Abstract
Introduction:
This two-part study explored the safety, feasibility, and efficacy of a mild-moderate resistance isometric leg exercise program in ambulatory boys with Duchenne Muscular Dystrophy (DMD).
Methods:
First, we used a dose escalation paradigm with varying intensity and frequency of leg isometric exercise to determine the dose response and safety in 10 boys. Second, we examined safety and feasibility of a 12-week in-home, remotely-supervised, mild-moderate intensity strengthening program in 8 boys. Safety measures included T2 MRI, creatine kinase levels, and pain. Peak strength and function (time to ascend/descend 4 stairs) were also measured.
Results:
Dose-escalation revealed no signs of muscle damage. Seven of the 8 boys completed the 12-week in-home program with a compliance of 84.9%, no signs of muscle damage, and improvements in strength (knee extensors p < 0.01; knee flexors p < 0.05) and function (descending steps p < 0.05).
Discussion:
An in-home, mild-moderate intensity leg exercise program is safe with potential to positively impact both strength and function in ambulatory boys with DMD.
Keywords: isometric exercise, T2 MRI, in-home exercise program, functional ability, Duchenne muscular dystrophy
INTRODUCTION
Only a few therapeutic interventions exist for Duchenne muscular dystrophy (DMD) which have limited impact on disease progression. Because of the enhanced fragility of dystrophic muscle, the thought of strengthening exercises in the DMD population has raised concerns due to potential for increasing muscle damage and injury1. The majority of this premise may be founded in prior mdx mouse studies focused on exercise inducing muscle injury, with results clearly demonstrating that dystrophic muscle is more easily damaged than healthy muscle when subjected to high mechanical forces aimed at promoting muscle injury, as occurs during eccentric muscle actions2. These preclinical results have contributed to clinical practice guidelines for those with neuromuscular disease (including DMD), where families are characteristically instructed to avoid strenuous physical activity for fears of inducing further muscle damage. Currently, many health care practitioners seeing patients with DMD caution against their participation in exercise or strength training due to the lack of definitive literature disputing these long-standing concerns of exercise exacerbating muscle damage.
Isometric muscle contractions, where force is generated without a change in length or joint angle, has been suggested as a possible mode of exercise in DMD since it diminishes the potential of exposing the muscle to damaging eccentric contractions 3. Another possible benefit of the isometric exercise may relate to findings showing that preconditioning isometric exercise conveys a protective effect to minimize subsequent muscle damage in healthy human muscle4. A recent study reported significant improvements in muscle function with isometric contractions in mdx mice3, however, this mode of exercise has not been investigated in boys with DMD.
Interestingly, early exploratory clinical studies showed that resistance exercise without an isometric focus did not cause physical deterioration in DMD5. Both Vignos6 and de Lateur et al.7 reported improvements from strength training without any evidence of overload weakness. While these early studies exhibit methodological weaknesses (i.e. very few subjects, heterogenous samples, etc.), they provide some limited support to the idea that strengthening exercise in humans with DMD may not be harmful and may in fact be beneficial. A clinical study in DMD demonstrated that assisted cycling training can be safely implemented and delays the loss of motor function8. However, this type of low load, dynamic exercise had no significant effect on the boys’ muscle strength. The literature currently contains insufficient evidence to support or refute the use of strengthening exercises in boys with DMD. A need exists to carefully and systematically examine the safety of using strength training in DMD.
Our objectives were: 1) Determine the dose response and safety of mild- to moderate-intensity isometric exercise in children with DMD and 2) Implement a pilot, remotely supervised home exercise intervention consisting of isometric strength training of the thigh musculature to examine its feasibility and safety.
METHODS
Participants:
Ambulatory boys with DMD were recruited to participate through online postings of Parent Project Muscular Dystrophy and the Muscular Dystrophy Association. Inclusion criteria were being 7-10.5 yrs of age, current use of corticosteroid therapy, and having the ability to walk 100m independently and ascend four steps. Exclusion criteria were any contraindication to an MR examination; presence of a secondary condition that would impact muscle function, metabolism, or motor control; and any behavioral problems that would create an inability to cooperate with exercise testing. For every participant in the study a parent gave written consent, and each subject provided written assent. All aspects of this research project were approved by the Institutional Review Board of the University of Florida.
Research Design:
Study 1:
In order to determine the dose response and safety of isometric leg strengthening exercise in boys with DMD, a first study was performed consisting of three experiments (Supplemental Fig 1) focused on a dose escalation paradigm with varying intensity and frequency of exercise: 1) an acute bout of isometric exercise at 30% of maximal volitional contraction (MVC) in four boys with DMD; 2) an acute bout of isometric exercise at 50% MVC in another group of four boys with DMD; and 3) three bouts of isometric exercise at either 30% or 50% MVC in two boys with DMD, depending on which intensity was found to be safe in at least three of four boys tested in the previous two experiments. On day one of Experiments 1 and 2, baseline assessments were performed, consisting of three safety measures [magnetic resonance imaging of leg muscles, serum creatine kinase (CK), and pain rating] and determination of peak strength (MVC) of both the knee extensors (KE) and knee flexors (KF) of the right leg. On day three, one exercise session was performed, with safety measures repeated 48 hours later. Because all four participants in experiment 1 showed no evidence of muscle damage, the second experiment assessing 50% MVC (Supplemental Fig 1b) was performed in another group of DMD boys. Experiment 3 (Supplemental Fig 1c) used an intensity of 50% MVC that was found to be safe in the two previous experiments.
