Abstract
Peripheral artery disease (PAD), the pathophysiologic narrowing of arterial blood vessels of the lower leg due to atherosclerosis, is a highly prevalent disease that affects more than 6 million individuals 40 years and older in the United States, with sharp increases in prevalence with age. Morbidity and mortality rates in patients with PAD range from 30% to 70% during the 5- to 15-year period after diagnosis and PAD is associated with poor health outcomes and reduced functionality and quality of life. Despite advances in medical, endovascular, and open surgical techniques, there is striking variation in care among population subgroups defined by sex, race and ethnicity, and socioeconomic status, with concomitant differences in preoperative medication optimization, amputation risk, and overall health outcomes. We reviewed studies from 1995 to 2021 to provide a comprehensive analysis of the current impact of disparities on the treatment and management of PAD and offer action items that require strategic partnership with primary care providers, researchers, patients, and their communities. With new technologies and collaborative approaches, optimal management across all population subgroups is possible.
1. Introduction
Peripheral artery disease (PAD), the pathophysiologic narrowing of blood vessels due to atherosclerosis, is estimated to affect nearly 6% of the US adult population older than 40 years. In a multiethnic estimation of prevalence using a cohort from the year 2000, Allison and colleagues [1] estimated that >78% of those with PAD were non-Hispanic White, 15.9% were African American, 3.8% were Hispanic, 1.5% were Asian, and 0.7% were American Indian. Patients with PAD can present at different stages of the disease spectrum: asymptomatic, with claudication pain on exertion, or with ischemic rest pain and/or wounds associated with chronic limb-threatening ischemia (CLTI). PAD is associated with poor health outcomes and reduced functionality and quality of life, not least due to the burden of amputation. Within the 5- to 15-year period after diagnosis with PAD, morbidity and mortality rates range from 30% to 70% [2]. Like other cardiometabolic diseases, social determinants of health influence all aspects of PAD, including prevalence, perioperative medical optimization, diagnostic testing use, and operative approach. This review will examine disparities in sex, race and ethnicity, and socioeconomic status, and provide action items to address each. Thoughtful consideration and elimination of these disparities is imperative for vascular specialists who aim to provide comprehensive and unbiased care, especially to vulnerable populations.
2. Methods
A comprehensive literature review was conducted to identify a wide and heterogeneous number of articles related to disparities in PAD prevalence, diagnosis, and management among minority and minoritized groups. With the guidance of an experienced research librarian, we designed and conducted an electronic web-based search on October 5, 2021 and January 5, 2022, using the following online databases: MEDLINE via PubMed, CINAHL, Cochrane, and Scopus Library. The search terms included: ((“Peripheral Vascular Diseases”[MeSH Terms] OR “Peripheral Artery Disease”[MeSH Terms]) AND (“health disparity, minority and vulnerable populations”[MeSH Terms] OR “Healthcare Disparities”[MeSH Terms] OR “Health Status Disparities”[MeSH Terms])) NOT “Review”[Publication Type].
The results underwent an initial two-person title and abstract review. Articles published June 1957 through January 2022 were screened for inclusion. The initial criterion for time-frame was intentionally broad and, upon review, it became evident that the first study to conceptualize disparities in PAD was published in 1995. Review studies; case reports and studies; book chapters; opinion pieces, letters, and comments; editorials; studies not published in English; and studies conducted outside the United States were excluded. A full-text review of the remaining studies was then conducted. Action items were shaped by the magnitude of representation in the reviewed literature, evidence of the effectiveness of comparable interventions in other fields, and expert knowledge (Fig. 1).
Fig, 1 –
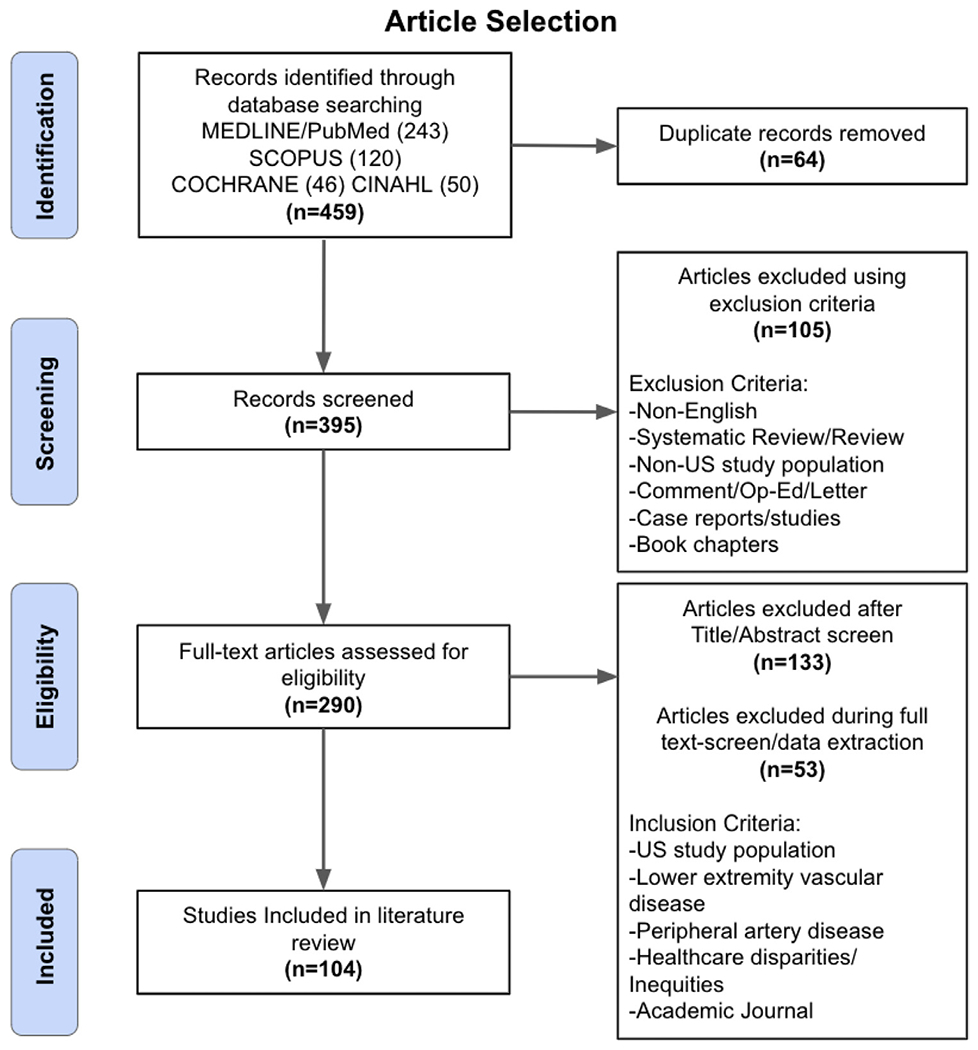
Article selection process and results.
3. Results
3.1. Sex-based disparities
Many clinical studies demonstrate strong associations between sex and PAD severity and outcomes. However, the underlying biological drivers that lead to differences in the manifestation of PAD in male and female individuals remain unclear. Contrary to popular belief, PAD is not significantly more prevalent in male individuals when defined by ankle-brachial index (ABI) <0.9 [3–7]. However, at initial presentation, female individuals are more likely to present with subclinical (asymptomatic) PAD. However, at the time of intervention, female patients are more likely to present at an older age and with the clinical diagnosis of CLTI [8–10]. Given that female patients are less likely to smoke or have elevated lipids, the etiology of the more severe presentation of CLTI remains to be elucidated [10]. Furthermore, the misperception of a higher incidence of PAD in male individuals is multifactorial. It includes issues such as sampling bias in population and clinical studies, sex-based differences in care-seeking behaviors, and biases related to screening for PAD [11].
Female patients with symptomatic PAD tend to report more significant disability compared with men. They report greater functional impairment, poorer outcomes after revascularization, and worse health-related quality of life, despite similar ABIs compared with male patients [12–15]. In addition, female individuals with PAD have slower walking speed, shorter 6-minute walk distance, and poorer leg strength, potentially contributing to reduced lower extremity functioning [13,16–18]. These walking limitations have been found even after enrollment in supervised exercise therapy (SET), a proven treatment modality for patients with PAD [19,20]. For reasons that remain unclear, female individuals were less likely to be enrolled in and less likely to benefit from an SET program [20]. For example, in a post-hoc analysis of the EXITPAD (Exercise Therapy in Peripheral Artery Disease) multicenter randomized trial to evaluate the efficacy of SET, researchers found that female participants had significantly shorter absolute walking distances after 12 months of follow-up compared with male participants [16].
In addition to potential biological and physiologic mechanisms, one possible reason for poorer functional status in female individuals with PAD relates to concomitant mental health burden. Major depression is a common comorbidity among patients with PAD [21–24]. In one cohort study of 1,635 participants with PAD, female participants were found to have an almost twofold greater prevalence of comorbid depression than male participants (46% v 26%) [21]. Younger female patients (ie, younger than 65 years) in particular appear to have a more persistent burden of depression, with up to fourfold greater odds of baseline and follow-up depressive symptoms after PAD diagnosis compared with male patients of the same age [22]. The higher rates of comorbid depression in female patients with PAD are particularly noteworthy because depressive symptoms are associated with more significant physical disability and functional decline over time, as evidenced by a greater annual decline in 6-minute walking distance and speed [25,26]. In addition, in a nationwide Veterans Affairs study, patients with PAD with comorbid depression had a greater long-term risk of limb loss after adjustment for covariates (hazard ratio, 1.13; 95% CI, 1.07–1.19) [27]. Moreover, patients with untreated depression (eg, not taking antidepressants) had an even greater risk of amputation (hazard ratio, 1.42; 95% CI, 1.27–1.58).
