ABSTRACT
Although caused by different pathogens, COVID-19 and influenza share many clinical features, as well as the potential for inflammatory, cardiovascular, and other long-term complications. During the 2020–2021 influenza season, COVID-19 mitigation efforts and a robust influenza vaccination campaign led to an unprecedented reduction in influenza cases. The lack of exposure to influenza, along with antigenic changes, may have reduced population immunity to influenza and set the stage for a high severity influenza season in 2021–2022. For the second consecutive season, the UK Department of Health and Social Care has expanded influenza vaccine eligibility to mitigate the impact of both COVID-19 and influenza. Continuation of clear policy decisions, as well as ongoing coordination between manufacturers, distributors, health authorities, and healthcare providers, is key to reducing the burden of influenza and COVID-19 and preventing large numbers of severe cases that can overwhelm the healthcare system.
KEYWORDS: Influenza, COVID-19, pandemic, vaccination, public health initiatives
Introduction
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic documented influenza cases have declined sharply in the UK (Figure 1) and across the world.1,2 Influenza viruses are generally less transmissible than SARS-CoV-2, and pandemic-mitigation tactics such social distancing, wearing masks, and hygienic practices—which lessened the impact of COVID-19—nearly eliminated influenza transmission.2,3 During the 2020–2021 season, the World Health Organisation (WHO) reported a 62% decrease in influenza virus shipments to WHO surveillance centers and 94% reduction in submission of genetic sequence data to the Global Initiative on Sharing All Influenza Data (GISAID).4 However, it is possible that lack of exposure to influenza may have lessened population immunity and, as a result, the 2021–2022 season may be more severe.3
Figure 1.
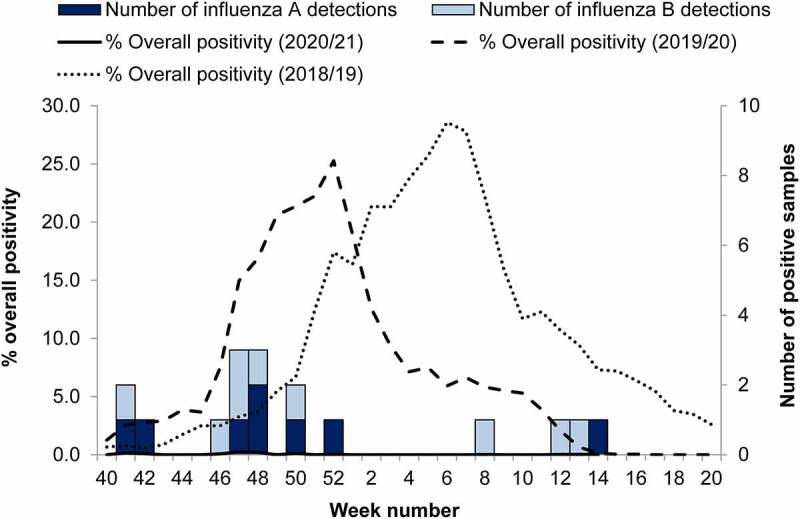
Weekly number of influenza A (dark blue bar) and B (pale blue bar) detections through Respiratory Datamart in England for the 2020–2021 season, with overall percent positivity for the 2018–2019 (dotted line), 2019–2020 (dashed line), and 2020–2021 (solid line) influenza seasons. Reprinted from Public Health England.1
The COVID-19 pandemic is the most severe global health crisis since the 1918 H1N1 influenza pandemic. Despite having different causes and occurring a century apart—in a world with vastly different medical understanding and resources—the two pandemics developed along parallel pathways. Their mortality waves share strikingly similar tracks, with a global average case fatality rate of ~2%, albeit with wide variation depending on country-specific factors.5,6 The virulence and transmissibility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) the virus that causes COVID-19—has overwhelmed healthcare systems at every level. Not only have emergency and intensive care wards been inundated with COVID-19 cases, but pandemic lockdowns and fears of COVID-19 transmission have prevented patients from seeking care for other conditions, which has led to worsening of overall physical and mental health.7 Whilst COVID-19 patients have flooded hospitals, policy makers have scrambled to stem the tide through public health mandates and restrictions. Meanwhile, just as in the 1918 pandemic, the public’s failure to adhere consistently to pandemic control measures, partly due to limited support for people self-isolating, has limited their effectiveness and led to further waves of infection, hospitalization, and death.5,8,9 This has so far proven to be the case; as of February 2022, the omicron wave has caused a third major peak in SARS-CoV2 infections, hospitalizations, and deaths since the start of the pandemic.
As economic and social pressures drive societies toward normal business, educational, and recreational routines—despite the ongoing pandemic—seasonal influenza cases will likely rise toward pre-pandemic levels, with potentially serious public health consequences. In high severity influenza seasons with high rates of serious illness, the global death toll may rise to 650,000, or 8 of every 100,000 persons.10 The 2017–2018 season was such a season, marked by high rates of hospitalizations and deaths across the Northern Hemisphere.11,12 In the UK, 22,000 deaths were attributed to influenza during that season, compared with 7000 in years with a lower influenza burden.11 However, even in less severe seasons, influenza may still have a serious impact on the economy in terms of lost productivity and on the healthcare system in terms of complications that require medical treatment and hospitalization.13,14–16,
Added to COVID-19, a high severity influenza season could take a heavy toll in the UK in 2021–2022.3,17 On an ongoing basis, should COVID-19 become a seasonal respiratory illness (as expected), the National Health Service (NHS) and the public will need to consider ongoing mitigation measures for both COVID-19 and influenza, including an increased focus on annual vaccination as the most effective means of preventing both illnesses. Other measures, such as mask mandates in some settings, may also be worthwhile.
