Abstract
In this study, the spatial variation of airborne bacteria in intensive care units (ICUs) was characterized. Fine particulate matter and several physical parameters were also monitored including temperature and relative humidity. The results showed that the total bacterial load ranged between 20.4 and 134.3 CFU/m3 across the ICUs. Bacterial cultures of the collected samples did not isolate any multi-drug-resistant Gram-negative bacilli indicating the absence of such aerosolized pathogens in the ICUs. Meanwhile, particulate matter levels in several ICUs were found to exceed the international guidelines set for 24-h PM exposure. Moreover, examining bacterial load contribution by size suggested that bacteria with sizes less than 0.65 µm contributed the least to the total bacterial loads, while those with sizes between 0.65 and 1.1 µm contributed the most. A multiple linear regression model was also built to predict the bacterial loads in the ICUs. The regression analysis explained 77% of the variability observed in the measured bacterial concentrations. The model showed that the level of activity in the ICU rooms as well as its occupancy level had strong positive correlations with bacterial loads, while distance away from the patient had a non-linear relationship with measured loads. No statistically significant correlation was found between bacterial load and particulate matter concentrations.
Keywords: Hospitals, Intensive care units, Indoor air quality, Airborne bacteria, Particulate matter
Introduction
Poor indoor air quality (IAQ) is associated with serious health implications. Some facilities, such as hospitals, are more critical than others in the presence of vulnerable patients. Physical and chemical characterizations of IAQ in hospitals have been widely reported (Lomboy et al. 2015; Loupa et al. 2016; Mohammadyan et al. 2018; Scheepers et al. 2017) with some efforts targeting bioaerosols (Asif et al. 2018; Baurès et al. 2018; Fu Shaw et al. 2018; Leung et al. 2016; Lindsley et al. 2010b; Blachere et al. 2009) that can cause respiratory infections requiring hospitalization (Kestler et al. 2018; Mullooly et al. 2007). The exposure of vulnerable patients to such pollutants can indeed negate the purpose of their hospital visit and is likely to extend hospital stays (Falsey et al. 2005; Lee et al. 2013; Volling et al. 2014). As such, several studies have probed into the health effects of compromised IAQ and highlighted both short-term (Tran et al. 2020) and long-term (Gola et al. 2019) hazards. The acute health effects range from mild irritations of the eyes and nose to severe syndromes such as headaches, throat conditions, asthma, and fatigue (Tran et al. 2020). Long-term exposure to poor air quality could also contribute to chronic respiratory and cardiac complications as well as increased risks of cancer (Gola et al. 2019). With the spread of the COVID-19 pandemic, the airborne transmission of bioaerosols has become more critical worldwide particularly that bioaerosols can remain in the air for a few hours (Morawska et al. 2020; Ningthoujam 2020) through droplets that are expelled by coughing and sneezing or through suspended aerosols (< 5 µm) (Kutter et al. 2018).
The ventilation system (natural, mechanical, or mixed) can play a key role in the transport of various pollutants in hospitals (Jung et al. 2015). Concurrently, temperature (T) and relative humidity (RH) are also known to affect the movement, decay, and settlement of various pollutants, especially bioaerosols (Murphy 2006). Variations in T and RH can activate or deactivate bioaerosols and exacerbate health risks (Vuorinen et al. 2020; Kameel and Khalil 2003; Murphy 2006). In turn, particulate matter (PM) contributes to the transport of bacterial and viral infections through coagulation (Annesi-Maesano et al., 2007) with several studies reporting high PM levels in hospitals (Ostro et al. 2009; Slezakova et al. 2012) due mostly to indoor-outdoor correlations, entraining outdoor pollutants to the indoors, and/or to inadequate air exchange, failing to filter indoor pollutants leading to their accumulation (Wang et al. 2006). Similarly, bacterial loads have been measured at different locations within hospitals (Cabo Verde et al. 2015; Asif et al. 2018), with one study only targeting patients’ rooms in intensive care units (ICUs) with measurements only done at a single location (O’Neil et al. 2017).
Empirical data ascertain that ventilation systems play a critical role in regulating IAQ. Božić and Ilić (2019) observed that natural ventilation and fan-coiled units aggravated the concentration of indoor PM and fungi, while air-handling units reduced their concentrations. These observations were consistent with earlier reported assertions that poorly maintained heating, ventilation, and air conditioning (HVAC) systems are primary catalysts for the multiplication of suspended microorganisms within offices (Burge et al. 2000; Wu et al. 2005; Maclntosh et al. 2006), implying that IAQ control strategies cannot be limited to the pollutant source removal alone but also to the modification and maintenance of HVAC systems (Božić and Ilić, 2019) or the deployment of active ionization technology (Sidhu 2018).
Recent work has underscored the importance of characterizing the spatio-temporal variability in the concentrations of microorganisms in the indoor air across a hospital. The temporal variability in indoor air quality has been well studied. For example, Zaman et al. (2021) highlighted the temporal variabilities in indoor PM concentrations across a year, with the colder season associated with higher PM, microorganisms, and CO2 levels as compared to the warmer season. Both Chamseddine et al. (2019) and Zaman et al. (2021) offered possible explanations for these seasonal variations; both cited that increased indoor activity during the cold season increased the rate of pollutants influx, ultimately causing the observed surges in concentrations. It should be noted that the impact of seasonality on indoor air quality is not limited to the hospital setting; several studies have reported similar observations for residential settings (Abdel-Salam 2021) and in learning institutions (Deng and Lau 2019; Stamp et al. 2022). In comparison, the spatial variability of microorganism levels in hospitals remains less studied. Yet, Lee et al. (2020) have reported that suspended PM and microorganism levels measured in more critical health units, such as ICUs, can often be higher than those measured in other open spaces like corridors and reception areas.
In this study, we present a first attempt at examining the spatial variation of airborne bacterial levels in ICU rooms and evaluate their variability as a function of particle size to provide an understanding of factors affecting bacterial loads in ICUs. Concurrently, we monitored particulate matter (PM10, PM2.5) and measured several physical parameters (temperature, relative humidity, distance away from patient, and level of activity). We then developed multivariate regression models (MLRs) using correlations between pollutant levels and physical parameters.
Materials and methods
Study design and monitoring program
The monitoring program was implemented for 10 different ICU patients during night hours. All samples were collected between 19:00 and 04:00. Sampling was done in one patient room per night and the total duration of sample collection in each room was approximately 1 h. All monitored ICUs were located at the American University of Beirut Medical Center (AUBMC). Sampling was done following receiving the approval of the University Institutional Review Board (IRB). The sampling period spanned from June to September 2019. Patients admitted to the ICU, mostly suffering from bacterial infection or physical traumas, were randomly selected. Nearly 70% of the approached patients accepted to take part in this study. The monitored parameters included the total bacterial load (TBL) with corresponding distribution of bacteria by their diameter size, the Gram-negative bacterial load, particulate matter (PM10 and PM2.5) concentrations, temperature (T), and relative humidity (RH). Additionally, the occupancy, the level of activity, and the room volume were recorded. Note that sampling was conducted in 4 ICU rooms. Three rooms were identical with an average volume of 40 m3 (L = 4.5 m, W = 3.5 m, H = 2.8 m), while the fourth was a suite room with a volume of 76 m3 (L = 7.8 m, W = 3.5 m, H = 2.8 m). The ventilation system in the hospital is fully mechanical with no windows in the ICU. The airflow within the ICU was reported by the mechanical department to range between 300 and 350 ft3/min, with an estimated average air change per hour (ACH) of 14. These are comparable to those reported in the literature (Saran et al. 2020).
