Abstract
Purpose
To assess refractive outcomes of a trifocal intraocular lens (IOL) in post-myopic laser refractive surgery eyes.
Methods
This was a retrospective chart review of 35 eyes (21 patients), with history of laser refractive surgery, who were implanted with a trifocal IOL. Surgeon’s standard procedure included femtosecond laser (FLACS), digital registration, and intraoperative aberrometry (IA). The primary outcome measure was absolute prediction error. Secondary measures were refractive outcomes, postoperative residual astigmatism (PRA), monocular uncorrected visual acuity at distance (UDVA; 4m), intermediate (UIVA; 60cm), and near (UNVA; 40cm), and monocular best-corrected visual acuity at distance (BCVA; 4m).
Results
At 3 months postoperatively, 71% and 68% of eyes had absolute prediction error 0.5 D or less with IA and preoperative planning respectively, which was not statistically significant (p > 0.05). The PRA was 0.5 D or less in 91% of eyes with IA and 56% of eyes with preoperative planning. The PRA differences between IA and preoperative planning were statistically significant (p < 0.002). The percentage of eyes 20/20 or better for monocular UCVA, BCVA, UIVA, and UNVA was 29%, 77%, 78%, and 66%, respectively. Absolute prediction error 0.5 D or less was significantly higher in post-LASIK eyes versus post-PRK eyes (p < 0.003), at 85% and 56% of eyes, respectively.
Conclusion
Implantation with a trifocal IOL can provide acceptable refractive and visual outcomes with minimal residual astigmatism in post-myopic LASIK and PRK eyes.
Keywords: PanOptix IOL, cataract surgery, intraoperative aberrometry, post-refractive
Plain Language Summary
Laser eye surgery is a common surgical procedure performed worldwide. It involves reshaping the front of the eye (cornea) to achieve excellent vision. However, this reshaping of the eye can make it difficult to select the appropriate power later in life during cataract surgery (where the natural opaque lens is replaced with an artificial intraocular lens). This study was a retrospective chart review, and designed to assess the refractive outcomes of a new intraocular lens in eyes that have had previous laser eye surgery. The results of this study indicate that eyes with previous laser eye surgery can have acceptable vision with this new intraocular lens, though selecting the appropriate lens power still remains a challenge.
Introduction
Laser vision correction (LVC) is a common elective procedure and is performed worldwide. Since LVC introduction more than 30 years ago, it has developed into three general types including laser in situ keratomileusis (LASIK), photorefractive keratectomy (PRK), and, more recently, small incision lenticule extraction (SMILE). Many patients who had LASIK or PRK have since developed cataracts and have a need for intraocular lens (IOL) implantation to see clearly. As the goal of LASIK and PRK is spectacle independence, post-LVC patients have high expectations of spectacle independence following cataract surgery.
Trifocal IOLs aim to reduce or eliminate the need for spectacles following cataract surgery by providing acceptable visual acuity at distance, intermediate, and near. This is accomplished by splitting incoming light into 3 distinct focal points, with 25% distributed for near vision, 25% for intermediate vision, and 50% for far vision.1 However, this redistribution of light can cause photic phenomena (such as glare and halos) and reduced contrast sensitivity.2,3 Given that post-LVC eyes may have high corneal aberrations, it can be challenging to implant trifocal IOLs in this patient cohort.4 In addition, predicting postoperative refraction in post-refractive surgery eyes is difficult, with reports of less than 70% of eyes within 0.5 D or less of their target refraction.5,6 Despite these obstacles, some studies have reported good visual outcomes with AT LISA tri 839MP (Carl Zeiss Meditec),7–9 FineVision Micro-F (PhysIOL),9 and FineVision Pod-F (PhysIOL)9 trifocal IOLs. However, studies of outcomes with other trifocal IOL models are lacking. The purpose of this study is to assess the refractive outcomes in post-myopic LASIK and PRK eyes after implantation with the PanOptix trifocal IOL (Alcon Vision, LLC).