Study 2:
The second study examined the safety and feasibility of an in-home, mild to moderate-intensity strengthening exercise program in ambulatory boys with DMD (Supplemental Fig 2). This study was performed following the determination of safety of one to three bouts of isometric exercise performed at 50% MVC (results from Study 1). Prior to the 12-week training program, baseline assessments were conducted at the University of Florida over a period of five days. On day one, safety measures, determination of peak strength for the bilateral KE and KF muscles, and measurement of functional ability (time to ascend and descend four steps) were performed. Following these assessments, an exercise session was performed later that day using both legs at the intensity deemed safe from Study 1 (50% MVC). A second exercise session was performed on day three, with safety measures re-assessed at day five. Following this assessment, a third exercise session was performed on day five. If safety measures were confirmed following these three exercise sessions, the study team would subsequently ship the exercise equipment to the participant’s home for the duration of the training program. The exercise training prescription parameters were to exercise (~1.5 hours/session) both legs three days per week at an intensity of 50% of the baseline MVC. Midway through the training (at 6 weeks), participants returned to the University of Florida for reassessment of safety, strength, and functional ability. The exercise intensity was then increased by 10% for the remaining 6 weeks of the exercise program. Patients returned to the University of Florida for a final assessment at the end of 12 weeks.
Safety Measures:
Three outcome measures were performed to assess the safety of the isometric exercise and to monitor the potential of any muscle damage from the exercise bout(s): 1) magnetic resonance (MR) proton transverse relaxation time (T2) of the KE and KF musculature, 2) serum CK levels, and 3) subjective rating of any pain. Muscle damage was determined to have occurred if the primary measure of T2 MR imaging and at least one of the other two safety measures were noted to be positive for indicating damage.
T2 MRI:
T2 weighted MRI has been extensively used as a construct of muscle damage and inflammation/edema, when performed 24-48 hours following exercise9–16. It detects acute muscle damage in vivo and has been correlated with histological markers to become a non-invasive, sensitive marker of muscle injury17–21. In this study, MRI was performed with a Philips 3.0T whole body scanner (Philips Achieva Quasar Dual 3T, Philips, Best, the Netherlands) with subjects positioned supine in the magnet. Multi-slice (6 axial slices) multi-echo (16 echoes with equal spacing from 20-320 ms) T2-weighted MR imaging was performed on the bilateral upper legs (thigh)22. T2 maps of the thigh muscles were created as previously done23. Using custom written software, regions of interest (ROI) were manually drawn on the T2 maps for eight KE and KF muscles (rectus femoris, vastus lateralis, vastus intermedius, vastus medialis, semitendinosus, semimembranosus, long head of the biceps femoris, and short head of the biceps femoris). All axial slices which had a clear representation and identifiable cross-sectional area were chosen for analysis. Mean T2 values for each of the eight KE and KF muscles were then calculated within each of the eight ROIs. An elevation of the mean T2 by 20% or greater (based on unpublished data) in any individual muscle was used as the threshold to indicate muscle damage had occurred.
Creatine Kinase levels:
Blood samples were collected for determination of CK levels. An elevation in CK of 7,000 U/L from baseline was considered the threshold for muscle damage. This was based on pilot data we collected in a longitudinal, natural progression study involving 10 boys with DMD (unpublished data) where 7,000 U/L was equal to two standard deviations. This amount of change in CK for an indicator of muscle damage is far more stringent than has been suggested by others when using CK levels to assess muscle damage post-intervention in DMD24.
Pain:
Pain was assessed using a Wong-Baker FACES Pain Rating Scale with faces and corresponding numbers ranging from 0 (No Hurt) to 10 (Hurts Worst)25. Subjects were asked to select one of the faces with its corresponding numerical rating and pain description. An increase in pain ≥4 was considered the threshold for muscle damage.
Muscle strength:
For strength assessment, isometric MVC was determined using a Biodex dynamometer as done previously (Biodex Medical Systems, Inc, Shirley, NY)22,26–28. The hip was set at 90 degrees of flexion while the KF and KE were both tested at 60 degrees and 30 degrees of knee flexion. Subjects were strongly encouraged to kick (KE) or pull (KF) as hard as possible for approximately five seconds with one minute of rest between trials for a minimum of five trials at both knee positions. The highest torque value was used as the MVC for each of the four tests.