SARS-CoV-2 and influenza as seasonal infections
Most respiratory viruses follow a seasonal pattern of infection with annual waves, and many experts believe SARS-CoV-2 will be no exception.18 As of February 2022, the WHO has identified five major variants of concern.19 Of these, the omicron strain is the most transmissible and has caused a resurgence of infections throughout the globe and is now the dominant variant in the UK. Although the available COVID-19 vaccines are effective against omicron in preventing severe disease and hospitalisations, they have not prevented infections as effectively as in the past. Immune escape in vaccinated and unvaccinated populations may contribute to SARS-CoV-2 becoming a worldwide endemic infection requiring regular booster inoculations.3,20
Seasonal influenza activity is highest during the winter in temperate regions, whereas outbreaks may occur any time in the tropics. In the UK, influenza activity typically peaks in January through February.1 If a COVID-19 wave occurs at the same time (as it did in early 2021), even a normal level of influenza activity could challenge health services in terms, not only of resource allocation, but disease management itself. Although the diseases are caused by different virus types, and the transmissibility and virulence of SARS-CoV2 is higher than influenza viruses, the two diseases share many clinical characteristics that could complicate prevention, diagnosis, and treatment, particularly in the case of co-infections.21,22 Both viruses may cause fever, breathing difficulties, fatigue, muscle aches, headache, gastrointestinal distress, coagulation disorders, inflammation, susceptibility to bacterial infections, multiorgan failure, and death.22 Early in the pandemic, many COVID-19 cases were mistaken for influenza or pneumonia, and differential diagnosis of illness (ideally with PCR confirmation, using a single swab which can be tested for a panel of respiratory infections) will be important to ensure appropriate treatment and isolation of the infected. Co-infection is also a serious concern, as it can increase symptom severity of both diseases.22 A study by Public Health England (PHE, which has since been succeeded by the United Kingdom Health Security Agency [UKHSA]) demonstrated that the risk of death in patients with both diseases was about 2 times higher than the mortality risk in patients with COVID-19 alone.23
Factors affecting the 2021–2022 influenza season
The 2019–2020 influenza season was mild, and COVID-19 mitigation measures essentially prevented the spread of influenza in 2020–2021.3 In addition, infection with SARS-CoV-2 may itself have provided some cross-protection against influenza via nonspecific mechanisms that stimulated antiviral defenses or primed adaptive responses to secondary infection with influenza.2 Although shipments of viruses and genetic samples to WHO and GISAID facilities were reduced, WHO reported no reduction in the number of influenza tests performed, so it appears unlikely that low activity is due to missed cases.2,4
Nevertheless, low levels of influenza activity may have diminished population immunity. Cross-reactive antibodies against influenza infection tend to expire within a year’s time.24 although T cells offer some long-term cross-protection against new strains of influenza, and memory cells may protect against more severe disease.25,26 Antigenic drift and shift over the past two years could lead to the emergence of new influenza strains, which could increase season severity (although it is also possible that the lower levels of transmission will mean fewer new variants will have arisen).3,11,12 These factors, along with resumption of daily economic and social routines, could have led to a doubling of influenza activity during 2021–2022 in the UK, with accompanying increases in hospitalizations and deaths.3–27–28–30 The same modeling studies predicted that increased influenza vaccination could reduce or even eliminate the extra impact of influenza (Figure 2).28–30
Figure 2.
