Abstract
LOX (Lysyl oxidase) and LOX like 1–4 (LOXL1–4) are amine oxidases that catalyse the cross-linking of elastin and collagen in the extracellular matrix (ECM). This activity can facilitate cell migration and the formation of metastases. Consequently, inhibition of these enzymes and, in particular of LOXL2, has been suggested as a therapeutic strategy to prevent breast cancer metastasis. Although medicinal chemistry studies have struggled to specifically inhibit LOXL2, the importance of selectivity in this context is not clear. To explore the role of each LOX in breast cancer and consequently their potential as biomarkers or therapeutic targets, a bioinformatic-based approach was followed. The expression profile of LOXs, the putative associations among mRNA expression from each LOX and clinical observations, the correlation between expression of LOX enzymes and other genes, and the association between expression of LOXs and the tumour infiltrates were assessed for breast cancer. Overall, the patient outcome and the characteristics of breast tumours with LOX, LOXL1 and LOXL2 upregulation is distinct from those with high expression of LOXL3 and LOXL4. Additionally, the expression correlation between LOXs and other genes involved in cellular processes relevant for cancer biology, also reveals a similar trend for LOX, LOXL1 and LOX2. This work further supports the relevance of LOXL2 as a breast cancer progression biomarker and therapeutic target. We speculate that while the impact of LOXL3 inhibition may vary with breast cancer subtype, the therapeutical inhibition of LOX, LOXL1 and LOXL2 but not of LOXL4 may be the most beneficial.
Keywords: lysyl oxidase, breast cancer, pharmacological inhibitors, gene expression, bioinformatics, immune infiltration
Introduction
Breast cancer is the most common type of cancer in women, with approximately 2.6 million cases diagnosed annually (Sung et al., 2021). Current therapeutic approaches to treat advanced breast cancer with distant organ metastases are not considered effective, resulting in very low patient survival rates. This strongly contrasts with early-stage non-metastatic disease where the available therapies are able to cure ∼70–80% of patients (Harbeck et al., 2019). Presently, treatment decisions take into account the high level of molecular heterogeneity of these tumours, consequently increasing patient life expectancy. In this context, the molecular heterogeneity of breast cancers should be taken into account when developing new therapeutic approaches (Testa et al., 2020).
The human Lysyl oxidase (LOX), and lysyl oxidase like-1 to 4 (LOXL1–LOXL4) belong to the lysyl oxidase family. The primary function of these enzymes is to catalyse the cross-linking of elastin and collagen in the extracellular matrix (ECM) (Rucker et al., 1998). Altered expression of genes from this family influences the remodelling of ECM components and consequently tissue stiffness. Therefore, these proteins can impact cancer cell proliferation, survival, invasion, migration, epithelial to mesenchymal transition, ultimately influencing tumour progression (Vallet et al., 2021). This is supported by data linking expression dysregulation of LOX enzymes with metastasis and tumour survival rates in some cancers (Ahn et al., 2013; Cox et al., 2015; Salvador et al., 2017; Yu et al., 2020; Ferreira et al., 2021). Consequently, LOX and LOXL1–4 enzymes have been suggested as potential druggable targets to prevent breast cancer metastasis (Cox et al., 2016; Ferreira et al., 2021). Various compounds have been developed with different inhibitory activities against LOXs (Ferreira et al., 2021). Depending on their structure, the inhibitors developed so far can be specific for LOXL2, dual inhibitors for LOX/LOXL2, dual inhibitors for LOXL2/LOXL3, or pan-LOX inhibitors (Ferreira et al., 2021). However, the importance of selectively inhibiting each of these enzymes in the various breast cancer subtypes is not clear.
Here we use The Cancer Genome Atlas (TCGA) Breast Invasive Carcinoma (BRCA) data (https://portal.gdc.cancer.gov/projects/TCGA-BRCA) to explore the relation between the expression of each LOX gene at the mRNA level with breast cancer patient survival, expression of genes involved in cellular mechanisms associated with cancer progression and tumour infiltrates in several breast cancer subtypes. Thus, providing a basis to clarify the potential usefulness of each LOX as progression biomarker or therapeutic target in breast cancer.
