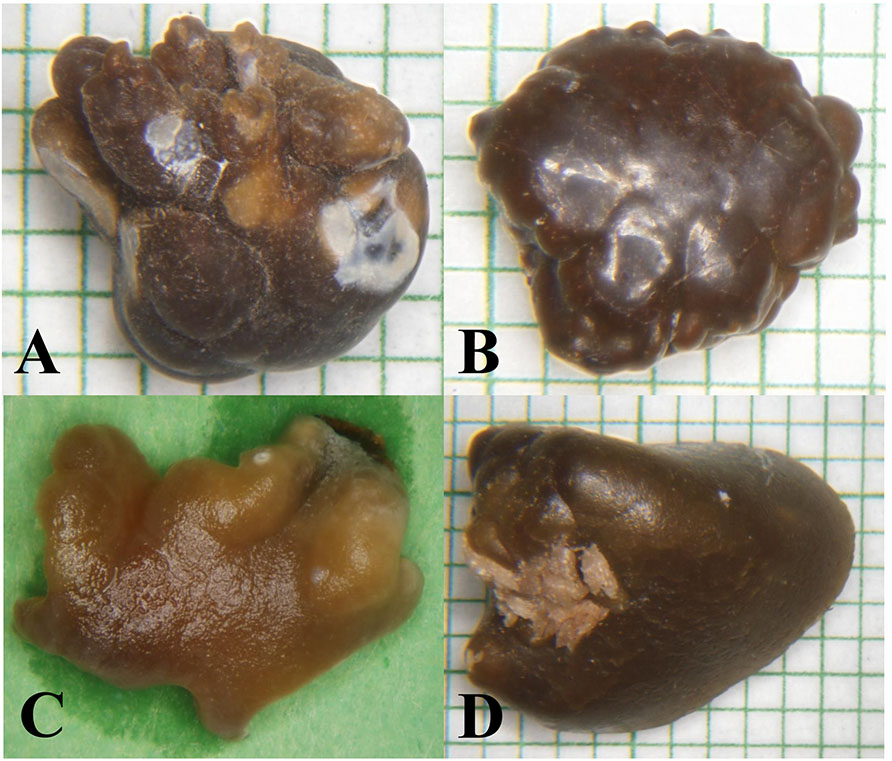
Figure 1 ∣.
Similar morphological appearance of naturally-occuring calcium oxalate uroliths from four different species: (A) human; (B) dog; (C) cat; (D) otter.
Figure 2 ∣.
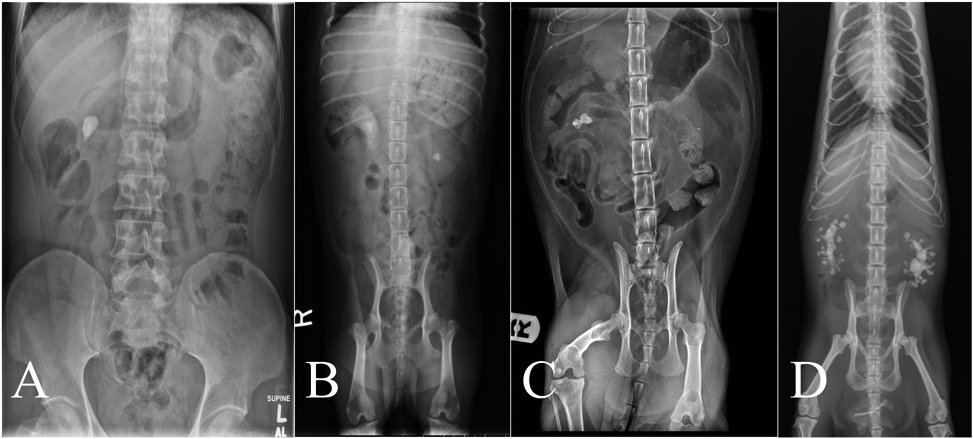
X-ray images of naturally-occuring calcium oxalate nephrolithisis in four different species: (A) human; (B) cat; (C) dog; (D) Asian small-clawed otter.
Humans
The vast majority of stones found in humans are calcium based. A review of >90,000 unselected stone analyses found that CaOx accounts for 61% of all stones, whereas 15% are basic calcium phosphate (for example, hydroxyapatite) and 3% are brushite14. The proportion of CaOx stones in first-time stone formers might be even greater, accounting for 76–83% of stones15,16. These statistics reflect the dominant stone type, but 59% of stones contain a mixture of mineral compositions.
Strong evidence suggests that genetics play a role in calcium-based kidney stone risk. Kidney stones are 2-3 times more likely to form in individuals with a positive family history, and heritability estimates for stone disease are 46–63%17-21. Monogenic disorders have been detected in 17–29% of young patients with calcium kidney stones or nephrocalcinosis diagnosed before the age of 25 years22-24. By contrast, most adult stones cases are thought to be polygenic; genome-wide association studies have discovered >20 genes with low-to-moderate effect sizes on stone risk, including ALPL, CLDN14, CYP24A1, SLC34A1, TRPV5, UMOD, and several genes predicted to influence signaling through the extracellular calcium receptor, CaSR25-30. Other undiscovered susceptibility genes of similar or greater effect are likely to exist. Additional risk factors in human calcium stones include older age, male sex, obesity, low fluid intake, and high-sodium diet2.
Hypercalciuria is the most common metabolic abnormality seen in calcium-based stone formers, occurring in 35–65% of patients31. Hypercalciuria can be associated with CaOx as well as calcium phosphate stones and often occurs in the presence of normal blood calcium concentrations, when it is termed ‘idiopathic hypercalciuria’. Idiopathic hypercalciuria can arise owing to increased intestinal calcium absorption, increased bone turnover, decreased renal calcium reabsorption, or a combination of these factors32. As with stone risk, idiopathic hypercalciuria has a strong inherited component, with heritability estimates of 40–50%33,34. Many of the genes implicated in monogenic and complex kidney stone disease have a role in regulating intestinal absorption or renal resorption of calcium and variants in these genes can, therefore, contribute to hypercalciuria35,36. Mutations in CYP24A1 were identified in 35% of a predominantly paediatric population with hypercalcemia of non-parathyroid aetiology; in this study, 19 of 20 patients with biallelic mutations had nephrocalcinosis or kidney stones37. CYP24A1 deficiency causes hypercalcemia and/or hypercalciuria owing to decreased inactivation of vitamin D metabolites37. Altered vitamin D inactivation has been reported in first-time calcium kidney stone formers, and genome-wide association studies have implicated CYP24A1 in kidney-stone risk and regulation of serum calcium and 25-hydroxyvitamin D concentrations29,38-41. Medical disorders that cause hypercalcemia and hypercalciuria are less common than idiopathic hypercalciuria, and include primary hyperparathyroidism, malignant hypercalcemia, sarcoidosis, and hyperthyroidism31.
Hypocitraturia is the second most common metabolic abnormality in calcium-based stone formers, occurring in 30–50% of patients42-45. Urine citrate reduces calcium oxalate crystallization by binding to calcium and forming soluble complexes46. Many mechanisms can contribute to hypocitraturia, including diet, acid-base balance, gastrointestinal malabsorption, genetic factors, and drugs47. In recurrent calcium-based stone formers, low urine potassium is the strongest predictor of hypocitraturia45.
Hyperoxaluria is another important risk factor specific to CaOx stone formation. Primary hyperoxaluria is due to an inherited enzyme deficiency caused by inactivating variants in AGXT, GRHPR, or HOGA148. Mutations in an oxalate transporter gene, SLC26A1, are also reported as a cause of CaOx nephrolithiasis in children49. Secondary hyperoxaluria is caused by increased dietary ingestion of oxalate or its precursors, as well as alterations in intestinal microflora48. Urinary excretion of oxalate is increased when calcium intake is low, owing to decreased CaOx complex formation in the gastrointestinal tract50. Thus, any medical condition that decreases availability of calcium for binding with oxalate in the intestinal tract is a risk factor for stone formation; such conditions include prior small bowel resection, gastric bypass surgery, and inflammatory bowel disease48. Decreasing dietary oxalate decreases oxalate levels in the urine and is, therefore, protective against CaOx stone formation.
Oxalobacter formigenes is a Gram negative, anaerobic bacterium that metabolizes oxalate in the intestinal tract. An estimated 30–40% of people in the USA are colonized with O. formigenes, but the prevalence is thought to be reduced (15–20%) in stone formers51. People who are not colonized with O. formigenes are 70% more likely to develop a kidney stone51. O. formigenes colonization is thought to decrease CaOx stone risk via two mechanisms: metabolism of gut oxalate in a calcium-dependent manner and promotion of oxalate secretion into the intestinal tract51-53. Growing evidence also suggests that other bacterial taxa have a role in oxalate metabolism and have differential abundance and metabolite profiles in the gut and urine of patients with kidney stones relative to healthy individuals54-57. Furthermore, antibiotic therapy might drive some of these alterations56.
In conjunction with supersaturation of the urine with calcium salts, the presence of a nidus is thought to be of critical importance in the pathophysiology of CaOx stone formation. Randall’s plaques were first discovered in the 1930s and were described as collections of subepithelial calcium phosphate deposits that serve as a nidus for calcium oxalate crystallization and stone formation58. Randall’s plaques preferentially form in the basement membranes of the ascending thin limbs of the loop of Henle, however relatively little is known about their precise mechanism of formation8,59. The relative supersaturation of calcium phosphate found in the urine of idiopathic calcium stone formers is thought to be a major mechanism of Randall’s plaque formation; another theory is that enhanced delivery of calcium out of the proximal tubule increases resorption of calcium in the thick ascending limb, where the calcium enters the interstitium and is transported to the to the deep medulla via the descending vasa recta59. The aetiology of calcium phosphate stone formation is less clearly linked to a nidus than CaOx stone formation but has been hypothesized to be associated with plugging of Bellini ducts and inner medullary collecting ducts with crystalline deposits.60 Such pathology has frequently been identified in renal biopsies from patients with kidney stones and systemic diseases such as primary hyperparathyroidism and renal tubular acidosis61,62.
Prevention and treatment of calcium stones in humans is multifaceted and includes dietary and pharmaceutical interventions and minimally invasive surgery63. Calcium stones cannot be managed with medical dissolution and, therefore, they require surgery if they are symptomatic and not amenable to passage64. Treatment for calcium stones is typically shock wave lithotripsy and subsequent passage, or with endoscopic lithotripsy through the urinary tract via either a retrograde or percutaneous antegrade approach63. Preventative measures are aimed at increasing urine volume to >2.5 liters daily and limiting intake of oxalate-rich foods, such as spinach, nuts, and chocolate, which increase urinary oxalate and stone risk and should therefore avoided50, as well as limiting dietary calcium and sodium65,66. Consumption of a normal amount of calcium (1000–1200 mg/day) is recommended, as decreasing calcium intake will increase urinary oxalate concentration50. Potassium citrate and thiazide diuretics are the most commonly prescribed medications to treat hypercalciuria, and are recommended for patients with high urine calcium and recurrent calcium stones65,66. Potassium citrate increases urinary citrate levels and binds to calcium in the urine, preventing CaOx complex formation. Thiazide diuretics decrease renal excretion of calcium and have been hypothesized to help minimize formation of Randall’s plaques in addition to improving bone mineral balance67.
Dogs
Calcium oxalate is one of the most commonly reported stone compositions in dogs. A review of >350,000 stone analyses in dogs found that 38% overall were composed of CaOx, and the proportion of CaOx stones in dogs has been increasing over the past 30 years; in 1981, only 5% of canine uroliths were composed of CaOx, rising to 41% in 2007 (Table 1)12. This increase might be due in part to dietary changes aimed at reducing the incidence of struvite stones in dogs, which have inadvertantly promoted hypercalciuria, hypocitraturia, and aciduria68. As is the case in humans, CaOx stone risk in dogs has a strong inherited component (Table 1). This heritability is illustrated by striking breed predispositions to stones; for example, the Bichon Frise, Miniature Schnauzer, and Shih Tzu have 10–24 times the risk of mixed-breed dogs68,69. This observation signifies familial aggregation of disease and supports the presence of major genetic risk factors, although causal variants have not yet been reported. Additional risk factors for CaOx stone formation in dogs mirror those seen in humans and include older age, male sex, and obesity (Table 1)68-71. Dietary risk factors are not well established in dogs. Retrospective studies have found that dry and canned formulations with low amounts of protein, sodium, and calcium were associated with increased stone risk72,73. By contrast, a prospective study found that feeding a low protein, sodium, and calcium diet reduced urine calcium and oxalate excretion in dogs with calcium oxalate stones74. Other calcium based stones account for <1% of canine stones12. Although stones composed predominently of calcium phosphate are rare in dogs, hydroxyapatite is often a minor component of mixed stones, detected in 38% of stones overall68.
Table 1 ∣.