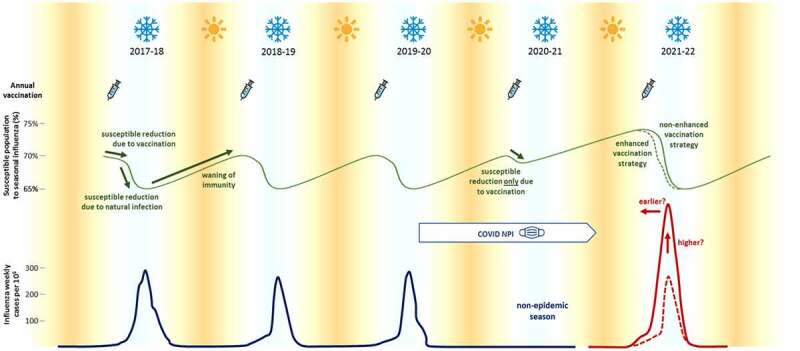
Historical and projected impact of influenza vaccinations on influenza during the 2021–2022 season. Mask-wearing and other COVID-19 mitigation measures reduced influenza infections to negligible levels in 2020–2021. Without enhanced vaccination efforts, influenza infections may double (solid red line) over typical levels (dotted red line). Reprinted from Sanz-Munoz 2021.28
Role of vaccination in overcoming the dual challenges of COVID-19 and influenza
COVID-19 vaccines
A rapid acceleration of vaccine technology—enabled by unprecedented collaboration between government and public health agencies, academia, industry, and regulatory authorities worldwide—has enabled development of over 200 COVID-19 vaccine candidates, 18 of which are either fully approved or granted an emergency use authorization in at least one country.31,32 These vaccines have helped reduce the impact of COVID-19, although vaccine resistance, the emergence of variants, and breakthrough infections continue to pose barriers to control.3–9−17–33–38
Vaccinations have so far been concentrated in the developed world. As of September 2021, >80% of UK residents had been fully vaccinated against SARS CoV-2, but in many countries, fewer than 1% had been vaccinated.6,39
Influenza vaccination during the 2020–2021 season
The COVID-19 pandemic had a dramatic impact on influenza vaccination in the UK in the 2020–2021 season. With some variation, influenza vaccination rates generally increased to the highest levels ever recorded, thanks to a robust immunisation policy set by the NHS, DHSC, and PHE that aimed for high influenza vaccine coverage rates and heightened public concern. In adults aged ≥65 years, influenza vaccination rates in 2020–2021 ranged from 77% to 81% across the British Isles and were 4 to 9 percentage points higher than in 2019–2020.1 One factor in this success was the NHS Investment and Impact Fund, which was introduced to incentivise general practice clinicians to increase overall levels of uptake by tracking the percentage of patients 65 years and older who received a seasonal flu vaccination.40 In addition, the UK Department of Health and Social Care (DHSC) expanded the free seasonal influenza vaccine programme to include all individuals aged 50–64 years to achieve maximum uptake of the influenza vaccine (previously, free vaccinations were available to individuals ≥65 years of age and those aged 50–64 years of age who were healthcare workers or had qualifying medical conditions).41 Overall, 35% of all those aged 50–64 years were vaccinated, although a higher proportion—66%—of this age group who were previously qualified received an influenza vaccine in 2020–2021. The rates of vaccination in children, adolescents, and younger adults in clinical risk groups also increased between the 2019–2020 and 2020–2021 seasons.42
The increased rates of vaccination were achieved not only by prompting general practitioners to increase vaccinations, but also by encouraging the public to obtain them. In October 2020, the DHSC initiated a public-facing marketing campaign to encourage uptake of the free influenza vaccine among eligible groups, including all children aged 2 to 15 years, those aged 6 months to <50 years in clinical risk groups, pregnant women, all adults aged ≥50 years, those in long-stay residential care homes, carers, close contacts of immunocompromised individuals, and frontline health and social care staff.41,43 Materials from the annual “Help Us Help You—Stay Well This Winter” campaign were produced centrally and disseminated to general practice surgeries and community pharmacy settings across the country,44 and the “Just the Flu” campaign included public service advertisements that ran on television, radio, and digital channels. A toolkit of adaptable campaign assets highlighting the protective benefits of influenza vaccination were made available to NHS Trusts and social care organisations to use in their own staff vaccination campaigns.41 The PHE also introduced a new national reminder call service—known as a call and recall service in the UK—to alert eligible segments of the public, which included mechanisms to remind them to book vaccination appointments. This effort was intended to support the work of local general practice surgeries and pharmacies, many of which already routinely invite registered eligible patients. The program advised healthcare providers on how to reassure patients that appropriate measures were in place to keep them safe from COVID-19 when they received the influenza vaccine, such as carefully structured appointment times to minimise waiting times and maintain social distancing; implementation of social distancing innovations such as drive-in vaccinations and ‘car as waiting room’ models, where possible; information for patients on what to expect during their appointment; proactive follow-up of at-risk individuals who miss scheduled vaccination appointments; and home visits for those on the NHS Shielded Patient List who were at high risk for COVID-19.45
Limiting infectious disease during the 2021–2022 season
Maintaining—or increasing—vaccination rates during the current influenza season will be a vital step in controlling the health impacts of both COVID-19 and influenza. A continuation of the NHS efforts to expand vaccination uptake through the Investment and Impact Fund and other initiatives is a primary goal. For the 2021–2022 season, the NHS and Joint Committee on Vaccination and Immunisation (JCVI) recommend adjuvanted quadrivalent influenza vaccine (aQIV) or high-dose quadrivalent influenza vaccine (HD-QIV) for those aged ≥65 years, either a cell-based quadrivalent influenza vaccine (QIVc) or a recombinant quadrivalent influenza vaccine (QIVr) for younger adults aged 18–64, and live attenuated vaccine to children aged 2–18 years or, if live attenuated vaccines are not suitable, QIVc in preference to vaccines developed using egg-based manufacturing.46 In agreement with the WHO, the following influenza strains should be included: A/Wisconsin/588/2019 (H1N1)pdm09-like virus, A/Cambodia/e0826360/2020 (H3N2)-like virus, B/Washington/02/2019 (B/Victoria lineage)-like virus, and B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.43,47
To foster vaccine uptake, the NHS further recommends providing influenza and COVID-19 vaccinations during the same visit.43 The JCVI has advised a policy of protecting the largest number of people rather than providing greater protection to a smaller segment of the population—a policy that has been vindicated. In late December 2020, UK officials extended the gap between first and second COVID-19 vaccine doses to provide some protection to more people in a shorter period of time.48 Initially, the decision was widely criticized based on concerns that it would leave vaccine recipients vulnerable to infection while they waited the extra time between doses. However, the JCVI’s decision at the time was based on experience with previous vaccines which suggested that longer prime-boost intervals are more effective. Subsequently, a vaccine effectiveness study of elderly people aged ≥80 years showed that a single dose of either the Pfizer (BNT162b2) or AstraZeneca (ChAdOx1) vaccines significantly reduced both symptomatic and severe disease, although protection against the delta variant was not as robust.49–51 In addition, preliminary findings from separate studies of these vaccines suggest that longer dosing intervals result in higher antibody titers than those seen with the standard intervals.52,53 NHS guidance on influenza vaccination permits the annual influenza vaccine to be given with a COVID-19 vaccine, regardless of whether it is a first, second, or booster dose if appropriate for the individual patient.54,55