In considering preoperative medical management, female patients are less likely to receive guideline-recommended high-intensity statin or any statin prescription compared with male patients [28,29]. In addition, female sex is associated with lower adherence to statin therapy, evidenced by the Patients and Provider Assessment of Lipid Management registry. When evaluating how patient beliefs contributed to lower adherence, authors found that female patients declined statin therapy more often, citing worries about adverse effects, preference for diet and exercise, and natural remedies [30]. Female patients were also less likely to believe in the safety and effectiveness of statins and had higher rates of perceived statin-associated adverse effects [28,29]. This treatment heterogeneity exists despite adjustment for patient-, facility-, and provider-level confounders.
Retrospective studies have revealed significant variations in the surgical treatment of PAD in female compared with male patients. For example, a 16-year review of the National Inpatient Sample (NIS) database found that female patients were 30% less likely to undergo surgery for PAD, suggesting that female patients were undertreated for the same disease [30]. Trends of hospital admissions and related diagnoses show that women are less likely to be admitted electively for lower extremity PAD, but more likely to be hospitalized emergently [31]. In addition, investigators have found that female patients are subject to different treatment decisions. Lo and colleagues [32] assessed sex differences in procedure utilization by indication using NIS and found that female patients remained more likely to undergo endovascular procedures for both claudication and CLTI. When assessing sex differences in the utilization of endovascular modalities, female patients were more likely to undergo percutaneous transluminal angioplasty alone and less likely to have atherectomy or stenting [33,34]. Reasons for these findings remain unclear, but anatomically, female patients have been shown to have significantly smaller reference vessel diameters compared with men, which may contribute to practitioner decision making during endovascular procedures [35]. Lastly, the prevalence of amputations also varies by sex, with Lo et al finding that between 1998 and 2009, major amputations declined more dramatically in female patients relative to male patients. Similar findings reported by Egorova et al [31] reported a 35% decrease in amputation rates seen in female patients compared with a 21% decrease in male patients during the study period.
A study that compiled 30-year outcome data from 1968 to 1998 for patients who underwent infrainguinal arterial reconstruction reported that perioperative mortality did not vary by sex [36]. In more recent years, follow-up studies have demonstrated mixed findings regarding apparent sex-related variations in mortality. The NIS study by Lo and colleagues [32] reported that although inpatient mortality declined during the study period for all patients, female patients had higher inpatient mortality after PAD intervention compared with male patients, regardless of disease severity and procedure type, even after adjusting for age and comorbidities. Another study corroborated this finding and concluded that among a cohort treated for lifestyle-limiting claudication, female patients had higher inpatient mortality than male patients after open, endovascular, and hybrid repair [37]. This was contradicted by Doshi et al [38], who evaluated NIS data from 2012 to 2014 looking specifically at endovascular procedures and found that in hospital mortality and 30-day mortality were comparable between female and male patients. The incongruity in the observed outcomes can be attributed to the severity of disease in the patient populations that were included in the study. Although the initial NIS study evaluated patients with claudication and CLTI and excluded patients who had a simple PAD diagnosis, the latter study did not stratify PAD diagnosis by disease severity. It is likely that a less advanced presentation contributed to more comparable outcomes between male and female patients. Furthermore, study time periods differed, potentially indicating improved outcomes for women in more recent years.
A multitude of retrospective studies have also found that female patients have higher rates of short-term complications after intervention, including early graft thrombosis, embolization, incisional site complications, cardiac events, stroke, and pulmonary complications irrespective of surgical technique compared with male counterparts [9,10,31,34,35,37,39–42]. Data on sex-based differences in long-term complications post intervention are much fewer, however. Of long-term studies described in the literature, one 1993 study of a relatively small cohort reported lower 3-year survival rates in female patients after infrainguinal bypass compared with male patients. Graft patency at that time was also significantly reduced in female patients [43]. A subsequent prospective surveillance study that followed patients after undergoing infrainguinal autogenous vein bypass grafting between 1988 and 1994 had similar findings of decreased 5-year survival rates in female patients [42].
3.1.1. Action items for sex-based disparities
Sex-based disparities exist at all stages of the PAD management spectrum. Addressing inequity begins preoperatively in the outpatient setting. Although they are less likely to have classic symptoms of claudication, female patients are more likely to attend preventative health care visits, suggesting that enhanced screening via the ABI could identify PAD in female patients early in the disease course [5]. Holistic and aggressive screening is critical when considering that female patients with PAD present for intervention later than their male counterparts. Once patients are identified, providers must be thorough in their medication reconciliation to ensure female patients are on the appropriate guideline-recommended statin prescription. Both vascular specialists and primary care providers play a part in discerning drug-related adverse effects from symptomatic claudication or other causes and reducing fluctuating adherence and premature discontinuation [29].
In addition to prescribing guideline-recommended treatment regimens, vascular specialists can help ensure that patients adhere to critical medical therapies. Research indicates medication adherence is more common in patients with both coronary artery disease (CAD) and PAD than PAD alone [44,45], suggesting that patients with PAD alone must be especially educated regarding the significance of their diagnosis and the associated benefit of cardiovascular medications in slowing the progression of PAD. A Centers for Disease Control and Prevention Grand Rounds in 2019 offered the following suggestions for improving medication adherence: ensure access to providers across the continuum of care; implement team-based care; educate and empower patients to understand their treatment regimen and its benefits; reduce barriers to obtaining medication, including cost reduction (prescribing generics over name brands); and reduce physical barriers for patients who have limited mobility or transportation access [46].
Another aspect of conservative management for PAD is SET. The benefits of exercise for the treatment of claudication are well known, but there are many potential contributors to the observed reduced benefit in female participants. The report by Gardner and colleagues suggests that walking distance may not be an adequate measure of functionality in female participants and that the development of other quantification tools is merited. A current clinical trial [47] studying the impact of aquatic versus land walking on vascular health and exercise tolerance in patients with PAD may elucidate further management recommendations for female individuals [42]. In addition, vascular providers are equipped to screen for depressive symptoms or coordinate care with mental health care providers to augment the management of PAD with concomitant depression [48,49].
Regarding surgical management of PAD in female patients, more research is needed to identify approaches that may lead to better amputation-free survival. Retrospective research showing better outcomes from endovascular therapy in female patients is interesting [50]. However, more robust mechanistic studies, combined with prospective evaluation through randomized trials, would provide more generalizable evidence of differences in treatment outcomes.
3.2. Racial and ethnic disparities
There appear to be multiple racial and ethnic disparities in the presentation, management, treatment, and outcomes of PAD in vascular patients. It is estimated that approximately 30% of Black patients will develop PAD in their lifetime, with a large burden of disease starting to appear around age 60 years [51]. The corresponding estimate for White and Hispanic patients is approximately 20%, with an increasing burden of disease appearing by age 70 years, 10 years later than in the Black population [51,52]. In the San Diego Population Study, participants were divided into four ethnic groups (non-Hispanic White, Black, Hispanic, and Asian). Of these groups, Black patients had the highest prevalence of PAD [53]. This finding was corroborated in the Multi-Ethnic Study of Atherosclerosis (MESA), which included more than 6,000 patients without symptomatic cardiovascular disease at enrollment. In this study, Black participants were 1.5 times more likely to have PAD than non-Hispanic White participants, after adjusting for traditional risk factors (ie, hypertension, diabetes, dyslipidemia, current smoker, less education and income, and body mass index) and novel ones (ie, homocysteine, C-reactive protein, interleukin-6, fibrinogen, and d-dimer) [54–61]. Using data from the National Health and Nutrition Examination Survey 1999-2000, Selvin et al [62] reported that after controlling for age, gender, and traditional cardiovascular risk factors, such as diabetes and hypertension, Black patients still had a significantly higher prevalence of PAD compared with non-Hispanic White participants, with odds ratios (ORs) of 2.83 and 2.39, respectively [34,61,62].
Data regarding prevalence of PAD among other racial and ethnic groups are more limited. The Strong Heart Study included participants from 13 tribes located in the Dakotas, Oklahoma, and Arizona, and reported the prevalence of PAD among American Indian patients as 5.3% across centers [1,63]. Hispanic and Latino communities have been found to have heterogeneous disease burdens. When stratified by country of origin, data from participants enrolled in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) showed that Cuban patients had nearly three times higher odds of developing PAD compared with Mexican or other Hispanic and Latino groups [64].
In consideration of the genetic association between PAD and ancestry, data from the MESA demonstrated that a higher percentage of American Indian ancestry reduced the prevalence of PAD in both Hispanic and Black patients (OR, 0.56; 95% CI, 0.36–0.96) [65]. Greater European Ancestry did not have a similar protective association [65]. A similar study using data from the Jackson Heart Study found that there may be unmeasured risk factors interacting with genetic mediators that play a role in the prevalence of more significant subclinical atherosclerosis seen in Black patients, reinforcing that there is much unknown about the interaction of genes, ancestry, and the environment in the pathogenesis of PAD [66].
Genetic epidemiological studies have identified a genetic locus on chromosome 11 associated with a low ABI value, and the odds of the association were increased in patients with a homozygous African genotype (OR, 1.59) [67]. Proteomic markers have also been associated with PAD. A multicenter study using the Genetic Epidemiology Network of Arteriopathy associated higher levels of myeloperoxidase, an enzyme secreted by neutrophils and implicated in the initiation and progression of atherosclerotic lesions, with lower ABIs and an increased prevalence of PAD in Black and non-Hispanic White individuals. These findings were sustained after adjusting for traditional risk factors of PAD, as well as history of myocardial infarction and stroke [68,69].
