ABSTRACT
Background:
In recent times, single-sitting root canal therapy has gained momentum over multiple-sitting root canal therapy due to its superior clinical outcome and less time required for treating the patient. Aim: Thus, the aim of current study was to compare the expression of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and high-sensitivity C-reactive protein (hs-CRP) in the serum of patients undergoing single-sitting and multiple-sitting root canal treatment.
Materials and Methods:
This cross-sectional experimental study was conducted on 300 subjects who were indicated for root canal treatment. Subjects were categorized into Group I (single visit) and Group II (multiple visits).Clinical data was obtained and serum samples were collected both before and after 1 week of treatment completion. Inclusion criteria were those patients (a) over 18 years of age, (b) without any disease of inflammatory etiology, and (c) who had not previously received endodontic treatment or any related therapeutic treatment. Exclusion criteria were those (a) without a complete clinical history, (b) with greater than one indicated tooth, (c) who did not complete their treatment, and (d) with any periodontal disease. Chi-square and Student’s t-test were applied.
Results:
It was found that in single-sitting root canal treatment, there was a statistically significant reduction in these inflammatory biomarkers, although no difference in clinical efficacy was observed.
Conclusion:
Single-visit root canal treatment is a better option for treatment of pulpitis compared to multiple-sitting treatment.
Keywords: High-Sensitivity C-Reactive Protein, interleukin-6, multiple sitting, tumor necrosis factor-α, serum, single sitting
Introduction
Pulpitis is one of the most common diseases in clinical dentistry.[1] It is associated with intense pain; hence, it impacts a person’s quality of life in good measure. It can be classified into (a) acute and (b) chronic types depending upon duration of history associated with the indicated tooth.[2] Both acute and chronic forms of pulpitis are due to bacteria resulting in infections. All patients almost commonly report with severe pain at the time of clinical presentation.[3,4]
At present, clinical management of pulpitis chiefly involves root canal treatment, wherein infectious canals are cleaned by using either mechanical or chemical methods. After the process of disinfection, the affected root canal is hermetically sealed, which blocks the connection up to the periapical tissues, thus preventing the progression of the disease.[5]
Conventionally performed root canal procedure involves multiple therapeutic applications for complete eradication of bacterial infection and usually takes a long period of time.[6]
Single-sitting or one-time root canal treatment for pulpitis has slowly gained attention of dental practitioners and its usage is gradually increasing momentum. This method of root canal treatment reduces the total number of dentists’ visits, thus saving both patient’s and doctor’s time. Additionally, single-visit root canal therapy causes reduction of repeated root canal infections that may be the result of microleakage of endodontic sealer in periapical tissues during sealing of root canal. Peripheral bodily fluids like gingival crevicular fluid, plasma, and serum have been identified as markers of acute and chronic periapical inflammatory processes.[7]
The acute-phase reactant response or high-sensitivity C-reactive protein (hs-CRP) reaction is a significant physiologically based phenomenon that accompanies the inflammatory process and has been associated with an increase in the activity of proinflammatory cytokines.[8] Its expression is seen during the episodes resulting due to trauma, burns, infections, inflammatory processes, and conditions in which there is pathologically related inflammatory response.[9] These proteinaceous markers of inflammation have routinely been studied as indicators of underlying physiological or pathological alterations.[10]
Thus, the aim of the present study was to compare the serum expressions of interleukin-6 (IL-6), tumor necrosis factor-a (TNF-α), and hs-CRP in patients who were undergoing single-visit and multiple sittings root canal treatment.
Materials and Methods
Clinical data collection
This retrospective study analysis was conducted on 300 pulpitis patients who were categorized as follows: (a) group I: single-sitting treatment group (n = 150) and (b) group II: multiple-visit treatment group (n = 150). Institutional ethical approval was obtained before commencing the study (EC/MDC/2020/1). The inclusion criteria of the subjects were (a) patients aged more than 18 years; (b) those without any other disease of inflammatory etiology, and (c) those who had not previously received endodontic treatment or any related therapeutic treatment. The exclusion criteria for the study were (a) subjects without any complete clinical treatment history, (b) patients with greater than a single indicated tooth, (c) patients who did not complete their treatment, and (d) those with any periodontal disease. The study was commenced after receiving signed informed consent from the patients.