Characteristics of naturally occurring calcium oxalate urolithiasis in different species.
| HUMANS | DOGS | CATS | OTTERS | |
|---|---|---|---|---|
| Renal anatomy | Multipapillary | Unipapillary and multipyramidal | Unipapillary | Reniculated |
| Stone characteristics | − 61% CaOx14 • 15% hydroxyapatite • 11% uric acid • 8% struvite • 3% brushite • 2% cystine |
− 41% CaOx12 • 39% struvite • 5% uric acid • 9% compound • 3% mixed • 1% cystine • 2% other |
− 41% CaOx12 • 49% struvite • 5% uric acid • 3% compound • 2 % other |
− 56% CaOx13 • 27% compound • 8% uric acid • 6% mixed • 2% calcium carbonate • 2% struvite |
| Genetics | Evidence of heritability17-19 and primary hyperoxaluria48 | Evidence of heritability68,69 and primary hyperoxaluria83,84,88 | Evidence of heritability91,92 and primary hyperoxaluria103 | Unknown |
| Metabolic abnormalities | Hypercalciuria - yes31,32 Hyperoxaluria - yes48 Hypocitraturia - yes42-45 Reduced 24-hydroxylation of vitamin D metabolites - yes38 Increased bone turnover - yes32 Obesity - yes2 |
Hypercalciuria - yes75-79 Hyperoxaluria - yes, rare83,84,88 Hypocitraturia - no76,78 Reduced 24-hydroxylation of vitamin D metabolites - yes81,238 Increased bone turnover - No – limited data80 Obesity - yes69,71 |
Hypercalciuria - yes75,99,100 Hyperoxaluria - yes, rare103,104 Hypocitraturia - Unknown Reduced 24-hydroxylation of vitamin D metabolites - Unknown Increased bone turnover - Unknown Obesity - Unknown |
Hypercalciuria - Unknown Hyperoxaluria - yes113 Hypocitraturia - Unknown Reduced 24-hydroxylation of vitamin D metabolites - Unknown Increased bone turnover - Unknown Obesity - Unknown |
| Microbiome | Lack of oxalobacter51-53 | Lack of oxalobacter87 | Unknown | Unknown |
| Pathology | Interstitial (Randall’s type) papillary plaque - yes6,58,59 Bellini duct plugs - yes60 |
Unknown | Interstitial (Randall’s type) papillary plaque - Yes Bellini duct plugs - unknown |
Unknown |
| Treatment and prevention | Increased hydration - yes63-67 Decreased oxalate consumption - yes50,63-67 Moderate calcium consumption - yes63-67 Reduced sodium consumption - yes63-67 Thiazides - yes63-67 Potassium citrate - yes63-67 |
Increased hydration - yes89 Decreased oxalate consumption - unknown Moderate calcium consumption - yes90 Reduced sodium consumption - unknown Thiazides - yes74,89 Potassium citrate - yes89 |
Increased hydration - yes89 Decreased oxalate consumption - unknown Moderate calcium consumption - unknown Reduced sodium consumption - unknown Thiazides – yes89 Potassium citrate - yes89 |
Increased hydration - Unknown Decreased oxalate consumption - unknown Moderate calcium consumption - unknown Reduced sodium consumption Thiazides - Unknown Potassium citrate - Unknown |
Paralleling human disease, idiopathic hypercalciuria is the most commonly identified urinary abnormality in dogs prone to forming CaOx stones (Table 1)75-79. The precise mechanisms of hypercalciuria in normocalcaemic dogs are unknown, although they might be similar to the absorption and excretion dysregulation mechanisms seen in humans. A bone resorption phenotype has not been identified in dogs; in fact, bone turnover in dogs with hypercalciuria and CaOx urolithiasis might be reduced80. A subset of dogs with hypercalciuria and CaOx stones have abnormalities in vitamin D metabolism with evidence for decreased 24-hydroxylation (deactivation) of calcitriol, as has also been reported in humans (Table 1)81. Hyperoxaluria seems to have a lesser role than hypercalciuria does in dogs; stone-forming dogs have generally been found to have either similar or lower urinary oxalate excretion as compared to controls77,78,82. Primary hyperoxaluria is seen rarely in specific dog breeds (Table 1)83,84. Urinary citrate excretion does not differ between healthy dogs and those with CaOx urolithiasis76,78,85. Primary hyperparathyroidism is present in <5% of dogs with CaOx stones, which is also associated with formation of hydroxyapatite stones77,86.
Also reflecting its presence in humans, enteric colonization with O. formigenes is more common in healthy stone-free dogs than in stone formers and could have a protective role in stone disease (Table 1)87.
Histopathological descriptions of CaOx nephrolithiasis in dogs are very limited and published data include only small case reports. Dogs with primary hyperoxaluria have been reported to have tubular necrosis with extensive deposition of oxalate crystals, but often without evidence of stone formation84,88. Further histopathological evidence detailing the formation of idiopathic CaOx nephrolithiasis is not available to date and remains an active area of research.
Treatment and prevention recommendations for CaOx urolithiasis in dogs are very similar to those for humans (Table 1). Guidelines focus on prevention of CaOx stone formation through dietary measures89. Dietary calcium restriction without concurrent oxalate restriction leads to an increase in CaOx crystallization, similar to that which is observed in humans90. Recommendations for CaOx stone prevention in dogs include a high-moisture diet and urine alkalinization, and — as in humans — potassium citrate and thiazide diuretics can be considered for dogs with persistent stone recurrence despite dietary modifications. Only renal stones large enough to cause recurrent infection, pain, or compression of the renal parenchyma should be considered for surgical removal. Ureteral stenting and stone destruction via extracorporeal shockwave lithotripsy or laser lithotripsy is recommended for obstructing ureteral stones and bladder stones associated with clinical disease89. Bladder stones are generally treated with cystotomy; however, minimally invasive techniques are recommended when possible89.
Cats
Similar to trends seen in domestic dogs, CaOx is one of the most common stone compositions observed in domestic cats (46% of nearly 100,000 feline stone analyses); the proportion of CaOx stones increased from 2% in 1981 to a peak of >60% in the 1990s to 41% in 2007 (Table 1)12. This increase in prevalence is again attributed to an increasingly acidic diet low in magnesium, which has been formulated to minimize struvite crystalluria; the decline over the past two decades might be due to additional reformation to minimize risk for calcium oxalate uroliths. Several feline breeds are particularly predisposed to CaOx stone formation, including Persian, Himalayan, Ragdoll, and Burmese cats (Table 1)91,92. Other risk factors in cats include age >10 years, male sex (even more so with neutered status), dehydration with associated low urine output, and aciduria91,93. Azotemia is seen at presentation of >80% of cats diagnosed with ureteroliths antemortem94, in 25–40% of cases, this is attributed to bilateral obstruction94,95. In cats with unilateral obstruction, the contralateral kidney is often small, suggesting chronic kidney disease. Chronic kidney disease is common in cats, and prevalence estimates range from 35% to 81% in geriatric feline populations and 56% of cats with uroliths in general (including all stone types and locations in the urinary tract)96,97. A positive association between nephrolithiasis and chronic kidney disease in cats has been reported; however, the directionality of this association has not been established96,97. Comparable to dogs, other calcium-based stones are rare, comprising <0.5% of feline stones12. Hydroxyapatite is less often a minor component of mixed stones in cats than in dogs and is only detected in 6% of all feline stones98.
Data regarding underlying metabolic disturbances in cats with CaOx stones are limited, but hypercalciuria has been documented (Table 1)75,99,100. Idiopathic hypercalcaemia was noted in one-third of cats with CaOx urolithiasis in a small study, but the origin of the hypercalcemia was not determined99. Overall, idiopathic hypercalcemia is uncommon in the general cat population with a prevalence estimated at 0.4%101,102. Hyperoxaluria can be seen in the setting of vitamin B6 deficiency; primary hyperoxaluria has been reported but is rare (Table 1)103,104. Current knowledge regarding the role of calcium and oxalate balance in the development of CaOx stones in cats requires considerable further investigation.
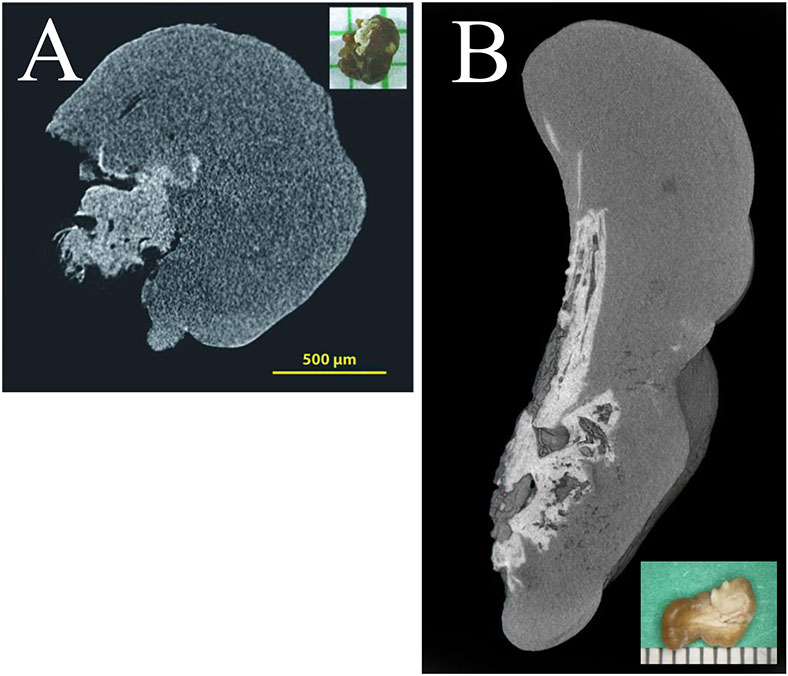
Histopathological descriptions of upper urinary tract CaOx stone formation in cats have been limited to case reports or small case series. Small studies have shown interstitial fibrosis, glomerular sclerosis, and oxalate crystals within the renal tubules, as well as generalized inflammation, although many of these animals had concurrent chronic kidney disease105-107. Parenchymal mineralization, similar to Randall’s plaques in humans, has been suggested as a mechanism for stone formation in cats (Fig 3; Table 1)108. To date, no widely used laboratory model is available for stone formation via a Randall’s plaque mechanism; thus, study of this mechanism in cats might offer a new understanding of the molecular events that lead to initial calcium phosphate plaque formation in humans.
Figure 3 ∣.
MicroCT scan of naturally-occuring calcium oxalate nephroliths from (A) human and (B) cat demonstrating a calcium oxalate composition (dark grey) surrounding a core of calcium phosphate (light grey), suggesting a common method of formation. Insets show the gross stone morphology.
Similar to dogs, the recommended treatment for urolithiasis in cats is primarily dietary management, and surgical treatment is offered only when the stone is obstructing (Table 1)89. Diets with high levels of protein, phosphorus, and magnesium with lower urine acidifying potential are associated with a decreased risk of CaOx stone recurrence109.
Asian small-clawed otters
Asian small-clawed (ASC) otters (Aonyx cinereus) are semiaquatic mammals native to South and Southeast Asia and are very often kept in captivity in zoos and aquariums throughout the world110,111. Based on necropsy studies, approximately two-thirds of captive ASC otters develop nephrolithiasis, which is often bilateral110. More than half of these stones are composed of calcium oxalate (Table 1)13. Risk factors for stone formation in this population are thought to be mainly nutritional, as only captive ASC otters in North America have a high proportion of stones. Diets of wild ASC otters are variable and depend on seasonal prey availability and location; wild diets consist predominantly of crabs, mollusks, snakes, fish, and insects112. Target nutrient ranges for captive ASC otters are based on those for domestic cats, and as such rarely resemble wild diets, as they are composed of fish, meats, and concentrates that do not vary throughout the year111,113. In a survey of captive ASC otter diets, crude protein intake was found to be a protective factor and high calcium content a risk factor for stone development111.
An analysis of six captive ASC otters during periods of controlled diet and fasting offered insight into urinary abnormalities of stone-forming otters and the consequences of diet113. All of these animals were known to have nephrolithiasis, later confirmed to be calcium oxalate stones. Urinary levels of calcium and phosphorus were increased during periods of food consumption, whereas oxalate levels were similar between the two states. These results were unable to conclusively determine the role of hypercalciuria in CaOx stone formation, as none of the animals tested were stone-free. However, in comparison to normal dogs and humans, these otters were hyperoxaluric (Table 1). The ratio of urinary oxalate to calcium during periods of food consumption was close to 1:1, a ratio that promotes maximal crystallization114. The hyperoxaluria observed was equal in feeding and fasting states, suggesting an endogenous mechanism for this imbalance. The pathophysiology of calcium stone disease in otters is not well understood.
Surgical stone treatment has been reported in ASC otters; however, no guidelines exist for its use115.
STRUVITE STONES
Struvite urolithiasis is also common in humans and companion animal species, where risk is driven largely by infection (as is the case in humans and dogs) or diet (as in cats and ferrets) that promotes urine alkalization and supersaturation of magnesium ammonium phosphate.
Humans
Struvite stones comprise 10–15% of renal stones in humans and are bilateral in 15% of patients116,117. They are often heterogeneous in composition and include components of magnesium ammonium phosphate and carbonate apatite118. Struvite stones in humans form exclusively in the setting of UTI with a urease-producing organism, such as Proteus, Staphylococcus, Pseudomonas, or Klebsiella species119. Urease is plasmid-encoded and can be transferred between species of microorganisms120; thus, other bacteria such as Escherichia coli have been found to produce urease in the setting of a UTI121. Urease splits urea into ammonia and carbon dioxide, which is further hydrolysed into ammonium and bicarbonate. This process promotes alkalinization of the urine and crystallization of magnesium ammonium phosphate. However, <20% of individuals with documented infection with a urease-producing bacteria will produce a struvite stone, implying the presence of other contributory factors in struvite stone formation122. Other risk factors include female sex, advanced age, medullary sponge kidney, and urinary tract malformations that can lead to urinary stasis, such as urinary diversion and neurogenic bladder119. Common medical comorbidities seen in this population include diabetes mellitus, dyslipidaemia, hypertension, and chronic kidney disease123. An inherited component to risk has not been reported.
Urinary abnormalities include high pH and hypocitraturia; however, hypercalciuria and hyperoxaluria can also be seen in mixed struvite stones124. In some cases a non-struvite stone might become a nidus for struvite stone overgrowth in the setting of infection, as nearly 90% of struvite stones are admixed with other compositions on stone analysis119. Both Randall’s plaque formation and Bellini duct plugging has been observed in struvite stone formers, along with substantial papillary inflammation on endoscopic evaluation125.
