ABSTRACT
Individuals who have shown recovery from coronavirus disease (COVID-19) are increasingly getting diagnosed with Mucormycosis or “Black fungus.” It is a difficult condition to diagnose as it has symptoms that are common among a variety of diseases. Hence, it is important to identify the presenting signs and understand the underlying pathogenesis of COVID-19 associated Mucormycosis. The incidence of these mycotic infections has shown a substantial increase in current times owing to an increase in the prevalence of immunocompromised subjects, human immunodeficiency virus (HIV) infection, and acquired immunodeficiency syndrome (AIDS). Any suspected case of mucormycosis requires rapid diagnosis and management due to its rapid progression as well as the destructive course of infection. This article reviews the taxonomy, pathogenesis, and clinical signs along with laboratory investigations that may play a vital role in the timely diagnosis of this condition as it is mostly fatal.
Keywords: COVID-19, diagnosis, management, mucormycosis, signs
Introduction
Phycomycosis, which is also known as “Zygomycosis” was first of all described by Paltauf in 1885.[1] It was later termed as “Mucormycosis” by Baker, an American pathologist in 1957.[1] It is an aggressive infectious condition which is caused by Rhizopus species and is an uncommon and fatal infection of fungal origin that usually affects individuals with altered immunological status.
The family Zygomycetes is comprised of two orders: “Mucorales” and “entomothorales.” The order “Mucorales” is responsible for Mucormycosis that mainly affects immunocompromised individuals. On the other hand, the order “Entomothorales” results in both superficial as well as mucocutaneous infections in immune-competent subjects.[2]
Mucoromycotina is saprophytic fungi that are found extensively all over the environment covering decayed organic matter, agricultural, as well as forest soils. These are not dimorphic organisms with fast growth and are characterized by large, ribbon-shaped, irregular, non-septate, or coenocytic or minimally septate with branching that often arises non-dichotomously at right angles. These are thermo-tolerant organisms and hence can grow at 37°C, while few of the species exhibit growth even at higher temperatures.
Phagocytes form a major host defensive mechanism that remains active against mucormycosis.[3,4] Also, treatment modality utilizing corticosteroid effects capability of macrophages in preventing germination process of spores of these fungal organisms. A particular hallmark of Mucormycosis infection is “presence of extensive angio-invasiveness with thrombosis of blood vessels and necrosis of tissues.”[5]
Mucormycosis involves organs like the nose, paranasal sinuses, orbit, central nervous system, lungs, gastrointestinal tract, skin, bones of jaws, joints, heart, kidneys, as well as mediastinum.
Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome-coronavirus (SARS-CoV-2) that is a non-segmented negative-sense RNA virus that results in significant lymphopenia. In the later stages of the infectious process, acceleration of the replication process of virus takes place due to which the integrity of epithelial-endothelial barrier gets compromised and there is an accentuation of inflammatory cell response and also, a triggering of the influx in monocytic and neutrophilic cells. Overall, disruption in the endothelial cell barrier, dysfunction in the transmission of oxygen between alveolus and capillaries, and impairment in the diffusion of oxygen form salient features indicative of COVID-19 infection.[6,7]
The novel coronavirus disease 2019 has been most often associated with a variety of symptoms like shortness of breath, cough, loss of smell, fever, along with fatigue. Patients suffering from preexisting health conditions, for example, hypertension, diabetes mellitus, and coronary artery disease demonstrate susceptibility to various compilations that may arise from COVID-19 infection. Patients with poor control of diabetes mellitus or those individuals who are immunocompromised have shown an increase in the risk of development of mucormycosis.[8]
Rhizopus is the commonest fungi recovered from patients who are diagnosed with mucormycosis in diabetic individuals. Rhizopus demonstrates an inability to sequestration of iron from iron-binding proteins.[9] Patients suffering from poorly controlled diabetes mellitus have chronically high blood glucose levels that ultimately lead to impairment of neutrophilic functioning. In diabetic ketoacidosis, hyperglycemia along with acidic pH can result in defects in the motility of phagocytic cells that impairs the destruction of bacteria and fungi by these cells.
