Abstract
Introduction :
Short-bowel syndrome (SBS) is a common cause of chronic intestinal failure and is associated with increased morbidity, mortality, poor quality of life, and an increased burden on healthcare costs.
Methods:
We used the US Nationwide Inpatient Sample database from 2005 to 2014. We identified adult SBS hospitalizations by using a combination of International Classification of Diseases, Ninth Revision, Clinical Modification codes. We studied the demographics of the patients with SBS and analyzed the trends in the number of hospitalizations, in-hospital mortality, and healthcare costs. We also identified the risk factors associated with in-hospital mortality.
Results:
A total of 53,040 SBS hospitalizations were identified. We found that SBS-related hospitalizations increased by 55% between 2005 (N = 4037) and 2014 (N = 6265). During this period, the in-hospital mortality decreased from 40 per 1000 to 29 per 1000 hospitalizations, resulting in an overall reduction of 27%. Higher mortality was noted in SBS patients with sepsis (6.7%), liver dysfunction (6.2%), severe malnutrition (6.0%), and metastatic cancer (5.4%). The overall mean length of stay (LOS) for SBS-related hospitalizations was 14.7 days, with a mean hospital cost of $34,130. We noted a steady decrease in the LOS, whereas the cost of care remained relatively stable.
Conclusions:
The national burden of SBS-related hospitalizations continues to rise, and the mortality associated with SBS has substantially decreased. Older SBS patients with sepsis, liver dysfunction, severe malnutrition, and metastatic cancer had the highest risk of mortality. Healthcare utilization in SBS remains high. Healthcare utilization; hospitalization trend; mortality; research and diseases; short-bowel syndrome
Introduction
Short-bowel syndrome (SBS) is a malabsorptive state that usually results after extensive surgical resection of the small intestine. Common indications for massive small-intestinal resection include inflammatory bowel disease, such as Crohn’s Disease; vascular complications, such as mesenteric ischemia, intra-abdominal trauma, or neoplasm; radiation injury; and small-bowel obstruction resulting from other etiologies.1,2 Symptoms of SBS usually vary, but commonly, patients present with abdominal pain, diarrhea, dehydration, and malnutrition.3 Patients with SBS are known to have macronutrient and micronutrient malabsorption, fluid and electrolyte losses, and vitamin and mineral deficiencies.4,5 For this reason, the majority of patients with SBS will require specialized nutrition support, and more than half of these patients develop permanent intestinal failure(IF), requiring lifelong parenteral nutrition (PN) support.2,6
SBS is a relatively rare disease. Because of its multifactorial etiology, the true incidence and prevalence of SBS in the US are unknown. Estimates of SBS prevalence are primarily based on the historical data published by Howard et al in 1992 who used the data from the North American Medicare Home Parenteral Nutrition registry and estimated a yearly prevalence of home PN use to be close to 120 per million population.1,7,8 Approximately a quarter of these patients were receiving PN because of SBS.7 A more recent US study that utilized the home PN data from the Sustain registry found similar results and reported that 24% of patients receiving home PN had SBS, which also made SBS the most common indication for home PN.9 Interestingly, the reported prevalence of both home PN use and SBS are higher in the US compared with other western countries in Europe. Although most SBS studies in the US reported data in terms of annual prevalence, European data are available in terms of point prevalence. A European multicenter study reported that the point prevalence of home PN in Europe is approximately 4 per million general population, and 35% of these patients were receiving PN because of SBS.10 As DiBaise et al noted earlier, these substantial differences in prevalence between these 2 continents are likely multifactorial and would partially be due to differences in methods of prevalence reporting (point prevalence in European studies vs annual prevalence in US studies), and it could also be because there are well-established and more easily accessible home PN resources in the US.1 This can result in a preferential shift toward an early transition to home PN, as it is more cost-effective in comparison with remaining in the hospital (or a skilled nursing facility) for PN in the US.1
Literature exploring large databases to study the epidemiology and healthcare utilization in SBS is lacking. Pant et al analyzed the US national database for pediatric patients called Kids’ Inpatient Database (KID) to estimate the in-hospital mortality and healthcare utilization in pediatric patients with SBS.11 They noted higher mortality, higher length of stay (LOS), and higher cost associated with SBS hospitalizations.11 Several studies have reported an increase in the use of home PN therapy in the US; however, changes in the trends of SBS-related hospitalizations have not been studied yet.12 In this study, we analyzed the Nationwide Inpatient Sample (NIS) database from 2005 to2014 to assess the trends in SBS-related hospitalizations and in-hospital mortality and to estimate the healthcare burden associated with SBS-related hospitalizations in the US.
Methods
Data Source
We used the US NIS database for >10 years, beginning from 2005 to 2014. This data base has been developed and is maintained by the Agency of Healthcare Research and Quality as a part of their initiative for the Healthcare Cost and Utilization Project (HCUP).13 This database is currently the largest publicly accessible, all-payer, inpatient, discharge-level healthcare database in the US, and it has been utilized in policy- and decision-making targeted at national, state, and community-level healthcare issues. The NIS database provides the deidentified data for ~7 million unweighted and ~35millionweightedannualUShospitalizations.Thislarge sample of discharge records is created from >4000 hospitals from all the states participating in HCUP and covers >97% of the US population. NIS provides comprehensive data comprising patient-level and hospital-level deidentified clinical and nonclinical information. Since multiple hospitalizations for a single patient are considered separate discharges, they are entered separately in the database. Patient-level clinical data elements are age, sex, race, principal discharge diagnosis and ≤29 secondary diagnoses, ≤15 inpatient procedures, patient’s insurance status and primary payer information, patient’s discharge disposition (in-hospital death or discharge to home or a nursing facility), hospital LOS, and hospital charges. Hospital data provided by the NIS include the region of the hospital, rural vs urban location, teaching or nonteaching status, and bed capacity of the hospital.13
Study Population
SBS does not have a unique International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) code; therefore, we used a previously described methodology that identified SBS via a combination of 2 diagnostic ICD-9 codes.11 We analyzed the data only for adult patients who were aged ≥18 years. We reviewed previously published literature to define the criteria to identify hospitalizations with SBS.11 Any hospital discharge with the presence of a combination of ICD-9 codes 579.3 (postsurgical nonabsorption) and 99.15 (parenteral infusion of concentrated nutrition substances) was considered an SBS hospitalization. We further excluded the cases with missing information for in-hospital mortality (N=40). Figure 1 shows the flow diagram of the case selection algorithm. The HCUP comorbidity software was used to identify patient comorbidities, such as liver dysfunction and metastatic cancer, along with a combination of ICD-9 diagnosis codes, which were used to identify patients with malnutrition and sepsis.14 ICD-9 codes are listed in Appendix 1.
Figure 1.
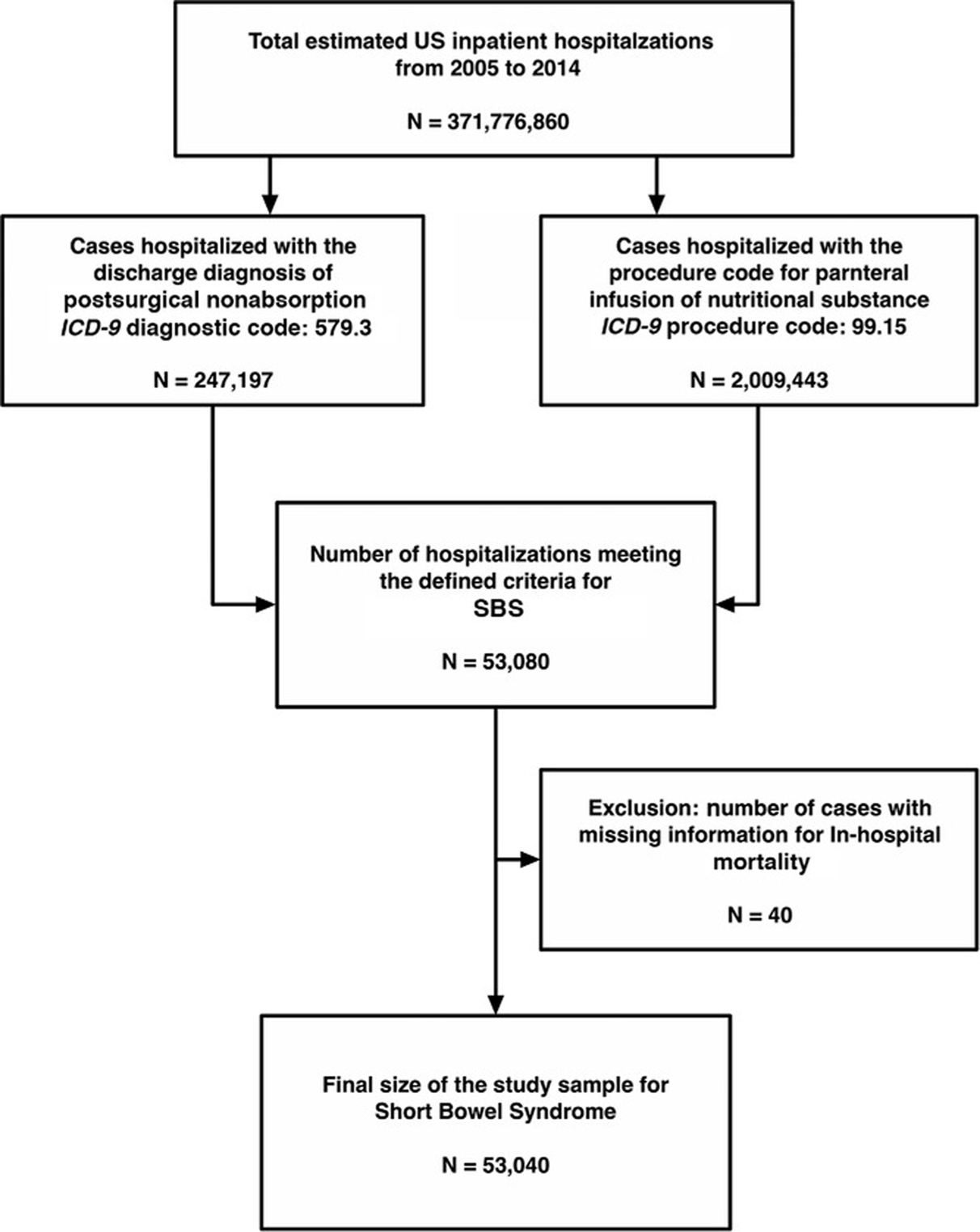
Flow diagram of case selection.