Functional Ability:
The time to ascend and descend four stairs was measured to assess functional ability29. Up to three trials were performed, and the fastest time was recorded for both ascent and descent.
Exercise Sessions:
For Study 1, all of the exercise sessions were done with the right leg using a Biodex dynamometer. For Study 2, all of the exercise sessions were done for the bilateral lower extremities using a custom-built exercise set-up consisting of a modified leg curl machine (Valor Fitness, Seminole, FL), load cell and transducer cable (Interface Inc, Scottsdale, AZ), and a laptop (Supplemental Fig 3a). Exercise was done with the same positioning as the strength assessments. For both Study 1 and 2, participants performed four sets of six repetitions of isometric knee extension and knee flexion at both 30 and 60 degrees of knee flexion with a one-minute rest between each set30. For all sessions done at the University of Florida, participants received both visual feedback from the computer monitor/laptop as well as verbal encouragement to hold the contraction for each repetition for three to five seconds. For the exercise sessions done at home for Study 2, real-time video conferencing via Skype (Skype Communications SARL, Luxembourg) was used to monitor each training session and provide verbal and visual feedback to the participants. Each laptop was also equipped with software that provided visual guidance for the subjects to achieve the target intensity. This target was a pair of horizontal lines within the software application that the research team individually placed on the laptop screen for each exercise session set at approximately ±10% of the desired intensity for each exercise (Supplemental Fig 3b). After the reassessment visit at 6 weeks, the exercise intensity was increased by 10% of the 6 weeks MVC and used for the exercise sessions from week six until week 12.
Statistical Analyses:
Descriptive statistics were used for all variables. Wilcoxon-signed rank tests (SPSS version 25) were used for assessing any changes over the course of the exercise intervention in Study 2. Significance was set at alpha < 0.05.
RESULTS
Ten boys with DMD enrolled in Study 1, and eight of these same 10 boys later enrolled in Study 2 (average ± SD time between enrollment in Study 1 and Study 2 was 1.0 ± 0.5 yrs). Table 1 provides the demographic information for all the participants at time of enrollment.
Table 1.
Demographics
| Study 1 (n=10) | Study 2 (n=8) | |
|---|---|---|
| Age (yrs) | 8.3 (±0.8) | 9.3 (±0.8) |
| Height (cm) | 122 (±6) | 125 (±7) |
| Weight (kg) | 27.5 (±7.3) | 30.0 (±9.2) |
| BMI (kg/m2) | 18.6 (±5.0) | 19.0 (±4.6) |
| KE strength (N*m) | 18.4 (±12.6) | 16.9 (±12.1) |
All values given in mean (±SD).
BMI = Body mass index; KE = Knee extensor musculature
KE strength is maximal volitional contraction assessed at 60 degrees of knee flexion at initial visit.
Study 1:
Four boys with DMD completed the first experiment at 30% MVC with no indications of muscle damage. Therefore, four more boys with DMD participated in the second experiment at 50% MVC, with no signs of muscle damage. Two additional participants with DMD completed the third experiment of exercising for three sessions at an intensity of 50% MVC. No evidence of muscle damage was observed. The mean T2 percentage change after exercise at 30% and 50% MVC was 1.1 [standard deviation (SD) 2.4] across all participants, and no meaningful increase in pain was reported by any of the boys. Figure 1 demonstrates the minimal impact acute isometric exercise at 50% MVC had on CK values.
Figure 1.
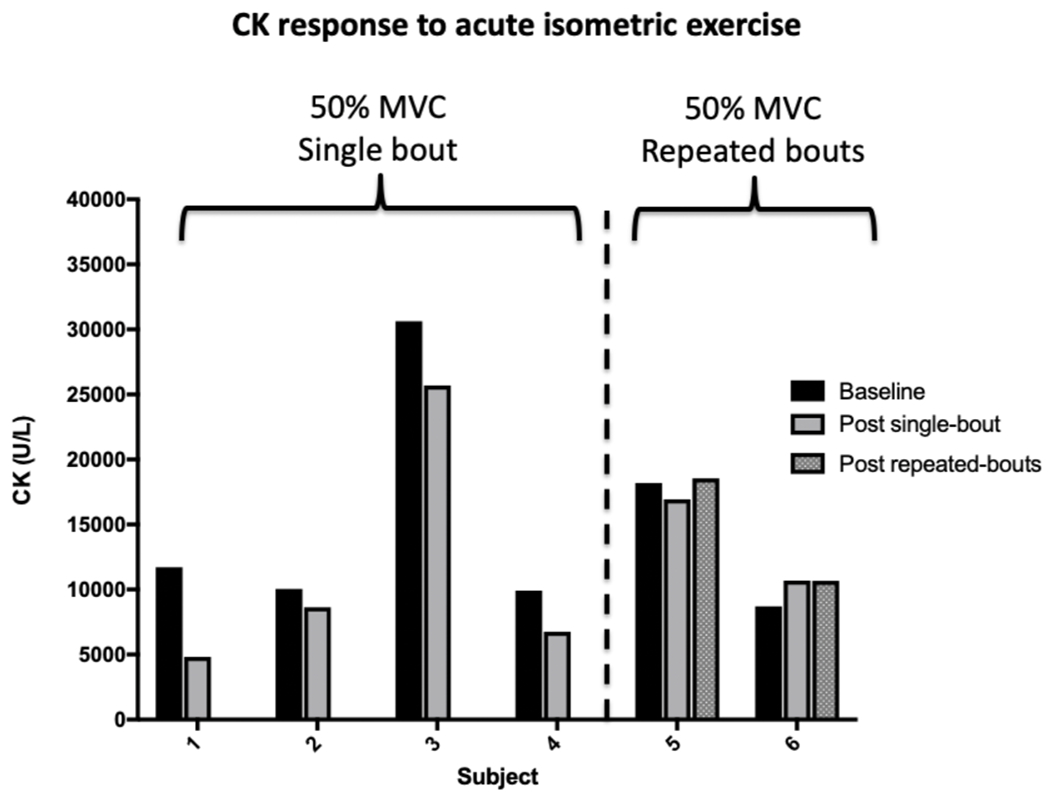
The six participants from Study 1 who performed single or repeated bouts of acute moderate intensity strengthening exercise did not demonstrate any clinically meaningful increases in creatine kinase (CK) levels. Values are shown before (Baseline) and after a single bout (Subjects 1-4 from Experiment 2) or after 3 bouts (Subjects 5 and 6 from Experiment 3) of exercise. Each bout consisted of four sets of six repetitions of isometric knee extensor and knee flexor exercise at both 30 and 60 degrees of knee flexion at an intensity of 50% maximal volitional contraction (MVC).
Study 2:
Based on the results from Study 1, the exercise training program was performed for the first six weeks at 50% MVC for Study 2. Seven of the eight boys with DMD enrolled in Study 2 completed the 12-week in-home, moderate intensity strengthening exercise program. The subject who withdrew from the study did so due to the burden of participation coupled with anxiety he was experiencing. For the boys who completed the study, compliance to perform the prescribed exercise sessions was high [84.9 (SD 9.0)%]. The primary safety outcome measure (MRI T2) did not indicate signs of muscle damage for any of the participants (Fig 2a). Only one participant had an increase in CK >7,000 U/L (8,397 U/L), but he reported no pain (0/10) and only had minor increases in T2 at 12 weeks (1.8 ms for KE and 0.4 ms for KF). Notably, strength and functional ability both improved after the exercise training program (Fig 2b), with all 7 participants who completed the program demonstrating strength gains.
Figure 2.
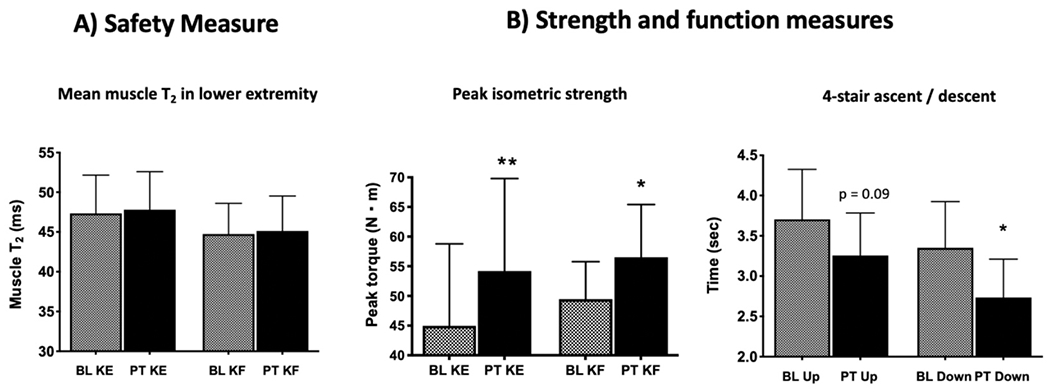
Baseline (BL) measures compared to post-training (PT) of the knee extensors (KE) and flexors (KF) after 12 weeks of moderate intensity isometric exercise: A) No increase in the primary safety measure of MRI T2 (a construct of muscle damage) was noted [non-significant increase in mean T2 from baseline to 12 weeks: KE = 2.3 (SD 3.6)% and KF = 0.4 (SD 4.6)%]; B) Significant improvements occurred for both outcome measures assessing strength (sum of KE or KF at both leg positions tested) and functional ability. Peak torque increased by 20.6% for KE and 14.3% for KF, and the time to ascend (13.5%) and descend (22.7%) four steps improved after 12 weeks of leg strengthening.
Differences noted between BL and PT (*p < 0.05, **p < 0.01).