It has been hypothesized that as pH turns acidic, dissociation of the iron-protein complex takes place, resulting in increased use of released iron by the fungal elements.[9]
Other predisposing factors are associated with various types of malignancies such as lymphomas, leukemias, renal failure, organ transplantation, administration of immune-suppressive therapy, cirrhosis of liver, burns, protein-energy malnutrition PEM), and acquired immune deficiency syndrome. Mucormycosis presents a variety of clinical manifestations like rhino-orbito-cerebral form, pulmonary involvement, gastro-intestinal form, mucormycosis involving the central nervous system, cutaneous mucormycosis, and involving miscellaneous organ systems bones, joints, cardiac, renal, and mediastinal involvement.[9,10]
In patients suffering from diabetes mellitus or type II diabetes, rhino-cerebral mucormycosis is the most frequent type of mucormycosis.[10] In the rhino-cerebral form of mucormycosis, this disease is characterized by the presence of characteristic necrosis of tissue due to angio-invasion which subsequently results in thrombosis. Clinically, it is presented as black as well as necrotic eschar tissue. These fungi enter a person’s respiratory system through inhalation into the paranasal sinuses and may undergo spread within the sphenoid sinus, palate, and/or cavernous sinus. Affected patients usually complain of signs and symptoms such as blurriness of vision, inflammation surrounding the orbit, sinusitis, facial pain or/and numbness, headache, proptosis, ophthalmoplegia, and periorbital cellulitis.[11,12]
Pathophysiological features of COVID-19
Certain distinct pathophysiological features of COVID-19 facilitate the development of secondary fungal infections which include a tendency for extensive pulmonary diseases and subsequently, development of alveolo-interstitial pathology resulting in enhancement for risk of contracting invasive type of fungal infestations. Second, immunological dysregulation that has been linked with COVID-19 infection such as reduction in numbers of T lymphocytic cells, CD4+T lymphocytes, and CD8+T lymphocytic cells significantly cause alteration in the innate immunological system.[13,14]
The development of an invasive variety of fungal infections causes alteration in the natural course of this disease.[15,16]
Developing an acidotic environment can cause impairment of phagocytic activity that can lead to an increase in free iron levels in serum via proton-mediated displacement of ferric iron form from transferring molecules leading to the growth of the fungal pathogenic organism.[17,18]
Similarly, injudicious as well as rampant usage of multivitamins containing zinc as well as iron (popularized as “immunity boosters”) might lead to an increase in levels of free iron levels. Hence, this over-the-counter use of these drugs should not be encouraged.
Other modes of transmission
Immunosuppression induced by glucocorticoids can result in hyperglycemia and the development of lymphocytopenia that predisposes an individual to pathogenic exposure to mucormycosis. Uncontrolled administration of glucocorticoids in patients suffering from COVID-19 without any hypoxemia or administering high doses of glucocorticoids must be avoided whenever it is possible. Ketonemia alongside ketoacidosis is observed among patients diagnosed with COVID-19 in absence of diabetes mellitus.[17]
The role of the hospital’s environment as the primary source of contacting Mucormycosis has to be assessed to address timely as well as successful implementation of infection control and various measures which can be followed. Fungal pathogenic organisms may be capable of persisting on bed bars as well as head posts, bed-side tables, taps, and numerous other surfaces in a hospital for many hours and even months.[19]
Therefore, adopting simple measures like hand-washing by the health care workers and performing decontamination of high-contact surfaces of the hospital are required to be emphasized in a greater measure.[19]
Continuous use of reusable oxygen humidifiers can also play a significant role in the transmissibility of potentially dangerous nosocomial disease-causing organisms via the generation of aerosol particles as they are capable of reaching deeper within the lung tissues immediately the following inhalation.[19]
The patient requiring mechanical support of oxygen within their home must ensure using clean and distilled water within oxygen concentrators. Over-zealous usage of steam inhalation along with well as the non-humidified type of oxygen may cause damage to respiratory mucosal epithelium which allows easy penetration of these Mucorales. Hence, these measures should be restricted to post-COVID patients only. Continuous usage of face masks might cause a reduction in chances of reinfection with SARS-CoV-2 and can also reduce the risk of inhalation of these fungal spores.[19]
However, reusing these masks for a prolonged duration of 2 to 3 weeks might cause an increase in the risk of a person acquiring mucormycosis. Optimal control of glycemia among individuals with preexisting or newly onset diabetes mellitus must also be ensured. At the time of discharge from the hospital after recovering from COVID-19 infection, these individuals must be provided knowledge regarding the earliest signs and/or symptoms of mucormycosis. These patients must be encouraged to consult a doctor for medical advice as soon as possible.[19]
Association of COVID-19 with Mucormycosis
Mishra et al.[7] in 2021 concluded in their observation that patients with COVID-19 infection are susceptible to mucormycotic infection due to reduction in host defensive barrier, dysfunctional phagocytes, as well as lymphocytes.
