Abstract
Dairy propionic acid bacteria, particularly the species Propionibacterium freudenreichii, play a major role in the ripening of Swiss type cheese. Isometric and filamentous bacteriophages infecting P. freudenreichii have previously been isolated from cheese. In order to determine the origin of these bacteriophages, lysogeny of P. freudenreichii was determined by isometric bacteriophage type analysis. The genomic DNA of 76 strains were hybridized with the DNA of nine bacteriophages isolated from Swiss type cheeses, and the DNA of 25 strains exhibited strong hybridization. Three of these strains released bacteriophage particules following UV irradiation (254 nm) or treatment with low concentrations of mitomycin C. A prophage-cured derivative of P. freudenreichii was readily isolated and subsequently relysogenized. Lysogeny was therefore formally demonstrated in P. freudenreichii.
Based on habitat, the genus Propionibacterium can be divided into two groups: cutaneous and dairy (or classic) propionic acid bacteria (dairy PAB). Dairy PAB are gram-positive, non-spore-forming, catalase-positive, nonmotile, facultatively anaerobic organisms (18). They are used in the manufacture of Swiss type cheeses to ensure eye formation and the development of a typical flavor (19).
Like members of many other bacterial genera, dairy PAB are infected by viruses (14). Two types of bacteriophages which can infect dairy PAB have been isolated in Swiss type cheeses. One type belongs to group B1 of Bradley's classification, and the other is, to our knowledge, the first infective filamentous virus found in gram-positive bacteria (16). The existence of bacteriophage DNA able to replicate and express itself in the genus Propionibacterium enabled us to determine the efficiency of electrotransfection for developing a cloning vector (13).
The isometric type of bacteriophages that infect dairy PAB have frequently been shown to be present in Swiss type cheeses; the levels of contamination range from 14 to 7 × 105 PFU/g of cheese, depending on the cheese and the indicator strain used for detection. These bacteriophages are produced during multiplication of dairy PAB during cheese ripening and may multiply on either endogenous or starter strains (15). In order to understand and control the multiplication of bacteriophages during cheese production, it is important to determine their source in the manufacturing plant. We have shown that raw milk can be a source of free bacteriophages (15), although lysogenic strains may also produce them.
Lysogeny is widespread in nature, but in the context of the genus Propionibacterium, only lysogeny of Propionibacterium acnes has been studied previously (33, 35).
The purpose of this work was to demonstrate lysogeny of dairy PAB. Studies of putative lysogeny are hindered by problems with finding the lysogen and sensitive strains. It is also difficult to determine the optimum amount of mutagenic agent and the optimum bacterial growth stage for massive prophage induction. There is some homology between the virulent and temperate bacteriophages which infect Lactococcus and Lactobacillus species (24, 26). The strategy which we chose was to hybridize the genomes of 76 dairy PAB strains with DNA from nine bacteriophages isolated from Swiss type cheeses. Some putative lysogenic strains of Propionibacterium freudenreichii subsp. shermanii harboring genomic sequences exhibiting homology with bacteriophages were chosen to determine the optimal induction parameters and further confirm lysogeny in this species.
(This research was conducted by C. Hervé in partial fulfillment of the requirements for a Ph.D.)
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains used for screening (Table 1) came from the American Type Culture Collection (ATCC), Rockville, Md.); from the Centre National de Recherches Zootechniques (CNRZ), Institut National de la Recherche Agronomique, Jouy en Josas, France; from the Collection du Laboratoire de Recherche de Technologie Laitière (TL), Institut National de la Recherche Agronomique, Rennes, France; from the Collection bactérienne de l'Institut Pasteur (CIP), Paris, France; from the National Collection of Food Bacteria (NCFB [=NCDO]), Aberdeen, United Kingdom; from the Deutsche Sammlung von Mikroorganismen (DSM), Braunschweig, Germany; from the National Collection of Industrial Bacteria (NCIB), Aberdeen, United Kingdom; from the Anaerobe Culture Collection of Virginia Polytechnic Institute (VPI), Blacksburg; from different university collections (T. Britz in South Africa and B. A. Glatz, in the United States); and from a commercial starter (industrial starter). Cells were stored at −80°C in YEL broth containing 15% glycerol. Prior to use, they were subcultured twice (2% inoculum) in YEL broth for 48 h at 30°C. Colonies were grown anaerobically (anaerocult A; Merck-Eurolab, Nogent sur Marne, France) on YEL agar (YELA) (12 g of agar [Merck-Eurolab] per liter) for 1 week at 30°C (17).
TABLE 1.