Differences exist across racial and ethnic groups and clinical presentation of PAD. Population health studies showed that Black patients with PAD often have a more significant comorbidity burden, present at younger ages, with higher disease severity, lower ABI values, and higher rates of novel risk factors [1,51,70–74]. The severity of the disease also depends on associated risk factors, with a 3.5- to 5-fold increase in lifetime risk of PAD when Black patients presented with current tobacco use, diabetes, and hypertension [51]. In addition, Black patients present with more significant mobility loss, defined as the inability to walk up and down a flight of stairs or walk one-quarter mile without assistance compared with White patients during an average 46-month follow-up period. These differences, however, were attenuated when controlling for socioeconomic status and general physical activity, suggesting the racial differences may be due to other factors [59].
Timely secondary prevention methods can avoid progression to CLTI and limb loss. Although high-intensity statins have been shown to decrease the risk of amputation and death, they are especially underused by patients with PAD. This finding is exacerbated in racially and ethnically minoritized patients [75]. The Reduction of Atherothrombosis for Continued Health (REACH) registry from 2003-2004 demonstrated that although Black and Hispanic patients were less frequently diagnosed with hypercholesterolemia, they were more likely to present with high cholesterol levels and less likely to be prescribed aspirin or statin, indicating poor disease management [76]. A multivariate analysis of Hispanic patients showed they were more likely to delay medical care due to employment and child care issues, worries about cost and concerns about interfacing with the health care system, and general nervousness about seeing a physician than non-Hispanic patients [76]. In addition, Hispanic patients with known PAD were less likely to adhere to cardiovascular medications recommended in clinical guidelines, including antiplatelet, lipid-lowering, and antihypertensive therapy. The overall prevalence was 31% taking antiplatelet therapy and 26% taking lipid-lowering therapy, and among hypertensive individuals, only 57% adhered to their antihypertensive medications. Of the various ethnic backgrounds, Mexican American patients have been reported to have the lowest use for all classes of cardiovascular medications [77]. Factors contributing to increased medication use were older age, number of doctor visits, and existing comorbid conditions, with patients having CAD and concurrent PAD more likely to use antiplatelets and statins than patients with PAD only [77].
Of note, Asian patients are frequently understudied in the literature, and elucidating subgroup effects within Asian populations can be difficult. Studies using data from the Vascular Quality Initiative database suggest that Asian patients present with more advanced disease, as measured by higher rates of tissue loss and acute limb ischemia requiring a distal bypass target [76,78]. However, there is little evidence of disparity in presentation, long-term primary patency, or mortality in this population [75,77]. Although there is increased risk of cardiovascular disease in Southeast Asian populations, there is limited research that clarifies disparities in PAD treatment or outcomes. One study using data from the NIS found that Asian or Pacific Islander patients had higher odds of in-hospital mortality (OR, 1.20; 95% CI, 1.01–1.43) compared with Black (OR, 0.81; 95% CI, 0.76–0.87) and Hispanic (OR, 0.84; 95% CI, 0.77–0.92) patients admitted for PAD [79]. Asian patients also had increased length of hospital stay, which was also observed in Black and Hispanic patients compared with American Indian and non-Hispanic White patients. Finally, average medical expenditures were greatest in Asian and Pacific Islander patients, and this group carried a greater economic burden than all other demographic groups included in this study [79]. Overall, studies better characterizing prevalence, treatment, and outcomes of PAD care that can address different Asian populations (eg, South Asian, East Asian, and Pacific Islander) are clearly needed.
The literature on racial and ethnic variations in operative management and outcomes provides a mixed picture. White race has been found to independently predict a greater likelihood of intervention in patients with CLTI [80]. Although studies from 1993-1996 had reported that Black patients were more likely than White patients to undergo surgical bypass grafting, subsequent Medicare data and the Atherosclerosis Risk in Communities (ARIC) study found that the excess of surgical bypass grafting could be attributed to the higher prevalence of PAD among Black patients [54–58]. A large population-based study of California hospitals found that among 41,507 individuals who underwent PAD intervention (61% endovascular and 39% open), Black and Hispanic patients had higher reintervention rates within 12 months and worse amputation-free survival rates [81]. Black patients have also been found to have a higher incidence of early (<1 year) graft failure than White patients, and there have not been significant differences found when comparing Hispanic patients with White patients [15,82].
Conversely, another study found improved outcomes in Black patients after peripheral vascular interventions, specifically lower rates of repeat ipsilateral percutaneous revascularization or bypass, and lower rates of major adverse vascular events compared with patients of other self-reported races [70]. Brothers et al also reported that despite disparities in prevalence and amputation burden in Black patients, Black patients who underwent lower extremity revascularization had higher survival rates than White patients [1,83,84]. This was contradicted recently by a study that evaluated the VQI database and found that the 1-year risk of major adverse limb event after elective infrainguinal revascularization among Non-Hispanic Black and Hispanic patients was 1.17 and 1.22 times the hazard among White patients, and even worse for amputation, with hazards ratios of 1.45 and 1.52, respectively [85]. Reasons for these discrepancies warrant additional study, but current studies are ultimately limited by evaluation of retrospective data involving different population sampling, potential differences in eliciting race categories, and factors that confound race.
Limb loss is the severe prognostic outcome of poorly managed PAD, and minority patients disproportionately experience the health and socioeconomic burden of amputation. Black and Hispanic patients are more likely to undergo amputations for PAD treatment, have an increased risk of frailty status and associated complications [15,86–88]. Black amputees were less likely to have any limb-related admission, toe amputation, or wound debridement before amputation compared with White patients, indicating less aggressive limb salvage care in Black patients [84]. Furthermore, an extensive retrospective analysis of the National Surgical Quality Improvement Program’s database between 2011 and 2017 yielded a notable racial disparity among Black patients with CLTI. Black patients with CLTI were 2.1 times more likely to undergo a below-knee amputation and 2.6 times more likely to undergo an above-knee amputation than White patients [89]. The higher burden of amputation in Black patients was strongly correlated with higher rates of diabetes and deep soft-tissue infections at presentation compared with White patients [89].
Studies have also shown that the risk of limb loss due to PAD in Hispanic patients is 2 to 3 times greater than in White patients [86,87]. For American Indian populations, Henry et al [90] demonstrated a 2.4 times increased odds of amputation compared with White patients. Regional variations were notable. For example, in one study, American Indian patients from Arizona demonstrated a fivefold increase in rates of amputation compared with Oklahoma and South and North Dakota populations, a finding thought to correlate with the higher rates of diabetes among Arizona’s American Indian population [63].
Postoperatively, Black patients are between 2 and 4 times more likely to undergo amputation after lower extremity bypass than non-Hispanic White and Hispanic patients [81,86,87,91]. This racial gap is evident even among the highest-performing providers, defined as vascular specialists in high-volume, urban teaching hospitals with angioplasty facilities [92,93]. Hispanic patients were 1.4 times more likely to experience amputation or death after infrainguinal bypass, with a median amputation-free survival of 1.3 years [94].
3.2.1. Action items for racial and ethnic disparities
Many studies have evaluated the impact of race on PAD development, progression, and treatment outcomes, some with mixed findings. This could point to the “race” variable being too ambiguous or a “catch-all” for multiple issues simultaneously. Investigators have found that after adjusting for atherosclerotic risk factors and social determinants of health, such as education level, the impact of race and ethnicity diminishes [60,95]. Although race is often used in the literature as an independent risk factor for disease, it is, in fact, a mediator of structural inequalities resulting from unjust policies and practices [96]. Emerging scholarship has introduced an alternative approach for understanding the impact of race by using a race-conscious approach to analyze the impact of structural racism, rather than race alone, on inequities in health care [97–99].
In a review by Hackler et al [100], the authors offer an illustration (Fig. 2) that comprehensively summarizes the historical structural inequities that contribute to the disparities observed between non-Hispanic White and Black patients. The figure accounts for the systematic ways that public policies and institutional practices, such as the criminal-legal system, education system, housing, and food insecurity, as well as cultural representations, disproportionally disadvantage people of color, resulting in the disparities observed in Black, Hispanic, and American Indian populations. Although it can be difficult to individually assess the impact of these factors, it creates a backdrop by which to understand how even after controlling for traditional risk factors of disease, non-White patients are inheriting inequities out of their immediate control that impact their health. A more complete understanding of the historic context of structural inequities empowers providers to practice informed and holistic care.