A Six Stage Microbial Andersen Cascade Impactor (TISH Environmental Model TE-10–800) was used for the fractionation of bacteria to simulate various stages of the human respiratory track. The diameter cut-offs for the 6 stages were 0.65, 1.1, 2.1, 3.3, 4.7, and 7 µm. A volumetric sampling approach was followed to measure the concentrations of viable bacterial loads inside the ICU rooms. The impactor’s 12 Volts vacuum pumps were calibrated to 28.3 L/min (1ft3/min) at the beginning of every sampling round using a rotameter airflow meter with a capacity of 60 L/min (~ 2.1 ft3/min). In each room, six samples were collected at the breathing level of 1.5 m for a duration of 20 min, as recommended by Hiwar et al. (2021). The sampling was consistent with the World Health Organization (2020) guidelines on sampling and analysis methods for indoor pollutants. Two simultaneous, 20-min samples were collected at 0.5 and 1.5 m away from the patient and tested for total bacteria load (TBL). Subsequently, another two simultaneous, 20-min samples at 1 and 2 m away from the patient were recorded. Two simultaneous, 20-min samples at 0.5 and 1.5 m were also taken and tested for Gram-negative Bacteria. Two additional samples at distance of 0.5 and 1.5 m were also collected in the last two ICU rooms to measure the concentration of total bacteria resistant to meropenem. Note that all distances were measured from the patient’s face to the center of the Andersen impactor horizontally, while the concentrations of TBL were estimated as the sum of colony forming units found across the six stages of the impactor. The percent bacterial load contribution (BLC) for each size was calculated by dividing each particle size concentration with its corresponding TBL. This number represents the percentage contribution of each size to the total concentration and allows for a standard comparison of sizes across different samples. The effectiveness of the sampling protocol has been reported in previous studies (Erdogan et al. 2009; Kim et al. 2010).
For each room, 24 glass petri dishes were prepared by pipetting 27 mL of Tryptic Soy Agar and autoclaving them, while an additional 12 petri dishes were filled with MacConkey agar for testing for Gram-negative bacteria. Following sample collection, the plates were incubated at 37 °C for 18–24 h after which the colonies formed were counted and reported. The final concentrations were adjusted based on the volume extracted during the sampling period of 20 min as expressed in Eq. 1.
| 1 |
where V = 566 L is the volume of air sampled in 20 min, C is the bacterial count, and TBL is the total bacterial load expressed in number of colony forming units (CFU) per 1 m3 of air.
During the sampling process in an ICU room in the hospital (Fig. 1), the door was always kept closed. Also, the pumps were activated from outside the rooms 10 min after they were installed in their sampling locations to minimize the effect of any disturbance that might be created during their installation. In rooms where nurses had to enter, the number of trips and occupancy inside the room was recorded (i.e., presence of a private nurse). Most ICU rooms were occupied by only one patient, except for two rooms (Room ID 3 and 6 in Fig. 2), where a private nurse was always present. The nurse was asked to remain seated during the sampling period. As for the regular hospital nurses’ trips, most were short (< 1 min).
Fig. 1.
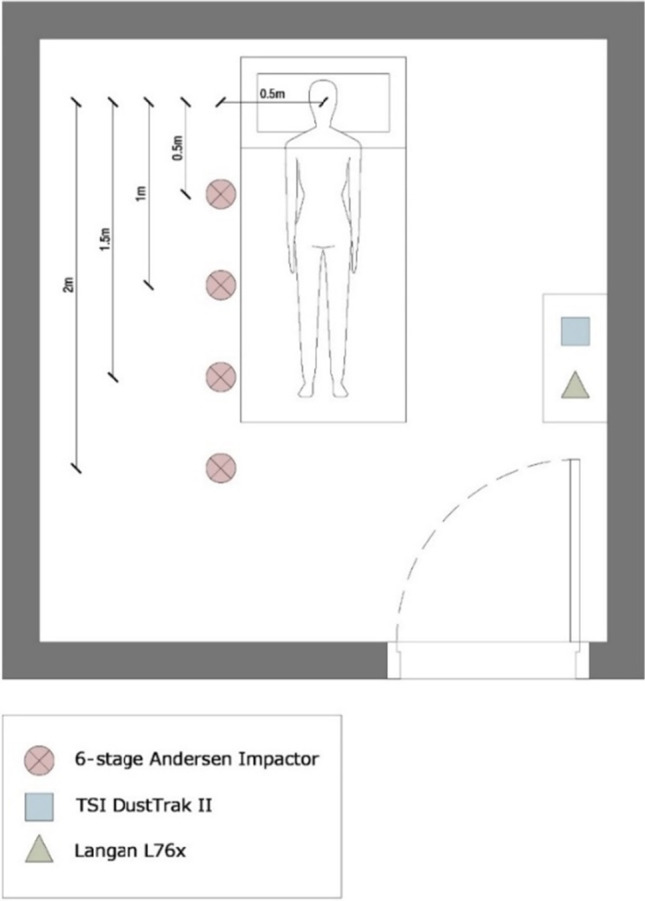
Top view of the 40 m.3 ICU rooms where most samples were collected
Fig. 2.
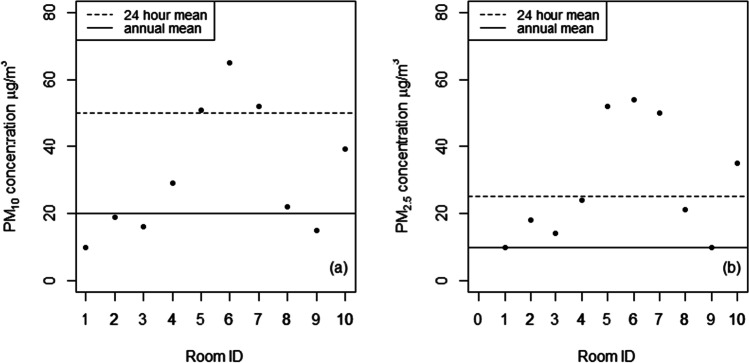
PM concentrations compared to WHO (2021) guidelines. a PM10. b PM2.5
The PM2.5 and PM10 levels were monitored using a factory-calibrated portable TSI DustTrak™ II Aerosol Monitor (Model 8532, TSI Corporation, Shoreview, USA) with a log interval of 1 min. The T and RH inside the patients’ rooms were recorded over a period of 20 min using a portable Langan analyzer (model L76n) with a log interval of 10 s. During the monitoring period, occupancy levels and the number of coughs were recorded to account for their potential effect on bacteria shedding, PM2.5 and PM10 concentrations, and airflow.
Statistical analysis
Correlations, ANOVA, and multiple regression were used to assess the importance of several factors in predicting bacterial concentrations in the ICU rooms. The average indoor PM2.5 and PM10 levels were compared with IAQ guidelines (WHO 2021). Pearson’s correlation coefficient was used to quantify the correlations between the indoor air quality variables (PM, TBL) and several predictors including T, RH, occupancy, and the number of nurses’ trips. A stepwise multiple linear regression model was also developed to predict TBL from the predictors measured in each room. The statistical analysis was performed using the R software (R Core Team 2022).