Several management options have been described for struvite stones. If a struvite stone is suspected, the patient should be treated with an initial regimen of antibiotics based on urine culture data. An attempt should then be made to render the patient completely stone free. Endoscopic treatments such as ureteroscopy or percutaneous nephrolithotomy are generally considered more effective treatments for such stones as they offer an increased chance for complete stone removal126. Conservative treatment with antibiotics alone can be considered in patients who are poor candidates for surgery127. Acetohydroxamic acid is a urease inhibitor that has been found to limit recurrence in those patients who are prone to rapid development of struvite stones or those who are not good surgical candidates; however, this agent is rarely used owing to its extensive adverse effect profile and limited availability in many countries128. Decreasing dietary calcium and phosphorus has limited success with stone dissolution and prevention65.
Dogs
Struvite stones account for ~40% of canine stones12. Female dogs are more likely than males to be affected (3:1 female-to-male ratio)68, presumably owing to their increased risk for urinary tract infections, as are smaller breeds, which might reflect anatomic (that is, smaller urethrae) or genetic risk factors68,129. Struvite stones in dogs are overwhelmingly associated with infection with a urease-producing bacteria, most commonly Staphylococcus and Proteus species130 however, struvite stones without infection have also been documented131,132. Canine struvite stones have no known genetic risk factors, but breed predispositions exist and are particularly strong for the minority of cases with sterile struvite stones (for example, pugs)132.
Urinary abnormalities in dogs affected with struvite stones include hyperammonuria, hyperphosphaturia, hypermagnesuria, and elevated pH130. As with other stone types, low urine volume is also a risk factor100,133. Studies on renal pathology associated with canine struvite nephroliths are lacking.
In contrast to humans, medical dissolution is an effective method of treatment for struvite stones in dogs130. This difference is largely due to stone location — most canine struvite stones are located in the bladder, enabling quick and easy dissolution owing to the volume of urine surrounding the stone. In addition, treatment of bladder struvite stones is not urgent if clinical signs can be managed; thus, prolonged medical management is allowable. Medical management is accomplished with antibiotics based on urine culture data, use of calculolytic diets, or treatment with urease inhibitors130,134,135. As struvite stones are radio-opaque, their presence and size can easily be monitored to guide treatment duration. The causative bacteria can be harboured within the stones and protected from antimicrobial activity in the urine,136 which can prolong the time required for antibiotic treatment. Dissolution is further aided by diets that acidify and dilute the urine. These diets often contain calcium sulphate and DL-methionine to achieve a target pH of 6.0, low concentrations of magnesium and phosphate, and a low protein content to reduce urinary urea concentration9. Low dietary urea further promotes the dilution of urine by reducing the medullary concentrating gradient. In dogs, the average time for dissolution of struvite stones using antibiotics and diet is ~3 months130. Dogs with stones comprised of a calcium phosphate shell are less likely to respond to these conservative treatment options, as are those with obstructive stones, which require removal with surgery or lithotripsy.
Cats
Struvite stones account for ~50% of feline urolithiasis12. Cats aged 2–10 years are at greatest risk for struvite stone formation, and these stones form more frequently in the bladder than in the upper urinary tract92. The overall proportion of struvite stones in cats has decreased since the 1980s, when almost 80% of stones in domestic cats were struvite137. This decrease has been attributed to the widespread adoption of a diet designed to prevent and dissolve struvite uroliths in domestic cats. In contrast to struvite stones in dogs, feline struvite stones are usually sterile with a less dramatic female predisposition (~3:2 female-to-male ratio)92. They have no known genetic risk factors, and breed predispositions are inconsistent across reports92,98.
Sterile struvite stones in cats can be dissolved by diet modification alone, without the use of antibiotics. This process is more rapid than struvite stone dissolution in dogs, and requires just 1–5 weeks for complete dissolution138. Diets with reduced phosphorus and magnesium help to decrease urinary pH, promoting struvite stone dissolution139,140.
Ferrets
Both pet and laboratory ferrets are known to develop urolithiasis, which can often be asymptomatic with diagnosis only made at time of necropsy141. Before 2010, sterile struvite was the predominant mineral type in uroliths found in ferrets142. Median age of stone formation in ferrets is 4.5 years and males are 3.6 times more likely to develop struvite uroliths than females, especially with neutered status142. Data regarding medical dissolution is lacking in this species, and stones are commonly removed with surgery. Since 2010, the incidence of cystine uroliths has been dramatically increased in North America, currently accounting for nearly all ferret submissions to the Minnesota Urolith Center [Lulich, J.P., unpublished data].
URIC ACID STONES
The general pathophysiology of uric acid stones differs between humans and non-primate species owing to species-specific differences in uric acid metabolism. Nevertheless, naturally-occurring uric acid stone formation in non-human animals informs genetic, metabolic, and dietary risk factors for uric acid excretion.
Humans
Uric acid nephrolithiasis accounts for 8–10% of kidney stones in countries such as the USA, Germany, Spain and Italy and has been estimated to be even more common in other regions of the world143. It is seen disproportionally in the diabetic population with prior studies suggesting that uric acid stones comprise over 33% of stones in patients with diabetes compared with 6% in those without diabetes144,145. Uric acid is the end product of purine metabolism in humans. It is excreted in the urine with relatively low solubility and quickly precipitates when urine pH drops below 5.5. Ammonium acid urate (AAU) stones, as opposed to pure uric acid stones, are rare in industrialized countries, overall accounting for 0.2% to 3.1% of stones146,147. In addition, AAU is more often found in compound or mixed stones than in a pure state148. Risk factors for AAU stones include inflammatory bowel disease, bowel diversion, laxative abuse, morbid obesity, and recurrent UTI148. In developing countries, rates of AAU stones has historically been higher due to a purine-rich, phosphate-poor diet, as well as decreased fluid intake and chronic diarrhoea149,150. Exact prevalence data for present day is unknown.
Individuals with diabetes or metabolic syndrome have decreased urine pH as a result of impaired ammonium excretion and increased net acid excretion151. Hyperuricosuria is another risk factor for the development of uric acid stones. Conditions that cause increased cell turnover, such as myeloproliferative disorders and haemolytic anaemia, or disorders of uric acid metabolism, such as Lesch–Nyhan syndrome and phosphoribosylpyrophosphate synthase overactivity, can increase uric acid levels in the serum and the urine152. Renal hypouricaemia is a rare autosomal recessive hereditary disorder associated with hyperuricosuria and is caused by genetic mutations in genes encoding renal uric acid transporters, SLC22A12 (which account for >90% of cases in Japan) or SLC2A9153,154.
Medical dissolution is first-line treatment for non-obstructing uric acid stones65,66. As low urine pH is the primary risk factor, alkalinization of the urine with potassium citrate increases the solubility of uric acid155. This approach both reduces the risk of uric acid stone formation and contributes to dissolution of existing uric acid stones. Sodium bicarbonate and sodium citrate are other alkalinizing agents that can be used in this setting155. As for all stone formers, increased fluid intake to increase urine volume is recommended. Restriction of dietary animal protein is recommended, particularly to patients with hyperuricosuria, and these patients might additionally benefit from treatment with a xanthine oxidase inhibitor such as allopurinol or feboxostat to decrease urinary uric acid levels65,66.
Dogs
Purine uroliths account for ~8% of stones in dogs, 97% of which are uric acid stones in Dalmatian dogs156. Most mammals, with the exception of the higher primates, are protected from the formation of uric acid stones by the conversion of uric acid to allantoin within the liver157. Allantoin is more soluble than uric acid and does not precipitate in the urine, even at low urine pH157. Dalmatian dogs have an inherited tendency to form uric acid stones owing to an autosomal-recessive mutation in SLC2A9, which has also been observed in humans158. The causal SLC2A9 variant also exists at low frequency in >30 additional dog breeds159. The uricase enzyme is present in affected dogs; however, uric acid cannot be effectively transported into the hepatocytes for metabolism to allantoin or resorbed in the proximal tubule160. The serum concentration of uric acid in Dalmatians is greater than that of other dog breeds and lower than that of humans, whereas the urinary uric acid concentration is similar to that of humans161,162. Amongst Dalmatians, males are disproportionately affected and the average age of diagnosis is 4.5 years156,163. Although >99% of Dalmatians are homozygous for the variant, only one-third of male Dalmatians over the age of 6 years are reported to form urate stones, suggesting the presence of modifying genetic or non-genetic factors164. Among those that do form stones, the recurrence rate is high at 33–50%156. Uric acid stones are also common in dogs with congenital portosystemic vascular anomalies owing to shunting that bypasses the liver and thereby reduces hepatic conversion of uric acid to allantoin161.
Medical dissolution protocols are similar to those for humans. Stone risk is decreased by feeding a low-protein or vegetarian diet, alkalinizing the urine with potassium citrate or sodium bicarbonate, and increasing urine volume165. Allopurinol can also be prescribed to dissolve or prevent stone formation in dogs with hereditary hyperuricosuria89.
Cats
Uric acid uroliths are rare in other domestic and wildlife species; however, they have been documented in cats. Approximately 5% of uroliths originating from cats are composed of urate166. Predisposition varies with breed, and risk is greatest between the ages of 4 and 7 years166. Additional risk factors in cats include neutered status, aciduria, high dietary protein intake, and liver disease166,167. Additional information about pathogenesis, dietary risk factors, and optimal treatment options have not been well-studied in this species.
Dolphins
Ammonium acid urate nephrolithiasis has been reported in captive bottlenose dolphins (Tursiops truncates), but it has not been observed in wild populations168. These stones can be obstructive and can result in hydronephrosis and infection169.
Captive dolphins consume a seafood diet high in purine, which correlates with hyperuricaemia and acidic urine170. Hypocitraturia is more likely to be found in captive populations and can be a risk factor for uric acid stone formation168. Captive dolphins are more likely to develop insulin resistance than wild dolphins, which has been demonstrated to be a risk factor for uric acid urolithiasis in humans171,172, and might also be a contributory factor in dolphins.
Medical dissolution is the initial treatment for purine stones in dolphins; options include hydration, allopurinol, potassium citrate, and sodium bicarbonate169. Frozen–thawed fish has lower water and higher purine content than fresh fish; thus, captive dolphins are recommended to feed on fresh fish when possible170,173,174. Successful treatment of obstructing stones with cystoscopic-guided ureteral stent placement and laser lithotripsy has been reported in one dolphin175.
CYSTINE STONES
In humans and companion animal species, cystine uroliths form secondary to monogenic disorders in genes encoding subunits of a renal dibasic amino acid transporter.
Humans
Cystine nephrolithiasis accounts for 1% of stone disease in adults and 6–8% in children176. Cystine stones and related renal injury are the only phenotypic manifestation of cystinuria, a disease caused by an inherited defect in the resorption of the dibasic amino acids cystine, ornithine, lysine, and arginine from the renal tubules176. Cystinuria is classified as Type A or B based on whether the causal variant resides in SLC3A1 or SLC7A9, respectively; these genes encode the subunits of the dibasic amino acid transporter. Type A cystinuria is inherited in an autosomal recessive pattern, whereas type B is autosomal dominant with incomplete penetrance and can seem recessive in some generations176,177. Modifier genes or epigenetic effects are thought to be responsible for the wide phenotypic variability of type B176. The estimated prevalence of cystinuria in the USA is about 1 in 10,000 but varies in other populations with prevalence as great as 1 in 2,500 in Israeli Jews of Libyan origin and as low as 1 in 100,000 in Sweden178. The degree of pathology varies; however, men have twice the rate of stone events than women179. Initial stone presentation most commonly occurs within the first two decades of life and is often bilateral180.
The goal of treatment is to prevent stone recurrence by decreasing urinary cystine concentrations to below the solubility limit of 250 mg/L, or by increasing the solubility of cystine65. Increasing fluid intake alone can be sufficient prevention in those with mild cystinuria. Other dietary interventions to reduce cystine excretion and increase urine pH include decreased sodium and animal protein intake. As cystine solubility is highly dependent on urine pH, urine alkalinisation with potassium citrate will decrease stone formation181. Some patients with more severe cystinuria might require treatment with a cystine-binding thiol drug, such as D-penicillamine or alpha-mercaptopropionyl glycine (tiopronin), to convert the poorly soluble cystine dimer into a more soluble cysteine monomer-thiol complex65. Up to 70% of patients with cystinuria can develop some form of chronic kidney disease182; thus, measures to prevent stone formation are imperative in all patients.
Dogs
The vast majority — 98% — of cystine urolithiasis in dogs occurs in males183. As with humans, cystinuria is caused by a hereditary defect in the renal resorption of dibasic amino acids. Breed predominance depends on the type of cystinuria present183,184. The classification system in dogs uses Roman numerals to indicate the inheritance pattern and letters to indicate the gene involved (as in the human system)185. Type IA cystinuria is an autosomal recessive disorder caused by variants in SLC3A1 and has been reported in Newfoundlands, Labradors, and Landseers. Type II is an autosomal dominant disorder caused by variants in either SLC3A1 (type IIA) or SLC7A9 (type IIB). Type II has been reported in Australian Cattle Dogs (type IIA) and Miniature Pinschers (type IIB). Type III cystinuria is androgen-dependent and is, therefore, sex-limited — it is only observed in intact male dogs and resolves with chemical or surgical castration185-187. Type III has not been molecularly characterized185. Type III cystinuria is thought to be most common in the Mastiff, English Bulldog, Scottish Deerhound, and Irish Terrier185,188. Additional breed predispositions exists, with the top breeds differing between countries, and Europe has the greatest overall proportion of canine uroliths composed of cystine (~4% compared to <1% in North America); these differences in prevalence might reflect differences breed popularity and in neutering culture and practices in different geographic locations183,186-188. This genetic heterogeneity provides an invaluable comparative model for cystinuria, as mutations in noncoding regions or regulatory sequences might have a role in the development of this disease in both dogs and humans, in particular in the highly variable penetrance of type B.
Cystine uroliths in dogs are amenable to medical dissolution189, which is achieved by increasing urine volume, increasing urinary pH, and reducing protein intake. Thiol-containing medications such as D-penicillamine and tiopronin can also aid with dissolution.