Study Objectives and Description of Variables
The primary objective of the study was to analyze the annual trends in the number of SBS hospitalizations and changes in the mortality associated with SBS during the defined study period. The secondary objectives were to identify the risk factors associated with in-hospital mortality and to assess the annual trends in healthcare resource utilization. Trends in healthcare resource utilization were measured by changes in the hospital LOS and the cost of care. We collected multiple potential confounding variables and accounted for them during the analysis. The variables that were collected and utilized were patient’s age; sex; race; insurance status and primary payer for the hospitalization; comorbidities, which included all 29 Elixhauser comorbidities; hospital characteristics, which included hospital region, hospital location and teaching status, and hospital bedsize.14–16 Data elements, including patients’ demographics, hospital LOS, and hospitalization charges, were obtained directly from the NIS database. HCUP also provides data files for cost analysis by means of hospital-specific cost-to-charge ratios files, which are based on all-payer inpatient costs and can be utilized to estimate variation across hospitals.17 In the NIS database, both hospital charges and hospital costs are presented in US dollars. As our study period extends over the period of a decade, it was necessary to adjust the cost for inflation to accurately analyze changes in the annual trends in hospital cost.18 We used consumer price index data, which are provided by the US Department of Labor Bureau of Labor Statistics, and converted the hospital cost to 2014 constant dollars.18 We assessed the comorbidity burden by utilizing Elixhauser’s comorbidity index, which is currently the most comprehensive method to estimate the burden of patient comorbidity based on ICD9-CM diagnosis codes found in administrative data.15,16
Statistical Analysis
All statistical analyses were performed by using IBM SPSS Statistics for Macintosh, version 25.0. (IBMCorp; Armonk, NY).We used descriptive statistics to study the demographic characteristics of patients hospitalized with SBS. We utilized the Pearson χ2 test and performed a bivariate analysis to compare the demographic variables and outcomes of interest between the study groups. We calculated and reported a 2-sided P-value for each analysis. A P-value of <.05 was considered to be statistically significant. We performed a multivariable logistic regression analysis to adjust for the confounding factors and identified the independent predictors associated with the risk of in-hospital mortality. We adjusted for the confounders, which included patient’s age, sex, race, and comorbidities (which included all 29 Elixhauser comorbidities, including liver disease, metastatic cancer, and congestive heart failure [CHF], as well as other comorbidities, such as inflammatory bowel disease, presence of severe malnutrition, and sepsis).14 We also adjusted for pertinent demographic factors, which included patient’s insurance status and primary payer during the hospitalization, and hospital factors, including hospital region, hospital location and teaching status, and hospital bed-size.
Results
Patient Demographics
We found a total of 53,080 adult hospitalizations matching our inclusion criteria. After excluding patients with missing information for in-hospital mortality (n=40), a final sample comprising data from 53,040 hospitalizations was included in the final analysis. A detailed flow diagram of the sample selection process is shown in Figure 1. The mean age of the study population was 56.6 years, and ~67.8 % of patients were aged >50 years. The majority of patients were women (68%), and the most common race was White (78.2%), followed by African American (11.7%). Approximately half of the patients had Medicare insurance (52.4%), and 31.2% of patients had private insurance. The highest number of hospitalizations occurred in the US South region (31.1%), followed by the Midwest (27.6%). The Northeast (20.5%) and West (20.8%) regions had a similar hospitalization burden. The majority of patients were admitted to urban teaching hospitals (62.7%) with a large hospital bed-size (70.6%). The demographic characteristics of SBS hospitalizations are summarized in Table S1.
Trends in SBS Hospitalizations and Clinical Characteristics
We found that the number of SBS-related hospitalizations increased from 4037 in 2005 to 6265 in 2014, resulting in an overall increase of 55% in the number of hospitalizations over a period of a decade. The number of hospitalizations for each year included in our study period are shown in Table 1.
Table 1.
Yearly Hospitalizations and All-Cause In-Hospital Mortality.
| Year | Number of hospitalizations | Number of deaths | In-hospital mortality, % |
|---|---|---|---|
| 2005 | 4037 | 160 | 4.00 |
| 2006 | 3663 | 167 | 4.60 |
| 2007 | 3826 | 179 | 4.70 |
| 2008 | 5368 | 260 | 4.80 |
| 2009 | 6010 | 210 | 3.50 |
| 2010 | 5517 | 181 | 3.30 |
| 2011 | 6064 | 207 | 3.40 |
| 2012 | 6040 | 245 | 4.10 |
| 2013 | 6250 | 225 | 3.60 |
| 2014 | 6265 | 180 | 2.90 |
| Overall | 53,040 | 2014 | 3.80 |
We found that fluid and electrolyte imbalances were reported in more than half (52.5%) of the cases, and the most common electrolyte abnormality was hypokalemia, which was reported in 21.3% of patients, followed by hyponatremia (reportedin13.7%of patients) and hypomagnesemia (reported in 10.1% of patients). We noted that 18.1% of patients were reported to have dehydration, ~15.4% of patients had a concurrent diagnosis of renal failure, and 4.1% received hemodialysis during their hospitalization.
Interestingly, a significant proportion of patients were noted to have weight loss (44.6%), and similarly, 40.1% of patients were diagnosed with some form of protein-energy malnutrition. Overall, 10% of patients with SBS had a concurrent diagnosis of severe malnutrition. We found that 38.3% of patients were diagnosed with anemia due to nutrition deficiencies, and 29.7% of patients received ≥1 unit of packed red blood cell transfusion during their hospitalization.
We found that infection-related complications, such as sepsis and bacteremia, were the leading comorbidities in these patients, with ~41.1% of patients having a concurrent diagnosis of sepsis or bacteremia, and a few patients (0.3%) were also reported to have a central catheter–associated, localized infection. Liver disease was reported as a diagnosed comorbid condition in 6.1% of these patients, and 5.4% of patients had metastatic cancer. We found that hypertension (32%), diabetes (13.7%), hypothyroidism (12.1%), and CHF (8.1%) were other common comorbidities in these patients. Interestingly, a significant proportion of SBS patients also had a concurrent diagnosis of psychiatric illness, with 18.4.%of patientswithdepressionand6.1%of patients with psychoses.
In-Hospital Mortality
The overall in-hospital mortality for SBS hospitalizations was3.8%.Between2005and2014,we noted a steady, consistent decline in the mortality, with the Pearson correlation of coefficient (r) for the 10-year mortality trend in our sample to be −0.682 (P = .03). Mortality for each year in our study period is summarized in Table 1.
We noted that SBS patients with sepsis had significantly higher mortality compared with patients without sepsis (6.7% vs 1.8%; P < .01). Similarly, patients with the concurrent diagnosis of liver disease also had significantly higher mortality in compared with patients without liver disease (6.2% vs 3.6%; P < .01). Patients with severe malnutrition (6.0% vs 3.5%; P < .01) and metastatic cancer (5.4% vs 3.7%; P < .01) also had significantly higher mortality compared with SBS patients without these comorbidities. Figure 2 shows a graphical presentation of the comparison of in-hospital mortality for these leading comorbidities in patients with SBS.
Figure 2.
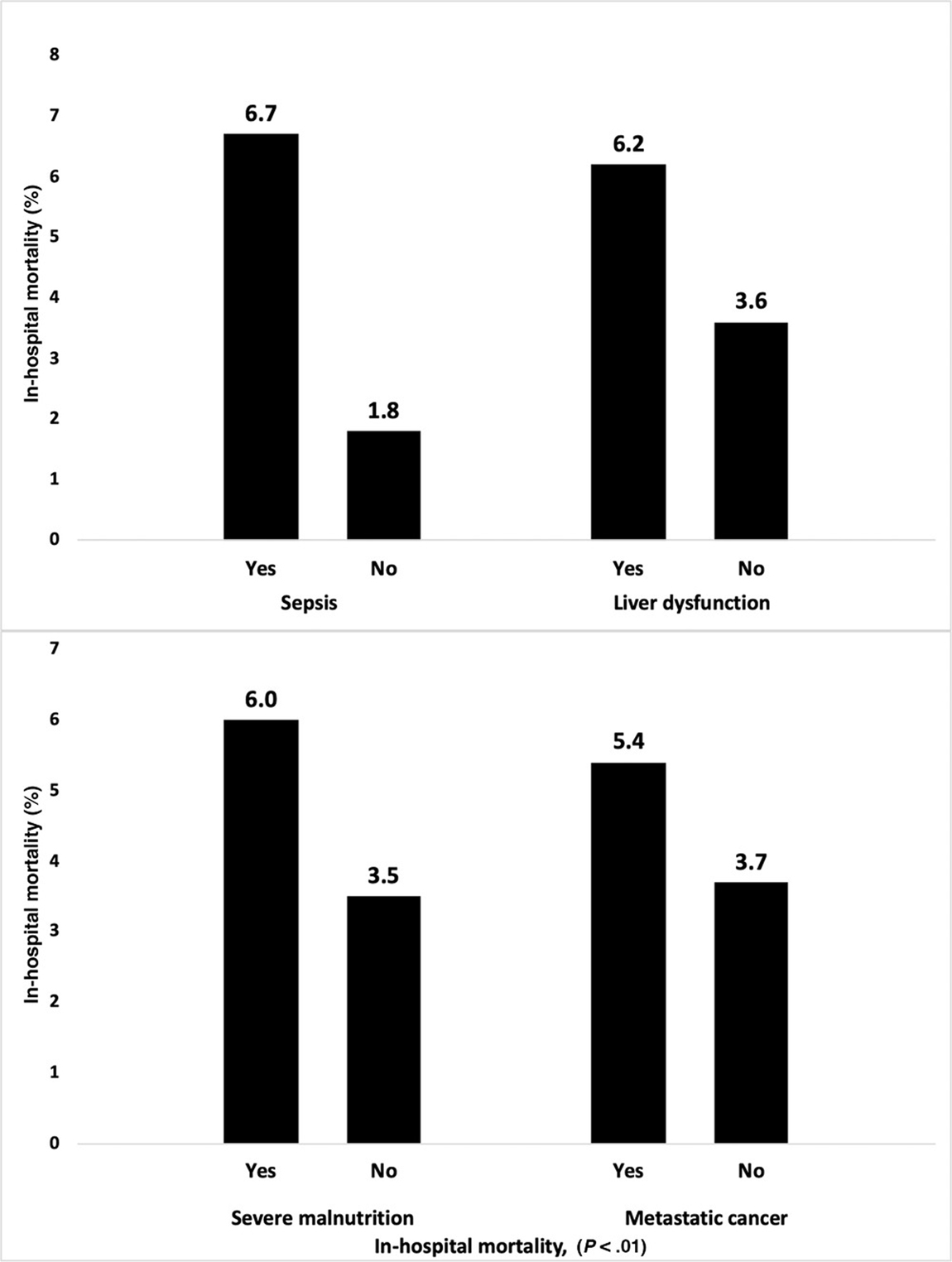
In-hospital mortality in leading comorbidities among patients admitted with short-bowel syndrome.
