Abstract
Purpose of Review
Advances in health care over time have led to an evolution in the epidemiology of invasive fungal infections. There is an increasing concern for antifungal resistance and emergence of less common fungal species for which optimal therapies are not well defined. The purpose of this review is to describe mechanisms of antifungal resistance and to evaluate the modern role of new and investigational antifungals.
Recent Findings
Isavuconazole and ibrexafungerp represent the two newest antifungal agents. Evidence from in vivo and in vitro studies has been published recently to help define their place in therapy and potential roles in treating resistant fungi. Isavuconazole is a broad-spectrum triazole antifungal with evidence to support its use in invasive aspergillosis and mucormycosis. Its utility in treating voriconazole-resistant Candida should be confirmed with susceptibility testing if available. Ibrexafungerp is an oral glucan synthase inhibitor with little cross-resistance among currently available antifungals, including echinocandins. It is a promising new agent for invasive candidiasis, including azole-resistant Candida species, and in combination therapy with voriconazole for aspergillosis. Multiple antifungals, some with novel mechanisms, are in development, including rezafungin, oteseconazole, olorofim, fosmanogepix, and opelconazole.
Summary
Both isavuconazole and ibrexafungerp are welcome additions to the arsenal of antifungals, and the prospect of more antifungal options in the future is encouraging. Such an array of antifungals will be important as antifungal resistance continues to expand alongside evolving medical practices. However, managing resistant fungal infections will grow in complexity as the unique role of each new agent is defined.
Keywords: Antifungal resistance, Ibrexafungerp, Isavuconazole, Candida, Aspergillus, Azole resistance
Introduction
The landscape of invasive fungal infections is progressively changing, and there are many factors that are contributing [1]. Advances in healthcare practices are imposing risk for invasive fungal infection in a greater number of patients. Increasing rates of multidrug resistance among bacteria are pressuring clinicians to prescribe broad-spectrum antibacterial therapies, a well-known risk for invasive candidiasis. Evolution in the use of surgical procedures, implantable medical devices, and other invasive interventions stands to increase the risk of invasive fungal infections. More specifically, organ transplantation is one such surgery which also imposes risk from immune suppressant medications used to prevent rejection. The development of novel chemotherapeutic medications and immune modulators to treat patients with cancer and rheumatologic conditions is broadening the spectrum of immune-compromised patients. Not to mention, the routine use of anti-mold prophylaxis in certain immune-compromised patients is selecting for fungi historically considered less common or even rare, e.g., Mucorales species [2]. Lastly, emerging fungi like Candida auris not only have the potential to impact infection prevention strategies but this fungus is also a concerning threat to the continued viability of our current antifungal options due to its propensity for multidrug resistance [3]. As such, it is of utmost importance that the medical community have a solid understanding of modern antifungals, both Food and Drug Administration (FDA)–approved agents and those in development, and the implications of antifungal resistance. The information contained in the following review aims to serve as a resource in this regard.
Antifungal Resistance
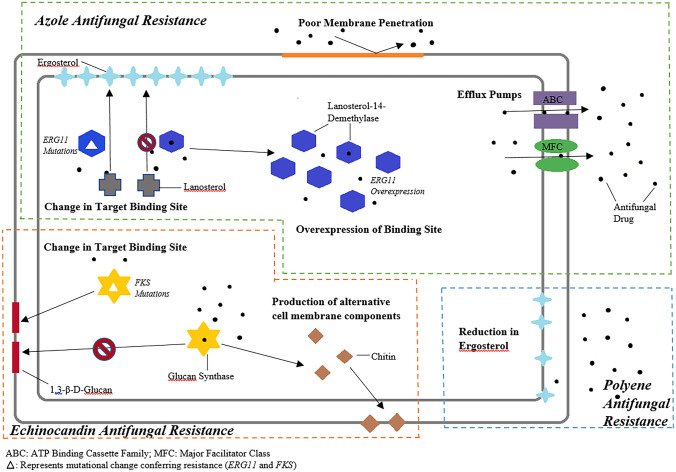
Effective treatment of invasive fungal infections has generally relied on three classes of systemic antifungals: azoles, echinocandins, and polyenes. Members of the current generation of echinocandins, including caspofungin, micafungin, and anidulafungin, are all fairly interchangeable in terms of spectrum, safety, and clinical utility, but they are also equally affected by relevant resistance mechanisms. Amphotericin B represents the lone member of the polyene class, and its use is complicated by a relatively unforgiving profile of side effects. So, while three classes of systemic antifungals may seem sufficient, resistance to even one of these antifungals has the potential to limit therapeutic options, especially if other factors like toxicities or drug interactions are at play. The following sections aim to describe major mechanisms of resistance within the three antifungal classes as well as clinical implications related to treatment of fungal infections. Figure 1 is a pictorial representation of these mechanisms of antifungal resistance.
Fig. 1.
Pictorial representation of mechanisms of antifungal resistance
Azole Resistance
Some of the most commonly prescribed azole antifungals include fluconazole, voriconazole, and posaconazole. Agents in this class inhibit fungal growth by interfering with the enzyme lanosterol 14α-demethylase which is responsible for converting lanosterol to ergosterol, a key component in the fungal cell wall. Resistance to azole antifungals has been identified via both acquired resistance and intrinsic resistance. Azole resistance is increasing over time, especially among non-albicans Candida species. The SENTRY Antifungal Surveillance Program reported a steady emergence of fluconazole- and echinocandin-resistant C. glabrata and C. tropicalis isolates, with the highest rates of fluconazole-resistant C. glabrata isolates in North America (10.6%). Additionally, all C. auris isolates collected in this study were noted to be fluconazole resistant [4].
Resistance to azole antifungals in Candida species can occur by multiple mechanisms, but the most common are expression of drug efflux pumps and upregulation or mutations in the gene ERG11, which is responsible for production of lanosterol 14α-demethylase. There are two types of drug efflux pumps, (1) ATP-binding cassette (ABC) transporter pumps encoded by CDR1 and CDR2 genes, and (2) major facilitator superfamily (MFS) pumps encoded by MDR1 and FLU1 genes. Isolates expressing multidrug efflux pumps have reduced intracellular accumulation of fluconazole and elevated fluconazole minimum inhibitory concentration (MIC). Induction of the ERG11 gene leads to excess ergosterol biosynthesis due to increased production of the azole target lanosterol 14α-demethylase. Mutations in ERG11 itself, by way of amino acid substitutions, have the potential to produce a mutated lanosterol 14α-demethylase that has reduced azole binding affinity. The ability of Candida to produce biofilms is yet another contributor to azole resistance. Biofilms harbor organisms in high density, some of which have reduced growth rate or upregulated efflux pumps, as well as extracellular matrix material which can act as a physical barrier to azole drug penetration [5–7].