DISCUSSION
Our results from Study 1 provide promising data that one to three bouts of mild- to moderate-intensity isometric exercise do not cause acute muscle damage in boys with DMD. Furthermore, results from Study 2 suggest a moderate-intensity isometric strengthening exercise program is not only safe but also promotes improvements in strength and function in ambulatory boys with DMD. To date, resistance exercise training is the only therapeutic strategy, other than corticosteroids, to increase muscle strength in boys with DMD. Our current study supports previous exploratory reports of improved muscle strength after a resistive exercise program6,7 and provides quantitative evidence of increased peak torque and improved function (time to descend 4 stairs). This pilot work supporting isometric strengthening exercise in children with DMD will assist in laying a foundation upon which much-needed future guidelines can be established for exercise prescription in patients with DMD.
Safety of acute isometric strengthening exercise for DMD:
Isometric strength training has never been assessed in DMD prior to our study despite its potential to induce strength gains without the risk of eccentric-associated muscle damage. Study 1 focused on developing isometric exercise at two different intensities, using a dose escalation paradigm similar to what is often used in Phase 1 pharmaceutical trials to determine a therapeutic dose for a new drug31. Our safety measures did not indicate any muscle damage as a result of either 30% or 50% MVC isometric exercise. This finding contrasts with previous reports that serum CK increases in boys with DMD after acute bouts of exercise32, thereby reflecting their greater susceptibility to muscle injury with exercise1. Variability in CK levels over time is known to be large in boys with DMD, with Jackson et al. reporting the coefficient of variation for CK to be approximately 35%33. We selected an increase in CK by 7,000 U/L, which is quite conservative relative to what others have used as a threshold for indicating exercise-induced muscle damage24. However, given variability in day-to-day levels of CK in DMD, other safety outcome measures reflecting potential muscle damage (i.e. T2 MRI) are advocated. Given its high day-to-day reproducibility34 and its sensitivity to detect change in inflammation/damage in muscle of boys with DMD22, T2 MRI was our primary safety measure and revealed no increase after acute exercise.
Safety and feasibility of isometric strength training as an intervention for DMD:
The moderate intensity 12-week exercise program was safely performed in-home three times per week at 50-60% MVC, with every exercise session monitored remotely by the research team. Various strategies were implemented to promote the excellent compliance obtained. The first three exercise sessions were done on site with the investigative team to build a rapport with the participants and their families using the same equipment that was shipped to their homes. For the subsequent weeks while exercising at home, the boys received visual feedback on their performance by watching the screen of the laptop. The laptop displayed in real time the amount of torque they were producing for each exercise repetition with lines individually placed on the screen for them to have a goal of achieving the desired intensity of MVC. We also used video conferencing, with a member of the research team instructing and encouraging the participants for the duration of every exercise session. Others have successfully used close supervision involving therapists, teachers, and parents to promote feasibility and compliance in exercise studies with DMD8,35. These methods, including parent engagement, mode of exercise, and ability to do the program in the participants’ home environment may have contributed to the successful feasibility and compliance in this study. These are important considerations for future exercise studies as feasibility and compliance can be quite challenging in this patient population36.
Efficacy of strength training in DMD:
Our results are notable in demonstrating improvements in muscle strength and function after 12 weeks of training. Being able to maintain and/or improve leg strength and walking ability may be the most important rehabilitation goals for ambulatory children with DMD35. While recent exercise studies have highlighted the potential for cycle ergometry to improve functional abilities in DMD, they did not have any impact on muscle strength8,35.
Interestingly, early exploratory studies assessing the value of exercise in DMD showed that strengthening exercise had a positive effect on strength with no evidence for overload weakness nor physical deterioration5–7. These studies used various range-of-motion, active-assisted or active-resistive strengthening exercises (not isometric). They concluded that strength exercise was not deleterious to patients with DMD and advocated for starting exercise early in the course of disease when there is a maximum amount of functional muscle. Despite these positive reports, advancement in the application of strength exercise to boys with DMD did not occur. These early studies did not provide details on what safety measures or outcomes were used to monitor muscle damage and thereby conclude that exercise had no ill-effects. Therefore, to build upon these studies, we utilized a systematic approach to assess the potential of exercise-induced muscle damage. Taken together, the results support the notion that appropriately dosed strengthening exercise has the potential to be beneficial and safe in DMD.
Isometric exercise was the mode used throughout the two studies. While the exact mechanisms as to how isometric exercise impacts muscle in a protective fashion are unknown, various explanations have been put forth37 including an apparent oxidant-mediated response to contractile activity38, an increase in heat shock proteins39, and neural adaptations38 such as improvement in neuromuscular junction function or excitation-contraction coupling3. Exploring the mechanisms of how isometric contractions protect muscle will be key for future exercise prescription guidelines in the DMD patient population.