Dairy PAB strains used for lysogeny screening
| Strain | Other designation(s) or source | Origin | Southern blot hybridization with bacteriophage genome(s)a |
|---|---|---|---|
| P. freudenreichii subsp. shermanii and P. freudenreichii subsp. freudenreichii strains | |||
| TL3 | CNRZ 81, ATCC 6207 | Swiss cheese | |
| TL4 | CNRZ 82, ATCC 4866 | Emmental cheese | φ110Elc, φ110D6 |
| TL18 | CNRZ 434 | ||
| TL19 | CNRZ 435 | ||
| TL20 | CNRZ 721 | Gruyere cheese | |
| TL21 | CNRZ 722 | Gruyere cheese | |
| TL22 | CNRZ 723 | Gruyere cheese | φ110B3, φ110E1t, φ110E1c |
| TL24 | CNRZ 725 | Gruyere cheese | φ110B3, φ110E1c, φ110D6 |
| TL26 | CNRZ 727 | Gruyere cheese | |
| TL27 | CNRZ 728 | Gruyere cheese | |
| TL28 | CNRZ 729 | Gruyere cheese | |
| TL34 | ATCC 9614 | Gruyere cheese | φ110Elt |
| TL37 | CIP 5932, NCDO 1073 | φ110Elt | |
| TL40 | ATCC 9616 | φ110Elt | |
| TL41 | ATCC 9617 | φ110B3, φ110D6 | |
| TL48 | DSM 20270 | Cheese | |
| TL56 | NCDO 1076 | ||
| TL61 | NCDO 1081 | ||
| TL63 | NCIB 9416 | ||
| TL100 | ATCC 6207 | Swiss cheese | φ110B3 |
| TL101 | φ110B3 | ||
| TL105 | GLATZ P93 | φ110B3, φ110Elc, φ110D6 | |
| TL106 | GLATZ P103 | φ110B3 | |
| TL110 | CNRZ 726 | Gruyere cheese | |
| TL146 | Private industrial collection | Industrial starter | φ110B3, φ110Elt, φ110E1c |
| TL153 | Private industrial collection | Industrial starter | φ110B3, φ110E1c, φ110D6 |
| TL154 | Private industrial collection | Industrial starter | φ110B3, φ110E1c, φ110D6 |
| TL157 | Private industrial collection | Industrial starter | |
| TL158 | Private industrial collection | Industrial starter | |
| TL159 | Private industrial collection | Industrial starter | |
| TL161 | Private industrial collection | Industrial starter | |
| TL164 | Private industrial collection | Industrial starter | φ110Elt |
| TL166 | Private industrial collection | Industrial starter | φ110Elt |
| TL167 | Private industrial collection | Industrial starter | φ110Elt |
| TL168 | Private industrial collection | Industrial starter | φ110Elt |
| TL170 | Private industrial collection | Industrial starter | φ110B3, φ110Elc, φ110D6 |
| TL171 | Private industrial collection | Industrial starter | |
| TL172 | Private industrial collection | Industrial starter | φ110B3, φ110E1c, φ110D6 |
| TL215 | Morbier cheese | φ110E1t | |
| TL216 | Morbier cheese | φ110B3 | |
| P. acidipropionici strains | |||
| TL2 | CNRZ 80, ATCC 4965 | ||
| TL9 | CNRZ 86, ATCC 4875 | Emmental cheese | |
| TL15 | CNRZ 287 | ||
| TL47 | ATCC 25562, DSM 4900 | ||
| TL151 | Private industrial collection | Industrial starter | |
| TL155 | Private industrial collection | Industrial starter | |
| P. jensenii strains | |||
| TL1 | CNRZ 79, ATCC 4870 | Emmental cheese | |
| TL7 | CNRZ 85, ATCC 4871 | Buttermilk | |
| TL43 | ATCC 14073 | Edam cheese | |
| TL46 | VPI 5166 | ||
| TL51 | ATCC 4869 | Gouda cheese | |
| TL55 | NCDO 1074 | ||
| TL57 | NCDO 1077 | ||
| TL58 | NCDO 1078 | ||
| TL60 | NCDO 1080 | ||
| TL62 | NCDO 1082 | ||
| TL103 | GLATZ P63 | ||
| TL104 | GLATZ P85 | ||
| TL107 | GLATZ P105 | φ110E1t | |
| TL109 | GLATZ P129 | ||
| TL219 | Appenzell cheese | φ110E1t | |
| TL246 | Ras Egyptian cheese | φ110E1t | |
| TL249 | Ras Egyptian cheese | ||
| TL328 | Raw milk | ||
| TL347 | Raw milk | ||
| TL350 | Raw milk | ||
| TL362 | Raw milk | ||
| TL396 | Raw milk | ||
| TL406 | Raw milk | ||
| P. thoenii strains | |||
| TL23 | CNRZ 724 | Gruyere cheese | |
| TL31 | CNRZ 732 | Gruyere cheese | |
| TL39 | ATCC 4872, CIP 6434 | Emmental cheese | |
| TL45 | VPI 5164 | ||
| TL59 | NCDO 1079 | ||
| TL115 | BRITZ 448 | ||
| TL220 | Appenzell cheese |
Bacteriophages which hybridized with strain genomes in Southern blot experiments.
Bacterial genome preparation: dot blot experiments.
Bacterial DNA were extracted from 500-ml cultures (optical density at 650 nm [OD650], 1 to 2). Cells were harvested, resuspended in 50 ml of buffer (10 mM Tris base, 2 mM EDTA, 100 mM NaCl, 5% Triton X100; (pH 8.0) containing 120 mg of lysozyme (CHR-Hansen, Arpajon, France), and then incubated for 30 min at 43°C. A longer incubation period (2 h) was sometimes necessary to achieve optimum lysis. Ten milligrams of proteinase K (Amresco, Solon, Ohio) and 2.5 ml of sarcosyl (5% [wt/vol]; Sigma Chemical Co., St. Louis, Mo.) were added to the cell material, and then each preparation was incubated at 40°C for 1 to 2 h. Proteins were eliminated by solvent extraction (28), and DNA was precipitated from the aqueous phase by adding 0.1 volume of 3 M sodium acetate and 0.7 volume of propan-2-ol. Nucleic acids were then dissolved in 5 ml of TE (10 mM Tris base, 1 mM EDTA; pH 8.0).
Pulsed-field gel electrophoresis (PFGE).
Dairy PAB cells were embedded in agarose to prepare a DNA insert (plug), as described by McClelland et al. (25). Ten milliliters of a culture was harvested at an OD650 of 0.3 and treated as previously described (15). The electrophoretic conditions employed are described in the figure legends.
Dot blot hybridization experiments.
A positively charged Hybond membrane (Appligen-Oncor, Illkirch, France) was humidified with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate, pH 7) and placed in a vacuum. The bacterial DNA was denatured for 10 min at 100°C and placed in ice for 10 min. One volume of 20× SSC was added. Bacterial DNA samples were placed in each well and subjected to a vacuum. The DNA was then fixed to the membrane for 5 min under UVc light (312 nm), and the membrane was dried at room temperature and stored at 4°C.