We studied the hospital factors associated with mortality and found that patients with SBS admitted to the hospitals in the Midwest had the lowest mortality (3.0%; P < .01) compared with other regions in the US. Overall in-hospital mortality was similar in SBS patients admitted to rural (4.1%) and nonteaching urban hospitals (4.1%). We found that the mortality was lower in SBS patients hospitalized in urban teaching hospitals (3.6%; P = .03). Figure 3 shows a graphical presentation of the comparison of in-hospital mortality between different hospital regions and hospital characteristics.
Figure 3.
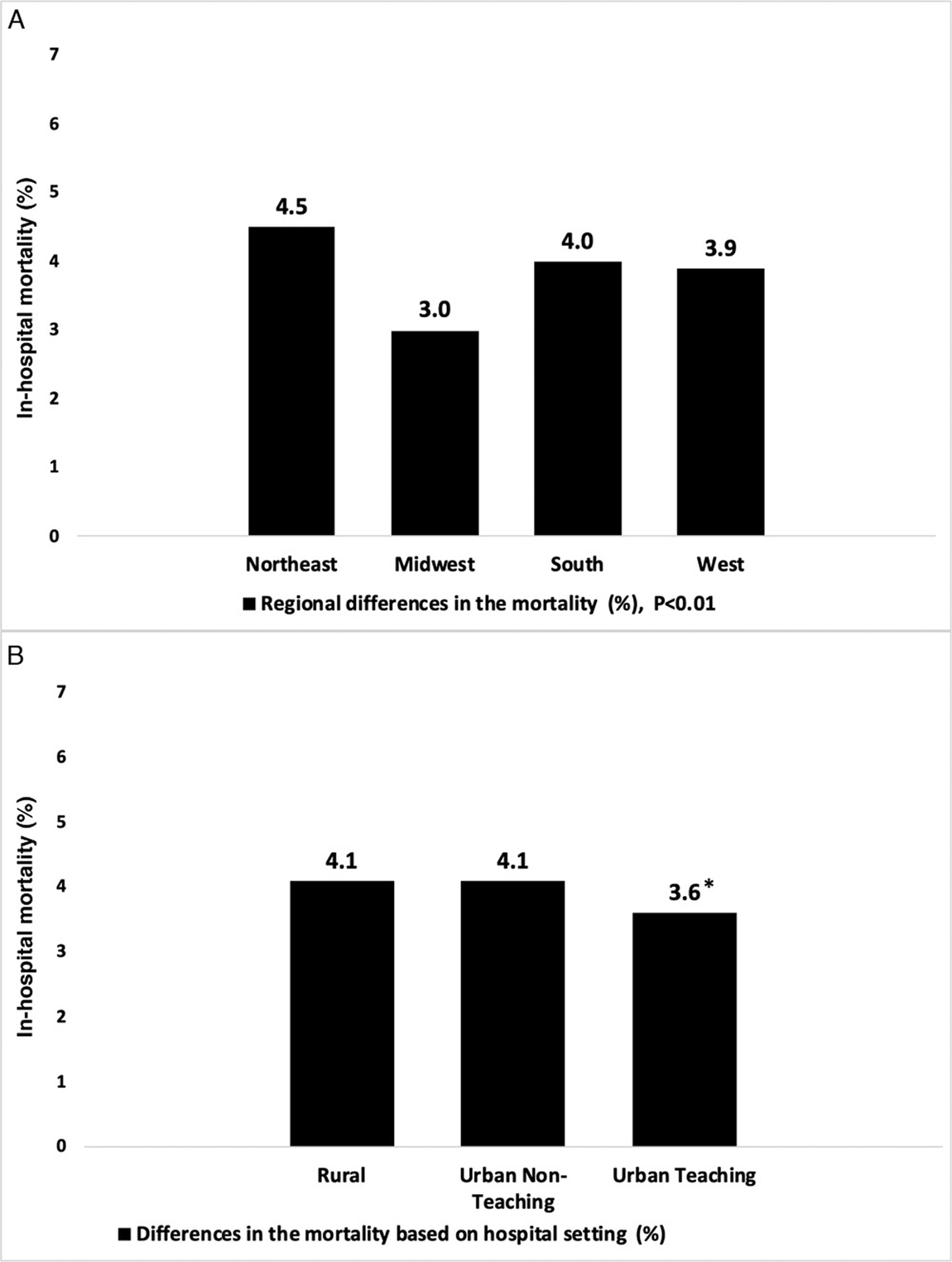
In-hospital mortality differences in geographic region and hospital setting among patients admitted with short-bowel syndrome. *P = .03
We performed the multivariable logistic regression analysis and identified the independent risk factors associated with in-hospital mortality in hospitalized SBS cases. We found that age >65 years (adjusted odds ratio [aOR], 3.49; 95% CI, 2.68–4.56; P < .001) and the presence of sepsis (aOR, 3.38; 95% CI, 3.02–3.78; P < .001) were the 2 most important risk factors for in-hospital mortality. Other independent risk factors that were associated with high in-hospital mortality were concurrent CHF (aOR, 2.64; P < .001), concurrent liver disease (aOR, 2.36; P < .001), and age 51–65 years (aOR, 2.18; P < .001). Patients with severe malnutrition (aOR, 1.64; P < .001) and metastatic cancer (aOR, 1.53; P < .001) also had a significantly higher risk of mortality. We did not find a significant association with mortality risk of other demographic factors, such as sex, race, patient’s insurance status, and hospital region or bed-size. There was no significant difference between mortality risk between urban teaching and nonteaching hospitals; however, we did note that the mortality risk was higher for patients with SBS who were admitted to rural hospitals (aOR, 1.41; P < .001). Results of our multivariable logistic regression analysis are listed in Table 2, and a graphical presentation with a forest plot of the most important predictors of mortality is shown in Figure 4.
Table 2.
Multivariable Logistic Regression Analysis of Factors Associated With In-Hospital Mortality in Cases Admitted With SBS.
| Variable | Adjusted odds ratio | 95% CI for adjusted odds ratio | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| Lower limit | Upper limit | ||||
| Age groups | 18–35 | R | - | - | |
| 36–50 | 1.355 | 1.034 | 1.775 | P = 0.028 | |
| 51–65 | 2.182 | 1.701 | 2.800 | P < 0.001 | |
| >65 | 3.496 | 2.688 | 4.566 | P < 0.001 | |
| Sex | Women | 1.016 | 0.912 | 1.132 | P = 0.769 |
| Men | R | - | - | ||
| Race | White | R | - | - | |
| Black | 1.060 | 0.899 | 1.250 | P = 0.490 | |
| Hispanic | 1.141 | 0.938 | 1.389 | P = 0.187 | |
| Asian or Pacific Islander | 0.615 | 0.356 | 1.062 | P = 0.081 | |
| Native American | 1.347 | 0.763 | 2.378 | P = 0.305 | |
| Others | 1.796 | 1.331 | 2.411 | P < 0.001 | |
| Sepsis | Yes | 3.383 | 3.025 | 3.784 | P < 0.001 |
| No | R | - | - | ||
| Liver disease | Yes | 2.369 | 1.996 | 2.816 | P < 0.001 |
| No | R | - | - | ||
| Metastatic cancer | Yes | 1.536 | 1.272 | 1.858 | P < 0.001 |
| No | R | - | - | ||
| Inflammatory bowel disease | Yes | 0.676 | 0.567 | 0.805 | P < 0.001 |
| No | R | - | - | ||
| Severe malnutrition | Yes | 1.642 | 1.414 | 1.908 | P < 0.001 |
| No | R | - | - | ||
| Chronic heart failure | Yes | 2.645 | 2.331 | 3.003 | P < 0.001 |
| No | - | - | - | ||
| Diabetes with chronic complications | Yes | 1.377 | 1.061 | 1.788 | P = 0.010 |
| No | - | - | - | ||
| Hospital: location/teaching status | Rural | 1.415 | 1.157 | 1.730 | P < 0.001 |
| Urban nonteaching | 1.001 | 0.893 | 1.122 | P = 0.987 | |
| Urban teaching | R | - | - | ||
| Hospital: bed-size | Small | 0.807 | 0.669 | 0.972 | P = 0.024 |
| Medium | 0.936 | 0.826 | 1.060 | P = 0.296 | |
| Large | R | - | - | ||
| Hospital: region | Northeast | 1.120 | 0.962 | 1.304 | P = 0.145 |
| Midwest | 0.837 | 0.708 | 0.988 | P = 0.036 | |
| South | 0.930 | 0.807 | 1.071 | P = 0.311 | |
| West | R | - | - | ||
| Primary expected payer | Medicare | R | - | - | |
| Medicaid | 1.004 | 0.829 | 1.216 | P = 0.967 | |
| Private insurance | 0.879 | 0.763 | 1.013 | P = 0.074 | |
| Self-pay | 2.261 | 1.574 | 3.250 | P < 0.001 | |
| Others | 1.734 | 1.237 | 2.432 | P < 0.001 | |
reference category (adjusted for demographic factors and Elixhauser comorbidities).