Study Limitations:
There are limitations to this work. First, there was no randomization nor a control group involved in this pilot study. Therefore, the results can not be generalized to ambulatory boys with DMD. A randomized controlled clinical trial is needed in order to confirm how this type of exercise program affects boys with DMD. However, it should be noted that all seven of the participants who completed Study 2 demonstrated an improvement in strength over the course of 12 weeks in spite of having a progressively debilitating disease. Another potential limitation to the exercise program in this study was the length of time to complete an exercise session. Each session was approximately 90 minutes in duration. While excellent compliance was noted, the sustainability of an exercise program for young boys should be more streamlined and efficient to better accommodate the patients’ attention spans as this kind of exercise may not be deemed “fun” from the children’s perspective. We recommend further investigation into means to shorten the amount of time for an exercise session to be no more than 30-45 minutes for these children. Last, there was no focus on exploring the mechanism(s) of the positive changes seen from exercise nor were measures included to assess functional changes beyond the timed stair test. Future work should include additional measures to assess the potential mechanisms for improvements from exercise and changes in functional mobility.
Conclusion:
The results of this work suggest an in-home, mild- to moderate-intensity isometric exercise program done 3x/week is safe and potentially has a positive effect on strength and function in ambulatory boys with DMD. No evidence of muscle damage was found after single bouts of isometric exercise nor after the 12-week isometric exercise training program. Boys who completed the exercise program had significantly greater leg strength and improved ability to descend stairs. Future research should include randomized controlled clinical trials with a larger sample to confirm the findings of this study and to optimize exercise parameters, especially in the context of a therapeutic study. In addition, other exercise paradigms (such as aerobic and/or combined aerobic and resistance) should be investigated with varying modes and parameters of exercise to determine optimal exercise prescription for boys with DMD.
Supplementary Material
ACKNOWLEDGEMENTS
This study was financially supported by the National Institutes of Health (NIAMS R21 AR064949- 01A1). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 & DMR-1644779 and the State of Florida. TT is supported by the US Department of Defense project W81XWH1910330. We appreciate both Parent Project Muscular Dystrophy and the Muscular Dystrophy Association for providing assistance with subject recruitment. We thank Wayne Lott (equipment design), Samuel Riehl, and Brandi Black (data processing & analyses) for their assistance during the course of the study. We are also extremely grateful to the boys and their families who supported this work.
ABBREVIATIONS
- BMI
Body mass index
- CK
Creatine kinase
- DMD
Duchenne muscular dystrophy
- KE
Knee extensor muscle group
- KF
Knee flexor muscle group
- MR
Magnetic resonance
- MVC
Maximal volitional contraction
- ROI
Region of interest
- SD
Standard deviation
- T2
Proton transverse relaxation time
Footnotes
Ethical publication statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflict of interest: None of the authors has any conflict of interest to disclose.
REFERENCES
- 1.Spaulding HR, Selsby JT. Is exercise the right medicine for dystrophic muscle? Med Sci Sports Exerc. 2018. Sep;50(9):1723–1732. doi: 10.1249/MSS.0000000000001639. Review. [DOI] [PubMed] [Google Scholar]
- 2.Markert CD, Case LE, Carter GT, Furlong PA, Grange RW. Exercise and Duchenne muscular dystrophy: where we have been and where we need to go. Muscle Nerve. 2012. May;45(5):746–51. doi: 10.1002/mus.23244. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay A, Larson AA, Verma M, Ervasti JM, Lowe DA. Isometeric resistance training increases strength and alters histopathology of dystrophin-deficient mouse skeletal muscle. J Appl Physiol (1985). 2019. Feb 1;126(2):363–375. doi: 10.1152/japplphysiol.00948.2018. Epub 2018 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HL, Nosaka K, Pearce AJ, Chen TC. Two maximal isometric contractions attenuate the magnitude of eccentric exercise-induced muscle damage. Appl Physiol Nutr Metab. 2012. Aug;37(4):680–9. doi: 10.1139/h2012-035. Epub 2012 May 11. [DOI] [PubMed] [Google Scholar]
- 5.Scott OM, Hyde SA, Goddard C, Jones R, Dubowitz V. Effect of exercise in Duchenne muscular dystrophy. Physiotherapy. 1981. Jun;67(6):174–6. [PubMed] [Google Scholar]