Voriconazole, posaconazole, isavuconazole, and itraconazole have demonstrated microbiologic activity against Aspergillus species. While A. fumigatus is the most common species involving invasive disease, other Aspergillus species have the potential to cause disease, including A. flavus, A. niger, and A. terreus. Non-fumigatus species of Aspergillus have been associated with breakthrough infections among patients receiving triazole prophylaxis, and many of these involve azole resistance [2, 8]. Resistance in Aspergillus is primarily mediated by mutations of the cyp51A gene that encodes for lanosterol 14α-demethylase, leading to reduced azole affinity for the enzyme. Overexpression of cyp51A is also possible in Aspergillus, leading to excess production of enzyme that overwhelms the azole antifungal at therapeutic concentrations. Upregulation of ABC transport proteins that reduce the intracellular concentrations of azoles through efflux is also possible [9]. Lastly, an emerging finding in Aspergillus species is the presence of cryptic or sibling species, which have been associated with reduced susceptibility to multiple antifungals [10•].
Echinocandin Resistance
Echinocandins (micafungin, caspofungin, and anidulafungin) represent a very important class of antifungals, particularly in the treatment of invasive candidiasis [11]. Echinocandin antifungals target the 1,3-β-D-glucan synthase causing a decrease in the synthesis of 1,3-β-D-glucan, an essential component in the fungal cell wall. This mechanism is distinct from that of azole antifungals, and for this reason, the echinocandins have generally retained activity against most azole-resistant Candida species. However, resistance to echinocandins is gaining momentum over time, especially among isolates of C. glabrata [12].
The most common form of resistance to echinocandins involves mutations in the FKS1 gene of 1,3-β-D-glucan synthase. The amino acid substitutions associated with these mutations result in significantly reduced echinocandin susceptibility, and unfortunately, these mutations cause cross-resistance among all members of the echinocandin class [13]. Other Candida species have naturally occurring polymorphisms of FKS genes which cause relatively higher MICs to echinocandins. For example, C. parapsilosis has the substitution P660A and C. guilliermondii has L633M and T634A, producing inherently higher MICs to the echinocandins compared to wild-type isolates of other species, such as C. albicans and C. tropicalis [14, 15•]. However, echinocandins are still able to inhibit glucan synthase in C. parapsilosis at therapeutic concentrations. Although this is not a sufficient reason to avoid echinocandins in treating infections caused by C. parapsilosis, it may support sequencing treatment to an azole antifungal if susceptibilities support this decision [16]. Another mechanism of echinocandin resistance involves upregulated chitin production. Like glucan, chitin is a structural component of the fungal cell wall. In response to inhibition of 1,3-β-D-glucan synthase by an echinocandin and resulting decrease in synthesis of glucan, the organism upregulates production of chitin, which is associated with reduced susceptibility to echinocandins.
Polyene Resistance
The past two decades have seen continued evolution of the better-tolerated triazole and echinocandin antifungals, such that amphotericin B, the only commercially available systemic polyene, is no longer considered a primary choice for certain fungal infections, including invasive aspergillosis and invasive candidiasis. Polyene antifungals alter cell membrane permeability by binding to ergosterol and causing leakage of intracellular contents. Acquired resistance to amphotericin B is rare and resistance usually develops by selecting inherently less susceptible strains of fungi. Organisms with intrinsically reduced susceptibility to amphotericin include less common (non-fumigatus) Aspergillus species, such as A. terreus, A. flavus, and A. nidulans, Fusarium species, and Scedosporium species [9]. Resistance to amphotericin B is characterized by a reduction in the ergosterol component of the fungal cell membrane. In a subset of Candida isolates, mutational defects in ERG genes have been shown to affect the synthesis of ergosterol [17]. Resistance mechanisms in Aspergillus remain less clear [9]. Amphotericin B continues to serve as a cornerstone therapy for cryptococcal meningitis, invasive Mucorales infections, and an alternative therapy for serious fungal infections involving azole-resistant molds, including breakthrough infections occurring in immune-compromised hosts receiving mold-active triazole prophylaxis [2, 8].
Candida auris
C. auris is an emerging pathogen that holds particular relevance as a resistant fungus. C. auris has been classified as a serious global threat according to the Centers for Disease Control and Prevention (CDC) [18]. What is most concerning is its ability to harbor multiple resistance determinants and to display transmission characteristics that are similar to bacteria. In this regard, it has the potential for nosocomial spread. Most isolates of C. auris in the USA have been resistant to azole antifungals [19], making the echinocandins critically important as a treatment modality for C. auris infections. A recent report describing two clusters of echinocandin-resistant C. auris strains, some of which were pan-resistant, raises concern about this species and its ability to spread within healthcare facilities [3]. Equally as concerning is the lack of treatment options that exist for infections due to pan-resistant C. auris.
Isavuconazole
At the time it was approved by the FDA, isavuconazole was the newest antifungal to hit the market in nearly a decade. Although mechanistically similar, isavuconazole provides clinical advantages to other triazoles such as lack of QTc prolongation and more consistent oral bioavailability [20], although drug interactions with isavuconazole are similar to those of other triazoles. Isavuconazole is FDA-approved to treat invasive forms of aspergillosis and mucormycosis [20]. In guidelines, isavuconazole is recommended as an alternative regimen for aspergillosis [21] and as a first-line agent in mucormycosis [22]. The following sections describe what is known about isavuconazole as a potential treatment of fungal infections involving resistant organisms.
Isavuconazole Against Resistant Candida
In the ACTIVE trial, a large, multicenter, phase 3, randomized, double-blind, double-dummy study of isavuconazole compared to caspofungin as initial therapy for invasive candidiasis, isavuconazole did not meet non-inferiority criteria [23••]. Echinocandins remain the preferred initial therapy for invasive candidiasis in guidelines [16]. However, in both arms of the ACTIVE trial, triazoles were utilized as oral step-down, isavuconazole and voriconazole respectively. So, while isavuconazole is not recommended as initial therapy of invasive candidiasis, it may still have purpose as oral step-down therapy, especially for infections involving resistant Candida species. Isavuconazole maintains in vitro activity against Candida species, including select fluconazole-resistant Candida. In a single-center study analyzing in vitro activity against bloodstream isolates, isavuconazole demonstrated activity against C. glabrata and C. krusei that are historically fluconazole-resistant [24]. Notably, the activity of isavuconazole against C. auris has been established. Arendrup and colleagues characterized the isavuconazole MIC distribution ranging from < 0.004 to 2 mg/L with an MIC50 of 0.125 mg/L against 122 C. auris isolates using EUCAST methodology [25]. Despite this in vitro data, one study noted its trailing effect with reduced but persistent fungal growth at isavuconazole concentrations above the MIC particularly with C. glabrata, C. albicans, and C. tropicalis, suggesting the possibility of variability in isavuconazole susceptibility depending on the method utilized, i.e., isolates with trailing effect may be reported as resistant though isavuconazole is still partially active against the isolate. Studies are still needed to determine whether such trailing has an impact on clinical outcomes when isavuconazole is utilized [26•].
Sanglard and Coste evaluated the activity of isavuconazole and other azoles against Candida isolates with known resistance mechanisms [27•]. Though to a lesser degree than fluconazole and voriconazole, isavuconazole MICs were increased in the presence of CDR gene efflux transporters. However, among isolates of C. albicans and C. glabrata, expression of the MDR1 transporter had no effect on isavuconazole or posaconazole MICs, unlike fluconazole and voriconazole. Isolates with multiple ERG11 mutations displayed 4- to 32-fold relative increases in isavuconazole MICs [27•]. Based on these findings, clinicians are advised to determine the isavuconazole MIC to guide decisions regarding use of isavuconazole as oral step-down in the treatment of invasive candidiasis caused by a fluconazole-resistant Candida species.