Methods
To explore the relevance of each LOX gene in BRCA and its subtypes [Basal-like, human epidermal growth factor receptor 2+ (HER2+), Luminal A (LumA) and Luminal B (LumB)], a bioinformatic approach was followed using the TCGA BRCA data set for mRNA expression. The expression profile of LOX family enzymes in BRCA and its subtypes and the association of this expression with patient survival were obtained using GEPIA2–Gene Expression Profiling Interactive Analysis (http://gepia2.cancer-pku.cn/#analysis) (Tang et al., 2019). Differential expression between normal and tumour tissues was determined by a one-way ANOVA test. To explore the association between LOXs individual expression levels and the prognosis of BRCA patients, Kaplan-Meier survival analysis with Log-rank test, and Cox Proportional Hazard (PH) Model were generated using the median cut-offs of tumour LOXs mRNA levels for both overall survival (OS) and disease-free survival (DFS). Cox proportional hazard ratios (HR) from low and high expressions were plotted in a heatmap. Differences between Kaplan-Meier curves were analysed by the Log rank test.
To study the relation between the expression of LOXs and other genes, the one hundred genes with the highest Pearson correlation for each LOX were obtained from the TCGA BRCA data set using both GEPIA2 and UALCAN, a web-portal to perform in-depth analyses of TCGA gene expression data (Chandrashekar et al., 2017) (http://ualcan.path.uab.edu). Non-protein coding mRNAs were excluded from this analysis and only genes commonly found in both platforms were included. To compare the correlation of each selected gene with the remaining LOXs, Pearson´s correlation values were obtained from GEPIA2. Genecard and Uniprot were used to categorize each selected gene based on their functional characterization. Genes were clustered into four groups: 1) cell migration, adhesion, and ECM regulation; 2) cell survival and proliferation; 3) angiogenesis and tumour proliferation; 4) others (other functions).
The association between expression of LOXs and abundance of immune infiltrates in BRCA and its subtypes was evaluated using the TCGA data-based platform TIMER2.0 (http://timer.cistrome.org/) (Li et al., 2020). Spearman’s correlations were calculated based on the algorithm EPIC (Estimating the Proportion of Immune and Cancer cells). This bioinformatic tool predicts the fraction of different cell types from bulk tumour gene expression data, by integrating gene expression profiles from each major non-malignant cell type and renormalizing based on cell-type-specific mRNA content (Racle et al., 2017). The Spearman correlations coefficients obtained were plotted in a heatmap.
Results and Discussion
Lysyl Oxidase Family Gene Expression and Breast Cancer Patient Survival
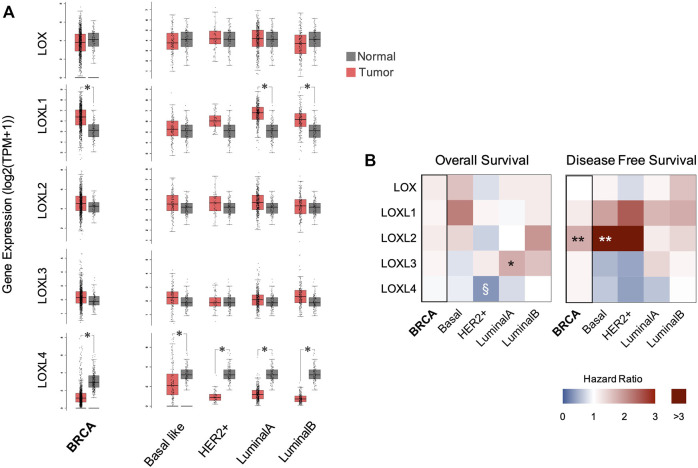
To characterize the expression levels of LOX and LOXL1-4 on normal and breast cancer tissues, the LOXs mRNA levels from TCGA BRCA data-set were analysed. While LOX expression is not significantly altered, higher average levels of LOXL1, LOXL2, and LOXL3 were found in breast cancer tissues when compared with normal tissues (Figure 1A). This is particularly evident in the LumA and LumB subtypes. Despite this, only LOXL1 showed significant differences between expression in normal and tumour tissues in BRCA and LumA and LumB subtypes. Other studies have reported an upregulation of LOXL2 in invasive/metastatic breast cancer cells when compared with poorly invasive/nonmetastatic breast cancer cells (Kirschmann et al., 2002). Thus, highlighting the important role of this protein in breast cancer progression (Salvador et al., 2017). Clinical and preclinical data also suggest that higher LOXL2 expression is associated with invasiveness of Basal-like breast cancer cells (Ahn et al., 2013). Unlike LOXL1-3, the levels of LOXL4 mRNA were significantly lower in breast cancer and in all analysed subtypes, when compared with normal tissue (Figure 1A).