Southern blot hybridization experiments.
Southern blotting was performed after PFGE. All DNA were transferred to a positively charged Hybond membrane (Appligen-Oncor) by capillary blotting (29). Each DNA was fixed to the membrane by treating it for 5 min with UVc light (312 nm).
For preparation of the bacteriophage genome probe, 25 ng of DNA was incubated at room temperature for 3 h with [α-32P]dCTP (3,000 Ci/mmol) in the presence of DNA polymerase (Amersham, Piscataway, N.J.). Labeling was halted by adding 150 μl of TE (10 mM Tris base, 1.0 mM EDTA; pH 8) and 25 μl of carrier DNA in a final volume of 200 μl; unbound labeled nucleotides were separated from labeled DNA by exclusion chromatography with a Sephadex G-50 column (Amersham). Immediately before use, the labeled DNA was denatured by boiling it for 5 min and then rapidly cooled in ice in the presence of 25 μl of herring sperm DNA.
The positive nylon membrane (Appligene-Oncor) was prehybridized in Denhardt solution for 4 h at 42°C in the presence of 250 μg of denatured herring sperm DNA. Hybridization was carried out for 18 h at 42°C. The membrane was then washed once for 30 min in 3× SSPE (1× SSPE is 0.15 M NaCl, 10 mM NaH2PO4 · H2O, and 1 mM EDTA [disodium salt]) containing 0.1% sodium dodecyl sulfate (SDS), twice for 30 min in 1× SSPE–0.1% SDS, and, if any noise remained, once for 30 min in 0.5× SSPE–0.1% SDS and twice for 30 min in 0.1× SSPE–0.1% SDS. The membrane was exposed overnight and read with a phosphoimager (Hewlett-Packard, Avondale, Pa.).
Prophage induction: spontaneous liberation.
In order to demonstrate spontaneous bacteriophage liberation, each bacterial culture obtained at the end of growth (48 to 72 h) was centrifuged at 10,000 × g for 20 min. The supernatants were treated with DNase I (final concentration, 1 μg/ml; Roche Diagnostics, Meylan, France) and RNase I (final concentration, 10 μg/ml; Roche Diagnostics) at 37°C for 1 h and then filtered though a 0.45-μm-pore-size filter (type HA; Millipore, Molsheim, France). The soft agar layer method described by Adams (1) was used to detect bacteriophages; 0.1 ml of each filtrate or 0.1 ml of a decimal dilution was added to 0.2 ml of a mid-log-stage sensitive (or potentially sensitive) bacterial culture. After incubation for 15 min at 30°C, 3 ml of YELA (8 g of Bacto Agar [Difco Laboratories, Detroit, Mich.] per liter) was added. The resulting mixture was spread on a YELA plate. After 2 to 3 days of anaerobic incubation at 30°C, the plaques were enumerated. In order to differentiate spontaneous liberation from the carrier stage, the lysogenic culture was reisolated three times in succession, and if bacteriophages were always present in the culture supernatant, spontaneous liberation was accepted.
UVc irradiation.
A mid-log-stage bacterial culture was centrifuged, washed in sterile water, and then resuspended in saline solution. Irradiation was performed with constant stirring by using a 254-nm UV lamp (Fisher-Bioblock Scientific, Illkirch, France). UV fluences were measured with a VLX-254 radiometer (Vilber-Lourmat, Marne la Vallée, France). Irradiated samples were then mixed in double-strength YEL broth. After incubation at 30°C for 24 h, the cells were pelleted, and the supernatant was treated with DNase I and RNase I at 37°C for 1 h. The supernatant was then filtered through a 0.45-μm-pore-size filter. A nonirradiated culture served as the control. The presence of bacteriophages in the supernatant was then determined as described above.
MC.
Different concentrations of mitomycin C (MC)(Roche Diagnostics) were added to a mid-log-stage bacterial culture. Turbidity (OD650) was monitored by spectrophotometry. After incubation at 30°C for 24 h, the cells were centrifuged and the supernatant was treated as described above for induction by UVc irradiation. MC-free cultures were used as controls. The presence of bacteriophages was determined as described above.
Purification and concentration of bacteriophages.
A lysis plaque was removed from the soft agar layer and incubated with 3 ml of an early-log-stage sensitive culture at 30°C for 24 h. After centrifugation, the supernatant was filtered with a 0.45-μm-pore-size filter and tested again with the sensitive strain. Following incubation, a plaque was removed and used to infect a culture of the same sensitive strain (designated the propagating strain). After titration, a new plaque was removed and propagated again on the propagating strain. The filtrate obtained was titrated and served as a bacteriophage stock suspension.
High-titer lysates (1010 to 1011 PFU/ml) were required for bacteriophage DNA extraction or electron microscopy observation. These high-titer lysates were prepared by infecting an early-log-stage sensitive culture (100 ml) with phage at multiplicity of infection of 0.1. The infected culture was incubated at 30°C for 24 h and centrifuged, and then the supernatant was treated as described above for spontaneous liberation.
In order to obtain the desired concentration of bacteriophages with this method or after treatment of bacterial cultures (100 ml) with mutagenic agents (MC, UVc), each filtrate was centrifuged at 30,000 × g for 4 h. The pellet was dissolved in 500 μl of TM buffer (50 mM Tris base, 10 mM MgSO4; pH 7.5) overnight.
Electron microscopy.
Bacteriophage particles purified on continuous CsCl gradients were stained with uranyl acetate 5 UA (2% [wt/vol], pH 4.5). Stained grids were observed with a Zeiss EM.10 electron microscope operating at 80 kV.