Patients and Methods
This study was a retrospective chart review of refractive outcomes in post-myopic LASIK and PRK eyes implanted with a trifocal IOL. Approval was obtained from an institutional review board (Advarra IRB, Aurora, ON, Canada; Pro00048102) and patient informed consent was obtained for the use of chart data. This study was conducted in agreement with International Harmonization (ICH) guidelines, Good Clinical Practice (GCP), and the tenets of the Declaration of Helsinki.
Inclusion criteria were eyes that underwent uncomplicated cataract surgery or refractive lens exchange (RLE) with either the PanOptix or PanOptix toric IOL, eyes that had previous myopic LASIK or PRK treatment, and patients highly motivated to increase spectacle independence. Note that RLE with either the PanOptix or PanOptix toric IOL is an off-label use. Chart data were excluded if there was ocular comorbidity that might hamper postoperative visual acuity, irregular corneal astigmatism or keratoconus, angle Kappa/chord mu ≥ 0.6, or higher order corneal aberrations indicative of irregular corneas: greater than 0.6 total RMS, greater than 0.3 coma, or greater than 0.3 trefoil.
Using the criteria above, the chart review identified 35 eyes (of 21 patients) for inclusion in this study. The surgeries took place between July 2018 and March 2021. All collected data were de-identified and consisted of preoperative and postoperative data on sex, refractive error, prediction error, and visual acuity. Visual acuities were recorded in Snellen and converted to the equivalent log of the minimum angle of resolution (logMAR) notation for statistical analysis. Pre-LVC data was not known and was not collected.
Preoperative biometry measurements were performed using the IOL Master 500 (Carl Zeiss Meditec). Preoperative tomography measurements were performed using the Pentacam (Oculus). Preoperative topography measurements were performed using the Atlas 9000 (Carl Zeiss Meditec). The preferred method for corneal incisions, capsulotomy, and lens fragmentation was using a LenSx femtosecond laser (Alcon Vision, LLC). One eye was not eligible for FLACS and was converted to conventional phacoemulsification. Intraoperative aberrometry (IA) was performed using the ORA System with Verifeye+ (Alcon Vision, LLC) to determine IOL power, cylinder power, and final axis of placement. The Holladay 2 formula was used with the Verion digital tracking system (with 3.0 software planner; Alcon Vision, LLC) for IOL toricity and axial alignment. Both the ASCRS IOL Power Calculator (Version 4.9) and Barrett True K formula were used to select IOL power. The postoperative regimen was the surgeon’s usual standard of care. All patients received topical moxifloxacin four times daily for one week, difluprednate ophthalmic emulsion 0.05% twice daily for two weeks, and nepafenac daily for six weeks. Acrysof IQ PanOptix non-toric and toric IOL models, were implanted (TFNT00, TFNT20, TFNT30, TFNT40, TFNT50, TFNT60; Alcon Vision, LLC). The Acrysof IQ PanOptix non-toric and toric IOLs are designed as single piece with diffractive optics. The lens material is an Acrylate/Methacrylate copolymer, the optic diameter is 6.0 mm, and the overall length is 13.0 mm.
The primary outcome measure of interest was the absolute prediction error using IA, specifically the percentage of eyes with absolute prediction error of 0.50 D or less at the 3-month postoperative visit. Prediction error was calculated as the difference between the predicted spherical equivalent refraction (by preoperative planning or IA) and the postoperative spherical equivalent refraction (manifest). Other outcome measures of interest included residual astigmatism, monocular uncorrected visual acuity at distance (UDVA; 4m), intermediate (UIVA; 60cm), and near (UNVA; 40cm) and monocular best-corrected visual acuity at distance (BCVA; 4m). Postoperative spherical equivalent refraction (both actual and predicted) and the type and power of the IOL implanted (and suggested), determined by preoperative planning and IA, were taken from the AnalyzOR database and used to calculate prediction error. Comparisons were performed for absolute prediction errors between preoperative planning and IA. Back-calculations were also performed to simulate residual astigmatism had the toric power suggested by preoperative planning been implanted. Back-calculations, as described by Hill et al,10 simulate removal of the implanted IOL and replacement with another IOL. Vector addition was used to perform all back calculations.11
The software program R (version 4.1.2; The R Foundation for Statistical Computing) was used for statistical analyses. A Wilcoxon signed-rank test was used to compare non-parametric paired variables, a Wilcoxon rank sum test was used to compare non-parametric non-paired variables, and Chi-square statistic were used to compare differences between categorical variables. A p-value ≤ 0.05 was considered significant.