Mehta and Pandey (2020) reported a case of a 60-year-old male subject who had a chronic history of diabetes diagnosed with COVID-19 infection contacted rhino-orbital mucormycosis during his treatment. The patient was positive by means of reverse-transcriptase polymerase chain reaction or RT-PCR for “severe acute respiratory syndrome coronavirus-2” or SARS-CoV-2. He was treated with parenteral meropenem and orally administered oseltamivir along with parenteral methyl-prednisolone. During the treatment, he showed signs of developing orbital cellulitis. Magnetic resonance imaging of the brain, orbital region, and paranasal sinuses demonstrated soft tissue swelling in right pre-septal, zygomatic or malar, pre-maxillary, and retro-bulbar regions with para-nasal sinusitis. Nasal biopsy was demonstrative of broad and aseptate filamentous fungal hyphae suggestive of “Mucormycosis” which was confirmed by performing a microbiological culture.[15]
The intra-cranial involvement of mucormycosis causes an increase in fatality rate up to 90%.[16] Also, an increase in rapidness of this organism’s dissemination (mucormycosis) is extraordinary, and the slightest delay of upto 12 h in diagnosis is fatal. Approximately 50% of cases of dissemination of mucormycosis are diagnosed after performing post-mortem autopsies.[17]
There is a variety of triggering factors that precipitate mucormycosis among people infected with COVID-19 and corticosteroids:
-
a)
Presence of Diabetes Mellitus with or without diabetic ketoacidosis results in an increase in the risk of catching mucormycosis. An individual suffering from type II diabetes or Diabetes mellitus has been associated with an increase in severity of COVID-19 infection. Uncontrolled hyperglycemia as well as precipitation of Diabetic Ketoacidosis has often been observed with an increase in intake of corticosteroids. Low pH as a result of acidosis acts as a suitable media for facilitating germination of mucor spores.
-
b)
Administration of steroid results in the reduction of phagocytic activities of white blood cells either as first line or second line of defense and also causes impairment in migration of broncho-alveolar macrophages, ingestive capacity, and phagolysosomal fusion mechanism, therefore, turning a diabetic individual exceptionally susceptible of developing mucormycosis.
-
c)
COVID-19 results in endothelialitis, damage to endothelial lining, development of thrombosis, lymphocytopenia, and decrease in numbers of CD4+ and CD8+ T lymphocytic cells. This results in a predisposition toward any secondary or opportunistic fungal-origin infection.
-
d)
Freely available iron is a perfect resource for the metabolism of mucormycosis. Production of hyperglycemia results in glycosylation of transferrin and ferritin molecules that causes a reduction in the capacity of iron-binding which allows an increase in free iron availability. In addition, an increase in levels of cytokines among patients with COVID-19 disease especially, interleukin-6 results in an increase in free iron by elevation of ferritin levels due to enhanced synthesis as well as a decrease in transport of iron. In addition, the development of acidosis causes an increase in available free iron by a similar mechanism and also, by decreasing the ability of transferrin for chelation of iron.
High levels of glucose, lower pH, freely available iron, and ketone bodies due to decrease in phagocytic functioning of white blood cells cause enhancement of growth and proliferation of mucor order. Also, it causes an increase in expression of glucose regulator protein-78 or glucose-regulated protein (GRP)-78 in endothelial cells and a fungal ligand spore-coating homolog protein which enables angio-invasion, hematogenous spread, and necrosis of tissue.