Others have performed in vitro studies of antifungal combinations with isavuconazole and an echinocandin against isolates of C. auris. These investigators discovered synergy in many of the isolates tested, suggesting that combination therapy with isavuconazole and an echinocandin is a treatment option to consider for pan-resistant C. auris [28, 29]. The combination of isavuconazole and an echinocandin has been studied in vitro against other Candida species, and similar results were demonstrated [30]. However, the threat of pan-resistance in these species is less than that of C. auris. Despite in vitro data demonstrating antagonistic activity with amphotericin B and isavuconazole combination therapy in C. glabrata isolates, one case report described successful combination therapy with liposomal amphotericin B (LAmB) and isavuconazole in a liver transplant patient with invasive C. glabrata infection [31]. The patient maintained persistent candidemia with each individual therapy as well as caspofungin monotherapy, but the antifungal combination successfully cleared blood cultures and stabilized the infection.
Isavuconazole Against Resistant Molds
Isavuconazole demonstrated non-inferiority to voriconazole in the SECURE trial leading to FDA approval for treatment of invasive aspergillosis [32]. In general, isavuconazole and voriconazole MICs among isolates of A. fumigatus have strong correlation, suggesting that clinicians can feel confident in using voriconazole as a surrogate for susceptibility to isavuconazole [33•]. Outside of Mucorales, there are a couple of exceptions for which isavuconazole is more active than voriconazole. These include A. lentulus and N. udagawae, both of which have lower MICs to isavuconazole compared with voriconazole [34]. Posaconazole MICs are not as well correlated with isavuconazole as some isolates with an increased isavuconazole MIC retain a relatively lower posaconazole MIC [33•].
As with other triazoles, the activity of isavuconazole against Aspergillus species is affected by mutations in the cyp51A gene that encodes for lanosterol 14α-demethylase, particularly in those isolates with multiple gene alterations [35–37]. For this reason, isavuconazole is not a viable option to treat invasive infection caused by voriconazole-resistant Aspergillus, unless the isavuconazole MIC is known to be within the wild-type range, generally ≤ 1 mg/L [38]. Although the recommended isavuconazole regimen includes fixed-dose loading and maintenance doses, there are evolving data to suggest potential benefit in adjusting (increasing) the dose of isavuconazole to overcome relatively higher MICs of resistant strains [33•, 39, 40]. Recommended dosing of isavuconazole is expected to produce adequate drug exposure for wild-type strains of Aspergillus [40], and so therapeutic drug monitoring of isavuconazole is not currently recommended. As more studies are performed to define the lower and upper limits of isavuconazole concentrations that optimize efficacy and safety, an approach to increase isavuconazole doses to overcome higher MICs may be employed in the future. Although combination therapy with isavuconazole and an echinocandin has demonstrated the potential for synergy against azole-resistant Candida, results of combination studies targeting Aspergillus have been variable [41, 42].
Mucormycosis is a severe infection with limited treatment options and intrinsic resistance to multiple antifungals. Historically, lipid formulations of amphotericin B have been considered preferred treatment options. Among the triazole antifungals, both isavuconazole and posaconazole have microbiologic activity against Mucorales. The efficacy of isavuconazole in mucormycosis was demonstrated in the VITAL trial, a matched case–control analysis that compared patients on isavuconazole to those in the FungiScope registry on amphotericin B [43••]. Outcomes were similar between the isavuconazole and amphotericin B groups. Of the 37 patients who received isavuconazole, only 1 (3%) experienced disease progression. Results of this trial led to FDA approval of isavuconazole for the treatment of invasive mucormycosis.
In practice, isavuconazole represents a primary treatment option for invasive mucormycosis, especially for patients at high risk for amphotericin-related adverse effects, or as step-down oral therapy after initial treatment with amphotericin B. While evidence from the VITAL trial supports isavuconazole for mucormycosis, not all Mucorales species are inhibited by isavuconazole at therapeutic concentrations. Two studies have been performed to characterize the in vitro activity of isavuconazole against Mucorales [44, 45]. A consistent pattern emerged from results of these studies. Isavuconazole MICs against the Lichtheimia, Rhizomucor, and Rhizopus species tested were similar to wild-type Aspergillus species. However, MICs were higher against Mucor circinelloides, raising concern as to how effective isavuconazole would be in treating infections caused by this member of the Mucorales order. According to the supplementary data of the VITAL trial, there was only one isolate of M. circinelloides (isavuconazole MIC = 32 µ/mL) among the 22 isolates that underwent susceptibility testing. Rhizopus and Rhizomucor species accounted for 17 of the isolates. Notably, posaconazole MICs against many of the Mucorales isolates were generally lower by 2–3 dilutions, including M. circinelloides.
Ibrexafungerp
With increasing resistance of fungi to the currently available antifungals and the threat of emerging pathogens like C. auris, there is an urgent need for new agents that will remain active in the face of the resistance mechanisms noted previously. One promising new antifungal agent, ibrexafungerp, gained its FDA approval in January 2021 for the treatment of vulvovaginal candidiasis. Ibrexafungerp is a triterpenoid antifungal that produces fungicidal activity in Candida by inhibiting 1,3-β-D-glucan synthase, similarly to the mechanism of action of echinocandins. Unlike the echinocandins, ibrexafungerp is orally bioavailable, approximately 50% in animal studies, making it the only oral glucan synthase inhibitor [46, 47].
Ibrexafungerp Against Resistant Candida
An initial study of ibrexafungerp demonstrated in vitro activity and efficacy against Candida species in murine models, including fluconazole-resistant strains [48]. Antifungal activity was also observed in 958 Candida isolates from blood cultures at a hospital in Spain. In this study, high rates of in vitro activity (99–100%) were demonstrated for the most common Candida species that cause disease in humans, including C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. krusei. Among isolates with a FKS mutation, all but one retained an ibrexafungerp MIC within the wild-type range, as did all fluconazole-resistant isolates [49]. These data suggest that ibrexafungerp is not affected by the resistance mechanisms that limit echinocandins, despite similar mechanisms of action [50, 51•]. However, other investigators have discovered certain FKS mutations that result in substantially increased ibrexafungerp MICs [52]. Lastly, ibrexafungerp has activity against C. auris, including isolates that are both azole and echinocandin resistant, making it an attractive choice for infections caused by pan-resistant C. auris [24, 53•].