Figure 4.
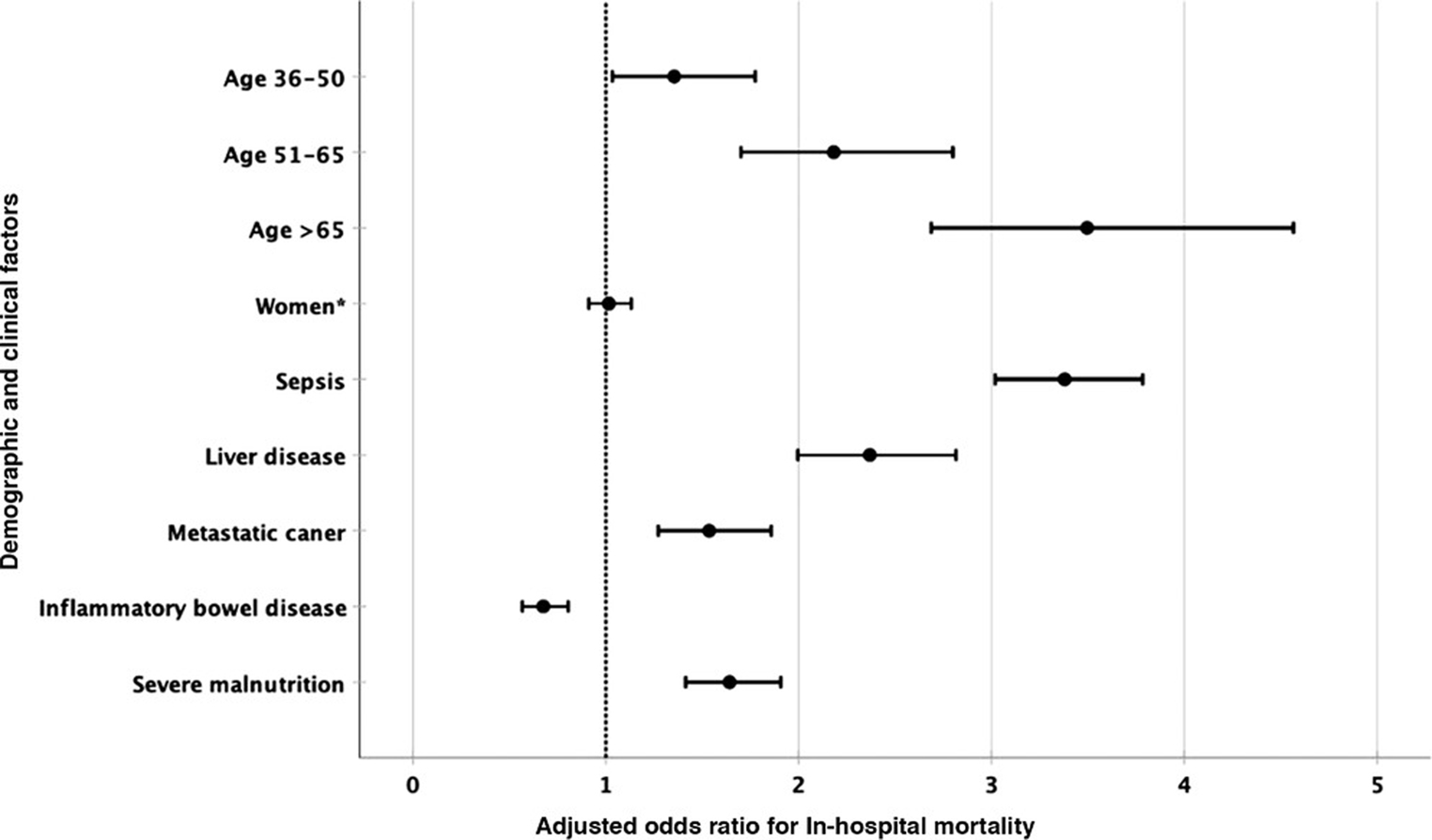
Forest plot for factors associated with in-hospital mortality. *P > .05.
Healthcare Resource Utilization
We found that the mean LOS in patients hospitalized with SBS was 14.7 days. With the exception of the years 2008 and 2009 when the reported LOS was >15 days (15.9 and 16.3 days, respectively), the overall mean LOS remained <15 days. Over the period of the last decade, we noted a gradual overall decrease in the LOS. No difference in LOS was noted between age groups; however, LOS was longer in men compared with women (15.5 vs 14.4 days; P < .01) and in Asian or Pacific Islander (17.6 days), Hispanic (17.0 days), and African American (16.5 days) patients compared with White patients (14.4 days; P < .01), as well as in those who had Medicaid insurance (17.3 days) compared with Medicare (14.5 days) and private insurance (13.8 days; P < .01). Patients with comorbidities, such as sepsis (18.1 vs 12.4 days; P < .01), liver disease (16.0 vs 14.6 days; P < .01), and severe malnutrition (17.9 vs 14.4 days; P < .01), also had longer a LOS. Figure 5 shows the trends in LOS during the study period.
Figure 5.
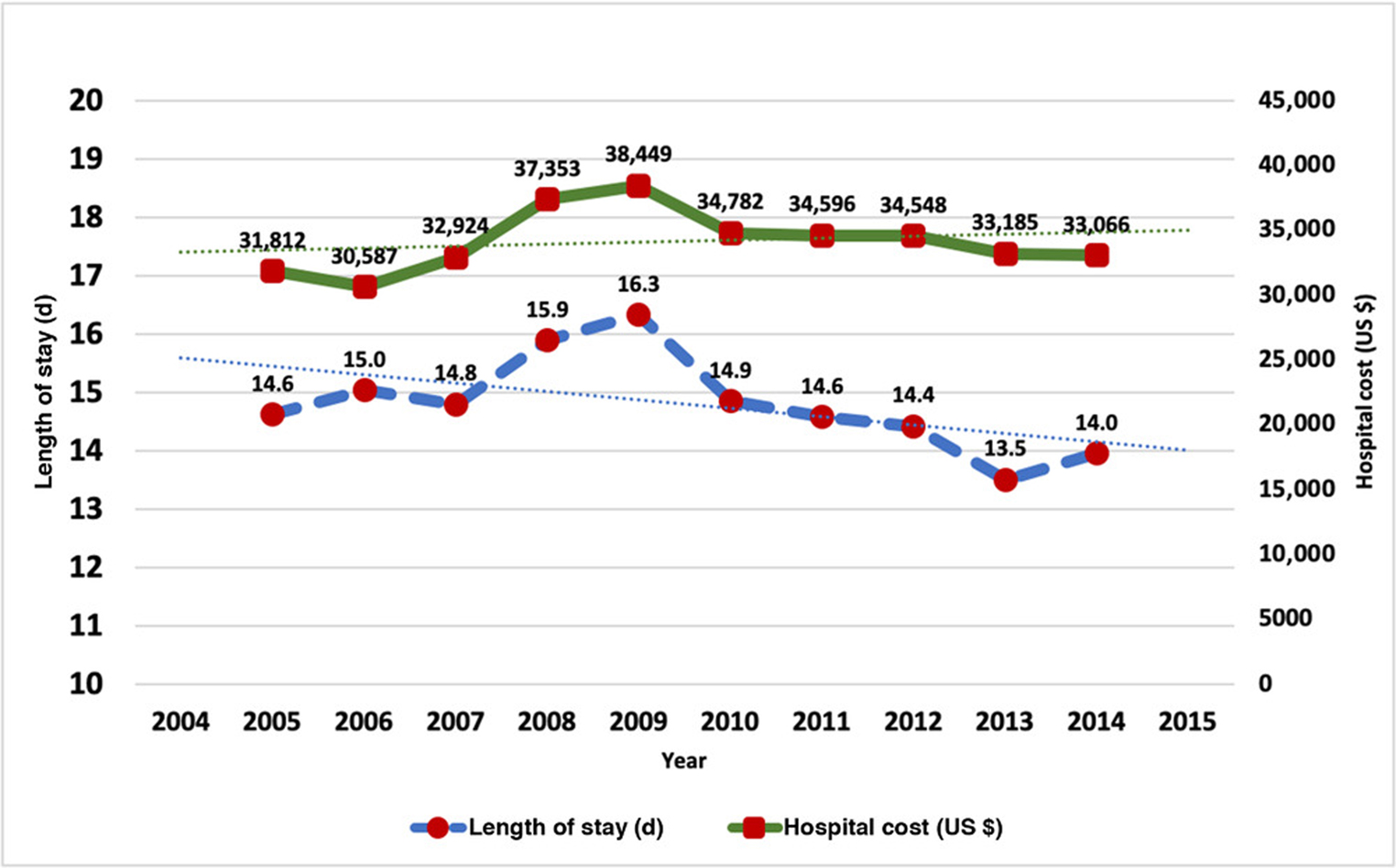
Trends in (A) hospital length of stay and (B) hospital cost.
The overall mean hospital cost for SBS hospitalizations was $34,130. This cost is in 2014 constant dollars after adjustments for the consumer price index. We noted that the hospital cost increased between 2005 and 2009 and thereafter remained stable in the last few years. Similar to our observation for LOS, we noted that cost of care was significantly higher in patients with sepsis ($41,502 vs $25,198; P < .01), liver disease ($38,136 vs $31,521; P < .01), and severe malnutrition ($39,639 vs $31,053; P < .01). Figure 5 shows the yearly trends in the hospital cost in 2014 constant dollars.
Discussion
Since the late 1960s, when a team of pioneers developed the technique for intravenous feeding to support patients with IF, there has been substantial progress in both medical and surgical management of SBS.19–22 Over the past decades, we have seen the development of home PN programs and nutrition support teams dedicated to providing care for patients with SBS.23 This has led to the development of several single and multicenter registries to study clinical outcomes in patients receiving home PN.7,9,24–26 However, there is limited literature exploring the hospitalization trends and inpatient outcomes in patients with SBS.27 Pant et al studied the KID and reported the epidemiology and healthcare utilization associated with hospitalized children with SBS; however, the adult hospitalization data from the NIS database have not yet been studied to analyze the epidemiologic trends and outcomes in SBS hospitalizations within the US.11 In the current study, we used 10 years of hospitalization data across the US and studied the trends in SBS hospitalizations, as well as assessed the burden of associated in-hospital mortality and healthcare resource utilization.