Extraction of bacteriophage DNA.
One hundred microliters of STEP buffer (50 mM Tris base, 0.4 M EDTA, 0.5% SDS, 1 mg of proteinase K per ml; pH 7.5) (22) was added to 500 μl of a high-titer bacteriophage suspension. The suspension was incubated at 55°C for 1 h and extracted twice with Tris-saturated phenol-chloroform-isoamyl alcohol (25:24:1). DNA was precipitated from the aqueous phase by adding 0.1 volume of 3 M sodium acetate (pH 7.0) and 0.7 volume of propan-2-ol. The precipitated DNA was recovered by centrifugation (20 min, 15,000 × g), washed in 70% (vol/vol) ethanol, dried, and resuspended in 30 μl of TE.
Restriction of bacteriophage DNA.
Digestion of bacteriophage DNA with restriction endonucleases was performed as recommended by the manufacturer (Roche Diagnostics). DNA fragments were separated on a 0.8% agarose gel (Life Technologies, Paisley, Scotland) in 1 × TBE buffer (89 mM Tris-borate, 89 mM boric acid, 2 mM EDTA; pH 8.3).
RESULTS AND DISCUSSION
Screening of putative lysogenic strains by dot blot hybridization.
Nine virulent bacteriophages infecting P. freudenreichii and representing the bacteriophages isolated from Swiss type cheeses were chosen for this study. Their genomes were used as probes. These bacteriophages (φ110E1t, φ110E1c, φ19E4, φ110D6, φ110B3, φ19E1, φ19B3, φ52, and φ142) belong to group B1 of Bradley's classification (4). They are similar to many of the bacteriophages which infect lactic acid bacteria, and the genome of each of them consists of a linear double-stranded DNA molecule that is 36 to 40 kb long. Cohesive ends were detected for one of the bacteriophages (φB22) (14). The host spectra of these bacteriophages differed and could be distinguished by using DNA restriction patterns (15). However, the bacteriophages exhibited a high degree of DNA homology (16).
First, bacteriophage DNA were hybridized in dot blots with the genomic DNA of 76 dairy PAB strains including 40 strains of P. freudenreichii, 7 strains of Propionibacterium thoenii, 23 strains of Propionibacterium jensenii, and 6 strains of Propionibacterium acidipropionici (Table 1). These strains were chosen because of their origins (starter strains, strains isolated from raw milk or cheeses, strains obtained from international collections). P. freudenreichii strains were more numerous because this species is the most frequently encountered species in Swiss type cheeses and is the principal dairy PAB species used as a cheese starter. Moreover, of the four dairy species, P. freudenreichii is the most resistant to heat during the manufacture of these cheeses (10). Only six strains of P. acidipropionici were used because our collection and other national and industrial collections do not contain many strains of this species. Certain strains were chosen because they are used in European dairy PAB starters (TL146, TL151, TL153, TL154, TL155, TL157, TL158, TL159, TL161, TL164, TL166, TL167, and TL168) or because they are sensitive to bacteriophages (TL18, TL19, TL21, TL105, and TL110) (15).
Of the 76 strains tested, 29 (38%) showed homology with the DNA of one or more bacteriophages. The four species studied produced different results. Three of seven P. thoenii (43%), 4 of 23 P. jensenii strains (17%), 22 of 40 P. freudenreichii (55%), and no P. acidipropionici strains produced positive results in dot blot experiments. Seven of 13 dairy industrial strains (54%) and one of five sensitive strains (20%) were positive for hybridization.
Probes from bacteriophages φ110B3, φ110E1c, φ110Elt, and φ110D6 hybridized with the genomes of numerous strains, whereas probes from bacteriophages φ19E1 and φ19E4 did not hybridize with bacterial DNA. Other bacteriophage probes hybridized with only one or a small number of bacterial DNA.
In terms of putative lysogeny with dairy PAB, the results are somewhat dubious because although they indicate that there is homology between some bacteriophages and bacterial genes, they do not formally prove that prophages are present on the bacterial chromosome. Indeed, the bacterial cultures employed during this study may have been contaminated with virulent or carrier stage bacteriophages, so that the bacterial DNA extracted could have been contaminated with DNA of bacteriophage which were not at the prophage stage.
In order to circumvent these problems with the dot blot procedure, Southern blot DNA hybridization was used to confirm our results.
Southern blot DNA hybridization.
Positive hybridization results obtained in the dot blot analysis were studied thoroughly by performing a Southern analysis. The 29 bacterial DNA exhibiting homology with one or more bacteriophage DNA were digested with endonuclease XbaI, which cut the dairy PAB chromosome into several fragments. These fragments were separated by PFGE. The patterns obtained were made up of 10 to 16 bands ranging in size from approximately 10 to 1,000 kb, which were easier to analyze (12).
The four bacteriophage genomes (φ110B3, φ110E1t, φ110E1c, and φ110D6) that exhibited homology with numerous bacterial DNA in dot blot experiments were used as probes.
Southern blot analysis confirmed the results obtained by dot blot hybridization. A total of 25 of the 29 strains studied (86%) exhibited homology on their chromosomes with the DNA of bacteriophages (Table 1). Only four bacterial DNA (DNA from P. jensenii TL1 and P. thoenii TL39, TL45, and TL115) which produced positive signals in the dot blot hybridization analysis did not hybridize with the corresponding bacteriophage DNA probes. When the strain patterns obtained by PFGE were similar, the strains were closely related and their hybridization patterns were identical (TL22 and TL24, TL166 and TL164, TL154 and TL170) (data not shown).
Spontaneous prophage induction.