Due to its aforementioned wide spectrum of in vitro activity, ibrexafungerp has become an attractive agent to investigate as treatment of various fungal infections, including those involving drug-resistant fungi. Currently, there are multiple studies focusing on evaluating ibrexafungerp. The FURI study is a multicenter, open-label clinical trial evaluating the efficacy and safety of ibrexafungerp in patients with a wide variety of fungal infections that are refractory or intolerant to standard of care antifungal treatment. Infections evaluated in FURI include invasive or cutaneous candidiasis, endemic mycoses, and different forms of aspergillosis. Outcome assessments include global response, recurrence of the baseline infection, and survival at days 42 and 84. An interim analysis was recently released summarizing the data of the initial 74 patients. Ibrexafungerp thus far has demonstrated a favorable clinical response in FURI with 62.1% of participants showing complete or partial response to therapy, 24.3% achieving stable disease, and 6.8% with progressive disease. Additionally, a subset analysis was performed for candidemia, intra-abdominal infections, Candida bone and joint infections, and oropharyngeal infections. For these diseases, a complete or partial response occurred in 72.7%, 58.3%, 62.5%, and 64.3% of cases, respectively [54]. Lastly, the CARES study is an ongoing multicenter, open-label, non-comparator, single-arm trial evaluating the use of ibrexafungerp in patients with infections caused by Candida auris. Participants will be assessed for efficacy by global success at the end of treatment with secondary outcomes aimed at evaluating adverse events.
Ibrexafungerp Against Resistant Molds
Ibrexafungerp has demonstrated fungistatic activity in vitro against a variety of Aspergillus species. An analysis of 71 isolates of four different Aspergillus species (A. flavus, A. fumigatus, A. niger, A. terreus) reported excellent in vitro activity against both wild-type and itraconazole-resistant isolates [55]. Additional studies have confirmed reliable activity against Aspergillus, including both cryptic species and CYP51A mutants, but its activity is variable against A. ustus complex and unreliable against A. alliaceus [56•]. With regard to non-Aspergillus molds, ibrexafungerp lacks microbiologic activity against Mucorales, Fusarium species, and Purpureocillium lilacinum, and it has marginal activity against Scedosporium apiospermum, S. prolificans, and Scopulariopsis species. Ibrexafungerp maintains potent activity against Paecilomyces variotii [57].
The SCYNERGIA study is a multicenter, randomized, double-blinded clinical trial evaluating the use of ibrexafungerp in combination with voriconazole in patients with invasive pulmonary aspergillosis. With an estimated enrollment of 60 participants, the primary outcome measures are based in safety, but secondary outcomes include a composite of the clinical, radiographical, and mycological response as well as mortality. The study’s design to utilize combination antifungal therapy is noteworthy. The evidence to support combination antifungal therapy is not robust. Nonetheless, there is room to improve on the efficacy of triazole monotherapy for invasive aspergillosis, and combination therapy with a drug like ibrexafungerp may help in this regard. The nature of all these studies evaluating ibrexafungerp implies potential for broadening its indications in the future. Table 1 provides a summary of key information about ibrexafungerp and other antifungals in the pipeline, as discussed in the following section.
Table 1.
Antifungals in the pipeline
| Antifungal agent | Class | Mechanism of action (novel*) | Target fungi | Stage of development | Advantages | Anticipated place in therapy |
|---|---|---|---|---|---|---|
|
Fosmanogepix (APX001) |
Gwt1 inhibitor | Inhibits fungal enzyme Gwt1* (mannoproteins) |
Candida spp. (not C. krusei) C. auris Cryptococcus Aspergillus spp. Fusarium spp. Scedosporium spp. L. prolificans |
Phase 2 | Active against resistant Candida spp.; broad mold activity (not Mucorales); encouraging CNS penetration | Candidiasis and IA, including treatment of azole- and echinocandin-resistant infections; cryptococcal meningitis; invasive mold infections other than Mucorales |
| Ibrexafungerp | Triterpenoid | Inhibits 1,3-beta-D-glucan synthase |
Candida spp. Aspergillus spp. |
FDA-approved (VVC) | Oral formulation; active against resistant Candida spp. | Treatment of candidiasis among patients with echinocandin-resistant Candida or when oral therapy is preferred for azole-resistant candidiasis; potential role in combination therapy of IA |
|
Olorofim (F901318) |
Orotomide | Inhibits dihydroorotate dehydrogenase* |
Aspergillus spp. Scedosporium spp. L. prolificans Endemic fungi |
Phase 2b | Limited toxicity; IV and oral formulation | IA and other mold infections with limited treatment options |
|
Opelconazole (PC945) |
Inhaled triazole | Inhibits 14-alpha demethylase | Aspergillus spp. | Phase 2b | Inhaled route avoids systemic toxicity | Antifungal prophylaxis in patients with lung transplant or cystic fibrosis; IA as combination therapy with systemic triazole |
|
Oteseconazole (VT-1161) |
Tetrazole | Inhibits 14-alpha demethylase | Candida spp. | FDA-approved (VVC) | Improved selectivity for fungal CYP450 (lower potential for toxicity and drug interactions); lower rates of recurrent VVC compared with fluconazole | Treatment of VVC among patients with history of multiple recurrences |
|
Rezafungin (CD101) |
Echinocandin | Inhibits 1,3-beta-D-glucan synthase |
Candida spp. C. auris P. jiroveci Aspergillus spp. |
Phase 3 | Single or weekly IV dosing; optimized pharmacokinetic-pharmacodynamic profile | Treatment of candidiasis, particularly when single/weekly dosing improves convenience of care; prophylaxis in immunocompromised patients |
CNS central nervous system, IA invasive aspergillosis, IV intravenous, VVC vulvovaginal candidiasis
Antifungals in the Pipeline
Rezafungin, (CD101) a novel echinocandin, is a promising medication in the antifungal pipeline both for its unique dosing strategy and its potential utility against echinocandin-resistant isolates. The addition of a choline moiety to an otherwise similar echinocandin structure allows for both prolonged half-life (133 h) and improved in vitro activity due to its higher affinity for 1,3-beta-D-glucan synthase [58•]. Most Candida species, including C. auris, have rezafungin MICs that are readily achievable with proposed dosing; however, C. parapsilosis is the least susceptible species with wild-type MICs ranging up to 4 mg/L, owing to the previously described FKS polymorphisms common among C. parapsilosis [59]. Likewise, FKS mutations result in increased rezafungin MICs in a similar pattern as demonstrated with other echinocandins [60]. The probability of pharmacokinetic-pharmacodynamic target attainment was analyzed in a study of C. albicans and C. glabrata [61]. Single-dose (400 mg) and weekly dosing (400 mg followed by 200 mg weekly for 5 weeks) of rezafungin were studied. Both regimens performed well against isolates with wild-type MICs, achieving ≥ 90% probability of target attainment. However, against isolates with higher rezafungin MICs, such as those of isolates with FKS mutations, the weekly dosing regimen was best as it was still able to maintain probability of target attainment of ≥ 90% throughout the 6-week duration of dosing [61].
In human trials, a phase 2 study called STRIVE was performed to compare the two rezafungin dosing regimens listed above with caspofungin as treatment of invasive candidiasis. Overall cure (resolution of signs of infection plus mycological eradication) and all-cause mortality were similar across the three groups [62]. The ReSTORE trial is an ongoing phase 3 extension of the STRIVE study comparing weekly rezafungin to daily caspofungin as treatment of invasive candidiasis. Topline results of the ReSTORE trial were presented in April 2022. Non-inferiority was demonstrated with regard to mortality at day 30 and global cure at day 14. Lastly, rezafungin also demonstrates microbiologic activity against Pneumocystis jiroveci and is currently being studied as prophylaxis in recipients of a bone marrow transplant. In this ongoing phase 3 trial called ReSPECT, weekly rezafungin is compared to the combination of sulfamethoxazole/trimethoprim and a triazole antifungal, and participants are assessed for invasive fungal infections, including PJP, Aspergillus species, and Candida species.