Fig. 2 –
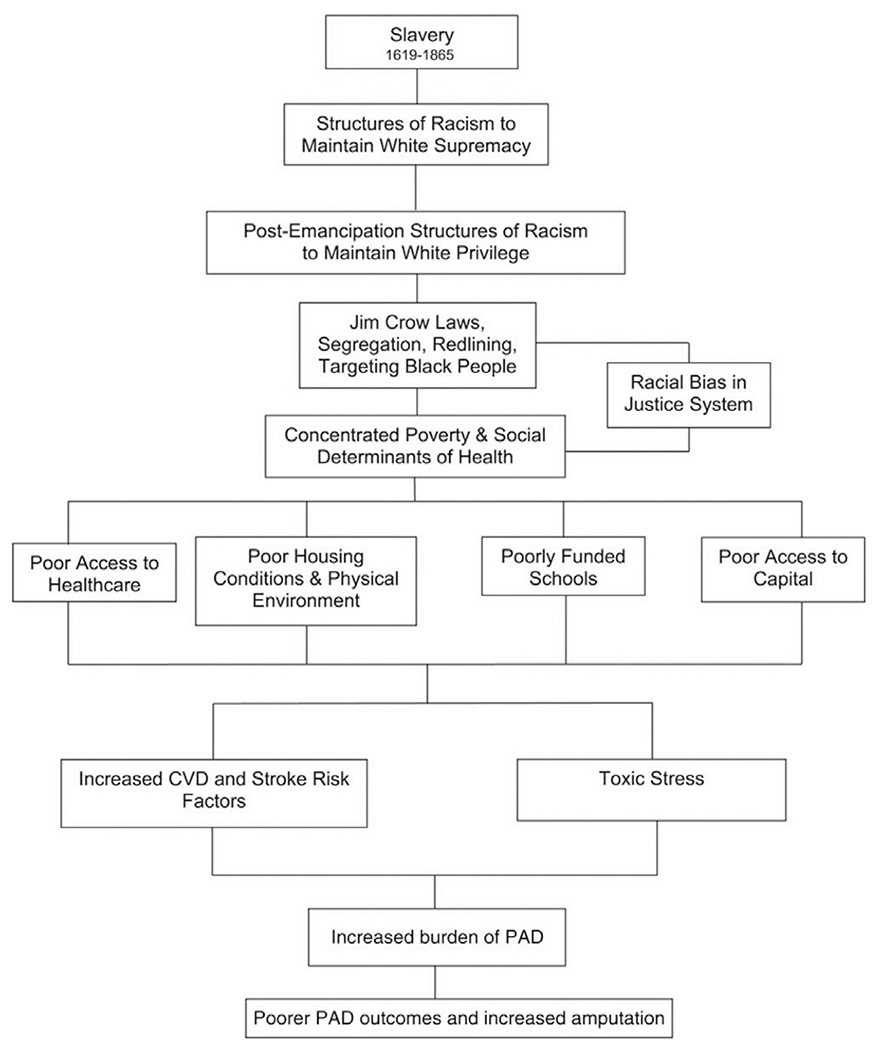
How structural racism leads to disparities in peripheral artery disease outcomes for Black Americans. CVD = cardiovascular disease [100].
It is also important to note that race-based medicine (ie, using race as an important aspect of medical decision making and medical research) has been challenged in several fields. For instance, further research into how such practices can harm racially and ethnically minoritized patients populations has led to substantially revising commonly used tools, such as the estimated glomerular filtration rate and vaginal birth after cesarean calculators [97–99]. Thus, when race is being used in care or research, caution is warranted and clinicians and researchers must have a clear understanding of what race as a variable or feature in a study represents and the implications of grouping patients and participants in this way. For example, when biologic and mechanistic studies are pursued, focusing on ancestral groupings, rather than self-reported race, may be more informative, given the genetic implications of ancestry and potential biological importance.
At times, PAD management strategies may need to be more tailored to specific subgroups. For example, a randomized controlled trial by Collins et al [101] found that motivational interviewing was not an effective intervention in improving walking distance in Black patients. In contrast, Patient-Centered Assessment and Counseling for Exercise successfully increased walking distance at 12 months in this group. Another study that used four different intervention strategies (ie, education and enhanced care, diet, physical activity, and meditation) found that education and enhanced care focus had the most considerable effect on Black patients [102]. Lastly, community-based intervention strategies that have been demonstrated to be effective in specific patient demographics, for example, a barbershop-targeted, pharmacist-directed intervention to significantly lower blood pressure in non-Hispanic Black men with hypertension, are viable interventions for early PAD diagnosis and management [103].
Another critical aspect of PAD management that needs to be explored and addressed is how comorbidity status interacts with race and ethnicity and subsequent decisions regarding whether to and how to treat symptomatic PAD. For example, because Black patients often present with a higher rate of comorbidities than other groups, they may appear “sicker,” affecting whether they receive revascularization at all. Even so, Zaitoun and colleagues [104] found substantial improvements in self-perceived physical and social functioning and quality of health in Black and White patients after revascularization, even though Black subjects had significantly higher diabetes and dialysis-dependent renal failure rates. Thus, finding ways to mitigate the risks comorbidities impose on revascularization outcomes may be of paramount importance in closing gaps in treatment disparities.
The vastly higher rates of amputation observed in Black and Hispanic patients are alarming. This could be addressed through a systematic surveillance protocol in managing treatment of Black and Hispanic patients to avoid limb loss [85]. Flores et al [105] and Armstrong et al [106] have published data showing improved surgical debridement and revascularization outcomes among patients who use their institutions’ comprehensive wound care centers, suggesting the benefit of a multidisciplinary approach. Thus, improving racially and ethnically minoritized patients access to wound care centers can be one approach to mitigate this disparity.
Another important aspect of making PAD care more equitable is addressing workforce diversity, which can help health care organizations improve the quality of patient care, health outcomes, and even financial cost [107]. When workforce diversity is lacking, it can have determinantal effects on patient trust, workplace experiences, and employee retention [108]. In a randomized trial by Alsan et al [109], the researchers’ findings suggested that pairing Black male patients with Black physicians could reduce the gap in cardiovascular mortality among Black and White patients by 19% because Black male patients treated by Black physicians were more likely to seek out preventative services. Although diversifying the workforce is essential to providing high-quality care to vascular patients, providing patient-centered, culturally competent care to patients of all cultural backgrounds is the work of all providers.
There is also evidence to show implicit bias is related to patient–provider interactions, treatment decisions, treatment adherence, and patient health outcomes [110,111]. Implicit biases can be difficult to acknowledge, and often reveal themselves in subtle, nondeliberate attitudes and behaviors, such as approaching patients with a condescending tone, failing to provide interpreters when needed, doing more or less thorough diagnostic work, and recommending treatment options based on assumptions about treatment adherence and capabilities [112]. Studies of implicit bias have demonstrated that most health care providers possess a positive attitude toward White patients and a negative bias toward Black, Hispanic, and dark-skinned patients [110]. Furthermore, women, regardless of their racial and ethnic identity, were found to be more likely than men to experience bias in their interactions with health care providers and treatment [113,114]. Cultural competence training helps health care providers develop the tools necessary to understand and respond to patients from diverse cultural backgrounds with humility and respect and, thus, better able to meet their needs [115,116].
Lastly, improving enrollment of underrepresented populations in clinical trials is another important component of reducing disparities in care. Research has shown disparities in the representation of racially and ethnically minoritized patients populations in clinical trials with patients of color being overrepresented in phase I safety studies and underrepresented in phase III trials, which typically offer a comparative benefit to patients [117]. Research efforts should focus on intentionally recruiting historically underserved racial and ethnic groups to elucidate and reduce disparities in the treatment and management of PAD. This task is made difficult by the many barriers that limit the participation of racially and ethnically minoritized patients in research, such as logistical issues, sociocultural factors, varying degrees of health literacy, and mistrust of researchers and the health care system [118–120]. Care Access, a decentralized research organization, developed the Bridge initiative in an effort to reduce the barriers that exclude underrepresented racial and ethnic minority groups participating in clinical trials, specifically in phase III, by bringing the clinical trial to the community where patients live and partnering with community members and community health centers [121]. These efforts also provide a framework with which a diverse research team is involved in the enrollment of patients. Another example of community engagement in research was demonstrated by a PAD study in England that showed success with targeted recruitment of Black participants via mailings, community events, and television advertisements in postal codes where the residents were >50% Black, and such an approach should be explored in future research efforts [122].
3.3. Socioeconomic disparities
Although studies have shown that lower socioeconomic status (SES) is associated with poorer outcomes in CAD, the association between SES and PAD has been less extensively studied [123–125]. The association between SES and PAD is multifaceted and requires investigation into intersections of SES with treatment, postoperative outcomes, and health at presentation in patients with PAD.
A prospective cohort study quantifying the association between PAD and SES in the United States reported that the risk of PAD-associated hospitalization is more than twice as high in a cohort with a mean household income (MHI) of <$12,000 compared with a cohort with an MHI of >$25,000, after controlling for age, sex, and race [126]. PAD-associated risk of hospitalization was also doubled when comparing groups with low educational attainment (less than high school) with those with high educational attainment (more than high school) [126]. These findings highlight the role of SES, while controlling for race, suggesting that lower SES plays an independent role in the risk of hospitalization for PAD.
Lower SES may also impact patients’ health at presentation with PAD. Patients with financial barriers, defined as underinsured or uninsured, presented with more severe PAD, with almost 60% of patients with financial barriers presenting more than 1 year after symptom onset. Patients facing financial barriers also had worse self-reported health status 12 months after treatment compared with patients without financial barriers [127]. Dual eligibility for Medicaid, an indicator of lower SES, is also associated with an increased all-cause mortality rate in patients with PAD after controlling for other factors [128]. One study of health behaviors in South Florida reported that, when compared with vascular surgery patients with insurance, uninsured patients were less likely to receive optimal medical management for PAD, highlighting room for improvement in the medical management of uninsured patients with PAD [129]. Interestingly, a retrospective cohort study found that the 2006 Massachusetts Health Care Reform resulted in a substantial diminishment in prior racial and socioeconomic disparities with regard to amputation, revascularization, and severity of disease in patients with PAD [130], suggesting that expansion of insurance at the state level is a possible solution to combatting the inequities in outcomes of patients with PAD.