Reagents used
Enzyme-linked immunosorbent assay (ELISA) kits for IL-6, TNF-α, and hs-CRP were used for the measurement of serum concentrations of these inflammatory markers.
Treatment protocols followed
Patients in both the study groups were treated for infected root canals. Patients underwent routine radiological imaging before treatment for analyzing the root canals which required endodontic intervention. Patients were provided with anesthesia by using 2% lignocaine with epinephrine. Following this, rubber dam was applied and access cavity was prepared, following which pulpal tissue from the coronal and radicular portions of the tooth was extirpated. Root canals were prepared by using a ProTaper nickel–titanium rotary expansion system. During the cleaning and shaping process, root canals were flushed by using 5.25% sodium hypochlorite solution.
In group 1, root canals were presealed using calcium hydroxide and zinc oxide and were packed using gutta percha, while in group B, cleaning and shaping and gutta percha obturation were performed in subsequent steps.
Analysis of serum expressions of IL-6, TNF-α, and hs-CRP
Both before and 1 week after completion of endodontic therapy, 5 mL of venous blood was obtained from patients and was centrifuged at 3000 rpm for duration of 10 min. The separated serum was then pipetted out and stored in Eppendorf tubes for subsequent analysis.
ELISA detection was carried out as per the instructions provided by the manufacturer.The separated serum was added to 50 mL of different concentrations of standardized solution in blank micro-wells. Fifty milliliters of antibodies was added to 50 mL of distilled water within the blank wells, which served as control. Forty milliliters of the serum sample was added to the remaining micro-wells, along with 10 mL of biotin-labeled antibody which was added afterward. Subsequent to this, the plate was incubated at 37°C for time duration of 30 min. While washing off the plate, the washing fluid within each well was filled fully without any overflow and was left standing for duration of 30 s, followed by which it was discarded. After this, the plate was blotted dry. Then, 50 mL of standard enzyme solution was added to each of the wells. Then, the plate was incubated at 37°C for up to 1 h. Following this, the micro-well plate was washed up to 5 times and was then blotted dry by using an absorbent paper following final wash. Horseradish peroxidase at a concentration of 100 mL per well was added, following which the plate was incubated at 37°C for a total time of 15 min in a dark room. Following this, 100 mL of chromogenic substrate (3,3′,5,5′-tetramethylbenzidine) was then added to each well. Then, the plate was incubated for duration of 20 min at room temperature in the dark. As a final step, 50 mL of stop solution was then added to each micro-well. Spectrophotometric detection was performed using an ELISA microplate reader within a time span of 15 min. The maximum absorption wavelength at 450 nm was determined in a time span of 15 min.
Indices used for observation of values
-
(a)
Clinical evaluation: The primary observation index was the clinical efficacy of root canal treatment, which was observed before and 1 week following the completion of treatment.
-
(b)
Evaluation of markers used: Changes in the serum levels of IL-6, TNF-α, and hs-CRP were observed both before and after endodontic treatment.
Statistical methods
The collected observation data were statistically analyzed by using Statistical Package for Social Sciences (SPSS) version 20.0 software (IBM Corp., Armonk, NY, USA).
Measurement data are recorded as mean ± standard deviation (SD) values. Chi-square test was employed to compare proportions. Values that were noted both before and after treatment were analyzed using a paired t-test. P values <0.05 were used to indicate statistically significant differences between the groups studied.
Results
On analyzing the clinical data regarding the efficacy of single-visit and multiple-visit root canal treatment and the serum levels of IL-6, TNF-α, and hs-CRP, the following observations were made.
-
(a)
Efficacy of both the study groups: It was found that in group I (single-sitting) root canal treatment, 95.12% cases demonstrated successful outcome, while 6.23% were failure cases. In group II (multiple sittings), 91.05% and 9.76% cases showed success and failure, respectively. On comparing the percentages of proportions using Chi-square test, it was found that there was no statistical significance (P = 0.42) between the clinical efficacy of both the treatment groups [Table 1].
