Abstract
Mitragyna speciosa (Rubiaceae) has traditionally been used in the tropical regions of Asia, Africa and Indonesia as a substitute for opium. Indole alkaloids are the most common compounds that have been isolated. We investigated the constituents of the leaves of M. speciosa that was grown at the University of Mississippi. Several alkaloids were isolated, including ajmalicine, corynantheidine, isomitraphylline, mitraphylline, paynantheine, isocorynantheidine, 7-hydroxymitragynine and mitragynine, but their percentages were lower than those in a commercial Thai sample of “kratom”. In addition, we isolated the flavonoid epicatechin, a saponin daucosterol, the triterpenoid saponins quinovic acid 3-O-β-d-quinovopyranoside, quinovic acid 3-O-β-d-glucopyranoside, as well as several glycoside derivatives including 1-O-feruloyl-β-d-glucopyranoside, benzyl-β-d-glucopyranoside, 3-oxo-α-ionyl-O-β-d-glucopyranoside, roseoside, vogeloside, and epivogeloside. This is the first report of the last group of compounds having been isolated from a Mitragyna species. Biological studies are currently underway to test these compounds for opioid activity.
Keywords: Mitragyna speciosa, alkaloids, glycoside derivatives, triterpenoid saponins
Mitragyna (Rubiaceae) is a small Afro-Asian genus consisting of nine species; four African and five Asian. They are mainly arboreal trees with characteristic mitriform stigmatic lobes and globular flowering heads [1]. In Thailand and Malaysia, M. speciosa, M. hirsuta, M. diversifolia, M. rotundifolia are commonly seen [2], but the most known species is M. speciosa or “kratom” in Thailand and “biak-biak” in Malaysia. The leaves of M. speciosa have been habitually used by natives and laborers for their euphoric effects at low doses, either by chewing the leaves or by infusing them (decoction) to make tea [3]. In traditional medicine, the plant has been used to treat diarrhea, cough, hypertension, and to relieve muscle pain. It is also commonly used as a substitute for opium [4].
The use of M. speciosa has been forbidden in Thailand since 1946 due its narcotic effects. It is also illegal in Australia, Malysia, and Myanmar. However, the availability of M. speciosa on the internet reflects a high demand for this material. In the United States, the use of Mitragyna is still legal, however, the DEA has put it in a list of drugs and chemicals of concern since 2005 [5]. Kratom is currently purchased by approximately 40 million Americans to self-manage the symptoms associated with opium withdrawal [6].
Phytochemical studies on M. speciosa [7a] showed that mitragynine, an indole alkaloid, is the major alkaloid [7b], constituting around 50% of the total alkaloidal content, and is responsible for the opioid effects. Recent pharmacological studies reported antinociceptive effects in animal models for an analogous alkaloid, 7-hydroxymitragynine [7c,7d].
Environmental factors play a vital role in modifying the alkaloidal content or even the presence of other classes of metabolites in the same species. For example, M. speciosa from Thailand has higher content in mitragynine than M. speciosa from Malaysia [7b,7d].
The exceptional properties of this medicinal plant have attracted many researchers in recent years [4,7]. In the course of our project to find therapeutic agents for pain, anxiety, and drug addiction [3], we investigated the secondary metabolites in the MeOH extract of the leaves of M. speciosa growing in the gardens of the University of Mississippi. Herein we report the isolation and identification of eighteen compounds including eight alkaloids: ajmalicine [8a], corynantheidine [8b], isomitraphylline [8c], mitraphylline [8c], paynantheine [7d], isocorynantheidine [8d], 7-hydroxymitragynine [7b], and mitragynine [7b]; a flavonoid (−)-epicatechin [7e]; a saponin daucosterol [9a]; two triterpenoid saponins: quinovic acid 3-O-β-d-quinovopyranoside [9b] and quinovic acid 3-O-β-d-glucopyranoside [9a]; two monoaryl glycosides: 1-O-feruloyl-β-d-glucopyranoside [10a] and benzyl-β-d-glucopyranoside [10b]; two cyclohexenone glycosides: 3-oxo-α-ionyl-O-β-d-glucopyranoside [10c] and (6S, 9R) roseoside [10d]; and two secoiridoid glycosides: vogeloside [10e] and epivogeloside [10e]. The structures of all isolated compounds were identified by interpretation of their spectral data including ESI-MS, 1H and 13C NMR, as well as by comparison of their spectral data with those reported previously in the related literature (Figure 1).