FIGURE 1.
LOX family gene expression and breast cancer patient survival. (A) LOXs mRNA expression profiling comparative analysis between BRCA (n = 1085) or its subtypes (Basal-like n = 135; HER2+ n = 66; LumA n = 415; and LumB n = 194) and normal (n = 112) tissue samples. *p < 0.01 (one-way ANOVA). (B) Overall survival and Disease-free survival Hazard ratio in patients with high tumour levels of LOXs mRNA calculated from Kaplain-Meier curves using the Cox PH Model. Hazard ratios using LOXs expression median were calculated for BRCA and its subtypes. *p < 0.05, **p < 0.01, § p = 0.051 (Logrank test).
Considering the role of LOX enzymes in cellular events related with cancer progression, the associations between LOXs mRNA levels and patient survival were assessed. In breast cancers, the correlations found were more pronounced in DFS than in OS (Figure 1B). While LOXL2 and to a minor extent LOXL1 increased expression was generally correlated with a poorer outcome as measured by an increased hazard ratio (HR) for DFS, the upregulation of LOXL4 was associated with lower HR values (Figure 1B). Effectively, our results concur with Ahn et al. (2013) that showed that dysregulation of LOXL2 expression in Basal-like breast cancer contributes to a poor prognosis and to appearance of distant metastasis.
Patients with low tumour mRNA levels of LOXL4 showed a reduction in OS and DFS HR, particularly in the HER2+ subtype (Figure 1B). LOXL4 expression dysregulation can have a progressive or repressive impact depending on the cancer type, the context and the tumour stage (Wong et al., 2011; Shao et al., 2019). Several studies show that LOXL4 downregulation is mostly associated with cellular events related with tumour progression and with enhanced tumour growth and metastasis in different cancer models (Wu et al., 2007; Shao et al., 2019) and specifically in breast cancer (Choi et al., 2017; Yin et al., 2020). Despite the unclear role of LOXL4 in tumour biology (Choi et al., 2017), a weak LOXL4 expression can lead to the remodelling of the ECM, induction of collagen synthesis, deposition, and to structural changes. These modifications can promote tumour growth and metastasis and are associated with poor clinical outcomes in triple-negative breast cancer (Choi et al., 2017).