Isolates collection and broth micro-dilution
Luria agar plates supplemented with 1 µg/mL of meropenem were prepared for the detection of the presence of meropenem-resistant microorganisms in the ICU rooms. The Viable 6-stage Andersen impactor was used to test the presence of airborne meropenem-resistant microorganisms. Minimum inhibitory concentrations were determined using the broth micro-dilution against ertapenem, meropenem, imipenem, gentamicin, ciprofloxacin, cefepime, vancomycin, and dalfopristin quinupristin (CLSI). Serial dilution of each antibiotic was prepared in Cation-adjusted Mueller Hinton broth in 96-well plates between columns 1 and 10. Column 1 had a concentration of 128 µg/mL while column 10 had a concentration of 0.25 µg/mL. Column 11 served as a positive control, while column 12 served as a negative control. Bacteria were adjusted in Cation-adjusted Mueller Hinton broth to a turbidity equal to that of the 0.5 McFarland standard, followed by a dilution to reach 5 × 106 CFU/mL. From the latter, 10 µL was added to each well between columns 1 and 11, leading to a final bacterial concentration of 5 × 105 CFU/mL. Each plate was then incubated at 37 °C for 18–24 h, and the results were determined based on turbidity.
Bacterial cultures were grown overnight on Columbia sheep blood agar (Becton Dickinson, Heidelberg, Germany) at 37 °C and subjected to ethanol-formic acid extraction according to the following protocol. One full 1 µL sterile loop of bacterial sample was suspended in 300 µL of sterile water and mixed with 900 µL of absolute ethanol. Samples were centrifuged at 12,000 g for 2 min and the supernatant was discarded. The pellet was re-suspended in 50 µL of 70% formic acid and 50 µL of acetonitrile (Sigma) and centrifuged at 12 000 g for 2 min. The supernatant was collected and stored at 20 °C. A 1 µL of each bacterial extract was spotted onto a MALDI target plate (MSP 96 target ground steel; Bruker Daltonics, Bremen, Germany) and air-dried at room temperature. Each spotted sample was then overlaid with 1 µL of a saturated matrix solution (α-cyano-4-hydroxy-cinnamic acid; Bruker Daltonics) in 50% acetonitrile and 2.5% trifluoroacetic acid then air-dried. Samples were measured on Microflex-LT system (Bruker Daltonik, Bremen, Germany).
Results and discussion
Monitoring program
The measured T in the ICU rooms ranged from 22.0 to 23.5 °C (mean = 22.5 °C, SD = 0.46 °C), while RH ranged from 57.2 to 63.5% (mean = 61.5%, SD = 1.94%). The low variability in T and RH is expected in ICU rooms due to the controlled mechanical ventilation and absence of natural ventilation.
PM10 levels ranged from 10 to 65 µg/m3 (mean = 33 µg/m3, SD = 17.8 µg/m3), while PM2.5 levels ranged from 10 to 54 µg/m3 (mean = 30 µg/m3, SD = 16.8 µg/m3). The measured values in several ICU rooms exceeded international guidelines for 24-h PM exposure (Fig. 2) (WHO 2021). The levels of PM10 and PM2.5 were found to be highly correlated (r = 0.98). Moreover, most of the measured PM10 was in the form of PM2.5 (mean of PM2.5/PM10 ratio = 0.90, SD = 0.1). Fine particles (PM2.5) tend to remain permanently suspended and slightly affected by resuspension (Hospodsky et al., 2012) which explains the low correlation (Table 1) observed between PM concentrations and the level of activity in each room (number of trips and occupancy rate). Note that the measured PM values fell within the range reported in the literature for measurements collected at different locations within hospitals (Table 2).
Table 1.
Correlation of PM concentrations with the level of activity
| Indicator | PM10 | PM2.5 |
|---|---|---|
| Number of trips |
ra = 0.30 p = 0.06b |
r = 0.26 p = 0.11 |
| Occupancy |
r = 0.13 p = 0.43 |
r = 0.1 p = 0.51 |
aPearson’s correlation coefficient
bSignificant to the 10% level
Table 2.
PM concentrations in hospitals
| Reference | PM2.5 (µg/m3) | PM10 (µg/m3) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| This study | 30 | 16.8 | 10–54 | 33 | 17.8 | 10–65 |
| Asghar et al. (2022) | 53 | 2.7 | - | 120 | 2 | - |
| Danesh Yazdi et al. (2021) | 10.2 | - | 31 | - | - | - |
| Baurès et al. (2018) | 1.6 | - | 0–45.4 | 12 | - | - |
| Scheepers et al. (2017) | 9.8 | - | - | - | - | - |
| Powell et al. (2015) | 51.5 | 21.6 | 15–122 | 91.8 | 61.3 | 28–186 |
| Jung et al. (2015) | 14.4 | 15.9 | - | 25.2 | 17.2 | - |
| Slezakova et al. 2012) | 23.4 | - | 10.5–41.9 | 30.8 | - | 13–58.8 |
| Wan et al. (2011) | 1.0 | - | 0.1–8.4 | 10 | - | 0.8–55.6 |
| Ostro et al. (2009) | 19 | 15 | 0–100 | - | - | - |
| Wang et al. (2006) | 128.1 | - | 61.7–250 | 99 | - | 40.9–214.9 |
| Nardini et al. (2004) | 1.6 | 0.9 | 0–110 | - | - | - |
The TBL concentrations (see Supplemental Material) ranged from 20.4 to 134.3 CFU/m3 (mean = 66.43 CFU/m3, SD = 35.20 CFU/m3). These values are at the lower end of reported literature measurement at different locations within hospitals (Table 3). Osman et al. (2018) conducted bimonthly (twice per month) sampling for a year in Egypt across different hospital units, including ICU wards. Their study reported TBL ranging from 118 to 1124 CFU/m3 (mean = 512 CFU/m3, SD = 425 CFU/m3), which is within the same order of magnitude, but higher than the TBL reported in this study. One possible reason for the difference is that Osman et al. (2018) sampled the ICU wards, which are more dynamic and less frequently cleaned as compared to ICU patient rooms. Other reasons could be (1) the difference in the sampling timing (day hours vs night hours), as day hours are usually more crowded with staff and visitors; and (2) the shorter sampling duration they adopted (5 min vs 20 min) that could lead to higher margins of error.
Table 3.