Figure 1:
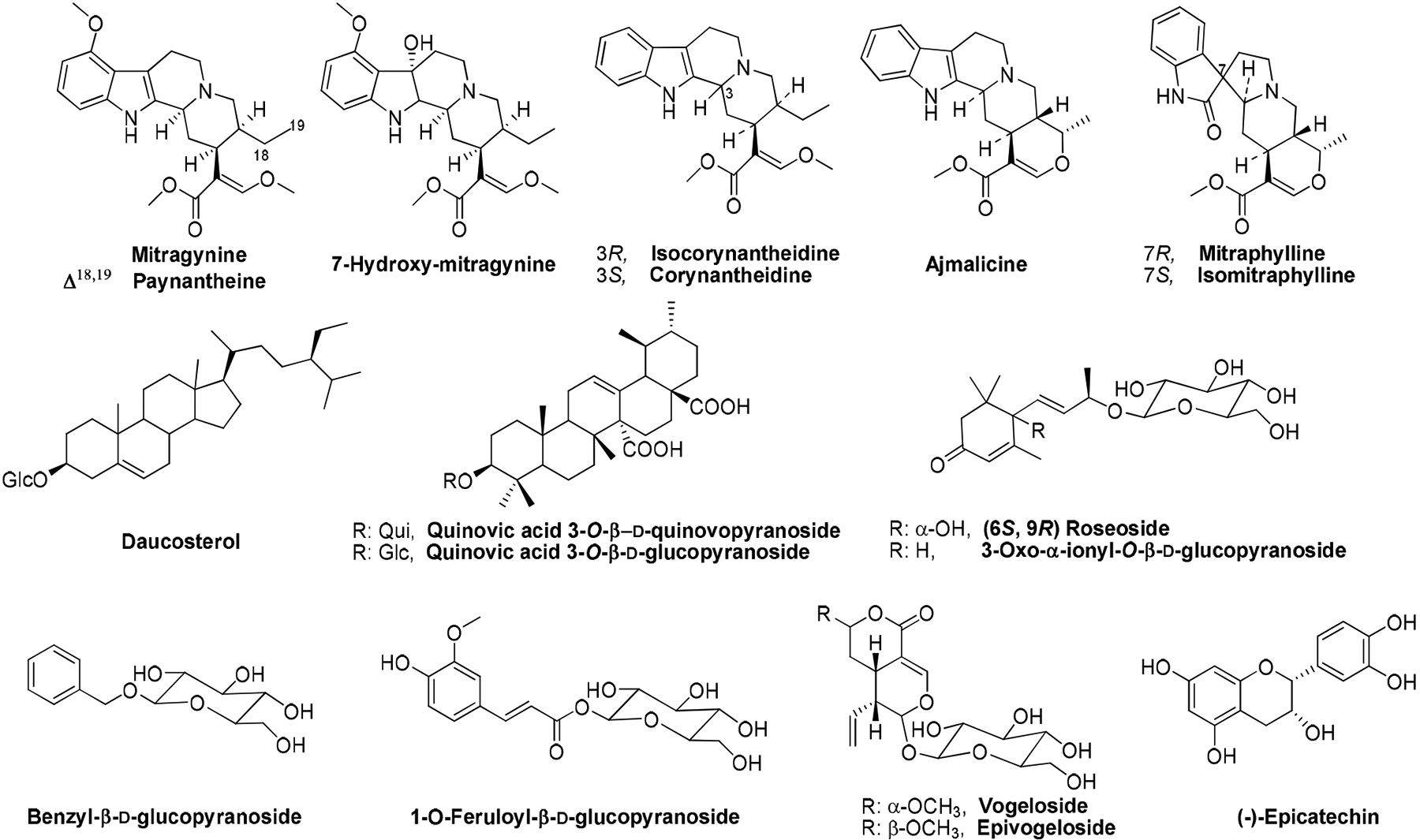
Chemical structures of compounds isolated from the leaves of Mitragyna speciosa.
The predominant alkaloid in M. speciosa leaves was mitraphylline, in contrast with Takayama et al [7b,7d] who reported mitragynine as the major alkaloid in M. speciosa from Thailand and Malaysia. Moreover, we observed less prevalence of C-9-methoxy indole alkaloids. These results reinforce the idea that there are different sub-varieties of M. speciosa from the viewpoint of plant chemotaxonomy, as proposed by Takayama et al. [7d]. On the other hand, Shellard [7e] proposed a biogenetic pathway in M. speciosa and reported that the amount of mitragynine is less in young plants than in old trees. This could be important because our samples were from trees younger than five years old, although environmental factors (temperature, soil composition, pressure, etc) could have had a major effect on the alkaloidal patterns. It is also worth mentioning that in our study we observed a significant decrease in the total amount of alkaloids compared to previous studies [7].