Three strains (TL4, TL146, and TL170) were chosen to study spontaneous prophage induction. They were selected because they had produced strong hybridization signals during the Southern blot analyses. Their hybridization profiles were made up of between three and six different bands. A hybridization band between 30 and 40 kb was observed in all cases, and this band could have corresponded to free bacteriophages in the culture. We therefore tried to demonstrate spontaneous bacteriophage liberation by using the sensitive strains in our collection (TL18, TL19, TL21, TL29, TL105, TL110, TL301, and TL302). A bacteriophage capable of infecting strain TL110 was detected in a culture of strain TL146. After three successive isolations of this strain on YELA, the bacteriophage (designated φ146) was still present. This result suggests that spontaneous liberation probably occurred. The concentration of temperate bacteriophages liberated at the beginning of the stationary stage was 106 PFU/ml of culture.
Optimization of prophage induction.
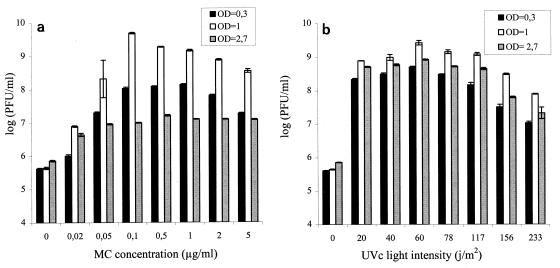
In order to optimize the conditions for prophage induction of mutagenic agents, UVc irradiation and MC were chosen because they are the mutagenic agents that are most commonly used in this type of study and because MC has been used successfully for P. acnes prophage induction (33). Strain TL146 was probably lysogenized with a bacteriophage which could be detected by sensitive strain TL110, so we decided to optimize the conditions under which UVc irradiation and MC were used with this strain. A culture of strain TL146 at three different growth stages was treated with several MC concentrations and different intensities of UVc irradiation (Fig. 1). Whatever level of mutagenic agent was used, a culture of strain TL146 at an OD650 of 1 (end of the log stage) exhibited the greatest bacteriophage liberation. Maximum liberation was obtained with an MC concentration of 0.1 μg/ml and a UVc intensity of 60 J/m2.
FIG. 1.
Optimization of induction of prophage φ146. Strain TL146 cultures were treated with different MC concentrations (a) and with different UVc intensities (b). The bacteriophages liberated were enumerated 24 h after induction treatment. Experiments were performed in quadriplicate. OD, optical density.
Differences in the physiological stage or cell density at the time of mutagen treatment could explain the variations in induction observed. In order to study the role of the physiological stage, three cultures of strain TL146 at different physiological stages (beginning and end of the log stage [OD650, 0.3 and 1] and stationary stage [OD650, 2.7]) were diluted with fresh YEL medium to obtain identical cell densities (OD650, 0.3). These cultures were treated with mutagenic agents under the optimum conditions determined previously (0.1 μg of MC per ml, 60 J of UVc irradiation per m2). Whatever the physiological stage of the cells at the time of mutagen treatment, bacteriophage φ146 liberation was the same (data not shown). Thus, for strain TL146, it appears that the efficacy of induction depended mainly on the number of cells capable of division after treatment.
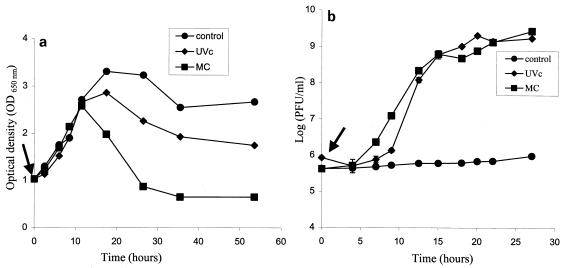
As a rule, massive prophage induction and liberation in a culture are accompanied by cell lysis. The culture of strain TL146 was treated under optimum conditions (UVc irradiation and MC) at the end of the log stage (OD650, 1.0) (Fig. 2a). In parallel, the temperate bacteriophages liberated were enumerated (Fig. 2b). During the first 12 h after treatment, no difference in OD650 was noted between controls and treated cultures. Thereafter, the OD650 values fell more markedly in cultures treated with mutagenic agents than in control cultures. A possible explanation for this difference is induction by MC of another undetected prophage.
FIG. 2.
(a) Growth of strain TL146 and liberation of temperate bacteriophage φ146 after mutagenic agent treatment (0.1 μg of MC per ml or 60 J of UVc irradiation per m2)]. The arrow indicates the time of treatment with mutagenic agents. (b) Kinetics of temperate bacteriophage φ146 liberation after treatment of a culture of strain TL146 with MC (0.1 μg/ml) or UVc irradiation (60 J/m2). The arrow indicates the time of treatment with mutagenic agents.
Bacteriophage φ146 liberation occurred 7 h after induction treatment. The majority of viruses had been liberated after 24 h (Fig. 2b).
The same induction parameters were applied successfully to two other strains (TL4 and TL170), which released bacteriophages φ4 and φ170.
Characterization of temperate bacteriophages φ4, φ146, and φ170. (i) Morphology.
Electron microscopy observations showed that three temperate bacteriophages (φ146, φ4, and φ170) had morphological characteristics similar to those of virulent bacteriophage φB22 isolated from an industrial cheese and described in a previous study (14; data not shown). These bacteriophages could be considered members of group B1 in Bradley's classification.
(ii) Genome.
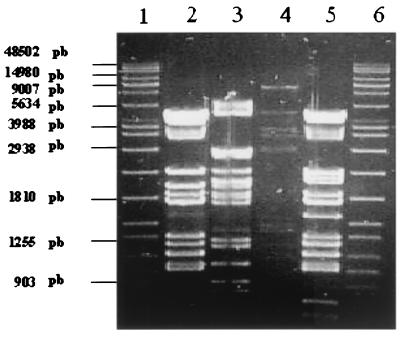
The genomes of temperate bacteriophages were digested with restriction endonuclease PstI, which was selected during a previous study (15) (Fig. 3). The lengths of the φ4, φ146, and φ170 genomes were 30, 32, and 34 kb, respectively, as calculated by adding the lengths of restriction fragments. The genome restriction profiles of temperate bacteriophage φ146 and virulent bacteriophage φB22 were found to be very closely related.