Cats
Cystine uroliths are rare in domestic cats (<0.1% of feline urolith submissions)12. Genetic investigation in affected cats has revealed one pathogenic variant in SLC3A1 and three different variants in SLC7A9, each present in a homozygous state, and consistent with an autosomal recessive inheritance pattern190,191.
Ferrets
Cystine is now the most common composition of uroliths retrieved from domestic ferrets. Prevalence has risen from 15% of ferret uroliths between 1981 and 2007 to 89% from 2010 to 2017 [Lulich, J.P., unpublished data from the Minnesota Urolith Center]. Male and female ferrets are equally affected. North American ferrets have relatively little genetic diversity192. Cystinuria in this species is presumed to be caused by a genetic defect in a founder with subsequent rise in frequency due to genetic selection, genetic drift, or changes in dietary factors increasing the risk for urolith development in cystinuric individuals193. No specific genetic causes have yet been identified.
Dissolution of cystine uroliths has not been reported in ferrets. This species is an obligate carnivore, and dietary protein restriction is not recommended194.
OTHER STONE TYPES
Other stone types in humans and domestic animals comprise those that form as a result of rare hereditary disorders (for example, xanthine and 2,8-dihydroxyadenine stones) or secondary to mineral or toxin ingestion (for example, silica and melamine stones).
Xanthine stones
Xanthine stones are formed in humans who have excess urinary excretion of the purine base xanthine. Hereditary xanthinuria is an autosomal recessive disorder resulting from a deficiency of the enzyme xanthine dehydrogenase (XDH), which metabolizes hypoxanthine and xanthine to uric acid. A deficiency in XDH activity can be caused by loss of function mutations in either the XDH gene (xanthinuria type 1) or in the molybdenum cofactor sulfurase gene (MOCOS) (xanthinuria type 2), which provides a cofactor necessary for XDH function195. The two types of hereditary xanthinuria are clinically indistinguishable and the combined prevalence is estimated to be 1 in 69,000 people worldwide195. However, the true incidence is probably much higher than this, as up to two-thirds of affected individuals are asymptomatic196,197. Use of allopurinol can cause iatrogenic xanthinuria and stone formation198. Recommendations to decrease stone formation in patients with xanthine uroliths include ensuring a high fluid intake and low purine diet. Alkalinization of the urine has little effect of on the solubility of xanthine199.
Xanthine uroliths comprise approximately 0.1% of canine stones188. Most of these are iatrogenic — 71% of xanthine uroliths originate from dogs with a history of allopurinol therapy188. The remainder of the cases are presumed to be caused by hereditary xanthinuria, as both XDH and MOCOS mutations have been reported in dogs200. Hereditary xanthinuria in dogs frequently presents as juvenile-onset end-stage renal disease caused by nephrolithiasis and obstructing ureteroliths201-205.
Xanthine uroliths comprise approximately 0.2% of feline uroliths12. In contrast to dogs, most cases are presumed to be hereditary206-209. Early onset of uroliths and kidney disease are common manifestations, as in other species.
2,8-DHA stones
Adenine phosphoribosyltransferase (APRT) deficiency is a rare recessive disorder of adenine metabolism. APRT converts adenine and 5-phosphoribosyl-1-pyrophsophate to 5-adenosine monophosphate, facilitating metabolism and excretion of adenine210. Decreased or absent function of APRT prevents the metabolism of adenine via this pathway, so instead it is converted to 2,8-dihydroxyadenine (2,8-DHA) by xanthine oxidase. This by-product crystallizes in the renal tubules and renal interstitium leading to stone production and renal failure. Precipitation is not dependent on urine pH, as 2,8-DHA remains insoluble at pH <8.5210. Most reported cases of APRT deficiency originate from Japan, where the estimated prevalence is 1 in 27,000211. The estimated prevalence in the white population is ~0.5–1 per 100,000, though it might be higher in Icelandic and French populations210. Age at presentation ranges from infancy to geriatric years210 Treatment of 2,8-DHA stones caused by APRT deficiency utilizes allopurinol or febuxostat, institution of a low purine diet, and high fluid intake.
Canine 2,8-DHA uroliths are extremely rare with only 9 cases reported and an estimated prevalence of less than 1 in 100,000 canine urolith submissions212. Most cases originate from a single rare breed, the Native American Indian Dog, and arise via a homozygous mutation in APRT212. Urinary tract obstructions and crystalline nephropathy are common in dogs with 2,8-DHA urolithiasis; however, subclinical disease has been reported212.
Silica stones
Silica uroliths are very rare in humans, accounting for <1% of all urinary tract stones213. Most of these have been documented in individuals who consume large quantities of magnesium-containing antacids213,214. Silica stones have also been associated with the consumption of water rich in silica and the use of various homeopathic remedies215,216. These stones are treated surgically and do not recur if consumption of silica remains low.
Similar to humans, silica uroliths account for <1% of stone disease in dogs217. They tend to form in a jackstone shape and are visible on radiographic imaging. Silica stones were first reported in dogs in the mid 1970s218. Initial presence of these stones was due to increased use of plant-based ingredients in dog food and the addition of plant-based fillers, such as rice and soybean hulls217. Plants have higher silica composition than animal-based products, and the addition of corn gluten feed as a high-protein ingredient to some low-quality dog foods might also increase silica consumption217. Silica stones are not amenable to medical dissolution and require surgical removal. Prevention is achieved by feeding a low-silica diet and increasing fluid intake217.
Melamine stones
Melamine is an industrially synthesized chemical used in a wide variety of household products. The presence of these stones in humans was first publicized in the late 2000s, precipitated by addition of melamine to infant formula in mainland China219. Among this population, young and preterm infants were most at risk for stone formation and renal failure219. On ingestion, melamine produces cyanuric acid diamide and cyanuric acid in a process that might be dependent on the presence of Klebsiella terrigena, a component of the normal gut flora220. The presence of melamine in combination with cyanuric acid forms a poorly soluble compound that precipitates in the renal tubules, leading to renal failure and kidney stones219. Melamine stones are often multiple and bilateral and can be combined with uric acid on stone analysis221. Melamine crystallizes under normal urinary conditions but this crystallization might be more be worse in the presence of a UTI and low urine pH222. The mainstay of treatment is elimination of melamine from the diet, increased water intake, and alkalinisation of the urine.
Melamine stones in companion animals were first described in the early 2000s during an outbreak of urolithiasis and renal failure in cats and dogs in Asia and North America. Many of these animals had ingested pet food that had been purposely contaminated with melamine in an attempt to deceptively increase the apparent protein content223. This outbreak foreshadowed the similar occurrence in children in China who ingested formula contaminated with melamine.
Opportunities for research
Dog models are well suited for gene discovery research. Most uroliths (for example, 86% of CaOx stones) occur in purebred dogs69. In contrast to people, dog breeds have relatively little genetic diversity, and disease traits are often controlled by a small number of variants with strong effect224. This reduced diversity enhances the ability to pinpoint major susceptibility genes in dog models225-227. Dogs and humans share susceptibility genes for several monogenic disorders associated with uroliths , including SLC2A9, SLC3A1, SLC7A9, XDH, MOCOS, and APRT158,185,200,212. In fact, SLC2A9 belongs to a family of glucose transporters, but it also has a role in uric acid transport, which was unknown until the discovery of its role in hereditary hyperuricosuria in Dalmatian dogs158. Thus, the dog is a biomedically relevant model that could help the discovery of novel susceptibility genes for urolith types, such as CaOx. The relatively low within-breed diversity could also benefit the discovery of modifier genes affecting uric acid stone formation, using the model of hereditary hyperuricosuria in Dalmatian dogs. In addition, dogs are well suited for microbiome research, as the dog gut microbiome has more similarilities to the human microbiome than mice or pigs; when gut microbiome sequencing is mapped to the human gut gene catalog, 63% of dog reads map compared to only 33% of pig reads and 20% of mouse reads228. The gut microbiome in dogs also undergoes alternations in response to dietary changes that parallel human studies . Alterations in the gut and urine microbiome and metabolome are linked to human stone risk54-57 and dogs could be used to investigate these bacterial networks and how they might be manipulated through diet or other intervention. Dogs additionally respond to many of the same pharmaceutical treatments used in humans for prevention and dissolution of various stone types, including penicillamine, tiopronin, allopurinol, potassium citrate and thiazide diuretics89,189. These parallels might enable further clinical studies randomizing a new therapy against a well-studied control to be performed in dogs before human studies begin.
Cats also share urolith susceptibility genes with humans, but, unlike in dogs, the majority of uroliths (for example, 74% of CaOx uroliths) occur in random bred cats92. Random bred cats have similar genetic diversity to humans and, therefore, lack the advantage for genetic research229. However, cats offer a unique model for research on the renal pathology associated with stones, as Randall’s plaques have been observed in cats with spontaneous calcium oxalate urolithiasis [Lulich, J.P., unpublished data]. This phenomenon fills a critical need in stone research as Randall’s plaques with adherent stone growth are largely absent in rodent models230 The procurement of gross kidney specimens from cats who were known stone formers could provide representations of stone disease from its very inception. In terms of treatment studies, the small ureteral size of cats prohibits the use of current endourological techniques to treat upper urinary tract stones; however, the cat could serve as a model for novel extracorporeal therapies for obstructing nephroureteroliths, such as burst wave lithotripsy231.
One unique aspect of the canine and feline models is the high prevalence of lower urinary tract stones12. Although bladder stones are relatively less common in humans, the pathogenesis of bladder stones seems to be shared with that of kidney stones. Individuals with a family history of kidney stones are at increased risk for bladder stones and vice versa21. Furthermore, in a study of men with bladder outlet obstruction secondary to benign prostatic hyperplasia, those with bladder stones were signficantly more likely to have a history of kidney stones (11 of 30 patients) compared to those without bladder stones (2 of 27, P <0.01)232. Bladder stones are presumed to initially form in the upper urinary tract and ultimately pass down into the bladder where they continue to grow and become symptomatic, particularly if outflow is obstructed232. In animals, the quadruped stance, urethral anatomy (tapering of the penile urethra and, in male dogs, the presence of an os penis) and, in some dogs, a relatively high residual urine volume (up to 3.4 ml/kg in healthy dogs), does not facilitate passage of these stones out of the bladder233-235. In dogs, upper urinary tract stones rarely cause clinical signs; thus,they often remain undetected. However, upper urinary tract stones are likely to be common in dogs with bladder stones, according to one small study in which screening with urinary tract ultrasonography revealed nephroliths in 6 of 7 dogs with active or historic CaOx bladder stones79. Similarly, upper and lower urinary tract stones often coexist in cats; one-third of cats with kidney stones have concurrent bladder stones.96 Thus, dogs and cats serve as a model for stone pathogenesis throughout the urinary tract, even when the predominant clinical presentation relates to lower urinary tract stones.
Other species, such as ferrets, otters, and dolphins, are less accessible than cats and dogs for laboratory research, but do have certain benefits when available. The utility of otters in human research stems primarily from the high proportion of upper urinary tract stones that are detected in this species110. This occurrence enables direct study of stone formation before the period of growth that is observed in stones that have descended to grow in the bladder. In the captive population, diets are easily manipulated and urine is simpler to collect than in wild otters. Ferrets are already commonly used in the laboratory setting for other research, such as respiratory tract disease (for example, influenza)236, and are relatively accessible for the study of de novo stone formation. Their growth of sterile struvite stones offers an opportunity to study struvite crystallization independently from the setting of chronic bacteriuria usually seen in humans who form struvite stones, and their high prevalence of cystine stones affords testing of novel therapies to prevent or dissolve this stone type. The dolphin kidney is of similar dimensions as a human kidney and could be an excellent model for novel surgical techniques or for better visualizing the initial pathogenic events of stone formation. In addition, altering the diet of captive dolphins and studying the results of new medications on blood or urine (collected via catheterization) electrolyte balances is simple237.
The use of companion animals in research promotes an ethical opportunity to study disease in animals without inflicting harm. Clinical trials in pet dogs and cats can reduce costs to their owners and provide the affected pets with access to novel therapies. In addition to benefits for the individual study participants, the results advance evidence-based veterinary medicine and contribute to the One Health collaborative mission to optimize care for humans and animals.
CONCLUSIONS
Various different species can provide naturally-occurring animal models for both common and rare urolith types, and each has advanges and limitations for use in translational research (Table 2).
Table 2.
Strengths and limitations of naturally occurring animal models for major stone types.
| ANIMAL MODEL |
STONE TYPE | OPPORTUNE RESEARCH AREAS |
LIMITATIONS |
|---|---|---|---|
| Dogs | Calcium oxalate | Genetics Supersaturation Microbiome Prevention (dietary and pharmaceutical) |
Most stones are located in the lower urinary tract; ureteral obstructions are uncommon. Urate stones are only observed with a genetic defect in urate transport or portosystemic vascular anomaly. |
| Struvite | Infection-induced struvite Medical dissolution (dietary and pharmaceutical) |
||
| Purine (uric acid) | Medical dissolution (dietary and pharmaceutical) Prevention (dietary and pharmaceutical) Genetics (modifiers) |
||
| Cystine | Effective of L-cystine dimethyl esters in prevention Effect of sex hormones on risk Medical dissolution (dietary and pharmaceutical) |
||
| Cats | Calcium oxalate | Pathology (Randall’s plaques) Supersaturation Treatment of obstructing nephroureteroliths Prevention (dietary and pharmaceutical) |
Ureteral diameter is prohibitively small (0.3 mm) for endourological study. |
| Struvite | Sterile struvite Medical dissolution (dietary and pharmaceutical) |
||
| Ferrets | Struvite | Sterile struvite | Less common |
| Cystine | Medical dissolution (dietary and pharmaceutical) | Molecular basis has not been established. | |
| Dolphins | Purine (urate) | Treatment of obstructing nephroureteroliths Medical dissolution (dietary and pharmaceutical) Prevention (dietary and pharmaceutical) |
Endangered species. |
| Otters | Calcium oxalate | Dietary risk factors Hyperoxaluria |
Endangered species. |
The use of naturally-occuring animal models could reduce the need for laboratory animals used in experimental research while providing models that are more physiologically relevant than rodent models. Furthermore, companion animals such as dogs, cats, and ferrets can be enrolled in clinical trials to test novel drugs and devices, benefiting both human and veterinary medicine. Naturally-occuring animal models also serve as sentinal species for the detection of environmental risk factors. Other benefits of these models include promotion of evidence-based veterinary medicine, reduced cost of veterinary care to owners whose pets are enrolled in clinical studies, and availability of leading edge veterinary care for animals who are enrolled in clinical studies. Limitations in the use of these naturally occurring models include spontaneous and unpredictable stone development, varying location of stones, and anatomical differences between the species in question and humans, including size and shape of the kidney.