-
(b)
Serum levels of IL-6, TNF-α, and hs-CRP: Inflammatory biomarkers IL-6, TNF-α, and hs-CRP were assessed in both the treatment groups. In group I, the mean ± SD levels (pg/g) of IL-6 before treatment and 1 week after completion of treatment were 156 ± 11.05 and 45.01 ± 21.04, respectively. On the other hand, the mean ± SD levels (mg/g) of TNF-α before and after 1 week of treatment were 6.12 ± 1.23 and 5.10 ± 0.21, respectively, while the serum hs-CRP levels (μg/L) before and following endodontic therapies were 2.13 ± 0.2 and 1.56 ± 0.11, respectively.
Table 1.
Efficacy of single-sitting and multiple-sitting endodontic treatment 6 months after completion of treatment
| Studied groups | Success (%) | Failure (%) | Chi-square values | P |
|---|---|---|---|---|
| Group I | 65 (95.12) | 5 (6.23) | 0.75 | 0.42 |
| Group II | 57 (91.05) | 6 (9.76) |
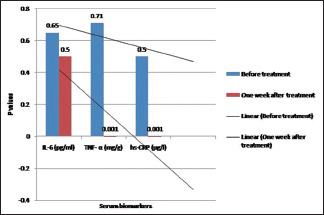
On the other hand, in group II, the mean ± SD values of IL-6 levels before and after 1 week of treatment completion were 143 ± 10.21 and 29.56 ± 12.01, respectively, whereas the mean ± SD values of TNF-α were 5.97 ± 1.0 and 3.12 ± 0.91, respectively. The mean ± SD levels of hs-CRP were 2.01 ± 0.01 and 1.12 ± 0.01, respectively. On applying paired t-test, the P values obtained on comparing IL-6 levels between groups I and II before treatment showed a nonsignificant difference of 0.65, whereas following treatment, a statistically significant P value, which was <0.05, was obtained. On comparing the TNF-α levels in both the study groups, P values of 0.71 (no significant difference) and <0.001 (extremely significant) were obtained before and following treatment, respectively. On comparing the hs-CRP levels both before and after treatment completion, P values of 0.5 (nonsignificant) and <0.001 (extremely significant) were obtained, respectively [Table 2 and Graph 1].
Table 2.
Serum IL-6, TNF-α, and hs-CRP levels before and after endodontic treatment
| Groups | IL-6 | TNF-α | hs-CRP | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before treatment | 1 week after treatment | Before treatment | 1 week after treatment | Before treatment | 1 week after treatment | |
| Group I | 156±11.05 | 45.01±21.04 | 6.12±1.23 | 5.10±0.21 | 2.13±0.2 | 1.56±0.11 |
| Group II | 143±10.21 | 29.56±12.01 | 5.97±1.0 | 3.12±0.91 | 2.01±0.01 | 1.12±0.01 |
| P | 0.65 | <0.5 | 0.71 | <0.001 | 0.5 | <0.001 |
hs-CRP=high-sensitivity C-reactive protein, IL=interleukin, TNF-α = tumor necrosis factor-alpha
Graph 1.
Serum biomarkers and P-values
Discussion
Endodontics has gained emphasis in the scientific community in recent years due to the increase in clinical and animal models studies focusing on endodontic medicine, which aims to evaluate the interrelationship between systemic and periapical tissues’ pathological conditions. These studies have shown that systemic changes can boost the pathogenesis of endodontic infection, favoring its development and progression.[11]
Apical periodontitis is a chronic asymptomatic disease. It results due to the pulpal infection caused by the formation of bacterial biofilm within the root canal systems of teeth. Various endodontic organisms and their by-products produce an inflammatory response that is an attempt for localization of infection and prevention of spread at the expense of apical periodontal tissues.[12]
This may result in the formation of apically located lesions of endodontic origin. Proinflammatory cytokine mediators IL-6, TNF-α, and hs-CRP have demonstrated significant inflammatory effects.
In the current study, no statistically significant difference was observed on comparing the clinical outcomes measured as efficacy. However, on comparison of the levels of inflammatory biomarkers (IL-6, TNF-α, and hs-CRP), statistically significant differences between single-sitting and multiple-sitting root canal treatments were noted. Our findings are supported by Bains et al.,[13] who evaluated hs-CRP levels in patients undergoing endodontic therapy before and after receiving treatment and found that the mean C-reactive protein (CRP; measured in mg/L) at the baseline level was 6.18 ± 3.72 and following treatment, the level was 3.92 ± 3.59. Thus, the mean change in CRP ± SD after follow-up at 30 days was 2.26 ± 3.04. This change was found to be statistically significant on using the paired t-test (t = 2.458; P = 0.034). Similarly, Lu et al.[14] compared the therapeutic efficacy of single-sitting root canal treatment with multiple-sitting root canal procedures among patients suffering from irreversible pulpitis. They reported that after 1 week of treatment, serum IL-6, TNF-α, and hs-CRP levels were significantly reduced in both the groups, though their levels were found to be significantly lesser in multi-visit group than in single-visit endodontic therapy.