Studies have also shown that SES disparities exist in the procedures that patients with PAD undergo in the context of CLTI. Patients with CLTI in the lower three income quartiles have significantly higher odds of undergoing a major amputation compared with patients in the highest income quartile [90]. Compared with patients on Medicare, patients with private insurance have lower odds of receiving a major amputation in the setting of CLTI [90]. This study also found that patients with lower MHI were less likely to receive an angiogram when seeking care for PAD, and undergoing a diagnostic angiogram was itself associated with 90% lower odds of requiring amputation [90]. In order to control for the presence of patients whose clinical presentation did not warrant an angiogram, such as patients with significant comorbidities or infection, the researchers conducted a subanalysis of patients who received angiogram. Even among patients in this study who did receive an angiogram, income and insurance status were still significantly associated with odds of major limb amputation.
The finding that patients with PAD with lower SES are more likely to receive amputation was corroborated in a recent retrospective analysis that showed that patients presenting with CLTI had decreased odds of undergoing a major amputation as their MHI quartile increased [131]. This trend was consistent in patients with Medicaid, Medicare, private insurance, and uninsured patients, demonstrating that lower SES results in an increased odds of undergoing a major amputation independent of insurance status [131]. Patients with Medicaid and uninsured patients were also more likely to undergo major amputation, and patients with private insurance were more likely to undergo limb-salvage procedures [131]. The impact of SES on the likelihood of undergoing amputation compared with a revascularization procedure has also been shown geographically. In Los Angeles County, California, patients with diabetes living in low-income neighborhoods had twice the amputation rate as those living within the same county in high-income neighborhoods [132].
Disparities in outcomes for patients with low SES also extend into the postoperative period after revascularization procedures. For example, a recent study found that lower SES (defined by MHI quartile by ZIP code) in patients undergoing revascularization procedures for PAD was associated with a significantly higher likelihood of requiring a major leg amputation after readmission to the hospital [133]. Similarly, there was a stepwise increase in the likelihood of requiring a leg amputation after a revascularization procedure during the same hospital admission as patients’ income quartile decreased [133]. These findings provide further evidence for the disproportionate burden of disease in low SES patients with PAD and illustrate the need for more research regarding the correlation between SES and PAD outcomes and the underlying factors contributing to the findings cited.
3.3.1. Unique considerations for rural populations
Patients from rural areas have unique considerations, such as physical access to care and geographic availability of specialist care. As such, rural patients were considered separately in the context of PAD. Although there is a paucity of data regarding the influence of rurality on patients with PAD, residents of rural areas are at higher risk of amputation in the setting of PAD [134]. Similarly, patients undergoing endovascular cardiac procedures who were residents of rural areas had higher rates of vascular complications, bleeding, and mortality [135]. In general, rural areas have been found to exhibit health disparities compared with urban areas [136–139], and more research concerning disparities in the management and outcomes of PAD in rural populations is needed.
3.3.2. Action items for socioeconomic disparities
Optimal interventions for addressing SES disparities require a multifaceted approach, including addressing underlying causal factors predominantly affecting patients of low SES with PAD and standardization of certain practices that may reduce limb loss, such as diagnostic angiogram for patients presenting with PAD. Optimization of medical management is a necessary and actionable solution to combat disparities in outcomes seen in patients with PAD. Furthermore, patients with PAD who are uninsured may require more robust assistance in smoking cessation, as these patients are less likely to quit smoking in the setting of symptomatic PAD [140]. Improving access to health care, guideline-recommended statin therapy, and increasing the number of primary care providers in low SES areas are potential steps toward decreasing the inequity seen in patients with PAD. Furthermore, a more nuanced understanding of the elements that drive the disparities in these outcomes would allow for a more tailored solution at the community, state, national, and hospital levels.
Action items related to combatting SES disparities seen in care include community outreach models, such as the “Taking Care of Your Feet” model used by the American Diabetes Association [141]. This model provides an outline for patients with diabetes for maintaining foot care, protecting their feet, and maintaining blood flow to their feet in a concise, easily intelligible modality. A similar model could be used for vascular surgery patients to guide home care, particularly in high-risk patients with PAD. This would allow patients with limited access to health care to improve their home care and provide them with guidance on when to seek emergency care, empowering patients with the knowledge to seek care earlier in their disease process, thereby reducing their severity of disease.
Similarly, telemedicine may be a viable option for increasing access to health care in patients with vascular disease and low SES and/or those who live in rural areas. Although telemedicine has various limitations, mainly due to the inability to complete a thorough vascular physical examination, telemedicine may be used to assess disease progression and assess barriers to medication adherence. For example, a recent prospective study demonstrated the feasibility of using photographs to assess diabetic foot injury in high-risk patients [142]. Unfortunately, patients of low SES and in rural areas may be unable to complete virtual care visits due to disparities in access to technology and internet capability. A recent study of telemedicine in oncology patients during the COVID-19 pandemic found that although patients with Medicaid, uninsured patients, and patients with lower MHI were less likely to complete virtual care visits, telephone visit completion was unaffected by insurance status or MHI [143]. Although assessment data lag behind implementation both nationally and internationally [144,145], Project ECHO (Extension for Community Health Care Outcomes) is an example of leveraging telemedicine in the management of complex disease that does not rely on individual patient access to technology or internet, instead using telementoring [146]. Both the utilization of telephone visits and enrichment of provider knowledge through a Project ECHO-type model could benefit low-SES patients and those in rural settings with different stages of vascular disease, while reducing the impact of technological disparities. These approaches represent possible avenues for assessing patients who have limitations in accessing care due to physical or technological barriers, allowing a subset of these patients to present earlier in their disease.
Related to telemedicine, mobile health is of increasing importance in health care today. With >85% of the general population owning smartphones, and notably 61% of US adults 65 years and older, smartphones provide a method for providing accessible care. An example of this in the vascular space is the mobile supervised exercise program developed and described by Ata et al [147]. Their platform can remotely monitor functional capacity in a validated way in the preoperative and postoperative setting. Mobile health tools empower patients to engage in healthier behaviors at home and in their community in ways that will profoundly impact their chronic disease management.
4. Conclusions
For the purpose of this review, we separated disparities in the treatment and management of PAD into three main categories and included a subsection for unique considerations relevant to patients in rural communities. We recognize that although this organizational structure is expedient for the purpose of our review, the identities of patients are multifaceted and intersectional, and that intersectionality deserves more robust examination in the literature. Thus, our review is limited by how these identity groups were stratified in the primary research.
Even so, a comprehensive review of the literature has identified key ways in which vascular specialists can help optimize health outcomes by applying principles of culturally informed and inclusive care (Fig. 3). To make a difference in outcomes, providers should aim to (1) provide care that is informed by an awareness of differences in sex, race and ethnicity, and SES status that can have significant impacts on disease presentation, medical management, and operative outcomes; (2) provide comprehensive care, working collaboratively with primary care practitioners and enlisting resources to address unique patient circumstances, and (3) be willing to develop and employ new technological tools that can bridge the gap between patients and providers and ultimately improve vascular outcomes across all patient subgroups.
Fig. 3 –
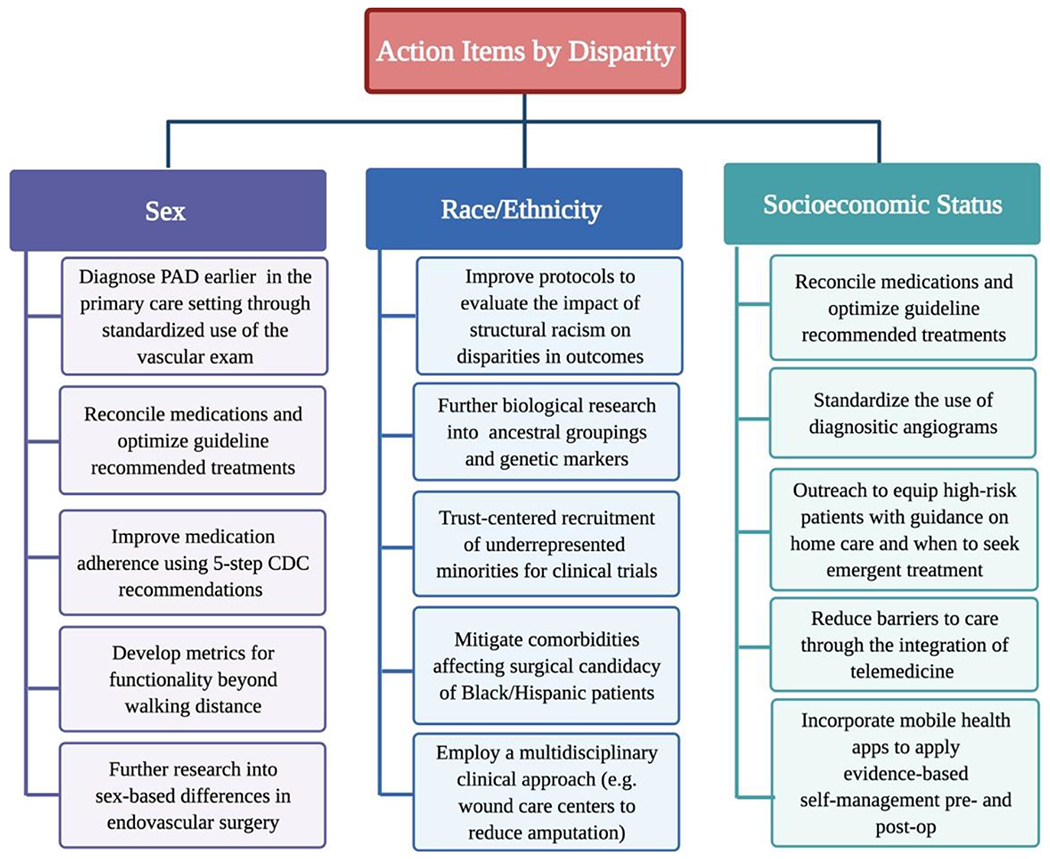
Summary of action items by disparity category. CDC, Centers for Disease Control and Prevention; PAD, peripheral artery disease.