Ultimately, recognizing the similarities in stone disease between these different populations and collaboration with our colleagues in veterinary medicine on a health issue that is relevant to both humans and animals will lead to improved care for all patients, both human and otherwise.
Key points.
Common and rare human urolith types also occur naturally in companion and captive animal species, offering diverse opportunities for research.
Calcium oxalate uroliths are common in dogs, cats, and Asian small-clawed otters; these models are uniquely suited for research on genetic risk factors, Randall’s plaque, and dietary hyperoxaluria, respectively.
Infection-induced struvite uroliths are common in dogs, whereas sterile struvite uroliths occur frequently in cats and ferrets; these models could be used to investigate medical dissolution therapy.
Natural animal models of uric acid uroliths are best suited to discovery of genetic modifiers (dogs), study of dietary hyperuricemia (dolphins), and treatment (dogs, cats, dolphins).
Other human urolith types occurring in domestic animals comprise those that form secondary to rare hereditary disorders (cystine, xanthine and 2,8-dihydroxyadenine) or mineral and toxin ingestion (silica and melamine).
Companion animal models of urolithiasis are also useful for discovering environmental and lifestyle risk factors and testing novel devices or therapeutics, which might simultaneously advance veterinary and human medicine.
Acknowledgements
Partial support for E.F. was provided by the Office of the Director, National Institutes of Health (NIH) under award number K01-OD019912.
Footnotes
Competing interests statement
The authors declare no competing interests.
REFERENCES
- 1.Sorokin I et al. Epidemiology of stone disease across the world. World J Urol 35, 1301–1320, doi: 10.1007/s00345-017-2008-6 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Scales CD Jr., Smith AC, Hanley JM & Saigal CS Prevalence of kidney stones in the United States. European urology 62, 160–165, doi: 10.1016/j.eururo.2012.03.052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero V, Akpinar H & Assimos DG Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 12, e86–e96 (2010). [PMC free article] [PubMed] [Google Scholar]
- 4.Rule AD et al. The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol 25, 2878–2886, doi: 10.1681/ASN.2013091011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan S in Animal Models for the Study of Stone Disease (ed Michael Conn P) 483–498 (Academic Press, 2013). [Google Scholar]
- 6.Liu J, Cao Z, Zhang Z, Zhou S & Ye Z A comparative study on several models of experimental renal calcium oxalate stones formation in rats. J Huazhong Univ Sci Technolog Med Sci 27, 83–87, doi: 10.1007/s11596-007-0124-z (2007). [DOI] [PubMed] [Google Scholar]
- 7.Evan AP et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int 68, 145–154, doi: 10.1111/j.1523-1755.2005.00388.x (2005). [DOI] [PubMed] [Google Scholar]
- 8.Evan AP et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111, 607–616, doi: 10.1172/jci17038 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syme HM Stones in cats and dogs: What can be learnt from them? Arab Journal of Urology 10, 230–239, doi: 10.1016/j.aju.2012.06.006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson MR, Norris RD, Sur RL & Preminger GM Urolithiasis: not just a 2-legged animal disease. J Urol 179, 46–52, doi: 10.1016/j.juro.2007.08.123 (2008). [DOI] [PubMed] [Google Scholar]
- 11.O'Kell AL, Grant DC & Khan SR Pathogenesis of calcium oxalate urinary stone disease: species comparison of humans, dogs, and cats. Urolithiasis 45, 329–336, doi: 10.1007/s00240-017-0978-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborne CA, Lulich JP, Kruger JM, Ulrich LK & Koehler LA Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: perspectives from the Minnesota Urolith Center. The Veterinary clinics of North America. Small animal practice 39, 183–197, doi: 10.1016/j.cvsm.2008.09.011 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Osborne CA et al. Quantitative analysis of 4468 uroliths retrieved from farm animals, exotic species, and wildlife submitted to the Minnesota Urolith Center: 1981 to 2007. The Veterinary clinics of North America. Small animal practice 39, 65–78, doi: 10.1016/j.cvsm.2008.09.005 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Mandel NS, Mandel IC & Kolbach-Mandel AM Accurate stone analysis: the impact on disease diagnosis and treatment. Urolithiasis 45, 3–9, doi: 10.1007/s00240-016-0943-0 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Singh P et al. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo Clinic proceedings 90, 1356–1365, doi: 10.1016/j.mayocp.2015.07.016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor EN & Curhan GC Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol 18, 2198–2204, doi: 10.1681/asn.2007020219 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Goldfarb DS, Fischer ME, Keich Y & Goldberg J A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int 67, 1053–1061, doi: 10.1111/j.1523-1755.2005.00170.x (2005). [DOI] [PubMed] [Google Scholar]
- 18.Resnick M, Pridgen DB & Goodman HO Genetic predisposition to formation of calcium oxalate renal calculi. N Engl J Med 278, 1313–1318, doi: 10.1056/nejm196806132782403 (1968). [DOI] [PubMed] [Google Scholar]
- 19.Goldfarb DS, Avery AR, Beara-Lasic L, Duncan GE & Goldberg J A Twin Study of Genetic Influences on Nephrolithiasis in Women and Men. Kidney Int Rep 4, 535–540, doi: 10.1016/j.ekir.2018.11.017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curhan GC, Willett WC, Rimm EB & Stampfer MJ Family history and risk of kidney stones. J Am Soc Nephrol 8, 1568–1573 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Hemminki K et al. Familial risks in urolithiasis in the population of Sweden. BJU Int 121, 479–485, doi: 10.1111/bju.14096 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Halbritter J et al. Fourteen monogenic genes account for 15% of nephrolithiasis/nephrocalcinosis. Journal of the American Society of Nephrology : JASN 26, 543–551, doi: 10.1681/asn.2014040388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun DA et al. Prevalence of Monogenic Causes in Pediatric Patients with Nephrolithiasis or Nephrocalcinosis. Clinical journal of the American Society of Nephrology : CJASN 11, 664–672, doi: 10.2215/cjn.07540715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daga A et al. Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int 93, 204–213, doi: 10.1016/j.kint.2017.06.025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorleifsson G et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41, 926–930, doi: 10.1038/ng.404 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Gudbjartsson DF et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet 6, e1001039, doi: 10.1371/journal.pgen.1001039 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urabe Y et al. A genome-wide association study of nephrolithiasis in the Japanese population identifies novel susceptible Loci at 5q35.3, 7p14.3, and 13q14.1. PLoS genetics 8, e1002541–e1002541, doi: 10.1371/journal.pgen.1002541 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oddsson A et al. Common and rare variants associated with kidney stones and biochemical traits. Nature Communications 6, 7975, doi: 10.1038/ncomms8975 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howles SA et al. Genetic variants of calcium and vitamin D metabolism in kidney stone disease. Nature Communications 10, 5175, doi: 10.1038/s41467-019-13145-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanikawa C et al. Novel Risk Loci Identified in a Genome-Wide Association Study of Urolithiasis in a Japanese Population. J Am Soc Nephrol 30, 855–864, doi: 10.1681/asn.2018090942 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S & Pearle MS Pathophysiology and management of calcium stones. The Urologic clinics of North America 34, 323–334, doi: 10.1016/j.ucl.2007.04.009 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Worcester EM & Coe FL New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol 28, 120–132, doi: 10.1016/j.semnephrol.2008.01.005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieske JC, Turner ST, Edeh SN, Smith JA & Kardia SL Heritability of urinary traits that contribute to nephrolithiasis. Clinical journal of the American Society of Nephrology : CJASN 9, 943–950, doi: 10.2215/cjn.08210813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter DJ et al. Genetic contribution to renal function and electrolyte balance: a twin study. Clinical science (London, England : 1979) 103, 259–265, doi:10.1042/ (2002). [DOI] [PubMed] [Google Scholar]
- 35.Palsson R, Indridason OS, Edvardsson VO & Oddsson A Genetics of common complex kidney stone disease: insights from genome-wide association studies. Urolithiasis 47, 11–21, doi: 10.1007/s00240-018-1094-2 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Sayer JA Progress in Understanding the Genetics of Calcium-Containing Nephrolithiasis. Journal of the American Society of Nephrology 28, 748, doi: 10.1681/ASN.2016050576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molin A et al. CYP24A1 Mutations in a Cohort of Hypercalcemic Patients: Evidence for a Recessive Trait. J Clin Endocrinol Metab 100, E1343–1352, doi: 10.1210/jc.2014-4387 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Ketha H et al. Altered Calcium and Vitamin D Homeostasis in First-Time Calcium Kidney Stone-Formers. PLoS One 10, e0137350, doi: 10.1371/journal.pone.0137350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang TJ et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376, 180–188, doi: 10.1016/s0140-6736(10)60588-0 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Seaghdha CM et al. Meta-analysis of genome-wide association studies identifies six new Loci for serum calcium concentrations. PLoS Genet 9, e1003796, doi: 10.1371/journal.pgen.1003796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang X et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun 9, 260, doi: 10.1038/s41467-017-02662-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudman D et al. Hypocitraturia in calcium nephrolithiasis. J Clin Endocrinol Metab 55, 1052–1057, doi: 10.1210/jcem-55-6-1052 (1982). [DOI] [PubMed] [Google Scholar]
- 43.Yagisawa T, Chandhoke PS & Fan J Metabolic risk factors in patients with first-time and recurrent stone formations as determined by comprehensive metabolic evaluation. Urology 52, 750–755, doi: 10.1016/s0090-4295(98)00340-9 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Pak CY, Poindexter JR, Adams-Huet B & Pearle MS Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med 115, 26–32, doi: 10.1016/s0002-9343(03)00201-8 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Domrongkitchaiporn S, Stitchantrakul W & Kochakarn W Causes of hypocitraturia in recurrent calcium stone formers: focusing on urinary potassium excretion. American journal of kidney diseases : the official journal of the National Kidney Foundation 48, 546–554, doi: 10.1053/j.ajkd.2006.06.008 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Nicar MJ, Hill K & Pak CY Inhibition by citrate of spontaneous precipitation of calcium oxalate in vitro. J Bone Miner Res 2, 215–220, doi: 10.1002/jbmr.5650020308 (1987). [DOI] [PubMed] [Google Scholar]
- 47.Zuckerman JM & Assimos DG Hypocitraturia: pathophysiology and medical management. Rev Urol 11, 134–144 (2009). [PMC free article] [PubMed] [Google Scholar]