By utilizing the largest all-payer hospitalization database in the US, extending over the period of a decade, we were able to study a comparatively large sample of 53,040 SBS hospitalizations. We noted that the average age of a hospitalized patient with SBS was 56.6 ± 15.8 years. Our findings are consistent with previously reported data from Sustain registry, which consisted of home PN data from 29 centers across the US and reported that the mean age at the time of enrollment in their database was 51.3 years.9 Similar findings were also reported in a multicenter European study of 688 patients, and the majority of them were patients with SBS with an overall mean age of 52.9 years.28 A study from France reported similar results for patients with nonmalignant SBS, and the mean age of their 268-patient cohort was 52.5 years.29 However, there are several differences in the reporting methods: longitudinal studies that analyzed data from home PN registries usually report patient demographics and age when patients were first enrolled into the registry, whereas the cross-sectional studies reported age when the cohorts are selected.9,30 One such recent study, which sampled the SBS cohort from inpatient and outpatient interventional radiology visits from the Japanese national database, reported the mean overall age of patients in the SBS cohort to be older (59.2 years).30 They also noted that patients who developed SBS as a consequence of malignancy were much older (67.2 years) than noncancer patients with SBS (51.5years).30 Thompson et al studied a retrospective cohort of 210 patients with SBS and reported that patients who developed SBS after undergoing bowel resection for intestinal obstruction or mesenteric ischemia were also relatively younger, with a mean age of 48 years.31 In the current study, we found that almost two-thirds of hospitalizations were >50 years. We also noted that more than two-thirds of all hospitalizations were women, which is consistent with a previously estimated higher prevalence of SBS among women.28,30–32 Nightingale et al pointed out earlier that the reason for the higher prevalence of SBS in women could be attributed to shorter bowel length in women.33 This sex correlation with bowel length was noted in other recent studies as well; however, they concluded that this correlation was likely due to differences in the heights and weights between the 2 sexes.34,35 We also found that the majority of hospitalizations were of patients who were White, which is consistent with the observations made earlier from the data from the Sustain registry.9 Interestingly, despite the age group of 51–65 years being the largest, we noted that Medicare was the payer for the majority of the hospitalizations, followed by private insurance. The reasons for this may be that according to section 5.07 of the Social Security Act, SBS is considered a condition with a disability, and therefore, these patients are eligible for Medicare benefits in the US despite being age <65 years.36 We observed that majority of the hospitalizations occurred at large urban hospitals, which is likely due to the multidisciplinary, multispecialty inpatient care required for these patients.
Our study found a consistent increase in the overall number of annual hospitalizations in adult patients with SBS in the US. We observed an overall 55% increase in the number of SBS hospitalizations. Our study confirms the trend that has been reported globally that the prevalence of patients utilizing home PN has considerably increased over the last few decades, a substantial proportion of which consists of patients with SBS.9,26,27 As Brandt et al speculated earlier, this finding likely reflects increased knowledge of SBS-associated IF among medical providers and awareness regarding the management tools that are readily accessible in developed countries, which includes home PN.26,37 The incidence and prevalence of medical comorbidities necessitating bowel resection are also on the rise, which include inflammatory bowel disease,38 the burden of vascular diseases and associated complications,39 intra-abdominal malignancies, specifically colorectal cancers,40 pancreatic cancers,41 and uterine42 and prostatic cancers,43 as well as the total number of patients receiving radiation therapy for these malignancies, has also increased.44 Additionally, the surgical catastrophes of postoperative vascular and obstructive complications leading to massive intestinal resection also seem to be increasing.31 However, this observed increase in hospitalizations can also be due to better outcomes in terms of prolonged survival in patients with SBS, as home PN has been reported to provide better long-term survival and quality of life at a lower cost.45–47 Overall 5-year survival in patients with SBS has been reported to be as high as 78%–87%.6,46,47 Although identification of causes for an increase in hospitalizations is beyond the scope of the current study, it seems that a significant proportion of patients are relatively younger when they are diagnosed with SBS and survive longer, which could be the cause of an increase in the number of hospitalizations.31,48 We noted that majority of patients hospitalized with SBS had higher severity of illness and (as Jeppesen had pointed out previously) that during last several years, the indication of home PN has been expanded to cover all causes of SBS, which includes benign and malignant causes in all age groups and specifically includes many elderly patients with malignant diseases.26,37 Several studies have reported that more patients with advanced malignancy are also being discharged with home PN.49,50 Consideration of these changes in the characteristics of patient population is important for better understanding of these demographic changes. It has also been reported that patients with chronic diseases of the gastrointestinal system with a higher comorbidity burden are also more likely to be hospitalized.27,51 During last several years, involvement of hospitalists and internists in the clinical care of patients receiving PN has also increased, and they play an active role in the initiation of PN, as well as the smooth transition to an outpatient PN care plan.52,53
We noted a gradual overall decrease in all-cause in-hospital mortality during our 10-year study period, which likely reflects an improvement in survival and other prognostic outcomes in patients with SBS.29,46,47 There has been a substantial development in both medical and surgical therapeutic interventions that are available for patients with SBS.24,54–60 For the last several decades, many scholars across the globe have reported and highlighted the importance of nutrition in these patients, as a result of which the nutrition support for SBS has seen a remarkable transformation.1,2,61 Home PN care is easily accessible in the majority of developed countries, including the US, and patients can self-care and infuse the PN at home.23,62 However, because of medical advancements, PN is no longer considered the final option for management of these patients, and newer medications, which include enterohormonal therapy with glucagon-like peptide analogs such as teduglutide, have shown promising results and are now available for management of SBS.63–66 Bowel-lengthening procedures, including serial transverse enteroplasty procedure and intestinal transplants, are also increasingly being utilized.57 There has been a substantial increase in the knowledge of complications associated with IF and PN use.67–69 Patients are closely monitored for some of these major complications, which include catheter-related infection and IF-associated liver disease (IFALD), and measures are taken to limit the consequences of them.70–72 The spectrum of care provided to these patients has further expanded and now encompasses many other aspects of their care, including quality of life and psychosocial support via multidisciplinary approaches.54,63,73–76 Several professional organizations from North America and Europe, such as the American Society for Parenteral and Enteral Nutrition and the European Society for Clinical Nutrition and Metabolism, and nonprofit organizations, such as the Oley Foundation, Crohn’s and Colitis Foundation, Short Bowel Syndrome Foundation, and National Organization of Rare Diseases, have also dedicated a significant amount of their resources to improve the outcomes in patients with SBS.77,78 Formations of online communities of medical professionals specializing in IF management have also streamlined various aspects of the medical and surgical care of these patients by bringing together several experts of the field on 1 platform.79 As a result of the combined impact of these efforts, the overall mortality in patients with SBS seems to have trended down despite the increase in the severity of their illness and comorbidities.26
We also identified the independent risk factors associated with in-hospital mortality by performing a multivariable logistic regression analysis and adjusted for demographic factors and all major patient comorbidities, including the Elixhauser comorbidities. We found that hospitalized older patients with SBS were at a significantly higher risk of mortality, with patients aged >65 years having the highest risk. Our findings are consistent with previously reported data from longitudinal studies.29,46 Amiot et al analyzed 10-year follow-up data of 268 patients with nonmalignant SBS and reported that the relative risk of death was significantly low in patients aged <60 years.29 In another study of 68 patients, Vantini et al found that home PN patients aged <45 years had 100% 4-year survival in contrast to only 44% 4-year survival in patients aged >45 years.46 Interestingly, apart from age, we did not find significant differences in mortality between other demographic factors, such as sex and ethnicity, and insurance status (Medicare, Medicaid, and private insurance).
Previous studies have reported that mortality in patients with SBS who have relatively shorter survival is likely due to their underlying disease, whereas in patients who have a longer survival, a substantial proportion of mortality is attributed to chronic IF and PN-related complications.26,29,46,47,80 We found that PN-related complications and patient comorbidities, such as sepsis, liver dysfunction, solid tumor with metastasis, and severe malnutrition, were associated with a significantly high independent risk of in-hospital mortality. Patients with SBS have an increased risk of sepsis because of several reasons, which include having a surgical site infection after gastrointestinal surgery, an ileostomy or an anastomotic bowel leak, intra-abdominal abscesses, and a central venous catheter for prolonged periods.81–84 Central line–associated bloodstream infections (CLABSIs) are one of the most common complications of home PN.84–86 Dreesen et al conducted a multicenter, multinational survey of home PN patients from 8 countries and found that catheter-related infection was perceived as the most important indicator of quality of their care by patients receiving home PN.87 During the last decade, many strategies have been implemented to reduce the rate of catheter-related infections, including utilization of tunneled catheters, ethanol, and taurolidine lock therapy in combination with extensive patient education surrounding hygienic practices paired with closer nursemonitoring.70,88,89 As a result of heightened awareness and continuous efforts for infection prevention, rates of CLABSI have significantly improved.70,84,90–92 Our study confirms that sepsis is the most important modifiable risk factor to prevent in-hospital mortality in patients with SBS, and therefore, home PN programs should continue their efforts to maintain the lowest possible infection rates.93,94
Liver disease is also a common complication in patients with IF.72 Studies have reported that abnormal levels of liver enzymes have been observed in many patients receiving home PN; however, specific diagnostic criteria for the diagnosis of IFALD is lacking, which has resulted in variability in the reported prevalence.95,96 A prospective cohort study by Cavicchi et al observed that the risk of PN-related liver disease increased with a longer duration of PN, and 65% of patients had chronic cholestasis after 6 months of home PN, whereas 41.5% of patients developed complicated liver disease with either extensive fibrosis or cirrhosis over 37 months of home PN.97 In addition to the duration of PN, other factors associated with a higher risk of IFALD are shorter length of the remaining bowel,98 small intestinal bacterial overgrowth,99,100 and overfeeding of glucose and lipid,101 as well as underfeeding, which could result in nutrient deficiencies, such as essential fatty-acid, choline, and vitamin E deficiencies.68,101 Several guidelines from nutrition societies are established for the prevention and management of IFALD, including frequent monitoring of liver function, cycled feeding, and limiting the dose of soybean oil–based lipid infusions, which can help clinicians in decision-making and can potentially decrease excess mortality.101–104 We found that mortality in patients who have SBS secondary to inflammatory bowel disease is relatively lower, which is consistent with reported higher survival rates in this patient population in recent multicenter studies.105 We found that severe malnutrition also increased the risk of mortality, which highlights the importance of early protein-energy optimization of these patients to decrease this excess mortality risk.