Results
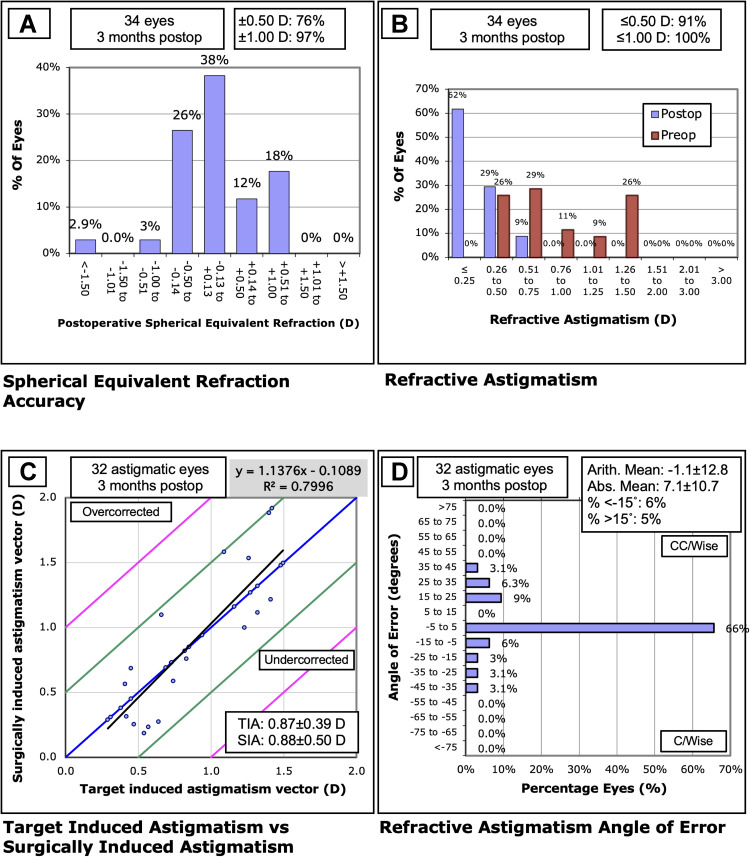
A total of 35 eyes of 21 subjects were identified during the chart review. This included similar numbers of male eyes (15) and female eyes (20), similar numbers of post-myopic LASIK eyes (15) and post-myopic PRK eyes (20), and similar cases of cataract removal (16 eyes) and RLE (19 eyes). Table 1 provides a summary of the preoperative and demographic data. Postoperative refractive outcomes are summarized in Figure 1. Postoperative mean manifest refraction spherical equivalent (MRSE) was 0.03 D ± 0.45 D.
Table 1.
Preoperative Patient Demographics
| Parameter | Mean ± SD (Range) |
|---|---|
| Number of Eyes (patients) | 35 (21) |
| Sex | |
| Female Eyes (n) | 20 |
| Male Eyes (n) | 15 |
| Post-Refractive Type | |
| LASIK (n) | 15 |
| PRK (n) | 20 |
| Surgery Type | |
| Cataract (n) | 16 |
| RLE (n) | 19 |
| IOL Type | |
| Non-Toric (n) | 10 |
| Toric (n) | 25 |
| Cylinder (D) | 0.84 ± 0.39 (0.29–1.50) |
| Axial Length (mm) | 24.86 ± 0.94 (23.47–27.05) |
Abbreviations: D, diopters; IOL, intraocular lens; LASIK, laser in situ keratomileusis; MRSE, manifest refraction spherical equivalent; n, sample size; PRK, photorefractive keratectomy; RLE, refractive lens exchange; SD, standard deviation.