- 48.Bhasin B, Urekli HM & Atta MG Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol 4, 235–244, doi: 10.5527/wjn.v4.i2.235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gee HY et al. Mutations in SLC26A1 Cause Nephrolithiasis. Am J Hum Genet 98, 1228–1234, doi: 10.1016/j.ajhg.2016.03.026 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmes RP, Knight J & Assimos DG Lowering urinary oxalate excretion to decrease calcium oxalate stone disease. Urolithiasis 44, 27–32, doi: 10.1007/s00240-015-0839-4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufman DW et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. Journal of the American Society of Nephrology : JASN 19, 1197–1203, doi: 10.1681/asn.2007101058 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatch M Intestinal adaptations in chronic kidney disease and the influence of gastric bypass surgery. Exp Physiol 99, 1163–1167, doi: 10.1113/expphysiol.2014.078782 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siener R et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int 83, 1144–1149, doi: 10.1038/ki.2013.104 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Stern JM et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44, 399–407, doi: 10.1007/s00240-016-0882-9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller AW, Choy D, Penniston KL & Lange D Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int 96, 180–188, doi: 10.1016/j.kint.2019.02.012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zampini A, Nguyen AH, Rose E, Monga M & Miller AW Defining Dysbiosis in Patients with Urolithiasis. Sci Rep 9, 5425, doi: 10.1038/s41598-019-41977-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie J et al. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiology 20, 41, doi: 10.1186/s12866-020-01734-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Randall A THE ORIGIN AND GROWTH OF RENAL CALCULI. Ann Surg 105, 1009–1027 (1937). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evan AP, Coe FL, Lingeman J, Bledsoe S & Worcester EM Randall's plaque in stone formers originates in ascending thin limbs. Am J Physiol Renal Physiol 315, F1236–f1242, doi: 10.1152/ajprenal.00035.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams JC Jr. et al. Papillary Ductal Plugging is a Mechanism for Early Stone Retention in Brushite Stone Disease. The Journal of urology 199, 186–192, doi: 10.1016/j.juro.2017.08.063 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evan AE et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int 74, 223–229, doi: 10.1038/ki.2008.161 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Evan AP et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int 71, 795–801, doi: 10.1038/sj.ki.5002113 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Ziemba JB & Matlaga BR Guideline of guidelines: kidney stones. BJU Int 116, 184–189, doi: 10.1111/bju.13080 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Khan SR et al. Kidney stones. Nat Rev Dis Primers 2, 16008, doi: 10.1038/nrdp.2016.8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearle MS et al. Medical management of kidney stones: AUA guideline. J Urol 192, 316–324, doi: 10.1016/j.juro.2014.05.006 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Turk C et al. EAU Guidelines on Diagnosis and Conservative Management of Urolithiasis. Eur Urol 69, 468–474, doi: 10.1016/j.eururo.2015.07.040 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Coe FL, Worcester EM & Evan AP Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol 12, 519–533, doi: 10.1038/nrneph.2016.101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Low WW, Uhl JM, Kass PH, Ruby AL & Westropp JL Evaluation of trends in urolith composition and characteristics of dogs with urolithiasis: 25,499 cases (1985–2006). J Am Vet Med Assoc 236, 193–200, doi: 10.2460/javma.236.2.193 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Lekcharoensuk C et al. Patient and environmental factors associated with calcium oxalate urolithiasis in dogs. J Am Vet Med Assoc 217, 515–519 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Lulich JP et al. Epidemiology of canine calcium oxalate uroliths. Identifying risk factors. The Veterinary clinics of North America. Small animal practice 29, 113–122, xi (1999). [DOI] [PubMed] [Google Scholar]
- 71.Kennedy SM, Lulich JP, Ritt MG & Furrow E Comparison of body condition score and urinalysis variables between dogs with and without calcium oxalate uroliths. Journal of the American Veterinary Medical Association 249, 1274–1280, doi: 10.2460/javma.249.11.1274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lekcharoensuk C et al. Associations between dry dietary factors and canine calcium oxalate uroliths. Am J Vet Res 63, 330–337, doi: 10.2460/ajvr.2002.63.330 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Lekcharoensuk C et al. Associations between dietary factors in canned food and formation of calcium oxalate uroliths in dogs. Am J Vet Res 63, 163–169, doi: 10.2460/ajvr.2002.63.163 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Lulich JP, Osborne CA, Lekcharoensuk C, Kirk CA & Allen TA Effects of hydrochlorothiazide and diet in dogs with calcium oxalate urolithiasis. J Am Vet Med Assoc 218, 1583–1586, doi: 10.2460/javma.2001.218.1583 (2001). [DOI] [PubMed] [Google Scholar]
- 75.Dijcker JC, Kummeling A, Hagen-Plantinga EA & Hendriks WH Urinary oxalate and calcium excretion by dogs and cats diagnosed with calcium oxalate urolithiasis. Vet Rec 171, 646, doi: 10.1136/vr.101130 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Stevenson AE, Robertson WG & Markwell P Risk factor analysis and relative supersaturation as tools for identifying calcium oxalate stone-forming dogs. J Small Anim Pract 44, 491–496 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Furrow E, Patterson EE, Armstrong PJ, Osborne CA & Lulich JP Fasting urinary calcium-to-creatinine and oxalate-to-creatinine ratios in dogs with calcium oxalate urolithiasis and breed-matched controls. J Vet Intern Med 29, 113–119, doi: 10.1111/jvim.12527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lulich JP, Osborne CA, Nagode LA, Polzin DJ & Parke ML Evaluation of urine and serum metabolites in miniature schnauzers with calcium oxalate urolithiasis. Am J Vet Res 52, 1583–1590 (1991). [PubMed] [Google Scholar]
- 79.Carr SV, Grant DC, DeMonaco SM & Shepherd M Measurement of preprandial and postprandial urine calcium to creatinine ratios in male Miniature Schnauzers with and without urolithiasis. J Vet Intern Med 34, 754–760, doi: 10.1111/jvim.15690 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luskin AC, Lulich JP, Gresch SC & Furrow E Bone resorption in dogs with calcium oxalate urolithiasis and idiopathic hypercalciuria. Research in veterinary science 123, 129–134, doi: 10.1016/j.rvsc.2019.01.001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groth EM, Lulich JP, Chew DJ, Parker VJ & Furrow E Vitamin D metabolism in dogs with and without hypercalciuric calcium oxalate urolithiasis. J Vet Intern Med 33, 758–763, doi: 10.1111/jvim.15442 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carvalho M, Lulich JP, Osborne CA & Nakagawa Y Defective urinary crystallization inhibition and urinary stone formation. International braz j urol : official journal of the Brazilian Society of Urology 32, 342–348; discussion 349 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Danpure CJ, Jennings PR & Jansen JH Enzymological characterization of a putative canine analogue of primary hyperoxaluria type 1. Biochimica et biophysica acta 1096, 134–138, doi: 10.1016/0925-4439(91)90051-a (1991). [DOI] [PubMed] [Google Scholar]
- 84.Vidgren G et al. Primary hyperoxaluria in Coton de Tulear. Animal genetics 43, 356–361, doi: 10.1111/j.1365-2052.2011.02260.x (2012). [DOI] [PubMed] [Google Scholar]
- 85.Stevenson AE, Blackburn JM, Markwell PJ & Robertson WG Nutrient intake and urine composition in calcium oxalate stone-forming dogs: comparison with healthy dogs and impact of dietary modification. Vet Ther 5, 218–231 (2004). [PubMed] [Google Scholar]
- 86.Kruger JM, Osborne CA & Lulich JP Canine calcium phosphate uroliths. Etiopathogenesis, diagnosis, and management. Vet Clin North Am Small Anim Pract 29, 141–159, xii, doi: 10.1016/s0195-5616(99)50009-0 (1999). [DOI] [PubMed] [Google Scholar]
- 87.Gnanandarajah JS, Abrahante JE, Lulich JP & Murtaugh MP Presence of Oxalobacter formigenes in the intestinal tract is associated with the absence of calcium oxalate urolith formation in dogs. Urol Res 40, 467–473, doi: 10.1007/s00240-011-0451-1 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Jansen JH & Arnesen K Oxalate nephropathy in a Tibetan spaniel litter. A probable case of primary hyperoxaluria. J Comp Pathol 103, 79–84 (1990). [DOI] [PubMed] [Google Scholar]
- 89.Lulich JP et al. ACVIM Small Animal Consensus Recommendations on the Treatment and Prevention of Uroliths in Dogs and Cats. J Vet Intern Med 30, 1564–1574, doi: 10.1111/jvim.14559 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stevenson AE, Hynds WK & Markwell PJ The relative effects of supplemental dietary calcium and oxalate on urine composition and calcium oxalate relative supersaturation in healthy adult dogs. Research in veterinary science 75, 33–41 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Thumchai R et al. Epizootiologic evaluation of urolithiasis in cats: 3,498 cases (1982-1992). Journal of the American Veterinary Medical Association 208, 547–551 (1996). [PubMed] [Google Scholar]
- 92.Lekcharoensuk C et al. Association between patient-related factors and risk of calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. Journal of the American Veterinary Medical Association 217, 520–525, doi: 10.2460/javma.2000.217.520 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Osborne CA et al. Feline urolithiasis. Etiology and pathophysiology. The Veterinary clinics of North America. Small animal practice 26, 217–232 (1996). [PubMed] [Google Scholar]
- 94.Kyles AE et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984-2002). J Am Vet Med Assoc 226, 932–936, doi: 10.2460/javma.2005.226.932 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Nesser VE, Reetz JA, Clarke DL & Aronson LR Radiographic distribution of ureteral stones in 78 cats. Vet Surg 47, 895–901, doi: 10.1111/vsu.12934 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Cleroux A, Alexander K, Beauchamp G & Dunn M Evaluation for association between urolithiasis and chronic kidney disease in cats. J Am Vet Med Assoc 250, 770–774, doi: 10.2460/javma.250.7.770 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Brown CA, Elliott J, Schmiedt CW & Brown SA Chronic Kidney Disease in Aged Cats: Clinical Features, Morphology, and Proposed Pathogeneses. Vet Pathol 53, 309–326, doi: 10.1177/0300985815622975 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Cannon AB, Westropp JL, Ruby AL & Kass PH Evaluation of trends in urolith composition in cats: 5,230 cases (1985–2004). J Am Vet Med Assoc 231, 570–576, doi: 10.2460/javma.231.4.570 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Lulich JP, Osborne CA, Lekcharoensuk C, Kirk CA & Bartges JW Effects of diet on urine composition of cats with calcium oxalate urolithiasis. J Am Anim Hosp Assoc 40, 185–191, doi: 10.5326/0400185 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Ross SJ et al. Canine and feline nephrolithiasis. Epidemiology, detection, and management. Vet Clin North Am Small Anim Pract 29, 231–250, xiii-xiv (1999). [DOI] [PubMed] [Google Scholar]
- 101.Midkiff AM, Chew DJ, Randolph JF, Center SA & DiBartola SP Idiopathic hypercalcemia in cats. Journal of veterinary internal medicine 14, 619–626 (2000). [DOI] [PubMed] [Google Scholar]
- 102.Coady M, Fletcher DJ & Goggs R Severity of Ionized Hypercalcemia and Hypocalcemia Is Associated With Etiology in Dogs and Cats. Front Vet Sci 6, 276, doi: 10.3389/fvets.2019.00276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKerrell RE et al. Primary hyperoxaluria (L-glyceric aciduria) in the cat: a newly recognised inherited disease. Vet Rec 125, 31–34 (1989). [DOI] [PubMed] [Google Scholar]
- 104.Gershoff SN, Faragalla FF, Nelson DA & Andrus SB Vitamin B6 deficiency and oxalate nephrocalcinosis in the cat. Am J Med 27, 72–80, doi: 10.1016/0002-9343(59)90062-2 (1959). [DOI] [PubMed] [Google Scholar]
- 105.Ross SJ, Osborne CA, Lekcharoensuk C, Koehler LA & Polzin DJ A case-control study of the effects of nephrolithiasis in cats with chronic kidney disease. J Am Vet Med Assoc 230, 1854–1859, doi: 10.2460/javma.230.12.1854 (2007). [DOI] [PubMed] [Google Scholar]
- 106.Chakrabarti S, Syme HM, Brown CA & Elliott J Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 50, 147–155, doi: 10.1177/0300985812453176 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Heiene R et al. Chronic kidney disease with three cases of oxalate-like nephrosis in Ragdoll cats. J Feline Med Surg 11, 474–480, doi: 10.1016/j.jfms.2008.11.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lulich J in American College of Veterinary Internal Medicine Forum (Denver, CO, 2016). [Google Scholar]
- 109.Lekcharoensuk C et al. Association between dietary factors and calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J Am Vet Med Assoc 219, 1228–1237, doi: 10.2460/javma.2001.219.1228 (2001). [DOI] [PubMed] [Google Scholar]
- 110.Calle PP Asian small-clawed otter (Aonyx cinerea) urolithiasis prevalence in North America. Zoo Biology 7, 233–242, doi:doi: 10.1002/zoo.1430070305 (1988). [DOI] [Google Scholar]
- 111.Yoong YT, Fujita K, Galway A, Liu MH & Cabana F UROLITH PREVALENCE AND RISK FACTORS IN ASIAN SMALL-CLAWED OTTERS ( AONYX CINEREUS). Journal of zoo and wildlife medicine : official publication of the American Association of Zoo Veterinarians 49, 863–869, doi: 10.1638/2018-0089.1 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Sivasothi N & Nor BHM A review of otters (Carnivora: Mustelidae: Lutrinae) in Malaysia and Singapore. Hydrobiologia 285, 151–170, doi: 10.1007/BF00005663 (1994). [DOI] [Google Scholar]
- 113.Petrini KR, Lulich JP, Treschel L & Nachreiner RF Evaluation of urinary and serum metabolites in Asian small-clawed otters (Aonyx cinerea) with calcium oxalate urolithiasis. Journal of zoo and wildlife medicine : official publication of the American Association of Zoo Veterinarians 30, 54–63 (1999). [PubMed] [Google Scholar]
- 114.Sutton RA & Walker VR Enteric and mild hyperoxaluria. Mineral and electrolyte metabolism 20, 352–360 (1994). [PubMed] [Google Scholar]
- 115.Sabater Gonzalez M, Osterwind M & Fernandez Colome J Management of nephrolithiasis by pyelotomy and pyeloscopy in an Asian small-clawed otter (Aonyx cinereus). Journal of the American Veterinary Medical Association 255, 1057–1063, doi: 10.2460/javma.255.9.1057 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Flannigan R, Choy WH, Chew B & Lange D Renal struvite stones—pathogenesis, microbiology, and management strategies. Nature Reviews Urology 11, 333, doi: 10.1038/nrurol.2014.99 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Koga S, Arakaki Y, Matsuoka M & Ohyama C Staghorn calculi--long-term results of management. British journal of urology 68, 122–124, doi: 10.1111/j.1464-410x.1991.tb15278.x (1991). [DOI] [PubMed] [Google Scholar]
- 118.Bichler KH et al. Urinary infection stones. International journal of antimicrobial agents 19, 488–498 (2002). [DOI] [PubMed] [Google Scholar]