Oteseconazole (VT-1161) is an oral tetrazole antifungal that targets lanosterol 14α-demethylase similarly to triazoles; however, its unique structure allows for increased affinity for CYP51 and improved selectivity for fungal CYP51 as opposed to human CYP450 enzymes, which may confer an improved safety and drug interaction profile compared with triazoles [63]. Oteseconazole demonstrates potent in vitro activity against the inherently azole-resistant Candida species C. krusei and C. glabrata, including isolates expressing FKS mutations [64, 65•]. Despite this, investigators have described reduced oteseconazole susceptibility in vitro among isolates expressing common forms of azole resistance, such as efflux pumps and ERG11 mutations [66]. More research is needed to determine whether these resistance mechanisms confer MICs above the concentrations achieved in humans with proposed dosing of oteseconazole.
Safety and efficacy of oteseconazole were demonstrated in a phase 2 trial comparing oteseconazole to fluconazole for acute vulvovaginal candidiasis (VVC) [65•]. By 6 months of follow-up, none of the patients who received oteseconazole experienced mycological recurrence compared to 46.1% of patients who received fluconazole. More recently, initial results of the phase 3 ultraViolet trial were presented [67]. In this study, oteseconazole was compared to fluconazole in the treatment of acute VVC among subjects with recurrent VVC. Oteseconazole achieved similar rates of VVC resolution (93.2% vs 95.8%), but the rate of recurrence by week 50 was significantly lower with oteseconazole (5.1% vs 42.2%). Breaking at the time of this writing (May 2022) is news that oteseconazole was approved by the FDA for the treatment of recurrent yeast infections in females who are not of reproductive potential [68].
Olorofim (F901318), the first agent in a novel class called orotomides, interrupts pyrimidine synthesis by inhibiting the enzyme dihydroorotate dehydrogenase, making it distinct in mechanism from all other currently available antifungals. In vitro studies have been performed to establish the spectrum of activity of olorofim. It lacks activity against Candida, Cryptococcus, and Mucorales. The activity of olorofim against Fusarium is variable, with some species, particularly F. solani, demonstrating relatively higher MICs [69, 70]. Where olorofim holds the most promise is against Aspergillus. Olorofim demonstrates potency against most Aspergillus species, including cryptic species, and appears to be unaffected by azole resistance [70, 71]. In addition, molds for which therapeutic options have been limited, such as Lomentospora prolificans and Scedosporium species, are generally inhibited by olorofim at relatively low MICs [72]. The microbiologic activity against these molds was confirmed in a study demonstrating efficacy of olorofim in treating a mouse model of infection [73]. Lastly, olorofim is reported to have activity against endemic fungi, including Histoplasma, Blastomyces, and Coccidioides.
Although there is limited human data with olorofim, phase I studies demonstrated its tolerability among healthy participants with no serious adverse events reported [74, 75]. Two notable clinical trials are underway to evaluate the safety and efficacy of olorofim. The FORMULA-OLS trial is a phase 2b study to evaluate its use for invasive fungal infections for patients without suitable alternatives, and the OASIS trial is a phase 3 study comparing olorofim to liposomal amphotericin B for invasive aspergillosis. Olorofim gained “Breakthrough Therapy” designation from the FDA for two fungal infections where treatment options are limited, including central nervous system coccidioidomycosis and invasive aspergillosis, as well as “Qualified Infectious Disease Product” designation for multiple fungal infections.
Fosmanogepix (APX001) is a first-in-class prodrug for the active compound manogepix (APX001A). Manogepix targets the fungal enzyme Gwt1, which is responsible for anchoring mannoproteins to the fungal cell wall. These mannoproteins facilitate adherence of the fungus to mucosal and epithelial cell surfaces in the host as part of the process for establishing infection. Manogepix has broad activity against both yeasts (Candida and Cryptococcus) and molds, including azole-resistant A. fumigatus, Fusarium species, Scedosporium species, and L. prolificans. Manogepix lacks activity against C. krusei and some Mucorales. Wild-type MICs for manogepix have been observed among Candida isolates demonstrating fluconazole resistance, but overall, fluconazole resistance is associated with relatively higher manogepix MICs, suggesting utility of manogepix susceptibility testing when considering fosmanogepix for treatment of fluconazole-resistant Candida infections [76]. Manogepix retains activity against C. auris, including multidrug-resistant isolates [77•]. Likewise, manogepix MICs of C. glabrata are generally unaffected by echinocandin resistance [78].
In a small open-label, non-comparative phase 2 study of 21 non-neutropenic patients with candidemia, fosmanogepix was successful in 80% of patients, and none of the patients experienced treatment-related serious adverse events or discontinuations [79]. The efficacy of fosmanogepix has been demonstrated in immunosuppressed murine models of pulmonary scedosporiosis and disseminated fusariosis [80]. Notably, a rabbit model study documented encouraging penetration of manogepix into cerebrospinal fluid and aqueous/vitreous humor, providing support for future studies of cryptococcal meningitis and Candida endophthalmitis [81]. Fosmanogepix is currently undergoing a phase 2 trial for patients with invasive aspergillosis. A trial evaluating its use for infections caused by C. auris was terminated early due to the COVID-19 pandemic.
Opelconazole (PC945) is a novel, long-acting, inhaled (nebulized) triazole antifungal designed to avoid systemic toxicity and to maximize drug concentration in the lungs. The spectrum of activity of opelconazole includes Candida species, including C. auris and A. fumigatus [82, 83]. Opelconazole is a promising agent as directed therapy or prophylaxis of fungal infections of the lungs, but it is not likely to have a role in the neutropenic host, either as treatment or prophylaxis, unless it is combined with a systemic antifungal. Tolerability of opelconazole has been demonstrated in a small study of healthy volunteers and subjects with mild asthma [84]. No episodes of bronchospasm or clinically significant changes in lung function occurred in association with opelconazole administration. Early clinical trials to evaluate opelconazole focused on unique patient populations, such as lung transplant recipients and patients with cystic fibrosis. However, some of these were terminated early due to the COVID-19 pandemic. A phase 3 trial of inhaled opelconazole when added to systemic antifungal therapy for treatment of refractory invasive pulmonary aspergillosis is planned. Proof of concept for this treatment modality was established in an in vitro model where synergy between inhaled opelconazole and systemic posaconazole or voriconazole was demonstrated [85].
Conclusions
With advances in medical practices and changing epidemiology of fungal infections, there is increasing need for expansion in antifungal therapy options. Making therapeutic decisions based on antifungal class assumptions or mechanism of action is no longer a reliable strategy, particularly when resistance to one or more agents is present or when the identified fungal species is less common. In this review, mechanisms of antifungal resistance are described alongside implications for antifungal activity. In vitro and clinical data for the two newest antifungal options, isavuconazole and ibrexafungerp, are examined, and their roles against resistant fungi are assessed. The most promising or unique antifungal agents under development are evaluated for their potential utility in the setting of antifungal resistance. As our understanding of antifungal resistance and the pace of adopting new antifungal therapies continue to grow, clinicians will be challenged by greater complexity in how fungal infections are managed. This review provides updated knowledge and awareness in this regard.