The NHS guidance urges providers to stock enough influenza vaccine doses to match or exceed the vaccine uptake of 2020–2021, including enough to cover populations eligible for influenza vaccination in 2021–2022, such as all adults aged 50–64 years and children in secondary school. In addition, the guidance advocates outreach efforts from pharmacies and social workers to ensure underserved populations receive equal access to vaccinations.43
Vaccine rollout efforts will also need to ensure adequate deployment of resources, including physical locations and personnel as well as information technology systems to record and track vaccines and vaccine recipients. In addition, provision of infrastructure for storage and transportation of vaccines, especially the mRNA COVID-19 vaccines, will be essential to program success. Differences in the supply chains, administration locations, and commissioners of the two vaccines should be resolved. In the past, COVID-19 vaccines have been procured and distributed centrally, and vaccination programmes relied primarily on mass vaccination clinics, with only some inoculations in general practice surgeries. In contrast, influenza vaccinations have historically been procured mainly by individual general practice surgeries and administered there. Management and synchronisation of distribution logistics will be vitally important if the two vaccines are to be co-administered during the same visit, or even given during the same month.
Communicating vaccine information, goals, and priorities to the public
Table 1 lists strategies for maximizing vaccine uptake in response to the challenges faced by public health officials. Vaccine hesitancy is one of the primary challenges to controlling infectious disease. Reluctance to receive vaccines frequently arises from a sensible desire to fully understand the safety and effects of any given medication. Patients have a right, and should be encouraged, to ask questions and express their concerns. In the UK, public health officials have worked hard to address questions related to both COVID-19 and influenza vaccines through efforts such as the Investment and Impact Fund and the Stay Well campaigns described above.40,44 The success of these efforts is supported by data from the Office for National Statistics (ONS), which show that only 4% of adults in Great Britain are reluctant or unwilling to obtain the COVID-19 vaccine, although vaccine hesitancy rates were 8% to 21% in economically deprived regions and among the unemployed and some ethnic minorities.56 Whilst vaccine hesitancy has not been as prevalent or politically charged in the UK as elsewhere, it remains a barrier to optimal public health. It is vital that government agencies, providers, and other stakeholders, including the media and advocacy groups, continue to convey the importance of both influenza and COVID-19 vaccinations.
Table 1.
Barriers to COVID and influenza vaccination uptake and efforts to overcome them.
| Challenge | Counter strategy |
|---|---|
| Vaccine coverage | Coadministration of COVID and influenza vaccines to eligible persons during same general practice or pharmacy visit Broadening of eligibility criteria (e.g., age groups) Extension of COVID dosing intervals |
| Logistics and distribution | Coordination between the NHS, manufacturers, distributors, and individual providers to ensure adequate supplies of both COVID and influenza vaccines at point of contact with individual vaccine recipients |
| Vaccine hesitancy due to: | |
| Misinformation | Public outreach and communication through multiple media channels (e.g., Stay Well campaign) Working with community leaders to disseminate factual information and educational materials |
| Concerns about vaccine effectiveness | Education on how vaccines reduce not only infections but disease severity in individuals and disease burden on society |
| Concerns about vaccine safety | Education that places vaccine side effect risk in context with risk of morbidity and mortality from COVID and/or influenza |
In a survey conducted in late 2020 in the UK, younger age and reliance on social media for information, alongside belief in COVID-19 conspiracy theories and antipathy for vaccines in general, were associated with vaccine hesitancy, similar to findings reported for the US.37,38 In March 2021, the ONS found that concerns about adverse effects and the safety of the COVID-19 vaccines, followed by the perception that the vaccine is unnecessary because of low personal risk, were the most frequently cited reasons for vaccine hesitancy.57 Influenza vaccine hesitancy is likewise associated with unfounded beliefs that influenza vaccines are unsafe or have too many adverse effects, that influenza is not a serious disease, influenza vaccines are ineffective, or even that they cause influenza.58–60
These concerns should be countered by putting information on vaccine effectiveness and safety into context of the burden of COVID-19 and influenza on individuals, communities, and especially healthcare systems, alongside the role vaccines play in fostering immunity in the wider population.61 It is important to actively dispel myths about COVID-19 as well as any misunderstandings the public may have about influenza.62–64 Neither condition should be dismissed as “just a cold”, as both can precipitate long-term complications as well as severe acute disease and death.22 Against the SARS-CoV-2 delta variant, a single dose of ChAdOx1 or BNT162b2 reduces infection rates by ~38%, and two doses reduce symptomatic cases by up to 88%.50 Over the past 5 years in the UK, influenza vaccine effectiveness against laboratory-proven infections ranged from 15% to 52%.11–68 Effectiveness varies depending on annual fluctuations in influenza activity and whether the vaccine and circulating virus strains match, yet even a 15% reduction in cases per influenza season—especially severe ones—would reduce the burden on healthcare resources and save lives. In addition, just as seen with COVID-19 vaccines, influenza vaccination lessens the severity of breakthrough infections,69–71 and “low” vaccine effectiveness estimates should be considered in this context. For example, even if influenza vaccination is only 50% effective against hospital admissions or death, tens or perhaps even hundreds of thousands of admissions or deaths would be prevented.