- 6.Vignos PJ Jr, Watkins MP. The effect of exercise in muscular dystrophy. JAMA. 1966. Sep 12;197(11):843–8. [PubMed] [Google Scholar]
- 7.de Lateur BJ, Giaconi RM. Effect on maximal strength of submaximal exercise in Duchenne muscular dystrophy. Am J Phys Med. 1979. Feb;58(1):26–36. [PubMed] [Google Scholar]
- 8.Jansen M, van Alfen N, Geurts AC, de Groot IJ. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial “no use is disuse”. Neurorehabil Neural Repair. 2013. Nov-Dec;27(9):816–27. doi: 10.1177/1545968313496326. Epub 2013 Jul 24. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc AD, Jaweed M, Evans H. Evaluation of muscle injury using magnetic resonance imaging. Clin J Sport Med. 1993. Jan;3(1):26–30. [DOI] [PubMed] [Google Scholar]
- 10.Sorichter S, Koller A, Haid C, Wicke K, Judmaier W, Werner P, Raas E. Light concentric exercise and heavy eccentric muscle loading: effects of CK, MRI, and markers of inflammation. Int J Sports Med. 1995. Jul;16)5):288–92. doi: 10.1055/s-2007-973007. [DOI] [PubMed] [Google Scholar]
- 11.Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA. MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol. 1999. Dec;87(6):2311–8. doi: 10.1152/jappl.1999.87.6.2311. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Bose P, Walter GA, Anderson DK, Thompson FJ, Vandenborne K. Changes in muscle T2 relaxation properties following spinal cord injury and locomotor training. Eur J Appl Physiol. 2006. Jun;97(3):355–61. doi: 10.1007/s00421-006-0199-4. [DOI] [PubMed] [Google Scholar]
- 13.Larsen RG, Ringgaard S, Overgaard K. Localization and quantification of muscle damage by magnetic resonance imaging following step exercise in young women. Scan J Med Sci Sports. 2007. Feb;17(1):76–83. doi: 10.1111/j.1600-0838.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 14.Fulford J, Eston RG, Rowlands AV, Davies RC. Assessment of magnetic resonance techniques to measure muscle damage 24 h after eccentric exercise. Scand J Med Sci Sports. 2015. Feb;25(1):e28–39. doi: 10.1111/sms.12234. Epub 2014 Apr 17. [DOI] [PubMed] [Google Scholar]
- 15.Maeo S, Ando Y, Kanehisa H, Kawakami Y. Localization of damage in the human leg muscles induced by downhill running. Sci Rep. 2017. Jul 18;7(1):5769. doi: 10.1038/s41598-017-06129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeo S, Saito A, Otsuka S, Shan X, Kanehisa H, Kawakami Y. Localization of muscle damage within the quadriceps femoris induced by different types of eccentric exercise. Scand J Med Sci Sports. 2018. Jan;28(1):95–106. doi: 10.1111/sms.12880. Epub 2017 Apr 6 [DOI] [PubMed] [Google Scholar]
- 17.Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandenborne K. Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve. 2005. Nov;32(5):605–12. [DOI] [PubMed] [Google Scholar]
- 18.Stekelenburg A, Oomens CW, Strijkers GJ, Nicolay K, Bader DL. Compression-induced deep tissue injury examined with magnetic resonance imaging and histology. J Appl Physiol. 2006. Jun;100(6):1946–54. doi: 10.1152/japplphsiol.00889.2005. [DOI] [PubMed] [Google Scholar]
- 19.Heemskerk AM, Drost MR, van Bochove GS, van Oosterhout MFM, Nicolay K, Strijkers GJ. DTI-based assessment of ischemia-reperfusion in mouse skeletal muscle. Magn Reson Med. 2006. Aug;56(2):272–81. doi: 10.1002/mrm.20953. [DOI] [PubMed] [Google Scholar]
- 20.Mathur S, Vohra RS, Germain SA, Forbes S, Bryant ND, Vandenborne K, Walter GA. Changes in muscle T2 and tissue damage after downhill running in mdx mice. Muscle Nerve. 2011. Jun;43(6):878–86. doi: 10.1002/mus.21986. Epub 2011 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye F, Mathur S, Liu M, Borst SE, Walter GA, Sweeney HL, Vandenborne K. Overexpression of insulin-like growth factor-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp Physiol. 2013. May;98(5):1038–52. doi: 10.1113/expphysiol.2012.070722. Epub 2013 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arpan I, Willcocks RJ, Forbes SC, Finkel RS, Lott DJ, Rooney WD, Triplett WT, Senesac CR, Daniels MJ, Byrne BJ, Finanger EL, Russman BS, Wang DJ, Tennekoon GI, Walter GA, Sweeney HL, Vandenborne K. Examinaton of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology. 2014. Sep 9;83(11):974–80. doi: 10.1212/WNL.0000000000000775. Epub 2014 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnard AM, Willcocks RJ, Finanger EL, Daniels MJ, Triplett WT, Rooney WD, Lott DJ, Forbes SC, Wang DJ, Senesac CR, Harrington AT, Finkel RS, Russman BS, Byrne BJ, Tennekoon GI, Walter GA, Sweeney HL, Vandenborne K. Skeletal muscle magnetic resonance biomarkers correlate with function and sentinel events in Duchenne muscular dystrophy. PLoS One. 2018. Mar 19;13(3):e0194283. doi: 10.1371/journal.pone.0194283. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vry J, Schubert IJ, Semler O, Haug V, Schönau E, Kirschner J. Whole body vibration training in children with Duchenne muscular dystrophy and spinal muscular atrophy. Eur J Paediatr Neurol. 2014. Mar;18(2):140–9. doi: 10.1016/j.ejpn.2013.09.005. Epub 2013 Oct 11. [DOI] [PubMed] [Google Scholar]