Also, Vidal et al.[15] showed a positive correlation between serum CRP levels and presence of periapical endodontic diseases. Garrido et al.[16] reported a positive correlation between IL-6 and CRP expression in apical periodontitis lesions.
Poornima et al.[17] conducted a study to assess the influence of root canal treatment on serum hsCRP levels in systemically healthy human adults. It was concluded that the root canal treatment can reduce serum hsCRP levels in systemically healthy individuals with apical periodontitis.
In contrast, the study conducted by Burgener et al.[18] in 2010 compared the total serum protein levels, interleukin-1 (IL-1), and IL-6 in gingival crevicular fluid samples that were collected from teeth associated with endodontic lesions with those from healthy, contralaterally placed teeth. They found that the protein levels in the samples collected from the affected teeth were higher when compared to those from normal control teeth, although there were no significant differences in the levels of IL-1 and IL-6.
Thus, single-visit root canal therapy effectively improves postoperative pain as well as the levels of inflammatory mediators in the serum of affected patients.
The biomarkers of inflammation serve as a useful indicator of a person’s overall metabolism. As most of the studies conducted are cross sectional, in accordance with the suggestions by van der Waal et al.,[19] the present paper attempted to incorporate a more appropriate model to assess the role of inflammatory biomarkers by taking samples from patients undergoing single- and multiple-visit root canal treatment.[13]
It was well established that the single-sitting root canal or endodontic treatment is more effective in reducing the inflammatory factors that are associated with the inflammatory etiology of pulpal diseases. Thus, treatment of inflammatory processes in the body is strongly associated with reduction of inflammatory markers.[20,21,22,23,24]
Conclusion
This study found that there was no statistically significant difference in the long-term follow-up of clinical efficacy between single-sitting root canal therapy and multiple-sitting root canal therapy. But on comparing the levels of inflammatory biomarkers in the serum samples, it was found that single-sitting root canal therapy was superior when compared to multiple-sitting root canal treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mohammadi Z, Abbott PV, Shalavi S. Postoperative pain following treatment of teeth with irreversible pulpitis:A review. N Y State Dent J. 2017;83:44–53. [PubMed] [Google Scholar]
- 2.Liu J, Shi C. Comparative study of pain releasing effect of two approaches to acute pulpitis in the night emergency. Shanghai Kou Qiang Yi Xue. 2017;26:669–71. [PubMed] [Google Scholar]
- 3.Karthikeson P, Gayathri R, Priya VV. Evaluation of salivary total proteins albumin, globulin, and A/G ratio among healthy individuals and patients with chronic pulpitis. Drug Invent Today. 2018;10:966–8. [Google Scholar]
- 4.Nelson-Filho P, Ruvie×re DB, de Queiroz AM. Comparative molecular analysis of gram-negative bacteria in primary teeth with irreversible pulpitis or periapical pathology. Pediatr Dent. 2018;40:259–64. [PubMed] [Google Scholar]
- 5.Pintor AVB, dos Santos MRM, Ferreira DM. Does smear layer removal influence root canal therapy outcome?A systematic review. J Clin Pediatr Dent. 2016;40:1–7. doi: 10.17796/1053-4628-40.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Parirokh M, Sadr S, Nakhaee N, Abbott PV, Manochehrifar H. Comparison between prescription of regular or on-demand ibuprofen on postoperative pain after single-visit root canal treatment of teeth with irreversible pulpitis. J Endod. 2014;40:151–4. doi: 10.1016/j.joen.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Linden GJ, McKinnell J, Shaw C, Lundy FT. Substance P and neurokinin A in gingival crevicular fluid in periodontal health and disease. J Clin Periodontol. 1997;24:799–803. doi: 10.1111/j.1600-051x.1997.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 8.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 9.Redker PM. Clinical application of C-Reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 10.Rasheed MK, Rasheed MK, Mahmood HG. Interleukin-6 and high sensitivity C-reactive protein correlation in atherosclerosis in Iraqi type 2 diabetic patients. Int J Drug Dev Res. 2013;5:106–14. [Google Scholar]