Correlation Between Lysyl Oxidases Expression and Other Genes in Breast Cancer
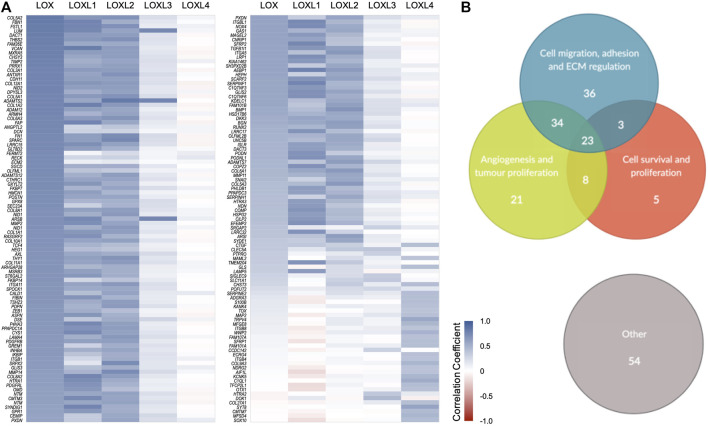
To explore the relation between the altered expression of LOXs and the mRNA levels of other genes in breast cancer, the genes more strongly correlated with LOXs expression in breast cancer were listed and their correlation coefficients plotted (Figure 2A). The genes gathered from this analysis were clustered according to their described functions. Interestingly, the great majority of the genes found have assigned biological functions related with tumour progression (Figure 2B). Considering the role of LOXs in ECM remodelling, it is not surprising that altered expression of several of these genes is associated with profound changes in the expression of genes related to cell migration, adhesion, ECM regulation and angiogenesis (Figure 2). The expression interdependency of some genes identified using our strategy have been previously demonstrated in breast cancer cell lines where LOXs were knocked down. Saatci O, et al. 2020 (Saatci et al., 2020) silenced LOX gene using siRNAs and observed a downregulation in fibronectin 1 (FN1) and integrin subunit alpha 5 (ITGA5) mRNA levels. In a different study, the knockdown of LOX lead to the reduction of Snail Family Transcriptional Repressor 2 (SNAI2) mRNA and protein expression levels (Boufraqech et al., 2016). The silencing of LOXL2 decreased the expression of cadherin 11 (CDH11) protein (Moreno-Bueno et al., 2011). These experimental observations are in agreement with the gene expression correlations found in the TCGA BRCA dataset.
FIGURE 2.
Correlation between the expression of LOXs and other genes in breast cancer. (A) Heat map showing Pearson’s correlation coefficients between mRNA expression of LOX family genes and other genes. For each LOX, the one hundred most strongly correlated genes in breast cancer were collected from GEPIA and UALCAN, and only those common to both platforms were selected. Blue gradient represents a positive Pearson’s correlation and red gradient a negative correlation. (B) Genes were clustered according to their described functions, highlighting specific groups that are relevant for breast cancer progression-related events.
The correlation trend observed between expression of LOX, LOXL1 and LOXL2 and the expression of the majority of genes analysed was often similar. Interestingly, LOXL4 presented an opposite trend in most cases. Additionally, the expression of LOXL3 appears not to strongly correlate with any group of genes in this context. These results are in line with the observed association between LOXs gene expression and patient survival.
Association Between Tumour Lysyl Oxidases Gene Expression and Breast Tumour Infiltrates
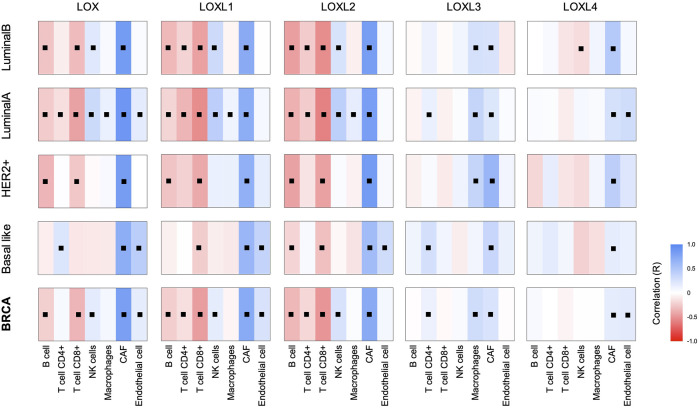
Tumour microenvironment is a key aspect of cancer biology that strongly controls tumour progression. Considering the numerous roles of this family of proteins that are associated with ECM remodelling, we hypothesise that LOXs have a great impact on tumour infiltrates. Effectively, previous works have demonstrated that LOX secreted in the hypoxic tumour environment of invasive breast cancer can contribute to the recruitment of inflammatory cells to a distant site, promoting the formation of premetastatic niches (Cox et al., 2012). To explore the relationship of LOXs expression on breast cancer tumour microenvironment, the prevalence of various tumour-infiltrating cells was calculated using immune deconvolution and marker gene-based methods (Figure 3). Data obtained revealed an association between the increased expression of LOX enzymes and the presence of Cancer-associated fibroblasts (CAFs) in all breast cancer subtypes. This increase in CAFs was more pronounced in the case of LOX, LOXL1 and LOXL2 upregulation, when compared with LOXL3 or LOXL4. Although with some exceptions, the expression of LOX, LOXL1 and LOXL2 was globally negatively associated with B, T CD4+ and T CD8+ cell infiltration. In breast cancer, less immunogenic tumours are typically associated with a poor prognosis (Dieci et al., 2021). This is in agreement with the worst outcome observed in Figure 1B for breast cancer patients with higher expression of LOXL1 and LOXL2 genes.