Bacterial concentrations in various hospital units
| Reference | Hospital unit |
Airborne bacteria concentration CFU/m3 |
||
|---|---|---|---|---|
| Mean | SD | Range | ||
| This study | ICU | 66.4 | 35.2 | 20.4–134.3 |
| Asif et al. (2018) | OT, ES, SW, OPD | 648.3 | NR | 20–3577 |
| Bielawska-Drózd et al. (2018) |
HED Ambulances Offices |
470c 300c 230c |
NR |
130–4200 130–1400 42–5000 |
|
Osman et al. (2018) (Private hospitals sampling) |
ICU ward OT AD |
512 75 1,127 |
425 73 763 |
118–1224 0–228 330–2638 |
| Shaw et al. (2018) | OT | 78 | 46.8 | 22–183 |
| Cabo Verde et al. (2015) | OT, ES, SW | NR | NR | 12–736 |
| Dai et al. (2015) | OT | 301 | NR | 87–585 |
| Mirhoseini et al. (2015) |
OT ward ICU ward SW |
396 222 537 |
NR |
45–1733 NR NR |
| Sudharsanam et al. (2012)a | Hospital ward | NR | NR | 1,120–168,560 |
| Sudharsanam et al. (2012)b | Hospital ward | NR | NR | 3,788–191,111 |
| Pasquarella et al. (2012) | Empty OT | 26.9 | 40 | 0–166 |
| Working OT | 140.14 | 163 | 0–798 | |
aImpingement sampling
bFilter sampling
cMedian reported
SD, standard deviation; ICU, intensive care unit; CFU, colony forming unit; BL, bacterial level; OT, operating theater; ES, emergency services; SW, surgical ward; OPD, out-patient department; HED, hospital emergency department; AD, admission department; NR, not reported
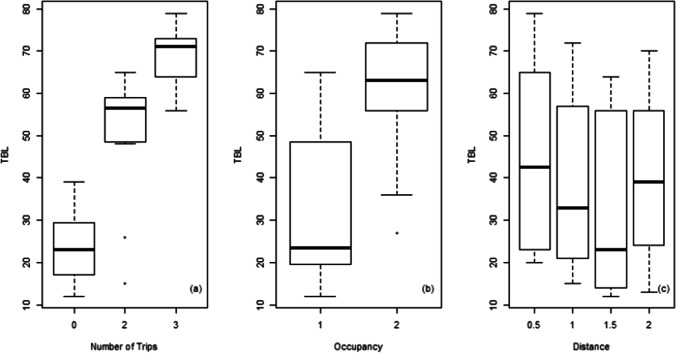
Correlations between TBL and the measured T (r = − 0.31, significant at the 10% level) and RH (r = − 0.15) (Table 4) were not statistically significant, as both physical parameters remained relatively constant in patient rooms as a result of mechanical ventilation. Note that Osman et al. (2018) reported negative correlations between TBL and physical parameters; yet these correlations could have been due to their sampling in less controlled areas within a hospital environment (i.e., ICU ward and admission department). As for the level of activity, the number of nurses’ trips was found to be highly correlated with the measured concentrations of airborne bacteria (r = 0.86; p-value < 0.05) (Fig. 3). Also, significant differences in the mean TBL were observed as a function of the number of trips (ANOVA F-value = 99.47; p-value < 0.05). The results from the multi-comparison t-tests, with Holm’s correction, showed that the mean concentration when no trips occurred was statistically lower than all other levels, where at least one trip was conducted (mean TBL for no trips was 39.87 CFU/m3, p-value < 0.05). Meanwhile, the mean concentration when three or more trips occurred was significantly higher than the rest (mean = 117.3 CFU/m3, p-value < 0.05). Similarly, the occupancy level was found to affect the measured bacterial concentrations in the air, since additional occupants can be bacteria sources and their activity may lead to the resuspension of settled bacteria. Hathway et al. (2011) conducted a 5-day air sampling campaign in a respiratory ward and reported that the ward activity level was highly correlated with the airborne concentrations of bacteria, while the presence of sedentary visitors was not. Pankhurst et al. (2012) reported that the number of people present in an operating theater significantly increases TBL. However, their study did not differentiate between occupancy and level of activity. In our study, the mean TBL in rooms with one versus two occupants was found to be statistically different (p-value < 0.05), with the mean level in the former measured at 31.7 CFU/m3, while the latter had a mean concentration of 59.8 CFU/m3. On the other hand, the correlation between TBL and the distance away from the patient was found to be negative as expected; however, it had a weak correlation (r = − 0.12; p-value = 0.47). TBL levels were found to show a constant drop up to 1.5 m away from the patient. Yet, TBL was found to increase again at 2 m (Fig. 3). This could be due to the proximity of the impactor to the door at 2 m, which could have resulted in the samples being affected by infiltration from the ICU common ward. Osman et al. (2018) reported high TBL in ICU wards (mean of 512 CFU/m3), while the literature consistently reported higher TBL in different hospitals wards and common area (Table 3) (Asif et al. 2018; Mirhoseini et al. 2015; Sudharsanam et al. 2012). In addition, Pankhurst et al. (2012) reported that TBL can increase by 50% when comparing a closed operating theater to an “open door” operating theater, supporting the fact that nearby wards can be a major source of airborne bacteria. It is also important to note that TBL and PM levels were not strongly correlated in this study, which may suggest that the two have different sources.
Table 4.
Bacterial load correlations with monitored parameters
| Indicator | TBL |
|---|---|
| Temperature | − 0.31a |
| Relative humidity | − 0.15 |
| PM10 | 0.22 |
| PM2.5 | 0.21 |
| Trips | 0.86 b |
| Occupancy | 0.61b |
| Distance | − 0.12 |
aSignificant to the 10% level
bSignificant to the 1% level
Fig. 3.
TBL as a function of a number of trips, b occupancy, and c distance away from patient
Bacterial load contribution by size
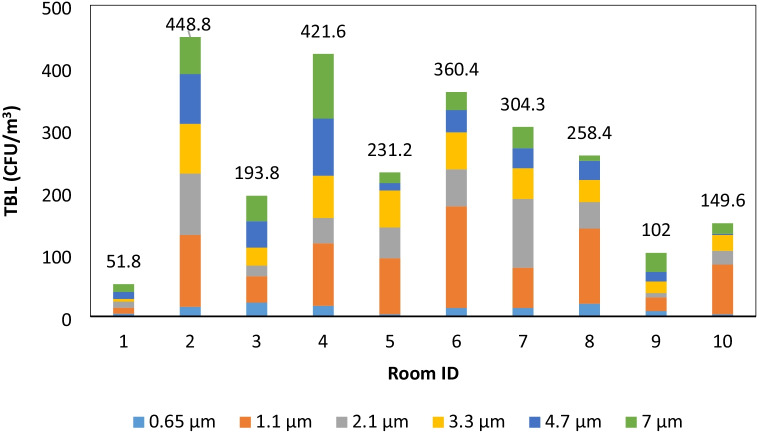
Examining the bacteria load contributions (BLC) for each size (see Supplemental Material), a significant difference in their mean contribution by size is evident (ANOVA F-value = 13.41; p-value < 0.05). The results from the multi-comparison t-tests with Holm’s correction showed that the mean BLC for the bacterial sizes less than 0.65 micron (mean BLC = 5%) was statistically lower than all other bacterial sizes (p-value < 0.05). The mean BLC for bacteria with sizes between 0.65 and 1.1 microns (mean BLC = 33%) was significantly higher than the rest of the bacterial size groups (p-value < 0.05) (Figs. 4 and 5). Consistent with literature-reported data (Clauß, 2015), the contributions of all other sizes did not exhibit a statistically significant difference in their mean contribution.
Fig. 4.
Bacteria concentration by size and room
Fig. 5.