FIG. 3.
Agarose gel electrophoresis of PstI fragments generated from the DNA of temperate bacteriophages. Lanes 1 and 6, size marker (Raoul; Appligene-Oncor); lane 2, φB22 DNA; lane 3, φ4 DNA; lane 4, φ170 DNA; lane 5, φ146 DNA. pb, base pairs.
Prophage-cured derivatives and relysogenization.
In a lysogenic bacterial culture, a fraction of the population was naturally prophage cured. There were few such cured derivatives, so it was necessary to reduce the number of lysogenic cells in order to isolate them. Treatment of a culture with one or more mutagenic agents was required to enable prophage induction. As the cured derivatives lost their immunity to infection, they became sensitive to a temperate bacteriophage and had to be isolated on solid agar medium. Finally, to complete the demonstration that lysogeny occurred, it was necessary to relysogenize the cured derivatives by using the same bacteriophage (21).
Temperate bacteriophages φ4 and φ170 could not be detected by a sensitive strain in our collection, so they may have been defective. In this case, identification of a cured derivative would have been impossible. We therefore decided to demonstrate lysogeny in dairy PAB by using strain TL146. Initially, strain TL146 cells in the log stage and a decimal dilution were plated directly onto YELA and then treated with a prophage-inducing dose of UVc irradiation. A mortality rate of 99% was obtained. One hundred clones that survived UV exposure were tested for sensitivity to the induction filtrate of strain TL146. Only one clone was found to be sensitive. When this variant was evaluated for spontaneous bacteriophage liberation or after MC treatment, no viruses were detected in the culture.
Relysogenization of the cured derivative.
In order to complete our formal demonstration of lysogeny, the prophage-cured derivative was relysogenized by using the same bacteriophage. The cured derivative was infected with temperate bacteriophage φ146, and bacteria from the center of a lysis plaque were plated onto YELA. Several colonies were screened for bacteriophage sensitivity. Seventeen clones were purified, and 15 were found to exhibit restored infectious immunity and spontaneous liberation.
Demonstration of prophage insertion.
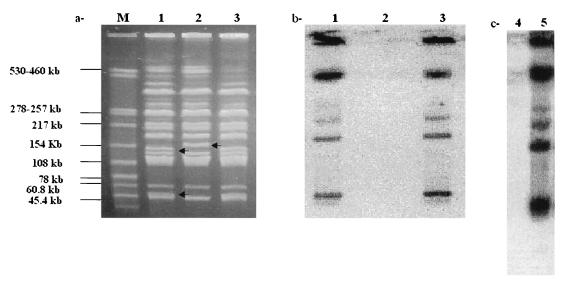
In order to obtain physical evidence that the clone isolated was a prophage-cured derivative of strain TL146, genomes of several isogenic strains were examined for the presence of φ146 DNA sequences. Genomic DNA from strain TL146, from a cured variant, and from one relysogenized clone were digested with the low- restriction enzyme XbaI, and the fragments were separated by PFGE. This analysis revealed that the two XbaI restriction fragments at 54 and 164 kb, which were present in the lysogenic strain, had disappeared in the cured variant and a new band at 186 kb had appeared (Fig. 4a). Bacteriophage φ146 DNA had one XbaI site, and its absence from the cured variant removed one XbaI site from the bacterial chromosome. The combined size of the two smaller fragments (54 and 164 kb) minus the size of the bacteriophage genome (32 kb) corresponded to the size of the largest fragment (186 kb). This result demonstrated that the integration site of prophage φ146 was on the bacterial chromosome. Restriction analysis of several relysogenized variants produced identical results, suggesting that there was a specific integration site for the genome of prophage φ146 on the chromosome of strain TL146. With this in mind, the DNA of φ146 was labeled with [α32P]dCTP and used as a hybridization probe to confirm that the insertion site really was on the bacterial chromosome (Fig. 4b). Hybridization signals were obtained for strain TL146 and the relysogenized clone. However, chromosomal XbaI fragments of the cured derivative did not exhibit homology with the DNA of bacteriophage φ146.
FIG. 4.
Demonstration of lysogeny. (a) PFGE patterns for dairy PAB genomes digested with restriction enzyme XbaI (3 to 30s, 200 V, migration for 21 h). Lane M, size marker; lane 1, strain TL146; lane 2, cured derivative; lane 3, cured derivative relysogenized with bacteriophage φ146. (b) Corresponding Southern blot hybridized with the 32P-labeled genome of bacteriophage φ146. The φ146 genome used as a positive control produced strong hybridization intensity (data not shown). (c) Southern blot of sensitive strain TL110 lysogenized by bacteriophage φ146. Lane 4, strain TL110; lane 5, strain TL110 lysogenized with bacteriophage φ146. Bacteriophage φ146 which was used as a positive control, hybridized strongly with the probe (data not shown).
These findings demonstrated that prophage φ146 was integrated in strain TL146 and relysogenized clonal chromosomes and was absent from the cured derivative. Thus, our data provide the first formal demonstration of lysogeny in the genus Propionibacterium.
The presence of a prophage in the bacterial genome endows the genome with certain properties. For instance, immunity to infection by other related bacteriophages is more widespread. In order to study this phenomenon, we infected strain TL146 and the cured derivative with four bacteriophages (φ110E1t, φB22, φ110D6, and φ110B3). All of the bacteriophages were able to propagate on the cured derivative, and none was able to propagate on strain TL146. This result suggested that there was homoimmunity among these bacteriophages.