- 119.Espinosa-Ortiz EJ, Eisner BH, Lange D & Gerlach R Current insights into the mechanisms and management of infection stones. Nat Rev Urol 16, 35–53, doi: 10.1038/s41585-018-0120-z (2019). [DOI] [PubMed] [Google Scholar]
- 120.Cook AR The elimination of urease activity in Streptococcus faecium as evidence for plasmid-coded urease. J Gen Microbiol 92, 49–58, doi: 10.1099/00221287-92-1-49 (1976). [DOI] [PubMed] [Google Scholar]
- 121.Collins CM & Falkow S Genetic analysis of Escherichia coli urease genes: evidence for two distinct loci. J Bacteriol 172, 7138–7144, doi: 10.1128/jb.172.12.7138-7144.1990 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Margel D et al. Clinical implication of routine stone culture in percutaneous nephrolithotomy--a prospective study. Urology 67, 26–29, doi: 10.1016/j.urology.2005.08.008 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Flannigan RK et al. Evaluating factors that dictate struvite stone composition: A multi-institutional clinical experience from the EDGE Research Consortium. Canadian Urological Association journal = Journal de l'Association des urologues du Canada 12, 131–136, doi: 10.5489/cuaj.4804 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cicerello E, Mangano M, Cova GD, Merlo F & Maccatrozzo L Metabolic evaluation in patients with infected nephrolithiasis: Is it necessary? Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica 88, 208–211, doi: 10.4081/aiua.2016.3.208 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Jaeger CD et al. Endoscopic and Pathologic Characterization of Papillary Architecture in Struvite Stone Formers. Urology 90, 39–44, doi: 10.1016/j.urology.2015.12.037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Assimos D et al. Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART I. J Urol 196, 1153–1160, doi: 10.1016/j.juro.2016.05.090 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Morgan TN et al. Conservative Management of Staghorn Calculi: When Is It Safe? J Endourol 32, 541–545, doi: 10.1089/end.2018.0002 (2018). [DOI] [PubMed] [Google Scholar]
- 128.Griffith DP et al. Randomized, double-blind trial of Lithostat (acetohydroxamic acid) in the palliative treatment of infection-induced urinary calculi. European urology 20, 243–247, doi: 10.1159/000471707 (1991). [DOI] [PubMed] [Google Scholar]
- 129.Okafor CC et al. Risk factors associated with struvite urolithiasis in dogs evaluated at general care veterinary hospitals in the United States. J Am Vet Med Assoc 243, 1737–1745, doi: 10.2460/javma.243.12.1737 (2013). [DOI] [PubMed] [Google Scholar]
- 130.Osborne CA et al. Medical dissolution and prevention of canine struvite urolithiasis. Twenty years of experience. Vet Clin North Am Small Anim Pract 29, 73–111, xi (1999). [DOI] [PubMed] [Google Scholar]
- 131.Bartges JW Recurrent sterile struvite urocystolithiasis in three related English Cocker Spaniels. Journal of the American Animal Hospital Association 28, 459–469 (1992). [Google Scholar]
- 132.Stiller AT, Lulich JP & Furrow E Urethral plugs in dogs. Journal of veterinary internal medicine 28, 324–330, doi: 10.1111/jvim.12315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palma D, Langston C, Gisselman K & McCue J Canine struvite urolithiasis. Compend Contin Educ Vet 35, E1; quiz E1 (2013). [PubMed] [Google Scholar]
- 134.Dear JD et al. Evaluation of a dry therapeutic urinary diet and concurrent administration of antimicrobials for struvite cystolith dissolution in dogs. BMC Veterinary Research 15, 273, doi: 10.1186/s12917-019-1992-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Krawiec DR, Osborne CA, Leininger JR & Griffith DP Effect of acetohydroxamic acid on dissolution of canine struvite uroliths. American journal of veterinary research 45, 1266–1275 (1984). [PubMed] [Google Scholar]
- 136.Fowler JE Bacteriology of Branched Renal Calculi and Accompanying Urinary Tract Infection. The Journal of Urology 131, 213–215, doi: 10.1016/S0022-5347(17)50311-0 (1984). [DOI] [PubMed] [Google Scholar]
- 137.Tarttelin MF Feline struvite urolithiasis: factors affecting urine pH may be more important than magnesium levels in food. The Veterinary record 121, 227–230, doi: 10.1136/vr.121.10.227 (1987). [DOI] [PubMed] [Google Scholar]
- 138.Osborne CA et al. Medical dissolution of feline struvite urocystoliths. Journal of the American Veterinary Medical Association 196, 1053–1063 (1990). [PubMed] [Google Scholar]
- 139.Torres-Henderson C, Bunkers J, Contreras ET, Cross E & Lappin MR Use of Purina Pro Plan Veterinary Diet UR Urinary St/Ox to Dissolve Struvite Cystoliths. Topics in companion animal medicine 32, 49–54, doi: 10.1053/j.tcam.2017.07.007 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Lulich JP et al. Efficacy of two commercially available, low-magnesium, urine-acidifying dry foods for the dissolution of struvite uroliths in cats. Journal of the American Veterinary Medical Association 243, 1147–1153, doi: 10.2460/javma.243.8.1147 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Nguyen HT, Moreland AF & Shields RP Urolithiasis in ferrets (Mustela putorius). Lab Anim Sci 29, 243–245 (1979). [PubMed] [Google Scholar]
- 142.Nwaokorie EE, Osborne CA, Lulich JP, Albasan H & Lekcharoensuk C Epidemiology of struvite uroliths in ferrets: 272 cases (1981–2007). Journal of the American Veterinary Medical Association 239, 1319–1324, doi: 10.2460/javma.239.10.1319 (2011). [DOI] [PubMed] [Google Scholar]
- 143.Trinchieri A & Montanari E Prevalence of renal uric acid stones in the adult. Urolithiasis 45, 553–562, doi: 10.1007/s00240-017-0962-5 (2017). [DOI] [PubMed] [Google Scholar]
- 144.Sakhaee K Epidemiology and clinical pathophysiology of uric acid kidney stones. Journal of nephrology 27, 241–245, doi: 10.1007/s40620-013-0034-z (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pak CY et al. Biochemical profile of stone-forming patients with diabetes mellitus. Urology 61, 523–527, doi: 10.1016/s0090-4295(02)02421-4 (2003). [DOI] [PubMed] [Google Scholar]
- 146.Soble JJ, Hamilton BD & Streem SB Ammonium acid urate calculi: a reevaluation of risk factors. J Urol 161, 869–873, doi: 10.1016/s0022-5347(01)61794-4 (1999). [DOI] [PubMed] [Google Scholar]
- 147.Pichette V et al. Ammonium acid urate crystal formation in adult North American stone-formers. Am J Kidney Dis 30, 237–242, doi: 10.1016/s0272-6386(97)90058-5 (1997). [DOI] [PubMed] [Google Scholar]
- 148.Lomas DJ, Jaeger CD & Krambeck AE Profile of the Ammonium Acid Urate Stone Former Based on a Large Contemporary Cohort. Urology 102, 43–47, doi: 10.1016/j.urology.2016.10.027 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Klohn M et al. Ammonium urate urinary stones. Urol Res 14, 315–318, doi: 10.1007/bf00262382 (1986). [DOI] [PubMed] [Google Scholar]
- 150.Hodgkinson A Composition of Urinary Tract Calculi from Some Developing Countries. Urologia Internationalis 34, 26–35, doi: 10.1159/000280246 (1979). [DOI] [PubMed] [Google Scholar]
- 151.Maalouf NM, Cameron MA, Moe OW & Sakhaee K Metabolic basis for low urine pH in type 2 diabetes. Clinical journal of the American Society of Nephrology : CJASN 5, 1277–1281, doi: 10.2215/CJN.08331109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abou-Elela A Epidemiology, pathophysiology, and management of uric acid urolithiasis: A narrative review. J Adv Res 8, 513–527, doi: 10.1016/j.jare.2017.04.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ichida K et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 15, 164–173, doi: 10.1097/01.asn.0000105320.04395.d0 (2004). [DOI] [PubMed] [Google Scholar]
- 154.Matsuo H et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 83, 744–751, doi: 10.1016/j.ajhg.2008.11.001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.York NE, Borofsky MS & Lingeman JE Risks associated with drug treatments for kidney stones. Expert Opin Drug Saf 14, 1865–1877, doi: 10.1517/14740338.2015.1100604 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Bartges JW et al. Canine urate urolithiasis. Etiopathogenesis, diagnosis, and management. The Veterinary clinics of North America. Small animal practice 29, 161–191, xii-xiii, doi: 10.1016/s0195-5616(99)50010-7 (1999). [DOI] [PubMed] [Google Scholar]
- 157.Folin O, Berglund H & Derick C THE URIC ACID PROBLEM: AN EXPERIMENTAL STUDY ON ANIMALS AND MAN, INCLUDING GOUTY SUBJECTS. Journal of Biological Chemistry 60, 361–471 (1924). [Google Scholar]
- 158.Bannasch D et al. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet 4, e1000246, doi: 10.1371/journal.pgen.1000246 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Donner J et al. Frequency and distribution of 152 genetic disease variants in over 100,000 mixed breed and purebred dogs. PLoS genetics 14, e1007361, doi: 10.1371/journal.pgen.1007361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Roch-Ramel F, Wong NL & Dirks JH Renal excretion of urate in mongrel and Dalmatian dogs: a micropuncture study. The American journal of physiology 231, 326–331, doi: 10.1152/ajplegacy.1976.231.2.326 (1976). [DOI] [PubMed] [Google Scholar]
- 161.Bannasch D & Henthorn PS Changing paradigms in diagnosis of inherited defects associated with urolithiasis. The Veterinary clinics of North America. Small animal practice 39, 111–125, doi: 10.1016/j.cvsm.2008.09.006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moulin B, Vinay P, Duong N, Gougoux A & Lemieux G Net urate reabsorption in the Dalmatian coach hound with a note on automated measurement of urate in species with low plasma urate. Canadian journal of physiology and pharmacology 60, 1499–1504, doi: 10.1139/y82-221 (1982). [DOI] [PubMed] [Google Scholar]
- 163.Albasan H, Lulich JP, Osborne CA & Lekcharoensuk C Evaluation of the association between sex and risk of forming urate uroliths in Dalmatians. Journal of the American Veterinary Medical Association 227, 565–569, doi: 10.2460/javma.2005.227.565 (2005). [DOI] [PubMed] [Google Scholar]
- 164.Bannasch DL, Ling GV, Bea J & Famula TR Inheritance of Urinary Calculi in the Dalmatian. Journal of Veterinary Internal Medicine 18, 483–487, doi: 10.1111/j.1939-1676.2004.tb02571.x (2004). [DOI] [PubMed] [Google Scholar]
- 165.Westropp JL et al. Evaluation of dogs with genetic hyperuricosuria and urate urolithiasis consuming a purine restricted diet: a pilot study. BMC veterinary research 13, 45, doi: 10.1186/s12917-017-0958-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Albasan H, Osborne CA, Lulich JP & Lekcharoensuk C Risk factors for urate uroliths in cats. Journal of the American Veterinary Medical Association 240, 842–847, doi: 10.2460/javma.240.7.842 (2012). [DOI] [PubMed] [Google Scholar]
- 167.Dear JD, Shiraki R, Ruby AL & Westropp JL Feline urate urolithiasis: a retrospective study of 159 cases. Journal of feline medicine and surgery 13, 725–732, doi: 10.1016/j.jfms.2011.07.001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Venn-Watson SK et al. Hypocitraturia in common bottlenose dolphins (Tursiops truncatus): assessing a potential risk factor for urate nephrolithiasis. Comp Med 60, 149–153 (2010). [PMC free article] [PubMed] [Google Scholar]
- 169.Venn-Watson S, Smith CR, Johnson S, Daniels R & Townsend F Clinical relevance of urate nephrolithiasis in bottlenose dolphins Tursiops truncatus. Diseases of aquatic organisms 89, 167–177, doi: 10.3354/dao02187 (2010). [DOI] [PubMed] [Google Scholar]
- 170.Le-Bert CR et al. Comparison of potential dietary and urinary risk factors for ammonium urate nephrolithiasis in two bottlenose dolphin ( Tursiops truncatus) populations. American journal of physiology. Renal physiology 315, F231–f237, doi: 10.1152/ajprenal.00606.2017 (2018). [DOI] [PubMed] [Google Scholar]
- 171.Wells RS et al. Evaluation of potential protective factors against metabolic syndrome in bottlenose dolphins: feeding and activity patterns of dolphins in sarasota bay, Florida. Frontiers in endocrinology 4, 139, doi: 10.3389/fendo.2013.00139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Venn-Watson S et al. Blood-Based Indicators of Insulin Resistance and Metabolic Syndrome in Bottlenose Dolphins (Tursiops truncatus). Frontiers in endocrinology 4, 136, doi: 10.3389/fendo.2013.00136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smith CR et al. Comparison of Nephrolithiasis Prevalence in Two Bottlenose Dolphin (Tursiops truncatus) Populations. Frontiers in endocrinology 4, 145, doi: 10.3389/fendo.2013.00145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ardente AJ et al. A Targeted Metabolomics Assay to Measure Eight Purines in the Diet of Common Bottlenose Dolphins, Tursiops truncatus. Journal of chromatography & separation techniques 7, doi: 10.4172/2157-7064.1000334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmitt TL & Sur RL Treatment of ureteral calculus obstruction with laser lithotripsy in an Atlantic bottlenose dolphin (Tursiops truncatus). Journal of zoo and wildlife medicine : official publication of the American Association of Zoo Veterinarians 43, 101–109, doi: 10.1638/2011-0002.1 (2012). [DOI] [PubMed] [Google Scholar]
- 176.Sahota A, Tischfield JA, Goldfarb DS, Ward MD & Hu L Cystinuria: genetic aspects, mouse models, and a new approach to therapy. Urolithiasis 47, 57–66, doi: 10.1007/s00240-018-1101-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martell HJ et al. Associating mutations causing cystinuria with disease severity with the aim of providing precision medicine. BMC Genomics 18, 550–550, doi: 10.1186/s12864-017-3913-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Goldstein B & Goldfarb DS Early Recognition and Management of Rare Kidney Stone Disorders. Urol Nurs 37, 81–102 (2017). [PMC free article] [PubMed] [Google Scholar]