- 25.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988. Jan-Feb;14(1):9–17. [PubMed] [Google Scholar]
- 26.Akima H, Lott D, Senesac C, Deol J, Germain S, Arpan I, Bendixen R, Lee Sweeney H, Walter G, Vandenborne K. Relationships of thigh muscle contractile and non-contractile tissie with function, strength, and age in boys in Duchenne muscular dystrophy. Neuromuscul Disord. 2012. Jan;22(1):16–25. doi: 10.1016/j.nmd.2011.06.750. Epub 2011 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathur S, Lott DJ, Senesac C, Germain SA, Vohra RS, Sweeney HL, Walter GA, Vandenborne K. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2010. Jul;91(7):1051–8. doi: 10.1016/j.apmr.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batra A, Harrington A, Lott DJ, Willcocks R, Senesac CR, McGehee W, Xu D, Mathur S, Daniels MJ, Rooney WD, Forbes SC, Triplett W, Deol JK, Arpan I, Bendixen R, Finkel R, Finanger E, Tennekoon G, Byrne B, Russman B, Sweeney HL, Walter G, Vandenborne K. Two-year longitudinal changes in lower limb strength and its relation to loss in function in a large cohort of patients with Duchenne muscular dystrophy. Am J Phys Med Rehabil. 2018. Oct;97(10):734–740. doi: 10.1097/PHM.0000000000000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martini J, Caromano FA, Carvalho EV, Goya PA, Hayasaka RM, Nakazune S, Fávero FM, Voos MC. Boys with Duchenne muscular dystrophy: 1-year locomotor changes in relation to a control group. Percept Mot Skills. 2018. Feb;125(1):40–56. doi: 10.1177/0031512517740684. Epub 2017 Nov 24. [DOI] [PubMed] [Google Scholar]
- 30.Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J Physiol. 1987. Oct;391:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angevin E, Spitaleri G, Rodon J, Dotti K, Isambert N, Salvagni S, Moreno V, Assadourian S, Gomez C, Harnois M, Hollebecque A, Azaro A, Hervieu A, Rihawi K, De Marinis F. A first-in-human phase I study of SAR125844, a selective MET tyrosine kinase inhibitor, in patients with advanced solid tumours with MET amplification. Eur J Cancer. 2017. Dec;87:131–139. doi: 10.1016/j.ejca.2017.10.016. Epub 2017 Nov 14. [DOI] [PubMed] [Google Scholar]
- 32.Pöche H, Hopfenmüller W, Hoffmann M. Detection and identification of myoglobin in serum by immunoblotting. Effect of exercise on patients with Duchenne muscular dystrophy. Clin Physiol Biochem. 1987;5(2):103–11. [PubMed] [Google Scholar]
- 33.Jackson MJ, Round JM, Newham DJ, Edwards RH. An examination of some factors influencing creatine kinase in the blood of patients with muscular dystrophy. Muscle Nerve. 1987. Jan;10(1):15–21. [DOI] [PubMed] [Google Scholar]
- 34.Forbes SC, Walter GA, Rooney WD, Wang DJ, DeVos S, Pollaro J, Triplett W, Lott DJ, Willcocks RJ, Senesac C, Daniels MJ, Byrne BJ, Russman B, Finkel RS, Meyer JS, Sweeney HL, Vandenborne K. Skeletal muscles of ambulant children with Duchenne muscular dystrophy: validation of multicenter study of evaluation with MR imaging and MR spectroscopy. Radiology. 2013. Oct;269(1):198–207. doi: 10.1148/radiol.13121948. Epub 2013 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alemdaroğlu I, Karaduman A, Yilmaz ÖT, Topaloğlu H. Different types of upper extremity exercise training in Duchenne muscular dystrophy: effects on functional performance, strength, endurance, and ambulation. Muscle Nerve. 2015. May;51(5):697–705. doi: 10.1002/mus.24451. Epub 2015 Mar 5. [DOI] [PubMed] [Google Scholar]
- 36.Hind D, Parkin J, Whitworth V, Rex S, Young T, Hampson L, Sheehan J, Maguire C, Cantrill H, Scott E, Epps H, Main M, Geary M, McMurchie H, Pallant L, Woods D, Freeman J, Lee E, Eagle M, Willis T, Muntoni F, Baxter P. Aquatic therapy for boys with Duchenne muscular dystrophy (DMD): an external pilot randomised controlled trial. Pilot Feasibility Stud. 2017. Mar 27;3:16. doi: 10.1186/s40814-017-0132-0. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostek MC, Gordon B. Exercise is an adjuvant to contemporary dystrophy treatments. Exerc Sport Sci Rev. 2018. Jan;46(1):34–41. doi: 10.1249/JES.0000000000000131. Review. [DOI] [PubMed] [Google Scholar]
- 38.McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004. Nov 15;561(Pt 1):233–44. Epub 2004 Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol. 2001. Mar;280(3):C621–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