Compliance of Ethical Standards
Conflict of Interest
All authors declare no competing or financial interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Antimicrobial Development and Drug Resistance
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ashley Logan and Amanda Wolfe contributed equally in authorship
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Seagle EE, Williams SL, Chiller TM. Recent trends in the epidemiology of fungal infections. Infect Dis Clin N Am. 2021;35:237–260. doi: 10.1016/j.idc.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamoth F, Chung SJ, Damonti L, Alexander BD. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin Infect Dis. 2017;64:1619–1621. doi: 10.1093/cid/cix130. [DOI] [PubMed] [Google Scholar]
- 3.Lyman M, Forsberg K, Reuben J, et al. Transmission of pan-resistant and echinocandin-resistant Candida auris in health care facilities – Texas and the District of Columbia, January-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1022–1023. doi: 10.15585/mmwr.mm7029a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ, Turnridge, et al. Twenty years of SENTRY Antifungal Surveillance Program: results for Candida species from 1997 – 2016. Open Forum Infect Dis. 2019;15:S79-S94. [DOI] [PMC free article] [PubMed]
- 5.Ramage G. Rajendran, Sherry L, Williams C. Fungal biofilm resistance Int J Microbiol. 2012;2012:528521. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8:1325–1337. doi: 10.2217/fmb.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nett JE, Crawford K, Marchillo K, Andes DR. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob Agents Chemother. 2010;54:3505–3508. doi: 10.1128/AAC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lionakis MS, Lewis RE, Kontoyiannis DP. Breakthrough invasive mold infections in the hematology patient: current concepts and future directions. Clin Infect Dis. 2018;67:1621–1630. doi: 10.1093/cid/ciy473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect. 2017;17:e383–e392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 10.• Alastruey-Izquierdo A, Mellado E, Pelaez T, et al. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP Study). Antimicrob Ag Chemother. 2013;57:3380–7. This study examined the prevalence of cryptic/sibling Aspergillus species throughout 29 different centers in Spain and discussed the higher levels of resistance these species may possess. [DOI] [PMC free article] [PubMed]
- 11.Gold JAW, Seagle EE, Nadle J, et al. Treatment practices for adults with candidemia at 9 active surveillance sites – United States, 2017–2018. Clin Infect Dis. 2021;73:1609–1616. doi: 10.1093/cid/ciab512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farmakiotis D, Tarrand JJ, Kontoyiannis DP. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis. 2014;20:1833–1840. doi: 10.3201/eid2011.140685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlin DS. Mechanisms of echinocandin antifungal drug resistance. Ann N Y Acad Sci. 2015;1354:1–11. doi: 10.1111/nyas.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudiuk C, Macedo D, Leonardelli F, et al. Molecular confirmation of the relationship between Candida guilliermondii Fks1p naturally occurring amino acid substitutions and its intrinsic reduced echinocandin susceptibility. Antimicrob Ag Chemother. 2017;61:e02644–e2716. doi: 10.1128/AAC.02644-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.• Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Ag Chemother. 2008;52:2305–12. This study identified the genetic polymorphisms responsible for the inherent reduction in susceptibility of Candida parapsilosis to echinocandins. [DOI] [PMC free article] [PubMed]
- 16.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hull CM, Bader O, Parker JE, et al. Two clinical isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B both harbor mutations in ERG2. Antimicrob Agents Chemother. 2012;56:6417–6421. doi: 10.1128/AAC.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Candida auris. Accessed March 21, 2022. Available at: https://www.cdc.gov/fungal/candida-auris/index.html.
- 19.Forsberg K, Woodworth K, Walters M, et al. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol. 2019;57:1–12. doi: 10.1093/mmy/myy054. [DOI] [PubMed] [Google Scholar]
- 20.Isavuconazole [package insert]. Northbrook, IL: Astellas Pharma US, Inc.; 2015.
- 21.Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guidance for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.•• Kullberg BJ, Viscoli C, Pappas PG, et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: the ACTIVE trial. Clin Infect Dis. 2019;36:1981–9. This phase 3, randomized, double-blind trial failed to demonstrate non-inferiority of isavuconazole compared with caspofungin followed by oral voriconazole in the treatment of candidemia. [DOI] [PubMed]
- 24.Seifert H, Aurbauch U, Stefanik D, Cornely O. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob Ag Chemother. 2007;51:1818–1821. doi: 10.1128/AAC.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arendrup MC, Jorgensen KM, Hare RK, Cowdhary A. In vitro activity of ibrexafungerp (SCY-078) against Candida auris isolates as determined by EUCAST methodology and comparison with activity against C. albicans and C. glabrata and with the activities of six comparator agents. Antimicrob Ag Chemother 2020;64:e02136–19. [DOI] [PMC free article] [PubMed]
- 26.• Marco-Zambrano LJ, Gomez A, Sanchez-Carrillo C, et al. Isavuconazole is highly active in vitro against Candida species isolates but shows trailing effect. Clin Microbiol Infect. 2018;24:1343.e1–4. This study highlights the potential trailing effect of isavuconazole against certain Candida species. This in vitro data has not been correlated with clinical outcomes at this time. [DOI] [PubMed]
- 27.• Sanglard D, Coste AT. Activity of isavuconazole and other azoles against Candida clinical isolates and yeast model systems with known azole resistance mechanisms. Antimicrob Ag Chemother. 2016;60:229–38. This is an excellent resource regarding isavuconazole’s in vitro activity as compared to other triazoles against resistant Candida species. [DOI] [PMC free article] [PubMed]
- 28.Nagy F, Toth Z, Nyikos F, et al. In vitro and in vivo interaction of caspofungin with isavuconazole against Candida auris planktonic cells and biofilms. Med Mycol. 2021;59:1015–1023. doi: 10.1093/mmy/myab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caballero U, Kim S, Eraso E, et al. In vitro synergistic interactions of isavuconazole and echinocandins against Candida auris. Antibiotics. 2021;10:355. doi: 10.3390/antibiotics10040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katragkou A, McCarthy M, Meletiadis J, et al. In vitro combination therapy with isavuconazole against Candida spp. Med Mycol. 2017;55:859–868. doi: 10.1093/mmy/myx006. [DOI] [PubMed] [Google Scholar]
- 31.Odysseos G, Mayr U, Bozsaki G, et al. Isavuconazole and liposomal amphotericin b as successful combination therapy of refractory invasive candidiasis in a liver transplant recipient: a case report and literature review. Mycopathologia. 2022;187:113–120. doi: 10.1007/s11046-021-00599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 33.• Buil JB, Bruggemann RJM, Wasmann RE, et al. Isavuconazole susceptibility of clinical Aspergillus fumigatus isolates and feasibility of isavuconazole dose escalation to treat isolates with elevated MICs. J Antimicrob Chemother. 2018;73:134–142. This study reviews the potential correlation or lack thereof between isavuconazole MICs and other triazole MICs in Aspergillus fumigatus isolates. It also demonstrates the possibility of using high-dose isavuconazole for Aspergillus species with elevated MICs.