- 179.Dello Strologo L et al. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol 13, 2547–2553, doi: 10.1097/01.asn.0000029586.17680.e5 (2002). [DOI] [PubMed] [Google Scholar]
- 180.Usawachintachit M et al. Clinical Outcomes for Cystinuria Patients with Unilateral Versus Bilateral Cystine Stone Disease. Journal of endourology 32, 148–153, doi: 10.1089/end.2017.0335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pareek G, Steele TH & Nakada SY Urological intervention in patients with cystinuria is decreased with medical compliance. J Urol 174, 2250–2252, discussion 2252, doi: 10.1097/01.ju.0000181817.89703.66 (2005). [DOI] [PubMed] [Google Scholar]
- 182.Kum F, Wong K, Game D, Bultitude M & Thomas K Hypertension and renal impairment in patients with cystinuria: findings from a specialist cystinuria centre. Urolithiasis 47, 357–363, doi: 10.1007/s00240-019-01110-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Osborne CA et al. Canine cystine urolithiasis. Cause, detection, treatment, and prevention. Vet Clin North Am Small Anim Pract 29, 193–211, xiii, doi: 10.1016/s0195-5616(99)50011-9 (1999). [DOI] [PubMed] [Google Scholar]
- 184.Roe K, Pratt A, Lulich J, Osborne C & Syme HM Analysis of 14,008 uroliths from dogs in the UK over a 10-year period. J Small Anim Pract 53, 634–640, doi: 10.1111/j.1748-5827.2012.01275.x (2012). [DOI] [PubMed] [Google Scholar]
- 185.Brons AK et al. SLC3A1 and SLC7A9 mutations in autosomal recessive or dominant canine cystinuria: a new classification system. J Vet Intern Med 27, 1400–1408, doi: 10.1111/jvim.12176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Florey J, Ewen V & Syme H Association between cystine urolithiasis and neuter status of dogs within the UK. The Journal of small animal practice 58, 531–535, doi: 10.1111/jsap.12707 (2017). [DOI] [PubMed] [Google Scholar]
- 187.Hesse A, Hoffmann J, Orzekowsky H & Neiger R Canine cystine urolithiasis: A review of 1760 submissions over 35 years (1979–2013). The Canadian veterinary journal = La revue veterinaire canadienne 57, 277–281 (2016). [PMC free article] [PubMed] [Google Scholar]
- 188.Lulich JP et al. Recent shifts in the global proportions of canine uroliths. The Veterinary record 172, 363, doi: 10.1136/vr.101056 (2013). [DOI] [PubMed] [Google Scholar]
- 189.Hoppe A & Denneberg T Cystinuria in the dog: clinical studies during 14 years of medical treatment. Journal of veterinary internal medicine 15, 361–367 (2001). [PubMed] [Google Scholar]
- 190.Mizukami K, Raj K, Osborne C & Giger U Cystinuria Associated with Different SLC7A9 Gene Variants in the Cat. PloS one 11, e0159247, doi: 10.1371/journal.pone.0159247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hilton S, Mizukami K & Giger U Cystinuria caused by a SLC7A9 missense mutation in Siamese-crossbred littermates in Germany. Tierarztl Prax Ausg K Kleintiere Heimtiere 45, 265–272, doi: 10.15654/TPK-160975 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gustafson KD et al. Founder events, isolation, and inbreeding: Intercontinental genetic structure of the domestic ferret. Evolutionary applications 11, 694–704, doi: 10.1111/eva.12565 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nwaokorie EE, Osborne CA, Lulich JP & Albasan H Epidemiological evaluation of cystine urolithiasis in domestic ferrets (Mustela putorius furo): 70 cases (1992–2009). Journal of the American Veterinary Medical Association 242, 1099–1103, doi: 10.2460/javma.242.8.1099 (2013). [DOI] [PubMed] [Google Scholar]
- 194.Johnson-Delaney CA Ferret nutrition. The veterinary clinics of North America. Exotic animal practice 17, 449–470, doi: 10.1016/j.cvex.2014.05.008 (2014). [DOI] [PubMed] [Google Scholar]
- 195.Ichida K, Amaya Y, Okamoto K & Nishino T Mutations associated with functional disorder of xanthine oxidoreductase and hereditary xanthinuria in humans. Int J Mol Sci 13, 15475–15495, doi: 10.3390/ijms131115475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sebesta I, Stiburkova B & Krijt J Hereditary xanthinuria is not so rare disorder of purine metabolism. Nucleosides Nucleotides Nucleic Acids 37, 324–328, doi: 10.1080/15257770.2018.1460478 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Yakubov R, Nir V, Kassem E & Klein-Kremer A [Asymptomatic classical hereditary xanthinuria type 1]. Harefuah 151, 330–331, 380 (2012). [PubMed] [Google Scholar]
- 198.Sighinolfi MC et al. Drug-Induced Urolithiasis in Pediatric Patients. Pediatric Drugs 21, 323–344, doi: 10.1007/s40272-019-00355-5 (2019). [DOI] [PubMed] [Google Scholar]
- 199.Seegmiller JE Xanthine stone formation. Am J Med 45, 780–783, doi: 10.1016/0002-9343(68)90210-6 (1968). [DOI] [PubMed] [Google Scholar]
- 200.Tate NM et al. P6030 Three diverse mutations underlying canine xanthine urolithiasis. Journal of Animal Science 94, 163–163, doi: 10.2527/jas2016.94supplement4163a (2016). [DOI] [Google Scholar]
- 201.Delbarre F, Holtzer A & Auscher C [Xanthine urinary lithiasis and xanthinuria in a dachshund. Deficiency, probably genetic, of the xanthine oxidase system]. Comptes rendus hebdomadaires des seances de l'Academie des sciences. Serie D: Sciences naturelles 269, 1449–1452 (1969). [PubMed] [Google Scholar]
- 202.van Zuilen CD, Nickel RF, van Dijk TH & Reijngoud DJ Xanthinuria in a family of Cavalier King Charles spaniels. The Veterinary quarterly 19, 172–174, doi: 10.1080/01652176.1997.9694766 (1997). [DOI] [PubMed] [Google Scholar]
- 203.Kucera J, Bulkova T, Rychla R & Jahn P Bilateral xanthine nephrolithiasis in a dog. The Journal of small animal practice 38, 302–305, doi: 10.1111/j.1748-5827.1997.tb03471.x (1997). [DOI] [PubMed] [Google Scholar]
- 204.Flegel T, Freistadt R & Haider W Xanthine urolithiasis in a dachshund. Veterinary Record 143, 420, doi: 10.1136/vr.143.15.420 (1998). [DOI] [PubMed] [Google Scholar]
- 205.Gow AG, Fairbanks LD, Simpson JW, Jacinto AM & Ridyard AE Xanthine urolithiasis in a Cavalier King Charles spaniel. The Veterinary record 169, 209, doi: 10.1136/vr.d3932 (2011). [DOI] [PubMed] [Google Scholar]
- 206.White RN, Tick NT & White HL Naturally occurring xanthine urolithiasis in a domestic shorthair cat. The Journal of small animal practice 38, 299–301, doi: 10.1111/j.1748-5827.1997.tb03470.x (1997). [DOI] [PubMed] [Google Scholar]
- 207.Tsuchida S, Kagi A, Koyama H & Tagawa M Xanthine urolithiasis in a cat: a case report and evaluation of a candidate gene for xanthine dehydrogenase. Journal of feline medicine and surgery 9, 503–508, doi: 10.1016/j.jfms.2007.03.012 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Furman E et al. Hereditary xanthinuria and urolithiasis in a domestic shorthair cat. Comp Clin Path 24, 1325–1329, doi: 10.1007/s00580-015-2072-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mestrinho LA, Goncalves T, Parreira PB, Niza MM & Hamaide AJ Xanthine urolithiasis causing bilateral ureteral obstruction in a 10-month-old cat. Journal of feline medicine and surgery 15, 911–916, doi: 10.1177/1098612x13477413 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bollee G et al. Adenine phosphoribosyltransferase deficiency. Clinical journal of the American Society of Nephrology : CJASN 7, 1521–1527, doi: 10.2215/cjn.02320312 (2012). [DOI] [PubMed] [Google Scholar]
- 211.Kamatani N, Terai C, Kuroshima S, Nishioka K & Mikanagi K Genetic and clinical studies on 19 families with adenine phosphoribosyltransferase deficiencies. Hum Genet 75, 163–168, doi: 10.1007/bf00591080 (1987). [DOI] [PubMed] [Google Scholar]
- 212.Furrow E, Pfeifer RJ, Osborne CA & Lulich JP An APRT mutation is strongly associated with and likely causative for 2,8-dihydroxyadenine urolithiasis in dogs. Mol Genet Metab 111, 399–403, doi: 10.1016/j.ymgme.2013.12.002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Haddad FS & Kouyoumdjian A Silica stones in humans. Urologia internationalis 41, 70–76, doi: 10.1159/000281165 (1986). [DOI] [PubMed] [Google Scholar]
- 214.Lee MH, Lee YH, Hsu TH, Chen MT & Chang LS Silica stone--development due to long time oral trisilicate intake. Scandinavian journal of urology and nephrology 27, 267–269, doi: 10.3109/00365599309181263 (1993). [DOI] [PubMed] [Google Scholar]
- 215.Nishizono T et al. Renal silica calculi in an infant. International Journal of Urology 11, 119–121, doi: 10.1111/j.1442-2042.2004.00746.x (2004). [DOI] [PubMed] [Google Scholar]
- 216.Flythe JE, Rueda JF, Riscoe MK & Watnick S Silicate nephrolithiasis after ingestion of supplements containing silica dioxide. American journal of kidney diseases : the official journal of the National Kidney Foundation 54, 127–130, doi: 10.1053/j.ajkd.2008.10.042 (2009). [DOI] [PubMed] [Google Scholar]
- 217.Osborne CA et al. Canine Silica Urolithiasis: Risk Factors, Detection, Treatment, and Prevention. Veterinary Clinics of North America: Small Animal Practice 29, 213–230, doi: 10.1016/S0195-5616(99)50012-0 (1999). [DOI] [PubMed] [Google Scholar]
- 218.Legendre AM Silica urolithiasis in a dog. Journal of the American Veterinary Medical Association 168, 418–419 (1976). [PubMed] [Google Scholar]
- 219.Guan X & Deng Y Melamine-associated urinary stone. Int J Surg 36, 613–617, doi: 10.1016/j.ijsu.2016.11.012 (2016). [DOI] [PubMed] [Google Scholar]
- 220.Zheng X et al. Melamine-Induced Renal Toxicity Is Mediated by the Gut Microbiota. Science translational medicine 5, 172ra122, doi: 10.1126/scitranslmed.3005114 (2013). [DOI] [PubMed] [Google Scholar]
- 221.Dalal RP & Goldfarb DS Melamine-related kidney stones and renal toxicity. Nature Reviews Nephrology 7, 267–274, doi: 10.1038/nrneph.2011.24 (2011). [DOI] [PubMed] [Google Scholar]
- 222.Lu X et al. Gender and urinary pH affect melamine-associated kidney stone formation risk. Urol Ann 3, 71–74, doi: 10.4103/0974-7796.82171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Osborne CA et al. Melamine and Cyanuric Acid-Induced Crystalluria, Uroliths, and Nephrotoxicity in Dogs and Cats. Veterinary Clinics of North America: Small Animal Practice 39, 1–14, doi: 10.1016/j.cvsm.2008.09.010 (2009). [DOI] [PubMed] [Google Scholar]
- 224.Lindblad-Toh K et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438, 803–819, doi: 10.1038/nature04338 (2005). [DOI] [PubMed] [Google Scholar]
- 225.Tsai KL, Clark LA & Murphy KE Understanding hereditary diseases using the dog and human as companion model systems. Mamm Genome 18, 444–451, doi: 10.1007/s00335-007-9037-1 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ostrander EA Epstein Lecture Franklin H.. Both ends of the leash--the human links to good dogs with bad genes. The New England journal of medicine 367, 636–646, doi: 10.1056/NEJMra1204453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Karlsson EK & Lindblad-Toh K Leader of the pack: gene mapping in dogs and other model organisms. Nature reviews. Genetics 9, 713–725, doi: 10.1038/nrg2382 (2008). [DOI] [PubMed] [Google Scholar]
- 228.Coelho LP et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 6, 72, doi: 10.1186/s40168-018-0450-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Alhaddad H et al. Extent of linkage disequilibrium in the domestic cat, Felis silvestris catus, and its breeds. PLoS One 8, e53537, doi: 10.1371/journal.pone.0053537 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu X-R Interstitial calcinosis in renal papillae of genetically engineered mouse models: relation to Randall's plaques. Urolithiasis 43 Suppl 1, 65–76, doi: 10.1007/s00240-014-0699-3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen TT, Samson PC, Sorensen MD & Bailey MR Burst wave lithotripsy and acoustic manipulation of stones. Curr Opin Urol 30, 149–156, doi: 10.1097/mou.0000000000000727 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Childs MA et al. Pathogenesis of bladder calculi in the presence of urinary stasis. The Journal of urology 189, 1347–1351, doi: 10.1016/j.juro.2012.11.079 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Borges NC, Pereira-Sampaio MA, Pereira VA, Abidu-Figueiredo M & Chagas MA Effects of castration on penile extracellular matrix morphology in domestic cats. J Feline Med Surg 19, 1261–1266, doi: 10.1177/1098612x16689405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Atalan G, Barr FJ & Holt PE Frequency of urination and ultrasonographic estimation of residual urine in normal and dysuric dogs. Res Vet Sci 67, 295–299, doi: 10.1053/rvsc.1999.0336 (1999). [DOI] [PubMed] [Google Scholar]
- 235.Thiel C, Häußler TC, Kramer M & Tacke S [Urethrolithiasis in the dog - a retrospective evaluation of 83 male dogs]. Tierarztl Prax Ausg K Kleintiere Heimtiere 47, 394–401, doi: 10.1055/a-1020-3359 (2019). [DOI] [PubMed] [Google Scholar]
- 236.Albrecht RA et al. Moving Forward: Recent Developments for the Ferret Biomedical Research Model. mBio 9, e01113–01118, doi: 10.1128/mBio.01113-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Smith CR et al. Pathophysiological and physicochemical basis of ammonium urate stone formation in dolphins. J Urol 192, 260–266, doi: 10.1016/j.juro.2014.01.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Okafor CC et al. Risk factors associated with calcium oxalate urolithiasis in dogs evaluated at general care veterinary hospitals in the United States. Preventive Veterinary Medicine 115, 217–228, doi: 10.1016/j.prevetmed.2014.04.006 (2014). [DOI] [PubMed] [Google Scholar]