Supplementary Material
Acknowledgements
The authors would like to thank Heather B. Blunt, research librarian, for her help conducting our literature search.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.semvascsurg.2022.05.003.
REFERENCES
- [1].Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the united states. Am J Prev Med 2007;32:328–33. [DOI] [PubMed] [Google Scholar]
- [2].Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006;47:1239–312. [DOI] [PubMed] [Google Scholar]
- [3].Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105. [DOI] [PubMed] [Google Scholar]
- [4].Moussa ID, Jaff MR, Mehran R, et al. Prevalence and prediction of previously unrecognized peripheral arterial disease in patients with coronary artery disease: the Peripheral Arterial Disease in Interventional Patients Study. Catheter Cardiovasc Interv 2009;73:719–24. [DOI] [PubMed] [Google Scholar]
- [5].Hirsch AT, Allison MA, Gomes AS, et al. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation 2012;125:1449–72. [DOI] [PubMed] [Google Scholar]
- [6].Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg 2007;45:1185–91. [DOI] [PubMed] [Google Scholar]
- [7].Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. [DOI] [PubMed] [Google Scholar]
- [8].Schramm K, Rochon PJ, Women’s health: gender differences in peripheral vascular disease. Semin Intervent Radiol 2018;35:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vouyouka AG, Egorova NN, Salloum A, et al. Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. J Vasc Surg 2010;52:1196–202. [DOI] [PubMed] [Google Scholar]
- [10].Jackson EA, Munir K, Schreiber T, et al. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardiol 2014;63:2525–30. [DOI] [PubMed] [Google Scholar]
- [11].Teodorescu VJ, Vavra AK, Kibbe MR. Peripheral arterial disease in women. J Vasc Surg 2013;57(4) suppl1 8S–26S. [DOI] [PubMed] [Google Scholar]
- [12].Collins TC, Suarez-Almazor M, Bush RL, et al. Gender and peripheral arterial disease. J Am Board Fam Med 2006;19:132–40. [DOI] [PubMed] [Google Scholar]
- [13].McDermott MMG, Greenland P, Liu K, et al. Sex differences in peripheral arterial disease: leg symptoms and physical functioning. J Am Geriatr Soc 2003;51:222–8. [DOI] [PubMed] [Google Scholar]
- [14].Enzler MA, Ruoss M, Seifert B, et al. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc Surg 1996;11:446–52. [DOI] [PubMed] [Google Scholar]
- [15].Nguyen LL, Hevelone N, Rogers SO, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation 2009;119:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gommans LNM, Scheltinga MRM, van Sambeek MRHM, et al. Gender differences following supervised exercise therapy in patients with intermittent claudication. J Vasc Surg 2015;62:681–8. [DOI] [PubMed] [Google Scholar]
- [17].McDermott MM, Ferrucci L, Liu K, et al. Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol 2011;57:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang GJ, Shaw PA, Townsend RR, et al. The associations between peripheral artery disease and physical outcome measures in men and women with chronic kidney disease. Ann Vasc Surg 2016;35:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135(12):e726–79. doi: 10.1161/CIR.0000000000000471/-/DC2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Divakaran S Carroll BJ, Chen S, et al. Supervised exercise therapy for symptomatic peripheral artery disease among Medicare beneficiaries between 2017 and 2018: participation rates and outcomes. Circ Cardiovasc Qual Outcomes 2021;14:7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim GY, Anderson MS, Brown CS, et al. Gender disparities in major depression among patients with peripheral artery disease and associations with mortality. J Vasc Surg 2021;74:e207–8. doi: 10.1016/J.JVS.2021.06.309. [DOI] [Google Scholar]
- [22].Smolderen KG, Spertus JA, Vriens PW, et al. Younger women with symptomatic peripheral arterial disease are at increased risk of depressive symptoms. J Vasc Surg 2010;52:637–44. [DOI] [PubMed] [Google Scholar]
- [23].Ruo B, Liu K, Tian L. et al. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosom Med 2007;69:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bruce ML, Seeman TE, Merrill SS, et al. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health 1994;84:1796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McDermott MM, Guralnik JM, Criqui MH, et al. The six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McDermott MMG, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA 2004;292:453–61. [DOI] [PubMed] [Google Scholar]
- [27].Arya S, Lee S, Zahner GJ, et al. The association of co-morbid depression with mortality and amputation in veterans with peripheral artery disease. J Vasc Surg 2018;68:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mahtta D, Ahmed ST, Ramsey DJ, et al. Statin prescription rates, adherence, and associated clinical outcomes among women with PAD and ICVD. Cardiovasc Drugs Ther 2020;34:745–54. [DOI] [PubMed] [Google Scholar]
- [29].Nanna MG, Wang TY, Xiang Q, et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes 2019;12(8):e005562. doi: 10.1161/CIRCOUTCOMES.118.005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McGinigle KL, Browder SE, Strassle PD, et al. Sex-related disparities in intervention rates and type of intervention in patients with aortic and peripheral arterial diseases in the National Inpatient Sample Database. J Vasc Surg 2021;73:2081–9 e7. doi: 10.1016/J.JVS.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Egorova N, Vouyouka AG, Quin J, et al. Analysis of gender-related differences in lower extremity peripheral arterial disease. J Vasc Surg 2010;51:372–8 e1 discussion 378–9. [DOI] [PubMed] [Google Scholar]
- [32].Lo RC, Bensley RP, Dahlberg SE, et al. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg 2014;59:409–18 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ramkumar N, Suckow BD, Brown JR, et al. Role of sex in determining treatment type for patients undergoing endovascular lower extremity revascularization. J Am Heart Assoc 2019;8(17):e013088. doi: 10.1161/JAHA.119.013088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ramkumar N, Suckow BD, Brown JR, et al. Sex-based assessment of patient presentation, lesion characteristics, and treatment modalities in patients undergoing peripheral vascular intervention. Circ Cardiovasc Interv 2018;11(1):e005749. doi: 10.1161/CIRCINTERVENTIONS.117.005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kohi MP, Brodmann M, Zeller T, et al. Sex-related differences in the long-term outcomes of patients with femoropopliteal arterial disease treated with the IN.PACT drug-coated balloon in the IN.PACT SFA randomized controlled trial: a post hoc analysis. J Vasc Interv Radiol 2020;31:1410–18 e10. [DOI] [PubMed] [Google Scholar]
- [36].Roddy SP, Darling RC, Maharaj D, et al. Gender-related differences in outcome: an analysis of 5880 infrainguinal arterial reconstructions, J Vasc Surg 2003;37:399–402. [DOI] [PubMed] [Google Scholar]
- [37].Miller SM, Sumpio BJ, Miller MS, et al. Higher inpatient mortality for women after intervention for lifestyle limiting claudication. Ann Vasc Surg 2019;58:54–62. [DOI] [PubMed] [Google Scholar]
- [38].Doshi R, Shah P, Meraj P, Gender disparities among patients with peripheral arterial disease treated via endovascular approach: A propensity score matched analysis. J Interv Cardiol 2017;30:604–11. [DOI] [PubMed] [Google Scholar]
- [39].Wang J, He Y. Shu C. et al. The effect of gender on outcomes after lower extremity revascularization. J Vasc Surg 2017;65:889–906 e4. [DOI] [PubMed] [Google Scholar]
- [40].Abando A, Akopian G, Katz SG, et al. Patient sex and success of peripheral percutaneous transluminal arterial angioplasty. Arch Surg 2005;140:757–61. [DOI] [PubMed] [Google Scholar]
- [41].Jain AK, Velazquez-Ramirez G, Goodney PP, et al. Gender-based analysis of perioperative outcomes associated with lower extremity bypass. Am Surg 2011;77:844–9. [PMC free article] [PubMed] [Google Scholar]
- [42].Mays BW, Towne JB, Fitzpatrick CM, et al. Women have increased risk of perioperative myocardial infarction and higher long-term mortality rates after lower extremity arterial bypass grafting. J Vasc Surg 1999;29:807–13. [DOI] [PubMed] [Google Scholar]
- [43].Magnant JG, Cronenwett JL, Walsh DB, et al. Surgical treatment of infrainguinal arterial occlusive disease in women. J Vasc Surg 1993;17:67–78. [PubMed] [Google Scholar]
- [44].Subherwal S, Patel MR, Kober L, et al. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation 2012;126:1345–54. [DOI] [PubMed] [Google Scholar]
- [45].Owens CD, Conte MS. Medical management of peripheral arterial disease: bridging the gap? Circulation 2012;126:1319–21. [DOI] [PubMed] [Google Scholar]
- [46].Neiman AB, Ruppar T, Ho M, et al. CDC grand rounds: improving medication adherence for chronic disease management—innovations and opportunities. MMWR Morb Mortal Wkly Rep 2019;66(45):1248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Impacts of Aquatic vs Land Walking on Vascular Health and Exercise Tolerance in Patients With Peripheral Artery Disease - Study Results. ClinicalTrials.gov. Accessed 19 December 2021. https://clinicaltrials.gov/ct2/show/results/NCT03849300
- [48].Callahan CM, Kroenke K, Counsell SR, et al. Treatment of depression improves physical functioning in older adults. J Am Geriatr Soc 2005;53:367–73. [DOI] [PubMed] [Google Scholar]
- [49].Blazer DG, Hybels CF. The association between successful treatment of depression and physical functioning in older people seeking primary care. J Am Geriatr Soc 2005;53:543–4. [DOI] [PubMed] [Google Scholar]
- [50].Hedayati N, Brunson A, Li CS, et al. Do women have worse amputation-free survival than men following endovascular procedures for peripheral arterial disease? An evaluation of the California state-wide database. Vasc Endovascular Surg 2015;49:166–74. [DOI] [PubMed] [Google Scholar]