Improper usage of oxygen cylinders
Another probable reason for the surge in post-COVID mucormycosis is the unhygienic delivery of oxygen or low-quality tubing system to these patients at the hospital intensive care units (ICUs), the oxygen cylinders with unclean masks, or using contaminated/tap water in humidifiers and prolonged usage of the same mask for more than two patients.[20]
Tocilizumab therapy
COVID-19 infection induces a dose-dependent production of IL-6 pro-inflammatory cytokine from bronchial epithelial cells. To control the levels of IL-6 in these particular patients, tocilizumab, an Food and Drug Administration (FDA)-approved drug, has been prescribed along with dexamethasone. Tocilizumab is a recombinant humanized anti-IL-6 receptor monoclonal antibody. Chronic usage of this therapy to control inflammation triggered by a SARS-CoV weakens the patient’s immune response. In this way, tocilizumab increases the risk of mucormycosis in post-COVID-19 patients.[20]
Identification of Mucormycosis
Clinical findings
The Gold standard for confirming the clinical diagnosis of mucormycosis according to Smith and Kirchner criteria include the following[21,22]:
Black and necrotic turbinates can be easily misdiagnosed for the presence of dried and crusted blood.
Blood-tinged discharge from the nose is accompanied by facial pain on the affected side.
Soft swelling around peri-orbital or peri-nasal area with discoloration along with the development of induration.
Ptosis of eye-lids, proptosis of eye-ball, as well as complete ophthalmoplegia.
Multiple palsies of cranial nerves.
Role of dentists in identifying signs of mucormycosis
Rhino-orbital-cerebral type of mucormycosis is defined as an infection that has an origin within the paranasal sinuses after inhalation of spores. This disease demonstrates the possibility of extension into the brain. Subsequently, involvement of the nose, sinuses, eyes, and/or brain takes place. The early-stage disease presents as pain in the sinus, congestion of the nose, pyrexia, swelling, or headache.
Ulceration in the nasal cavity may be present which if untreated causes extension within surrounding tissues, thrombosis, and necrosis resulting in a painful and brownish-black colored eschar in the maxilla or within nasal mucosa or palate. A dentist plays an important role in identifying these signs and aiding in quick management.[23]
Microbiological assessment of mucormycosis
Microbiological identification of hyphal filaments of Mucorales is completely based upon filamentous diameter, presence/absence of Hyphal septa, branching angulation whether right-angled or acutely branched, and type of pigmentation produced by the organism. These features help in differentiating mucormycosis from other types of fungal infections.
Histopathological findings in mucormycosis
Histopathological examination of suspected tissue with mucormycosis shows necrotic tissue association with inflammatory components comprising neutrophils, lymphocytes, macrophages, and giant cells. There is evidence of inflamed granulation tissue, hemorrhagic elements, and extravasation. The necrotic tissue demonstrates the presence of broad and non-septate hyphal forms [Figure 1]. Use of Periodic Acid Schiff and Grocott-Gomori methenamine silver staining delineates the fungal hyphal forms.[8]
Figure 1.
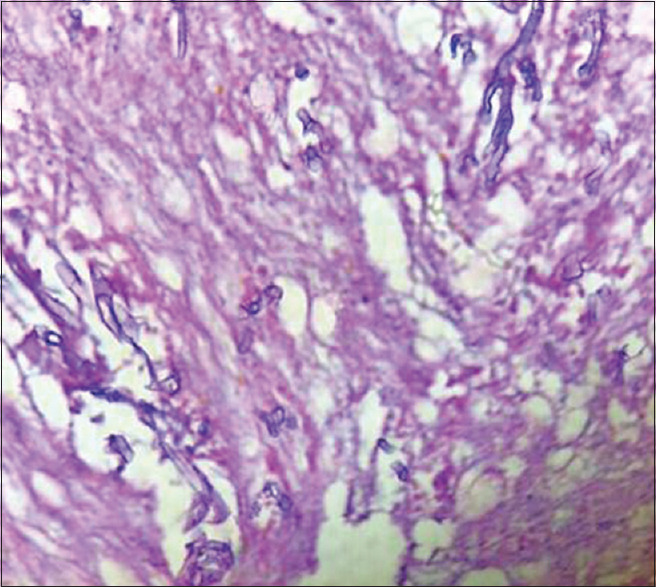
Histopathological picture of necrotic tissue with branching and aseptate fungal hyphae
Challenges in managing mucormycoses in COVID-19 patients
The most frequently used treatments for mucormycosis include management using the anti-fungal agent, Amphotericin B. Amphotericin B acts by targeting sterols and lipid components that are found within cellular membranes of both human as well as fungal cells.