We did not find significant differences in mortality between hospitals of different bed sizes and teaching vs nonteaching hospitals. However, mortality in rural hospitals was higher compared with urban hospitals. This could perhaps be because these patients might not have a personal desire or a potential of benefitting from further advanced surgical and intestinal rehabilitation that would be available in an urban tertiary center. Interestingly, we also found that mortality in the Midwest was relatively lower compared with other regions in the US. Our study is not designed to explore this further; however, it should be noted that there is a substantially higher number of centers for SBS specialty care located in this region compared with other geographic regions in the US.106 Alternatively, this perceived lower mortality could also be due to a higher rate of hospitalizations in this geographic region.
We found that hospitalizations in patients with SBS were associated with a substantially high healthcare utilization, with the average hospital LOS and hospitalization cost being 14.7 days and $34,130 (in 2014 constant US dollars), respectively. Thus, the average healthcare utilization in patients with SBS is 3-fold higher compared with the overall mean LOS and hospital cost reported for the hospitalizations across the US.107 We found that the LOS in patients with SBS is gradually decreasing, which likely reflects a trend toward the early transition from hospital to home care because well-established home PN resources are now easily accessible across the US.108 We noted that the inflation-adjusted mean hospital cost for SBS hospitalizations has remained relatively stable during the last several years. This trend is particularly promising, since the inflation-adjusted mean cost per hospitalization for all other inpatient stays has increased by 12.7% during the same time period.109 This decrease in healthcare utilization and hospital costs, with a concurrent decrease in in-hospital mortality, suggests an improvement in overall healthcare delivery in patients with SBS in the US.
There are several limitations to our study. This is a retrospective, large-database study consisting of discharge-level medical records of inpatient hospitalizations; therefore, individual chart review is not possible, and validity of analysis depends on the accuracy of diagnoses and procedures documented by utilizing the ICD-9 coding system at the time of hospitalization. Although te accuracy of discharge-level data is high, ranging from 80%–95% sensitivity,110 it is susceptible to inaccurately entered and missing codes, which could decrease its accuracy for comorbidities.111 The HCUP provides limited data elements; therefore, clinical information, such as length of remaining bowel, type and specifics of PN formula, and type of intravenous catheters, were not available. It is possible that our case selection criteria could underestimate the true number of SBS cases and may not account for patients with SBS who are not receiving PN. It should be noted that ICD-9 code 579.3 is a heterogenous group, and it could include patients with limited small-bowel resection or gastrectomy or patients who underwent bariatric operative procedures. Also, some comorbidities, such as hypomagnesemia, are more likely to be susceptible to undercoding, and our estimates may not reflect exact prevalence of these electrolyte disturbances. The NIS database does not provide access to the laboratory values at the time of hospitalization; therefore, accurate estimation of these electrolytes abnormalities could not be made. We limited our analysis until 2014 because the ICD-10 coding system was introduced in 2015, and therefore, the data from 2015 have a mixture of codes from both coding systems. To maintain a higher reliability in measuring yearly trends, it was important to limit this study to a single uniform coding system throughout the study period. Since this is an observational study, an association does not imply a causal relationship between the variables. Our mortality analysis is limited to in-hospital mortality, and we were not able to estimate a 30- or 90-day mortality because the NIS data do not follow patients after their discharge from the hospital. Although we utilized a robust regression model and adjusted for all of the possible confounders, there is still the risk of residual confounding.
Despite these limitations, this study has many strengths. Our study consists of 10 years of hospitalization data from across the US, which enabled us to study a large sample size with a higher statistical power. The utilization of the national data also eliminates the bias generated from a single-center or small multicenter study, and therefore, our findings are more generalizable. This is also the first study in the literature that utilizes the NIS data to study the hospitalization outcomes in adult patients with SBS. The HCUP data also enabled us to study the trends in mortality as well as healthcare utilization, including the LOS and hospital cost of care.
In conclusion, by analysing 10 years of national data, our study finds an increase in the number of hospitalizations in patients with SBS and a decrease in their in-hospital mortality. Demographic and clinical factors, such as older age, sepsis, liver dysfunction, severe malnutrition, and metastatic cancer, were associated with a higher risk of mortality. We found a trend that reflected an overall improvement in the healthcare delivery in patients hospitalized with SBS, which was evident from decreased healthcare utilization in terms of decreasing LOS and of relatively stable cost of care.
Supplementary Material
Clinical Relevancy Statement.
Clinical care of short-bowel syndrome (SBS) has substantially evolved during the last few decades. Several studies on single-center and multicenter registries of home parenteral nutrition have reported an overall increase in the number of patients with SBS concurrently with improved survival. We report the first study in the literature that analyzes the demographic trends in hospitalizations and in-hospital mortality over a 10-year study period. We used the Nationwide Inpatient Sample database, which is the largest database containing US inpatient hospitalization records. Our study assesses the effectiveness of the overall healthcare delivery to patients with SBS. We also identified the risk factors associated with in-hospital mortality, which can help clinicians in identifying patients at higher risk of mortality.
Appendix 1. List of ICD-9 Diagnosis and Procedure Codes
Multivariable Logistic Regression Analysis of Factors Associated With In-Hospital Mortality in Cases Admitted With SBS
| Diagnosis or Procedure Name | ICD-9th-CM Codes |
|---|---|
| Postsurgical nonabsorption | 579.3 |
| Parenteral infusion of nutrition substances | 99.15 |
| Congestive heart failure | 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4–425.9, 428.x |
| Diabetes, complicated | 250.4–250.9 |
| Sepsis | 038.0, 038.10, 038.11, 038.12, 038.19, 038.2, 038.3, 038.40, 038.41, 038.42, 038.43, 038.44, 038.49, 038.8, 038.9, 995.91, 995.92, 999.39, 790.7, 785.52, 112.5, 112.81, 999.31, 999.32, 999.33 |
| Liver disease | 070.22, 070.23, 070.32, 070.33, 070.44, 070.54, 070.6, 070.9, 456.0–456.2, 570.x, 571.x, 572.2–572.8, 573.3, 573.4, 573.8, 573.9, V42.7 |
| Metastatic cancer | 196.x–199.x |
| Fluid and electrolyte disorders | 253.6, 276.x |
| Psychoses | 293.8, 295.x, 296.04, 296.14, 296.44, 296.54, 297.x, 298.x |
| Depression | 296.2, 296.3, 296.5, 300.4, 309.x, 311 |
Footnotes
Financial disclosure: None declared.
Conflicts of interest: None declared.
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.DiBaise JK, Young RJ, Vanderhoof JA. Intestinal rehabilitation and the short bowel syndrome: part 1. Am J Gastroenterol. 2004;99(7):1386–1395. [DOI] [PubMed] [Google Scholar]
- 2.Purdum PP 3rd, , Kirby DF. Short-bowel syndrome: a review ofthe role of nutrition support. JPEN J Parenter Enteral Nutr. 1991;15(1):93–101. [DOI] [PubMed] [Google Scholar]
- 3.Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr. 2014;38(4):427–437. [DOI] [PubMed] [Google Scholar]
- 4.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology. 1997;113(5):1767–1778. [DOI] [PubMed] [Google Scholar]
- 5.Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38(1_suppl):8S–13S. [DOI] [PubMed] [Google Scholar]
- 6.Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117(5):1043–1050. [DOI] [PubMed] [Google Scholar]
- 7.Howard L, Ament M, Fleming CR, Shike M, Steiger E. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology. 1995;109(2):355–365. [DOI] [PubMed] [Google Scholar]
- 8.Howard L, Heaphey L, Fleming CR, Lininger L, Steiger E. Four years of North American registry home parenteral nutrition outcome data and their implications for patient management. JPEN J Parenter Enteral Nutr. 1991;15(4):384–393. [DOI] [PubMed] [Google Scholar]
- 9.Winkler MF, DiMaria-Ghalili RA, Guenter P, et al. Characteristics of a Cohort of Home Parenteral Nutrition Patients at the Time of Enrollment in the Sustain Registry. JPEN J Parenter Enteral Nutr. 2016;40(8):1140–1149. [DOI] [PubMed] [Google Scholar]
- 10.Bakker H, Bozzetti F, Staun M, et al. Home parenteral nutrition in adults: a European multicentre survey in 1997. ESPEN-Home Artificial Nutrition Working Group. Clin Nutr. 1999;18(3):135–140. [DOI] [PubMed] [Google Scholar]
- 11.Pant C, Sferra TJ, Fischer RT, Olyaee M, Gilroy R. Epidemiology and Healthcare Resource Utilization Associated With Children With Short Bowel Syndrome in the United States. JPEN J Parenter Enteral Nutr. 2017;41(5):878–883. [DOI] [PubMed] [Google Scholar]
- 12.Ireton-Jones C, DeLegge M. Home parenteral nutrition registry: a five-year retrospective evaluation of outcomes of patients receiving home parenteral nutrition support. Nutrition. 2005;21(2):156–160. [DOI] [PubMed] [Google Scholar]
- 13.HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2005–2014. Agency for Healthcare Research and Quality. www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed April 1, 2020. [Google Scholar]
- 14.Elixhauser ASC, Kruzikas D. Comorbidity Software Documentation, January 2004. HCUP Method Series Report # 2004–01. U.S. Agency for Healthcare Research and Quality. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Published 2004. Accessed April 1, 2020. [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):827. [DOI] [PubMed] [Google Scholar]
- 16.Ondeck NT, Bohl DD, Bovonratwet P, McLynn RP, Cui JJ, Grauer JN. Discriminative Ability of Elixhauser’s Comorbidity Measure is Superior to Other Comorbidity Scores for Inpatient Adverse Outcomes After Total Hip Arthroplasty. J Arthroplasty. 2018;33(1):250–257. [DOI] [PubMed] [Google Scholar]
- 17.HCUP National In-patient Sample. Cost-to-Charge Ratio Files. https://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. Accessed May 25, 2020.