In the UK, PHE, other public health officials, NHS staff, and the media have worked hard to make clear that both influenza and COVID-19 vaccines have been safely administered to millions of individuals worldwide, and serious adverse effects are extremely rare and barely more common than in the general population. Ongoing discussions of vaccine effectiveness and safety should continue to emphasize these points. For instance, vaccine-induced thrombotic thrombocytopenia (VITT) occurs at a rate of 0.9–3.6 per million people after vaccination with an adenovirus-based COVID-19 vaccine, whereas the rate of cerebral vein thrombosis is 2.4 per million in the general population and 207 per million in patients hospitalized with COVID-19.72 Put another way, even if all VITT were fatal, the risk of death would be 4 in a million, versus a 2 out of 100 chance of dying from COVID-19 or even a 1 out of a 1000 chance of dying from influenza.5,6,10 Communicating these facts may be particularly important for UK residents aged 50–64 years who are unaccustomed to receiving an influenza vaccine and may not be aware of their eligibility for one. Because this group may also be unvaccinated for COVID and thus at heightened risk of severe COVID-19, preventing a co-infection with influenza is of prime importance.
Future outlook
The COVID-19 pandemic revealed many of the best aspects of the healthcare system in the UK and worldwide, but it also highlighted numerous areas in need of improvement. The speed with which vaccines were developed and rolled out—at least in the UK, where the majority of the population was fully vaccinated by July 2021—are achievements on par with the evacuation of Dunkirk. However, education about infectious respiratory diseases (in general and specifically COVID-19 and influenza), as well as inequities in healthcare access and outcomes (due to socioeconomic, geographic, personal, or social vulnerabilities),73 are gaps that need to be addressed.
A first step will be to identify and proactively address not only clinical but also policy questions related to the pandemic. For instance, how has the COVID-19 vaccination campaign altered the public’s understanding of vaccinations? How has the campaign affected preferences for vaccines or vaccine technologies? How have attitudes toward vaccination safety and effectiveness changed, and has the success of the COVID-19 vaccination program fostered a more positive outlook toward influenza vaccination? These inquiries can help guide future education and information efforts and, importantly, counter misinformation.
On the clinical side, PHE has taken the lead in COVID epidemiological research, including the impact of coinfections with influenza, which confer a 6-fold increase in mortality risk, as noted above.23 In a vaccine effectiveness study, the PHE estimated that COVID vaccines prevented over 7 million infections and 27,000 deaths during the first six months of 2021.74 Recently, the JCVI advised giving COVID-19 boosters to individuals at risk of serious COVID disease, including all adults aged ≥50 years, residents of care homes for older adults, health and social care workers, people 16–49 years of age with underlying conditions putting them at high risk, immunocompromised individuals 12 years and older, and adult household contacts of immunocompromised individuals.39,75 Ongoing surveillance and data analysis will inform our understanding of the role of vaccine boosters in overcoming waning immunity and the emergence of new variants. Studying best practices in the logistics and distribution and administration of COVID-19 vaccines during the same period in which influenza vaccines are also administered will provide data on important logistical, clinical, and public health questions, including the effectiveness and safety of co-administration.
Another important question will be to evaluate COVID-19 vaccine development and rollout as a model for development and eventual distribution of new influenza vaccine formulations. Investigational influenza vaccines include those based on mRNA, DNA, viral vectors, and recombinant technologies, all aimed at shortening vaccine development and production timelines. For example, with standard egg-based manufacture, vaccine production must begin several months before each influenza season starts, creating the potential for mismatch between the vaccine and virus strains should circulating viruses change between seasons. In addition, egg-adaptive changes to vaccine viruses frequently occur during egg-based vaccine production.76 An mRNA-based approach could permit large quantities of influenza vaccines to be produced soon after identification of predominant circulating strains.77 This would not only ensure a closer match between vaccine and virus strains at the start of the season but also permit the vaccine supply to “evolve” in response to mid-season changes in predominant strains. On the other hand, many inherent challenges with these novel approaches must be overcome, from proof of concept in humans, to storage requirements, to the potential for adverse effects.77 Careful review of various COVID-19 vaccine programs will provide important insight into new approaches for influenza. In the meantime, current egg-based, cell-based, and recombinant influenza vaccine manufacturing platforms remain vitally important to ensuring adequate vaccine supplies across the world, and advancing cell-based and recombinant influenza vaccine technology may address shortcomings of egg-based influenza vaccine production.