- 34.Datta K, Rhee P, Byrnes E, et al. Isavuconazole activity against Aspergillus lentulus, Neosartorya udagawae, and Cryptococcus gattii, emerging fungal pathogens with reduced azole susceptibility. J Clin Microbiol. 2013;51:3090–3093. doi: 10.1128/JCM.01190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard SJ, Lass-Florl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob Ag Chemother. 2013;57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castanheira M, Collingsworth TD, Davis AP, Deshpande LM, Pfaller MA. Isavuconazole nonwildtype Aspergillus fumigatus isolates from a global surveillance study display alterations in multiple genes involved in the ergosterol biosynthesis pathway not previously associated with resistance to other azoles. Mycoses. 2021;64:1279–1290. doi: 10.1111/myc.13267. [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen KM, Astvad KMT, Hare RK, Arendrup MC. EUCAST susceptibility testing of isavuconazole: MIC data for contemporary clinical mold and yeast isolates. Antimicrob Ag Chemother. 2019;63:e00073–e119. doi: 10.1128/AAC.00073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arendrup MC, Meletiadis J, Mouton JW, et al. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect. 2016;22:571.e1–571.e4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Seyedmousavi S, Bruggemann RJM, Meis JF, Melchers JG, Verweij PE, Mouton JW. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrbo Agents Chemother. 2015;59:2855–2866. doi: 10.1128/AAC.04907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Box H, Livermore J, Johnson A, et al. Pharmacodynamics of isavuconazole in a dynamic in vitro model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2016;60:278–287. doi: 10.1128/AAC.01364-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buil JB, Bruggemann RJM, Denardi LB, Melchers WJG, Verweij PE. In vitro interaction of isavuconazole and anidulafungin against azole-susceptible and azole-resistant Aspergillus fumigatus. J Antimicrob Chemother. 2020;75:2592–2586. doi: 10.1093/jac/dkaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffetin A, Courbin V, Jullien V, Dannaoui E. In vitro combination of isavuconazole with echinocandins against azole-susceptible and -resistant Aspergillus spp. Antimicrob Agents Chemothe. 2018;62:e01382–e1417. doi: 10.1128/AAC.01382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.•• Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–37. The VITAL trial provided evidence to both support the use of isavuconazole and lead to FDA approval of isavuconazole in invasive mucormycosis. [DOI] [PubMed]
- 44.Pfaller MA, Rhomberg PR, Wiederhold NP, et al. In vitro activity of isavuconazole against opportunistic fungal pathogens from two mycology reference laboratories. Antimicrob Agents Chemother. 2018;62:e01230–e1318. doi: 10.1128/AAC.01230-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arendrup MC, Jensen RH, Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucroales order. Antimicrob Agents Chemother. 2015;59:7735–7742. doi: 10.1128/AAC.01919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brexafemme®. Package insert. Scynexis, Inc; 2021.
- 47.McCarthy WM. Pharmacokinetics and pharmacodynamics of ibrexafungerp. Drugs R D. 2022;22:9–13. doi: 10.1007/s40268-021-00376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker SS, Xu Y, Triantafyllou I, et al. Discovery of a novel class of orally active antifungal beta-1,3-D-glucan synthase inhibitors. Antimicrob Agents Chemother. 2011;55:5099–5106. doi: 10.1128/AAC.00432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mesquida A, Díaz-García J, Sánchez-Carrillo C, et al. In vitro activity of ibrexafungerp against Candida species isolated from blood cultures. Determination of wild-type populations using the EUCAST method. Clin Microbiol Infect. 2022;28:140.e1–140.e4. [DOI] [PubMed]
- 50.Jiménez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother. 2014;58:1248–1251. doi: 10.1128/AAC.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.• Nunnally NS, Etienna KA, Angulo D, Lockhart SR, Berkow EL. In vitro activity of ibrexafungerp, a novel glucan synthase inhibitor against Candida glabrata isolates with FKS mutations. Antimicrob Agents Chemother. 2019;63:e01692–19. This study demonstrated microbiologic activity of ibrexafungerp against Candida glabrata with resistance to echinocandins and showed the FKS mutations that cause echinocandin resistance likely do not have the same effect on ibrexafungerp, despite similar mechanisms of action. [DOI] [PMC free article] [PubMed]
- 52.Jimenez-Ortigosa C, Perez WB, Angulo D, Borroto-Esoda K, Perlin DS. De novo acquisition of resistance to SCY-078 in Candida glabrata involves FKS mutations that both overlap and are distinct from those conferring echinocandin resistance. Antimicrob Agents Chemother. 2017;61:e00833–e917. doi: 10.1128/AAC.00833-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.• Wiederhold NP, Najvar LK, Olivo M, et al. Ibrexafungerp demonstrates in vitro activity against fluconazole-resistant Candida auris and in vivo efficacy with delayed initiation of therapy in an experimental model of invasive candidiasis. Antimicrob Agents Chemother. 2021;65:e02694–20. This study shows the activity of ibrexafungerp against fluconazole-resistant Candida auris, an emerging and concerning pathogen with the potential for multidrug resistance. [DOI] [PMC free article] [PubMed]
- 54.Pappas PG. Oral ibrexafungerp outcomes by fungal disease in patients from an interim analysis of a phase 3 open-label study (FURI). Presented at: IDWeek 2021; September 29, 2021; virtual.