- [51].Matsushita K, Sang Y, Ning H, et al. Lifetime risk of lower-extremity peripheral artery disease defined by ankle-brachial index in the United States. J Am Heart Assoc 2019;8(18):e012177. doi: 10.1161/JAHA.119.012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Soden PA, Zettervall SL, Deery SE, et al. Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J Vasc Surg 2018;67:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego population study. Circulation 2005;112(17):2703–7. [DOI] [PubMed] [Google Scholar]
- [54].Guadagnoli E, Ayanian JZ, Gibbons G, et al. The influence of race on the use of surgical procedures for treatment of peripheral vascular disease of the lower extremities. Arch Surg 1995;130:381–6. [DOI] [PubMed] [Google Scholar]
- [55].Lamorte WW, Scott TE, Menzoian JO. Relationship of cardiovascular risk factors to racial differences in femoral bypass surgery and abdominal aortic aneurysmectomy in Massachusetts. Ann N Y Acad Sci 1996;800:25–35. [DOI] [PubMed] [Google Scholar]
- [56].Tunis SR, Bass EB, Klag MJ, et al. Variation in utilization of procedures for treatment of peripheral arterial disease: a look at patient characteristics. Arch Intern Med 1993;153:991–8. [PubMed] [Google Scholar]
- [57].Pandit V, Nelson P, Kempe K, et al. Racial and ethnic disparities in lower extremity amputation: assessing the role of frailty in older adults. Surgery 2020;168:1075–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kalbaugh CA, Kucharska-Newton A, Wruck L, et al. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among Medicare Fee-for-Service beneficiaries in the Atherosclerosis Risk in Communities (ARIC) Study. J Am Heart Assoc 2017;6(5):e003796. doi: 10.1161/JAHA.116.003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McDermott MM, Polonsky TS, Kibbe MR, et al. Racial differences in functional decline in peripheral artery disease and associations with socioeconomic status and education. J Vasc Surg 2017;66:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rucker-Whitaker C, Greenland P, Liu K, et al. Peripheral arterial disease in African Americans: clinical characteristics, leg symptoms, and lower extremity functioning. J Am Geriatr Soc 2004;52:922–30. [DOI] [PubMed] [Google Scholar]
- [61].Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2006;48:1190–7. [DOI] [PubMed] [Google Scholar]
- [62].Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation 2004;110:738–43. [DOI] [PubMed] [Google Scholar]
- [63].Fabsitz RR, Sidawy AN, Go O, et al. Prevalence of peripheral arterial disease and associated risk factors in American Indians: The Strong Heart Study. Am J Epidemiol 1999;149:330–8. [DOI] [PubMed] [Google Scholar]
- [64].Allison MA, Gonzalez F, Raij L, et al. Cuban Americans have the highest rates of peripheral arterial disease in diverse Hispanic/Latino communities. J Vasc Surg 2015;62:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Allison MA, Peralta CA, Wassel CL, et al. Genetic ancestry and lower extremity peripheral artery disease in the Multi-Ethnic Study of Atherosclerosis. Vasc Med 2010;15:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gebreab SY, Riestra P, Khan RJ, et al. Genetic ancestry is associated with measures of subclinical atherosclerosis in African Americans: the Jackson Heart Study. Arterioscler Thromb Vasc Biol 2015;35:1271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Scherer ML, Nalls MA, Pawlikowska L, et al. Admixture mapping of ankle–arm index: identification of a candidate locus associated with peripheral arterial disease. J Med Genet 2010;47:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ali Z, Sarcia P, Mosley TH, et al. Association of serum myeloperoxidase with the ankle-brachial index and peripheral arterial disease. Vasc Med 2009;14:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kullo IJ, Turner ST, Kardia SLR, et al. A genome-wide linkage scan for ankle-brachial index in African American and non-Hispanic white subjects participating in the GENOA study. Atherosclerosis 2006;187:433–8. [DOI] [PubMed] [Google Scholar]
- [70].Alasaad M, Zaitoun A, Szpunar S, et al. Association of race with long-term outcomes in patients undergoing popliteal and infra-popliteal percutaneous peripheral arterial interventions. Cardiovasc Revasc Med 2019;20:649–53. [DOI] [PubMed] [Google Scholar]
- [71].Nelson K, Reiber G, Kohler T, et al. Peripheral arterial disease in a multiethnic national sample: the role of conventional risk factors and allostatic load. undefined. Ethn Dis 2007;17:669–75. [PubMed] [Google Scholar]
- [72].Ix JH, Allison MA, Denenberg JO, et al. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans. The San Diego Population Study. J Am Coll Cardiol 2008;51(24):2347–54. [DOI] [PubMed] [Google Scholar]
- [73].Khawaja FJ, Bailey KR, Turner ST, et al. Association of novel risk factors with the ankle brachial index in African American and non-Hispanic white populations. Mayo Clin Proc 2007;82:709–16. [DOI] [PubMed] [Google Scholar]
- [74].Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA). J Vasc Surg 2007;45:319–27. [DOI] [PubMed] [Google Scholar]
- [75].Arya S, Khakharia A, Binney ZO, et al. Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation 2018;137:1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tan TW, Urbina D, Hsu CH, et al. Disparities of health care access among Hispanics at risk of lower extremity amputation. J Vasc Surg 2021;74:e209. doi: 10.1016/J.JVS.2021.06.311. [DOI] [Google Scholar]
- [77].Hua S, Isasi CR, Kizer JR, et al. Underuse of cardiovascular medications in individuals with known lower extremity peripheral artery disease: HCHS/SOL. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2020;9(16):e015451. doi: 10.1161/JAHA.119.015451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Aalberg JJ, Erlichman ZD, Blank M, et al. Race-based disparities in presentation and outcomes in infrainguinal bypass patients. Int Angiol 2021;40:105–11. [DOI] [PubMed] [Google Scholar]
- [79].Chen L, Zhang D, Shi L, et al. Disparities in peripheral artery disease hospitalizations identified among understudied race-ethnicity groups. Front Cardiovasc Med 2021;8:692236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Amaranto DJ, Abbas F,Krantz S, et al. An evaluation of gender and racial disparity in the decision to treat surgically arterial disease. J Vasc Surg 2009;50:1340–7. [DOI] [PubMed] [Google Scholar]
- [81].Loja MN, Brunson A, Li CS, et al. Racial disparities in outcomes of endovascular procedures for peripheral arterial disease: an evaluation of California hospitals, 2005-2009. Ann Vasc Surg 2015;29:950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Selvarajah S, Black JH, Haider AH, et al. Racial disparity in early graft failure after infrainguinal bypass. J Surg Res 2014;190:335–43. [DOI] [PubMed] [Google Scholar]
- [83].Brothers TE, Zhang J, Mauldin PD, et al. Better survival for African and Hispanic/Latino Americans after infrainguinal revascularization in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg 2017;65:1062–73. [DOI] [PubMed] [Google Scholar]
- [84].Holman KH, Henke PK, Dimick JB, et al. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg 2011;54:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kalbaugh CA, Witrick B, Laksika M, et al. Non-Hispanic Black and Hispanic patients have worse outcomes than White patients within similar stages of peripheral artery disease. J Am Heart Assoc 2022;11:23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dillingham TR, Pezzin LE, MacKenzie EJ, Racial differences in the incidence of limb loss secondary to peripheral vascular disease: a population-based study. Arch Phys Med Rehabil 2002;83:1252–7. [DOI] [PubMed] [Google Scholar]
- [87].Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008;89:422–9. [DOI] [PubMed] [Google Scholar]
- [88].Lefebvre KM, Metraux S. Disparities in level of amputation among minorities: implications for improved preventative care. J Natl Med Assoc 2009;101:649–55. [DOI] [PubMed] [Google Scholar]
- [89].Traven SA, Synovec JD, Walton ZJ, et al. Notable racial and ethnic disparities persist in lower extremity amputations for critical limb ischemia and infection. J Am Acad Orthop Surg 2020;28:885–92. [DOI] [PubMed] [Google Scholar]
- [90].Henry AJ, Hevelone ND, Belkin M, et al. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg 2011;53:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yang Y, Lehman EB, Aziz F. African Americans are at a higher risk for limb loss but not mortality after lower extremity bypass surgery. Ann Vasc Surg 2019;58:63–77. [DOI] [PubMed] [Google Scholar]
- [92].Regenbogen SE, Gawande AA, Lipsitz SR, et al. Do differences in hospital and surgeon quality explain racial disparities in lower-extremity vascular amputations? Ann Surg 2009;250:424–30. [DOI] [PubMed] [Google Scholar]
- [93].O’Donnell TFX, Powell C, Deery SE, et al. Regional variation in racial disparities among patients with peripheral artery disease. J Vasc Surg 2018;68:519–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Oh-Park M, McGinn A, Lipsitz E, et al. Racial disparity in amputation-free survival after infrainguinal bypass procedure: contribution of socioeconomic status. Am J Phys Med Rehabil 2009;88:986–94. [DOI] [PubMed] [Google Scholar]
- [95].Collins TC, Petersen NJ, Suarez-Almazor M, et al. Ethnicity and peripheral arterial disease. Mayo Clin Proc 2005;80:48–54. [DOI] [PubMed] [Google Scholar]
- [96].Cerdeña JP, Plaisime MV, Tsai J, et al. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet 2020;396(10257):1125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].UW Medicine to exclude race from calculation of eGFR (measure of kidney function). Department of Medicine, University of Washington. Accessed 10 January 2022. https://medicine.uw.edu/news/uw-medicine-exclude-race-calculation-egfr-measure-kidney-function [Google Scholar]