Although triterpenoid saponins are common in most of the Rubiaceae family [9c] and have been encountered in several members of the genus Mitragyna [9a,9d], this is the first report of their presence in M. speciosa.
It is rare to find secoiridoids or monoterpenes in Rubiaceae. Also there are few examples of compounds reported that belong to the vogeloside or roseoside chemical type [10f]. To the best of our knowledge there are no reports of monoaryl glycosides derivatives from Mitragyna species.
According to the above results, we suggest that our USA grown M. speciosa displays a different chemotype than the Asian-African plants.
Experimental
General Experimental Procedures:
1H and 13C NMR spectra were obtained on Bruker model AMX-500 and 400 NMR spectrometers with standard pulse sequences, operating at 500 MHz and 400 MHz in 1H and 125 MHz and 100 MHz in 13C. CDCl3, Acetone-d6, CD3OD, and CD3CN were used as solvents and TMS as internal standard. The High-Resolution Mass Spectra (HRMS) were recorded on a Micromass Q-Tof Micro mass spectrometer with a lock spray source. Column chromatography was carried out on silica gel (70–230 mesh, Merck) and Sephadex LH-20. Fractions obtained from column chromatography were monitored by TLC (silica gel 60 F254) and preparative TLC was carried out on silica gel 60 PF254+366 plates (20 × 20 cm, 1 mm thick).
Plant Material:
Young plants of M. speciosa were obtained in January of 2005, identified by Rita Moraes from the National Center for Natural Products Research, and grown in the gardens of the University of Mississippi, University, MS, USA. Fresh leaves were collected in August 2008 and extracted.
A voucher (MISS 76727) specimen has been deposited in the herbarium of the University of Mississippi.
Extraction and isolation:
Fresh leaves (70 g) were powdered and extracted with hot MeOH in a Soxhlet apparatus for 72 h. The extract was evaporated and gave a residue (18 g), which was subjected to column chromatography on Si gel using n-hexane-EtOAc/MeOH gradient to afford one hundred fractions. Fractions (60–85) contained alkaloids (as indicated by Dragendorff’s reagent). These fractions were combined and chromatographed on Silica gel using hexane/EtOAc gradient until 100% EtOAc followed by EtOAc/MeOH gradient until 20%, to give 20 sub-fractions (200 mL/fraction). The sub-fractions were compared using analytical TLC (CHCl3/Acetone 9:1 and 8:2) to yield four substantive fractions. Fraction 1 was chromatographed on Sephadex LH-20 using hexane/DCM/MeOH (1:1:1) and silica gel column using petroleum ether/acetone gradient until 50% to give 20 sub-fractions, the first sub-fractions (1–10)were combined and rechromatographed on preparative TLC with CHCl3/acetone (8:2), and toluene/acetone (9:1) (two elutions) to yield ajmalicine (3 mg, 1.6 × 10−4 %), mitragynine (8 mg, 4.4 × 10−4 %), corynantheidine (4 mg, 2.2 × 10−4 %). The last ten sub-fractions were combined and purified using preparative TLC using benzene/EtOAc (7:3) and CHCl3/acetone (75:25) to yield the alkaloids 7-hydroxymitragynine (1.2 mg, 6.6 × 10−5 %), isomitraphylline (7 mg, 3.8 × 10−4 %), and mitraphylline (4 mg, 2.2 × 10−4 %). Fraction 2 was chromatographed by column chromatography using a silica gel column eluted with CHCl3/acetone gradient to obtain 40 sub-fractions. Sub-fractions 1–8 were combined and chromatographed by preparative TLC eluted with DCM/acetone (8:2) to obtain paynantheine (2 mg, 1.1 × 10−4 %). The sub-fractions 9–20 were combined and a precipitate was formed and recrystallized to give mitraphylline (20 mg, total 24 mg, 1.3 × 10−3 %). Sub-fractions 21–25 were chromatographed using silica gel column with CHCl3/MeOH gradient, followed with Sephadex LH-20 using DCM/MeOH (1:1) to give the compounds isocorynantheidine (3 mg, 1.6 × 10−4 %), and a mixture of saponins that was separated by Sephadex LH-20 using MeOH to yield daucosterol (30 mg, 1.6 × 10−3 %) as the major product, and quinovic acid 3-O-β-d-quinovopyranoside (15 mg, 8.3 × 10−4 %). In the last subfractions 26–40, there was a major product that was purified by Sephadex LH-20 using CHCl3/MeOH (1:1) as mobile phase to yield epicatechin (12 mg, 6.6 × 10−4 %). Fraction 3 was chromatographed by silica gel column using EtOAc/MeOH gradient, to obtain twenty fractions which were combined in three fractions (a-c). The fraction (a) was rechromatographed by Sephadex LH-20, to yield quinovic acid 3-O-β-d-glucopyranoside (10 mg, 5.5 × 10−4%) as a major product. Fraction (b) was purified by preparative TLC using toluene/IPA (8:2) and CHCl3/IPA (7:3) to obtain 1-O-feruloyl-β-d-glucopyranoside (8 mg, 4.4 × 10−4 %). The last fraction (c) was chromatographed by Sephadex LH-20 and silica gel column using CHCl3/IPA to obtain benzyl-β-d-glucopyranoside (7 mg, 3.8 × 10−4 %). Fraction 4 was chromatographed by column of silica gel using EtOAc with increasing polarity with MeOH to obtain twenty fractions. The sub-fractions 5–10 were chromatographed over Sephadex LH-20 using CHCl3/MeOH (1:1), then further purified by preparative TLC using EtOAc/MeOH (10%) and CHCl3/IPA (9:1) several times to yield the compounds (+)-3-oxo-α-ionyl-O-β-d-glucopyranoside (3.2 mg, 1.7 × 10−4 %), roseoside (4 mg, 2.2 × 10−4 %), epivogeloside (2 mg, 1.1 × 10−4 %), and vogeloside (5 mg, 2.7 × 10−4 %).
Acknowledgments-
This investigation was conducted in a facility constructed with support from research facilities improvement program C06 RR-14503-01 from the NIH National Center for Research Resources and research support by the NIH-NCRR 5P20RR021919. This paper’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- [1].Razafimandimbison SG, Bremer B. (2002) Phylogeny and classification of Naucleeae s.l. (Rubiaceae) inferred from molecular (ITS, RBCL, and TRNT-F) and morphological data. American Journal of Botany, 89, 1027–1041. [DOI] [PubMed] [Google Scholar]
- [2].Sukrog S, Zhu S, Ruangrusgsi N, Phadungcharoen T, Palanuvej C, Komatsu K. (2007) Molecular analysis of the genus Mitragyna existing in Thailand based on rDNA ITS sequences and its applications to identify a narcotic species: Mitragyna speciosa. Biological & Pharmaceutical Bulletin, 30, 1284–1288. [DOI] [PubMed] [Google Scholar]
- [3].Babu KM, McCurdy CR, Boyer EW. (2008) Opioid receptors and legal highs: Salvia divinorum and Kratom. Clinical Toxicolgy, 46, 146–152. [DOI] [PubMed] [Google Scholar]
- [4].Jansen KL, Prast CJ. (1988) Ethnopharmacology of kratom and the Mitragyna alkaloids. Journal of Ethnopharmacology, 23, 115–119. [DOI] [PubMed] [Google Scholar]
- [5].U.S. Department of Justice, Drug Enforcement Administration, (2006) Microgram Bulletin, 39, 30–32. (www.usdoj.gov/dea/programs/forensicsci/microgram/mg0306/mg0306.html) [Google Scholar]
- [6].Boyer E, Babu K, Macalino G, Compton W. (2007) Self-treatment of opioid withdrawal with a dietary supplement, kratom. American Journal on Addictions, 16, 352–356. [DOI] [PubMed] [Google Scholar]