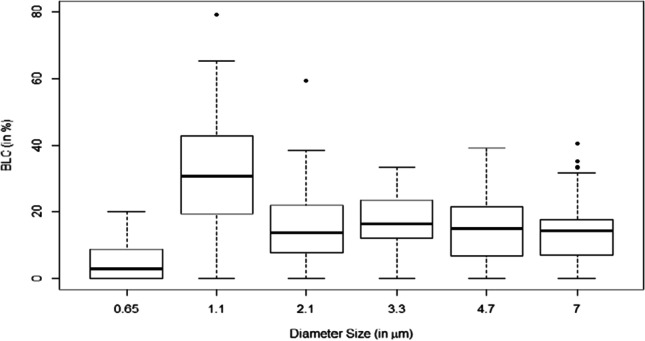
Distribution of bacteria by diameter size
A correlation analysis was conducted between measured bacterial loads by size and the different physical, PM, and occupancy variables measured in each ICU. The bacteria were divided into 3 categories, namely the small size category with diameters < 2.1 µm, the medium size category with diameters between 2.1 and 4.7 µm, and the large category for those with diameters > 4.7 µm. There was no correlation between the concentrations of small bacterial particles and distance away from the patient (r = 0), suggesting that small diameter bacterial concentration is constant across the room (well-mixed). A weak negative correlation was found with the bacteria in the larger bacterial size group (Table 5). These results indicate that the heavier the bacteria, the higher the probability that it will settle with distance. This is consistent with the fact that gravitational velocity is proportional to the squared particle diameter (Seinfeld and Pandis 2006) . As for the correlation between the bacterial concentrations and the number of trips by the nursing staff, the lowest correlation was found for the small-sized bacteria, which also supports the idea that these light particles tend to be well mixed and are the least affected by resuspension. Strong positive correlations were found between the number of trips on one hand and the medium- and large-sized particles concentrations on the other. This highlights the potentially important role that resuspension (due to increased activity) may have on these two sizes. The occupancy rate had a positive correlation with bacterial concentration irrespective of size. The correlation between bacterial concentrations on one hand and T and RH on the other showed that these were not significant because of the small fluctuations of the latter in the ICU rooms. The correlations between the concentrations of the different bacterial sizes and the measured PM concentrations were low for the same reasons discussed previously. Table 6 summarizes the correlations between the bacterial concentrations by size and the measured physical parameters.
Table 5.
Correlations of bacterial concentrations with physical parameters
| Indicator | Concentration of small particles (< 2.1 µm) | Concentration of medium particles (2.1 < d < 4.7 µm) | Concentration of large particles (> 4.7 µm) |
|---|---|---|---|
| Temperature | − 0.24 b | − 0.13 | − 0.11 a |
| Relative humidity | − 0.05 | − 0.02 | − 0.20 |
| Distance | 0.001 | − 0.08 | − 0.15 |
| Number of trips | 0.34 c | 0.57 c | 0.50 c |
| Occupancy | 0.18 | 0.38 c | 0.49 c |
| PM10 | 0.14 | 0.18 | − 0.30 c |
| PM2.5 | 0.16 | 0.27 b | − 0.28 b |
aSignificant to the 10% level
bSignificant to the 5% level
cSignificant to the 1% level
Table 6.
Regression model parameters for total bacterial load (TBL)
| Variable | Unit | Estimate | t-statistic | p-value |
|---|---|---|---|---|
| Intercept | CFU/m3 | 57.12 c | 3.298 | 0.00234 |
| Distance (D) | m | − 66.003 b | − 2.41 | 0.0217 |
| Distance squared (D2) | m2 | 25.785 b | 2.372 | 0.02369 |
| Number of trips (Tp) | 21.487 c | 8.05 | 2.74E − 09 | |
| Occupancy (O) | 15.597 b | 2.111 | 0.04245 | |
| R2 = 0.774 | ||||
| TBL = 57.120 – 66.003 D + 25.875 D2 + 21.487 Tp + 15.597 O | ||||
aSignificant to the 10% level
bSignificant to the 5% level
cSignificant to the 1% level
TBL regression analysis
A regression model was developed to predict the measured TBL levels as a function of the room characteristics and occupancy levels (Table 6). The model showed that distance away from the patient and its squared value (to account for the increase in concentrations at 2 m) along with its occupancy level and the number of trips to the room were strong predictors of TBL. Consistent with the reported literature, the number of trips was the most significant factor due to the potential increase in resuspension and its impact on the airborne bacterial concentration (Chen 2009; Hospodsky et al. 2012). In addition, O’Neil et al. (2017) analyzed different activities within ICUs and identified those that can generate significant amounts of airborne bacteria. Nurses could be a bacteria source and thus their presence may increase TBL. Additional work on the DNA of the collected bacteria is needed to determine their actual sources. Occupancy was also found to be a significant predictor of TBL which is expected given that an additional occupant could emit bacteria through breathing, coughing, sneezing, or talking. As for distance away from the patient, the relationship is expected to be negative as the concentration should decrease when moving further away from the patient. Yet in our results, we had to account for a non-linear relationship with distance (distance squared term) to account for the observed increase in concentrations at 2 m that is probably attributed to bacteria entering from the ICU common ward. Overall, the performance of the model was good with an adjusted R2 of 0.77 and showed no bias (0%) (Table 5). Note that the model did not account for the fact that each patient had a different shedding rate. Measuring the shedding rate of each can be done by taking surface samples from the patient’s mouth or having the patient exhale on an agar plate which were outside the scope of this study. Another source of uncertainty was the lack of continuous data on the airflow in each room, as the management was only able to provide a general range for the airflow (300–350 ft3/min) within the hospital, which can significantly affect the spatial distribution of PM and airborne bacteria by creating regions of accumulation and dilution.
Microbiological characteristics for resistant bacteria
The samples collected on MacConkey agars did not yield bacterial growth; hence, Gram-negative bacteria were absent from the indoor environment in the ICU. As for the sampling of resistant bacteria in the last two rooms, twelve isolates were obtained from the Luria agar plates supplemented with 1 µg/mL of meropenem. Using MALDI-TOF mass spectrometry, four isolates were identified as Staphylococcus hominis, 4 as Staphylococcus haemolyticus, 2 as Staphylococcus epidermidis, 1 as Corynebacterium afermentans, and 1 as Brevundimonas diminuta (Table 7).
Table 7.
Identification of isolates
| Isolate code | Species | Isolate code | Species |
|---|---|---|---|
| 1 | Corynebacterium afermentans | 7 | Staphylococcus haemolyticus |
| 2 | Staphylococcus hominis | 8 | Staphylococcus haemolyticus |
| 3 | Staphylococcus epidermidis | 9 | Staphylococcus haemolyticus |
| 4 | Staphylococcus epidermidis | 10 | Staphylococcus hominis |
| 5 | Staphylococcus hominis | 11 | Staphylococcus haemolyticus |
| 6 | Staphylococcus hominis | 12 | Brevundimonas diminuta |
Broth micro-dilution results showed that 50% of the isolates were susceptible to gentamicin, 60% were susceptible to ciprofloxacin, and 100% were susceptible to vancomycin and dalfopristin quinupristin. However, for meropenem, ertapenem, imipenem, and cefepime, their breakpoints were not specified according to the CLSI and EUCAST guidelines (Table 8). Coagulase-negative staphylococci (CoNS), such as Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus haemolyticus, are considered part of the skin normal flora (Garza-González et al. 2011). However, these species are among the most causative agents of hospital-acquired infections in ICUs (Fitzpatrick et al. 2002). Among the recovered isolates, gentamicin and ciprofloxacin resistance was detected. Such resistance imposes a serious threat, if one of these isolates were acquired by a patient from a healthcare worker. Therefore, healthcare professionals must take good care of their skin hygiene, to halt a possible transmission of resistant skin flora microorganism to their patients.
Table 8.