- [98].Abolish race-based medicine in kidney disease and beyond. The San Francisco Examiner. Accessed 10 January 2022. https://www.sfexaminer.com/opinion/abolish-race-based-medicine-in-kidney-disease-and-beyond/ [Google Scholar]
- [99].Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight–reconsidering the use of race correction in clinical algorithms. N Engl J Med 2020;383:874–82. [DOI] [PubMed] [Google Scholar]
- [100].Hackler EL, Hamburg NM, White Solaru KT, Racial and ethnic disparities in peripheral artery disease. Circ Res 2021;128:1913–26. [DOI] [PubMed] [Google Scholar]
- [101].Collins TC, Lu L, Ahluwalia JS, et al. Efficacy of community-based exercise therapy Among African American patients with peripheral artery disease: a randomized clinical trial. JAMA Netw Open 2019;2(2):e187959. doi: 10.1001/JAMANETWORKOPEN.2018.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Eastridge DK, An integrative review of interventions to reduce peripheral arterial disease risk factors in African Americans. J Vasc Nurs 2009;27(2):31–45. [DOI] [PubMed] [Google Scholar]
- [103].Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in Black barbershops. N Engl J Med 2018;378:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zaitoun A, Al-Najafi S, Musa T, et al. The association of race with quality of health in peripheral artery disease following peripheral vascular intervention: the Q-PAD Study. Vasc Med 2017;22:498–504. [DOI] [PubMed] [Google Scholar]
- [105].Flores AM, Mell MW, Dalman RL, et al. Benefit of multidisciplinary wound care center on the volume and outcomes of a vascular surgery practice. J Vasc Surg 2019;70:1612–19. [DOI] [PubMed] [Google Scholar]
- [106].Armstrong DG, Bharara M, White M, et al. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev 2012;28:514–18. [DOI] [PubMed] [Google Scholar]
- [107].Gomez LE, Bernet P, Diversity improves performance and outcomes. J Natl Med Assoc 2019;111:383–92. [DOI] [PubMed] [Google Scholar]
- [108].Rotenstein LS, Reede JY, Jena AB, Addressing workforce diversity–a quality-improvement framework. N Engl J Med 2021;384:1083–6. [DOI] [PubMed] [Google Scholar]
- [109].Alsan M, Garrick W, Graziani GC. Does diversity matter for health? Experimental evidence from Oakland. National Bureau of Economic Research; 2018. Published July 9 https://www.nber.org/papers/w24787. [Google Scholar]
- [110].Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health 2012;102:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Penner LA, Dovidio JF, West TV, et al. Aversive racism and medical interactions with Black patients: a field study. J Exp Soc Psychol 2010;46:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Govender V, Penn-Kekana L, Gender biases and discrimination: a review of health care interpersonal interactions. Glob Public Health 2008;3(1):90–103 suppl. [DOI] [PubMed] [Google Scholar]
- [114].Verdonk P, Benschop YWM, de Haes HCJM, et al. From gender bias to gender awareness in medical education. Adv Health Sci Educ Theory Pract 2009;14:135–52. [DOI] [PubMed] [Google Scholar]
- [115].Handtke O, Schilgen B, Mösko M. Culturally competent healthcare–a scoping review of strategies implemented in healthcare organizations and a model of culturally competent healthcare provision. PLoS One 2019;14(7):e0219971. doi: 10.1371/JOURNAL.PONE.0219971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. FOCUS 2020;18(1):49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health 2011;101:2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Farmer DF, Jackson SA, Camacho F, Hall MA. Attitudes of African American and low socioeconomic status white women toward medical research. J Health Care Poor Underserved 2007;18:85–99. [DOI] [PubMed] [Google Scholar]
- [119].Thompson HS, Valdimarsdottir HB, Jandorf L, et al. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Educ Couns 2003;51:217–27. [DOI] [PubMed] [Google Scholar]
- [120].Scharf DP, Mathews KJ, Jackson P, et al. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved 2010;21:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].BRIDGE Initiative. Accessed 24 April 2022. https://bridgeinitiative.com/ [Google Scholar]
- [122].Love B, Nwachokor D, Collins T. Recruiting African Americans with peripheral artery disease for a behavioral intervention trial. Vasc Med 2016;21:345–51. [DOI] [PubMed] [Google Scholar]
- [123].Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88(4 Pt 1):1973–98. [DOI] [PubMed] [Google Scholar]
- [124].Patrick WL, Bojko M, Han JJ, et al. Neighborhood socioeconomic status is associated with differences in operative management and long-term survival after coronary artery bypass grafting [published online ahead of print August 19, 2020]. J Thorac Cardiovasc Surg doi: 10.1016/J.JTCVS.2020.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bashinskaya B, Nahed BV, Walcott BP, et al. socioeconomic status correlates with the prevalence of advanced coronary artery disease in the United States. PLoS One 2012;7(9):e46314. doi: 10.1371/JOURNAL.PONE.0046314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Vart P, Coresh J, Kwak L, et al. Socioeconomic status and incidence of hospitalization with lower-extremity peripheral artery disease: Atherosclerosis Risk in Communities Study. J Am Heart Assoc 2017;6(8). doi: 10.1161/JAHA.116.004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jelani QUA, Jhamnani S, Spatz ES, et al. Financial barriers in accessing medical care for peripheral artery disease are associated with delay of presentation and adverse health status outcomes in the United States. Vasc Med 2020;25:13–24. [DOI] [PubMed] [Google Scholar]
- [128].Narcisse DI, Ford CB, Weissler EH, et al. The association of healthcare disparities and patient-specific factors on clinical outcomes in peripheral artery disease. Am Heart J 2021;239:135–46. [DOI] [PubMed] [Google Scholar]
- [129].Yeh D, Jones M, Schulman C, et al. Uninsured South Florida vascular surgery patients are less likely to receive optimal medical management than their insured counterparts. J Vasc Surg 2010;51(4) suppl 4S–8S. [DOI] [PubMed] [Google Scholar]
- [130].Loehrer AP, Hawkins AT, Auchincloss HG, et al. Impact of expanded insurance coverage on racial disparities in vascular disease: insights from Massachusetts. Ann Surg 2016;263:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hughes K, Mota L, Nunez M, et al. The effect of income and insurance on the likelihood of major leg amputation. J Vasc Surg 2019;70:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower extremity amputations in low income regions of California. Health Aff (Millwood) 2014;33(8):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hughes K, Olufajo OA, White K, et al. The influence of socioeconomic status on outcomes of lower extremity arterial reconstruction. J Vasc Surg 2022;75:168–76. [DOI] [PubMed] [Google Scholar]
- [134].Barnes JA, Eid MA, Creager MA, et al. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol 2020;40:1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Baljepally VS, Wilson DC. Gender-based disparities in rural versus urban patients undergoing cardiac procedures. Cureus 2021;13(7):e16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Henley SJ, Anderson RN, Thomas CC, et al. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in non-metropolitan and metropolitan counties–United States. MMWR Surveill Summ 2019;66(14):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Meit M, Knudson A, Gilbert T, et al. The 2014 Update of the Rural-Urban Chartbook. Published 2014. Accessed 24 April 2022. https://ruralhealth.und.edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf
- [138].Onega T, Hubbard R, Hill D, et al. Geographic access to breast imaging for US women. J Am Coll Radiol 2014;11:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Huang HC, Smart MH, Zolekar A, et al. Impact of socioeconomic status and rurality on cancer-specific survival among women with de novo metastatic breast cancer by race/ethnicity [published online ahead of print April 23, 2022]. Breast Cancer Res Treat doi: 10.1007/S10549-022-06603-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Huen KH, Chowdhury R, Shafii SM, et al. Smoking cessation is the least successful outcome of risk factor modification in uninsured patients with symptomatic peripheral arterial disease. Ann Vasc Surg 2015;29:42–9. [DOI] [PubMed] [Google Scholar]
- [141].Taking Care of Your Feet. American Diabetes Association. Accessed 9 January 2022. https://professional.diabetes.org/pel/taking-care-your-feet-english [Google Scholar]
- [142].Hazenberg CEVB, Bus SA, Kottink AIR, et al. Telemedical home-monitoring of diabetic foot disease using photographic foot imaging—a feasibility study. J Telemed Telecare 2012;18:32–6. [DOI] [PubMed] [Google Scholar]
- [143].Tam S, Wu VF, Williams AM, et al. Disparities in the uptake of telemedicine during the COVID-19 surge in a multidisciplinary head and neck cancer population by patient demographic characteristics and socioeconomic status. JAMA Otolaryngol Head Neck Surg 2021;147:209–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].McBain RK, Sousa JL, Rose AJ, et al. Impact of Project ECHO models of medical tele-education: a systematic review. J Gen Intern Med 2019;34:2842–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Faherty LJ, Rose AJ, Chappel A, et al. Assessing and expanding the evidence base for Project ECHO and ECHO-Like Models: findings of a technical expert panel. J Gen Intern Med 2020;35:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Cuttriss N, Bouchonville MF, Maahs DM, et al. Tele-rounds and case-based training: Project ECHO telementoring model applied to complex diabetes care. Pediatr Clin North Am 2020;67:759–72. [DOI] [PubMed] [Google Scholar]
- [147].Ata R, Gandhi N, Rasmussen H, et al. Clinical validation of smartphone-based activity tracking in peripheral artery disease patients. npj Dig Med 2018;1(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