Conclusions
High rates of infection, severe disease, and deaths with COVID-19 have challenged healthcare systems worldwide, and the 2021–2022 influenza season threatens to further strain resources. If, as expected, influenza cases resurge over the winter, maximizing vaccination coverage for both conditions will be the most effective means of reducing morbidity, mortality, and economic impacts from the pandemic. Efforts by the DHSC, PHE, and NHS during the 2020–2021 season to mitigate the impact of both COVID-19 and influenza have set a model for the world. Continuation of clear policy decisions, planning and implementation, and communication from manufacturers, distributors, health authorities, and healthcare providers will be the key to reducing the burden of influenza and COVID-19 in 2022.
Acknowledgments
We thank consultants C. Gordon Beck and Amanda M. Justice for editorial assistance in the preparation of this article.
Funding Statement
This work was supported by Seqirus Inc.
Disclosure statement
MA and SR are employees of Seqirus, London, Ltd.
References
- 1.Public Health England . Surveillance of influenza and other seasonal respiratory viruses in the UK: winter 2020 to 2021. London: Public Health England; 2021. [Google Scholar]
- 2.Karlsson EA, Mook PAN, Vandemaele K, Fitzner J, Hammond A, Cozza V, et al. Review of global influenza circulation, late 2019 to 2020, and the impact of the COVID-19 pandemic on influenza circulation. Releve Epidemiologique Hebd. 2021;96:241–8. [Google Scholar]
- 3.Academy of Medical Sciences . COVID-19: preparing for the future: looking ahead to winter 2021/22 and beyond. London: Academy of Medical Sciences; 2021. [Google Scholar]
- 4.Owen J. WHO warns that averting flu pandemic may be harder as surveillance switches to covid-19. BMJ. 2020;369:m2441. doi: 10.1136/bmj.m2441. [DOI] [PubMed] [Google Scholar]
- 5.He D, Zhao S, Li Y, Cao P, Gao D, Lou Y, Yang L. Comparing COVID-19 and the 1918–19 influenza pandemics in the United Kingdom. Int J Infect Dis. 2020;98:67–70. doi: 10.1016/j.ijid.2020.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns Hopkins University Coronavirus Resource Center.
- 7.Mansfield KE, Mathur R, Tazare J, Henderson AD, Mulick AR, Carreira H, Matthews AA, Bidulka P, Gayle A, Forbes H, et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Health. 2021;3(4):e217–e30. doi: 10.1016/S2589-7500(21)00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Public Health England . Coronavirus (COVID-19) in the UK.
- 9.Petherick A, Goldszmidt R, Andrade EB, Furst R, Hale T, Pott A, Wood A. A worldwide assessment of changes in adherence to COVID-19 protective behaviours and hypothesized pandemic fatigue. Nat Hum Behav. 2021;5(9):1145–60. doi: 10.1038/s41562-021-01181-x. [DOI] [PubMed] [Google Scholar]
- 10.Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England . Surveillance of influenza and other respiratory viruses in the UK: winter 2019 to 2020. London: Public Health England; 2020. [Google Scholar]
- 12.Rolfes MA, Flannery B, Chung J, O’-Halloran A, Garg S, Belongia EA, Gaglani M, Zimmerman RK, Jackson ML, Monto AS, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis. 2019;69(11):1845–53. doi: 10.1093/cid/ciz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichol KL, D’-Heilly SJ, Greenberg ME, Ehlinger E. Burden of influenza-like illness and effectiveness of influenza vaccination among working adults aged 50–64 years. Clin Infect Dis. 2009;48(3):292–98. doi: 10.1086/595842. [DOI] [PubMed] [Google Scholar]
- 14.Putri W, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960–66. doi: 10.1016/j.vaccine.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 15.Keech M, Scott AJ, Ryan PJ. The impact of influenza and influenza-like illness on productivity and healthcare resource utilization in a working population. Occup Med (Lond). 1998;48(2):85–90. doi: 10.1093/occmed/48.2.85. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiology. 2016;1(3):274–81. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burki TK. Circulation of influenza, RSV, and SARS-CoV-2: an uncertain season ahead. Lancet Respir Med. 2021;9(10):e103. doi: 10.1016/S2213-2600(21)00364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar M, Mazumder P, Mohapatra S, Kumar Thakur A, Dhangar K, Taki K, Mukherjee S, Kumar Patel A, Bhattacharya P, Mohapatra P, et al. A chronicle of SARS-CoV-2: seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J Hazard Mater. 2021;405:124043. doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Tracking SARS-CoV-2 variants; 2022. [PubMed]
- 20.Mahase E. Covid-19: omicron and the need for boosters. BMJ. 2021;375:n3079. doi: 10.1136/bmj.n3079. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. 2020;371:m4677. doi: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khorramdelazad H, Kazemi MH, Najafi A, Keykhaee M, Zolfaghari Emameh R, Falak R. Immunopathological similarities between COVID-19 and influenza: investigating the consequences of co-infection. Microb Pathog. 2021;152:104554. doi: 10.1016/j.micpath.2020.104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stowe J, Tessier E, Zhao H, Guy R, Muller-Pebody B, Zambon M, Andrews N, Ramsay M, Lopez Bernal J. Interactions between SARS-CoV-2 and influenza, and the impact of coinfection on disease severity: a test-negative design. Int J Epidemiol. 2021;50(4):1124–33. doi: 10.1093/ije/dyab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucharski AJ, Lessler J, Cummings DAT, Riley S, Rowland-Jones S. Timescales of influenza A/H3N2 antibody dynamics. PLoS Biol. 2018;16(8):e2004974. doi: 10.1371/journal.pbio.2004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19(10):1305–12. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 26.Hayward AC, Wang L, Goonetilleke N, Fragaszy EB, Bermingham A, Copas A, Dukes O, Millett ERC, Nazareth I, Nguyen-Van-Tam JS, et al. Natural T Cell–mediated protection against seasonal and pandemic influenza. Results of the flu watch cohort study. Am J Respir Crit Care Med. 2015;191(12):1422–31. doi: 10.1164/rccm.201411-1988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joint Committee on Vaccination and Immunisation . JCVI interim advice: potential COVID-19 booster vaccine programme winter 2021 to 2022; 2021.