- 18.Department U.S. of Labor Bureau of Labor Statistic. Consumer Price Index (CPI) Databases. https://www.bls.gov/cpi/data.htm. Accessed May 25, 2020.
- 19.Dudrick SJ. Intravenous hyperalimentation. Surgery. 1970;68(4):726–727. [PubMed] [Google Scholar]
- 20.Dudrick SJ, Steiger E, Long JM, Rhoads JE. Role of parenteral hyperalimentation in management of multiple catastrophic complications. Surg Clin North Am. 1970;50(5):1031–1038. [DOI] [PubMed] [Google Scholar]
- 21.Steiger E, Daly JM, Allen TR, Dudrick SJ, Vars HM. Postoperative intravenous nutrition: effects on body weight, protein regeneration, wound healing and liver morphology. Surgery. 1973;73(5):686–691. [PubMed] [Google Scholar]
- 22.Steiger E. Jonathan E Rhoads lecture: experiences and observations in the management of patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2007;31(4):326–333. [DOI] [PubMed] [Google Scholar]
- 23.Kirby DF, Corrigan ML, Hendrickson E, Emery DM. Overview of Home Parenteral Nutrition: An Update. Nutr Clin Pract. 2017;32(6):739–752. [DOI] [PubMed] [Google Scholar]
- 24.Shatnawei A, Parekh NR, Rhoda KM, et al. Intestinal failuremanagementattheClevelandClinic.ArchSurg.2010;145(6):521–527. [DOI] [PubMed] [Google Scholar]
- 25.Wanden Berghe C, Gomez Candela C, Chicharro L, et al. Home parenteral nutrition registry in Spain for the year 2010: NADYA-SENPE Group. Nutr Hosp. 2011;26(6):1277–1282. [DOI] [PubMed] [Google Scholar]
- 26.Brandt CF, Hvistendahl M, Naimi RM, et al. Home Parenteral Nutrition in Adult Patients With Chronic Intestinal Failure: The Evolution Over 4 Decades in a Tertiary Referral Center. JPEN J Parenter Enteral Nutr. 2017;41(7):1178–1187. [DOI] [PubMed] [Google Scholar]
- 27.Fuglsang KA, Brandt CF, Scheike T, Jeppesen PB. Hospitalizations in Patients With Nonmalignant Short-Bowel Syndrome Receiving Home Parenteral Support. Nutr Clin Pract. 2020. [DOI] [PubMed] [Google Scholar]
- 28.Pironi L, Hebuterne X, Van Gossum A, et al. Candidates for intestinal transplantation: a multicenter survey in Europe. Am J Gastroenterol. 2006;101(7):1633–1643; quiz 1679. [DOI] [PubMed] [Google Scholar]
- 29.Amiot A, Messing B, Corcos O, Panis Y, Joly F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr. 2013;32(3):368–374. [DOI] [PubMed] [Google Scholar]
- 30.Wing VK, Song Y, Xiang C, et al. Incidence of catheter-related complications among Japanese patients with central venous catheters as well as patients with short bowel syndrome. Clin Exp Gastroenterol. 2018;11:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson JS, DiBaise JK, Iyer KR, Yeats M, Sudan DL. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201(1):85–89. [DOI] [PubMed] [Google Scholar]
- 32.Nightingale JM, Lennard-Jones JE, Gertner DJ, Wood SR, Bartram CI. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33(11):1493–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nightingale J, Woodward JM, Small B, Nutrition Committee of the British Society of G. Guidelines for management of patients with a short bowel. Gut. 2006;55 (Suppl 4):iv1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raines D, Arbour A, Thompson HW, Figueroa-Bodine J, Joseph S. Variation in small bowel length: factor in achieving total enteroscopy? Dig Endosc. 2015;27(1):67–72. [DOI] [PubMed] [Google Scholar]
- 35.Hounnou G, Destrieux C, Desme J, Bertrand P, Velut S. Anatomical study of the length of the human intestine. Surg Radiol Anat. 2002;24(5):290–294. [DOI] [PubMed] [Google Scholar]
- 36.Social Security Administration. Disability Evaluation Under Social Security. 5.00 Digestive System - Adult. https://www.ssa.gov/disability/professionals/bluebook/5.00-Digestive-Adult.htm. Published 2020. Accessed July 20, 2020.
- 37.Jeppesen P. Short bowel syndrome: Definition, Classification, Etiology, Epidemiology, Survival, and Costs. In: John K, DiBaise CRP, Jon S. Thompson, ed. Short bowel syndrome: Practical approach to management, CRC Press, Taylor and Francis Group; 2017:1–10. [Google Scholar]
- 38.Collaborators GBDIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020;158(2):341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10(1):10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henley SJ, Miller JW, Dowling NF, Benard VB, Richardson LC. Uterine Cancer Incidence and Mortality - United States, 1999–2016. MMWR Morb Mortal Wkly Rep. 2018;67(48):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawla P. Epidemiology of Prostate Cancer. World J Oncol. 2019;10(2):63–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahase SS, D’Angelo D, Kang J, Hu JC, Barbieri CE, Nagar H. Trends in the Use of Stereotactic Body Radiotherapy for Treatment of Prostate Cancer in the United States. JAMA Netw Open. 2020;3(2):e1920471. [DOI] [PubMed] [Google Scholar]
- 45.Richards DM, Irving MH. Cost-utility analysis of home parenteral nutrition. Br J Surg. 1996;83(9):1226–1229. [DOI] [PubMed] [Google Scholar]
- 46.Vantini I, Benini L, Bonfante F, et al. Survival rate and prognostic factors in patients with intestinal failure. DigLiverDis.2004;36(1):46–55. [DOI] [PubMed] [Google Scholar]
- 47.Pironi L, Joly F, Forbes A, et al. Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut. 2011;60(1):17–25. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe K, Sasaki I, Fukushima K, et al. Long-term incidence and characteristics of intestinal failure in Crohn’s disease: a multicenter study. J Gastroenterol. 2014;49(2):231–238. [DOI] [PubMed] [Google Scholar]
- 49.Vashi PG, Dahlk S, Popiel B, Lammersfeld CA, Ireton-Jones C, Gupta D. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer. 2014;14:1, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sowerbutts AM, Lal S, Sremanakova J, et al. Discharging Women with Advanced Ovarian Cancer on Home Parenteral Nutrition: Making and Implementing the Decision. Nutrients. 2020;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen NH, Khera R, Ohno-Machado L, Sandborn WJ, Singh S. Annual burden and costs of hospitalization for high-need, high-cost patients with chronic gastrointestinal and liver diseases. Clin Gastroenterol Hepatol. 2018;16(8):1284-1292 e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mundi MS, Nystrom EM, Hurley DL, McMahon MM. Management of Parenteral Nutrition in Hospitalized Adult Patients [Formula: see text]. JPEN J Parenter Enteral Nutr. 2017;41(4): 535–549. [DOI] [PubMed] [Google Scholar]
- 53.Kirkland LL, Kashiwagi DT, Brantley S, Scheurer D, Varkey P. Nutrition in the hospitalized patient. J Hosp Med. 2013;8(1):52–58. [DOI] [PubMed] [Google Scholar]
- 54.Parrish CR, DiBaise JK. Managing the adult patient with short bowel syndrome. Gastroenterol Hepatol (N Y). 2017;13(10):600–608. [PMC free article] [PubMed] [Google Scholar]
- 55.Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol. 2011;17(4):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yildiz BD. Where are we at with short bowel syndrome and small bowel transplant. World J Transplant. 2012;2(6):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abu-Elmagd KM, Armanyous SR, Fujiki M, et al. Management of five hundred patients with gut failure at a single center: surgical innovation versus transplantation with a novel predictive model. Ann Surg. 2019;270(4):656–674. [DOI] [PubMed] [Google Scholar]
- 58.Bharadwaj S, Tandon P, Gohel TD, et al. Current status of intestinal and multivisceral transplantation. Gastroenterol Rep (Oxf). 2017;5(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bharadwaj S, Tandon P, Rivas JM, et al. Update on the management of intestinal failure. Cleve Clin J Med. 2016;83(11):841–848. [DOI] [PubMed] [Google Scholar]
- 60.Jeppesen PB. The Long Road to the Development of Effective Therapies for the Short Gut Syndrome: A Personal Perspective. Dig Dis Sci. 2019;64(10):2717–2735. [DOI] [PubMed] [Google Scholar]
- 61.DiBaise JK, Young RJ, Vanderhoof JA. Intestinal rehabilitation and the short bowel syndrome: part 2. Am J Gastroenterol. 2004;99(9):1823–1832. [DOI] [PubMed] [Google Scholar]
- 62.Kirby DF, Corrigan ML, Speerhas RA, Emery DM. Home parenteral nutrition tutorial. JPEN J Parenter Enteral Nutr. 2012;36(6):632–644. [DOI] [PubMed] [Google Scholar]
- 63.Chen K, Mu F, Xie J, et al. Impact of Teduglutide on Quality of Life Among Patients With Short Bowel Syndrome and Intestinal Failure. JPEN J Parenter Enteral Nutr. 2020;44(1): 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puello F, Wall E, Herlitz J, Lozano ES, Semrad C, Micic D. LongTerm Outcomes With Teduglutide From a Single Center. JPEN J Parenter Enteral Nutr. 2020. [DOI] [PubMed] [Google Scholar]
- 65.Pape UF, Iyer KR, Jeppesen PB, et al. Teduglutide for the treatment of adults with intestinal failure associated with short bowel syndrome: pooled safety data from four clinical trials. Therap Adv Gastroenterol. 2020;13:1756284820905766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeppesen PB. Gut hormones in the treatment of short-bowel syndrome and intestinal failure. Curr Opin Endocrinol Diabetes Obes. 2015;22(1):14–20. [DOI] [PubMed] [Google Scholar]
- 67.Mundi M, Kuchkuntla AR, Hurt RT. Metabolic Complications of Home Parenteral Nutrition and Short Bowel Syndrome: Metabolic Bone Disease, Hyperglycemia, Dehydration, and D-Lactic Acidosis. In: Corrigan M, Roberts K, Steiger E, eds. Adult Short bowel syndrome: Nutritional, Medical, and Surgical Management. Elsevier Academic Press; 2018:109–127. [Google Scholar]
- 68.Huijbers A, Wanten GJA. Hepatobiliary Complications of Intestinal Failure and Home Parenteral Nutrition. In: Corrigan M, Roberts K, Steiger E, eds. Adult short bowel syndrome: nutritional, medical, and surgical management. Elsevier Academic Press; 2018: 129–145. [Google Scholar]
- 69.Opilla M. Catheter-Related Complications of Home Parenteral Nutrition. In: Corrigan M, Roberts K, Steiger E, eds. Adult short bowel syndrome: Nutritional, Medical, and Surgical Management. Elsevier Academic Press, 2018:147–163. [Google Scholar]
- 70.John BK, Khan MA, Speerhas R, et al. Ethanol lock therapy in reducing catheter-related bloodstream infections in adult home parenteral nutrition patients: results of a retrospective study. JPEN J Parenter Enteral Nutr. 2012;36(5):603–610. [DOI] [PubMed] [Google Scholar]
- 71.Norsa L, Nicastro E, Di Giorgio A, Lacaille F, D’Antiga L. Prevention and Treatment of Intestinal Failure-Associated Liver Disease in Children. Nutrients. 2018;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130(2):S70–77. [DOI] [PubMed] [Google Scholar]
- 73.McFadden MA, DeLegge MH, Kirby DF. Medication delivery in the short-bowel syndrome. JPEN J Parenter Enteral Nutr. 1993;17(2):180–186. [DOI] [PubMed] [Google Scholar]
- 74.Rhoda KM, Parekh NR, Lennon E, et al. The multidisciplinary approach to the care of patients with intestinal failure at a tertiary care facility. Nutr Clin Pract. 2010;25(2):183–191. [DOI] [PubMed] [Google Scholar]
- 75.Winkler MF, Smith CE. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38(1_suppl):32S–37S. [DOI] [PubMed] [Google Scholar]
- 76.Saqui O, Fernandes G, Allard JP. Quality of life analysis during transition from stationary to portable infusion pump in home parenteral nutrition patients: a Canadian experience. Nutr Clin Pract. 2014;29(1):131–141. [DOI] [PubMed] [Google Scholar]
- 77.Short Bowel Syndrome Foundation. Short Bowel Syndrome Foundation. http://shortbowelfoundation.org/. Accessed July 20, 2020.