Isolates MIC against various antibiotics
| MIC (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Isolates | Mer | Ert | Imi | Cef | Gen | Cip | Van | DQ |
| 2 | 0.5 | 4 | < 0.125 | 4 | < 0.125 (S) | < 0.125 (S) | 1 (S) | < 0.125 (S) |
| 3 | 1 | 16 | 0.5 | 8 | < 0.125 (S) | 0.25 (S) | 4 (S) | < 0.125 (S) |
| 4 | > 128 | 8 | 0.5 | 4 | > 128 (R) | 0.5 (S) | 4 (S) | < 0.125 (S) |
| 5 | 4 | 128 | 16 | 128 | 0.25 (S) | < 0.125 (S) | 2 (S) | < 0.125 (S) |
| 6 | 8 | > 128 | 16 | 128 | < 0.125 (S) | 0.25 (S) | 2 (S) | < 0.125 (S) |
| 7 | 32 | > 128 | 1 | > 128 | 128 (R) | 8 (R) | 2 (S) | < 0.125 (S) |
| 8 | 4 | 16 | < 0.125 | 16 | 8 (I) | 1 (S) | 0.5 (S) | < 0.125 (S) |
| 9 | 16 | > 128 | 32 | > 128 | 128 (R) | 8 (R) | 2 (S) | < 0.125 (S) |
| 10 | 4 | 32 | < 0.125 | 4 | < 0.125 (S) | 4 (R) | 2 (S) | < 0.125 (S) |
| 11 | 32 | > 128 | 128 | > 128 | 128 (R) | 128 (R) | 2 (S) | < 0.125 (S) |
MIC, minimum inhibitory concentration; Mer, meropenem; Ert, ertapenem; Imi, imipenem; Cef, cefepime; Gen, gentamicin; Cip, ciprofloxacin; Van, vancomycin; DQ, dalfopristin quinupristin; S, susceptible I, intermediate R, resistance.
While we did not isolate multi-drug-resistant Gram-negative bacilli indicating the absence of such aerosolized pathogens in the ICUs, the results still raise concerns (mostly around nosocomial infections) requiring mitigation measures that can reduce concentrations of airborne contaminants.
Conclusion
We present a first attempt at characterizing the spatial variation of total bacterial load in ICU rooms while concurrently monitoring particulate matter (PM2.5, PM10), temperature (T), and relative humidity (RH) at several distances from patients. In parallel, we recorded the room occupancy and the number of nurses’ trips inside the room. The latter was found to be an important potential contributor affecting airborne bacterial concentrations along with the distance from the source. Several antibiotic-resistant bacterial species were identified, and a regression model was developed to predict concentrations at several points in a typical ICU room. Several mitigation measures can be implemented to better control biological air quality within ICUs including regular surface cleaning to remove settled PM and bioaerosols, along with reducing air disturbance within the room through limited occupancy and in–out trips. Medical protocols should encourage nurses to perform as many activities as they can through one room entry, which can significantly reduce the resuspension of bacteria. In addition, hospitals must adhere to well-established best practices and standards through regular maintenance of ducts, replacement of filters, monitoring of air quality, and UV disinfection.
Managing IAQ is an integrated approach that encompasses various stakeholders towards developing an environmental management plan with adequate resources for monitoring and feedback and to raise awareness of hospital’s staff regarding the importance of IAQ in protecting patients and occupants’ health. A common challenge to a proper implementation of such a plan in hospitals is the lack of standards for IAQ although benchmark guidelines have been reported (Capolongo et al. 2017) .
Additional work to build on our findings can target CFD model simulations along with DNA analysis to identify sources of resistant bacteria and enhance the understanding of bioaerosols movement within hospitals in general and ICUs in particular.
Acknowledgements
Special thanks to Dar Al-Handasah (Shair & Partners) Endowment for its support to the graduate programs in Engineering at the American University of Beirut.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Declarations
Ethics approval
This study involves human subjects and was approved by the Institutional Review Board (IRB) at the American University of Beirut Medical Center. All patients provided written informed consents prior to indoor air sample collection. The field sampling did not replace or obstruct routine medical care procedures and institutional protocols at the hospital.
Conflict of interest
The authors declare that they have no known conflict of interests that could influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Salam MM. Seasonal variation in indoor concentrations of air pollutants in residential buildings. J Air Waste Manag Assoc. 2021;71(6):761–777. doi: 10.1080/10962247.2021.1895367. [DOI] [PubMed] [Google Scholar]
- American Society of Heating, Refrigerating and Air-Conditioning Engineers. Standard 62.1, 2016. ASHRAE standard : standards for natural and mechanical ventilation.
- Annesi-Maesano IFF, Kunzli N, Brunekref B. Particulate matter, science and EU policy. Eur Respir J. 2007;29:428–431. doi: 10.1183/09031936.00129506. [DOI] [PubMed] [Google Scholar]
- Asghar K, Ali A, Tabassum A, Nadeem SG, Hakim ST, Amin M, Raza G, Bashir S, Afshan N, Usman N and Aurangzeb N. 2022 Assessment of particulate matter (PM) in ambient air of different settings and its associated health risk in Haripur city, Pakistan. Brazilian J Biol 84 [DOI] [PubMed]
- Asif A, Zeeshan M, Hashmi I, Zahid U, Bhatti MF. Microbial quality assessment of indoor air in a large hospital building during winter and spring seasons. Build Environ. 2018;135:68–73. doi: 10.1016/j.buildenv.2018.03.010. [DOI] [Google Scholar]
- Baurès E, Blanchard O, Mercier F, Surget E, le Cann P, Rivier A, Gangneux J, Florentin A. Indoor air quality in two French hospitals: measurement of chemical and microbiological contaminants. Sci Total Environ. 2018;642:168–179. doi: 10.1016/j.scitotenv.2018.06.047. [DOI] [PubMed] [Google Scholar]
- Bielawska-Drózd A, Cieślik P, Bohacz J, Korniłłowicz-Kowalska T, Żakowska D, Bartoszcze M, Wlizło-Skowronek B, Winnicka I, Brytan M, Kubiak L, Skopińska-Różewska E, Kocik J. Microbiological analysis of bioaerosols collected from Hospital Emergency Departments and ambulances. Ann Agric Environ Med. 2018;25(2):274–279. doi: 10.26444/aaem/80711. [DOI] [PubMed] [Google Scholar]
- Blachere FM, Lindsley WG, Pearce TA, Anderson SE, Fisher M, Khakoo R, Meade BJ, Lander O, Davis S, Thewlis RE, Celik I, Chen BT, Beezhold DH. Measurement of airborne influenza virus in a hospital emergency departmet. Clin Infect Dis. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- Božić J, Ilić P, (2019) Indoor air quality in the hospital: the influence of heating, ventilating and conditioning systems. Brazilian Archives Biol Technol 62
- Burge HA, Pierson DL, Groves TO, Strawn KF, Mishra SK. Dynamics of airborne fungal populations in a large office building. Curr Microbiol. 2000;40(1):10–16. doi: 10.1007/s002849910003. [DOI] [PubMed] [Google Scholar]