FIGURE 3.
LOXs expression and breast cancer tumour infiltrates. The correlations between expression of LOX family members and the abundance of immune infiltrates in BRCA and its subtypes were calculated based on the EPIC (Estimating the Proportion of Immune and Cancer cells) algorithm. Blue gradient represents a positive Spearman’s correlation and red gradient a negative correlation, ■ p < 0.05 (Spearman). Number of samples in each group: BRCA = 1100; Basal-like = 191; Her2+ = 82; LumA = 568; LumB = 219.
The relation between LOXL3 and LOXL4 expression and immune infiltrates is more modest, especially for B and T cells. Despite that, a positive correlation between LOXL4 expression and macrophage infiltration was found, as also observed by Yin et al. (2020). Globally, tumours with LOX, LOXL1 and LOX2 upregulation have a distinct tumour cell infiltrating profile from tumours with LOXL3 or LOXL4 increased gene expression.
Equally to LOXs gene expression and correlation with genes related to breast cancer progression, the patterns of tumour cell infiltration observed for LOX, LOXL1 and LOXL2 are comparable, and distinct from those of LOXL3 and LOXL4.
Future Implications
Many of the molecular mechanisms involved in breast cancer invasion and metastasis are still unclear. Considering the impact of breast cancer metastasis in patient outcome, increased therapeutic specificity that accounts for the molecular heterogeneity of the tumour is highly desirable. In that sense, LOX and LOXL1-4 are interesting candidates as novel targets to modulate breast cancer progression. Data presented here highlight the importance of Lysyl oxidases gene expression and its association with breast cancer patient survival and relapse. Specifically, the increased expression of LOX, LOXL1 and LOXL2 appears to be correlated with similar trends in terms of patient survival, tumour infiltrates and correlation with expression of genes involved in tumour-related processes. Contrarily to LOXL3, for which strong correlations were not found, or to LOXL4 that was associated with opposite trends. Previous studies have demonstrated that, contrarily to LOXL2, LOXL4 overexpression has an inhibitory effect on cancer proliferation and progression-related events in different cancer models (Wu et al., 2007; Choi et al., 2017; Shao et al., 2019). Thus, the results obtained are in agreement with the limited available data describing the impact of LOXs in cancer.
Several authors have proposed the LOX family proteins, mostly LOXL2 as therapeutic targets in breast cancer treatment (Moreno-Bueno et al., 2011), in accordance with the data presented here. The current targeting strategy focuses on inhibiting the enzymatic activity of LOX proteins. The small molecule inhibitors developed so far present distinct selectivity towards different enzymes of the LOXs family. However, a rational basis to pursue a specific selectivity profile in the drug development process, tailored for each potential therapeutic use, is still missing. The work presented here contributes to fill this gap for the case of breast cancer. Overall, we speculate that while the impact of LOXL3 inhibition may vary with breast cancer subtype, the specific therapeutical inhibition of both LOXL1 and LOXL2 but not of LOXL4 may be beneficial in breast cancer. Therefore, these data provide a rational basis for the drug development process of novel LOXs inhibitors aimed for breast cancer treatment.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (TCGA, BRCA dataset).
Author Contributions
SR and SF: Data collection and analysis, Visualization, Writing Original draft preparation. AF: Conceptualization, Reviewing and Editing. NS: Conceptualization, Writing Original draft preparation, Reviewing and Editing, Supervision.