- 78.Andolina JM, Metzger LC, Bishop J. The Oley Foundation and Consumer Support Groups. Gastroenterol Clin North Am. 2019;48(4):625–635. [DOI] [PubMed] [Google Scholar]
- 79.Learn Intestinal Failure TeleECHO Clinic. Learn Intestinal Failure TeleECHO Clinic. https://liftecho.org/web/resources. Accessed July 20, 2020.
- 80.Schalamon J, Mayr JM, Hollwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2003;17(6):931–942. [DOI] [PubMed] [Google Scholar]
- 81.Choudhuri AH, Uppal R. Predictors of septic shock following anastomotic leak after major gastrointestinal surgery: An audit from a tertiary care institute. Indian J Crit Care Med. 2013;17(5):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.GlobalSurg C. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis. 2018;18(5):516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang MK, Li LT, Avellaneda A, Moffett JM, Hicks SC, Awad SS. Outcomes and predictors of incisional surgical site infection in stoma reversal. JAMA Surg. 2013;148(2):183–189. [DOI] [PubMed] [Google Scholar]
- 84.Ross VM, Guenter P, Corrigan ML, et al. Central venous catheter infections in home parenteral nutrition patients: Outcomes from Sustain: American Society for Parenteral and Enteral Nutrition’s National Patient Registry for Nutrition Care. Am J Infect Control. 2016;44(12):1462–1468. [DOI] [PubMed] [Google Scholar]
- 85.O’Connor A, Hanly AM, Francis E, Keane N, McNamara DA. Catheter associated blood stream infections in patients receiving parenteral nutrition: a prospective study of 850 patients. J Clin Med Res. 2013;5(1):18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dreesen M, Foulon V, Spriet I, et al. Epidemiology of catheter-related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr. 2013;32(1):16–26. [DOI] [PubMed] [Google Scholar]
- 87.Dreesen M, Pironi L, Wanten G, et al. Outcome Indicators for Home Parenteral Nutrition Care: Point of View From Adult Patients With Benign Disease. JPEN J Parenter Enteral Nutr. 2015;39(7):828–836. [DOI] [PubMed] [Google Scholar]
- 88.Hon K, Bihari S, Holt A, Bersten A, Kulkarni H. Rate of catheter-related bloodstream infections between tunneled central venous catheters versus peripherally inserted central catheters in adult home parenteral nutrition: a meta-analysis. JPEN J Parenter Enteral Nutr. 2019;43(1):41–53. [DOI] [PubMed] [Google Scholar]
- 89.Klek S, Szczepanek K, Hermanowicz A, Galas A. Taurolidine lock in home parenteral nutrition in adults: results from an open-label randomized controlled clinical trial. JPEN J Parenter Enteral Nutr. 2015;39(3):331–335. [DOI] [PubMed] [Google Scholar]
- 90.Wouters Y, Causevic E, Klek S, Groenewoud H, Wanten GJA. Use of Catheter Lock Solutions in Patients Receiving Home Parenteral Nutrition: A Systematic Review and Individual-Patient Data Meta-analysis. JPEN J Parenter Enteral Nutr. 2020;44(7):1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pichitchaipitak O, Ckumdee S, Apivanich S, Chotiprasitsakul D, Shantavasinkul PC. Predictive factors of catheter-related blood-stream infection in patients receiving home parenteral nutrition. Nutrition. 2018;46:1–6. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Wang B, Wang J, Yang Q. Ethanol locks for the prevention of catheter-related infection in patients with central venous catheter: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2019;14(9):e0222408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leiberman D, Stevenson RP, Banu FW, Gerasimidis K, McKee RF. The incidence and management of complications of venous access in home parenteral nutrition (HPN): A 19 year longitudinal cohort series. Clin Nutr ESPEN. 2020;37:34–43. [DOI] [PubMed] [Google Scholar]
- 94.Bond A, Teubner A, Taylor M, et al. Catheter-related infections in patients with acute type II intestinal failure admitted to a national centre: Incidence and outcomes. Clin Nutr. 2019;38(4):1828–1832. [DOI] [PubMed] [Google Scholar]
- 95.Salvino R, Ghanta R, Seidner DL, Mascha E, Xu Y, Steiger E.Liver failure is uncommon in adults receiving long-term parenteral nutrition. JPEN J Parenter Enteral Nutr. 2006;30(3):202–208. [DOI] [PubMed] [Google Scholar]
- 96.Sasdelli AS, Agostini F, Pazzeschi C, Guidetti M, Lal S, Pironi L. Assessment of Intestinal Failure Associated Liver Disease according to different diagnostic criteria. Clin Nutr. 2019;38(3):1198–1205. [DOI] [PubMed] [Google Scholar]
- 97.Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132(7):525–532. [DOI] [PubMed] [Google Scholar]
- 98.Cazals-Hatem D, Billiauws L, Rautou PE, et al. Ultra-short bowel is an independent risk factor for liver fibrosis in adults with home parenteral nutrition. Liver Int. 2018;38(1):174–182. [DOI] [PubMed] [Google Scholar]
- 99.Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol. 2006;4(1):11–20. [DOI] [PubMed] [Google Scholar]
- 100.Fialho A, Fialho A, Thota P, McCullough AJ, Shen B. Small Intestinal Bacterial Overgrowth Is Associated with Non-Alcoholic Fatty Liver Disease. J Gastrointestin Liver Dis. 2016;25(2): 159–165. [DOI] [PubMed] [Google Scholar]
- 101.Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35(2):247–307. [DOI] [PubMed] [Google Scholar]
- 102.Beath SV, Kelly DA. Total Parenteral Nutrition-Induced Cholestasis: Prevention and Management. Clin Liver Dis. 2016;20(1):159–176. [DOI] [PubMed] [Google Scholar]
- 103.Raman M, Almutairdi A, Mulesa L, Alberda C, Beattie C, Gramlich L. Parenteral Nutrition and Lipids. Nutrients. 2017;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arenas Villafranca JJ, Nieto Guindo M, Alvaro Sanz E, Moreno Santamaria M, Garrido Siles M, Abiles J. Effects of cyclic parenteral nutrition on parenteral-associated liver dysfunction parameters. Nutr J. 2017;16(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joly F, Baxter J, Staun M, et al. Five-year survival and causes of death in patients on home parenteral nutrition for severe chronic and benign intestinal failure. Clin Nutr. 2018;37(4):1415–1422. [DOI] [PubMed] [Google Scholar]
- 106.Short Bowel Syndrome Foundation. Short Bowel Syndrome Foundation: List of Centers of Excellence. http://shortbowelfoundation.org/centers-of-excellence/ Accessed July 20, 2020.
- 107.Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief #180. In. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD), 2014. [Google Scholar]
- 108.Kumpf VJ, Tillman EM. Home parenteral nutrition: safe transition from hospital to home. Nutr Clin Pract. 2012;27(6):749–757. [DOI] [PubMed] [Google Scholar]
- 109.McDermott K, Elixhauser A, Sun R. Trends in Hospital InpatientStays in the United States, 2005–2014.HCUP Statistical Brief #225. Agency for Healthcare Research and Quality. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.pdf. Published 2017. Accessed July 20, 2020. [Google Scholar]
- 110.Stavrou E, Pesa N, Pearson SA. Hospital discharge diagnostic and procedure codes for upper gastro-intestinal cancer: how accurate are they? BMC Health Serv Res. 2012;12:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8Suppl):IV-26–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