Another property found in lysogenic bacteria is conversion by the integrated prophage (20, 23, 31). In order to study whether conversion was present in strain TL146 because of bacteriophage φ146 integration, several metabolic activities (API 50 CH strips) and growth kinetics on YEL medium were compared in the lysogenic strain and the cured derivative. No differences were observed between the two cell types.
To complete this work, we attempted to lysogenize sensitive strain TL110 with bacteriophage φ146. Twelve clones were isolated from a lysis plaque. All of these clones were resistant to φ146 and liberated it spontaneously. Genomic DNA from strain TL110 and one clone lysogenized with φ146 and digested with the low-restriction enzyme XbaI produced PFGE patterns similar to those produced by the cured derivative and strain TL146, respectively. One restriction fragment present in strain TL110, the XbaI fragment at 186 kb, was not present in the lysogenized derivative, while two bands appeared at 54 and 164 kb (data not shown). The gel was Southern blotted, and the φ146 genome was used as a hybridization probe. Identical hybridization patterns were obtained for the lysogenized clone of TL110 and strain TL146, indicating that similar integration sites were present in strains TL110 and TL146 (Fig. 4b and c).
Conclusion and perspectives.
Obtaining a cured derivative and relysogenization of this derivative with the same bacteriophage enabled us to demonstrate lysogeny for the first time in P. freudenreichii subsp. shermanii. Spontaneous induction could be detected with prophage φ146. We identified a temperate bacteriophage-sensitive strain system which allowed optimization of the conditions for prophage induction. The MC concentrations used were similar to or lower than those used for P. acnes, Lactococcus spp., Leuconostoc oenos, Lactobacillus spp., and Streptococcus thermophilus (2, 3, 5, 6, 8, 9, 27, 33). When UVc irradiation was used, the optimum intensities used for dairy PAB were higher than those employed for lactococcal and Lactobacillus strains (7, 8, 11). This result agreed with the antimutagenic and reactive activities against UVc irradiation described for dairy PAB (32).
The incidence of lysogeny in dairy PAB is higher than those observed in P. acnes, S. thermophilus, and Lactobacillus spp. (5, 33, 34). However, it appears to be lower than those observed in Leuconostoc oenos and lactococcal strains (9, 30). Our data may have been underestimates, because only bacteriophages capable of infecting some of our strains could be used as probes. These bacteriophages were closely related and thus undoubtedly not representative of the true diversity present in the environment (16).
It was difficult to obtain a true idea of prophage diversity because only three strains were studied during this work; extension of the lysogeny study to a larger number of strains was not possible because few sensitive strains were available. It was therefore very difficult to show clearly which bacteriophages were liberated. Electron microscopy is the only technique which can be used to detect the presence of bacteriophages following induction.
The discovery of a prophage on P. freudenreichii is of fundamental interest because it should contribute to a broader knowledge of the genome of this bacterial species. It may also be of value in terms of potential applications (e.g., in the context of constructing an integrative cloning vector). Indeed, an Escherichia coli-P. freudenreichii shuttle vector has been constructed in our laboratory (16). The integrase and attP site of the temperate bacteriophage enabled construction of this genetic tool. The implications of prophages for the manufacture of Swiss type cheese were not studied here. It is highly probable that these bacteriophages do not disrupt the microbial ecosystem during cheese ripening. Indeed, if bacteriophages are released while cheese is in the warm room (when dairy PAB develop [19]), thought to be where spontaneous prophage induction occurs, the compact structure of the cheese prevents diffusion of the bacteriophages and therefore destruction of sensitive strains present in the cheese. Nevertheless, studying lysogeny of dairy PAB may also be of value in terms of use of these bacteria for lysis control. Indeed, if high levels of spontaneous induction occur, lysis of lysogenic strains could accelerate the ripening process by releasing intracellular enzymatic potential into the curd.
ACKNOWLEDGMENTS
This work was supported by funds provided to C. Hervé by the Brittany Region.
We thank Françoise Michel for assistance with the electron microscopy observations and Jane Hall for correcting the English in the manuscript.