- 55.Pfaller MA, Messer SA, Motyl MR, et al. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother. 2013;57:1065–8. [DOI] [PMC free article] [PubMed]
- 56.• Rivero-Mendez O, Soto-Debran JC, Cuence-Estrella M, Alastruey-Izquierdo A. In vitro activity of ibrexafungerp against a collection of clinical isolates of Aspergillus, including cryptic species and Cyp51A mutants, using EUCAST and CLSI methodologies. J Fungi. 2021;7:232. This is an in vitro analysis demonstrating the activity of ibrexafungerp on various Aspergillus isolates, including those with resistance to azole antifungals. [DOI] [PMC free article] [PubMed]
- 57.Lamoth F, Alexander BD. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother. 2015;59:4308–4311. doi: 10.1128/AAC.00234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.• Toth Z, Forgacs L, Locke JB, et al. In vitro activity of rezafungin against common and rare Candida species and Saccharomyces cerevisiae. J Antimicrob Chemother. 2019;74:3505–10. The article provides detailed information regarding the activity of rezafungin against multiple Candida species. [DOI] [PMC free article] [PubMed]
- 59.Arendrup MC, Meletiadis J, Zaragoza O, et al. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin Microbiol Infect. 2018;24:1200–1204. doi: 10.1016/j.cmi.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother. 2016;71:2868–73. [DOI] [PMC free article] [PubMed]
- 61.Bader JC, Laokta EA, Flanagan S, et al. Overcoming the resistance hurdle: pharmacokinetic-pharmacodynamic target attainment analyses for rezafungin (CD101) against Candia albicans and Candida glabrata. Antimicrob Ag Chemother. 2018;62:e02614–e2617. doi: 10.1128/AAC.02614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson GR, Sariano A, Skoutelis A, et al. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis. 2021;73:e3647–e3655. doi: 10.1093/cid/ciaa1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453–1461. doi: 10.2217/fmb-2021-0173. [DOI] [PubMed] [Google Scholar]
- 64.Schell WA, Jones AM, Garvey EP, Hoekstra WJ, Scotzinger RJ, Alexander BD. Fungal CYP51 inhibitors VT-1161 and VT-1129 exhibit strong in vitro activity against Candida glabrata and C. krusei isolates clinically resistant to azole and echinocandin antifungal compounds. Antimicrob Agents Chemother. 2017;61:e01817–6. [DOI] [PMC free article] [PubMed]
- 65.• Brand SR, Sobel JD, Nyirjesy P, Ghannoum MA, Schotzinger RJ, Degenhard TP. A randomized phase 2 study of VT-1161 for the treatment of acute vulvovaginal candidiasis. Clin Infect Dis. 2021;73:e1518–24. This manuscript describes favorable safety and efficacy results for a phase 2 study of oteseconazole compared to fluconazole for VVC. [DOI] [PMC free article] [PubMed]
- 66.Monk BC, Keniya MV, Sabherwal M, et al. Azole resistance reduces susceptibility to the tetrazole antifungal VT-1161. Antimicrob Agents Chemother. 2018;63:e02114–e2118. doi: 10.1128/AAC.02114-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martens MG, Maximos B, Degenhardt T, et al. A phase 3, randomized, double-blind study to evaluate the efficacy and safety of oteseconazole (VT-1161) oral capsules versus fluconazole and placebo in the treatment of acute vulvovaginal candidiasis episodes in subjects with recurrent vulvovaginal candidiasis (ultraViolet) Open Forum Infect Dis. 2021;8(Suppl 1):S66–S67. doi: 10.1093/ofid/ofab466.107. [DOI] [Google Scholar]
- 68.Mycovia Pharmaceuticals. FDA approves Mycovia Pharmaceuticals’ Vivjoa™ (oteseconazole), the first and only FDA-approved medication for recurrent vulvovaginal candidiasis (chronic yeast infection). Accessed May 6, 2022. Available at: www.mycovia.com/news.
- 69.Badali H, Canete-Gibas C, Patterson H, et al. In vitro activity of olorofim against clinical isolates of the Fusarium oxysporum and Fusarium solani species complexes. Mycoses. 2021;64:748–752. doi: 10.1111/myc.13273. [DOI] [PubMed] [Google Scholar]
- 70.Thyssen Astvad KM, Jorgensen KM, Hare RK, Datcu R, Arendrup MC. Olorofim susceptibility testing of 1,423 Danish mold isolates obtained in 2018–2019 confirms uniform and broad-spectrum activity. Antimicrob Agents Chemother. 2021;65:e01527–e1620. doi: 10.1128/AAC.01527-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J Antimicrob Chemother. 2019;74:1586–1590. doi: 10.1093/jac/dkz078. [DOI] [PubMed] [Google Scholar]
- 72.Wiederhold N, Law D, Birch M. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother. 2017;72:1977–1980. doi: 10.1093/jac/dkx065. [DOI] [PubMed] [Google Scholar]
- 73.Seyedmousavi S, Chang YC, Youn JH, et al. In vivo efficacy of olorofim against systemic scedosporiosis and lomentosporiosis. Antimicrob Agents Chemother. 2021;65:e00434–e521. doi: 10.1128/AAC.00434-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kennedy T, Graham A, Steiner J, et al. Multiple dose pharmacokinetics of an immediate-release tablet formulation of F901318 in healthy male and female subjects (abstract P1710). In: Proceedings of the 27th European congress of clinical microbiology and infectious diseases; 22–25 April 2017; Vienna.
- 75.Kennedy T, Graham A, Steiner J, Heep M, Birch M. Assessment of the duration of infusion on the tolerability and repeat dose pharmacokinetics of F901318 in health-volunteers (abstract P1711). In: Proceedings of the 27th European congress of clinical microbiology and infectious diseases; 22–25 April 2017; Vienna.
- 76.Arendrup MC, Chowdhary A, Astvad KMT, Jorgensen KM. APX001A in vitro activity against contemporary blood isolates and Candida auris determined by the EUCAST reference method. Antimicrob Agents Chemother. 2018;62:e01225–e1318. doi: 10.1128/AAC.01225-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.• Zhu Y, Kilburn S, Kapoor M, Chaturvedi S, Shaw KJ, Chaturvedi V. In vitro activity of manogepix against multidrug-resistant and panresistant Candida auris from the New York outbreak. Antimicrob Agents Chemother. 2020;64:e01124–20. The in vitro activity of manogepix against multidrug-resistant Candida auris isolates was established in this study. [DOI] [PMC free article] [PubMed]
- 78.Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. In vitro activity of APX001A (manogepix) and comparator agents against 1,706 fungal isolates collected during an international surveillance program in 2017. Antimicrob Agents Chemother. 2019;63:e00840–e919. doi: 10.1128/AAC.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pappas P, Kullberg BJ, Vazquez JA, et al. Clinical safety and efficacy of noval antifungal, fosmanogepix, in the treatment of candidemia: results from a phase 2 proof of concept trial. Open Forum Infect Dis. 2020;7(Suppl 1):S203–S204. doi: 10.1093/ofid/ofaa439.457. [DOI] [Google Scholar]
- 80.Alkhazraji S, Gebremariam T, Alqarihi A, et al. Fosmanogepix (APX001) is effective in the treatment of immunocompromised mice infected with invasive pulmonary scedosporiosis or disseminated fusariosis. Antimicrob Agents Chemother. 2020;64:e01735–e1819. doi: 10.1128/AAC.01735-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petraitiene R, Petraitis V, Maung BBW, et al. Efficacy and pharmacokinetics of fosmanogepix (APX001) in the treatment of Candida endophthalmitis and hematogenous meningoencephalitis in nonneutropenic rabbits. Antimicrob Agents Chemother. 2021;65:e01795–e1820. doi: 10.1128/AAC.01795-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colley T, Alanio A, Kelly SL, et al. In vitro and in vivo antifungal profile of a novel and long-acting inhaled azole, PC945, on Aspergillus fumigatus infection. Antimicrob Agents Chemother. 2017;61:e02280–e2316. doi: 10.1128/AAC.02280-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudramurthy SM, Colley T, Abdolrasouli A, et al. In vitro antifungal activity of a novel topical triazole PC945 against emerging yeast Candida auris. J Antimicrob Chemother. 2019;74:2943–2949. doi: 10.1093/jac/dkz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cass L, Murray A, Davis A, et al. Safety and nonclinical and clinical pharmacokinetics of PC945, a novel inhaled triazole antifungal agent. Pharmacol Res Perspect. 2021;9:e00690. doi: 10.1002/prp2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colley T, Sehra G, Daly L, et al. Antifungal synergy of a topical triazole, PC945, with a systemic triazole against respiratory Aspergillus fumigatus infection. Sci Rep. 2019;9:9482. doi: 10.1038/s41598-019-45890-w. [DOI] [PMC free article] [PubMed] [Google Scholar]