- Cabo Verde S, Almeida SM, Matos J, Guerreiro D, Meneses M, Faria T, Botelho D, Santos M, Viegas C. Microbiological assessment of indoor air quality at different hospital sites. Res Microbiol. 2015;166:557–563. doi: 10.1016/j.resmic.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Capolongo, S., Settimo, G., & Gola, M..( 2017) Indoor air quality in healthcare facilities. E-book library [online]. Available at: https://link.springer.com/book/10.1007%2F978-3-319-49160-8 (Accessed: 15 February 2020)
- Chamseddine A, Alameddine I, Hatzopoulou M, El-Fadel M. Seasonal variation of air quality in hospitals with indoor–outdoor correlations. Build Environ. 2019;148:689–700. doi: 10.1016/j.buildenv.2018.11.034. [DOI] [Google Scholar]
- Chen QaHL. The effects of human activities on exposure to particulate matter and bioaerosols in residential homes. Environ Sci Technol. 2009;43(13):4641–4646. doi: 10.1021/es802296j. [DOI] [PubMed] [Google Scholar]
- Clauß M. Particle size distribution of airborne microorganisms in the environment – a review. Landbauforschung Appl Agric Forestry Res. 2015;65(2):77–100. [Google Scholar]
- Dai C, Zhang Y, Ma X, Yin M, Zheng H, Gu X, Xie S, Jia H, Zhang L, Zhang W. Real-time measurements of airborne biologic particles using fluorescent particle counter to evaluate microbial contamination: results of a comparative study in an operating theater. Am J Infect Control. 2015;43(1):78–81. doi: 10.1016/j.ajic.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Danesh Yazdi M, Wang Y, Di Q, Wei Y, Requia WJ, Shi L, Sabath MB, Dominici F, Coull BA, Evans JS, Koutrakis P. Long-term association of air pollution and hospital admissions among Medicare participants using a doubly robust additive model. Circulation. 2021;143(16):1584–1596. doi: 10.1161/CIRCULATIONAHA.120.050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Lau J. Seasonal variations of indoor air quality and thermal conditions and their correlations in 220 classrooms in the Midwestern United States. Build Environ. 2019;157:79–88. doi: 10.1016/j.buildenv.2019.04.038. [DOI] [Google Scholar]
- Erdogan M, Yurtseven E, Erginoz E, Vehid S, Koksal S, Yuceokur A. Total volatile organic compounds (TVOC), carbon monoxide (CO), carbon dioxide (CO2) concentrations in the hospital building of a medical faculty in Istanbul. Turkey Nobel Medicus. 2009;18:66–72. [Google Scholar]
- Falsey A, Hennessey P, Formica M, Cox C, Walsh E, (2005) Respiratory syncytial virus infection in elderly and high-risk adults. The New England J Med 352(1749) [DOI] [PubMed]
- Fitzpatrick F, Humphreys H, Smyth E, Kennedy CA, O’Gara JP. Environmental regulation of biofilm formation in intensive care unit isolates of Staphylococcus epidermidis. J Hosp Infect. 2002;52(3):212–218. doi: 10.1053/jhin.2002.1309. [DOI] [PubMed] [Google Scholar]
- Garza-González E, Morfin-Otero R, Martínez-Vázquez MA, Gonzalez-Diaz E, González-Santiago O, Rodríguez-Noriega E. Microbiological and molecular characterization of human clinical isolates of Staphylococcus cohnii, Staphylococcus hominis, and Staphylococcus sciuri. Scand J Infect Dis. 2011;43(11–12):930–936. doi: 10.3109/00365548.2011.598873. [DOI] [PubMed] [Google Scholar]
- Gola M, Settimo G, Capolongo S, (2019) Indoor air quality in inpatient environments: a systematic review on factors that influence chemical pollution in inpatient wards. J Healthcare Eng Volume 2019: Article ID 8358306 10.1155/2019/8358306 [DOI] [PMC free article] [PubMed]
- Hathway A, Noakes C, Sleigh A, Fletcher LA. CFD simulation of airborne pathogen transport due to human activities. Build Environ. 2011;46:2500–2511. doi: 10.1016/j.buildenv.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwar W, King MF, Shuweihdi F, Fletcher LA, Dancer SJ, Noakes CJ. What is the relationship between indoor air quality parameters and airborne microorganisms in hospital environments? A systematic review and meta-analysis. Indoor Air. 2021;31(5):1308–1322. doi: 10.1111/ina.12846. [DOI] [PubMed] [Google Scholar]
- Hospodsky D, Qian J, Nazaroff W, Yamamoto N, Bibby K, Rismani-Yazdi H, Peccia J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE. 2012;7(4):e34867. doi: 10.1371/journal.pone.0034867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CC, Wu PC, Tseng CH, Su HJ. Indoor air quality varies with ventilation types and working areas in hospitals. Build Environ. 2015;85:190–195. doi: 10.1016/j.buildenv.2014.11.026. [DOI] [Google Scholar]
- Kameel RA and Khalil EE, (2003) Thermal comfort vs air quality in air-conditioned healthcare applications, 36th AIAA Thermophysics Conference, Orlando, FL; US.
- Kestler M, Muñoz P, Mateos M, Adrados D, Bouza E. Respiratory syncytial virus burden among adults during flu season: an underestimated pathology. J Hosp Infect. 2018;100:463–468. doi: 10.1016/j.jhin.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Kim KY, Kim YS, Kim D. Distribution characteristics of airborne bacteria and fungi in the general hospitals of Korea. Ind Health. 2010;48:236–243. doi: 10.2486/indhealth.48.236. [DOI] [PubMed] [Google Scholar]
- Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Lee KH and Kim DK, (2020) Evaluation and comparison of the indoor air quality in different areas of the hospital. Medicine 99(52) [DOI] [PMC free article] [PubMed]
- Lee N, Lui G, Wong K, Li T, Tse E and Chan J, et al (2013) High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infectious Dis 57(1069) [DOI] [PubMed]
- Leung NH, Zhou J, Chu DK, Yu H, Lindsley WG, Beezhold DH, Yen HL, Li Y, Seto WH, Peiris JS and Cowling BJ, (2016) Quantification of influenza virus RNA in aerosols in patient rooms. PLoS One 11(2) [DOI] [PMC free article] [PubMed]
- Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, Palmer JE, Clark KE, Fisher MA, Khakoo R and Beezhold DH, (2010b) Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One 5(11) [DOI] [PMC free article] [PubMed]
- Lomboy M, Quirit L, Molina V, Dalmacion G, Schwartz J, Suh H and Baja E, (2015) Characterization of particulate matter 2.5 in an urban tertiary care hospital in the Philippines. Building Environ 92(432–439)
- Loupa G, Zarogianni A, Karali D, Kosmadakis I, Rapsomanikis S. Indoor/outdoor PM 2.5 elemental composition and organic fraction medications, in a Greek hospital. Sci Total Environ. 2016;550:727–735. doi: 10.1016/j.scitotenv.2016.01.070. [DOI] [PubMed] [Google Scholar]
- Maclntosh DL, Brightman HS, Baker BJ, Myatt TA, Stewart JH, McCarthy JF. Airborne fungal spores in a cross-sectional study of office buildings. J Occup Environ Hyg. 2006;3(7):379–389. doi: 10.1080/10543400600760438. [DOI] [PubMed] [Google Scholar]
- Mirhoseini SH, Nikaeen M, Khanahmad H, Hatamzadeh M and Hassanzadeh A, (2015) Monitoring of airborne bacteria and aerosols in different wards of hospitals-particle counting usefulness in investigation of airborne bacteria. Ann Agric Environ Med 22(4) [DOI] [PubMed]
- Mohammadyan M, Keyvani S, Bahrami A, Yetilmezsoy K, Heibati B, Godri Pollitt KJ. Assessment of indoor air pollution exposure in urban hospital microenvironments. Air Qual Atmos Health. 2018;12(2):151–159. doi: 10.1007/s11869-018-0637-6. [DOI] [Google Scholar]