- [7].(a) Shellard EJ. (1974) The alkaloids of Mitragyna with special reference to those of Mitragyna speciosa, Korth. Bulletin on Narcotics, 26, 41–55; [PubMed] [Google Scholar]; (b) Takayama H, Kurihara M, Kitajima M, Said IM, Aimi N. (1998) New indole alkaloids from the leaves of Malaysian Mitragyna speciosa. Tetrahedron, 54, 8433–8440; [Google Scholar]; (c) Ponglux D, Wongseripipatana S, Takayama H, Kikuchi M, Kurihara M, Kitajima M, Aimi N, Sakai SI. (1994) A new indole alkaloid, 7α-hydroxy-7H-mitragynine, from Mitragyna speciosa in Thailand. Planta Medica, 60, 580–581; [DOI] [PubMed] [Google Scholar]; (d) Matsumoto K, Horie S, Takayama H, Ishikawa H, Aimi N, Ponglux D, Murayama T, Watanabe K. (2005) Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sciences, 78, 2–7; [DOI] [PubMed] [Google Scholar]; (e) Houghton PJ, Said IM. (1986) 3-dehydromitragynine: an alkaloid from Mitragyna speciosa. Phytochemistry, 25, 2910–2912; [Google Scholar]; (f) Shellard E6J, Houghton PJ, Resha M. (1978) The Mitragyna species of Asia, part XXXII. Planta Medica, 34, 253–263. [Google Scholar]
- [8].(a) Wenkert E, Chang CJ, Chawla HPS, Cochran DW, Hagaman EW, King JC, Orito K. (1974) General methods of synthesis of indole alkaloids. Short routes of construction of Yohimboid and ajmalicinoid alkaloid system and their 13C Nuclear magnetic resonance spectral analysis. Journal of the American Society, 98, 3645–3655; [Google Scholar]; (b) Staerk D, Lemmich E, Christensen J, Kharazmi A, Olsen CE, Jaroszewski JW. (2000) Leishmanicidal, antiplasmodial and cytotoxic activity of indole from Corynanthe pachyceras. Planta Medica, 66, 531–536; [DOI] [PubMed] [Google Scholar]; (c) Seki H, Takayama H, Aimi N, Sakai SI, Ponglux D. (1993) A nuclear magnetic resonance study on the eleven stereoisomers of heteroyohimbine-type oxindole alkaloids. Chemical & Pharmaceutical Bulletin, 41, 2077–2086; [Google Scholar]; (d) Lounasmaa M, Jokela R, Laine C, Hanhinen P. (1998) Preparation of (±)-hirsutine and (±)-isocorynantheidine. Heterocycles, 49, 445–450. [Google Scholar]
- [9].(a) Bishay DW, Che CT, Gonzalez A, Pezzuto JM, Kinghorn AD, Farnsworth NR. (1988) Further chemical constituents of Mitragyna inermis stem bark. Fitoterapia, 59, 397–398; [Google Scholar]; (b) Ahmad VU, Uddin S, Bano S. (1990) Saponins from Zygophyllum propinquum. Journal of Natural Products, 53, 1193–1197; [Google Scholar]; (c) Quin GW. (1998) Some progress on chemical studies of triterpenoid saponins from Chinese medicinal plants. Current Organic Chemistry, 2, 613–625; [Google Scholar]; (d) Kang W, Hao X. (2006) Triterpenoid saponins from Mitragyna rotundifolia. Biochemical Systematics and Ecology, 34, 585–587. [Google Scholar]
- [10].(a) Baderschneider B, Winterhalter P. (2001) Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. Journal of Agricultural and Food Chemistry, 49, 2788–2798; [DOI] [PubMed] [Google Scholar]; (b) DeRosa S, DeGuilio A, Tommonaro G. (1996) Aliphatic and aromatic glycosides from the cell cultures of Lycopersicon esculentum. Phytochemistry, 42, 1031–1034; [DOI] [PubMed] [Google Scholar]; (c) Cui B, Nakamura M, Kinjo J, Nohara T. (1993) Chemical constituents of Astragali semen. Chemical & Pharmaceutical Bulletin, 41, 178–181; [Google Scholar]; (d) Yamano Y, Ito M. (2005) Synthesis of optically active vomifoliol and roseoside stereoisomers. Chemical & Pharmaceutical Bulletin, 53, 541–546; [DOI] [PubMed] [Google Scholar]; (e) Recio-Iglesias MC, Marston A, Hostettmann K. (1992) Xanthones and secoiridoid glucosides of Halenia campanulata. Phytochemistry, 31, 1387–1389; [Google Scholar]; (f) Kitajima M, Fujii N, Yoshino F, Sudo H, Saito K, Aimi N, Takayama H. (2005) Camptothecins and two new monoterpene glucosides from Ophiorrhiza liukiuensis. Chemical & Pharmaceutical Bulletin, 53, 1355–1358. [DOI] [PubMed] [Google Scholar]