- 11.Cintra LTA, Gomes MS, da Silva CC, Faria FD, Benetti F, Cosme-Silva L, et al. Evolution of endodontic medicine:A critical narrative review of the interrelationship between endodontics and systemic pathological conditions. Odontology. 2021 doi: 10.1007/s10266-021-00636-x. doi:10.1007/s10266-021-00636-x. [DOI] [PubMed] [Google Scholar]
- 12.Tibúrcio-Machado CS, Michelon C, Zanatta FB, Gomes MS, Marin JA, Bier CA. The global prevalence of apical periodontitis:A systematic review and meta-analysis. Int Endod J. 2021;54:712–35. doi: 10.1111/iej.13467. [DOI] [PubMed] [Google Scholar]
- 13.Bains R, Tikku AP, Ali W, Verma P, Pandey P. Pre- and post-treatment levels of serum high-sensitivity C-reactive protein in patients with lesions of endodontic origin:A clinical pilot study. Asian J Oral Health Allied Sci. 2020;10:2–7. [Google Scholar]
- 14.Lu Y, Liu Z, Huang J, Liu C. Therapeutic effect of one-time root canal treatment for irreversible pulpitis. J Int Med Res. 2020;48:1–11. doi: 10.1177/0300060519879287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidal F, Fontes TV, Marques TV, Gonçalves LS. Association between apical periodontitis lesions and plasmatic levels of C-reactive protein, interleukin 6 and fibrinogen in hypertensive patients. Int Endod J. 2016;49:1107–15. doi: 10.1111/iej.12567. [DOI] [PubMed] [Google Scholar]
- 16.Garrido M, Dezerega A, Bordagaray MJ, Reyes M, Vernal R, Melgar-Rodriguez S, et al. C-reactive protein expression is upregulated in apical lesions of endodontic origin in association with interleukin-6. J Endod. 2015;41:464–71. doi: 10.1016/j.joen.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Poornima L, Ravishankar P, Abbott PV, Subbiya A, PradeepKumar AR. Impact of root canal treatment on high-sensitivity C-reactive protein levels in systemically healthy adults with apical periodontitis-a preliminary prospective, longitudinal interventional study. Int Endod J. 2021;54:501–8. doi: 10.1111/iej.13444. [DOI] [PubMed] [Google Scholar]
- 18.Burgener B, Ford AR, Situ H, Fayad MI, Hao JJ, Wenckus CS. Biologic markers for odontogenic periradicular periodontitis. J Endod. 2010;36:1307–10. doi: 10.1016/j.joen.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Der Waal SV, Lappin DF, Crielaard W. Does apical periodontitis have systemic consequences?The need for well-planned and carefully conducted clinical studies. Br Dent J. 2015;218:513–6. doi: 10.1038/sj.bdj.2015.340. [DOI] [PubMed] [Google Scholar]
- 20.Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–8. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- 21.Huang GT, Do M, Wingard M, Park JS, Chugal N. Effect of interleukin-6 deficiency on the formation of periapical lesions after pulp exposure in mice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:83–8. doi: 10.1067/moe.2001.115025. [DOI] [PubMed] [Google Scholar]
- 22.de Sa AR, Pimenta FJ, Dutra WO, Gomez RS. Immunolocalization of interleukin 4 interleukin 6, and lymphotoxin alpha in dental granulomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:356–60. doi: 10.1016/s1079-2104(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 23.Gomes MS, Blattner TC, Sant'Ana Filho M, Grecca FS, Hugo FN, Fouad AF, et al. Can apical periodontitis modify systemic levels of inflammatory markers?A systematic review and meta-analysis. J Endod. 2013;39:1205–17. doi: 10.1016/j.joen.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Gutmann JL, Baumgartner JC, Gluskin AH, Hartwell GR, Walton RE. Identify and define all diagnostic terms for periapical/periradicular health and disease states. J Endod. 2009;35:1658–74. doi: 10.1016/j.joen.2009.09.028. [DOI] [PubMed] [Google Scholar]