Funding
This work is funded by FCT-Foundation for Science and Technology (UIDB/04567/2020 and UIDP/04567/2020 to CBIOS), and by Universidade Lusófona/ILIND grant program Fazer+ (ILIND/F+/EI/01/2020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LOX, lysyl oxidase; LOXL1-4, LOX like1-4; ECM, extracellular matrix; TCGA, the cancer genome atlas; BRCA, breast invasive carcinoma; GEPIA, gene expression profiling interactive analysis; HER2+, human epidermal growth factor receptor 2+; LumA, luminal A; LumB, luminal B; OS, overall survival; DFS, disease free survival; HR, hazard ratio; CAFs, cancer-associated fibroblasts.
References
- Ahn S. G., Dong S. M., Oshima A., Kim W. H., Lee H. M., Lee S. A., et al. (2013). LOXL2 Expression Is Associated with Invasiveness and Negatively Influences Survival in Breast Cancer Patients. Breast Cancer Res. Treat. 141 (1), 89–99. 10.1007/s10549-013-2662-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boufraqech M., Zhang L., Nilubol N., Sadowski S. M., Kotian S., Quezado M., et al. (2016). Lysyl Oxidase (LOX) Transcriptionally Regulates SNAI2 Expression and TIMP4 Secretion in Human Cancers. Clin. Cancer Res. 22 (17), 4491–4504. 10.1158/1078-0432.CCR-15-2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar D. S., Bashel B., Balasubramanya S. A. H., Creighton C. J., Ponce-Rodriguez I., Chakravarthi B. V. S. K., et al. (2017). UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 19 (8), 649–658. 10.1016/J.NEO.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. K., Kim H. S., Jin T., Moon W. K. (2017). LOXL4 Knockdown Enhances Tumor Growth and Lung Metastasis through Collagen-dependent Extracellular Matrix Changes in Triple-Negative Breast Cancer. Oncotarget 8 (7), 11977–11989. 10.18632/ONCOTARGET.14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. R., Gartland A., Erler J. T., Erler J. T. (2012). The Pre-metastatic Niche: Is Metastasis Random? Bonekey Rep. 1, 80. 10.1038/bonekey.2012.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. R., Gartland A., Erler J. T. (2016). Lysyl Oxidase, a Targetable Secreted Molecule Involved in Cancer Metastasis. Cancer Res. 76 (2), 188–192. 10.1158/0008-5472.CAN-15-2306 [DOI] [PubMed] [Google Scholar]
- Cox T. R., Rumney R. M. H., Schoof E. M., Perryman L., Høye A. M., Agrawal A., et al. (2015). The Hypoxic Cancer Secretome Induces Pre-metastatic Bone Lesions through Lysyl Oxidase. Nature 522 (7554), 106–110. 10.1038/NATURE14492 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dieci M. V., Miglietta F., Guarneri V. (2021). Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 10 (2), 223. 10.3390/CELLS10020223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S., Saraiva N., Rijo P., Fernandes A. S. (2021). LOXL2 Inhibitors and Breast Cancer Progression. Antioxidants (Basel) 10 (2), 312. 10.3390/antiox10020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., et al. (2019). Breast Cancer. Nat. Rev. Dis. Prim. 5 (1), 66–31. 10.1038/s41572-019-0111-2 [DOI] [PubMed] [Google Scholar]
- Kirschmann D. A., Seftor E. A., Fong S. F., Nieva D. R., Sullivan C. M., Edwards E. M., et al. (2002). A Molecular Role for Lysyl Oxidase in Breast Cancer Invasion. Cancer Res. 62 (15), 4478–4483. https://cancerres.aacrjournals.org/content/62/15/4478. [PubMed] [Google Scholar]