Ergosterol which is a component of fungal cellular membranes is highly sensitive to Amphotericin B, an anti-fungal drug when compared with cholesterol. Although, the administration of amphotericin B gets limited by toxicity related to infusion which is the aftereffect of the production of pro-inflammatory cytokines as a result of COVID-19 infection. Apart from this, the use of corticosteroids during treatment using Amphotericin B causes imbalances in metabolism such as hypokalemia that further complicates the medical treatment in cases of COVID-19 with Mucormycosis as a complication.[24]
Conclusion
There is an increase in the number of mucormycosis cases as evident nowadays. These are most frequently seen in COVID-19 patients who are suffering from comorbid conditions such as diabetes mellitus or type II diabetes. This has also been reported from patients who have recovered from COVID-19 or who were hospitalized in ICUs for a long period. Hence, it is essential to identify the risk factors, their clinical presentation, and investigations required for quick intervention as these are fatal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19:A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15:102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribes JC, Vanover-Sans CL. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser. 1989;47:243–71. [PubMed] [Google Scholar]
- 4.Waldorf AR, Ruderman N, Diamond RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense mechanisms against Rhizopus. JClin Invest. 1984;74:150–60. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchara JP, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur J Cell Biol. 1996;70:76–83. [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission diagnosis, and treatment of coronavirus disease 2019 (COVID-19):A review. JAMA. 2020;324:782–93. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 7.Mishra N, Mutya VSS, Thomas A, Rai G, Reddy B, Mohanan AA, et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7:867–70. [Google Scholar]
- 8.Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12:85–9. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rammaert B, Lanternier F, Poiree S, Kania R, Lortholary O. Diabetes and mucormycosis:A complex interplay. Diabetes Metab. 2012;38:193–204. doi: 10.1016/j.diabet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Ali Asghar S, Majid Z, Tahir F, Qadar LT, Mir S. Rhinooculo cerebral mucormycosis resistant to amphotericin B in a young patient with diabetic ketoacidosis. Cureus. 2019;11:e4295. doi: 10.7759/cureus.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley TT, Muzny CA, Swiatlo E, Legendre DP. Breaking the mold:A review of mucormycosis and current pharmacological treatment options. Ann Pharmacother. 2016;50:747–57. doi: 10.1177/1060028016655425. [DOI] [PubMed] [Google Scholar]
- 12.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis:An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–21. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagtap SV, Jagtap SS, Nagar V, Varshney K. Invasive mucormycosis in post-COVID-19 infection:Case report with review. IP Arch Cytol Histopathol Res. 2021;6:135–9. [Google Scholar]
- 14.Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–4. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19 associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia:An observational study from Pakistan. Mycoses. 2020;63:766–70. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutsch PG, Whittaker J, Prasad S. Invasive and non-invasive fungal rhinosinusitisda review and update of the evidence. Medicina. 2019;55:319. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maartens G, Wood MJ. The clinical presentation and diagnosis of invasive fungal infections. J Antimicrob Chemother. 1991;28:17e44. doi: 10.1093/jac/28.suppl_a.13. [DOI] [PubMed] [Google Scholar]
- 18.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients:A prospective study. Clin Infect Dis. 2020:ciaa1065. doi: 10.1093/cid/ciaa1065. doi:10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935–41. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhogireddy R, Krishnamurthy V, Jabaris S SL, Pullaiah CP, Manohar S. Is mucormycosis an inevitable complication of Covid-19 in India? Braz J Infect Dis. 2021;25:101597. doi: 10.1016/j.bjid.2021.101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee M, Pal R, Bhadada SK. Intercepting the deadly trinity of mucormycosis, diabetes and COVID-19 in India. Postgrad Med J. 2021 doi: 10.1136/postgradmedj-2021-140537. postgradmedj-2021-140537. doi:10.1136/postgradmedj-2021-140537. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim AS, Spellberg B, Edwards J. Iron acquisition:A novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620–5. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:e10726–31. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID-19:We should be prepared. J Mycol Med. 2020;30:100971. doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]