Figure 1.
Postoperative refractive outcomes: (A) Spherical equivalent refraction, (B) Refractive astigmatism, (C) Target-induced astigmatism vs surgically induced astigmatism, and (D) Refractive astigmatism angle of error.
Abbreviations: D, diopters; SEQ, spherical equivalent refraction; SIA, surgically induced astigmatism; TIA, target-induced astigmatism.
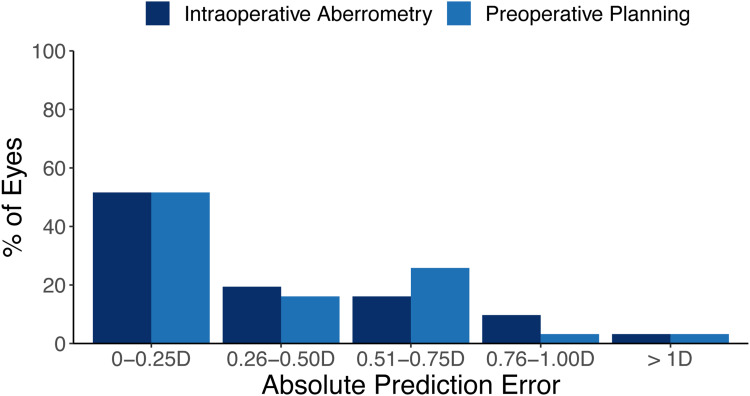
Figure 2 summarizes the postoperative absolute prediction errors. Of the 35 eyes included in this study, 4 were missing absolute prediction error data and were not included in this analysis, for a remaining total of 31 eyes. At 3 months postoperatively, mean absolute prediction error for IA was 0.35 ± 0.35 D (range 0–1.46 D). In addition, 71.0% of eyes (22/31) had absolute prediction error 0.5 D or less, 25.8% (8/31) were between 0.5 D and 1 D, and 3.2% of eyes (1/31) had prediction error greater than 1 D. The mean absolute prediction error for preoperative planning was 0.36 ± 0.33 D (range 0–1.50 D), while 67.7% of eyes (21/31) had absolute prediction error 0.5 D or less, 29.0% (9/31) were between 0.5 D and 1 D, and 3.2% of eyes (1/31) had prediction error greater than 1 D. These differences between IA and preoperative planning were not statistically significant (p > 0.05).
Figure 2.
Histogram of the postoperative absolute refractive prediction errors (n=31).
Abbreviation: D, diopters.
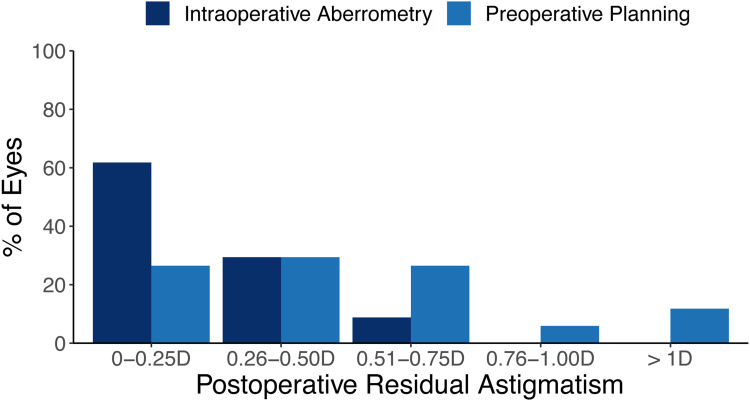
Figure 3 summarizes the results of postoperative residual astigmatism (PRA). Of the 35 eyes included in this study, 1 was missing PRA data and was not included in this analysis, for a remaining total of 34 eyes. At 3 months postoperatively, mean PRA for IA was 0.26 ± 0.26 D (range 0–0.75 D). In addition, 91.2% of eyes (31/34) had PRA 0.5 D or less, 8.8% (3/34) were between 0.5 D and 1 D, and 0% of eyes (0/34) had PRA greater than 1 D. In contrast, the mean PRA using back-calculations for preoperative planning was 0.50 ± 0.36 D (range 0–1.21 D), while 55.9% of eyes (19/34) had absolute prediction error 0.5 D or less, 32.4% (11/34) were between 0.5 D and 1 D, and 11.8% of eyes (4/34) had prediction error greater than 1 D. These differences between IA and preoperative planning were statistically significant (p < 0.002). Post hoc power analysis indicated a power of 96.2%.