- Morawska L, Tang J, Bahnfleth W, Bluyssen P, Boerstra A and Buonanno Gea (2020) How can airborne transmission of COVID-19 indoors be minimised? Environ Int 142 [DOI] [PMC free article] [PubMed]
- Mullooly J, Bridges C, Thompson W, Chen J, Weintraub E and Jackson L, et al (2007) Influenza- and RSV-associated hospitalizations among adults. Vaccine, 25(846) [DOI] [PubMed]
- Murphy J. Temperature and humidity control in surgery rooms. Health Care HVAC ASHRAE J. 2006;48:18–25. [Google Scholar]
- Nardini SCR, Invernizzi G, Ruprecht A, Boffi R, Formentini S. (2004) Monaldi archive for chest diseases. 61(3) 183 192 [DOI] [PubMed]
- Ningthoujam R. COVID 19 can spread through breathing, talking, study estimates. J Curr Med Res Practice. 2020;10(3):132–133. doi: 10.1016/j.cmrp.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil C, Li J, Leavey A, Wang Y, Hink M, Wallace M, et al. Characterization of aerosols generated during patient care activities. Clin Infect Dis. 2017;65(8):1342–1348. doi: 10.1093/cid/cix535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman ME, Ibrahim HY, Yousef FA, Elnasr AA, Saeed Y, Hameed AA. A study on microbiological contamination on air quality in hospitals in Egypt. Indoor and Built Environ. 2018;27(7):953–968. doi: 10.1177/1420326X17698193. [DOI] [Google Scholar]
- Ostro B, Roth L, Malig B, Marty M. The effects of fine particle components on respiratory hospital admissions in children. Environ Health Perspective. 2009;117(3):475–480. doi: 10.1289/ehp.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst LJ, Taylor J, Cloutman-Green EA, Hartley JC, Lai KM. Can clean-room particle counters be used as an infection control tool in hospital operating theatres? Indoor Built Environ. 2012;2012(21):381–391. doi: 10.1177/1420326X11409467. [DOI] [Google Scholar]
- Pasquarella C, Vitali P, Saccani E, Manotti P, Boccuni C, Ugolotti M, Signorelli C, Mariotti F, Sansebastiano G, Albertini R. Microbial air monitoring in operating theatres: experience at the University Hospital of Parma. J Hosp Infect. 2012;81(1):50–57. doi: 10.1016/j.jhin.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Powell H, Krall JR, Wang Y, Bell ML, Peng RD. Ambient coarse particulate matter and hospital admissions in the Medicare Cohort Air Pollution Study, 1999–2010. Environ Health Perspect. 2015;123(11):1152–1158. doi: 10.1289/ehp.1408720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Saran S, Gurjar M, Baronia A, Sivapurapu V, Ghosh P, Raju G, & Maurya I (2020) Heating, ventilation and air conditioning (HVAC) in intensive care units. Critical Care 24(1) [DOI] [PMC free article] [PubMed]
- Seinfeld JH, Pandis SN. Atmospheric chemistry and physics: from air pollution to climate change, 2nd;3rd; edn. Hoboken, N.J.: John Wiley & Sons; 2006. [Google Scholar]
- Scheepers P, Van Wel L, Beckmann G, Anzion R. Chemical characterization of the indoor air quality of a university hospital: penetration of outdoor air pollutants. Int J Environ Res Public Health. 2017;14(5):497. doi: 10.3390/ijerph14050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LF, Chen IH, Chen CS, Wu HH, Lai LS, Chen YY, Der Wang F. Factors influencing microbial colonies in the air of operating rooms. BMC Infect Dis. 2018;18(1):4. doi: 10.1186/s12879-017-2928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu S, 2018. Maintaining indoor air quality in hospitals. [online] Biospectrumindia.com. Available at: <https://www.biospectrumindia.com/features/70/12163/maintaining-indoor-air-quality-in-hospitals.html> [Accessed 22 May 2022].
- Slezakova K, da ConceiçãoAlvim-Ferraz M, do Carmo Pereira M. Elemental characterization of indoor breathable particles at a Portuguese urban hospital. J Toxicol Environ Health A. 2012;75(13–15):909–919. doi: 10.1080/15287394.2012.690707. [DOI] [PubMed] [Google Scholar]
- Stamp S, Burman E, Shrubsole C, Chatzidiakou L, Mumovic D, Davies M. Seasonal variations and the influence of ventilation rates on IAQ: a case study of five low-energy London apartments. Indoor and Built Environ. 2022;31(3):607–623. doi: 10.1177/1420326X211017175. [DOI] [Google Scholar]
- Sudharsanam S, Swaminathan S, Ramalingam A, Thangavel G, Annamalai R, Steinberg R, Srikanth P. Characterization of indoor bioaerosols from a hospital ward in a tropical setting. Afr Health Sci. 2012;12(2):217–225. doi: 10.4314/ahs.v12i2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran VV, Park D, Lee YC. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. Int J Environ Res Public Health. 2020;17(8):2927. doi: 10.3390/ijerph17082927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volling C, Hassan K, Mazzulli T, Green K, Al-Den A and Hunter P, et al (2014) Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infectious Diseases 14(665) [DOI] [PMC free article] [PubMed]
- Vuorinen V, Aarnio M, Alava M, Alopaeus V, Atanasova N, Auvinen M, Balasubramanian N, Bordbar H, Erasto P, Grande R, Hayward N, Hellsten A, Hostikka S, Hokkanen J, Kaario O, Karvinen A, Kivisto I, Korhonen M, Kosonen R, Kuusela J, Lestinen S, Laurila E, Nieminen HJ, Peltonen P, Pokki J, Puisto A, Raback P, Salmenjoki H, Sironen T, Osterberg M. Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Saf Sci. 2020;130:104866. doi: 10.1016/j.ssci.2020.104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan GH, Chung FF, Tang CS. Long term surveillance of air quality in medical center operating rooms. Am J Infect Control. 2011;39(4):302–308. doi: 10.1016/j.ajic.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Wang X, Bi X, Sheng G, Fu J. Hospital indoor PM10/PM2.5 and associated trace elements in Guangzhou. China Sci Total Environ. 2006;366(1):124–135. doi: 10.1016/j.scitotenv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2020. Methods for sampling and analysis of chemical pollutants in indoor air: supplementary publication to the screening tool for assessment of health risks from combined exposure to multiple chemicals in indoor air in public settings for children. WHO Regional Office for Europe, Copenhagen, Denmark
- World Health Organization, 2021. WHO global air quality guidelines 2021. Available at https://www.google.com/search?client=firefox-b-d&q=WHO+global+air+quality+guidelines+2021 [Accessed May 28, 2022].
- Wu PC, Li YY, Chiang CM, Huang CY, Lee CC, Li FC, Su HJ. Changing microbial concentrations are associated with ventilation performance in Taiwan’s air-conditioned office buildings. Indoor Air. 2005;15(1):19–26. doi: 10.1111/j.1600-0668.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- Zaman SU, Yesmin M, Pavel M, Sarkar R, Jeba F, Salam A. Indoor air quality indicators and toxicity potential at the hospitals’ environment in Dhaka. Bangladesh Environ Sci Pollut Res. 2021;28(28):37727–37740. doi: 10.1007/s11356-021-13162-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).