- 28.Sanz-Muñoz I, Tamames-Gómez S, Castrodeza-Sanz J, Eiros-Bouza JM, de Lejarazu-Leonardo RO. Social distancing, lockdown and the wide use of mask; a magic solution or a double-edged sword for respiratory viruses epidemiology? Vaccines. 2021;9(6):595. doi: 10.3390/vaccines9060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krauland MG, Galloway DD, Raviotta JM, Zimmerman RK, Roberts MS. Agent-based investigation of the impact of low rates of influenza on next season influenza infections. medRxiv. 2021. doi: 10.1101/2021.08.18.21262185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K, Jalal H, Raviotta JM, Krauland MG, Zimmerman RK, Burke DS, et al. Predicting the impact of low influenza activity in 2020 on population immunity and future influenza season in the United States. medRxiv. 2021. doi: 10.1101/2021.08.29.21262803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndwandwe D, Wiysonge CS. COVID-19 vaccines. Curr Opin Immunol. 2021;71:111–16. doi: 10.1016/j.coi.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comirnaty (COVID-19 Vaccine, mRNA) prescribing information . New York: Pfizer Inc.; 2021. [Google Scholar]
- 33.Mishra S, Mindermann S, Sharma M, Whittaker C, Mellan TA, Wilton T, Klapsa D, Mate R, Fritzsche M, Zambon M, et al. Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England. E Clin Med. 2021;39:101064. doi: 10.1016/j.eclinm.2021.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hills S, Eraso Y. Factors associated with non-adherence to social distancing rules during the COVID-19 pandemic: a logistic regression analysis. BMC Public Health. 2021;21(1):352. doi: 10.1186/s12889-021-10379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrams EM, Szefler SJ. COVID-19 and the impact of social determinants of health. Lancet Respir Med. 2020;8(7):659–61. doi: 10.1016/S2213-2600(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman SM, Smith LE, Sim J, Amlôt R, Cutts M, Dasch H, Rubin GJ, Sevdalis N. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVaccs), a nationally representative cross-sectional survey. Hum Vaccines Immuno Ther. 2021;17(6):1612–21. doi: 10.1080/21645515.2020.1846397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allington D, McAndrew S, Moxham-Hall V, Duffy B. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol Med. 2021:1–12. doi: 10.1017/s0033291721001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz JB, Bell RA. Predictors of intention to vaccinate against COVID-19: results of a nationwide survey. Vaccine. 2021;39(7):1080–86. doi: 10.1016/j.vaccine.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Public Health England . JCVI issues updated advice on COVID-19 booster vaccination; 2021.
- 40.National Health Service England . Investment and impact fund 2020/21: guidance. London: National Health Service; 2020. [Google Scholar]
- 41.Public Health England, Department of Health and Social Care . The national flu immunisation programme 2020 to 2021- update. London: National Health Service; 2020. [Google Scholar]
- 42.Public Health England . Seasonal influenza vaccine uptake in GP patients: winter season 2020 to 2021: final data for 1 September 2020 to 28 February 2021. London: Public Health England; 2021. [Google Scholar]
- 43.National Health Service England . Guidance: national flu immunisation programme 2021 to 2022 letter; 2021.
- 44.National Health Service England . We’re here to help you stay well this winterwell winter. London: Public Health England; 2020. [Google Scholar]
- 45.Edwards A. Public health England. Health matters: delivering the flu immunisation programme during the COVID-19 pandemic; 2020.
- 46.National Health Service England . Guidance, appendix C: recommended influenza vaccines; 2021.
- 47.World Health Organization . Recommended composition of influenza virus vaccines for use in the 2021-2022 northern hemisphere influenza season; 2021.
- 48.Iacobucci G, Mahase E. Covid-19 vaccination: what’s the evidence for extending the dosing interval? BMJ. 2021;372:n18 doi: 10.1136/bmj.n18. [DOI] [PubMed] [Google Scholar]
- 49.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O'Doherty M, et al. Effectiveness of the Pfizer-BioNtech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373(n1088). doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–94. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.English PM. The UK approach to COVID-19 vaccination: why was it so different? Drugs Context. 2021;10:10. doi: 10.7573/dic.2021-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parry H, Bruton R, Stephens C, Brown K, Amirthalingam G, Hallis B, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv 2021:2021.05.15.21257017. [DOI] [PMC free article] [PubMed]
- 53.Flaxman AD, Marchevsky N, Jenkin D, Aboagye J, Aley PK, Angus BJ, et al. Tolerability and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 (AZD1222); 2021.