Figure 3.
Histogram of the postoperative residual astigmatism (n=34).
Abbreviation: D, diopters.
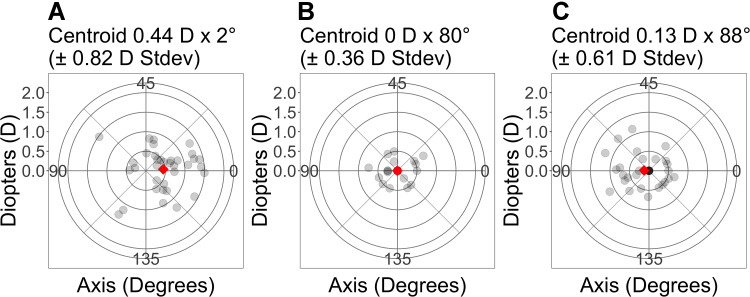
Figure 4 shows double-angle vector plots for preoperative astigmatism, IA, and back-calculations using preoperative planning. The standard deviation for IA and back-calculations using preoperative planning were 0.4 D and 0.6 D, respectively, and were lower compared to the preoperative astigmatism (0.8 D). The standard deviation for IA was also lower than that of the back-calculations using preoperative planning.
Figure 4.
Double angle vector plots of astigmatism vectors for (A) Preoperative, (B) Intraoperative aberrometry, and (C) Preoperative planned power. Each ring represents 0.5 D. The diamond represents the centroid.
Abbreviations: N, 34 eyes. D, diopters; Stdev, standard deviation.
Table 2 summarizes the data on postoperative visual acuity. All eyes had monocular visual acuities 20/40 or better at all distances. The percentage of eyes 20/20 or better for UCVA, BCVA, UIVA, and UNVA was 28.6% (10/35 eyes), 77.1% (27/35 eyes), 77.8% (21/27 eyes), and 65.6% (21/32 eyes), respectively.
Table 2.
Postoperative Visual Acuity
| Visual Acuity | n | Mean ± SD (Range) logMAR | 20/40 or Better (%) | 20/30 or Better (%) | 20/25 or Better (%) | 20/20 or Better (%) |
|---|---|---|---|---|---|---|
| UCVA | 35 | 0.09 ± 0.08 (−0.12–0.3) | 100 | 97.1 | 74.3 | 28.6 |
| BCVA | 35 | 0.02 ± 0.05 (−0.12–0.2) | 100 | 100 | 97.1 | 77.1 |
| UIVA | 27 | 0.01 ± 0.12 (−0.12–0.3) | 100 | 92.6 | 85.2 | 77.8 |
| UNVA | 32 | 0.05 ± 0.10 (−0.12–0.3) | 100 | 90.6 | 90.6 | 65.6 |
Abbreviations: BCVA, best corrected distance visual acuity; n, sample size; SD, standard deviation; UDVA, uncorrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; UNVA, uncorrected near visual acuity.
We conducted a sub-analysis comparing absolute prediction errors in post-myopic LASIK eyes and post-myopic PRK eyes. At 3 months postoperatively, mean absolute prediction error in post-LASIK eyes was 0.12 ± 0.12 D (range 0.02–0.32 D). In addition, 100% of eyes had absolute prediction error 0.5 D or less. The mean absolute prediction error in post-PRK eyes was 0.49 ± 0.39 D (range 0.07–1.50 D), while 50.0% of eyes had absolute prediction error 0.5 D or less, 44.4% were between 0.5 D and 1 D, and 5.6% of eyes had prediction error greater than 1 D. These differences between post-LASIK eyes and post-PRK eyes were statistically significant (p < 0.003). Post hoc power analysis revealed a power of 96.6%. We also conducted a subgroup analysis comparing prediction error in RLE and cataract eyes, and found no significant differences between these groups.