- Li T., Fu J., Zeng Z., Cohen D., Li J., Chen Q., et al. (2020). TIMER2.0 for Analysis of Tumor-Infiltrating Immune Cells. Nucleic Acids Res. 48 (W1), W509–W514. 10.1093/nar/gkaa407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Bueno G., Salvador F., Martín A., Floristán A., Cuevas E. P., Santos V., et al. (2011). Lysyl Oxidase-like 2 (LOXL2), a New Regulator of Cell Polarity Required for Metastatic Dissemination of Basal-like Breast Carcinomas. EMBO Mol. Med. 3 (9), 528–544. 10.1002/EMMM.201100156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racle J., de Jonge K., Baumgaertner P., Speiser D. E., Gfeller D. (2017). Simultaneous Enumeration of Cancer and Immune Cell Types from Bulk Tumor Gene Expression Data. Elife 6, e26476. 10.7554/ELIFE.26476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker R. B., Kosonen T., Clegg M. S., Mitchell A. E., Rucker B. R., Uriu-Hare J. Y., et al. (1998). Copper, Lysyl Oxidase, and Extracellular Matrix Protein Cross-Linking. Am. J. Clin. Nutr. 67 (5), 996S–1002S. 10.1093/ajcn/67.5.996S [DOI] [PubMed] [Google Scholar]
- Saatci O., Kaymak A., Raza U., Ersan P. G., Akbulut O., Banister C. E., et al. (2020). Targeting Lysyl Oxidase (LOX) Overcomes Chemotherapy Resistance in Triple Negative Breast Cancer. Nat. Commun. 11 (1), 2416–2417. 10.1038/s41467-020-16199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador F., Martin A., López-Menéndez C., Moreno-Bueno G., Santos V., Vázquez-Naharro A., et al. (2017). Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 77 (21), 5846–5859. 10.1158/0008-5472.CAN-16-3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J., Lu J., Zhu W., Yu H., Jing X., Wang Y. L., et al. (2019). Derepression of LOXL4 Inhibits Liver Cancer Growth by Reactivating Compromised P53. Cell Death Differ. 26 (11), 2237–2252. 10.1038/s41418-019-0293-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71 (3), 209–249. 10.3322/CAAC.21660 [DOI] [PubMed] [Google Scholar]
- Tang Z., Kang B., Li C., Chen T., Zhang Z. (2019). GEPIA2: an Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 47 (W1), W556–W560. 10.1093/nar/gkz430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa U., Castelli G., Pelosi E. (2020). Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-specific Treatments. Med. Sci. (Basel) 8 (1), 18. 10.3390/medsci8010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet S. D., Berthollier C., Salza R., Muller L., Ricard-Blum S. (2021). The Interactome of Cancer-Related Lysyl Oxidase and Lysyl Oxidase-like Proteins. Cancers 13 (1), 71. 10.3390/cancers13010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. C., Gilkes D. M., Zhang H., Chen J., Wei H., Chaturvedi P., et al. (2011). Hypoxia-inducible Factor 1 Is a Master Regulator of Breast Cancer Metastatic Niche Formation. Proc. Natl. Acad. Sci. U.S.A. 108, 16369–16374. 10.1073/pnas.1113483108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Guo Z., Chang X., Kim M. S., Nagpal J. K., Liu J., et al. (2007). LOXL1 and LOXL4 Are Epigenetically Silenced and Can Inhibit Ras/extracellular Signal-Regulated Kinase Signaling Pathway in Human Bladder Cancer. Cancer Res. 67 (9), 4123–4129. 10.1158/0008-5472.CAN-07-0012 [DOI] [PubMed] [Google Scholar]
- Yin H., Wang Y., Wu Y., Zhang X., Zhang X., Liu J., et al. (2020). EZH2-mediated Epigenetic Silencing of miR-29/miR-30 Targets LOXL4 and Contributes to Tumorigenesis, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Theranostics 10 (19), 8494–8512. 10.7150/THNO.44849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Ding J., Zhu H., Jing Y., Zhou H., Tian H., et al. (2020). LOXL1 Confers Antiapoptosis and Promotes Gliomagenesis through Stabilizing BAG2. Cell Death Differ. 27 (11), 3021–3036. 10.1038/s41418-020-0558-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (TCGA, BRCA dataset).