- 54.Public Health England . Flu vaccination programme 2021 to 2022: information for healthcare practitioners, 2021.
- 55.UK Health Security Agency . When can I get my flu vaccine? Flu vaccinaiton winter 2021 to 2022; 2021.
- 56.Office for National Statistics . Coronavirus and vaccine hesitancy, Great Britain: 9 August 2021; 2021.
- 57.Office for National Statistics . COVID-19 vaccine refusal, UK: February to March 2021; 2021.
- 58.Schmid P, Rauber D, Betsch C, Lidolt G, Denker ML, Cowling BJ. Barriers of iInfluenza vaccination intention and behavior – a systematic review of influenza vaccine hesitancy, 2005 – 2016. PLoS One. 2017;12(1):e0170550. doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smedley J, Poole J, Waclawski E, Stevens A, Harrison J, Watson J, Hayward A, Coggon D. Influenza immunisation: attitudes and beliefs of UK healthcare workers. Occup Environ Med. 2007;64(4):223–27. doi: 10.1136/oem.2005.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrikrishna D, Williams S, Restrick L, Hopkinson NS. Influenza vaccination for NHS staff: attitudes and uptake. BMJ Open Respir Res. 2015;2(1):e000079–e. doi: 10.1136/bmjresp-2015-000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’-Souza G, Dowdy D. What is herd immunity and how can we achieve it with COVID-19? 2021.
- 62.Krelle H, Tallack C. One year on: three myths about COVID-19 that the data proved wrong. London: The Health Foundation; 2021. [Google Scholar]
- 63.Public Health England . The flu vaccination: Who should have it this winter and why; 2021.
- 64.Williams L, Gallant AJ, Rasmussen S, Brown Nicholls LA, Cogan N, Deakin K, Young D, Flowers P. Towards intervention development to increase the uptake of COVID-19 vaccination among those at high risk: outlining evidence-based and theoretically informed future intervention content. Br J Health Psychol. 2020;25(4):1039–54. doi: 10.1111/bjhp.12468. [DOI] [PubMed] [Google Scholar]
- 65.Pebody R, Djennad A, Ellis J, Andrews N, Marques DFP, Cottrell S, et al. End of season influenza vaccine effectiveness in adults and children in the United Kingdom in 2017/18. Euro Surveill. 2019;24:1800488. doi: 10.2807/1560-7917.ES.2019.24.31.1800488. [DOI] [Google Scholar]
- 66.Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. End-Of-Season influenza vaccine effectiveness in adults and children, United Kingdom, 2016/17. Euro Surveill. 2017;22:17–00306. doi: 10.2807/1560-7917.Es.2017.22.44.17-00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill. 2016;21:30348. doi: 10.2807/1560-7917.Es.2016.21.38.30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Public Health England . Surveillance of influenza and other respiratory viruses in the UK: winter 2018 to 2019. London: Public Health England; 2019. [Google Scholar]
- 69.Deiss RG, Arnold JC, Chen WJ, Echols S, Fairchok MP, Schofield C, Danaher PJ, McDonough E, Ridoré M, Mor D, et al. Vaccine-Associated reduction in symptom severity among patients with influenza A/H3N2 disease. Vaccine. 2015;33(51):7160–67. doi: 10.1016/j.vaccine.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson MG, Pierse N, Sue Huang Q, Prasad N, Duque J, Claire Newbern E, Baker MG, Turner N, McArthur C. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine. 2018;36(39):5916–25. doi: 10.1016/j.vaccine.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 71.Godoy P, Romero A, Soldevila N, Torner N, Jané M, Martínez A, Caylà JA, Rius C, Domínguez A. Influenza vaccine effectiveness in reducing severe outcomes over six influenza seasons, a case-case analysis, Spain, 2010/11 to 2015/16. Euro Surveillance : Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 2018;23(43). doi: 10.2807/1560-7917.ES.2018.23.43.1700732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barnes GD, Cuker A, Piazza G, Siegal D. Vaccine-induced thrombotic thrombocytopenia (VITT) and COVID-19 vaccines: what cardiovascular clinicians need to know; 2021.
- 73.Public Health England . NHS population screening: identifying and reducing inequalities; 2021.
- 74.Public Health England . Direct and indirect impact of the vaccination programme on COVID-19 infections and mortality. London: Public Health England; 2021. [Google Scholar]
- 75.Joint Committe on Vaccination and Immunisation . Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination. London: Department of Health and Social Care; 2021. [Google Scholar]
- 76.Rajaram S, Van Boxmeer J, Leav B, Suphaphiphat P, Iheanacho I, Kistler K. Retrospective evaluation of mismatch from egg-based isolation of influenza strains compared with cell-based isolation and the possible implications for vaccine effectiveness. Open Forum Infect Dis. 2018;5(suppl_1):X69. doi: 10.1093/ofid/ofy209.164. [DOI] [Google Scholar]
- 77.Rockman S, Laurie KL, Parkes S, Wheatley A, Barr IG. New technologies for influenza vaccines. Microorganisms. 2020;8(11):1745. doi: 10.3390/microorganisms8111745. [DOI] [PMC free article] [PubMed] [Google Scholar]