Discussion
Prediction accuracy in post-LVC eyes remains a challenge with cataract surgery and IOL implantation. This makes it difficult to get both happy patients and happy surgeons with this patient cohort. In this study, we compared the prediction accuracy of preoperative planning to IA in post-myopic LASIK and post-myopic PRK eyes. We did not find any significant difference in absolute prediction error between preoperative planning (mean 0.36 ± 0.33 D) and IA (mean 0.35 ± 0.35 D). These results are different than those reported by Fram et al,12 who studied 1067 post-myopic LASIK and post-myopic PRK eyes. The authors observed that using IA resulted in significantly more accurate refractive outcomes (as measured by absolute prediction error) compared to the Barrett True-K formula. Other studies of eyes with no history of LVC, have reported mixed results when comparing prediction accuracy between preoperative planning and IA. A couple studies of large datasets reported that IA resulted in a higher percentage of patients with absolute prediction error 0.5 D or less compared to preoperative planning.13,14 However, there are also reports of minimal difference in absolute prediction error between preoperative planning and IA.15–18 In addition, the percentages of eyes with absolute prediction error of 0.5 D or less was greater in this study for both IA and preoperative planning compared to other studies of post-LVC eyes with non-trifocal6,19 and trifocal7,8 IOLs. However, the prediction error results in this study are comparable to the study by Cobo-Soriano et al9 that evaluated a large number of post-LVC eyes implanted with a trifocal IOL.
In this study, there was a significant difference between IA and preoperative planned power for PRA in post-LVC eyes. The percentage of eyes with 0.5 D or less of PRA was 91% for IA and 56% for preoperative planned power, a difference of 35%. Other studies have shown similar results in eyes without a history of LVC.18,20,21 Therefore, it would appear that the use of IA results in less postoperative astigmatism than preoperative planning alone in post-LVC eyes. With less PRA, both surgeons and patients benefit as there is less need for an enhancement procedure postoperatively. With the results of this study in mind, surgeons may reduce enhancement procedures by 35 out of every 100 toric IOL implantations by using both preoperative planning and IA together.
The percentage of eyes with absolute prediction error 0.5 D or less were 100% for post-LASIK (100%) and 50% for post-PRK eyes, a difference of 50%. This is an interesting result, though we acknowledge the small sample size in each group (15 for post-LASIK and 20 for post-PRK). To the best of our knowledge, this is the first study to compare and report absolute prediction errors between post-LASIK and post-PRK eyes. We are not certain as to why there was a significant difference in our study. There were no differences in power calculation between post-LASIK and post-PRK eyes. A systemic review uncovered minimal differences between LASIK and PRK in terms of higher order aberrations and photic phenomena.22 Some studies have reported that postoperative refraction with PRK is less stable than LASIK,23,24 which could be a possible explanation. It is also possible that post-PRK eyes have more irregular astigmatism, which is more difficult to correct with a toric IOL,25 however, we observed minimal differences in the preoperative higher order corneal aberrations between groups. It is also not clear whether prediction error is higher in post-PRK eyes in general, or if prediction error is higher when combined with trifocal IOL implantation. A limitation of this comparison is that the pre-LVC refraction data was not available and there may be differences between the pre-LASIK and pre-PRK eyes. Future studies are needed to confirm our results and to draw definitive conclusions.
The primary limitation of this study was the sample size. Multifocal IOL implantation in post-refractive eyes is not that common since predicting the refraction is challenging and there is a perceived risk of exacerbating any photic phenomena present after refractive surgery. Not many of these implantations were done at the single site (private practice) in this study. Retrospective analysis revealed 35 eyes that fit the inclusion criteria over a 32-month period. Of these eyes, 8 eyes were missing postoperative UIVA, and 3 eyes had incomplete postoperative refraction at 3 months postoperatively. Despite these limitations, there was still a reasonable sample size to make comparisons between IA and preoperative planning and to draw conclusions.