REFERENCES
- 1.Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers; 1959. [Google Scholar]
- 2.Arendt E K, Lonvaud A, Hammes W P. Lysogeny in Leuconostoc oenos. J Gen Microbiol. 1991;137:2135–2139. doi: 10.1099/00221287-137-9-2135. [DOI] [PubMed] [Google Scholar]
- 3.Barefoot S F, McArthur J L, Kidd J K, Grinstead D A. Molecular evidence for lysogeny in Lactobacillus acidophilus and characterization of a temperate bacteriophage. J Dairy Sci. 1990;73:2269–2277. [Google Scholar]
- 4.Bradley D E. Ultrastructure of bacteriophages and bacteriocins. Bacteriol Rev. 1967;31:230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carminati D, Giraffa G. Evidence and characterization of temperate bacteriophage in Streptococcus salivarius subsp. thermophilus St8. J Dairy Res. 1992;59:71–79. doi: 10.1017/s0022029900030260. [DOI] [PubMed] [Google Scholar]
- 6.Caso J L, Clara G, de Los Reyes-Gavilan C G, Herrero M, Montilla A, Rodriguez A, Suarez J E. Isolation and characterization of temperate and virulent bacteriophages of Lactobacillus plantarum. J Dairy Sci. 1995;78:741–750. [Google Scholar]
- 7.Chopin M C, Rouault A, Rousseau M. Elimination d'un prophage dans des souches mono-et multilysogènes de streptocoques du groupe N. Lait. 1983;63:102–115. [Google Scholar]
- 8.Cluzel P J, Veaux M, Rousseau M, Accolas J P. Evidence for temperate bacteriophages in two strains of Lactobacillus bulgaricus. J Dairy Res. 1987;54:397–405. doi: 10.1017/s0022029900025577. [DOI] [PubMed] [Google Scholar]
- 9.Cuesta P, Suarez J E, Rodriguez A. Incidence of lysogeny in wild lactococcal strains. J Dairy Sci. 1995;78:998–1003. [Google Scholar]
- 10.Fessler D, Casey M G, Puhan Z. Identification of propionibacteria isolated from brown spots of Swiss hard and semi-hard cheeses. Lait. 1999;79:211–216. [Google Scholar]
- 11.Gasson M J, Davies F L. Prophage-cured derivatives of Streptococcus lactis and Streptococcus cremoris. Appl Environ Microbiol. 1980;40:964–966. doi: 10.1128/aem.40.5.964-966.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautier M, Mouchel N, Rouault A, Sanséau P. Determination of genome size of four Propionibacterium species by pulsed-field gel electrophoresis. Lait. 1992;72:421–426. [Google Scholar]
- 13.Gautier M, Rouault A, Lemée R. Electrotransfection of Propionibacterium freudenreichii TL 110. Lett Appl Microbiol. 1995;20:125–129. [Google Scholar]
- 14.Gautier M, Rouault A, Lortal S, Leloir Y, Patureau D. Characterization of a phage infecting Propionibacterium freudenreichii. Lait. 1992;72:431–435. [Google Scholar]
- 15.Gautier M, Rouault A, Sommer P, Briandet R. Occurrence of Propionibacterium freudenreichii bacteriophages in Swiss cheese. Appl Environ Microbiol. 1995;61:2572–2576. doi: 10.1128/aem.61.7.2572-2576.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautier M, Rouault A, Hervé C, Sommer P, Leret V, Jan G, Fraslin J M, Prévot F, Coste A. Bacteriophages of dairy propionibacteria. Lait. 1999;79:93–104. [Google Scholar]
- 17.Hettinga D H, Vedamuthu E R, Reinbold G W. Pouch method for isolating and enumerating propionibacteria. J Dairy Sci. 1968;57:1707–1709. doi: 10.3168/jds.S0022-0302(68)87259-5. [DOI] [PubMed] [Google Scholar]
- 18.Hettinga D H, Reinbold G W. The propionic-acid bacteria, a review. I. 1972. Growth. J. Milk Food Technol. 35:295–301. [Google Scholar]
- 19.Langsrud T, Reinbold G W. Flavor development and microbiology of Swiss cheese—a review. III. Ripening and flavor production. J Milk Food Technol. 1973;36:593–609. [Google Scholar]
- 20.Lee C Y, Iandolo J J. Mechanism of bacteriophage conversion of lipase activity in Staphylococcus aureus. J Bacteriol. 1985;164:288–293. doi: 10.1128/jb.164.1.288-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lwoff A. Lysogeny. Bacteriol Rev. 1953;17:269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 23.Mason R E, Allen W E. Characteristics of Staphylococcus aureus associated with lysogenic conversion to loss of beta-hemolysin production. Can J Microbiol. 1975;21:1113–1116. doi: 10.1139/m75-161. [DOI] [PubMed] [Google Scholar]
- 24.Mata M, Trautwetter A, Luthaud G, Ritzenthaler P. Thirteen virulent and temperate bacteriophages of Lactobacillus bulgaricus and Lactobacillus lactis belong to a single DNA homology group. Appl Environ Microbiol. 1986;52:812–818. doi: 10.1128/aem.52.4.812-818.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClelland M, Jones R, Patel Y, Nelson M. Restriction endonucleases for pulsed-field mapping of bacterial genomes. Nucleic Acids Res. 1987;15:5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Relano P, Mata M, Bonneau M, Ritzenthaler P. Molecular characterization and comparison of 38 virulent and temperate bacteriophages of Streptococcus lactis. J Gen Microbiol. 1987;133:3053–3063. doi: 10.1099/00221287-133-11-3053. [DOI] [PubMed] [Google Scholar]
- 27.Reyrolle J, Chopin M C, Letellier F, Novel G. Lysogenic strains of lactic acid streptococci and lytic spectra of their temperate bacteriophages. Appl Environ Microbiol. 1982;43:349–356. doi: 10.1128/aem.43.2.349-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 30.Tenreiro R, Santos R, Brito L, Paveira H, Vieira G, Santos M A. Bacteriophages induced by mitomycin C treatment of Leuconostoc oenos strains from Portuguese wines. Lett Appl Microbiol. 1993;16:207–209. [Google Scholar]
- 31.Thomas J M, Kay W W. Effect of bacteriophage P1 lysogeny on lipopolysaccharide composition and the lambda receptor of Escherichia coli. J Bacteriol. 1984;159:1047–1052. doi: 10.1128/jb.159.3.1047-1052.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorobjeva L I, Khodjaev E Y, Cherdinceva T A. Antimutagenic and reactivative activities of dairy propionibacteria. Lait. 1995;75:473–487. [Google Scholar]
- 33.Webster G F, Cummins C S. Use of bacteriophage typing to distinguish Propionibacterium acnes types I and II. J Clin Microbiol. 1978;7:84–90. doi: 10.1128/jcm.7.1.84-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokokura T, Kodaira S, Ishiwa H, Sakurai T. Lysogeny in lactobacilli. J Gen Microbiol. 1974;84:277–284. doi: 10.1099/00221287-84-2-277. [DOI] [PubMed] [Google Scholar]
- 35.Zierdt C H. Properties of Corynebacterium acnes bacteriophage and description of an interference phenomenon. J Virol. 1974;14:1268–1273. doi: 10.1128/jvi.14.5.1268-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]