Conclusions
The results of this study demonstrate that implantation with the PanOptix IOL can provide acceptable refractive and visual outcomes with minimal residual astigmatism in post-myopic LASIK and PRK eyes. The use of IA resulted in less PRA compared to preoperative planning alone, although there were no significant differences in absolute prediction error. Absolute prediction error was significantly lower in post-myopic LASIK compared to post-myopic PRK eyes.
Funding Statement
This study was supported with an investigator-initiated study grant (60521371) from Alcon, Fort Worth, TX, USA.
Disclosure
Dr John F Blaylock reports grants from Alcon Canada, during the conduct of the study; personal fees from Alcon Canada, outside the submitted work; Dr Brad J Hall reports personal fees from Valley Laser Eye Centre, during the conduct of the study; personal fees from Ace Vision Group, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Garcia-Perez JL, Gros-Otero J, Sanchez-Ramos C, Blazquez V, Contreras I. Short term visual outcomes of a new trifocal intraocular lens. BMC Ophthalmol. 2017;17:72. doi: 10.1186/s12886-017-0462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schallhorn JM. Multifocal and extended depth of focus intraocular lenses: a comparison of data from the United States food and drug administration premarket approval trials. J Refract Surg. 2021;37:98–104. doi: 10.3928/1081597X-20201111-02 [DOI] [PubMed] [Google Scholar]
- 3.Hovanesian JA, Jones M, Allen Q. The PanOptix trifocal IOL vs the ReSTOR 2.5 active focus and ReSTOR 3.0-add multifocal lenses: a study of patient satisfaction, visual disturbances, and uncorrected visual performance. Clin Ophthalmol. 2021;15:983–990. doi: 10.2147/OPTH.S285628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khor WB, Afshari NA. The role of presbyopia-correcting intraocular lenses after laser in situ keratomileusis. Curr Opin Ophthalmol. 2013;24:35–40. doi: 10.1097/ICU.0b013e32835ab457 [DOI] [PubMed] [Google Scholar]
- 5.Ianchulev T, Hoffer KJ, Yoo SH, et al. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology. 2014;121:56–60. doi: 10.1016/j.ophtha.2013.08.041 [DOI] [PubMed] [Google Scholar]
- 6.McCarthy M, Gavanski GM, Paton KE, Holland SP. Intraocular lens power calculations after myopic laser refractive surgery: a comparison of methods in 173 eyes. Ophthalmology. 2011;118:940–944. doi: 10.1016/j.ophtha.2010.08.048 [DOI] [PubMed] [Google Scholar]
- 7.Chow SSW, Chan TCY, Ng ALK, Kwok AKH. Outcomes of presbyopia-correcting intraocular lenses after laser in situ keratomileusis. Int Ophthalmol. 2019;39:1199–1204. doi: 10.1007/s10792-018-0908-0 [DOI] [PubMed] [Google Scholar]
- 8.Li QM, Wang F, Wu ZM, et al. Trifocal diffractive intraocular lens implantation in patients after previous corneal refractive laser surgery for myopia. BMC Ophthalmol. 2020;20:293. doi: 10.1186/s12886-020-01556-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobo-Soriano R, Ortega-Usobiaga J, Rodriguez-Gutierrez B, et al. Trifocal intraocular lens implantation in eyes with previous corneal refractive surgery for myopia and hyperopia. J Cataract Refract Surg. 2021;47:1265–1272. doi: 10.1097/j.jcrs.0000000000000637 [DOI] [PubMed] [Google Scholar]
- 10.Hill W, Osher R, Cooke D, et al. Simulation of toric intraocular lens results: manual keratometry versus dual-zone automated keratometry from an integrated biometer. J Cataract Refract Surg. 2011;37:2181–2187. doi: 10.1016/j.jcrs.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 11.Alpins NA. A new method of analyzing vectors for changes in astigmatism. J Cataract Refract Surg. 1993;19:524–533. doi: 10.1016/S0886-3350(13)80617-7 [DOI] [PubMed] [Google Scholar]
- 12.Fram N, Davidson J, Gu X, Babu R, Breen M. Refractive prediction accuracy using intraoperative aberrometry vs. Barrett true k in post–corneal refractive surgery eyes. Paper presented at: American Academy of Ophthalmology Congress; November 13, 2021; New Orleans, USA. [Google Scholar]
- 13.Cionni RJ, Dimalanta R, Breen M, Hamilton C. A large retrospective database analysis comparing outcomes of intraoperative aberrometry with conventional preoperative planning. J Cataract Refract Surg. 2018;44:1230–1235. doi: 10.1016/j.jcrs.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 14.Cionni RJ, Breen M, Hamilton C, Williams R. Retrospective analysis of an intraoperative aberrometry database: a study investigating absolute prediction in eyes implanted with low cylinder power toric intraocular lenses. Clin Ophthalmol. 2019;13:1485–1492. doi: 10.2147/OPTH.S191887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raufi N, James C, Kuo A, Vann R. Intraoperative aberrometry vs modern preoperative formulas in predicting intraocular lens power. J Cataract Refract Surg. 2020;46:857–861. doi: 10.1097/j.jcrs.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 16.Davison JA, Potvin R. Preoperative measurement vs intraoperative aberrometry for the selection of intraocular lens sphere power in normal eyes. Clin Ophthalmol. 2017;11:923–929. doi: 10.2147/OPTH.S135659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaylock JF, Hall B. Clinical outcomes of a diffractive trifocal intraocular lens with femtosecond laser, digital tracking, and intraoperative aberrometry. Can J Ophthalmol. 2021. doi: 10.1016/j.jcjo.2021.05.014 [DOI] [PubMed] [Google Scholar]
- 18.Blaylock JF, Hall BJ. Clinical outcomes of monofocal toric IOLs using digital tracking and intraoperative aberrometry. Clin Ophthalmol. 2021;15:3593–3600. doi: 10.2147/OPTH.S322523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gouvea L, Sioufi K, Brown CE, Waring IG, Chamon W, Rocha KM. Refractive accuracy of Barrett true-K vs intraoperative aberrometry for IOL power calculation in post-corneal refractive surgery eyes. Clin Ophthalmol. 2021;15:4305–4315. doi: 10.2147/OPTH.S334489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaylock JF, Hall B. Astigmatic results of a diffractive trifocal toric IOL following intraoperative aberrometry guidance. Clin Ophthalmol. 2020;14:4373–4378. doi: 10.2147/OPTH.S285711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodcock MG, Lehmann R, Cionni RJ, Breen M, Scott MC. Intraoperative aberrometry versus standard preoperative biometry and a toric IOL calculator for bilateral toric IOL implantation with a femtosecond laser: one-month results. J Cataract Refract Surg. 2016;42(6):817–825. doi: 10.1016/j.jcrs.2016.02.048 [DOI] [PubMed] [Google Scholar]
- 22.Shortt AJ, Allan BD, Evans JR. Laser-assisted in-situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for myopia. Cochrane Database Syst Rev. 2013;CD005135. doi: 10.1002/14651858.CD005135.pub3 [DOI] [PubMed] [Google Scholar]
- 23.Durrie DS, Slade SG, Marshall J. Wavefront-guided excimer laser ablation using photorefractive keratectomy and sub-Bowman’s keratomileusis: a contralateral eye study. J Refract Surg. 2008;24:S77–S84. doi: 10.3928/1081597X-20080101-14 [DOI] [PubMed] [Google Scholar]
- 24.Hersh PS, Abbassi R. Surgically induced astigmatism after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 1999;25:389–398. doi: 10.1016/S0886-3350(99)80088-1 [DOI] [PubMed] [Google Scholar]
- 25.Smolek MK, Oshika T, Klyce SD, Maeda N, Haight DH, McDonald MB. Topographic assessment of irregular astigmatism after photorefractive keratectomy. J Cataract Refract Surg. 1998;24:1079–1086. doi: 10.1016/S0886-3350(98)80101-6 [DOI] [PubMed] [Google Scholar]